?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
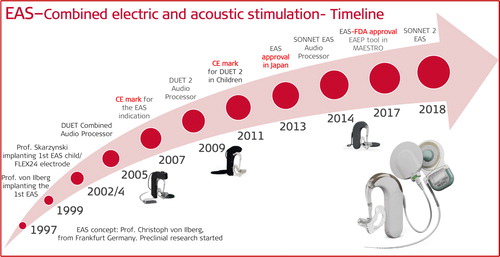
Electric-acoustic stimulation (EAS) is a special treatment modality for those patients who are profoundly deaf in the high-frequency (HF) region and retain usable hearing in the low-frequency (LF) region. Combining the electric stimulation with cochlear implant (CI) in the HF and acoustic amplification of residual hearing using a conventional hearing aid (HA) in the LF region defines EAS. The EAS concept was first proposed by C. von Ilberg from Frankfurt, Germany in the year 1997. In association with MED-EL, all the necessary safety studies were performed in non-human subjects before the first patient received it in 1997. In association with MED-EL, all the necessary safety studies were performed in non-human subjects before the first patient received it in 1999. For the patient to successfully use the EAS concept, the residual hearing needs to be preserved to a high extent and for several years. This requires a highly flexible electrode array in safeguarding the intra-cochlear structures during and after the CI electrode array insertion. Combining the HA unit with the audio processor unit of the CI was necessary for the convenient wearing of the unified audio processor. Fitting of the unified audio processor is another important factor that contributes to the overall success of the EAS treatment. The key translational research efforts at MED-EL were on the development of flexible electrodes, a unified audio processor, innovations in the fitting process, intra-operative monitoring of cochlear health during electrode insertion, pre-operative soft-ware tool to evaluate the cochlear size and electrode selection and some new innovations tried within EAS topic. This article covers the milestones of translational research from the first concept to the widespread clinical use of EAS.
Graphical Abstract
Chinese abstract
对于那些在高频(HF)区域严重失聪并在低频(LF)区域保持可用听力的患者, 电声刺激(EAS)是一种特殊的治疗方式。对于HF, 采用电刺激;对于LF区域, 采用常规助听器(HA)进行残余听力的声学放大。两者结合即可形成EAS。 EAS概念最早是由德国法兰克福的冯·伊尔伯格(von Ilberg)教授于1997年提出的。与MED-EL联合, 所有必要的安全性研究都是在非人类受试对象中进行的。后来, 于1999年安全性研究被用于人类。为使患者成功使用EAS概念, 应始终保留LF残余听力。这需要高度灵活的电极阵列, 以便在CI电极阵列插入期间和之后保护耳蜗内结构。为了方便使用统一音频处理器, 必须将HA单元与CI的音频处理器单元组合在一起。统一音频处理器的安装是有助于EAS治疗总体成功的另一个重要因素。本章介绍了MED-EL在柔性电极、统一音频处理器的开发和装配过程中的创新, 电极插入时术中对耳蜗健康的监测, 术前评估耳蜗大小电极选择的工具, 以及EAS主题中尝试的一些创新。
2.1. Introduction
The science behind human hearing is a fascinatingly complex process, and the last decades have seen outstanding achievements with mimicking nature to achieve more natural hearing in cochlear implant (CI) patients. To understand even a small portion of the sound’s journey in human hearing, it is crucial to interrelate each of the journey’s detailed properties. In this chapter, however, the focus will lay on the portion between the oval window (OW) and the brainstem, and the relevant realised milestones.
It is now understood that once the sound hits the OW, it creates an intracochlear vibration, and more precisely, it causes a vibration of the basilar membrane (BM) – all the way from OW to its apical end, the helicotrema. The travelling wave namely passes through different frequencies which are logarithmically distributed along the BM – from high at the OW, to lower towards the apex. Now depending on the cochlear health, inner-hair cells on stimulated pitch regions during the BM vibration () get excited and fulfil their function as mechanoreceptor cells by transforming the mechanical force received from the BM underneath them into electric signals. This mechanical force actuates the inner-hair cells to bend against the tectorial membrane, which is covering them. The bending opens small channels in the inner-hair cells, allowing ions in the surrounding fluid (endolymph of the scala media) to rush in and convert the physical movement to an electrochemical signal which excites the auditory nerve, and which then sends the electric signals to the brainstem – and after subsequent auditory functionalities, the patient eventually perceives a relevant sound [Citation1]. The outer-hair cells are different group that mechanically amplify low-level sound that enters the cochlea and such amplification may be powered by the movement of their hair bundles.
Figure 1. Morphology of inner-hair cells in three different conditions (A) [Citation2]. Typical audiogram of a partially deaf patient with severe to profound HL in the HF region: indication from the earlier times when the functional LF residual hearing cut-off was kept at 500 Hz which was extended to 1,500Hz under expanded indication criteria (indication 2) (B). Image (A) reproduced by permission of www.davidsonhearingaids.com.
![Figure 1. Morphology of inner-hair cells in three different conditions (A) [Citation2]. Typical audiogram of a partially deaf patient with severe to profound HL in the HF region: indication from the earlier times when the functional LF residual hearing cut-off was kept at 500 Hz which was extended to 1,500Hz under expanded indication criteria (indication 2) (B). Image (A) reproduced by permission of www.davidsonhearingaids.com.](/cms/asset/a31ad5fa-2c11-4ed2-9def-3fc0847e1ced/ioto_a_1888477_f0001_c.jpg)
In some patients, the high frequency (HF) responsible inner-hair cells are permanently damaged. This may occur due to variety of reasons, including ageing, noise-related hearing loss (HL), genetics, medication side effects and different diseases, causing severe to profound HL in the HF region () [Citation2]. However, the low frequency (LF) residual hearing with mild to moderate HL could still be utilised in such patients through a sound amplification device, like hearing aid (HA). The exact frequency range and the degree to which the HL occurs can be detected from the pure tone audiogram of the patient, tested in the quiet condition. is a typical audiogram of an extended indication (indication 2) of a partially deaf patient with severe to profound HL in the HF region which extends from 1,500–8,000Hz, and mild to moderate HL from LF to mid-frequencies in the range between 125–1,500Hz. A normal-hearing is referred to when the hearing threshold is within twenty-five decibels (dB) of HL across all frequencies.
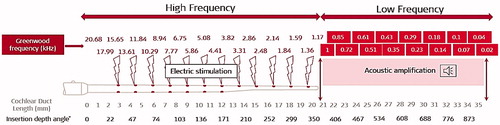
In the late ‘90 s, according to Niskar et al., 14.9% of the US children had some degree of LF HL of at least sixteen decibels HL in one or both ears [Citation3]. To accommodate this unique but relatively common partial deafness, the technology which combines both, electric stimulation of HF region and acoustic amplification of LF region, was developed as the EAS™ (Electric Acoustic Stimulation Hearing Implant System). shows the electric stimulation provided by implanting the CI electrode array to cover the HF region and acoustic amplification of the LF region. Where the electric stimulation of the HF region and acoustic amplification of the LF region shall cross-over, depends on the patient’s hearing condition and the history of progressiveness of the HL.
Figure 2. Schematic representation of electric stimulation in the HF region and acoustic amplification in the LF region in an average-sized cochlea (image courtesy of MED-EL).
The successful implementation of this treatment modality in partially deaf (PD) patients requires consideration of the below points:
highly flexible CI electrode array design
an extra safe surgical procedure in placing the CI electrode array with minimal, if not zero, damage to the intracochlear structures
corticosteroids to minimise the inflammation reaction following the electrode array insertion
an efficient audio processor that combines acoustic stimulation from the HA module with the electric stimulation from the CI electrode array
optimised fitting strategy
To address the above points, we will canvass through a brief history of MED-EL’s electric acoustic stimulation (EAS) journey beginnings in the late ’90s, followed by its early research works that supported the development of the first EAS™ system. This article covers the key clinical studies that evaluated the safety and effectiveness of unified EAS™ audio processors from the first generation until the most recent generation so far, along with patients’ overall hearing performance with the system. This article will also address some EAS-relevant topics, such as the effective hearing preservation (HP) classification system in general, and how it may be mathematically calculated in a uniform manner. The article will walk us through the topic advancements, including identification of patient-specific LF cut-off region, effective preservation of residual hearing, long electrode arrays in EAS, and electrocochleography to monitor inner ear function during the electrode insertion process. Advancements in genetic testing to predict HP results will be discussed, as well as the current EAS indication criteria, and studies that supported MED-EL in obtaining its EAS™ device approval by the notified bodies in the USA, EU and Japan. Also, this article will give a short overview of the annually held Hearing and Structure Preservation (HSP) workshop.
2.2. Beginning of MED-EL’s EAS journey
In 1997, MED-EL’s EAS journey began with Prof. von Ilberg’s (EAS inventor and patent holder) suggestion to create a concept which would combine electric and acoustic stimulation as a mode of treating partially deaf patients ). EAS applies to patients with LF functional hearing, to patients who will undergo HP surgery, and postoperatively, to patients who would use both, electric stimulation and acoustic amplification. At the time, the below questions on the safety and efficacy of such treatment option were raised by Prof. von Ilberg himself, his colleagues, and MED-EL.
Figure 3. Prof. Christoph von Ilberg, Head of the ENT department, from Johann Wolfgang Goethe University Hospital Frankfurt, Germany, the inventor of the EAS concept. US patent number: 6231604B1.

Does the simultaneous EAS interfere with physiological discharge patterns of the auditory system?
Is a chronic electric stimulation hazardous to residual hair cells?
Is a simultaneous EAS beneficial to patients with severe high-frequency HL?
The physiological discharge patterns of the auditory system in response to EAS were explained through an experiment involving non-human subjects with acute electric stimulation in their normal-hearing ears [Citation4]. Under anaesthesia, normal hearing adult subjects underwent nerve exposure through posterior fossa with a ball electrode, fixed at the RW for electric stimulation. Single-fibre action potentials were conventionally recorded from the auditory nerve in response to acoustic stimuli, delivered to the eardrum through a condenser microphone in a closed system. The response area of the single fibre was tested for acoustic stimuli, electric stimuli and combined EAS. demonstrates the effect on an HF fibre with acoustic tuning curve before () and after () simultaneous EAS. The random distribution of spikes in the subtraction plot presents no major differences compared to the original tuning curves (). With the simultaneous EAS (), there is an increase in the overall activity, but the shape of the tuning curve remains unchanged. By plotting the difference between acoustic stimulation (AS) vs EAS, it is apparent that the electric stimulation reduces the number of acoustically evoked spikes (). In the subtraction plot of EAS–AS (), a slight decrease in spike activity in the response area may be seen, and the electrically driven activity becomes apparent. This acute experiment demonstrates that the electric stimulation in a normal hearing ear does not substantially interfere with natural acoustic hearing.
Figure 4. Responses of an HF single fibre (18.1 kHz) in a normal-hearing subject during different stimulation conditions. (a) Response areas evoked by acoustic stimulation, recorded before EAS (ASb): 0 dB equals to approximately 110 dB sound pressure level (SPL). (b) Same stimulation after combined EAS (Asa). (C) Subtraction plot of acoustically evoked response areas (ASb–Asa): no differences appear. (d) Response area evoked under EAS. (e) Subtraction plot of response areas (AS–EAS). (f) Subtraction plot of response areas (EAS–AS) [Citation4]. Reproduced by permission of Karger AG, Basel.
![Figure 4. Responses of an HF single fibre (18.1 kHz) in a normal-hearing subject during different stimulation conditions. (a) Response areas evoked by acoustic stimulation, recorded before EAS (ASb): 0 dB equals to approximately 110 dB sound pressure level (SPL). (b) Same stimulation after combined EAS (Asa). (C) Subtraction plot of acoustically evoked response areas (ASb–Asa): no differences appear. (d) Response area evoked under EAS. (e) Subtraction plot of response areas (AS–EAS). (f) Subtraction plot of response areas (EAS–AS) [Citation4]. Reproduced by permission of Karger AG, Basel.](/cms/asset/7f7d56e2-140e-4651-bda4-bca1224d250e/ioto_a_1888477_f0004_c.jpg)
To examine the effect of chronic electric stimulation on the hair cells, normal hearing adult non-human subjects were used for chronic experiments with gold ball electrodes bilaterally implanted at the RW [Citation4]. The left side underwent a chronic stimulation, and the right side was kept unstimulated and served as a control ear. The stimulation was continuously running with biphasic charge-balanced pulses (30 Hz, 200µs/phase) at currents of approximately 100 µA for 24 h/day and compound action potential (CAP) audiograms were measured once a week on both ears by placing the subjects under sedation. Acoustic stimuli, using tone pips from 300–64,000Hz, were used for measuring the thresholds of acoustically evoked CAP, whilst the auditory brainstem response (ABR) and the threshold of electrically evoked auditory brainstem response (eABR) of the chronically stimulated side were both determined using the standard averaging procedures and were compared with the prestimulation values.
compares CAP audiograms from subjects at three different time points, including before stimulation, shortly after the onset of stimulation, and after eighty-five days of electric stimulation in both, stimulated and the control ear. The results showed no significant differences between the two ears, suggesting that the hearing thresholds were not negatively affected by the continuous chronic suprathreshold extracochlear electric stimulation.
Figure 5. CAP audiograms in normal-hearing non-human subjects before and after chronic electric stimulation: square points refer to the time before stimulation, triangle points refer to the time shortly after the onset of stimulation, and circle points refer to 85 days after stimulation, for both stimulated and the control ear. No major differences were identified between the two ears [Citation4]. Reproduced by permission of Karger AG, Basel.
![Figure 5. CAP audiograms in normal-hearing non-human subjects before and after chronic electric stimulation: square points refer to the time before stimulation, triangle points refer to the time shortly after the onset of stimulation, and circle points refer to 85 days after stimulation, for both stimulated and the control ear. No major differences were identified between the two ears [Citation4]. Reproduced by permission of Karger AG, Basel.](/cms/asset/ab45382f-7e63-4b34-be1a-f8713c0b08be/ioto_a_1888477_f0005_b.jpg)
2.3. First EAS concept application in human
In 1999, the two experiments which showed no adverse effects of electric stimulation in normal hearing ears [Citation4], inspired Prof. von Ilberg to apply the combined EAS treatment modality to a patient with a history of slowly progressive bilateral HL [Citation4]. The patient was fitted with bilateral high-power HA from Phonak, and the CI was implanted when the patient was fifty years of age. The implanted system was MED-EL’s COMBI 40+ with the STANDARD electrode array of 31.5 mm length, inserted only 20 mm intracochlearly and via cochleostomy surgical approach. This was to ensure electric coverage until 1,000Hz starting from the RW entrance, leaving the LFs from 1,000Hz towards the apex with acoustic amplification. Audiological tests were performed two months postoperatively, where the hearing scores from Göttingen sentence test showed an increase in hearing performance with CI alone and in the combined EAS mode, in comparison with the HA alone (control ear). Also, with an increased number of stimulating channels activated in the first/basal turn of the cochlea, the hearing performance was improving.
summarises the acute results of speech understanding in a patient with LF residual hearing. With eight basal channels covering the centre frequency range from 300–5,500Hz, the speech scores resulted in 92% correct with combined EAS (HA + CI), and 88% with CI alone mode. The scores dropped to 22.9% and 0%, respectively, when only two stimulating channels were kept active.
Table 1. Acute results of the speech understanding (Göttingen sentence test) in a single patient with LF residual hearing after CI implantation [Citation4].
This was the first study to evaluate the synergistic effect of combined EAS concept with MED-EL CI in an adult patient with severe-to-profound HF HL and preserved functional LF hearing. The utilisation of a separate HA unit and behind-the-ear (BTE) speech processor of CI posed some practical challenges to the patient in the ease of using two separate audio processors. This impelled the authors to recommend the development of a unified speech processor which would combine both, electric stimulation and acoustic amplification.
In 2002, the same team of specialists chose to apply the combined EAS treatment method to further eight patients [Citation5]. Patients were included based on their pure-tone audiograms with a hearing threshold between 30–60dB in the frequency range between 0.25–1kHz and >60dB above 1 kHz in the ear to be implanted with MED-EL’s COMBI 40+ device and TEMPO + BTE audio processor. The STANDARD CI electrode array was inserted intracochlearly through a 1 mm diameter cochleostomy. For amplification of the acoustic hearing on the ipsilateral ear, all patients used high power in-the-ear (ITE) HA from Resound®. The implantation of CI electrode preserved residual hearing to within 10 dB HL in four out of eight patients, which was considered as complete hearing preservation. In two further patients, it was preserved partially with up to 30 dB HL, while the remaining two patients lost their residual hearing after implantation. presents the pre- and post-operative results of the Freiburg monosyllable word test.
Figure 6. Preoperative Freiburg monosyllabic word scores, tested with the ipsilateral HA and in the best-aided condition at optimal loudness. Postoperative monosyllabic word score with CI alone at 70 dB presentation level, and with CI + HA in the ipsilateral ear (n = 4), as well as CI + HA in the optimal condition—either ipsi-, contra-, or bi-lateral at 70 dB. Histogram created from the data given in Kiefer et al. [Citation5].
![Figure 6. Preoperative Freiburg monosyllabic word scores, tested with the ipsilateral HA and in the best-aided condition at optimal loudness. Postoperative monosyllabic word score with CI alone at 70 dB presentation level, and with CI + HA in the ipsilateral ear (n = 4), as well as CI + HA in the optimal condition—either ipsi-, contra-, or bi-lateral at 70 dB. Histogram created from the data given in Kiefer et al. [Citation5].](/cms/asset/e2f263a8-6076-4997-85f6-9fbc52d44198/ioto_a_1888477_f0006_b.jpg)
The preoperative performance of the individual optimal loudness in the best-aided condition with ipsilateral HA at 70 dB and in the best-aided condition did not exceed 15% correct answers, whereas with the CI alone mode, the hearing performance reached 53% correct answers and it increased to 78% with the addition of HA (). This was a clear demonstration of the synergistic effect between electric and acoustic stimulation in the HF and LF regions, respectively. In terms of LF hearing preservation in these patients, complete preservation was possible in 50%, and at least partial preservation in 75% of those who were implanted with a partial insertion of the STANDARD electrode array. The insertion depth of approximately 22 mm out of the total 31.5 mm ensured at least eight channels intracochlearly for a fully functioning CI, as well as attempt to preserve residual hearing in the LF region.
In the same year, the combined EAS concept extended to neighbouring Poland to reach a twenty-five-year-old prelingually HF deaf patient, who was fitted with HA at the age of four [Citation6]. The patient was implanted with MED-EL’s COMBI 40+ system with STANDARD electrode array, inserted 18–20mm intracochlearly through the RW entrance, ensuring angular insertion depth of 360° with eight stimulating channels inside the cochlea. Electrode insertion through the RW opening at that time was very special as Cochleostomy approach was the common practice among the surgeons. A postoperative pure-tone audiogram showed a decrease in hearing threshold sensitivity of 15 dB across the LF region, compared to the preoperative condition. Speech comprehension was performed using Pruszewicz monosyllabic word test (). Before the CI surgery, with HA alone, the patient was able to score 23% and <5% in quiet and in noise, respectively. One week past the first fitting, the results increased slightly – to 30% and 5%, respectively – but at three weeks, the hearing performance improved significantly and reached 90% and 65% under the combined effect of both, electric and acoustic stimulations. This relatively rapid monosyllabic word test score increase, otherwise considered one of the most difficult in the standard audiological practice, is clear evidence of how the auditory system positively embraces the EAS. The results proved another favourable outcome of the EAS technique in providing the restoration of normal hearing in partially deaf patients.
Figure 7. Clinicians from the Warsaw Institute of Physiology and Pathology of Hearing, Poland, treated the first Polish EAS-indicated patient with MED-EL device. Results of monosyllabic speech understanding after CI in quiet surroundings and noise [Citation6].
![Figure 7. Clinicians from the Warsaw Institute of Physiology and Pathology of Hearing, Poland, treated the first Polish EAS-indicated patient with MED-EL device. Results of monosyllabic speech understanding after CI in quiet surroundings and noise [Citation6].](/cms/asset/127cdc5d-fc27-48d7-914c-b5b44cd89cc2/ioto_a_1888477_f0007_c.jpg)
In 2002, Prof. Skarzynski and his colleagues reported the first ever child patient with residual hearing implanted with MED-EL’s COMBI 40+ device and was using EAS post-operatively with a HA for acoustic amplification and CI audio processor for the electric stimulation. This patient was implanted in the year 2000 at the age of 8 years.
In 2003, Prof. Skarzynski and his colleagues introduced the concept of treating partial deafness with cochlear implantation (PDCI). Many of these partially deaf (PD) patients would not have been considered as CI candidates in the past because their speech recognition was either borderline or better than the criteria for standard CI. However, children with PD display different speech development and language acquisition patterns when compared to normal-hearing children or children with severe-to-profound sensorineural hearing loss [Citation7].
2.4. Dedicated electrode array design and surgical procedures, supporting the EAS
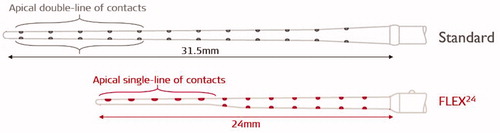
The year 2004 was quite a busy year for the clinicians from Frankfurt in Germany and Vienna in Austria in further exploring the combined EAS concept in more patients, and in parallel, trying to understand more about the intracochlear structure preservation with the placement of the CI electrode array through cochleostomy drilling. For MED-EL, it was an important year with the introduction of FLEX24™ electrode array, which offered a significant design change from its predecessor, the STANDARD electrode array ().
Figure 8. Illustration of STANDARD electrode array with apical double-lined channels and FLEX24™ electrode array with apical single-lined channels (image courtesy of MED-EL).
In parallel, specialists from both, Goethe University Frankfurt in Germany and the Medical University of Vienna in Austria implanted fourteen patients who had residual hearing thresholds in the ear to be implanted at <60dB in at least two of the frequencies (125-, 250-, or 500-Hz), and at >60dB at ≥1kHz [Citation8] ().
Figure 9. CI surgeons from 1 Johann Wolfgang Goethe University Hospital Frankfurt, Germany, and 2 Medical University of Vienna, Austria, who implanted MED-EL EAS™ device in patients with measurable LF residual hearing.

All patients were implanted with MED-EL COMBI 40+ system with the limited electrode insertion depths of 19 mm, and up to eight channels of the STANDARD electrode array were placed inside the cochlea through a 1 mm diameter cochleostomy. The study aimed to understand how well the LF residual hearing can be preserved with the insertion of a CI electrode array to basal turn with only above-described conditions [Citation8]. shows pure tone audiogram of fourteen individual patients (13 adults, 1 paediatric) along with the average plot for both, preoperative and three months postoperative conditions. The average preoperative threshold in the frequency range between 125–1,000Hz was 60 dB, and it increased to 75 dB three weeks after the operation. A 15 dB drop in hearing after surgery with cochleostomy drilling was still considered good conservation of residual hearing by the authors of the study.
Figure 10. Individual audiograms and mean values (bold black line) with pre-op and three months post-op pure-tone thresholds in the ear chosen for implantation [Citation8]. Reproduced by permission of Taylor and Francis Group.
![Figure 10. Individual audiograms and mean values (bold black line) with pre-op and three months post-op pure-tone thresholds in the ear chosen for implantation [Citation8]. Reproduced by permission of Taylor and Francis Group.](/cms/asset/1f3e6cee-ce2d-4ae3-9c93-20433568d3d6/ioto_a_1888477_f0010_c.jpg)
One of the critical factors in the EAS process is the preservation of intracochlear structures, which is directly related to the preservation of the LF residual hearing. Any disturbance to the intracochlear structures or the cochlear physiology would disrupt the residual hearing, and therefore both, the CI electrode and the surgical approach shall be as atraumatic as possible.
In 2004, by the same group of specialists from Frankfurt, another laboratory test was piloted on eight cadaveric temporal bones to understand the intracochlear level of trauma caused by each of the two surgical approaches with MED-EL’s STANDARD electrode array – RW and cochleostomy approach. Histological evaluation unveiled basal cochlear trauma in almost 30% of the implanted human temporal bones, associated with the bony cochleostomy drilling. On the other hand, the RW approach revealed smoother insertion, consequently manifesting a deeper and more atraumatic introduction of the array to scala tympani (ST) [Citation9]. The latter result was one of the key motivations going forward towards a gradual shift from the cochleostomy to RW approach.
In all previously combined EAS implantations, the STANDARD electrode was inserted to accommodate only eight channels intracochlearly, leaving the four basal channels extracochlearly and at the time, MED-EL CI device was used as an off-label device in EAS implantations. To have an ideal electrode array choice for the EAS solution, MED-EL developed FLEX24™ electrode array with 24 mm length, and with apical five channels in a single-channel configuration, as illustrated in . In comparison – the STANDARD electrode array has all twelve channels in a double-lined configuration.
Figure 11. Prof. Oliver Adunka from Johann Wolfgang Goethe University Hospital Frankfurt, Germany, performed an in-vitro evaluation of FLEX24™ electrode array in the year 2004.

Prof. Adunka and his colleagues were instrumental in evaluating the FLEX24™ electrode array (in the year 2004), both in the laboratory and in the clinical setup . Inserting an electrode array exerts a certain force on the intracochlear structures, and any consequential trauma depends mostly on the array stiffness [Citation10]. In a laboratory setting and with a plastic ST model, the insertion force with FLEX24™ measured on average 22 mN, while with the STANDARD electrode array it increased to 35 mN on average, when reaching the intracochlear insertion depth of 24 mm with both (). Lower insertion force indicates the flexible nature of the electrode array. The volume of FLEX24™ electrode array, as measured from its 3 D computerised model, is 7mm3, which is almost four times less than the volume of ST measured from RW entrance to the most apical point, the helicotrema () – this was a later finding and reported in the year 2020 by Dr Dhanasingh from MED-EL [Citation11]. In the same year (2004), Prof. Adunka and his colleagues from the Johann Wolfgang Goethe University Hospital Frankfurt showed the importance of round window membrane (RWM) approach of electrode insertion in achieving an atraumatic CI surgery. The superiority of the approach was proved in eight fresh human cadaveric temporal bones to which FLEX24™ electrode was inserted through RWM approach to reach an average insertion depth of 382.5° with no damage to the intracochlear structures. The histological analysis revealed that this electrode array was positioned entirely inside the ST with no deviation to scala vestibuli (SV), preserving the organ of Corti () [Citation9]. The authors concluded that a combination of the flexible electrode array with an ideal array length of 24 mm, along with the RW approach with surgical placement inside the ST, ensures the preservation of intracochlear structures, which is a prerequisite for the successful acoustical amplification of LF residual hearing.
Figure 12. Force measurement data is showing 40% lower values for FLEX24™ electrode array in comparison with the STANDARD electrode array (A) (Courtesy of MED-EL). Mean ST volume compared against STANDARD and FLEX24™ electrode arrays—a later finding from the year 2020 (B) [Citation11]. Histological evaluation of FLEX24™ in human cochlea, showing complete ST placement (C) [Citation9]. Histological image—Courtesy of Freiburg Medical University, Germany, Study sponsored by MED-EL.
![Figure 12. Force measurement data is showing 40% lower values for FLEX24™ electrode array in comparison with the STANDARD electrode array (A) (Courtesy of MED-EL). Mean ST volume compared against STANDARD and FLEX24™ electrode arrays—a later finding from the year 2020 (B) [Citation11]. Histological evaluation of FLEX24™ in human cochlea, showing complete ST placement (C) [Citation9]. Histological image—Courtesy of Freiburg Medical University, Germany, Study sponsored by MED-EL.](/cms/asset/126dbee4-1da5-42f6-9ceb-57f0d63d4d5a/ioto_a_1888477_f0012_c.jpg)
2.5. Unified audio processor unit, combining acoustic and electric stimulation
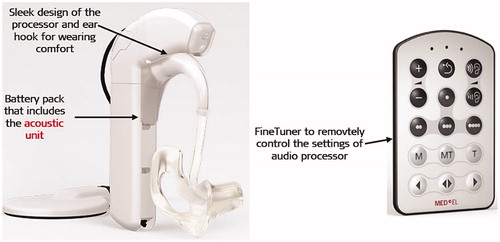
A unified audio processor that technically combines HA unit to the CI audio processor is another important technological advancement which was needed at the time for complete acceptance of the EASTM technology among the partially deaf patients. The challenges included practical handling of two separate devices with different types of batteries and battery life spans, insufficient amplification with ITE HA in the frequencies below 500 Hz, and posed challenges with the fitting of these two devices separately. Internally at MED-EL, Dipl. Ing. Schmidt and his colleagues were strongly engaged in the development of the DUETTM unified audio processor .
In 2005 November, as the world’s first hearing implant company to combine HA with CI audio processor, MED-EL introduced DUET™audio processor in order to overcome all the practical issues with having two separate devices as mentioned above (). The DUET™ audio processor featured a single microphone for the TEMPO + audio processor (using the continuous interleaved sampling (CIS+) strategy) and a two-channel HA, allowing 40 dB gain through 1,800Hz in one unit. The ear received the acoustic amplification through the ear mould positioned inside the external ear canal, receives an acoustic amplification from the processor. The processor unit controls both the HA and the CI speech processor, which is powered by a single battery pack. The DUET™ system was designed to amplify acoustic hearing between 125–1,500Hz and between 30–75dB.
Prof. Skarzynski and his colleagues from the Institute of Physiology and Pathology of Hearing in Warsaw in Poland, supported by Dr Polak from MED-EL, evaluated the effectiveness of DUET™ EAS™ audio processor for the first time in partially deaf patients [Citation12]. The study comprised of eleven partially deaf adults, implanted with MED-EL COMBI 40+ device and STANDARD electrode array, inserted 18–22mm and with eight stimulating channels intracochlearly. After at least one year of CI use, these patients were fitted with DUET™ audio processor for at least one month before their hearing performance was analysed. The CI was fitted with a frequency range between 0.3–8.5 kHz. Control group comprised of twenty-two adult CI patients implanted with MED-EL COMBI 40+ in combination with STANDARD electrode array inserted to its full length of 31.5 mm and twenty normal-hearing adult patients participated for comparison of the hearing performance of partially deaf patients, treated with the EAS™ technology. The mean duration of CI and DUET™ use before audiological testing was 22.3 months and 3.4 months, respectively. Audiological tests, including pure-tone audiograms in three different listening conditions, Pruszewicz monosyllable (Polish) word tests in quiet at a signal-to-noise ratio (SNR) of 10- and 0-dB, and the Polish Hochmair-Schulz-Moser (HSM) sentence test at 10 dB SNR, were conducted across all patient groups. shows an almost complete return of normal hearing thresholds across all frequencies with the EAS™ hearing solution, which was not the case with only acoustic amplification of HA unit from DUET™ audio processor. The HA alone from the DUET™ audio processor certainly did help patients improve their hearing thresholds in the LF region, but that was not enough to bring back the HF hearing. The monosyllabic word test results in the partially deaf patient group tested in quiet, at 10- and 0-dB SNR, the condition DUET™ only and best-aided (plus contralateral HA), were significantly higher than in the CI only patient group. The significantly higher scores obtained with the conditions DUET™ only over the CI only condition suggests that the application of additional HA allows the utilisation of the LF hearing to a greater extent. For the best-aided condition, the patients scored 91.4% in quiet on average, and 78% at 10 dB SNR (). Although DUET™ processor was used only for a short duration of 3.4 months, the data given in placed the partially deaf patients treated with EAS™ and DUET™ in an intermediate position between CI only and NH group ().
Figure 15. Mean audiograms for the implanted ear in three different listening conditions (unaided, HA alone from DUET™, and CI + HA) (A). Pruszewicz monosyllable test results in quiet at 10- and 0-dB SNR and Polish HSM sentence test results at 10 dB SNR for the group of partially deaf patients (n = 11). Mean values for the conditions DUET™ only (CI + HA), DUET™ HA only, CI only, and best-aided (plus contralateral ear) are shown with W (word test) and S (sentence test) (B). Comparison of Pruszewich monosyllable test results for three groups of patients: (1) CI patients (n = 22) tested with their CI (contralateral ear was unplugged), (2) partially deaf patients using the EAS™ (n = 11) (tested in three conditions: CI only (contralateral ear plugged), DUET™ only (contralateral ear plugged), and best-aided (plus contralateral ear)), and (3) NH group (n = 20) tested in both ears. The red shaded area shows the hearing performance gap between EAS™ and normal hearing, and the grey shaded area shows the hearing performance gap between the CI and EAS™ (C) [Citation12]. Statistical analysis: ANOVA single-factor test was used to compare speech data between three groups (p < .05). Graphs and histogram created from raw data provided by Dr Polak (MED-EL) one of the authors of Lorens et al. [Citation12].
![Figure 15. Mean audiograms for the implanted ear in three different listening conditions (unaided, HA alone from DUET™, and CI + HA) (A). Pruszewicz monosyllable test results in quiet at 10- and 0-dB SNR and Polish HSM sentence test results at 10 dB SNR for the group of partially deaf patients (n = 11). Mean values for the conditions DUET™ only (CI + HA), DUET™ HA only, CI only, and best-aided (plus contralateral ear) are shown with W (word test) and S (sentence test) (B). Comparison of Pruszewich monosyllable test results for three groups of patients: (1) CI patients (n = 22) tested with their CI (contralateral ear was unplugged), (2) partially deaf patients using the EAS™ (n = 11) (tested in three conditions: CI only (contralateral ear plugged), DUET™ only (contralateral ear plugged), and best-aided (plus contralateral ear)), and (3) NH group (n = 20) tested in both ears. The red shaded area shows the hearing performance gap between EAS™ and normal hearing, and the grey shaded area shows the hearing performance gap between the CI and EAS™ (C) [Citation12]. Statistical analysis: ANOVA single-factor test was used to compare speech data between three groups (p < .05). Graphs and histogram created from raw data provided by Dr Polak (MED-EL) one of the authors of Lorens et al. [Citation12].](/cms/asset/a2b117a3-e035-4b10-8300-61cc9971a99b/ioto_a_1888477_f0015_c.jpg)
Overall, the study showed the efficacy of EAS™ hearing solution with DUET™ processor in partially deaf patients. It had also revealed a hearing performance gap (red shaded area in ) between prosthetic (EAS) patients and normal-hearing patients, and at the same time, EAS group showed better hearing performance compared to the CI group (grey shaded area in ). An important factor to note is that those with good preoperative hearing reached better hearing on average than the CI patients.
In parallel to Prof. Skarzynski’s study mentioned above, Priv.-Doz. Dr med. Helbig, Prof. Baumann and their colleagues (), also evaluated the efficacy of MED-EL’s DUET™ audio processor in nine partially deaf patients [Citation13].
Figure 16. A team of ENT surgeons and audiologist from Johann Wolfgang Goethe University Hospital Frankfurt, Germany, evaluated the effectiveness of DUET™ audio processor.

Before the study, patients were using MED-EL’s TEMPO + audio processor unit, controlling the CI and during the study, they received an additional ITE HA to amplify the LF signal. The study also revealed the same practical challenges with the usage of two separate controlling devices. All patients underwent the Freiburg monosyllables speech perception test at 70 dB SPL, and the HSM sentence testing in quiet and in noise (+10dB, +5dB and 0 dB SNR) before switchover, and again at two and eight months after switching to the new EAS™ ().
Figure 17. Mean results of Freiburg monosyllables in quiet at 70 dB (A), HSM sentences at 70 dB with + 10 dB SNR (B), HSM sentences at 70 dB with +5dB SNR (C), and HSM sentences at 70 dB with 0 dB SNR (D). Statistical analysis: Parametric Student’s t-test was used to detect discrepancies between the test intervals (p < .05). Histogram created from data given in Helbig et al. [Citation13].
![Figure 17. Mean results of Freiburg monosyllables in quiet at 70 dB (A), HSM sentences at 70 dB with + 10 dB SNR (B), HSM sentences at 70 dB with +5dB SNR (C), and HSM sentences at 70 dB with 0 dB SNR (D). Statistical analysis: Parametric Student’s t-test was used to detect discrepancies between the test intervals (p < .05). Histogram created from data given in Helbig et al. [Citation13].](/cms/asset/1cd23310-a2a1-4d0e-b74b-2ab170cf1517/ioto_a_1888477_f0017_c.jpg)
Testing for monosyllables with DUET™ system at both, two and eight months of EAS™ use, revealed a significant benefit with the mean values’ increase of 14%, compared to before the switchover testing with CI only (). The cohort achieved a mean result of 77% correct answers after two months, and the result increased to 78% at eight months after moving to the new device. With the HSM sentence test, the patients achieved better results with the DUET™ system, compared to the CI only condition when tested in noise at the +10dB, +5dB, and 0 dB SNR. At +10dB SNR, the mean result increased from 55% to 84% after two months and kept a similar rate at eight months, with 81% correct answers (). Testing at the +5dB SNR increased from a mean value of 26% in the CI only condition to 53% after two months and resulted in 51% at eight months (). At difficult listening conditions at 0 dB SNR, the mean results increased from 5% to 14% after two months, and after eight months they resulted in 8% ().
Both of the abovementioned studies point out the major benefit of the unified audio processor for better speech understanding in quiet and noisy situations. This was underlined with patients’ high scores in difficult listening conditions, as well as with reported user comfort improvements, compared to the experience before switching [Citation12,Citation13].
In 2007, MED-EL became the world’s first hearing implant company to CE-mark its EAS hearing system with indication criteria of at least 65 dB HL in LF frequencies at up to 750 Hz. The abovementioned studies from Germany/Austria [Citation8], Poland [Citation12] and Germany [Citation13] were highly instrumental in demonstrating the value of MED-EL’s EAS™ hearing system.
2.6. Acceptance of the unified audio processor by the patients
The success of any technology may be claimed by its wide user acceptance. For DUET™ audio processor, the patient acceptance depended mainly on the preservation and successful acoustic amplification of LF residual hearing, in combination with effective CI stimulation of HFs. Prof. Baumann and Priv.-Doz. Dr med. Helbig studied the acceptance of the HA part of the DUET™ device in fifteen patients who underwent EAS™ surgery at their clinic [Citation14]. Eleven out of fifteen patients accepted DUET™ processor and were using it in their daily life, whereas four rejected acoustic amplification due to insufficient benefit and were using electric stimulation only. The mean pure tone audiometric thresholds of both groups are given in . Within the frequency range of up to 500 Hz, the DUET™ audio processor users showed hearing thresholds of maximum 75 dB, while the nonusers of the device showed increased hearing thresholds of up to 105 dB. Both groups revealed a maximum HL at the maximum hearing threshold of 120 dB at frequencies above 500 Hz.
Figure 18. Mean pure-tone audiometric results of DUET™ users (n = 11) and DUET™ nonusers (n = 4) (A). Speech audiometry results of the four patients who rejected DUET™ and used the CI processor: the Freiburg monosyllable word test correct answers with 66% (mean) (B) and HSM sentence correct answers with 62% at 10 dB SNR (mean) (C). Graph and histograms created from data given in Helbig et al. [Citation14].
![Figure 18. Mean pure-tone audiometric results of DUET™ users (n = 11) and DUET™ nonusers (n = 4) (A). Speech audiometry results of the four patients who rejected DUET™ and used the CI processor: the Freiburg monosyllable word test correct answers with 66% (mean) (B) and HSM sentence correct answers with 62% at 10 dB SNR (mean) (C). Graph and histograms created from data given in Helbig et al. [Citation14].](/cms/asset/0e408f10-2f9d-45aa-880a-e91d921ebbe0/ioto_a_1888477_f0018_c.jpg)
With electric stimulation only, the four patients who were not using DUET™ audio processor scored only 66% in monosyllable word testing (group’s mean value, ), and 62% in sentence testing at 10 dB SNR (). The study revealed that patients with preserved residual hearing and who had hearing thresholds better than 75 dB in the frequency region of ≤500Hz experienced optimised benefits offered by the EAS™. The conclusion also indicates the importance of atraumatic electrode array design and surgical technique in preserving the LF residual hearing. However, it shall be bared in mind that factors such as certain genetic predispositions, could still cause progressive HL over subsequent time, irrespectively of atraumatic electrode design and surgical techniques.
2.7. The second-generation unified audio processor
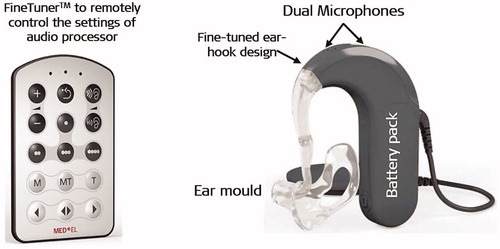
The year 2009 marked ten years of MED-EL’s EAS™ hearing system research efforts that resulted in the second generation of DUET™ EAS™ audio processor, which was named as DUET-2™ (). The DUET-2™ audio processor utilises dedicated parallel signal processing for both, acoustic and electric stimulation, using an omnidirectional microphone which allows each signal to be optimised for maximum efficiency. The acoustic amplification was raised to over 43 dB, and the acoustic frequency range was optimised to between 125–1,700Hz. DUET-2™ also features the FineTuner™ remote control, which allows adjustment of the settings without any hearing interruption. DUET-2™ applies automatic sound management (ASM), enabling users to experience optimal hearing by automatic adjustment of the audio processor setting based on the sound environment and background noise without removing the BTE speech processor for manual adjustment, based on the environment, background noise, or both. Safety features include continuous static electricity self-monitoring of the device (SoundGuard™) and relevant automatic stimulation stop. In terms of battery, the low battery alert feature was introduced as well. Overall, DUET-2™ weighs fourteen grams less than the DUET™ audio processor.
Figure 19. DUET-2™ EAS™ audio processor with its remote control FineTuner™ (image courtesy of MED-EL).
User acceptance of DUET-2™ audio processor was evaluated by Prof. Lorens and Prof. Skarzynski [Citation15], involving ten just under forty-three years old on average, experienced DUET™ users who had been using the device twenty-five months on average. DUET-2™ was offered as part of the processor upgrade, and the fitting map from DUET™ was simply transferred to DUET-2™ to evaluate the overall benefits straight after the upgrade, and at one- and three-months intervals. Pruszewicz monosyllabic word testing and user questionnaire showed that DUET-2™ is either similar or slightly better than DUET™ in terms of hearing performance and general acceptance of the processor by the patients.
The monosyllabic word test result did not show any significant differences between DUET™ and DUET-2™ processors (). Visual analogue scale (VAS) satisfaction with the sound quality for speech and music stimuli was 69% for DUET-2™ at upgrade (interval I) which increased to 75% at the second interval and reached 80% at the third. These results revealed statistical superiority of the second generation with p = .014 (), and the study concluded that the conversion from DUET™ to DUET-2™ improved patient satisfaction and the subjective benefits.
Figure 20. Mean Pruszewicz monosyllabic word recognition in background noise with an SNR of +10dB (A) and mean subjective report on sound quality satisfaction of music stimuli (B) [Citation15]. Statistical tests: One-way repeated measures (RM) ANOVAs were used to assess the improvement of DUET™ and DUET-2™ and the level of user satisfaction across three-time intervals. Reproduced by permission of Taylor and Francis Group.
![Figure 20. Mean Pruszewicz monosyllabic word recognition in background noise with an SNR of +10dB (A) and mean subjective report on sound quality satisfaction of music stimuli (B) [Citation15]. Statistical tests: One-way repeated measures (RM) ANOVAs were used to assess the improvement of DUET™ and DUET-2™ and the level of user satisfaction across three-time intervals. Reproduced by permission of Taylor and Francis Group.](/cms/asset/e414bc7b-18b3-44f9-89ee-17a56d2c1866/ioto_a_1888477_f0020_b.jpg)
In 2010, the focus expanded towards music perception by the EAS™ users, as assessed with the Music Sounds in Cochlear Implants (Mu.S.I.C) test by Dr Brockmeier from the Technical University of Munich in Germany and her colleagues from other CI centres in Europe [Citation16]. Thirteen patients met the EAS inclusion criteria and underwent soft surgery to receive MED-EL COMBI 40+ CI with a STANDARD electrode and CIS + speech coding strategy. The Mu.S.I.C test battery consists of six objective subsets assessing aspects of pitch, rhythm, melody, harmony, chord and timbre perception. The patients were tested under EAS condition, and the results were compared with those of CI and normal hearing (NH) participants. The EAS patients performed better than the CI participants on pitch and melody discrimination, but poorer when compared to NH participants. No significant difference was found in the three groups with chord and rhythm discrimination. With instrument detection, both EAS and CI participants performed significantly lower on instrument detection than NH participants, but a positive trend was observed for EAS over CI participants in xylophone, soprano, flute and double bass instruments ().
Figure 21. Instrument identification. Scores on instrument identification according to instruments for all three groups. Histogram created from data given in Brockmeier et al. [Citation16].
![Figure 21. Instrument identification. Scores on instrument identification according to instruments for all three groups. Histogram created from data given in Brockmeier et al. [Citation16].](/cms/asset/b4fdb34c-ea90-44ea-89f2-68dc8f4939fa/ioto_a_1888477_f0021_c.jpg)
This was an encouraging preliminary result showing the added benefit of acoustic amplification when it comes to music perception.
In 2011, MED-EL CE-marked its EAS™ hearing system in combination with DUET-2™ audio processor as a treatment option for children with partial deafness. To restore hearing in the paediatric population with the named technology was another important milestone in MED-EL’s EAS™ journey.
2.8. Evolution of surgical approaches in EAS
While technological advancements in optimising audio processors and implants were the focus at MED-EL, expert CI surgeons were focusing on fine-tuning the surgical procedure which is seen as an essential factor influencing the HP results in EAS patients. In the late ‘70 s, when Prof. Burian from the Medical University of Vienna, performed the first MED-EL CI implantation, the RW approach was used for accessing the cochlea for the electrode array insertion. Then the trend shifted to cochleostomy drilling on the cochlear promontory which was later adopted also in EAS surgery.
In 2003, Prof. Skarzynski performed successful HP surgery with RW approach, and the soft surgical techniques were later described in the year 2007 [Citation17].
In 2004, Prof. Kiefer described the cochleostomy surgical technique, which was applied in the EAS cases at the time [Citation8].
In 2009, the Hearing and Structure Preservation (HSP) consensus meeting that took place in Vienna in Austria, hosted by Prof. Baumgartner and Prof. Gstöttner, resulted in the recommendation of prioritising RW approach over the cochleostomy approach – not just in HP/EAS surgery but in every CI surgery in general. The expert CI surgeons who were the panellists approving this recommendation, were Prof. Baumgartner and Prof. Gstöttner from Medical Faculty of the University of Vienna in Austria, Prof. Lenarz from Hannover Medical School in Germany, Prof. Rask-Andersen from Uppsala University in Sweden, Prof. Skarzynski from the Institute of Physiology and Pathology of Hearing in Warsaw, Poland, and Prof. Van de Heyning from Antwerp University Hospital in Belgium.
In 2010, Prof. Skarzynski reported on the HP results obtained from fifteen EAS paediatric patients implanted with MED-EL CI device with STANDARD/FLEXSOFT™ electrode array, using RW approach [Citation18]. These fifteen patients underwent HP surgery between the years 2004 and 2007 using RW technique to increase the likelihood of better HP results. Pure tone audiograms and Polish version of monosyllabic word test were performed at various time points, including before surgery and one, three, six and twelve months after surgery to follow up on the HP and the hearing performance results. HP immediately after surgery was achieved in all patients; however, three patients were considered as having non-functional partial preservation. The average hearing thresholds measured before surgery and one to four years thereafter showed no statistical significance across any of the frequencies measured, as demonstrated in . The monosyllabic word testing under noisy conditions is shown in . The post-implantation scores after one year of CI use exceeded the preimplant scores in all patients.
Figure 22. Preoperative and postoperative audiograms showing the mean hearing level for each frequency for the CI implanted group (A). Monosyllable scores overtime under the noisy condition for patients with PD. Graph and histogram created from data given in Skarzynski et al. [Citation18].
![Figure 22. Preoperative and postoperative audiograms showing the mean hearing level for each frequency for the CI implanted group (A). Monosyllable scores overtime under the noisy condition for patients with PD. Graph and histogram created from data given in Skarzynski et al. [Citation18].](/cms/asset/3f3d7b3f-5b82-4fc8-a5d7-b40b40702a9e/ioto_a_1888477_f0022_c.jpg)
The results presented in this study indicate the possibility of preserving good LF hearing when using the RW approach with an insertion depth of between 20–30mm
With the introduction of EAS™, the intracochlear structure preservation became an important topic even in cases with no LF residual hearing. In order to make every CI surgery highly atraumatic to the intracochlear structures, MED-EL introduced FLEX28™ electrode array () in the year 2011. It measures 28 mm in implantable length and belongs to the FLEX Series, which aims to preserve the intracochlear structures with deep insertion, especially in cases with expected progressive HL. The suggestion for this electrode came from Prof. Harold Pillsbury from the University of North Carolina as he thought that a slightly shorter than STANDARD electrode would be good for several US surgeons to achieve full insertion offering electric stimulation covering the entire frequency range.
Figure 23. FLEX28™ electrode array with an implantable array length of 28 mm, along with five apical channels in a single line and extra slim configuration (Image courtesy of MED-EL).

In 2019, The first simultaneous bilateral EAS surgery in the world was performed by Prof. Usami and his colleagues from the Matsumoto University in Japan in an adult patient of age 31 who chose MED-EL EAS™ hearing system. FLEX28 electrode was chosen in this patient [Citation19].
2.9. Reimplantation with EAS™ and residual hearing preservation
Reimplantation is an important CI topic in general, as device failure due to variety of reasons could potentially occur. If that shall happen in an EAS™ case, the explantation and subsequent reimplantation should avoid any trauma to the intracochlear structures to ensure successful application of the acoustic amplification of LF residual hearing through the HA unit of EAS™ audio processor – even after the revision surgery. The first report on achieved hearing preservation, following a reimplantation surgery in an EAS patient, was published in 2011 [Citation20] by Dr Hoffman and his colleagues from New York Eye and Ear Infirmary in the US ().
Figure 24. First report on hearing preservation after reimplantation by Dr Ronald Hoffman from New York Eye and Ear Infirmary, New York, USA.

The patient was a forty-three-year-old male who had a sensation of bilateral non-pulsatile tinnitus for many years and wearing HA with decreasing satisfaction. The patient was implanted with MED-EL’s EAS™ hearing system in combination with the FLEX24™ electrode array, inserted via RW opening with ten channels intracochlearly. Three months postoperative unaided audiogram evaluation revealed good preservation of residual hearing, improvement in hearing thresholds and in aided consonant-nucleus-consonant (CNC) word scores. At six months postoperatively, the patient complained of air accumulation under the skin flap, which he tried to remove by rubbing the area with his knuckles, and that resulted in electrode lead’s wire breakage. The CI was explanted ten months postoperatively and reimplanted with FLEX24™ once again. The follow-up audiometric evaluation at three months post-reimplantation revealed good preservation of auditory thresholds, and the patient reported wearing DUET™ audio processor approximately sixteen hours per day maximum, as well as he expressed general satisfaction with the reimplantation.
In 2012, an EAS™ reimplantation of two cases was reported from the University of Western Australia [Citation21] ().
Figure 25. CI surgeons from the University of Western Australia (in 2012) reported on hearing preservation after CI reimplantation surgery.

The first case was a ten-year-old girl with bilateral severe-to-profound mid-to-high frequency sensorineural HL, who was implanted with FLEX24™ electrode array, with eleven out of twelve channels intracochlearly. At eighteen months postoperatively, a suspected device failure was reflected by fluctuating impedances found during the fitting and the patient was re-implanted with FLEX24™ with full cochlear insertion. The patient retained complete hearing preservation after reimplantation, as evident at the three months follow-up pure tone audiogram ().
Figure 26. Pure-tone audiometry results of case 1 (child) and case 2 (adult) with pre-op (grey line), post-1-year CI (black line) and post-re-implantation (red line) audiogram results [Citation21]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 26. Pure-tone audiometry results of case 1 (child) and case 2 (adult) with pre-op (grey line), post-1-year CI (black line) and post-re-implantation (red line) audiogram results [Citation21]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/671f4d44-1428-4dff-b6e8-6be183060cdf/ioto_a_1888477_f0026_c.jpg)
The second patient was a fifty-year-old man with a fifteen-year history of progressive bilateral moderate-to-severe downward sloping sensorineural HL who underwent the first implantation with FLEX24™ electrode array and with eleven contacts intracochlearly. Device failure was detected at thirteen months postoperatively with a subjective sensation of a: ‘Double voice, crackling sound, and an echo.’ The patient was re-implanted with the FLEX28™ electrode array, fully intracochlearly and with no noticeable insertion resistance, resulting in complete hearing preservation, as seen at post-reimplantation pure tone audiogram ().
In 2013, a joint case report from three different centres, in which ENT surgeons shared their findings with hearing preservation during reimplantations, was published [Citation22]. Demographic data of the three patients who had, on average, 23 mm of electrode array inserted intracochlearly during their first implantation, is given in .
Table 2. Demographic data of the three patients with preserved residual hearing after undergoing reimplantation [Citation22].
Before the revision surgery, pure-tone audiogram average of all cases showed considerable LF residual hearing. Hearing thresholds were at least 65 dB at frequencies up to 250 Hz, 85 dB at 500 Hz, and 105 dB at 1,000Hz. Reasons for revision surgery were mainly infection and implant failure, and the patients were reimplanted with FLEX28™, FLEX20™ and FLEX24™ electrode arrays, respectively in three cases as given in . Post reimplantation, the residual hearing in all patients was preserved completely within frequencies up to 250 Hz. This was the first EAS™ patient group reimplantation report, operated by three different ENT surgeons from three different locations, which was, concurrently, concluded with complete hearing preservation ().
Figure 27. Pure-tone audiometric thresholds determined preoperatively and postoperatively in three patients, implanted with EAS™ [Citation22]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 27. Pure-tone audiometric thresholds determined preoperatively and postoperatively in three patients, implanted with EAS™ [Citation22]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/d37763ef-c634-4474-970c-55bb40c1266b/ioto_a_1888477_f0027_c.jpg)
In 2018, Prof. Brown and his colleagues from the University of North Carolina in the US demonstrated the HP in a single patient who underwent CI reimplantation surgery after nine years and with a MED-EL CI device, featuring FLEX24™ electrode array in both instances [Citation23]. The patient had audiometric testing and speech perception test with CNC words in quiet and noise at various time points, including nine years after implantation after the first implanted device failed due to electrode wire breakage and three months after the reimplantation. shows postoperative audiometric test results at various time points, including at the time of device failure and three months post-reimplantation with preservation of LF hearing with thresholds similar to the preoperative findings. Postoperative speech perception testing demonstrated improved performance with EAS as compared with the preoperative performance (). The patient reported a gradual change in sound quality and a significant decline in communication abilities at nine years after the first implantation, with aided speech perception decrease from 90% to 48% in CNC words. Audiometric testing was performed with three months follow-up intervals, and the patient’s residual hearing was unchanged, which demonstrated restoration of aided speech perception performance that matched his best performance with the initial device. This was the first reported case to show normal LF HP after nearly ten years of EAS™ device use and two CI procedures in the same ear.
Figure 28. Unaided pure-tone audiometric hearing thresholds at various time points, including at the device failure time point, and three months post-reimplantation (A). Speech perception test results at various time points (B). Graph and histogram created from data given in Thompson et al. [Citation23].
![Figure 28. Unaided pure-tone audiometric hearing thresholds at various time points, including at the device failure time point, and three months post-reimplantation (A). Speech perception test results at various time points (B). Graph and histogram created from data given in Thompson et al. [Citation23].](/cms/asset/520186fd-9709-45ca-b199-f610d21d7fae/ioto_a_1888477_f0028_b.jpg)
All these reports are supporting the fact that hearing preservation in EAS™ cases is possible even after CI reimplantation, helping the patients to benefit additionally from the acoustic amplification of their LF residual hearing.
2.10. Consensus on the method of hearing preservation classification
Until 2011, the method of calculating the rate of hearing preservation postoperatively in patients with measurable LF residual hearing was simply subtracting the preoperative hearing thresholds from the postoperative hearing thresholds. Typically, if the difference was within 10 dB HL, then the result was considered as complete hearing preservation. This may be making sense if the preoperative audiogram is in the normal to mild HL range in the LF. However, if the patient’s preoperative hearing in LF is in the range of 80 dB or worse, then postoperatively, with the same 10 dB loss, the patient would have no hearing at all, but this could still be considered as complete hearing preservation, which may be misleading.
In 2013, the HEARRING group (www.hearring.com), an independent organisation formed by a group of expert CI surgeons and audiologists, came up with a new classification system in calculating the hearing preservation based on what the patients can actually hear postoperatively, rather than reporting on how much hearing was lost [Citation24]. The HEARRING group proposed the following formula for the hearing preservation classification:
PTA post represents the pure-tone average measured postoperatively, PTA pre is a pure-tone average measured preoperatively, and PTA max is the limit of the audiometer. This equation is representing relative change as a percentage of HL and is applicable for all CI users with measurable preoperative residual hearing (PTA: 0–120dB) across the frequency range measurable with an audiometer. The HL is then converted to hearing preservation by calculating 100% minus the relative change in percent:
S represents hearing preservation on a numerical scale. The numerical scale may be converted to a categorical scale for ease of reporting, as given in .
Table 3. Scale for the proposed hearing preservation classification system [Citation24].
The authors of the report recommended the above formulas to be generally used by clinicians in their clinical practice and their scientific reports, with deciding on electrode array types, and for better evidence-based practice in the CI field ().
Table 4. Clinicians from the HEARRING group who were involved in establishing the method of HP classification.
2.11. EAS™ in unilaterally deaf patients
CI surgery with preservation of LF residual hearing helps partially deaf patients to achieve more natural hearing as a result of combining acoustic amplification in the LF region and the electric stimulation in the HF region. Unilaterally implanted patients with preserved acoustic hearing at LF in the implanted ear will most likely be making use of bilateral LF acoustic amplification if the contralateral ear has sufficient acoustic hearing in the LF region. This is because the hearing preservation in the LF region helps the EAS patients to use their interaural time difference (ITD) and interaural level difference (ILD) cues to separate the target and noise when speech and noise originate from different spatial locations.
In 2013, a multicentric study from the USA and Poland demonstrated indirect benefits of binaural hearing by the preservation of LF residual hearing after CI treatment in unilateral deaf patients [Citation25] ().
Figure 29. Team of clinicians from USA (1Vanderbilt University, 2Arizona State University, 3Mayo Clinic, Rochester, 4University of Texas Southwestern, 5University of North Carolina), Poland (6International Center for Hearing and Speech) and 7MED-EL demonstrated the benefits of binaural hearing by preserving the LF residual hearing during CI procedure in the ipsilateral ear.

Twenty-one native speakers of English and seventeen of Polish language participated in the named study. English speakers were unilaterally implanted with CI from various CI brands, whereas Polish speakers were unilaterally implanted with MED-EL’s 31.5 mm long electrode array (STANDARD) in eleven, and 24 mm of the STANDARD’s array in six patients. All Polish patients were implanted via RW surgical approach, while all English patients were implanted via cochleostomy. In order to understand the binaural benefits, including the squelch effect, head shadow effect and loudness summation effect, the speech recognition in noise experiments were conducted with patients surrounded by eight loudspeakers in a circular pattern. The speech stimuli always originated from the speaker placed at 0° azimuth and the noise was fixed at 72dBA (A is a type of calibration), originating from all eight loudspeakers, which would imitate noise occurring at a large gathering or a noisy restaurant. The speech stimuli in English and Polish language were presented to these two groups of patients at a fixed +6dB and +2dB SNR, as reported in this study. However, personal communication from the authors of the study declares that the Polish patients were actually tested at 0 dB SNR and not at +2dB SNR.
The speech recognition was assessed for all thirty-eight patients in the best-aided EAS condition (CI + binaural acoustic hearing), as well as in bimodal condition with the ipsilateral ear occluded with foam or earplug. The results of the fixed SNR testing at +6dB and +2dB SNR for both English and Polish speakers are given in . For the English speakers implanted with various CI brands, the mean performance at +6dB SNR was 48.7% in bimodal, and 58.3% in the best-aided EAS conditions. At +2dB SNR, the mean performance dropped to 40% in bimodal, and to 50.2% in the best-aided EAS conditions. For the Polish speakers implanted with MED-EL CIs, the mean performance resulted in 79.4% in bimodal, and 85.1% in the best-aided condition at +6dB SNR. At +2dB SNR (which is actually 0 dB SNR, according to the personal communication from the authors), the mean performance dropped to 64.7% in bimodal, and to 74.2% in the best-aided EAS condition. The results obtained from the Polish speakers at 0 dB SNR should be seen much superior compared to the English speaker results obtained at +2dB SNR (typically, +2dB SNR corresponds to approximately 6% of the mean speech recognition improvement). shows normalised benefit as a function of the LF PTA in dB HL for the implanted ear.
Figure 30. Individual and mean speech recognition scores (% correct) for fixed level SNR of +6dB and +2dB for both groups under two different listening conditions and the participant numbers inside the red boxes correspond to MED-EL implanted devices (A). The Polish group given under +2dB SNR was actually tested at 0 dB SNR as per the personal communication from the authors. Normalised EAS benefit for speech recognition at +6dB and +2dB SNR as a function of low-frequency pure-tone average in dB HL (note: Polish group was tested at 0 dB SNR and not at +2dB SNR as mentioned in this study, according to the personal communication from the authors) (B) [Citation25]. Reproduced by permission of Wolters Kluwer, Inc.
![Figure 30. Individual and mean speech recognition scores (% correct) for fixed level SNR of +6dB and +2dB for both groups under two different listening conditions and the participant numbers inside the red boxes correspond to MED-EL implanted devices (A). The Polish group given under +2dB SNR was actually tested at 0 dB SNR as per the personal communication from the authors. Normalised EAS benefit for speech recognition at +6dB and +2dB SNR as a function of low-frequency pure-tone average in dB HL (note: Polish group was tested at 0 dB SNR and not at +2dB SNR as mentioned in this study, according to the personal communication from the authors) (B) [Citation25]. Reproduced by permission of Wolters Kluwer, Inc.](/cms/asset/712eb44e-3517-48d7-aa86-8af68a36a480/ioto_a_1888477_f0030_c.jpg)
An interesting twist of a tale was the finding showing CI insertion depth of >20mm being an effective treatment option for patients with considerable LF acoustic hearing in both ears. The amount of postoperative hearing preservation benefit was seen as the largest at the most difficult listening condition (speech recognition at +2dB SNR). The degree of normalised EAS benefit was also significantly correlated with postoperative LF PTA in the implanted ear, and it in part explains the preservation of ITD cues, responsible for better hearing scores. The advantage of the head shadow effect in the best-aided condition is another possible explanation for the better hearing scores. ILD cues are present for LF stimuli, generally in the range of 2 dB or less and considering the experiment performed in the study, it was hypothesised that ILD cues were present and utilised by the unilaterally CI implanted listeners with binaural acoustic hearing. These data not only provide evidence of functional efficacy for hearing preservation in the implanted ear, but also for the expansion of the CI criteria to include individuals with LF thresholds in even normal to the near-normal hearing range. The study suggests that MED-EL’s EAS™ users have better hearing in noisy situations.
The difference in hearing performance between English and Polish speaking group could have been caused either by the device or the unified audio processor fitting methods. One of the key differences amongst the fitting methods was the selection of cut-off frequency between electric and acoustic stimulation. While MED-EL patients were fitted with cut-off frequency obtained from unaided audiogram at 65 dB HL, for Cochlear™ patients, the fitting method included a selection of cut-off frequency typically at 80 dB from unaided audiogram [Citation26].
2.12. The third-generation unified audio processor
In 2014, MED-EL introduced SONNET®, the third generation EAS™ audio processor with new features, enabling users to enjoy close to natural hearing ().
The acoustic gain from the HA unit of the audio processor was increased from 43 dB in DUET-2™ to 48 dB and maximum power output of 118 dB SPL across all frequencies in SONNET®. Battery life was increased to up to sixty hours, and the volume of acoustic amplification was made adjustable together with the electric stimulation via the same volume control in the FineTuner™. Directionality function of the dual microphones helps to focus on sounds that are coming from the front of the listener and attenuates the background noise. Wind noise reduction was another new feature which minimises the continuous wind noise for improved listening in outdoor environments.
2.13. Clinical trials in Japan and the USA
In the same year, an important clinical trial results published from Japan that was sponsored by MED-EL to evaluate hearing preservation results and speech discrimination outcomes of hearing preservation surgeries using medium electrodes in Japanese-speaking patients [Citation27] . The official clinical trial period was from 1st August 2010 till 1st April 2014. The first patient was implanted on 20th August 2010 and the last patient that was implanted was on 16th November 2012. FLEX24 electrode array was implanted in twenty-five patients.
Figure 32. Team of CI surgeons from Japan: 1Shinshu University School of Medicine, 2Toranomon Hospital, Tokyo, 3Kobe City Medical Center General Hospital, 4Nagasaki University Graduate School of Biomedical Sciences, and 5Miyazaki University School of Medicine were involved in the clinical evaluation of EAS™ hearing system.

The results of the study were highly valuable for approval of MED-EL’s EAS™ hearing system by the Japanese authority which is similar to Food and Drug Administration (FDA) in the USA. In the named study, the hearing preservation surgeries were performed in twenty-nine ears of twenty-seven patients whom all had late or postlingual onset of HF sensorineural HL with a very good functional LF hearing. All patients fulfilled the audiological criteria for EAS and were implanted with FLEX24™ electrode array. The audiometric evaluation in the range between 125–8,000Hz was performed preoperatively and at one, three, six and twelve months after the initial EAS™ stimulation. Pure-tone hearing was evaluated four weeks postoperatively, at the time of CI and EAS™ fitting, as well as at three, six and twelve months. The audiograms of twenty-nine ears are shown in with LF (250–1,000Hz). After the initial deterioration of the pure-tone thresholds at the first CI activation at one month postoperatively, it remained highly stable at the same level for an additional eleven months. Postoperative audiogram measured at twelve months from each of the operated ear is depicted in red color in .
Figure 33. Pure-tone audiograms of each of the twenty-nine operated ears measured at various time points. Black continuous lines correspond to the preoperative time points and the red continuous lines correspond to the twelfth month post-surgery. Shadow indicates the audiological criteria for EAS clinical trial. The average audiogram of all ears is shown within the red outlined section [Citation27]. Reproduced by permission of Taylor and Francis Group.
![Figure 33. Pure-tone audiograms of each of the twenty-nine operated ears measured at various time points. Black continuous lines correspond to the preoperative time points and the red continuous lines correspond to the twelfth month post-surgery. Shadow indicates the audiological criteria for EAS clinical trial. The average audiogram of all ears is shown within the red outlined section [Citation27]. Reproduced by permission of Taylor and Francis Group.](/cms/asset/c2b3cd84-2702-4a37-8db9-92621b44d87b/ioto_a_1888477_f0033_c.jpg)
Improvement of speech discrimination and perception scores are given in . The average monosyllable discrimination score in quiet was improved from 24.1% preoperatively with hearing aid (AS) to 67.4% with EAS 12-months after the first fitting. This postoperative improvement occurred gradually from 48.4% at 1 month to 67.4% at 12 months and was mainly based on the adaptation of electric stimulation, because in a comparison of monosyllable discrimination scores in three conditions (acoustic stimulation only (AS only), electric stimulation only (ES only), and EAS), acoustic stimulation scores changed only slightly from 13.8% to 18.1% at 12 months after the first fitting, but electric stimulation improved from 35.0% to 55.4%. Also, the EAS condition showing the best performance for monosyllable discrimination revealed that acoustic stimulation combined with electric stimulation increases perception ability (EAS results were significantly better than ES only; p < .001) (). Similar results were observed in monosyllable, word, and sentence perception tests in noise. The results for monosyllable perception in noise were improved from 21.0% preoperatively with hearing aids to 60.2% with EAS 12-months after the first fitting. This postoperative improvement occurred gradually from 36.9% at 1 month to 60.2% at 12 months. Also, EAS results (60.2% correct) were significantly better than AS only (13.9% correct) and ES only (46.0% correct) results (p < .001 and p = .009) (). The average word and sentence perception test score in noise improved from 35.8%, and 51.3% to 77.0%, and 88.2%, respectively (). In both word and sentence perception tests, EAS showed the better results. EAS results were significantly better than the ES only results (p = .002 for word and p = .01 for sentence)
Figure 34. Mean values for speech discrimination and perception scores at different time points and under different listening conditions [Citation27]. Monosyllable word test in quiet (A), in noise (+10dB SNR) (B), word test in noise (+10dB SNR) (C), and sentence test in noise (+10dB SNR) (D). Statistical analysis: paired t-test. Reproduced by permission of Taylor and Francis Group.
![Figure 34. Mean values for speech discrimination and perception scores at different time points and under different listening conditions [Citation27]. Monosyllable word test in quiet (A), in noise (+10dB SNR) (B), word test in noise (+10dB SNR) (C), and sentence test in noise (+10dB SNR) (D). Statistical analysis: paired t-test. Reproduced by permission of Taylor and Francis Group.](/cms/asset/6794efe7-de8e-4fcf-ab8c-aa52a996967f/ioto_a_1888477_f0034_b.jpg)
The study concluded that EAS™ is beneficial also for Japanese-speaking patients with less residual hearing at lower frequencies, indicating that the indication criteria could be expanded for EAS.
In 2018, a multicentric FDA clinical trial study, sponsored by MED-EL, was published (. The first and the last patient within this clinical trial was implanted in April 2007 and in December 2014 respectively. The study results were collected until February 2016.
The study included sixteen different CI centres within USA to evaluate the safety and effectiveness of the EAS™ system in adults with residual LF hearing and severe-to-profound HL in the mid-high frequencies. Also, evaluating the speech perception in quiet and noise was part of the study objective [Citation28]. Altogether, seventy-three patients who met the EAS inclusion criteria were part of the study, and they were implanted with the EAS™ system with FLEX24™ electrode array for an insertion depth of approximately 20 mm. Access to the cochlea was achieved via the RW approach in fifty-five (75.3%), and via the cochleostomy approach in seventeen (23.3%) patients, while the approach was unspecified in one patient (1.4%). Postoperatively, the patients were fitted with DUET audio processor, combining electric stimulation and acoustic amplification. In total, 67 of the 73 patients completed the audiometric testing and effectiveness outcome tested preoperatively and three, six and twelve months. Speech perception was assessed at these intervals using the City University of New York (CUNY) sentences in noise and CNC words in quiet.
Figure 35. Clinicians from CI clinics across the USA who were involved in the FDA clinical trial study evaluating the safety and effectiveness of MED-EL EAS™ system. 1University of North Carolina, 2Kansas University Medical Center, 3Hospital of the University of Pennsylvania, 4Miller School of Medicine of the University of Miami, 5Medical College of Wisconsin, 6Stanford University, 7New York Eye and Ear Infirmary, 8Duke University, 9University of Texas Southwestern Medical Center, 10Indiana University, 11Swedish Neuroscience Institute, 12Oregon Health Sciences University, 13Michigan University, 14Boys Town National Research Hospital, Nebraska and 15MED-EL.

An initial decrease in unaided thresholds was shown by 3 months post-activation, and thresholds remained stable (within 2 dB HL) through the 12-month interval (). Mean LF-PTA increased by 24.1 dB in sixty-seven patients who were tested at both, the preoperative and at the twelfth-month, post-activation intervals. Out of these, fifty-three patients (79.1%) experienced a LF-PTA shift of less than 30 dB HL. Eight patients (11.9%) had profound or total HL, as determined by a LF-PTA of >90dB HL. A total of sixty-five out of sixty-seven patients (97.0%) were able to use EAS through DUET™ audio processor at twelfth-month post-activation.
Figure 36. Average pure-tone unaided thresholds. Lines show mean audiograms obtained preoperatively (grey, solid line, diamonds), at three months (grey, long-dashed line, circles), at six months (grey, short-dash line, stars) and twelve months post-activation (black line, triangles). Error bars indicating standard deviation from the mean are shown for pre-op and twelve months interval (A). Speech recognition scores for all patients followed-up until the twelfth-month post-activation interval. Scores for CUNY sentences in noise (B) and CNC words in quiet (C) are represented by filled circles for patients using EAS and open triangles for those tested in CI alone condition. A solid reference line is shown on both figures, indicating no change in score from the preoperative to the twelfth-month post-activation interval. Dashed lines are shown at ±10% of the solid reference line to indicate scores that may fall within test-retest variability [Citation28]. Statistical analysis: paired t-tests and Wilcoxon signed-rank test, with least-square means used to estimate change from the preoperative interval. Reproduced by permission of Wolter Kluwer Health, Inc.
![Figure 36. Average pure-tone unaided thresholds. Lines show mean audiograms obtained preoperatively (grey, solid line, diamonds), at three months (grey, long-dashed line, circles), at six months (grey, short-dash line, stars) and twelve months post-activation (black line, triangles). Error bars indicating standard deviation from the mean are shown for pre-op and twelve months interval (A). Speech recognition scores for all patients followed-up until the twelfth-month post-activation interval. Scores for CUNY sentences in noise (B) and CNC words in quiet (C) are represented by filled circles for patients using EAS and open triangles for those tested in CI alone condition. A solid reference line is shown on both figures, indicating no change in score from the preoperative to the twelfth-month post-activation interval. Dashed lines are shown at ±10% of the solid reference line to indicate scores that may fall within test-retest variability [Citation28]. Statistical analysis: paired t-tests and Wilcoxon signed-rank test, with least-square means used to estimate change from the preoperative interval. Reproduced by permission of Wolter Kluwer Health, Inc.](/cms/asset/abf850ee-ee71-4907-ab2e-08742d5309e7/ioto_a_1888477_f0036_b.jpg)
summarizes the improvement in hearing scores as measured by CUNY sentences in noise and CND words between the preoperative and postactivation time points. Such an improvement was seen in both EAS and electric only mode.
Table 5. Summary of primary and secondary effectiveness endpoints.
Individual speech perception outcomes of CUNY sentences in noise and CNC words in quiet are shown in respectively for all sixty-seven patients reaching the twelfth-month study endpoint, with percentage correct shown as a function of the preoperative score. Patients with speech perception scores greater than 10% above the reference line were classified as performing better. Scores within ±10% of the reference line were classified as results with similar performance, and scores >10% below the reference were classified as results with worse performance. With CUNY sentences in noise, fifty-seven out of sixty-seven patients (85.1%) performed better at twelfth-month in the EAS condition, compared with the preoperative aided condition, when tested in the implanted ear. With CNC words in quiet, fifty-nine out of sixty-seven patients (88.1%) performed similarly or better at twelfth-month in the CI alone condition (full-frequency electric map) compared with the preoperative aided condition. Four out of sixty-seven patients (6.0%) performed worse on both tests (CNC and CUNY) at twelfth-month with electric stimulation only.
This FDA clinical trial data illustrated a successful application of combined electric and acoustic stimulation in adult CI recipients with LF residual hearing. Patients experienced additional performance and subjective benefits from the EAS, beyond those of electric stimulation alone, reconfirming the advantages of the EAS, particularly in difficult listening conditions. The FDA approval for implantation with a thin, flexible long electrode and combined EAS provides an effective treatment option for individuals with low-frequency acoustic hearing who do not meet traditional CI candidacy.
In 2013 and 2017, MED-EL received approval in Japan and FDA approval in the USA for its EAS™ hearing system, which was a significant – and at the time unprecedented amongst all CI brands – milestone.
Cochlear Corporation, another CI manufacturer received FDA approval of its Hybrid hearing system in the year 2014 [Citation29]. Cochlear recruited 50 patients in their clinical trial study and a 16 mm long electrode array was inserted through a small cochleostomy drilling inferior to the RW entrance. They evaluated the hearing benefits using CNC word test at six-month postactivation whereas MED-EL evaluated at 12-month postactivation. compares the results of Cochlear Hybrid hearing system with MED-EL EAS hearing system.
Table 6. Summary of primary outcomes (CNC word scores) comparing MED-EL EAS and Cochlear Hybrid hearing system.
While the word scores at the pre-operative acoustic only mode and postactivation are more or less similar in both the clinical trials, it is important to note the electrode array length, which is 20 mm with MED-EL EAS whereas it was 16 mm with Cochlear Hybrid. Within 6 months of postactivation, 22 out of 50 patients (44%) of the patients from the Cochlear Hybrid group lost the residual hearing while it was only 8 out of 67 patients (11.9%) from MED-EL EAS group at 12-months postactivation. This can only be explained for the flexible nature of MED-EL’s electrode incorporating wavy wires conserving the intra-cochlear structures which would not have been the case with Cochlear’s thin electrode that incorporates straightened metal wires bunched together giving the rigid property. The cochleostomy approach was predominantly used in all the patients in the Cochlear’s clinical trial while it was mainly the RW approach in majority of the patients and cochleostomy approach in some patients in MED-EL’s clinical trial.
2.14. Long-term hearing preservation results from around the world
It is evident that the integration of residual acoustic hearing can increase speech perception in noise as well as music appreciation in candidates for electric stimulation with a CI [Citation4–6,Citation8,Citation12–17]. Despite optimal electrode array design, surgical technique and corticosteroid treatment, long-term HL may occur due to intracochlear immunocompetent reactions to the electrode array, or due to blood components or other elements that lead to an inflammatory response. Furthermore, genetically driven HL progression is discussed as a potential cause. The question remains whether residual hearing can be preserved for a long time after HP surgery or not, and therefore, long-term results in the EAS patient population are of great importance. Over the years, several CI specialising groups around the world have reported on the long-term results observed from their centres, and this section will cover most of those pieces of evidence.
In 2010, Prof. Skarzynski and his colleagues shared their clinical experience in treating partially deaf (PD) patients [Citation30].
Ten years of management of PD adults and children, with varying levels of preservation of residual hearing, using combined stimulation (EAS).
Seven years of follow-up of PD adults who retained 93.2% of good low-frequency hearing after implantation, complemented electrically (EC).
Nearly five years of follow-up of PD children, who retained 100% of good LF hearing after implantation, complemented electrically (EC).
Seventeen years of experience using RW approach, gained since the initial stages of the Warsaw CI program already.
In 2011, Dr Helbig and her colleagues from Johann Wolfgang Goethe University Hospital Frankfurt in Germany reported on thirty-three months follow-up of HP results from twenty-two severely to profoundly deaf patients with measurable residual hearing preoperatively, implanted with MED-EL’s FLEXSOFT™ electrode array using RW approach [Citation31]. Pure tone audiograms were measured for these patients at three different time points, including at preoperative stage, at thirty-three months post-surgery and one measurement was performed intermediately. shows the dimensions of FLEXSOFT™ electrode array and the mean pure-tone audiometric results of patients with preserved hearing at three time points. A statistically significant drop in the hearing was observed between preoperative and postoperative measurements, but not between intermediate and long-term measurements. The study demonstrated that postoperative residual hearing is stable in most cases in medium to long term (six to thirty-three months follow-up if implanted with a flexible electrode array).
Figure 37. FLEXSOFT™ electrode array with its dimensions in millimetres (image courtesy of MED-EL). Mean pure-tone audiograms measured at three different time points [Citation31]. Statistical analysis: Nonparametric Wilcoxon signed-rank test to look for the difference between test intervals (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 37. FLEXSOFT™ electrode array with its dimensions in millimetres (image courtesy of MED-EL). Mean pure-tone audiograms measured at three different time points [Citation31]. Statistical analysis: Nonparametric Wilcoxon signed-rank test to look for the difference between test intervals (p < .05). Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/dd295262-a5ea-46f5-881c-6b14981b726a/ioto_a_1888477_f0037_b.jpg)
In 2013, Prof. Atlas and his colleagues from the University of Western Australia studied the long-term HP rates (>24 months) in thirteen CI recipients implanted with MED-EL EAS™ and FLEX24™, who had measurable LF residual hearing before surgery [Citation32]. depicts the HP rate, and at under three months post-operation time, complete HP was observed in 42.9%, partial HP in 50% and minimal HP in 7.1% of the patients. Between six and twelve months, complete HP reduced to 22.2% patients, partial HP was seen in 66.7% and minimal HP in 11.1% of the patients. Between twelve and twenty-four months, the complete HP slightly increased to 33.3%, partial HP to 22.2% and minimal HP to 44.4%. Until the twenty-fourth month, there was no case reported with complete loss of residual hearing. However, beyond twenty-four months of follow-up, 12.5% of patients had a complete loss of residual hearing, whereas 25% maintained complete HP. The study concluded that because of the electric stimulation from the long electrode array there was no difference in the quality of life witnessed between EAS and non-EAS users (not shown in ), and if the LF hearing is preserved, patients will enjoy the added benefits of EAS, including more natural hearing and music appreciation.
Figure 38. Prof. Marcus Atlas studied the long-term HP results from his patient population implanted with MED-EL EAS™ system. Results are summarised in the above graph: hearing preservation over time and the linear trendline summarises changes over time—statistical analysis: 1-way ANOVA test. Histogram created from the data given in Santa-Maria et al. [Citation31].
![Figure 38. Prof. Marcus Atlas studied the long-term HP results from his patient population implanted with MED-EL EAS™ system. Results are summarised in the above graph: hearing preservation over time and the linear trendline summarises changes over time—statistical analysis: 1-way ANOVA test. Histogram created from the data given in Santa-Maria et al. [Citation31].](/cms/asset/f865d187-ca89-466a-a436-9732364aecc9/ioto_a_1888477_f0038_c.jpg)
In 2014, the first long-term results on the HP in the range of ten years follow-up were reported by Prof. Van de Heyning and his colleagues from Antwerp University Hospital in Belgium, in which they studied nine post-lingually partially deaf EAS patients who underwent HP surgery [Citation33]. HP rates were evaluated preoperatively and three, six, twelve, eighteen and twenty-four months postoperatively, as well as annually after that. None of the patients had progressive HL, autoimmune disease, nor both. The electrode array types MEDIUM, FLEX28™ or FLEXSOFT™ were used, and HP formula proposed by the HEARRING group was applied for calculating the HP rates. quantifies the degrees of HP, showing complete HP obtained in ‘subject 2′ (left ear, 85%) and ‘subject 5′ (100%) up to six years postoperatively. Partial HP was achieved in ‘subject 1′ (47%), ‘subject 2′ (right ear, 56%), ‘subject 4′ (left ear, 34%; right ear, 56%), and ‘subject 6′ (44%) up to six years postoperatively. There was only minimal RH in ‘subject 7′ (13%) and ‘subject 3′ (19%) six years after surgery. In summary, the study conveys that long-term HP in EAS users after HP surgery is possible, although there is a small continuous decline of 3% HP per year. Not shown in , the long-term speech perception results from the study showed a continuous statistically significant improvement for monosyllables in quiet, sentences in quiet, and sentences in noise. Also, the subjective benefit was found already three months after the implantation.
Figure 39. Hearing preservation for each patient using the HP numerical scale [Citation33]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 39. Hearing preservation for each patient using the HP numerical scale [Citation33]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/27fc0f33-ef86-452c-aa28-82793ba1819a/ioto_a_1888477_f0039_b.jpg)
In 2016, Prof. Usami and his colleagues from Shinshu University School of Medicine and International University of Health and Welfare, both in Tokyo in Japan, evaluated the long-term threshold changes in the LF hearing region by comparing patient groups with stable hearing and progressive HL [Citation34]. Altogether, seventeen individuals were enrolled and received MED-EL EAS™ implant with FLEX24™ electrode array through RW approach along with an intraoperative/systemic infusion of dexamethasone. Postoperative HP rates were calculated using the HEARRING HP numerical scale. Under the stable hearing group (), two patients had complete HP over the five years, whereas six patients had either partial or minimal HP. Within the progressive HL group (), four patients had complete HP until the second year after surgery, and after that, it migrated to partial HP. Within this group, another four patients who had partial HP until the second year shifted to mild HP after that. In short, the HP rates remained stable over time within the stable hearing group, whereas it declined over time in the progressive HL group (). The authors concluded that EAS™ provided better speech perception scores (data not shown in ) in those patients with a larger degree of residual hearing compared with those who had at least minimal hearing preserved in the LF hearing region. Furthermore, the authors suggested that EAS™ can provide improvement in hearing ability over the long term even if the residual hearing is lost to some extent over time.
Figure 40. The linear regression coefficient for the decline in HP score of the stable hearing group, as categorised from the average linear regression coefficient of the decrease in hearing preservation score (A). The linear regression coefficient for the decline in HP score of the progressive HL group (B) [Citation34]. The dotted line in black in both graphs, indicates the average for the contralateral ear. Reproduced by permission of Taylor and Francis Group.
![Figure 40. The linear regression coefficient for the decline in HP score of the stable hearing group, as categorised from the average linear regression coefficient of the decrease in hearing preservation score (A). The linear regression coefficient for the decline in HP score of the progressive HL group (B) [Citation34]. The dotted line in black in both graphs, indicates the average for the contralateral ear. Reproduced by permission of Taylor and Francis Group.](/cms/asset/3d593568-5692-41c3-86a4-8020a7f3462c/ioto_a_1888477_f0040_c.jpg)
In 2016, another report on the long-term HP (up to twenty-four months) was published. Ninety-six EAS patients (one hundred and three ears) implanted with MED-EL EAS™ with FLEX24™ were included in the study in which the HP techniques were applied by Dr Helbig and her colleagues from the Johann Wolfgang Goethe University Hospital Frankfurt in Germany [Citation35]. Forty-seven out of ninety-six patients had a history of progressive HL in the HF, and unknown aetiology was reported in twenty-four patients. The remaining patients had other aetiology, including sudden HL, viral infection, autoimmune disease and other. Immediate postoperative results showed that 25% of the patients had complete HP, and 60% partial HP. At twelve months after surgery, 27% had complete HP, and 55% maintained partial HP. At long-term follow-up (up to twenty-four months), the complete HP rate dropped to 12%, and partial HP was maintained in 53% of the patients. Regarding the complete loss of residual hearing, a slight inclination in the number of cases from the immediate postoperative stage (4%) to long-term (15%) was observed (). The authors concluded that long-term residual LF HP is feasible in a subset of patients implanted with EAS™. From the residual hearing cohort, eighty-two out of ninety-five patients (85.3%) could utilise acoustic amplification post-operatively, fifty-eight out of sixty-six (87.9%) after twelve months, and thirty-eight out of forty (95.0%) in the long-term outcomes.
Figure 41. HP with regards to shifts of pure-tone averages in low frequencies (125-, 250- and 500-Hz). Results are shown for postoperative, 12 months, and long-term shifts (>24 months) from pre-operative measurement. Histogram created from data given in Helbig et al. [Citation35].
![Figure 41. HP with regards to shifts of pure-tone averages in low frequencies (125-, 250- and 500-Hz). Results are shown for postoperative, 12 months, and long-term shifts (>24 months) from pre-operative measurement. Histogram created from data given in Helbig et al. [Citation35].](/cms/asset/0ec195a4-5c3d-4fd3-95af-ce91c283c47a/ioto_a_1888477_f0041_c.jpg)
In 2020, Prof. Sprinzl and his colleagues from the University Clinic St. Pölten in Austria studied the long-term HP results (>12 months) from eight patients (ten ears) implanted with MED-EL EAS™ and FLEX24™ at their centre [Citation36] ().
Figure 42. Clinicians from University Clinic St. Pölten, Austria, who investigated the long-term HP in EAS™ CI users.
They reported that in the long-term, complete HP was achieved in 50% of the ears, partial HP in 40%, and minimal HP in 10% of the ears (). They further mentioned that none of the patients lost the residual hearing completely. They concluded that the combination of acoustic and electric stimulation via the EAS™ system is a safe, effective, and most importantly, stable treatment option for patients with normal-to-moderate HL in the LFs and severe-to-profound HL in the HFs.
Table 7. Eight patients (ten ears) with long-term follow-up data [Citation36].
All these pieces of scientific evidence show encouraging results, pointing to the direction of long-term HP possibility in the majority of the EAS patients implanted with a flexible electrode array. Inflammatory and immunocompetent reactions, as well as individual genetic background, are assumed to be the main influences of counteracting HP in some cases.
The University of North Carolina, USA is in the process of publishing (at the time of writing this chapter) their findings on long-term hearing preservation results from the patient population who were a part of MED-EL EAS FDA clinical trial. FDA required a post-approval study following subjects from the original EAS clinical trial for at least five years post-implantation. Fifty subjects returned for follow-up in the post-approval study, with more than half of the subjects (68%) having six years or more of experience with their EAS device. At the long-term interval, all subjects continued to experience improvement over pre-operative speech perception scores on at least one measure. Of the 50 subjects in the long-term study, 43 maintained aidable low-frequency residual hearing (at least one threshold better than or equal to 80 dB between 125 and 1000 Hz). Further data analysis will be completed and published in the future.
2.15. Optimised fitting procedure in EAS patients
With the unified EAS™ audio processor that combines acoustic amplification and electric stimulation in one ear, there was a need to develop an optimised fitting strategy. Typically, the EAS candidates have much higher expectations of hearing performance after implantation in comparison to standard CI candidates. The EAS users usually reach the ceiling effect in speech test in quiet and expect greater improvements with the speech in noise test.
In 2002, Prof. Wilson (with his colleagues) from the Center for Auditory Prosthesis Research, Research Triangle Park in the US was the first person to find out that electric and acoustic overlap is beneficial [Citation37].
In 2005, Prof. Kiefer and his colleagues from Johann Wolfgang Goethe University Hospital Frankfurt in Germany fitted thirteen EAS patients with CI processor and a HA on the implanted side and based on their findings they proposed that the acoustic amplification cut-off frequency be obtained from the unaided audiogram at 65 dB HL [Citation38].
In 2008, Dr Vermeire and her colleagues from the Antwerp Medical University in Belgium fitted four EAS patients with CI processor and a HA on the implanted side and evaluated the HA amplification [Citation39]. The authors reconfirmed that reduced electric and acoustic overlap is beneficial to reach better EAS benefit. However, questions remained on how much overlapping between electric stimulation and acoustic amplification in EAS patient is needed to reach the most optimised benefit and secondly, how with the combined audio processor, the acoustic and electric parameters interact.
In 2010, Dr Polak from MED-EL, together with clinicians from multiple centres, evaluated twenty-four EAS patients fitted with DUET™ audio processor that combined both acoustic amplification and electric stimulation [Citation40]. The authors evaluated (i) when to fit electric and acoustic modality, (ii) important acoustic and electric parameters for DUET™, (iii) modified half gain rule by use of DUET™, (iv) optimal gain, (v) effect of cut-off frequency in EAS and electric-only mode, and (vi) they identified optimised benefit for EAS patients using the unified audio processor. DUET™ HA was fitted with half gain rule and compared with the individual gain adaptations. Electric stimulation was tested for full frequency range and minimum frequency obtained from unaided audiogram at 50-, 65- and 80-dB HL. Tested acoustic and electric parameters parameters included compression threshold (40–70dB), low cut slope (Th500-Th250)/2, 0, 18 dB/octave), compression threshold (1:1 − 1:2) and lower electric frequency (200 Hz from unaided audiogram – at 50, 65, and 80 dB HL) The experiments were performed at electric-only or EAS-only mode. For each test condition, patients were asked to switch their processor to the testing condition for either two hours or one day, depending on the test difficulty. The authors observed a difference in speech performance between both CI frequency ranges in Electric-only and EAS-only conditions. Therefore, it was proposed to fit both modalities (acoustic and electric) at the same time. Overall, the half gain rule was satisfactory; however, it was necessary to adjust the optimal gain for each patient. 81% of the patients reached the best score when the acoustic cut-off frequency was obtained at 65 dB HL from unaided audiogram. The cut-off frequency difference of 50 Hz from the optimised value influenced the outcomes. To reach optimised benefit, electric and acoustic overlap was needed. Selecting the correct cut-off frequency had the highest impact on the optimised benefit. For the tested group, single parameter changes from optimised value degraded the benefit by 32.3% in EAS mode. Other parameters influencing the EAS performance were low cut slope, compression threshold and compression rate. A single change in any of these parameters from the optimal value degraded the overall speech benefit from 20.5% (for low cut slope) to 23.0% (for compression). depicts optimised benefits of the tested groups. EAS improvement for monosyllable in quiet was 47%, while EAS benefit over electric-only mode varied from 10% to 15% depending on speech tests. Data showed a relatively small benefit for HA only condition. Immense synergistic benefits when adding CI to the acoustic hearing and when adding ipsilateral (implanted ear) HA to the CI was seen.
Figure 43. Speech results of all patients with the DUET™ fitting parameters [Citation40]. CI = electric only; HA = acoustic only; DUET = EAS; (image courtesy of Dr Polak from MED-EL).
![Figure 43. Speech results of all patients with the DUET™ fitting parameters [Citation40]. CI = electric only; HA = acoustic only; DUET = EAS; (image courtesy of Dr Polak from MED-EL).](/cms/asset/1d7ff2a3-f5a4-4823-b6eb-fca26f919d69/ioto_a_1888477_f0043_c.jpg)
Together with the development of a surgical pre-planning tool for individualised electrode array selection, there was a need to develop an EAS fitting strategy with deep electrode array insertion. This was especially important with several clinics beginning to implant patients with better residual hearing and with long electrode arrays, such as with STANDARD, FLEX28™ or FLEXSOFT™ with full intracochlear insertion.
In 2016, at the XV Hearing and Structure Preservation Workshop, Oct 20–23, Paris, Dr Polak, Prof. Skarzynski and Prof. Lorens introduced the so-called Natural Based EAS fitting [Citation41]. In patients utilising both, acoustic and electric modality, one of the important aspects is to find out how many electrodes are inserted in the region with the residual hearing, and in patients with the anatomical gap, to find out how many electrodes are missing to reach the identified acoustic region. Having such information, the electrodes residing in the acoustic region may be turned off, and the remaining electrodes may be fitted according to the frequency tonotopicity for electric stimulation. Acoustic amplification is performed for the low frequencies with the cut-off frequency obtained from the unaided audiogram, typically at 65 dB-HL. Another approach may propose to keep all electrodes residing in the acoustic region on and at the same time to keep the frequency tonotopicity for the remaining electrodes residing in the electric region. This notion is based on outcomes reported by Prof. Lorens and colleagues [Citation42], showing no degradation in speech benefit if all electrodes are activated (including the electrodes in the acoustic region); the reason for this may be that the electric and acoustic stimulation provide a frequency match. If the electrodes residing in the acoustic region remain turned off, with ongoing hearing deterioration (i.e. from clinical observation at the Institute of Physiology and Pathology of Hearing in Warsaw, Poland, the natural drop of hearing in EAS users is 2–3dB per year), the electrode in the apical region may be turned on once the hearing in this region is not functional anymore. The advantage of such a fitting is that the electrodes responsible for the higher frequencies will not change their frequency allocation, so with possible acoustic hearing deterioration, no adjustments to any parameter changes are required.
Additionally, all EAS patients utilise their complete fine structure information. If patients can utilise only limited fine structure information via acoustic amplification, the remaining higher frequency fine structure information is supplied via fine structure channels (i.e. FS4 strategy, MED-EL). Therefore, the Natural Based EAS fitting may utilise both principles, the phase-locked temporal information for the low frequencies and the organised firing of groups of nerve fibres at higher frequencies.
This method of natural-based fitting was recently applied by Prof. Usami and his colleagues in EAS patients implanted with longer electrode arrays and found to be beneficial [Citation43].
2.16. EAS in paediatric patients
Paediatric patients are a select group, as additional care needs to be taken during all phases of CI treatment. Children with partial deafness display different speech development and language acquisition patterns when compared to normal-hearing children or children with severe-to-profound sensorineural HL [Citation44]. Not every CI surgeon is willing to perform the delicate surgery in children and especially in children with partial deafness, as any minor degree deviation from soft surgical techniques could compromise the LF residual hearing – which is crucial for speech and language development. This section will list the key surgeons who implanted EAS™ in paediatric patients and will also cover the consensus statement on the identification of paediatric EAS criteria.
In 2000, Prof. Kiefer and his colleagues performed the first surgery in a child who had a measurable residual hearing at 125-, 250-, 500- and 1,000-Hz. The patient was implanted with MED-EL CI for electric stimulation of the HF region starting at 1,000Hz, and acoustic amplification of LF below 1,000Hz was amplified with conventional HA. This was an important milestone in MED-EL’s EAS journey.
In 2004, Prof. Skarzynski and his colleagues implanted the first PDCI paediatric patient in Poland by applying the HP techniques with MED-EL EAS™ CI device.
In 2012, the youngest child at the time to receive EAS™ with HP surgical technique was a thirteen months old infant, operated by Dr Kuthubutheen and Prof. Rajan from the University of Western Australia.
In 2018, Prof. Usami and his colleagues implanted an eleven months old infant – considered the youngest child to date at the time – with EAS™ and with HP technique.
In 2018, amongst the HEARRING members, a consensus was made for paediatric EAS patients, based on which criteria for identification of children with partial deafness was established – as in past experiences, the latter represented a challenge due to lack of verbal feedback in audiological assessment in children. Regarding the age-dependent language development, four groups of children were identified, and recommendation of an assessment tool based on age was proposed ().
Table 8. Recommended age-specific assessment tools. [Citation44].
In summary, partial deafness in children is a hearing disability which needs to be identified early to avoid the risk of permanent speech and language deficits. The consensus included on strong recommendation that every child undergoing CI shall be implanted using the HP surgical technique, irrespective of the level of residual hearing.
2.17. Advancements in EAS
Like any other field, EAS has also been complemented with several advancements in terms of technological, surgical and audiological aspects in the last years, and this section will list the key published pieces of evidence in this regard.
2.17.1. Combination of cochlear duct length and Greenwood’s frequency map
It is known since the 1930s from Dr Hardy’s work that the cochlear duct length (CDL) varies among the human population, from a minimum of 25.26 mm to a maximum of 35.45 mm – facts established through histological assessment of cadaveric human cochleae [Citation45]. The fundamental question of how the individual patient’s CDL could be estimated from the preoperative radiographic images has been a great motivation for MED-EL to conduct extensive researches since 2010. The first research began with the collaboration between MED-EL engineers and Dr Alexiades, intending to establish mathematical equations which would estimate the CDL along the organ of Corti and the tonotopic insertion depths ().
Figure 44. ENT surgeon from New York, USA, and engineers from MED-EL established the mathematical function in the estimation of patient-specific CDL.

The reason for estimating the CDL along the organ of Corti is that the lateral wall electrodes, once placed inside the ST, would position precisely under the BM and therefore it is the most reasonable to establish the CDL along the organ of Corti. Moreover, Greenwood’s frequency function may be further applied to estimate the patient-specific frequency map along the organ of Corti [Citation46]. By combining the data from Dr Hardy [Citation45] and Dr Escude [Citation47], the resulting mathematical equations [Citation48] would take a single cochlear measurement (the cochlear diameter, or so-called A-value) in the oblique coronal radiological plane as the only input from the preoperative computed tomography (CT) images, as given in .
Figure 45. Mathematical equations to estimate the CDL along the organ of Corti (Image courtesy of MED-EL).
Combining CDL [Citation48] and the Greenwood’s function [Citation46], as given in , would present the picture to clinicians with cochlear insertion depth, including the identification of where the LF residual hearing starts – consequently helping in choosing the most suitable electrode array length. This opened a new door towards the even more individualised approach with taking into consideration each individual and unique cochlear anatomy, providing anatomy-based CI treatment to each patient [Citation49]. This was a concept MED-EL proposed in the year 2011 and developed the research-based CDL software in 2014 (link to download).
Figure 46. Applying the CDL value in Greenwood’s frequency function would result in patient-individual frequency map. From this map, the starting point of LF residual hearing is possible to identify (image courtesy of MED-EL) [Citation49].
![Figure 46. Applying the CDL value in Greenwood’s frequency function would result in patient-individual frequency map. From this map, the starting point of LF residual hearing is possible to identify (image courtesy of MED-EL) [Citation49].](/cms/asset/99e3109e-b0b0-49ff-b5b0-23aa22ff5979/ioto_a_1888477_f0046_c.jpg)
In 2018, MED-EL joined hands for a specific project with CAScination AG, a Swiss company which developed a tablet-based otological planning software OTOPLAN® (www.otoplan.ch). With dedicated cooperation in this domain, the tool incorporated much of MED-EL’s research efforts as shown in and , and further fine-tuned it to develop an even more sophisticated tool which utilises the cochlear parameters as an input to estimate the CDL, along with the corresponding frequency mapping and the visualisation of MED-EL’s electrode portfolio, tailored for specific patient anatomies. It also allows 3 D segmentation of delicate anatomical structures of the temporal bone, as well as it can precisely calculate safe trajectory, for either conventional or robotic surgery with its minimally invasive surgical system, HEARO®. Dr Zoka Assadi from MED-EL was highly instrumental logistically in the joint development of OTOPLAN® software.
To summarise, combining cochlear parameters measured from the preoperative radiological imaging with Greenwood’s frequency function, it is possible to establish the exact location of the functional acoustic region towards the lower frequencies in a partially deaf cochlea. This also helps to choose the optimal electrode array length and to foresee different varieties in finding the tonotopic frequency match, as shown in .
Figure 47. Illustration of Greenwood’s frequency map for an average CDL of 35 mm with an assumption of low frequency functional residual hearing, starting at 1,000Hz. Visualisation of different electrode array lengths shows how many channels would be in the acoustic amplification zone (image courtesy of MED-EL).
2.17.2. Long electrode arrays in classic EAS candidates
It has become a trend to use short electrode arrays in EAS patients to preserve the LF residual hearing. The latter was reflected in literature until 2010, describing electrode insertion depth to up to 18–22mm as a standard in clinical practice amongst clinicians globally. An important question that accompanied the EAS topic was residual HL over time or during surgery, and more precisely, would a short electrode array suffice in providing full electric coverage in such situations. Clinicians believed that implanting a longer electrode array, which physically reaches well beyond the basal turn of the cochlea and still effectively preserves the LF residual hearing, would be the most desirable option. If the LF residual hearing deteriorates over time, a long electrode array will substitute it with electric coverage – with an eventual extension over the entire frequency range. Studies have shown that if EAS patients lose residual hearing over time and if they had symmetric hearing preoperatively, then the synergistic EAS effect is still preserved, i.e. if the contralateral ear retains residual hearing. In such a case, the healthy ear would still offer support to the implanted ear, despite its residual HL [Citation12,Citation24].
In 2010, Prof. Staecker and his colleagues from University of Kansas in the US moved forward with implanting a long electrode array in eighteen patients with measurable LF residual hearing – but not EAS candidates – and evaluated the effect of CI electrode array insertion depth on HP [Citation50]. A total of eighteen patients were implanted with the soft surgical technique with either MEDIUM (24 mm electrode array length) or STANDARD (31.5 mm electrode array length). Electrode arrays reached intracochlear insertion depth in the range of 20–28mm with a near-complete cochlear frequency coverage. The LF residual hearing was well preserved, as shown in , and the PTAs were calculated for the frequencies 250-, 500-, and 750-Hz, and plotted against the electrode insertion depth. The graph in demonstrates no clear relationship between the electrode insertion depth and the amount of residual hearing preserved, indicating that the apical region of the cochlea may be reached without compromising hearing thresholds (r2=.091). This was one of the early studies that demonstrated the possibility of implanting long electrode arrays in patients with functional LF residual hearing.
Figure 48. Example audiogram of pre-op (open-circle) and post-op (crossed circle) with the MEDIUM performed with a MED-EL STANDARD electrode array. The HP remained stable over eighteen months (A). Effect of electrode insertion depth on postoperative change in hearing (B). Using the RW approach, there was no clear relationship between implant insertion depth and post-operative PTA [Citation50]. Reproduced by permission of Journal of American Academy of Audiology.
![Figure 48. Example audiogram of pre-op (open-circle) and post-op (crossed circle) with the MEDIUM performed with a MED-EL STANDARD electrode array. The HP remained stable over eighteen months (A). Effect of electrode insertion depth on postoperative change in hearing (B). Using the RW approach, there was no clear relationship between implant insertion depth and post-operative PTA [Citation50]. Reproduced by permission of Journal of American Academy of Audiology.](/cms/asset/e3fbd54e-79b2-4e21-a8ef-32c35153ffcb/ioto_a_1888477_f0048_b.jpg)
In 2011, Prof. Skarzynski and his colleagues reported on the possibility of LF hearing preservation with the deep insertion of MED-EL’s electrodes (STANDARD and FLEXSOFT™), using the RW surgical approach [Citation51]. The study included forty-two patients (implanted until the year 2008) with 85 dB HL or better at 500 Hz, and 80 dB or better at 125 Hz and 250 Hz. Pure tone audiograms were taken at different time points, including preoperatively and postoperatively at three, six and thirteen months. Three patients lost their residual hearing immediately after the surgery, and additional three patients lost their hearing progressively between three and thirteen months postoperatively. shows the mean audiograms for the implanted ear with significant differences between preoperative and postoperative thresholds for all measured audiometric thresholds.
Figure 49. Mean audiograms of the implanted ear at four testing time points (pre-op and post-op at 3, 6, and 13 months). Error bars depict standard deviations [Citation51]. Statistical test: ANOVA two-factor-without-replication test was used for comparison of hearing thresholds at various time points (p < .05). Reproduced by permission of Taylor and Francis Group.
![Figure 49. Mean audiograms of the implanted ear at four testing time points (pre-op and post-op at 3, 6, and 13 months). Error bars depict standard deviations [Citation51]. Statistical test: ANOVA two-factor-without-replication test was used for comparison of hearing thresholds at various time points (p < .05). Reproduced by permission of Taylor and Francis Group.](/cms/asset/a1ef7f0e-7814-4398-b9a9-5da2b4a65c34/ioto_a_1888477_f0049_c.jpg)
In 2013, a similar report came from Dr Mick and his colleagues from Sunnybrook Health Sciences Centre at the University of Toronto in Canada, with which they proved a high degree of residual hearing preservation at two years postoperatively in patients with the history of progressive HL, implanted with FLEXSOFT™ [Citation52].
In 2014, another encouraging report was published, demonstrating the preservation of residual hearing in the LF in profoundly deaf patients who were implanted with FLEXSOFT™ [Citation53]. In the study conducted by Prof. Green and his colleagues from the University of Manchester in the UK, thirteen out of fourteen patients preserved their residual hearing in an average follow-up period of two years.
In 2019, Prof. Lenarz reported on a new concept of partial insertion of a longer length flexible electrode with the aim of preserving LF residual hearing in potential EAS patients [Citation54]. This was a conservative approach of minimizing the loss of residual hearing following EAS treatment. Six EAS patients were implanted with a partially inserted (FLEX24, FLEX28) electrode from MED-EL, with basal most two contacts left outside the cochlea. Median preoperative and postoperative air-conduction thresholds at six-month post-activation are shown in . All patients had preserved functional residual hearing defined HL ≤ 80 dB HL at 250 Hz at first activation and 6 months post first activation. In no case a complete hearing loss (>30 dB) occurred.
Figure 50. Air-conducted hearing thresholds at 6 months postactivation for 16 mm insertion (n = 3) and 20 mm insertion (n = 3) [Citation54]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 50. Air-conducted hearing thresholds at 6 months postactivation for 16 mm insertion (n = 3) and 20 mm insertion (n = 3) [Citation54]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/1136194e-8246-4426-9197-c9f1bef34a58/ioto_a_1888477_f0050_b.jpg)
Part of the partial electrode insertion concept is that there is no need for the overall implant replacement when the residual hearing losses over time, instead with a minor revision surgery, the partially inserted electrode array can be further pushed inside the cochlea. The justification for the partial electrode insertion is the high probability of hearing preservation, however the patients need to be informed about the possible revision surgery later.
In 2020, Prof. Usami and his colleagues reported on their experiences with implanting FLEX28™ in ten EAS-indicated patients [Citation43]. The confidence for implanting FLEX28™ in EAS-indicated patients came from their earlier experience that showed the possibility of HP with FLEXSOFT™ and FLEX28™ in non-EAS-indicated – but with measurable LF residual hearing – patients [Citation55]. shows preoperative and six months postoperative audiogram with very minimal threshold shifts, as illustrated by the red dotted arrow marks. The argument for the longer length flexible electrode by the authors is that even if the residual hearing deteriorates over time, the electric stimulation covering the entire frequency range would continue offer good hearing benefit to the patients. They further recommended genetic testing in every patient to find out if there will be any deterioration of LF residual hearing over time.
Figure 51. Average air-conduction hearing thresholds. The dashed and solid lines indicate pre-op and six months post-op, respectively. Grey and black lines show the individual and mean results [Citation43]. Reproduced by permission of Taylor and Francis Group.
![Figure 51. Average air-conduction hearing thresholds. The dashed and solid lines indicate pre-op and six months post-op, respectively. Grey and black lines show the individual and mean results [Citation43]. Reproduced by permission of Taylor and Francis Group.](/cms/asset/cecaf72b-2d90-4ba5-b8d7-85c89f9d7d8d/ioto_a_1888477_f0051_c.jpg)
All presented studies in this section show that the RW approach enables the reduction of electrode insertion trauma (EIT), thereby preserving the LF hearing, even with long electrode array insertion. An inherent factor with implanting long electrode arrays is that if the LF hearing is lost over time, then the electric coverage of most, if not all, of the frequencies is possible, helping patients in optimising their hearing potential. Patients with a good preoperative hearing level in the LF retain residual hearing postoperatively, even with long electrode arrays.
The hearing preservation rates with various length electrode arrays varies from clinic to clinic as it is evident from these published reports and it essential to have a detailed preoperative counselling with the candidates explaining them the probabilities of hearing preservation and outcomes with different lengths of electrode arrays and treatment concepts.
2.17.3. Electrocochleography in the monitoring of inner ear functions during and after surgery
Preservation of residual hearing is successful in many but not all cases. Online monitoring of hearing during CI surgery is one way of ensuring the presence of residual hearing during and at the end of the surgery. Intracochlear recording of electric potentials from sensory cells in response to acoustic stimulation during CI electrode array insertion is also known as intracochlear electrocochleography (ECochG) [Citation56]. ECochG recorded signals have several components, including compound action potential (CAP), summation potential, cochlear microphonics (CM), and auditory neurophonics (ANN). CMs are regarded to reflect the status of hair cells, indirectly measuring cochlear health. In case of a deterioration of CMs during the intraoperative ECochG measurement, the operating surgeon could immediately adapt the insertion, thus improving the preservation of residual hearing. Caution needs to be taken as the ECochG does not necessarily reflect the actual hearing of the patient as the pure-tone audiometry does. MED-EL recently came up with a concept of online biofeedback system which measures the ECochG involving minimal manual steps by the surgeon during the CI electrode array insertion.
In 2012, under a research software platform from MED-EL, a novel concept of Electric and Acoustic Evoked Potential (EAEP) was introduced, allowing for acoustic-only and synchronous acoustic and electric stimulation. EAEP tool includes ECochG recordings. The recording methodology to determine the status of cochlear health was developed by Dr Polak (US patent numbers: 8862220 and 8170678).
In 2014/15, Prof. Adunka and his colleagues from the University of North Carolina in the US conducted several studies in human and non-human subjects to characterise the ECochG signals and to detect cochlear trauma during electrode insertion. A non-human subject proved that with normal hearing within its species, the CMs were more sensitive than the CAPs when detecting a cochlear trauma induced by electrode insertion [Citation57]. The in-human study using external ECochG measurement system showed that it is possible to measure it extracochlearly at the RW membrane, and intracochlearly by placing a temporary, flexible electrode array inside the cochlea intraoperatively in patients with good functional/measurable LF residual hearing [Citation58]. shows a sample response measurement recorded extracochlearly at the RW membrane, and intracochlearly.
Figure 52. Prof. Oliver Adunka, who led the study in characterizing ECoghG signals. Example comparison of extracochlear (at the RW membrane) and intracochlear (just over the RW membrane) ECochG recordings [Citation57]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 52. Prof. Oliver Adunka, who led the study in characterizing ECoghG signals. Example comparison of extracochlear (at the RW membrane) and intracochlear (just over the RW membrane) ECochG recordings [Citation57]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/e39861f1-ff38-4c42-bf41-8bae31399e69/ioto_a_1888477_f0052_b.jpg)
In 2016, Prof. Rajan and his colleagues from the University of Western Australia were the first to measure the CM during cochlear implantation procedure using a MED-EL implant electrode array [Citation59]. During the intraoperative monitoring, a prototype software algorithm was used to communicate with the standard implant interface and the CI via an external coil. The acoustic stimulus used was a 500 Hz tone pip, and the most apical electrode channel obtained the recordings in a millisecond time window. shows the results of CM measurement from a patient who was implanted with FLEX24™ (full intracochlear electrode insertion) – the results are given intraoperatively, and ten days, three weeks, three months and six months postoperatively. Due to no intracochlear structural damage, postoperative responses are similar to the intraoperative, indicating preservation of hair cells function.
Figure 53. Prof. Gunesh Rajan was the first one to measure CM during CI procedure. Post-op CM measurements (from the top) at intra-op, and ten days, three weeks, three months, and six months post-op [Citation59]. Reproduced by permission of Wolters Kluwer Health, Inc.
![Figure 53. Prof. Gunesh Rajan was the first one to measure CM during CI procedure. Post-op CM measurements (from the top) at intra-op, and ten days, three weeks, three months, and six months post-op [Citation59]. Reproduced by permission of Wolters Kluwer Health, Inc.](/cms/asset/10abbe53-6fc4-45df-8bea-4671efd9e10f/ioto_a_1888477_f0053_c.jpg)
In 2019, Prof. Lorens and his colleagues from the Institute of Physiology and Pathology of Hearing in Poland recorded the CM directly from the CI in CI recipients with measurable residual hearing [Citation56]. This was achieved in sixteen CI recipients implanted with MED-EL devices with different electrode arrays (FLEX20™, FLEX24™, MEDIUM, FLEX28™, FLEXSOFT™ or STANDARD). For the acoustic stimuli, either tone pips at frequencies 0.25-, 0.5-, 1-, 2- and 4-kHz, or 1 ms clicks were used. The duration of the tone pips was chosen to be sufficiently long to identify the CM in the response. A prototype software algorithm (Research Evoked Potentials Software from MED-EL) allowed the recording window to be increased to up to 20 ms and to communicate with the standard implant interface and the CI via an external coil. is an example CM measured from the first, third and fifth electrode channels in response to tone pips of 500 Hz acoustic stimulus. This study aimed to find the most sensitive stimuli to which ECochG can be recorded during intraoperative and postoperative monitoring. In the HP patients, the most sensitive stimuli were 500 Hz and 1 kHz tone pips and 1 ms click.
Figure 54. Prof. Artur Lorens led the study in measuring CM directly from the CI during CI surgery. Example of intracochlear ECochG recordings for tone pips and clicks from electrode channels 1, 3, and 5 [Citation56].
![Figure 54. Prof. Artur Lorens led the study in measuring CM directly from the CI during CI surgery. Example of intracochlear ECochG recordings for tone pips and clicks from electrode channels 1, 3, and 5 [Citation56].](/cms/asset/c5fc8559-7377-4b08-b0bf-8653518cc031/ioto_a_1888477_f0054_c.jpg)
In 2019, Prof. Lenarz and his colleagues from Hannover Medical School in Germany measured ECochGs using MED-EL’s research software in combination with FLEX24™ and FLEX28™ electrode arrays, implanted in ten patients with identifiable residual hearing [Citation60]. The ECochG recordings were performed both extracochlearly and intracochlearly with the acoustic stimulus of tone bursts at 250-, 500-, and 1,000-Hz. shows an example recording of ECochGs measured intracochlearly with a CI electrode array inserted 20–22mm with an acoustic stimulus of 1,000Hz at a loudness of 70 dB and 80 dB. The amplitudes of intracochlear ECochG were detected higher than the extracochlear.
Figure 55. Clinicians from Hannover Medical School. Example of an intraoperative ECochG recording just after the electrode insertion process. Data is shown for 1,000Hz tone burst [Citation59]. Image courtesy of Dr Sabine Haumann, Hannover, Germany.
![Figure 55. Clinicians from Hannover Medical School. Example of an intraoperative ECochG recording just after the electrode insertion process. Data is shown for 1,000Hz tone burst [Citation59]. Image courtesy of Dr Sabine Haumann, Hannover, Germany.](/cms/asset/eb37a7b1-4738-4943-88b9-dd0d4c8848a8/ioto_a_1888477_f0055_c.jpg)
All pieces of evidence seen in this section show encouraging results of the possibility of monitoring the cochlear health during and after CI electrode insertion. However, the current status of ECochG research is at its early stages before it moves toward potentially becoming a part of a clinical routine with the EAS™ surgery, HP surgery, or both. EAEP offers the possibility to stimulate the cochlea electrically with the CI electrode array and with acoustic amplification with a short delay between the two stimuli. EAEP opens the door to several research possibilities, for example, to how the cochlea responds to electric stimulation if the acoustic amplification is masked and vice versa.
In 2017, the EAEP tool was added to the MED-EL’s fitting software MAESTRO and was CE-marked for official use in clinical practice.
2.17.4. Genetic screening in predicting hearing loss
Half of the congenital HL is genetic with more than four hundred known syndromes with HL as a feature and more than one hundred known genes that have HL as the only clinical manifestation. Most cases of congenital HL are identified soon after birth via newborn hearing screening (NBHS). However, many HL cases only become apparent later in life due to the expression of late-onset HL mutations or following an environmental insult, like antibiotic use or head trauma, in the genetically predisposed patient. A genetic screening panel that incorporates a population’s common HL genes could hence represent an effective adjunct test to newborn screening, improving time of diagnosis and treatment [Citation61].
In 2020, Dr Yoshimura, Prof. Usami and their colleagues carried out genetic testing that has the potential to impact HP, following CI [Citation19]. Forty-four patients (forty-one families) with age at implantation above six and with measurable residual hearing in the LF with a threshold less than 80 dB HL were implanted with FLEX24™, FLEX28™ or FLEXSOFT™. To define the extent of hearing deterioration following CI, they measured auditory thresholds before surgery and six months after initial activation using the HEARRING HP scale. The aim of the study was to investigate the predictive factors, including the aetiology of HL as a patient-related factor, influencing residual HP after CI. Genetic testing was performed to identify the responsible genes for HL. They identified the cause of HL in twenty-one families, and nineteen patients out of those received a genetic diagnosis, with the CDH23 gene most frequently implicated, followed by ACTG1, mit1555A > G, MYO7A, MYO15A, SLC26A4, and TMPRSSS3. Additionally, two patients were diagnosed with otosclerosis and congenital diaphragmatic hernia ().
Figure 56. Aetiology of patients with residual acoustic hearing. (A) (n = 41): orange indicates genetic causes of HL; yellow indicates other causes; grey indicates unknown. (B): comparison of HP scores in each group [Citation19]. Reproduced by permission of Taylor and Francis Group.
![Figure 56. Aetiology of patients with residual acoustic hearing. (A) (n = 41): orange indicates genetic causes of HL; yellow indicates other causes; grey indicates unknown. (B): comparison of HP scores in each group [Citation19]. Reproduced by permission of Taylor and Francis Group.](/cms/asset/11c97bec-27c9-4a00-a6b5-1e557965cea6/ioto_a_1888477_f0056_c.jpg)
Out of all the abovementioned genes, they found that patients who had pathogenic variants in the CDH23, MYO7A, or MYO15A gene showed statistically better HP scores compared with patients with HL due to other causes (). Between these two groups, the age was comparable (33.5 vs 37.4 years). In summary, these results reveal that genetic testing facilitates not only the diagnosis of patients with HL but also the prediction of HP after CI.
In the same year, a report on concurrent hearing and genetic screening of neonates by Prof. Dai, Prof. Han and their colleagues from the two biggest hospitals in Beijing, China was published, showing the importance of genetic screening in the early identification of late development of hearing loss in children [Citation62] ().
Figure 57. Clinicians from two biggest Hospital in Beijing, China, who undertook the concurrent hearing and genetic screening of 180,469 neonates with follow-up.

The study included 180,469 infants born in Beijing between April 2013 and March 2014 with the last follow-up on February 24, 2018. Hearing screening was performed using Transiently evoked otoacoustic emission (TEOAE) and dried blood spots were collected for genetic screening using DNA microarray platform to identify nine variants in four genes, GJB2, SLC26A4, mtDNA 12SrRNA, and GJB3. The important finding from this mega-study is that infants with pathogenic combinations of GJB2 variants and SLC26A4 variants may pass newborn hearing screening, and most of them will develop hearing loss at an early age (<5 years old).
These two recent studies show that newborn genetic screening clearly shortens time to diagnosis and intervention, reveals the aetiology of genetic deafness and ensures timely habilitation of infants and young children. Also, the genetic screening predicts the possibility of preserving residual hearing in patients with LF hearing following CI procedure.
2.17.5. Electro-natural stimulation (ENS)
The EAS topic has come a long way since its introduction in the year 1997. It started with implanting patients with LF residual hearing from ∼500Hz, which was then expanded to 1,500Hz with the advancements in the flexible electrode array design and soft surgical skills. Combination of acoustic amplification of LF region and electric stimulation in the HF region has shown to be highly beneficial for the patients. However, some patients who have natural or near-natural LF residual hearing with HL only in the HF region may not fall under the eligibility criteria for CI. These patients may become the candidates for CI in the future with the developing advancements in the EAS topic.
In 2019, Prof. Skarzynski and his colleagues published their findings on the long-term HP results in a select group of patients (n = 12) who had a natural hearing in the LF until 1,500Hz [Citation63] ().
Figure 58. Clinicians from the Institute of Physiology and Pathology of Hearing, Warsaw who introduced the Electro-Natural Stimulation in partial deafness treatment of adults CI users.

Nine out of twelve patients were implanted – with soft surgical HP technique – with MED-EL CI device in combination with MEDIUM or FLEX24™ electrode array, partially inserted to reach an intracochlear depth of 20- and 21-mm. displays the average preoperative and postoperative air conduction hearing thresholds. The HP rate evaluated one month after the CI surgery indicated that seven out of nine patients maintained complete HP. In the long-term follow-up (thirty-six months), five out of nine patients still maintained complete HP, whereas the remaining four had partial HP. None of the patients experienced minimal HP or complete loss of hearing. The aetiology of all nine patients was unknown. Prof. Skarzynski is well known in the CI field for his outstanding soft surgical skills, and the excellent HP results reported in the study could be due to his surgical expertise – while the same outcomes may not be obtained for sure if the same surgeries would be performed with a less experienced surgeon. In conclusion, the authors reported that soft surgical technique could lead to excellent HP in patients who have normal hearing to up to 1,500Hz and HF HL.
Figure 59. Average pre-op and post-op air conduction hearing thresholds for operated and non-operated ear [Citation62]. Reproduced by permission of Karger AG, Basel.
![Figure 59. Average pre-op and post-op air conduction hearing thresholds for operated and non-operated ear [Citation62]. Reproduced by permission of Karger AG, Basel.](/cms/asset/8a7cb27e-7647-4da5-9eea-756f26feab65/ioto_a_1888477_f0059_c.jpg)
2.17.6. Electrode selection based on pre-operative residual hearing level
There is some research work underway looking at the preoperative level of residual hearing in predicting the likelihood of the patient to use the acoustic component of the EAS system post-operatively. Prof. Lenarz and his colleagues from Hannover Medical School in Germany are currently (in the year 2020) investigating the effect of pre-operative residual hearing level and predicting the likelihood of the patient using the acoustic component post-operatively and selecting the electrode array length accordingly.
2.18. Distinct HP surgical techniques
Soft surgical technique was originally proposed by Lehnhardt et al. [Citation64], is one of the critical factors which affect the HP results. The proposed methods remain in clinical practice to this day with little change. This section lists some of the key surgical approaches in order to achieve optimal HP results [Citation65]:
route of electrode insertion through RW membrane opening whenever possible
mild hypothermia
avoidance of drilling directly over the cochlear promontory to prevent vibration-related trauma
avoidance of blood entry into the scala tympani to minimise fibrosis formation
avoidance of bone dust in the cochlea to prevent new bone formation
application of steroid at the cochlear entrance and inside the cochlea to minimise foreign body inflammation and to heal intracochlear trauma (if any)
avoidance of perilymph leakage and suctioning to prevent an abrupt change in cochlear pressure
slow electrode insertion at a speed of 15mm of the electrode array length per minute to enable full electrode insertion, to minimise electrode insertion-related trauma, and cochlear pressure change [Citation66]
avoidance of filling the middle ear space with fascia to prevent impeding the ossicular chain movement.
2.19. Current (the year 2021) indication for EAS and its audiogram
The following conditions are considered safe for treatment with MED-EL EAS™ hearing system:
no age restrictions
air-bone gap ≤10dB for two or more frequencies (0.5-, 1-, 2- and 4-kHz) in each ear
pre-operative CNC monosyllables score ≤60% in the best-aided condition
radiologic evidence of bilateral patent cochleae
no rapid hearing loss
fulfilling the audiogram criteria as shown in
2.20. Reimbursement from the healthcare system
All the pieces of scientific evidence presented in this chapter have shown that MED-EL EAS™ hearing system is safe and effective in restoring both, HF and LF hearing in partially deaf patients. Also, it has been approved by the notified bodies from the EU, the USA, Japan, Canada and Australia for clinical use in patients, making it eligible for reimbursement of the treatment costs from the healthcare system.
2.21. Hearing and structure preservation workshop
The Hearing and Structure Preservation’s (HSP) workshop came into existence with the initiative by the group of expert CI surgeons in hearing preservation (HP) and is supported logistically by MED-EL. Its primary focus/aim is on advancing the CI field in general, HP and EAS in particular, strengthening the scientific evidence base, and generating a platform for exchange between research groups. It was originally started as HP workshop in the year 2002 hosted by Prof. Miyamoto from Indiana University, USA, which changed to HSP in the year 2013. shows all the HSP meetings that took place so far (until the year 2019) along with the clinicians who have hosted it.
Figure 60. Clinicians from around the world who have hosted the HSP meeting between 2002–19. 1Indiana University, USA; 2Johann Wolfgang Goethe University Hospital Frankfurt, Germany; 3UT Southwestern Medical Center, USA; 4Institute of Physiology and Pathology of Hearing, Poland; 5University of North Carolina, USA; 6Antwerp Medical University, Belgium; 7Kansas University Medical Center, USA; 8Medical University of Vienna, Austria; 9University of Miami Ear Institute, USA; 10 St. Thomas Hospital, UK; 11Sunnybrook Health Sciences Centre, Canada; 12Heidelberg Medical University, Germany; 13Shinshu University, Japan; 14Vanderbilt University, USA; 15University Hospital of North Paris, France; 16University of Western Australia, Australia; 17Uppsala University, Sweden; 18New York Eye and Ear Infirmary, USA.

Although MED-EL has been supporting the logistical side of the workshops so far, it is the host clinician who is fully responsible for the scientific side of the workshop. It has been the tradition that the clinicians present their latest research findings related to HSP topic in this workshop before submitting it to any scientific journals for publication, making the workshop highly interesting for the participants. Internally at MED-EL, it is Dr Polak, the Head of Electrophysiology for Assessment, Research and Development, who is responsible for the whole scientific program and Dr Garnham for the life science part of the EAS and HP program ().
2.22. Conclusion
The indication criteria for CI have expanded over the years from severe to profound HL over the entire frequency range to patients with near-normal hearing in the LF region. Thanks to the advancements in the CI electrode array design, soft surgical techniques, fitting techniques, as well as the audio processor advancements, combining the acoustic unit of HA to the CI became a treatment option for partially deaf patients with the EAS™ hearing system. Most importantly, the cochlear condition with its unique anatomy should be addressed in detail and individual manner from infants to geriatric patients. Preserving the LF residual hearing in the CI ear gives the possibility to use the natural ITD and ILD cues to enjoy the benefits of binaural hearing if the contralateral ear has a natural hearing or if it is aided with the HA for acoustic amplification of the LF hearing. With all these clinical evidences showing the added benefit of EAS in comparison to electric stimulation only, or acoustic stimulation only modes, the combined EAS should become the standard treatment option for patients who do not benefit enough with the HA alone. Good understanding of partially deaf patients’ hearing history before surgery may help the surgeons to choose the optimal electrode array length. If the hearing history shows progressive HL, then choosing a long electrode array length with flexible feature would ensure electric stimulation over the entire frequency range. Genetic testing to understand the chances of progressive HL and the intraoperative ECochG method to monitor the electrode insertion related trauma are both considered to be the future trends in combined EAS treatment. Corticosteroid treatment of the inner ear is another key topic that supports the HP by suppressing the inflammation reaction caused by the introduction of a foreign body, which is the electrode array insertion trauma. This is addressed in detail in chapter 6 of this compendium.
Looking back, it is evident that a strong international scientific collaboration between clinicians and engineers from MED-EL made it possible to master every aspect in the advancements of the technological, surgical and fitting sides of the EAS™ system. Results of the several laboratory experiments and clinical trials helped MED-EL to develop the EAS™ hearing system and to make it commercially available as a product to treat partially deaf patients. EAS™ is yet another example of the translational science path MED-EL took to bring a unique concept from laboratory settings to patients.
Acknowledgments
The authors would gratefully like to acknowledge the key contributors to the development of the subject matter. Their contributions are outlined in this article. The authors further acknowledge Marek Polak from MED-EL for his valuable input and comments during several rounds of review meetings that contributed to the final version of this article.
Disclosure statement
This article is sponsored by MED-EL and has not undergone the regular peer-review process of Acta Oto-Laryngologica. Both the authors are affiliated with MED-EL.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Ekdale EG. Form and function of the mammalian inner ear. J Anat. 2016;228(2):324–337.
- Brigande JV, Heller S. Quo Vadis, hair cell regeneration. Nat Neurosci. 2009;12(6):679–685.
- Niskar AS, Kieszak SM, Holmes A, et al. Prevalence of hearing loss among children 6 to 19 years of age: the third national health and nutrition examination survey. JAMA. 1998;279(14):1071–1075.
- von Ilberg C, Kiefer J, Tillein J, et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61(6):334–340.
- Kiefer J, Tillein J, von Illberg C. Fundamental aspects and first clinical results of the clinical application of combined electric and acoustic stimulation of the auditory system. In: Kubo T, Iwaki Tm Takagashi Y, editors. Advances in cochlear implants. The Hague: Kugler Publications. p. 569–576.
- Skarzynski H, Loren A, Piotrowska A. A new method of partial deafness treatment. Med Sci Monit. 2003;9(4):CS20–CS204.
- Tzifa K, Hanvey K. Cochlear implantation in asymmetrical hearing loss for children: our experience. Cochlear Implants Int. 2013;14(Suppl 4):S56–S61.
- Kiefer J, Gstoettner W, Baumgartner W, et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124(3):272–280.
- Adunka O, Unkelbach MH, Mack M, Hambek M, et al. Cochlear implantation via the round window membrane minimizes trauma to cochlear structures: a histologically controlled insertion study. Acta Otolaryngol. 2004;124(7):807–817.
- Adunka O, Kiefer J, Unkelbach MH, et al. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope. 2004;114(7):1237–1241.
- Dhanasingh A. The rationale for FLEX (cochlear implant) electrode with varying array lengths. World J Otorhinolaryngol Head Neck Surg. 2020;7(1):45–53.
- Lorens A, Polak M, Piotrowska A, et al. Outcomes of treatment of partial deafness with cochlear implantation: a Duet study. Laryngoscope. 2008;118(2):288–294.
- Helbig S, Baumann U, Helbig M, et al. A new combined speech processor for electric and acoustic stimulation-eight months experience. ORL J Otorhinolaryngol Relat Spec. 2008;70(6):359–365.
- Helbig S, Baumann U. Acceptance and fitting of the DUET device – a combined speech processor for electric acoustic stimulation. Adv Otorhinolaryngol. 2010;67:81–87.
- Lorens A, Zgoda M, Skarzynski H. A new audio processor for combined electric and acoustic stimulation for the treatment of partial deafness. Acta Otolaryngol. 2012;132(7):739–750.
- Brockmeier SJ, Peterreins M, Lorens A, et al. J. Music perception in electric acoustic stimulation users as assessed by the Mu.S.I.C test. Adv Otorhinolaryngol. 2010;67:70–80.
- Skarzynski H, Lorens A, Piotrowska A, et al. Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Otolaryngol. 2007;127(1):41–48.
- Skarzynski H, Lorens A. Electric acoustic stimulation in children. Adv Otorhinolaryngol. 2010;67:135–143.
- Yoshimura H, Moteki H, Nishio SY, et al. Genetic testing has the potential to impact hearing preservation following cochlear implantation. Acta Otolaryngol. 2020;140(6):438–444.
- Kamat A, Goldin L, Hoffman RA. Unusual electroacoustic device failure and electroacoustic reimplantation with hearing preservation. Otol Neurotol. 2011;32(6):948–950.
- Jayawardena J, Kuthubutheen J, Rajan G. Hearing preservation and hearing improvement after reimplantation of pediatric and adult patients with partial deafness: a retrospective case series review. Otol Neurotol. 2012;33(5):740–744.
- Helbig S, Rajan GP, Stöver T, et al. Hearing preservation after cochlear reimplantation. Otol Neurotol. 2013;34(1):61–65.
- Thompson NJ, Dillon MT, Bucker AL, et al. Electric-acoustic stimulation after reimplantation: hearing preservation and speech perception. Otol Neurotol. 2019;40(2):e94–e98.
- Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Oto-Laryngologica. 2013;133(sup564):3–13.
- Gifford RH, Dorman MF, Skarzynski H, et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear. 2013;34(4):413–425.
- Gifford RH, Davis TJ, Sunderhaus LW, et al. Combined electric and acoustic stimulation with hearing preservation: effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear Hear. 2017;38(5):539–553.
- Usami S-I, Moteki H, Tsukada K, et al. Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngol. 2014;134(7):717–727.,.
- Pillsbury HC, Dillon MT, Buchman CA, 3rd, et al. Multicenter US clinical trial with an electric-acoustic stimulation (EAS) system in adults: final outcomes. Otol Neurotol. 2018;39(3):299–305.
- Roland JT, Jr, Gantz BJ, Waltzman SB, et al. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope. 2016;126(1):175–181.
- Skarzynski H, Lorens A. Partial deafness treatment. Cochlear Implants Int. 2010;11(sup1):29–41.
- Helbig S, Bauman U, Hey C, et al. Hearing preservation after complete cochlear coverage in cochlear implantation with the free-fitting FLEX electrode carrier. Otol Neurotol. 2011;32(6):973–979.
- Santa Maria PL, Domville-Lewis C, Sucher CM, et al. Hearing preservation surgery for cochlear implantation-Hearing and quality of life after 2 years. Otol Neurotol. 2013;34(3):526–531.
- Mertens G, Kleine PA, Cochlet E, et al. P. Long-term follow-up of hearing preservation in electric-acoustic stimulation patients. Otol Neurotol. 2014;35(10):1765–1772.
- Moteki H, Nishio SY, Miyagawa M, et al. Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Otolaryngol. 2017;137(5):516–521.
- Helbig S, Adel Y, Rader T, et al. Long-term hearing preservation outcomes after cochlear implantation for electric-acoustic stimulation. Otol Neurotol. 2016;37(9):e353-359–e359.
- Sprinzl GM, Schoerg P, Edlinger SH, et al. Long-term hearing preservation in electric acoustic cochlear implant candidates. Otol Neurotol. 2020;41(6):750–757.
- Wilson B, Wolford R, Lawson D, et al. Speech processors for auditory prostheses. Third quarterly progress report. NIH project N01-DC-2-1002.
- Kiefer J, Pok S, Adunka O, et al. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurootol. 2005;10(3):134–144.
- Vermeire K, Anderson I, Flynn M, et al. The influence of different speech processor and hearing aid settings on speech perception outcomes in electric acoustic stimulation patients. Ear Hear. 2008;29(1):76–86.
- Polak M, Lorens A, Helbig S, et al. Fitting of the Hearing System Affects Partial Deafness Cochlear Implant Performance. Cochlear Implants Int. 2010;11(sup1):117–121.
- Polak M, Lorens A, Furmanek M, et al. Electrode estimation in the acoustic region of the human cochlea. Acta Otolaryngol. 2020;140(6):487–496.
- Lorens A, Zgoda M, Polak M. Stimulus and place overlap on PDT-EC and PDT-EAS patients after deep electrode insertion. 32nd World Congress of Audiology, Processedings May 3–7th, 2014. 347:75.
- Yoshimura H, Moteki H, Nishio SY, et al. Electric-acoustic stimulation with longer electrodes for potential deterioration in low-frequency hearing. Acta Otolaryngol. 2020;140(8):632–638.
- Rajan G, Tavora-Vieira D, Baumgartner W-D, et al. Hearing preservation cochlear implantation in children: the HEARRING group consensus and practical guide. Cochlear Implants Int. 2018;19(1):1–13.
- Hardy M. The length of the organ of corti in man. Am J Anat. 1938;62(2):291–311.
- Greenwood DD. Critical bandwidth and consonance: their operational definitions in relation to cochlear nonlinearity and combination tones. Hear Res. 1991;54(2):209–246.
- Escude B, James C, Deguine O, et al. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurotol. 2006;11(1):27–33.
- Alexiades G, Dhanasingh A, Jolly C. Method to estimate the complete and two-turn cochlear duct length. Otol Neurotol. 2015;36(5):904–907.
- Dhanasingh A. Research software in cochlear duct length estimation, Greenwood frequency mapping and CI electrode array length simulation. World J Otorhinolaryngol Head Neck Surg. 2020;7(1):17–22.
- Prentiss S, Sykes K, Staecker H. Partial deafness cochlear implantation at the University of Kansas: techniques and outcomes. J Am Acad Audiol. 2010;21(3):197–203.
- Skarzynski H, Lorens A, Zgoda M, et al. Atraumatic round window deep insertion of cochlear electrodes. Acta Otolaryngol. 2011;131(7):740–749.
- Mick P, Amoodi H, Shipp D, et al. Hearing preservation with full insertion of the FLEX SOFT electrode. Otol Neurotol. 2013;35:e40–e44.
- Bruce IA, Felton M, Lockley M, et al. Hearing preservation cochlear implantation in adolescents. Otol Neurotol. 2014;35(9):1552–1559.
- Lenarz T, Timm ME, Salcher R, et al. Individual Hearing Preservation Cochlear Implantation Using the Concept of Partial Insertion. Otol Neurotol. 2019;40(3):e326–e335.
- Usami SI, Moteki H, Suzuki N, et al. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Otolaryngol. 2011;131(4):405–412.
- Lorens A, Walkowiak A, Polak M, et al. Cochlear microphonics in hearing preservation cochlear implantees. J Int Adv Otol. 2019;15(3):345–351.
- Choudhury B, Adunka OF, Awan O, et al. Electrophysiologic consequences of flexible electrode insertions in gerbils with noise-induced hearing loss. Otol Neurotol. 2014;35(3):519–525.
- Calloway NH, Fitzpatrick DC, Campbell AP, et al. Intracochlear electrocochleography during cochlear implantation. Otol Neurotol. 2014;35(8):1451–1457.
- Acharya AN, Tavora-Vieira D, Rajan GP. Using the implant electrode array to conduct real-time intraoperative hearing monitoring during pediatric cochlear implantation: preliminary experiences. Otol Neurotol. 2016;37(2):e148–e153.
- Haumann S, Imsiecke M, Bauernfeind Büchner A, et al. Monitoring of the inner ear function during and after cochlear implant insertion using electrocochleography. Trends Hear. 2019;23:2331216519833567
- Rudman J, Liu XZ. Genetics of hearing loss. Hear J. 2019;4:6–9.
- Dai P, Huang L-H, Wang G-J, et al. Concurrent hearing and genetic screening of 180,469 neonates with follow-up in Beijing, China. Am J Hum Genet. 2019;105(4):803–812.
- Skarzynski H, Lorens A, Dziendziel B, et al. Electro-natural stimulation in partial deafness treatment of adult cochlear implant users: long-term hearing preservation results. ORL J Otorhinolaryngol Relat Spec. 2019;81(2–3):63–72.
- Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41(7):356–359.
- Friedland DR, Runge-Samuelson C. Soft cochlear implantation: rationale for the surgical approach. Trends Amplif. 2009;13(2):124–138.
- Rajan GP, Kontorinis G, Kuthubutheen J. The effects of insertion speed on inner ear function during cochlear implantation: a comparison study. Audiol Neurootol. 2013;18(1):17–22.