Abstract
Background: It has been proposed that irritable bowel syndrome (IBS) is a low-grade mucosal inflammatory disease.
Objective: To characterize the intestinal inflammatory profile in IBS patients with or without fructose intolerance.
Design: Patients referred to colonoscopy with IBS complaints were screened for participation. IBS patients diagnosed according to the Rome II criteria and with no organic gastrointestinal disease were included in the study. One subgroup was patients included in a fructose-reduced diet study for 2 months with effects based on VAS symptom scores. Healthy controls were subjects under investigation of colorectal cancer screening with no IBS or other gastrointestinal diseases. All patients included had normal histology from rectum. Mucosal cytokines, chemokines and growth factors were measured by multiplex technology.
Results: Of 27 inflammatory markers tested in the mucosal tissue, 13 were significantly increased and none was significantly decreased in IBS as compared to controls. Significantly increased were the proinflammatory cytokines tumor necrosis factor, the typical TH1 markers IFNγ, IL-1β, IL-2 and RANTES, the typical TH2 markers IL-5 and IL-9, the TH17 marker IL-17, TNF, the pleiotropic IL-15, and the growth factors bFGF and GM-CSF. In IBS patients with fructose intolerance only IL-5 was significantly increased compared to patients without fructose intolerance.
Conclusions: A dysregulated mucosal inflammatory profile with an increased level of TH1, TH2 and TH17 markers, and growth factors were observed in bowel mucosa in of IBS patients when compared to healthy controls.
Introduction
Irritable bowel syndrome (IBS) is characterized as a functional disease as there are no well-documented causal pathophysiological mechanisms. Typically, in IBS, there is a hypersensitivity with low response threshold to various external stimuli, first documented by Ritchie [Citation1]. This hypersensitivity of afferent neurons is activated by stimuli such as distension of hollow organs and chemical mediators such as proinflammatory and lipotoxic molecules (for review, see reference [Citation2]). The underlying mechanisms behind visceral hypersensitivity in IBS is unknown, but the most referred hypothesis is that the visceral hypersensitivity in IBS is caused by an aberrant neuroimmune interaction (for review, see [Citation2–7]).
One of the first reports suggesting IBS as a low-grade mucosal inflammatory disease was by Collins [Citation8], but this hypothesis is still controversial [Citation9]. Two features support this hypothesis: IBS is frequently seen both after a gastrointestinal infection [Citation10] and in inflammatory bowel disease in remission [Citation11]. However, the documentation of visceral hypersensitivity as a neuroimmune dysregulation in IBS is poor. At the level of mucosal immune cells, several reports describing increased number of T lymphocytes [Citation12–14], mast cells [Citation15,Citation16] and degranulated mast cells [Citation14,Citation17]. Somewhat contradictory, decreased levels of T cells and mast cells have been reported [Citation18] in post infectious and classical IBS. Moreover, various results have been reported for cytokines and chemokines at mucosal level: increased transcript levels of IL-1β [Citation19] has been reported, decreased transcript levels were observed for IL-10 [Citation20,Citation21] and for the chemokines IL-8, CXCL-9 and MCP1 [Citation22], an imbalance between TH1 and TH2 cytokines was observed [Citation23], whereas no changes were observed for the proinflammatory TNF alpha, IL-6 and IL-beta [Citation22]. Finally, in contrast to these reports, no significant differences between classical, non-postinfectious IBS and healthy control were found neither at the mucosal levels of immune cells nor at transcript levels [Citation24,Citation25]. Taken together, there are contradictory reports concerning the type of immune dysregulation involved if IBS can be explained as a low-grade inflammatory bowel disease.
The aim of this study was to identify the inflammatory profile of various relevant cytokines, chemokines and growth factors in colon biopsies from patients with IBS, including the subgroup with self-reported fructose intolerance, compared to healthy controls.
Materials and methods
Subject groups
The patients were recruited from three cohorts: from patients referred to colonoscopy due to IBS symptoms, from the FINN study (Fructose malabsorption In North Norway [Citation25,Citation26]), and patients in colorectal cancer screening. The recruited patients were first interviewed for a complete medical record to ensure they fulfilled Rome II criteria for IBS diagnosis. The inclusion criteria were patients who fulfilled Rome II criteria and were willing to participate. They then underwent an individual diagnostic workup including, but not mandatory, blood tests, stool samples, breath tests, endoscopy, histological examination, X-ray or ultrasound investigations to exclude organic disease or other malabsorption diseases including lactose intolerance and food allergy. A standard screening laboratory test including test for celiac disease was performed. According to the question of food allergy and lactose intolerance, the patient was carefully asked for food allergy and lactose intolerance and was excluded if typical symptoms. In some cases with uncertainty, food allergy blood tests and lactose breath test were performed. The exclusion criteria were patients with other gastrointestinal diseases, including post-infectious IBS, use of laxatives due to constipation, patients with severe medical disorders such as diabetes mellitus, cancer, severe cerebral, lung or heart diseases and finally patients with severe immunological diseases such as rheumatoid arthritis, SLE, etc. A subgroup of the IBS patients were further included in the FINN study. They performed a diagnostic test for self-reported dietary fructose intolerance (12-week fructose reduced diet (FRD) followed by 1 week high-fructose provocation test) [Citation27]. Finally, patients with no IBS symptoms according to Rome II criteria represented the healthy control group. All patients performed colonoscopy, 20 biopsies were obtained from rectum and stored at –70 °C until analysis. Biopsies were also obtained for ordinary histological examinations (haematoxylin and eosin staining). The IBS group and the healthy control group were included in the study if no endoscopic nor histological sign of pathology, and no other gastrointestinal disease including post-infectious IBS and severe medical disorders. Moreover, in all subjects, VAS registrations for pain and bloating (0–100 mm, 0 mm for no symptoms and 100 mm for maximal symptom score) were performed, and the number of stools and registration of stool quality on a scale from 1 to 7 (Bristol scale [Citation27]).
Analyses of cytokines, chemokines and growth factors
The mucosal profile of various relevant cytokines, chemokines and growth factors were determined by multiplex technology. Homogenization of tissue was performed with the following protocol: a mix of 495 µL CytoBuster Protein Extraction Reagent (Novagen, San Diego, CA) and 5 µL Protease Inhibitor cocktail set 1 (Calbiochem, Darmstadt, Germany) was added to 50 mg of tissue sample and homogenized with Xiril Dispomix. After completion, the samples were incubated for five minutes on ice and thereafter centrifuged at 2500×g for 20 min at 4 °C. The supernatants were transferred to Nunc tubes and stored at –70 °C. The samples were analyzed using a multiplex cytokine assay (Bio-Plex Human Cytokine 27-Plex Panel; Bio-Rad Laboratories Inc., Hercules, CA) containing the following interleukins (ILs), chemokines and growth factors: IL-1β, IL-1 receptor antagonist (IL1-ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, eotaxin, basic fibroblast growth factor (bFGF), granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), interferon (IFN)-γ, interferon-inducible protein (IP-10), monocyte chemotactic protein (MCP-1), macrophage inflammatory protein (MIP)-1α, MIP-1β, platelet derived growth factor-BB (PDGF-BB), regulated upon activation T cell expressed and secreted (RANTES), tumor necrosis factor (TNF) and vascular endothelial growth factor (VEGF). The samples were analyzed on a Multiplex Analyzer (Bio-Rad Laboratories Inc., Hercules, CA) according to instructions from the manufacturer.
Ethics and registrations
The study was approved by the Regional Committee of Medical Ethics North Norway (ID: 136/2006), and the study was registered in clinicaltrials.gov NCT00555191.
Statistical analysis
Baseline characteristics and individual mediator measurements were explored using parametric tests; in most cases, it was necessary to perform a logarithmic transformation to obtain a normal distribution. Chi-square test was used for contingency tables. A principal component analysis was performed on the mediator readings from all subject groups. Further details in ‘Results’ section.
Results
Subject groups
A total of 42 IBS patients were included, 14 patients performing FRD where eight patients had fructose intolerance based on the criteria of effect of fructose restricted diet (<2 g fructose/meal) and positive provocation test on fructose-rich meals [Citation27] and six patients had no fructose intolerance. All IBS patients had a combination of diarrhea and constipation so subgrouping could not be performed. The demographics are shown in .
Table 1. Demographic and baseline variables for patients included.
Mucosal intestinal inflammatory profiles in IBS patients versus healthy controls
Of the 27 mediators measured, 13 was significantly (p < .05) increased and none were decreased in the IBS patients compared to controls ().
Table 2. Mucosal levels of cytokines, chemokines and growth factors in patients with IBS and in healthy controls.
Cytokines
Most of the proinflammatory cytokines were increased at significant levels such as the TH1 cytokines IFNγ, IL-1β, IL-2, the pleiotropic IL-15, the TH17 cytokine IL-17, TNF, but not IL-6, IL-7 or IL-8. The TH2 cytokines IL-4 and IL-9, but not IL-5, was significantly increased compared to the healthy control group ().
Chemokines
Of the chemokines analyzed, only RANTES and eotaxin were significantly increased when compared to the control group ().
Growth factors
Of the various growth factors analyzed, bFGF, PDGFBB and GM-CSF were significantly increased, but not the other growth factors when compared to the control group ().
Intestinal immune profile in subgroups of IBS
IL-5 was significantly increased in IBS patients with self-reported fructose intolerance only (0.4 pg/mL [0.2–0.6] n = 6) compared to patients without fructose intolerance (0.1 pg/mL [0.0–0.3] n = 8) p=.02 (data not shown for the rest).
Principal component analysis
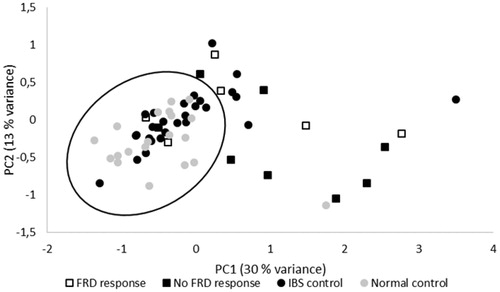
Principal component analysis of the dataset extracted three components with a cumulative explained variance of 55% (KMO 0.69; Bartlett’s test of sphericity Chi-square = 1835; df = 351; p = 4.9E–199). The rotated solution showed that PC1 (30% variance explained) had highest factor loadings on the mediators TNF, IL-4, IFNγ, IL-17 and IL-2 corresponding to a pro-inflammatory cytokine axis. PC2 (13% variance explained) had highest factor loadings on IL-8, IL-6, IL-9, FGF basic and MIP-1α corresponding to a pro-inflammatory chemokine axis. Finally, PC3 (11% variance explained) had highest loadings on VEGF, IL-10, IL-12 (p70), IL-7 and IL-5 the direction of which is not entirely clear. shows factor loadings of PC1 and PC2 for the individual observations in the study groups. Many IBS subjects are located among the normal controls. However, some degree of immune activation may exist in subgroups of IBS.
Figure 1. Principal component analysis of 27-plex mediator measurement in rectal mucosa from subjects with IBS w/wo fructose intolerance, IBS controls and normal controls. Three PCs were extracted accounting for a cumulated 55% of the variance. The plot shows individual factor loadings of the two first components in a rotated solution. PC1 (30% variance) represents an adaptive pro-inflammatory component with high contribution from mediators like TNF, IFNγ, IL-17, IL-4 and IL-2. PC2 (13% variance) represents an early response pro-inflammatory component with high contribution from mediators like IL-8, IL-6, IL-9, bFGF and MIP-1α.
Discussion
In this study, we have found increased mucosal levels of proinflammatory TH1 cytokines IFN-γ, IL-1b, 1L-2; the TH1 chemokine RANTES, the TH 17 cytokine IL-17 cytokine, the TH2 and allergy-associated cytokines IL-5, TNF and IL-9; the growth factors FGF basic and GM-CSF, when compared to healthy control without IBS symptoms. Our study indicates that in IBS with no sign of infiltrating leucocytes at HE histology examinations represents a disease of immune dysregulation whereas an apparent minor immune dysregulation may be due to the fructose intolerance.
The mucosal levels of some of the mucosal cytokines TH1, TH2 and TH17 were significantly increased in IBS compared to the healthy control group. These cytokines which is generally increased in IBD, although at much higher levels, has to some extent been studied in IBS [Citation28,Citation29]. Thus, various differences to mucosal levels in healthy controls have been reported in some publications [Citation19,Citation23,Citation30] but not in others [Citation21,Citation24,Citation25]. Also of interest to note was the mucosal TNF as one of the main mediators of IBD [Citation28], was increased in this study but not in other studies [Citation20,Citation22]. This may be due to methodological differences, especially considering the low-level increase registered. The TNF-alpha G/A polymorphism at position 308 has been observed in IBS patients [Citation31] and may have pathophysiological role in IBS.
The two TH2 cytokines IL-5 and IL-9 were increased compared to the healthy control group. Both cytokines secreted from mast cells that has been proposed to be one of the main mediators of hypersensitivity in IBS [Citation32]. Our IL-5 and IL9 data are in agreement with previous reports in IBS [Citation33,Citation34]. Therefore, all these data taken together indicate that in IBS a mucosal TH2 dysregulation exists, which mediates a low-grade inflammation with activation of inflammatory cells such as mast cells [Citation3,Citation32].
The mucosal level of the TH1 chemokine RANTES and eotaxin was significantly increased in IBS. First, the eotaxin result fits well with the documentation of mast cell activation in IBS [Citation16] and the mast cell activation of eotaxin [Citation35]. Moreover, our data agree with our findings of increased levels of the TH1 cytokines and TNF as cytokines with strong effect on activation of chemotaxis. In another report, CXCL-9 and MCP1 were even lower in IBS than in the healthy controls [Citation22]. These discrepancies are hard to explain. The chemokines are responsible for the leukocyte migration by creating a chemical gradient from the vascular endothelium to the infected cells and by selective expressions of different chemokine receptors on the leukocytes [Citation36]. It is interesting to speculate that a lack of a more complete and global chemokine response may be one explanation of the lack of a tissue invasion of leucocytes measured by conventional diagnostic procedures.
Of the various growth factors measured, there were significant increases in the growth factors bFGF, PDGFBB and GM-CSF. As far as we know, there are no previous reports of these two mucosal growth factors in IBS. Other growth factors such as neural growth factor have been shown to be overexpressed in intestinal mucosa in IBS [Citation37]. In general, growth factors play a role to maintain the mucosa integrity [Citation38] are proposed to have anti-inflammatory effects. In chronic inflammation such as in IBD, these mediators play a role in the mucosal healing.
In IBS patients with self-reported fructose intolerance, only IL-5 was significantly increased compared to IBS patients without fructose intolerance indicating a possible allergy-like mechanism. The pathophysiological mechanisms behind this condition is unknown, but it has been proposed to be due to reduced intestinal absorption capacity of fructose [Citation39]. Whether an allergic mechanism could be involved, has to be further investigated.
In general, IBS is most likely a heterogeneous disease where the main proposed contributing pathophysiological factors are neuroendocrine abnormalities, low grade inflammation, failure of the gut barrier, increased fecal bile content, abnormal visceral hypersensitivity and psychosocial stress. Moreover, stress as a causal factor of immune dysregulation in the gut has been known for long in animals [Citation40] and in humans (for review, see [Citation41]). The heterogeneity is supported by the various contradictory reports concerning the mucosal immunophenotype between our and other reports (for review, see [Citation4,Citation7,Citation42,Citation43]). The pathophysiological mechanism behind IBS can either be abnormal interactions between neurons/nerves and immune cells [Citation43], also referred to as the ‘neuroimmune’ synapse [Citation6]. Moreover, though many of the IBS subjects in the present study show a pro-inflammatory tendency, a substantial part of the IBS subjects falls within the ‘normal control’ sphere (), which underlines the impression that IBS is a disorder of heterogenic pathogenesis. Finally, we should be aware that the suggested immunological disturbances in IBS is still least controversial and described by some as a possible myth [Citation44].
The strength of this study is the broad mucosal characterization of cytokines, chemokines and growth factors that would be potentially involved in IBS as an immunological active disease. The weakness of the study that should initiate further studies is that whole biopsy extracts is relatively crude. A mucosal immunologic characterization at cellular level would give more comprehensive data. Moreover, IBS is most likely a heterogeneous disease concerning the potential etiological factors and the various clinical phenotypes. Therefore, further studies are needed focusing on subgrouping defined by subgroup phenotypes of IBD, as well as precipitating factors such as infection, stress and antibiotics. Moreover, potentially, the inflammatory biomarkers could be explained by changes in the fecal microbiota, and microbiotic phenotypes related to diet including fiber intake [Citation45,Citation46]. This awaits further studies.
In conclusion, a dysregulated mucosal immune profile with a mixed TH1, TH2, TH17 and growth factor profile is observed in IBS patients when compared to healthy control. This indicates that IBS is a low-grade inflammatory bowel disease with an apparent incomplete chemotactic stimulus that may preclude recruitment of leucocytes from the general circulation.
| Abbreviations | ||
| bFGF | = | basic fibroblast growth factor |
| FRD | = | fructose reduced diet |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| IBS | = | irritable bowel syndrome |
| IL | = | interleukin |
| IP | = | interferon gamma individual protein |
| MCP | = | monocyte chemoattractant protein |
| MIP | = | macrophage inflammatory protein |
| PDGF-BB | = | platelet derived growth factor-BB |
| RANTES | = | regulated on activation, normal T expressed and secreted |
| TH | = | T helper cell |
| TNF | = | tumor necrosis factor |
Acknowledgements
The authors thank for our colleagues at the Departments of Gastroenterology at Hospital of Helgeland, Rana; Nordlandssykehuset, Bodø; and University Hospital North Norway, Tromsø, Norway in support in recruiting patients. Guarantor of article: Jon Florholmen.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14(2):125–132.
- Feng B, La JH, Schwartz ES, et al. Irritable bowel syndrome, mechanisms, and pathophysiology. Neural and neuro-immune mechanism of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302(10):G1085–G1098.
- Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46(4):421–431.
- Bashashati M, Rezaei N, Andrews CN, et al. Cytokines and irritable bowel syndrome: where do we stand? Cytokine. 2012;57(2):201–209.
- Hughes PA, Zola H, Penttila IA, et al. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108(7):1066–1074.
- Vanner S, Greenwood-Van Meerveld G, Mawe GM, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2016;150(6):1280–1291.
- Boeckxstaens GE, Wouters MM. Neuroimmune factors in functional gastrointestinal disorders: a focus on irritable bowel syndrome. Neurogastroenterol Motil. 2017;29(6):e13007–e13010.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;27(Suppl. 192):102–105.
- Thompson JR. Is irritable bowel disease an infectious disease? World J Gastroenterol. 2016;22(4):1331–1334.
- Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47(6):804–811.
- Barbara G, Cremon VC, Stanghellini V. Inflammatory bowel disease and irritable bowel syndrome: similarities and differences. Curr Opin Gastroenterol. 2014;30(4):352–358.
- Spiller RC, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136(6):1979–1988.
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122(7):1778–1783.
- Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104(2):392–400.
- O’Sullivan MA, O’Morain C. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457.
- Krammer L, Sowa AS, Lorentz A. Mast cells in irritable bowel syndrome: a systematic review. J Gastrointestin Liver Dis. 2019;28(4):463–472.
- Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702.
- Braak B, Klooker TK, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: is there any relationship? Am J Gastroenterol. 2012;107(5):715–726.
- Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52(4):523–526.
- Bennet SM, Polster A, Tornblom H, et al. Global cytokine profiles and association with clinical characteristics in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;111:1165–1176.
- Chang L, Adeyemo M, Karagiannidis I, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107(2):262–272.
- Macsharry J, O'Mahony L, Fanning A, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43(12):1467–1476.
- Chen J, Zhang Y, Deng Z. Imbalance shift of cytokine expression between T helper 1 and T helper 2 (TH1/TH2) in intestinal mucosa of patients with post-infectious irritable bowel disease. BMC Gastroenterol. 2012;12(1):91.
- Mearin F, Perello A, Balboa A, et al. Pathogenetic mechanisms of postinfectious functional gastrointestinal disorders: results 3 years after gastroenteritis. Scand J Gastroenterol. 2009;44(10):1173–1185.
- Wouters MM, Van Wanrooy S, Nguyen A, et al. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut. 2016;65(8):1279–1288.
- Berg LK, Fagerli E, Martinussen M, et al. Effect of fructose-reduced diet in patients with irritable bowel disease and its correlation to a standard fructose breath test. Scand J Gastroenterol. 2013;48(8):936–943.
- Berg LK, Fagerli E, Myhre AO, et al. Self-reported dietary fructose intolerance in irritable bowel syndrome: proposed diagnostic criteria. World J Gastroenterol. 2015;21(18):5677–5684.
- Florholmen J, Fries W. Candidate mucosal and surrogate predictive and prognostic biomarkers of inflammatory bowel disease in the era of new technology. Scand J Gastroenterol. 2011;46(12):1407–1417.
- Soufli I, Toumi R, Rafa H, et al. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7(3):353–360.
- Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103(10):2570–2576.
- Van de Veek PP, van den Berg M, de Kroon YE, et al. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel disease. Am J Gastroenterol. 2005;100(11):2510–2516.
- Wouters MM, Vicario M, Santos J. The role of mast cells in functional disorders. Gut. 2016;65(1):155–168.
- Kindt S, Van Oudenhove L, Broekaert D, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21(4):389–398.
- Li M, Zhang L, Lu B, et al. Role of dendritic cell-mediated abnormal immune response in visceral hypersensitivity. Int J Clin Exp Med. 2015;8(8):13243–13250.
- Miyagawa Y, Murakami A, Ebihara N. The proteolytic effect of mast cell tryptase to eotaxin-1/CCL11·eotaxin-2/CCL24 and eotaxin-3/CCL26 produced by conjunctival fibroblasts. Jpn J Ophthalmol. 2019;63(2):215–220.
- Sallusto F, Mackay CR. Chemoattractants and their receptors in homeostasis and inflammation. Curr Opin Immunol. 2004;16(6):724–731.
- Xu XJ, Zhang YL, Liu L, et al. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Aliment Pharmacol Ther. 2017;45(1):100–114.
- Playford RS, Gosh S. What is the role of growth factors in IBD? Inflamm Bowel Dis. 2008;14(2):S119–S120.
- Rumessen JJ, Gudmand-Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27(10):1161–1168.
- Aarstad HJ, Kolset SO, Seuelid R. The effect of stress in vivo on the function of mouse macrophages in vitro. Scand J Immunol. 1991;33(6):673–681.
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut. Pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62(6):591–599.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel disease. Nat Rev Dis Primers. 2016;2(1):16014.
- Ng QX, Soh AYS, Loke W, et al. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345–349.
- Sinagra E, Pompei Tomasello G, Cappello F, et al. Inflammation in irritable bowel syndrome. Myth or new treatment target. World J Gastroenterol. 2016;22(7):2242–2255.
- Canakis A, Haroon M, Weber HC. Irritable bowel syndrome and gut microbiota. Curr Opin Endocrinol Diabetes Obes. 2020;27(1):28–35.
- El-Salhy M, Ystad SO, Mazzawi T, et al. Dietary fiber in irritable bowel syndrome. Int J Mol Med. 2017;40(3):607–613.

