Abstract
Background: Electric-acoustic stimulation (EAS) has emerged as a standard treatment for patients with high-frequency hearing loss. EAS is usually performed with shorter electrodes of 16–24 mm in length. As most EAS recipients gradually lose residual acoustic hearing in the implanted ear over time, EAS with longer electrodes without causing significant intra-cochlear damage might be ideal.
Objective: The aim of this study was to investigate hearing preservation (HP) results after EAS surgery with longer electrodes.
Methods: Ten patients (11 ears) with partial deafness that met the indications for EAS with a MED-EL FLEX28 electrode were included in this study. Auditory thresholds before and at 6 months after activation were examined.
Results: In 100% of cases, HP was comfortably achieved, indicating that all patients could utilize acoustic amplification combined with electric stimulation.
Conclusion: EAS with longer electrodes can offer broader cochlear coverage, resulting in natural frequency matching in comparison with shorter electrodes, even in EAS cases. The combination of advanced surgical techniques and flexible, long, straight electrodes permits deep insertion that reaches the apical region with little or no insertion trauma.
Chinese abstract
背景:电声刺激(EAS)已经成为高频听力损失患者的标准治疗方法。 EAS通常使用长度为16–24mm的较短电极进行。大多数EAS接受者会随时间而逐渐失去植入耳中的残余听觉, 因此使用较长电极而不引起耳蜗内损伤的EAS可能是理想的选择。
目的:本研究的目的是调查使用较长电极的EAS手术后的听力保存(HP)结果。
方法:本研究招纳10例部分耳聋的患者(11耳), 这些患者符合MED-EL FLEX28电极的EAS适应症。在激活之前和激活之后6个月检查听觉阈值。
结果:100%的病例都获得了HP, 表明所有患者都可以结合利用声音放大和电刺激。
结论:具有较长电极的EAS可以提供更大的耳蜗覆盖范围, 与较短电极相比, 即使在EAS情况下, 也可以实现自然的频率匹配。先进的外科手术技术与灵活的长而直的电极相结合, 可进行深度插入, 从而到达尖端区域, 而很少或无插入创伤。
Introduction
Cochlear implantation (CI) is a standard treatment for restoring hearing ability in patients with profound deafness across all frequencies, while patients with severe high-frequency hearing loss and residual low-frequency hearing have previously been left untreated. To address this issue, von Ilberg and colleagues first described the concept of combined electric-acoustic stimulation (EAS) in a single device as a promising treatment for patients with ski-slope hearing loss in 1999 [Citation1]. Since then, numerous clinical studies have demonstrated the feasibility of EAS in clinical settings in terms of speech perception and music appreciation when compared to electrical stimulation (ES) alone [Citation2–4].
To permit advantages in connection through better speech perception in noise and improvements in sound quality when listening to music as well as sound localization, preservation of the residual acoustic hearing is very important for EAS recipients [Citation5]. More advanced and less traumatic surgical techniques and improved electrode design have increased the rate of hearing preservation (HP). In recent years, limiting the insertion depth of the electrode array was preferred for EAS surgery in order not to overlap with the residual hearing region of the cochlea [Citation4]. To reduce the insertion depth of the electrode so as to reach the 1-kHz area according to the mapping of the basilar membrane [Citation6], thin, straight, flexible and ‘shorter’ electrodes of 16, 20, and 24 mm in length were developed to preserve residual hearing and to cover the high-frequency range of the cochlea for ES [Citation4]. In contrast, for patients with profound deafness, longer electrodes provide significantly better speech perception [Citation7]. Additionally, other studies have shown that a larger insertion angle results in better speech perception for ES-only [Citation8].
Most EAS recipients may gradually lose residual acoustic hearing in the implanted ear over time [Citation2]. In these cases, EAS eventually has to be converted into pure ES via CI, increasing disadvantages for patients treated with a shorter electrode if the residual hearing is lost or if it is not suitable for EAS. To avoid this dilemma, EAS using longer electrodes without causing significant intra-cochlear damage would be ideal. In this study, we sought to use longer electrodes, FLEX28 (28 mm) developed by MED-EL (Innsbruck, Austria), for patients with residual hearing that met the indications for EAS with a hearing loss of below 65 dBHL in the low frequencies between 125 and 500 Hz [Citation1]. To our knowledge, EAS with longer electrodes has not yet been evaluated in patients meeting the aforementioned criteria.
Here, we present the following: (1) auditory test results obtained pre-operatively versus those at 6 months postoperatively, (2) frequency matching between the intra-cochlear channel position and the location of the characteristic frequency at the organ of Corti, and (3) EAS fitting on the deterioration of residual hearing.
Materials and methods
Patients studied
We performed EAS surgery for 10 patients (11 ears) who met the audiological criteria for EAS at the Department of Otorhinolaryngology, Shinshu University Hospital. The inclusion criteria were as follows: (1) pure-tone hearing levels bilaterally at ≤65 dBHL for 125, 250, and 500 Hz; ≥80 dBHL at 2000 Hz; and ≥85 dBHL at 4000 and 8000 Hz; as well as minimal benefit from conventional hearing aids; that is, monosyllable scores in quiet under 60% even in the best-aided condition, and (2) FLEX28 electrode array, developed by MED-EL (Innsbruck, Austria), implantation. provides a summary of the patients’ demographic information. The median age at implantation was 33.1 years (range 9–64 years). This study was approved by the Ethics Committee of Shinshu University School of Medicine.
Table 1. Summary of subject characteristics and hearing preservation outcomes.
CI surgical procedure
As described previously [Citation9], all patients underwent a less-invasive surgical procedure for HP. The round window membrane was widely exposed by removing the bony overhang of the round window with a low-speed drill. Subsequently, the electrode was fully inserted carefully and slowly via the round window membrane. Intra- and post-operative steroids were systemically administered.
HP assessment
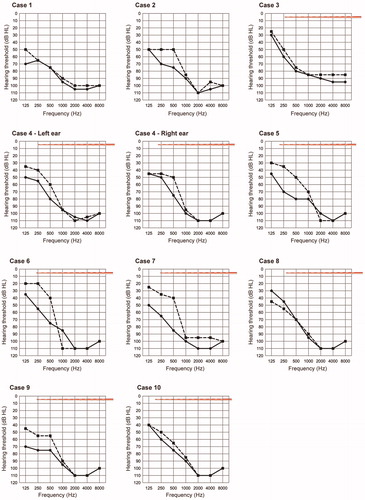
To investigate the extent of hearing deterioration following CI, we measured the auditory thresholds before and at 6 months after activation. Subsequently, to assess HP rate, we utilized the classification system provided by Skarzynski and the HEARRING group [Citation10] with the HP scores (%) categorized as follows: complete HP, defined as greater than 75%; partial HP, 25–75%; minimal HP, 1–25%; and complete loss of hearing; 0%.
Genetic analysis
We performed genetic testing for 10 patients as described previously [Citation11]. In brief, Amplicon libraries were constructed using an Ion AmpliSeqTM Custom Panel (Thermo Fisher Scientific, Rockford, IL) in accordance with the manufacturer’s protocols for 63 genes reported to cause non-syndromic genetic HL [Citation11]. Subsequently, emulsion PCR and sequencing were conducted with an Ion 200 sequencing kit and Ion PGM sequencer or Ion HiQ Chef kit and Ion Proton sequencer. The sequence data were mapped on the human genome sequence (build GRCh37/hg19), and the DNA variant regions were piled up with Torrent Variant Caller plug-in software. The effects of the detected variants were subsequently analyzed using ANNOVAR software. The missense, nonsense, insertion/deletion and splicing variants were determined, and the variants were next selected as less than 1% of (1) the 1000 genome database, (2) the 6500 exome variants, (3) the Human Genetic Variation Database (dataset for 1208 Japanese exome variants), and (4) the 333 in-house Japanese normal hearing controls. For missense variants, functional prediction software, including Sorting Intolerant from Tolerant (SIFT), Polymorphism Phenotyping (PolyPhen2), Likelihood Ratio Test (LRT), Mutation Taster, and Mutation Assessor, were used to assess pathogenicity. Direct sequencing was utilized to confirm the candidate variants identified, and segregation analysis was performed for each proband and their family members. The pathogenicity of the identified variants was evaluated based on the ACMG (American College of Medical Genetics) standards and guidelines. This system classified variants into five categories; pathogenic, likely pathogenic, uncertain significance, likely benign, and benign. Additionally, we referred to Inter Var for the evaluation of the variants. A combined annotation-dependent depletion (CADD) was also used to prioritize potential causal variants.
Cochlear duct length measurement and pre-operative simulation by OTOPLAN
Cochlear duct length (CDL) was measured using OTOPLAN software (version 2.0) developed by CAScination (Bern, Switzerland) in cooperation with MED-EL. The DICOM files from patient CT images were utilized. This software measured the CDL based on cochlear diameter, width and height for each patient. CDL was shown for three levels; the organ of Corti, spiral ganglion and the average between them. Rather than pre-curved modiolar-hugging electrodes, straight lateral-wall electrodes are believed to stimulate the nerve fiber endings of the auditory neurons at the organ of Corti. Therefore, we used the length of the organ of Corti in this study. Using individualized data, we compared insertion depth and tonotopic pitch matching for each MED-EL electrode array (FLEX28 or FLEX24) by OTOPLAN. The insertion depth angle was confirmed by post-operative X-rays.
Results
Achievement of HP in EAS recipients with longer electrodes
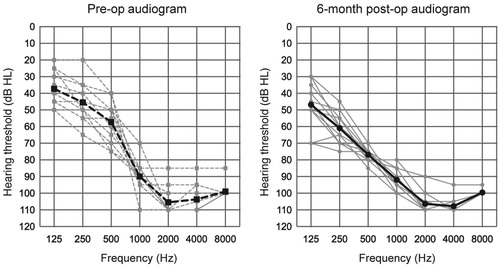
All subjects underwent EAS surgery with longer electrodes (FLEX28). Individual audiograms () indicated that residual low-frequency hearing was well-preserved in all cases, suggesting that all cases could utilize AS combined with ES. The position of the intra-cochlear electrode, measured using OTOPLAN, is also described in and suggests that HP could be achieved even if the electrode tip reached the residual hearing region (Note: it was impossible to calculate the accurate cochlear duct length in Case 1 and 2 due to incomplete partition type II). The pre-operative and 6-month postoperative audiograms are shown in . According to the HP classification system reported by Skarzynski and the HEARRING group [Citation10], we evaluated HP scores at 6 months after activation: with complete HP in 36.4% (4 of 11 cases) and partial in 63.6% (7 of 11 cases) of cases. Additionally, none of the patients experienced significant hearing deterioration, which is indicated by minimal HP or no measurable hearing.
Deep insertion with longer electrodes allowed for better place pitch matching
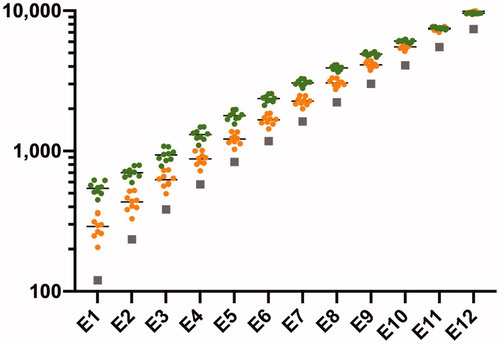
OTOPLAN software allowed us to estimate CDL based on pre-operative CT scans for each case. The average CDL was 33.9 ± 1.0 mm. Subsequently, this software showed each contact position with each MED-EL electrode array, FLEX28 and FLEX24 (24 mm). Compared with FLEX24, the tonotopic pitch matching for FLEX28 was theoretically closer to the default center frequency ().
Cases with different mapping strategies
Case 4
A 33-year-old female. Hearing loss was detected at 15 years of age. As her hearing loss was progressive, she started to wear hearing aids (HAs). After that, she visited our department at the age of 30 due to insufficient hearing with HAs. Bilateral simultaneous EAS (MED-EL synchrony FLEX28) was provided at the age of 31. Her CDL estimate was 33.3 mm, and the insertion depth angle was thought to be 540 degrees (). At activation, the crossover frequency, where the hearing loss crosses the 65 dBHL line, was 400 Hz. Therefore, the coverage of the frequency range with ES was automatically assigned from 400 Hz to 8.5 kHz for all electrodes. However, she preferred maps with ‘deactivated’ apical electrodes located at the residual hearing region (<400 Hz). Therefore, we set the frequency range with ES to cover from 70 Hz to 8.5 kHz as the default and turned off the two most apical electrodes (E1 and E2). Her hearing then gradually deteriorated and E2 was switched on based on her audiogram ().
Figure 4. Case 4: the right ear. (A) Audiograms at activation and 1 year later. Electrode array illustrations represent insertion depth. (B) Post-op X-ray findings. Numbers indicate channels. (C) Imaging for each electrode location and the reference tonotopic map.
Note: (C) Copyright license agreement was accepted [Citation9].
![Figure 4. Case 4: the right ear. (A) Audiograms at activation and 1 year later. Electrode array illustrations represent insertion depth. (B) Post-op X-ray findings. Numbers indicate channels. (C) Imaging for each electrode location and the reference tonotopic map. Note: (C) Copyright license agreement was accepted [Citation9].](/cms/asset/85f10272-6848-456f-9691-57f44cb7d458/ioto_a_1760351_f0004_c.jpg)
Case 8
A 14-year-old girl. She had not undergone newborn hearing screening. At the age of 5, HL was suspected at an elementary school wellness check-up, and she was diagnosed with high-frequency hearing loss and started wearing HAs. Subsequently, hearing deterioration was observed and she visited our department at the age of 13. Genetic testing covered by social health insurance revealed pathogenic variants in the CDH23 gene; therefore, we selected a longer electrode in terms of the prospective nature of her hearing loss caused by the CDH23 mutations. She received EAS (MED-EL synchrony FLEX28) for the left ear at the age of 14. The residual hearing was completely preserved 6 months after surgery. Unlike Case 4, she preferred mapping with ‘activated’ apical electrodes traversing the region of residual hearing at that time. In other words, she was satisfied with the acoustic amplification obtained with ES in the low frequencies ().
Figure 5. Case 8. (A) Pre- and 6 months post-op audiograms. Electrode array illustrations represent insertion depth. (B) Post-op X-ray findings. Numbers indicate channels. (C) Imaging for each electrode location and the reference tonotopic map.
Note: (C) Copyright license agreement was accepted [Citation9].
![Figure 5. Case 8. (A) Pre- and 6 months post-op audiograms. Electrode array illustrations represent insertion depth. (B) Post-op X-ray findings. Numbers indicate channels. (C) Imaging for each electrode location and the reference tonotopic map.Note: (C) Copyright license agreement was accepted [Citation9].](/cms/asset/a416de83-46d8-4ff3-9b09-abedb48eb541/ioto_a_1760351_f0005_c.jpg)
Discussion
For EAS users to fully benefit from their EAS, the conservation of residual hearing is crucial in terms of speech perception. This has given rise to the concept of hearing preservation (HP), which has been gradually refined. Several studies demonstrate that due to recent developments, including the round window insertion, slow insertion with lateral-wall electrodes and the application of intrascalar and systemic steroids, a high degree of HP is possible [Citation3,Citation12]. Regarding electrode design, thin, flexible, straight and shorter electrodes of 16, 20 and 24 mm in length were developed to minimize trauma during implantation and to cover the high-frequency range of the cochlea up to 1 kHz for ES [Citation13]. In most EAS patients, however, residual hearing in the low-frequency region deteriorates over time due to the natural course of the underlying disease or other unknown causes [Citation2], suggesting that deep insertion with longer electrodes would provide a higher percentage of cochlear coverage and allow much better place pitch matching than any shorter electrodes [Citation4]. Our earlier studies demonstrated that it was possible to preserve acoustic hearing even in the presence of a longer 28- or 31.5-mm electrode covering the residual hearing region [Citation3,Citation9,Citation14]. Here, we first report that in cases meeting the EAS criteria with longer electrodes, the residual hearing could be preserved in all patients and that this preservation was stable up to the 6-month follow up. The HP scores with longer electrodes were comparable to those with shorter electrodes previously reported [Citation3]. Additionally, we previously demonstrated that the risk of significant post-operative hearing loss is independent of electrode length and insertion depth angle [Citation14,Citation15]. Collectively, irrespective of the electrode length, HP can be comfortably achieved.
Deep insertion with longer electrodes allows for the range of electrical stimulation to be extended into the apical region, resulting in the production of low-frequency pitches, which are essential for the application of fine structure strategies. Compared to shorter electrodes (FLEX24), longer electrodes (FLEX28) offered broader cochlear coverage, leading to better place pitch matching. The provision of optimized pitch perception allowed improved speech perception in noise and enhanced sound quality, which becomes particularly obvious when listening to music [Citation4,Citation13]. Consistent with this observation, Schatzer et al. reported that in single-sided deafness CI users, the acoustic pitch of low-frequency tones can be recreated most reliably via a cochlear implant by using a combination of rate coding and stimulation in the second turn, indicating that deeper insertion is desirable [Citation16]. Moreover, our findings indicated that EAS with longer electrodes allows for two types of mapping strategy. If the inserted electrodes overlap with the residual hearing region, EAS patients can comfortably utilize ES (Case 8). Alternatively, they have the opportunity to turn off some of the apical contacts in situations where it does not provide any benefit to them. Further, if hearing deteriorates, all of the contacts can be later switched on to provide better pitch matching (Case 4). Taken together, EAS with longer electrodes allowed users to optimize mapping toward a more natural hearing.
We previously reported that genetic testing could provide not only the etiology of hearing loss but also further information regarding the state of residual hearing [Citation17]. For example, most patients carrying CDH23 mutations initially present with high-frequency hearing loss. Subsequently, their residual hearing in the low frequencies gradually deteriorates over time [Citation18], suggesting that the clinician should provide longer electrodes for broader coverage of the frequency range. Additionally, even when the causative gene of hearing loss is not identified, ongoing low-frequency hearing loss in the implanted ear or in both ears is sometimes observed, indicating that longer electrodes should be selected as well. Taken together, the clinician should select the proper array length for each individual according to the ‘future’ (not current) status of residual acoustic hearing based on the etiology and/or the natural course of hearing loss.
In cases in which the loss of residual hearing progresses over time, partial insertion of a longer electrode is an alternative option reported by Lenarz and colleagues [Citation19]. The authors demonstrated that partially inserted longer electrodes showed good hearing preservation in EAS patients and, if hearing loss progressed, the electrode could be inserted more deeply, allowing the patients to benefit from deeper insertion. One limitation of this scenario is that additional surgery was required, raising some concerns about potential side effects (i.e., infection).
In conclusion, we found that longer electrodes can offer broader cochlear coverage, resulting in more natural frequency matching compared to shorter electrodes, even in EAS cases. The benefits of deep insertion should not, however, be compromised by structural damage during insertion. Our findings first revealed that the combination of advanced surgical techniques and flexible, long, straight electrodes permits deep insertion that reaches the apical region with little or no insertion trauma. As a result, all EAS patients were able to utilize AS in this study. Reliable HP after deep insertion with longer electrodes could extend the indications for CI to cases with much less severe deafness and more residual hearing.
Acknowledgments
The authors thank the probands and their family members who participated in this study.
Disclosure statement
No potential conflicts of interest were reported by the author(s).
Additional information
Funding
References
- von Ilberg C, Kiefer J, Tillein J, et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61(6):334–340.
- Moteki H, Nishio SY, Miyagawa M, et al. Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Otolaryngol. 2017;137(5):516–521.
- Usami S, Moteki H, Tsukada K, et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngol. 2014;134(7):717–727.
- von Ilberg CA, Baumann U, Kiefer J, et al. Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiol Neurotol. 2011;16(s2):1–30.
- Gstoettner W, Helbig S, Settevendemie C, et al. A new electrode for residual hearing preservation in cochlear implantation: first clinical results. Acta Otolaryngol. 2009;129(4):372–379.
- Greenwood DD. A cochlear frequency-position function for several species–29 years later. J Acoust Soc Am. 1990;87(6):2592–2605.
- Buchner A, Illg A, Majdani O, et al. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One. 2017;12(5):e0174900.
- Buchman CA, Dillon MT, King ER, et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779.
- Usami S, Moteki H, Suzuki N, et al. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Otolaryngol. 2011;131(4):405–412.
- Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl. 2013; 133(564):3–13.
- Nishio SY, Usami S. Deafness gene variations in a 1120 nonsyndromic hearing loss cohort: molecular epidemiology and deafness mutation spectrum of patients in Japan. Ann Otol Rhinol Laryngol. 2015;124(1):49S–60S.
- Welch C, Dillon MT, Pillsbury HC. Electric and acoustic stimulation in cochlear implant recipients with hearing preservation. Semin Hear. 2018;39(04):414–427.
- Hochmair I, Hochmair E, Nopp P, et al. Deep electrode insertion and sound coding in cochlear implants. Hear Res. 2015;322:14–23.
- Moteki H, Nishio SY, Miyagawa M, et al. Feasibility of hearing preservation for residual hearing with longer cochlear implant electrodes. Acta Otolaryngol. 2018;138(12):1080–1085.
- Yoshimura H, Moteki H, Nishio SY, et al. Genetic testing has the potential to impact hearing preservation following cochlear implantation. Acta Otolaryngol. 2020;5:1–7.
- Schatzer R, Vermeire K, Visser D, et al. Electric-acoustic pitch comparisons in single-sided-deaf cochlear implant users: frequency-place functions and rate pitch. Hear Res. 2014;309:26–35.
- Usami S, Miyagawa M, Nishio SY, et al. Patients with CDH23 mutations and the 1555A > G mitochondrial mutation are good candidates for electric acoustic stimulation (EAS). Acta Otolaryngol. 2012;132(4):377–384.
- Miyagawa M, Nishio SY, Usami S. Prevalence and clinical features of hearing loss patients with CDH23 mutations: a large cohort study. PLoS One. 2012;7(8):e40366.
- Lenarz T, Timm ME, Salcher R, et al. Individual hearing preservation cochlear implantation using the concept of partial insertion. Otol Neurotol. 2019;40(3):e326–e335.