Abstract
Background: Recent advances in less-invasive surgery and electrode design allow for a high degree of hearing preservation (HP) after cochlear implantation (CI), although residual hearing still deteriorates in some patients. To date, the factors predictive of preserving residual hearing remain a controversial topic.
Objective: The aim of this study was to investigate the predictive factors, including the etiology of hearing loss (HL) as a patient-related factor, influencing residual HP after CI.
Methods: Forty-four patients (50 ears, 41 families) with residual acoustic hearing who underwent CI were included. Auditory thresholds before and at 6 months after initial activation were measured. Genetic testing was performed to identify the responsible genes for HL.
Results: We identified the cause of HL in 21 families (51.2%). HP was marginally correlated with age at implantation, while it was independent of pre-operative low-frequency hearing thresholds, cochlear duct length, and electrode length. We found that patients who had pathogenic variants in the CDH23, MYO7A, or MYO15A gene showed statistically better HP scores compared with patients with HL due to other causes (p = .002).
Conclusions: Identification of the etiology of HL using genetic testing is likely to facilitate the prediction of HP after implant surgery.
Chinese abstract
背景:近年来在微创手术和电极设计方面的进展使得人工耳蜗植入术(CI)后听力得到了很好的维护, 尽管有些患者的残余听力仍在恶化。到目前为止, 预测保留残余听力的因素仍然是一个有争议的话题。
目的:探讨影响CI术后残余HP的预测因素, 包括作为患者相关因素的听力损失(HL)的病因。
方法:对44例(50个耳, 41个家庭)听力残存患者行CI治疗。分别于术前和术后6个月测听阈。对HL的相关基因进行了基因检测。
结果:明确了21个家庭(51.2%)HL的病因。HP与植入时的年龄有一些的相关性, 而与术前低频听阈、耳蜗导管长度(CDL)和电极长度无关。我们发现, 在CDH23、MYO7A或MYO15A基因中有致病性变异的患者与其他原因导致的HL患者相比, 在统计学上表现出更好的HP评分(p=.002)。
结论:应用基因检测鉴定HL的病因有助于预测植入术后HP的发生。
Introduction
Cochlear implantation (CI) remains a successful treatment option for patients with severe-to-profound hearing loss (HL). In the last few decades, the indication criteria for implantation have been expanded to patients with ski slope-type HL. In such individuals who have severe-to-profound high-frequency HL with only mild-to-moderate HL in the low frequencies, hearing aids cannot offer any great benefit in terms of understanding speech. To address this issue, von Ilberg and colleagues developed the concept of electric–acoustic stimulation (EAS) providing low-frequency simulation via residual hearing together with electrical stimulation (ES) in the high frequencies by an inserted electrode in a single device [Citation1,Citation2]. The benefits of EAS in the implanted ear are well established and include improved speech recognition in noise compared with ES alone, indicating that the preservation of residual hearing in the low frequencies is important in EAS patients [Citation1,Citation2].
Hearing preservation (HP) was originally thought to be only necessary for EAS patients who could benefit from their residual hearing post-operatively. That being said, the benefits of HP surgical techniques are currently also recognized for all CI patients as the concept of HP leads to the preservation of cochlear structures, which is important in expanding future treatment strategies such as gene therapy or regeneration therapy, particularly for infants.
In terms of HP surgery, a round window approach is preferable as (1) less drilling reduces acoustic trauma, (2) it ensures insertion into the Scala tympani, and (3) preserves vestibular function [Citation3], and we first clearly demonstrated that HP can be achieved even with the electrode beneath the basilar membrane [Citation3]. In addition to the improved surgical approach, it is necessary to optimize the electrode array design. The development of thinner and super flexible straight electrode arrays can allow for round window insertion and offer better structure and HP more comfortably [Citation4]. Using such flexible atraumatic electrodes, we previously demonstrated that HP was achievable together with improved speech perception after EAS surgery [Citation5].
Despite advances in less-invasive surgical techniques and electrode design, residual hearing still deteriorates in a certain number of patients after CI. Various groups have attempted to identify the factors that impact HP, with insertion depth angle, implant age, degree of residual hearing and cochlear duct length (CDL) being identified as potentially important factors [Citation6–9]. While these reports are noteworthy, the factors predictive of preserving residual hearing remain a controversial topic. As patient-related factors, we hypothesized that the etiology of HL may be an important factor related to HP. In our series of studies, we performed comprehensive genetic testing, and reported that genetic etiogy is a major cause of HL in CI/EAS patients [Citation10 for review]. To evaluate the relative impact of the aforementioned variables, we approached this issue from a single institution approach with CI recipients having residual hearing in the low frequencies pre-operatively. Given the natural course of HL itself, we measured the low-frequency pure-tone average (PTA) at 6-month post-activation in comparison with the pre-operative PTA. Herein, we present our findings regarding the predictive factors impacting HP post-operatively in patients with residual hearing.
Materials and methods
Patients studied
Forty-four patients (50 ears, 41 families) who underwent implant surgery at the Department of Otorhinolaryngology, Shinshu University Hospital met the inclusion criteria for this study. The inclusion criteria were as follows: (1) age at implantation above 6 years old for the reliable assessment of pure-tone audiometric test; (2) FLEXsoft (31.5 mm), FLEX28 (28 mm) or FLEX24 (24 mm) electrode array implantation developed by MED-EL (Innsbruck, Austria); and (3) measurable residual hearing in the low frequency with a threshold less than 80 dBHL for the average of values at 125, 250, and 500 Hz before surgery. provides a summary of patient demographic information. The median age at implantation was 36.4 years (range 6–70 years). This study was approved by the Ethics Committee of Shinshu University School of Medicine.
Table 1. Characteristics of patients evaluated in this study (N = 44).
CI surgical procedure
Patients included 20 males and 24 females, with 6 patients receiving implantation with a FLEXsoft electrode, 17 patients with a FLEX28 electrode, and 27 patients with a FLEX24 electrode. The CI surgery was performed as described previously [Citation3]. Briefly, the round window approach was applied to insert the electrode array. The bony overhang of the round window was removed with a low-speed drill and the round window membrane was widely exposed. The electrode was fully inserted carefully and slowly via the round window membrane [Citation3]. All participants underwent surgery by the same experienced surgeon (S.U.) and received steroids intra- and post-operatively.
HP assessment
To define the extent of hearing deterioration following CI, we measured auditory thresholds before and at 6 months after initial activation. Subsequently, to assess HP rate, we used the classification provided by Skarzynski et al. and the HEARRING group [Citation11] with the HP scores (%) categorized as follows: complete HP, defined as greater than 75%; partial HP, 25–75%; minimal HP, 1–25%; and complete loss of hearing; 0%.
Genetic analysis
Genetic testing was performed for 41 families as described previously [Citation12]. In brief, Amplicon libraries were constructed using an Ion AmpliSeqTM Custom Panel (Thermo Fisher Scientific, Rockford, IL) in accordance with the manufacturer’s instructions for 68 genes reported to cause non-syndromic hereditary HL [Citation12]. Subsequently, emulsion PCR and sequencing were performed with an Ion 200 sequencing kit and Ion PGM sequencer or Ion HiQ Chef kit and Ion Proton sequencer. The sequence data were mapped on the human genome sequence (build GRCh37/hg19), and the DNA variant regions were piled up with Torrent Variant Caller plug-in software. The effects of the detected variants were subsequently analyzed using ANNOVAR software. The missense, nonsense, insertion/deletion, and splicing variants were determined, and the variants were next selected as less than 1% of (1) the 1000 genome database, (2) the 6500 exome variants, (3) the Human Genetic Variation Database (dataset for 1208 Japanese exome variants), and (4) the 333 in-house Japanese normal hearing controls. For missense variants, functional prediction software, including Sorting Intolerant from Tolerant (SIFT), Polymorphism Phenotyping (PolyPhen2), Likelihood Ratio Test (LRT), Mutation Taster, Mutation Assessor, were used to assess the pathogenicity. Direct sequencing was utilized to confirm the candidate variants identified, and segregation analysis was performed for each proband and their family members. The pathogenicity of the identified variants was evaluated based on the ACMG (American College of Medical Genetics) standards and guidelines. This system classified variants into five categories; pathogenic, likely pathogenic, uncertain significance, likely benign, and benign. Additionally, we referred to Inter Var for evaluation of the variants. A combined annotation-dependent depletion (CADD) was also used to prioritize potential causal variants.
Cochlear duct length measurement
CDL was measured by ‘OTOPLAN’ software for otological surgical planning developed by CAScination (Bern, Switzerland) in cooperation with MED-EL. The DICOM files from patient CT images were uploaded. We next highlighted a number of cochlear parameters, including the round window, the center of the cochlear apex and so on, according to the manufacturer’s instructions. As a result, the software calculated the CDL based on cochlear diameter, width, and height for each patient.
Assessment of correlation between each factor and HP
To identify factors that impact HP, all subjects were evaluated for: (1) age at implantation; (2) pre-operative low-frequency hearing thresholds at 125, 250, and 500 Hz; (3) etiology of HL using genetic testing; and (4) CDL.
Statistical analysis
Sample sizes are noted in the figure legends. Hearing threshold data are presented as mean ± standard deviation (SD). Statistical analysis was performed using the Prism 8 software package (GraphPad, San Diego, CA). Two groups were compared using the Mann–Whitney test. For comparisons of more than two groups, one-way ANOVA was performed, followed by post-hoc test with Bonferroni correction of pairwise group differences. A p value <.05 was considered statistically significant.
Results
Genetic testing facilitates the diagnosis of patients with ski slope-type hearing loss
To investigate the etiology of HL, massively parallel sequencing was performed for 41 families (44 patients) with residual acoustic hearing. We identified the cause of HL in 21 families (51.2%). Of them, 19 patients received a genetic diagnosis, with the CDH23 gene most frequently implicated, followed by ACTG1, Mit1555A > G, MYO7A, MYO15A, SLC26A4, and TMPRSS3 (). Additionally, two patients were diagnosed with otosclerosis and congenital diaphragmatic hernia ().
Figure 1. Etiology of patients with residual acoustic hearing. Orange indicates genetic causes of hearing loss; yellow, other causes; grey, unknown (n = 41).
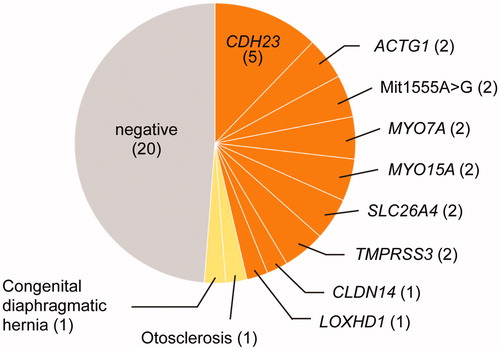
Table 2. Summary of the clinical features and information of the variants identified as likely causative in this study.
Table 3. Univariate analysis of variables and hearing preservation scale.
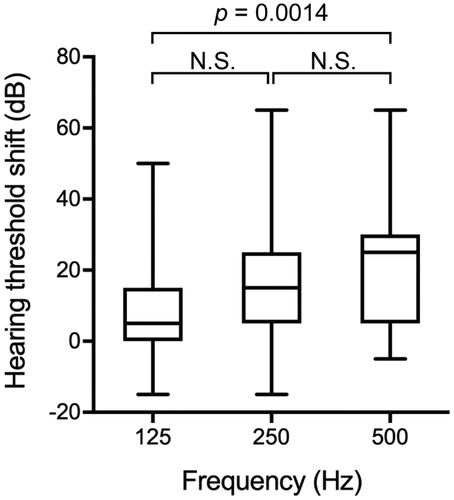
Low-frequency hearing threshold shift and hearing preservation scale
To define the extent of hearing deterioration following CI, we measured auditory thresholds before and at 6 months after activation. The average pre-operative air conduction hearing thresholds at 125, 250, and 500 Hz were 36.7 ± 17.2, 46.8 ± 20.8, and 62.6 ± 21.8 dB, respectively. The mean LF threshold shift was 9.7 dB at 125 Hz, 15.5 dB at 250 Hz, 21.0 dB at 500 Hz, indicating that the hearing deterioration was statistically significant at 500 Hz as compared to that at 125 Hz (). To calculate HP, we used the classification reported by Skarzynski et al. and the HEARRING group [Citation11]. The HP evaluated at 6 months after activation indicated that 20 of the 50 (40%) implanted ears completely maintained their preoperative hearing thresholds, followed by 19 with partial (38%), 8 with minimal (16%) and 3 with no measurable hearing (6%).
Assessment of predictive factors influencing residual hearing preservation
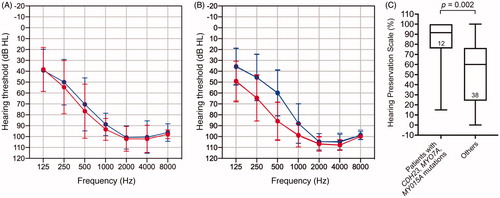
To investigate predictors that impact HP after surgery, we examined some patient-related factors (age, pre-operative LFA, CDL, and cause of HL) as well as the length of the inserted CI (FLEXsoft [31.5 mm], FLEX28 [28 mm], FLEX24 [24 mm]). We measured CDL using OTOPLAN software, and found that the mean CDL was 32.9 mm, which was independent of age. Univariate analysis of variables and the HP scale are shown in and suggest that a younger age at implantation was moderately associated with better HP scores as compared with older patients (R2=0.186), while other variables (pre-operative LFA, CDL) demonstrated negative correlations with HP scores (R2=0.013, 0.0004, respectively). Additionally, the results of one-way ANOVA demonstrated no statistically significant differences in HP among the three types of electrode array used (), indicating that HP was not dependent on inserted electrode length. As described above, eight responsible genes were found in the patients. Of them, we found that patients who had pathogenic variants in the CDH23, MYO7A, or MYO15A gene showed statistically better HP scores compared with patients with HL due to other causes (p = .002) (), while the age at implantation in these groups was comparable (33.5 vs. 37.4 years).
Figure 3. Comparison of HP scores among three types of CI electrode array. Median, interquartile, minimum and maximum scores for the HP scale. Statistical analysis by one-way ANOVA with Bonferroni’s correction. NS: statistically not significant.
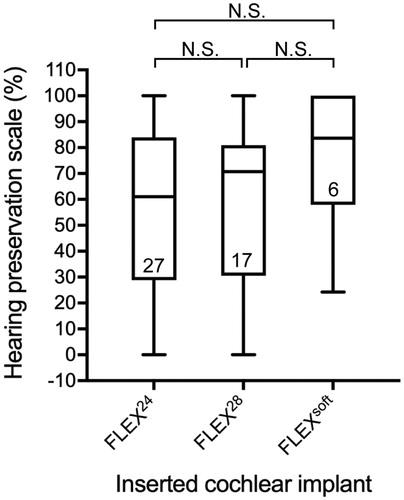
Figure 4. Average pre- (blue line) and post-operative (red line) air conduction hearing thresholds for patients with (A) pathogenic variants in the CDH23, MYO7A, or MYO15A gene, and (B) hearing loss due to other causes. Error bars represent the SD. (C) A comparison of HP scores in each group. Median, interquartile, minimum and maximum scores for the HP scale. Statistical analysis by Mann–Whitney test.
Discussion
In recent years, minimally invasive surgery and flexible electrode arrays have allowed residual hearing to be preserved, leading to the preservation of cochlear structures, although the HP results remain variable on an individual level. Therefore, we analyzed the factors that have an impact on the auditory function after CI in this study. To this end, we compared pre- and 6-month post-operative auditory thresholds in patients with residual acoustic hearing. Based on the HP classification [Citation11], complete HP was observed in 40% of patients, partial HP in 38%, minimal HP in 16% and a total loss of hearing in another 6%, which is similar to the results of previous studies [Citation13].
We found that CI surgery impaired hearing at 500 Hz as compared to that at 125 Hz. This phenomenon was observed in another study using shorter electrode arrays (20 mm) [Citation14], so that irrespective of the length of inserted electrode arrays, the intrinsic structural vulnerability of the basal-apical turn may potentially explain this. Additionally, the HP outcomes were independent of the electrode length (24 mm vs. 28 mm vs. 31.5 mm), which was consistent with the findings that there were no differences in HP score among the insertion depth angles in our and other previous reports [Citation7,Citation8]. Conversely, Suhling et al. reported that 20- or 24-mm electrodes resulted in better HP than did those 28 mm in length [Citation15]. The exact reason for this discrepancy is unclear; however, the previous studies [Citation4,Citation16] provided evidence that longer electrodes could offer the closest to a natural frequency match, resulting in better speech perception, which encourages surgeons to choose longer electrodes.
As described in our previous report [Citation7], we demonstrated that a younger age at the time of implantation led to statistically better HP, indicating that the implant age is one of the key predictors influencing residual hearing in CI surgery (). On the other hand, we did not find that HP was associated with pre-operative LFA or CDL, which contradicts some previous studies that suggest there is a positive impact on post-operative hearing [Citation8].
Among the studies on the predictive factors for HP in CI surgery, this report is unique as the etiology of HL was analyzed as a patient-related factor. Our data showed that the use of next-generation sequencing provided a diagnostic rate of 51.2% in this study, suggesting that genetic testing facilitates the accurate diagnosis of patients with residual hearing in the low frequencies. Interestingly, we observed statistically better HP scores in patients who had pathogenic variants in the CDH23, MYO7A, or MYO15A gene, which are only expressed in the stereocilia of the inner and outer hair cells (HCs) [17 for review]. In such subjects, the stereocilia function is thought to be a key component in residual hearing. Conversely, other responsible genes identified in this study are associated with other components of the cochlea; ACTG1 with HCs and supporting cells [Citation17], SLC26A4 with outer sulcus cells [Citation17], TMPRSS3 with HCs and spiral ganglion neurons [Citation17], CLDN14 with the organ of Corti, Reissner’s membrane, the spiral limbus and the stria vascularis [Citation17], and LOXHD1 with HCs and spiral ganglion neurons [Citation17]. Based on an animal study, Reiss et al. reported that a number of inner and outer HCs were intact after implant surgery in guinea pigs, although the presynaptic ribbon and postsynaptic receptor were damaged [Citation18], implying that CI insertion may not have an impact on the residual function of HCs. We speculated that these observations could possibly explain the phenomenon in which residual acoustic hearing after CI was well-preserved in patients with HL caused by genes related to HCs only in comparison with patients with HL caused by mutations in other genes. As the number of subjects in this study was limited, a study employing a larger cohort will be performed to validate the present findings.
In summary, we show that HP was marginally correlated with age at implantation, which is consistent with previous reports, while HP was independent of pre-operative LFA, CDL, and electrode length. Likewise, identification of pathogenic variants in the CDH23, MYO7A, or MYO15A gene resulted in better HP after surgery. We reasoned that these genes are known to be expressed in the stereocilia of the inner and outer HCs of the cochlea, which may not be related to implant-induced HL. We also demonstrated that the diagnostic rate was 51.2% in patients with ski slope-type HL. Taken together, these results reveal that genetic testing facilitates not only the diagnosis of patients with HL but also the prediction of HP after CI.
Acknowledgments
The authors thank the probands and their family members who participated in this study. We would also like to thank Sachiko Matsuda and Fumiko Tomioka for their technical assistance.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- von Ilberg CA, Baumann U, Kiefer J, et al. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiol Neurotol. 2011;16(s2):1–30.
- Welch C, Dillon MT, Pillsbury HC. Electric and acoustic stimulation in cochlear implant recipients with hearing preservation. Semin Hear. 2018;39(04):414–427.
- Usami S, Moteki H, Suzuki N, et al. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Otolaryngol. 2011;131(4):405–412.
- Hochmair I, Hochmair E, Nopp P, et al. Deep electrode insertion and sound coding in cochlear implants. Hear Res. 2015;322:14–23.
- Usami S, Moteki H, Tsukada K, et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngol. 2014;134(7):717–727.
- Wanna GB, O'Connell BP, Francis DO, et al. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope. 2018;128(2):482–489.
- Moteki H, Nishio SY, Miyagawa M, et al. Feasibility of hearing preservation for residual hearing with longer cochlear implant electrodes. Acta Otolaryngol. 2018;138(12):1080–1085.
- Helbig S, Adel Y, Leinung M, et al. Hearing preservation outcomes after cochlear implantation depending on the angle of insertion: indication for electric or electric-acoustic stimulation. Otol Neurotol. 2018;39(7):834–841.
- Takahashi M, Arai Y, Sakuma N, et al. Cochlear volume as a predictive factor for residual-hearing preservation after conventional cochlear implantation. Acta Otolaryngol. 2018;138(4):345–350.
- Usami S, Nishio SY, Moteki H, et al. Cochlear implantation from genetic background viewpoints. Anat Rec. 2020;303(3):563–593.
- Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl. 2013;133(564):3–13.
- Nishio SY, Usami S. Deafness gene variations in a 1120 nonsyndromic hearing loss cohort: molecular epidemiology and deafness mutation spectrum of patients in Japan. Ann Otol Rhinol Laryngol. 2015;124(1):49S–60S.
- Skarzynski H, Lorens A, Dziendziel B, et al. Electro-natural stimulation in partial deafness treatment of adult cochlear implant users: long-term hearing preservation results. ORL J Otorhinolaryngol Relat Spec. 2019;81(2–3):63–72.
- Skarzynski PH, Skarzynski H, Dziendziel B, et al. Hearing preservation with the use of flex20 and flex24 electrodes in patients with partial deafness. Otol Neurotol. 2019;40(9):1153–1159.
- Suhling MC, Majdani O, Salcher R, et al. The impact of electrode array length on hearing preservation in cochlear implantation. Otol Neurotol. 2016;37(8):1006–1015.
- Buchman CA, Dillon MT, King ER, et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779.
- Nishio SY, Attori M, Moteki H, et al. Gene expression profiles of the cochlea and vestibular endorgans: localization and function of genes causing deafness. Ann Otol Rhinol Laryngol. 2015;124(1):6S–48S.
- Reiss LA, Stark G, Nguyen-Huynh AT, et al. Morphological correlates of hearing loss after cochlear implantation and electro-acoustic stimulation in a hearing-impaired Guinea pig model. Hear Res. 2015;327:163–174.