Abstract
Objective
Irritable bowel syndrome (IBS) is a gut-brain disorder associated with increased gut permeability. Zonulin has been suggested to regulate the gut barrier and claimed to be pre-haptoglobin 2 (pre-HP2) and circulating zonulin is often used as a proxy for gastrointestinal permeability. This study investigated the correlation between colonic paracellular permeability and levels of circulating zonulin and pre-HP2.
Materials and Methods
Colonic biopsies from 32 patients with IBS and 15 healthy controls (HC) were used to measure permeability in Ussing chambers and levels of zonulin (Cusabio ELISA). Zonulin was also measured in blood samples from 40 HC, 78 patients with IBS and 20 patients with celiac disease (CeD), before and after a gluten-free diet. In addition, we verified HP genotype and circulating pre-HP2 using a monoclonal pre-HP2 antibody (Bio-Rad) by ELISA.
Results
Increased colonic paracellular permeability correlated positively with zonulin levels in IBS biopsies, but negatively with plasma zonulin. We found no agreement between circulating zonulin and pre-HP2. Genotyping revealed non-specificity of the zonulin kit, as all pre-HP2 non-producers presented detectable levels. Patients with CeD displayed higher pre-HP2 and zonulin levels compared to HC. A gluten-free diet in patients with CeD led to lower serum zonulin and pre-HP2 concentrations.
Conclusions
Our study suggests that neither circulating zonulin nor pre-HP2 mirror colonic permeability. Our data corroborate previous reports showing the inability of the Cusabio zonulin kit to target zonulin and highlights that the results of studies using this kit must be re-examined with caution.
Introduction
Irritable bowel syndrome (IBS) is one of the most common disorders of the gut–brain axis mainly characterized by abdominal pain associated with changes in bowel habits (constipation/diarrhea) [Citation1]. Altered gut-immune activation, intestinal and colonic microbiome, and intestinal permeability have also been identified in patients with IBS [Citation2–9].
Impaired gut barrier function is a key pathogenic component in the etiology of several chronic diseases, including IBS [Citation10] following acute gastroenteritis [Citation5,Citation11,Citation12]. The intestinal barrier maintains the balance between absorption of ions and nutrients, secretion of fluids, and protection from microorganisms and dietary antigens present in the lumen [Citation13]. The passage across the epithelium occurs mainly via the paracellular route under the control of the apical junctional complex which is partly formed by the tight junctions (TJ) [Citation13,Citation14]. Little is known about the precise mechanisms operating TJ, however, several factors such as food products, pathogens, cytokines and growth factors modulate TJ, thus influencing gut permeability [Citation15].
Zonulin has been advertised as a master regulator of endothelial and epithelial TJ, modulating both blood–brain and gut barriers [Citation16]. In conditions such as celiac disease (CeD), zonulin would be upregulated and released into the gut lumen [Citation17–19], temporarily dissembling TJ and increasing permeability [Citation20]. Zonulin has been identified as pre-haptoglobin 2 (pre-HP2) [Citation21]. Due to a polymorphism of the haptoglobin (HP) gene, represented by HP1 and HP2 alleles, three different genotypes/phenotypes known as HP1-1, HP2-1 and HP2-2 can be expressed [Citation22]. Thus individuals expressing HP1.1 produce haptoglobin1, and those bearing HP2.2 genotype synthesize HP2 exclusively [Citation23].
Researchers have spent time and money on commercial zonulin enzyme-linked immunoassay (ELISA) kits. However, recent studies [Citation24,Citation25] showed that these kits (for instance Immundiagnostik and Cusabio), do not detect zonulin, but haptoglobin and complement factor C3 [Citation25]. Other studies have shown that zonulin failed to identify conditions where small intestinal permeability is a known feature [Citation24–26]. Intriguingly, no study has addressed both human colonic permeability and the levels of zonulin in parallel.
We sought to investigate if colonic and circulating zonulin levels were altered in patients with IBS presenting increased colonic paracellular permeability [Citation10,Citation27] compared to healthy controls (HC). We also verified the HP genotype in a larger group and their respective zonulin and pre-HP2 circulating levels. Serum from patients with CeD before and after the gluten-free diet were analyzed as a comparison.
Materials and methods
Subjects
Seventy-eight IBS patients, 9 men, with median age 30 years (range 18–56 years) and 40 HC, 4 men, with median age 32 years (range 20–55 years) were included in the study. HC had no medical history of gastrointestinal symptoms or complaints and were recruited by public advertisement. The IBS patients fulfilled Rome III criteria [Citation28] and were recruited from the Gastroenterology Department, University Hospital, Linköping. The Rome III IBS-stool consistency distribution of the patients revealed 18 patients with IBS-diarrhea, 17 with IBS-constipation and 43 with IBS-mixed. The IBS Severity Scoring System (IBS-SSS) was used for assessing self-reported IBS symptom-severity. At the time for inclusion, patients had moderate to severe symptoms according to IBS-SSS, median score 330 (interquartile range (IQR) 282–380). None of the patients related the onset of their symptoms to infectious gastroenteritis. Exclusion criteria for both groups included self-reported allergy, organic gastrointestinal disease, metabolic or neurological disorders as well as nicotine or nonsteroidal anti-inflammatory drug (NSAID) intake. The committee of human ethics, Linköping, approved the study (Dnr 2013/506-32) and all subjects gave their written informed consent. Twenty patients with CeD (8 men, 12 women), median age 56 (range 32–76) were included in the study as a disease control group.
Collection of blood samples
Venous blood samples were collected from 40 HC, 78 patients with IBS, and 20 patients with CeD. Blood was centrifuged and plasma or serum was redrawn and frozen in −80 °C until further analysis. For patients with CeD, blood samples were obtained before and after 1 year of a gluten-free diet.
Collection of biopsies
Biopsies from 32 patients with IBS and 15 HC were taken by a flexible sigmoidoscopy from the sigmoid colon with forceps without a central lance. Biopsies for permeability studies were directly put in ice-cold oxygenated Krebs buffer (115 mM NaCl, 1.25 mM CaCl2, 1.2 mM MgCl2, 2 mM KH2PO4, and 25 mM NaHCO3, pH 7.35) and biopsies for measurements of zonulin were snap-frozen and stored at −80 °C. Biopsies were taken at the same time point as blood samples.
Ussing chamber experiments
Colonic biopsies were mounted in Ussing chambers (Harvard apparatus Inc., Holliston, MA, USA) as previously described [Citation29]. After equilibration for 40 min, the paracellular probe 51Chromium (Cr)-EDTA (Perkin Elmer, MA, USA) was added to the mucosal side of the tissues to a final concentration of 34 µCi/ml. Samples from the serosal sides of the chambers were collected at 0, 60 and 120 min and passage was measured in a gamma-reader (1282 Compugamma, Sweden). Further, 51Cr-EDTA permeability was calculated during the 60–120 min period and results are presented as Papp (apparent permeability coefficient; cm/s × 10−6). The transepithelial potential difference (PD) and the transepithelial resistance (TER) were monitored throughout the experiments to ensure tissue viability.
Zonulin concentration in plasma, serum and biopsies by ELISA
To measure zonulin levels, a human zonulin ELISA kit was used following the manufacturer’s instructions (Cusabio Biotech Co, PA, USA). Biopsies were lyzed in RIPA-buffer (Pierce, Thermo Scientific, Sweden), containing protease inhibitors (Roche, Germany) and nuclease (Pierce). Samples were further homogenized on Tissue-Lyser II (Qiagen, Sweden). Supernatants were redrawn and protein concentration was measured with Bio-Rad DC protein assay (Bio-Rad, Sweden).
Plasma or serum (undiluted), biopsy lysates (diluted 1:2), and standard samples were added in duplicates to plates pre-coated with primary antibody and incubated for 2 h in 37 °C. A secondary-biotinylated antibody was added and after 1 h in 37 °C, streptavidin-horseradish peroxidase was added followed by repeated incubation. Tetramethylbenzidine enzyme-substrate (TMB) was added and after 30 min of incubation, 2 M HCl was added for stopping the reaction. Absorbance was measured at 450 nm in VERSAmax Tunable Microplate Reader (Molecular Devices, San Diego, CA, USA) using Softmax pro 5 (Molecular Devices). The software generated a standard curve based on the standard points, from which the concentrations of the samples were calculated.
Genotyping of HP1 and HP2 alleles
We verified the distribution of HP1 and HP2 alleles by polymerase chain reaction (PCR) [Citation30]. DNA was extracted from blood using a QIAamp DNA blood kit (Qiagen). Using two pairs of primers, specific DNA segments were amplified representing the alleles HP 1 and HP 2 from genomic DNA. To amplify a 1757-bp HP 1 allele and a 3481-bp HP 2 allele sequences the following oligonucleotide primers were used: A (5′-GAGGGGAGCTTGCCTTTCCATTG-3′) and B (5′-GAGATTTTTGAGCCCTGGCTGGT-3′). A 349-bp HP2 allele was amplified using the primers C (5′-CCTGCCTCGTATTAACTGCACCAT-3′) and D (5′-CCGAGTGCTCCACATAGCCATGT-3′). PCR reactions contained 2 U of Taq polymerase (Thermo Fisher), 1–100 ng of DNA, and 200 µM each of dATP, dCTP, dGTP, and dTTP (Invitrogen) and PCR buffer with no supplements added. We used a T100 Thermal Cycler Bio-Rad for PCR reactions. After initial denaturation at 95 °C for 2 min, the two-step thermocycling procedure consisted of denaturation at 95 °C for 1 min and annealing and extension at 69 °C for 2 min (in the presence of primers A and B or primers A, B, C and D) or 1 min (in the presence of primers C and D only), repeated for 35 cycles, and followed by a final extension at 72 °C for 7 min. PCR products were separated in 0.7% agarose gels for genotyping determination.
Pre-HP2 concentration in plasma or serum by ELISA
Because zonulin levels by ELISA and genotyping of HP genes revealed the non-specificity of the Cusabio kit, we employed a quantitative sandwich ELISA using a monoclonal antibody specific for pre-HP2 kindly provided by Bio-Rad (France), as described in Flanagan and colleagues [Citation31]. This antibody was developed to target serum or plasma pre-HP2, and it did not work with biopsy lysates. Standards and plasma or serum samples (diluted 1:5), were transferred into wells coated with specific pre-HP2 monoclonal antibody to capture any pre-HP2 present. After washing, a biotinylated antibody specific for pre-HP was added to the wells; following washing, horseradish peroxidase (HRP)-conjugated streptavidin was added to the wells; after one final wash, a TMB substrate was added to the wells and the reaction was stopped by adding sulfuric acid 1 M. Absorbance was measured as aforementioned.
Statistics
No statistical methods were used to determine sample size. Comparisons between two groups were analyzed by Mann–Whitney t-test (unpaired, two-tailed) or non-parametric t-test Wilcoxon matched-pair rank when appropriated; tests involving more than two groups were analyzed by one-way ANOVA Kruskal–Wallis followed by Dunn’s multiple comparison post hoc test. We employed non-parametric Spearman’s rank for correlational analysis. Data were presented as median (IQR), unless otherwise indicated. Statistical significance displayed as p < .05*, p < .01**, p < .001*** and p < .0001****.
Results
Tissue viability in ussing chambers
The PD was stable in all biopsies after 40 min of equilibration, confirming tissue viability. As previously published [Citation10], there was a lower TER in biopsies from patients with IBS compared to HC (data not shown). There was no correlation between TER and levels of zonulin measured in the corresponding biopsy lysates (data not shown).
Higher colonic permeability and zonulin levels in IBS biopsies compared to HC
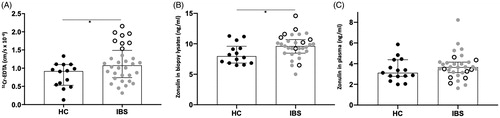
There was a higher permeability to 51Cr-EDTA in patients with IBS compared to HC, p < .05 (), data partly published in Gastroenterology [Citation10]. No significant difference in permeability between subgroups could be found (data not shown). In the present study, we wanted to investigate whether this increase was related to levels of zonulin in biopsy lysates and in plasma. Results of measurements using the Cusabio ELISA zonulin kit showed increased concentrations of zonulin in biopsy lysates of patients with IBS compared to HC, p < .05, but not in plasma (). There was no significant difference in zonulin levels between subgroups, neither in plasma nor in biopsy lysates (data not shown).
Figure 1. Paracellular permeability in colonic biopsies mounted on Ussing chambers and their corresponding zonulin colonic and circulating levels. (A) Passage of 51Chromium (Cr)-EDTA in the colonic mucosa of 15 healthy controls (HC) and 32 patients with irritable bowel syndrome (IBS). (B) Zonulin levels measured in biopsy lysates of HC and patients with IBS. (C) Circulating levels of zonulin measured in the plasma of HC and patients with IBS. Open scatter dots depict samples showing leakier mucosa. Data was analyzed by Mann–Whitney U test (unpaired, two-tailed) and presented as median (interquartile range) *p < .05.
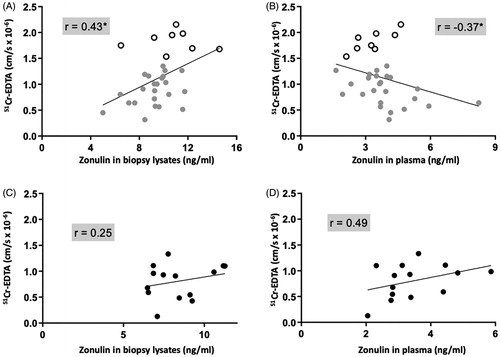
Colonic paracellular permeability does not correlate with circulating zonulin levels
There was a cluster of samples from IBS patients presenting higher paracellular permeability (open dots in ). Even though the more permeable colonic samples showed some parallel with zonulin levels detected in the biopsy lysates (open dots in ), the Cusabio ELISA kit failed to detect them in the corresponding plasma samples (open dots in ).
Correlation analyses revealed a positive correlation between paracellular permeability through IBS colonic biopsies and zonulin levels measured in the same tissue, p < 0.05 (). Correlational analysis of only biopsies from IBS patients expressing high paracellular permeability (n = 8, identified as open dots in ), with circulating zonulin was considerably high, r = 0.8, p < 0.05. The correlation between paracellular permeability and circulating zonulin was negative, p < .05 (). In HC, no significant correlations were found, neither in biopsies () nor in plasma ().
Figure 2. Colonic paracellular permeability correlates negatively with circulating zonulin and positively with zonulin in biopsy lysates of patients with irritable bowel syndrome (IBS). (A) Correlation of the passage of 51Chromium (Cr)-EDTA in the colonic mucosa of 32 patients with IBS with their corresponding tissue zonulin levels. (B) Correlation of the passage of 51Cr-EDTA in the colonic mucosa of 32 patients with IBS with their corresponding plasma zonulin levels. (C) Correlation of the passage of 51Cr-EDTA in the colonic mucosa of 15 healthy controls (HC) with their corresponding tissue zonulin levels (D) Correlation of 51Cr-EDTA permeability in the colonic mucosa of 15 HC with their corresponding plasma zonulin levels. Data was analyzed by non-parametric Spearman’s correlation and presented as the actual values of each parameter. Open scatter dots depict samples showing leakier mucosa. Correlation coefficient is presented as r = −1 to 1, statistical significance is indicated as *p < .05.
Genotyping of haptoglobin genes reveals the inefficacy of zonulin ELISA kit manufactured by Cusabio
Genotyping was performed on 15 HC samples and 32 samples of patients with IBS that were assayed on Ussing chambers. One HC sample and 4 IBS samples were identified as Hp 1.1 genotyping (non-pre-HP2producers). Using the ELISA kit manufactured by Cusabio, these samples showed zonulin levels (HC: 2.87 ng/ml and IBS 3.11 ng/ml) within the average distribution of their groups, indicating false-positive zonulin detection by the kit, as the target protein could not possibly be present in those samples. Genotyping and zonulin were assessed in a larger number of samples (). Hp 1.1 distribution within the 40 HC and 78 patients IBS, was 5 (12.2%) and 13 (16.7%), respectively.
Table 1. Haptoglobin (Hp) genes distribution within the healthy control (HC) group and the group of patients with irritable bowel syndrome (IBS) and their average zonulin levels as assayed by Cusabio ELISA kit.
Colonic paracellular permeability does not correlate to circulating pre-HP2 levels
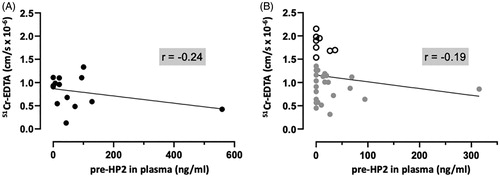
Since genotyping showed detectable zonulin in plasma from pre-HP2 non-producing subjects, we performed an additional ELISA using a specific monoclonal pre-HP2 antibody. Results showed no correlation with paracellular permeability, neither in HC () nor in IBS ().
Figure 3. Colonic paracellular permeability does not correlate with pre-haptoglobin 2 (pre-HP2) in plasma of healthy controls (HC) and patients with irritable bowel syndrome (IBS). (A) Correlation of 51Chromium (Cr)-EDTA permeability in the colonic mucosa of 15 HC with their corresponding plasma pre-HP2 levels. (B) Correlation of 51Cr-EDTA permeability in the colonic mucosa of 32 patients with IBS with their corresponding plasma pre-HP2 levels. Data was analyzed by non-parametric Spearman’s correlation and presented as the actual values of each parameter. Open scatter dots depict samples showing leakier mucosa. Correlation coefficient is presented as r = –1 to 1.
Pre-HP2 levels assessed by ELISA using a specific monoclonal pre-HP2 antibody (Bio-Rad), were also performed in the larger sample material (). None of the Hp 1.1 samples showed detectable levels of pre-HP2, suggesting that the antibody does not target any protein other than pre-HP2.
Table 2. Haptoglobin (Hp) genes distribution within the healthy control (HC) group and the group of patients with irritable bowel syndrome (IBS) and their average pre-HP2 levels as assayed by ELISA using a specific monoclonal pre-HP2 antibody (Bio-Rad).
Circulating zonulin and pre-HP2 levels measured by ELISA showed no agreement
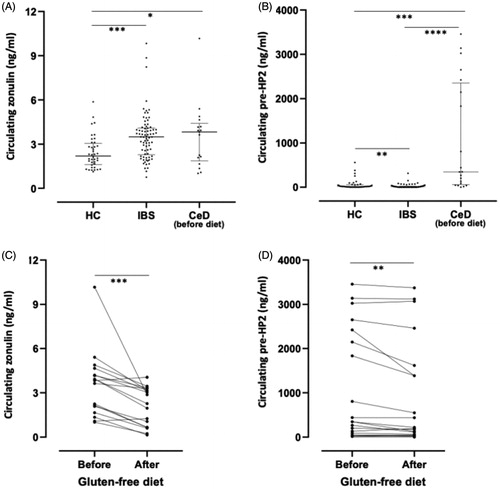
Results from zonulin and pre-haptoglobin, as measured by ELISA in plasma from HC and patients with IBS, showed no agreement between them. Zonulin levels, measured by Cusabio ELISA kit, were increased in the IBS group compared to HC, p < .001 () while pre-HP2 levels were lower in IBS compared to HC, p < .01 (). Zonulin levels in the disease control group, CeD, were higher compared to HC, p < .05 (), but similar to the IBS group (), which was surprising since CeD is an inflammatory disorder known to have high circulating levels of zonulin [Citation32]. In contrast, pre-HP2 values were considerably high in many of the patients within the CeD group, clearly distinguishing it from HC and IBS, p < .0001 (). Interestingly, both zonulin () and pre-HP2 () levels in patients with CeD presented a significant drop in circulating concentrations after 1 year on a gluten-free diet.
Figure 4. Circulating levels of zonulin and pre-haptoglobin 2 (HP2) in healthy controls (HC), patients with irritable bowel syndrome (IBS) and patients with celiac disease (CeD) before and after gluten-free diet. (A) Zonulin concentrations measured by Cusabio ELISA kit in plasma from 40 HC and 78 patients with IBS and in serum from 17 patients with CeD (B) Pre-HP2 concentrations measured by ELISA using pre-HP2 monoclonal antibody (Bio-Rad) in plasma from 40 HC and 78 patients with IBS and in serum from 20 patients with CeD. (C) Zonulin concentrations measured by Cusabio ELISA in serum from17 patients with CeD before and after gluten-free diet. (D) Pre-HP2 concentrations measured by ELISA using pre-HP2 monoclonal antibody (Bio-Rad) in serum from 20 patients with CeD before and after gluten-free diet. Data is expressed as median with interquartile range (A, B) or as mean values (C,D). One-way ANOVA Kruskal–Wallis followed by Dunn’s multiple comparison post hoc test (A,B), or non-parametric Wilcoxon matched-pair rank (C,D) were used for comparisons, *p < .05; **p < .01; ***p < .001 and ****p < .0001.
Discussion
Zonulin has been advertised as a peripheral marker of intestinal permeability [Citation33], however, studies addressing both colonic paracellular permeability and zonulin levels in humans are lacking. In the present study, we initially aimed to determine mucosal and circulating zonulin levels in HC and patients with IBS and correlate them to colonic paracellular permeability. We used the zonulin ELISA kit manufactured by Cusabio since it has been used previously by our group [Citation34]. Our findings showed a positive correlation between colonic permeability and zonulin levels in the biopsies of IBS patients, but a negative correlation between permeability and plasma zonulin. Even though permeability and zonulin in biopsy lysates showed a correlative pattern, the samples presenting higher permeability were not exclusively found among those with higher zonulin content. Of note, no sample showed levels low enough to be undetectable.
By the time we were performing the current study, Scheffler and colleagues [Citation24] reported the inability of an ELISA kit, manufactured by Immundiagnostik, to detect zonulin, raising concerns over other commercially available zonulin ELISA kits. As the genetic ability of an individual to synthesize zonulin, also known as pre-HP2, can be verified by genotyping [Citation22], we looked at the distribution of haptoglobin genes (HP1 and HP2) in IBS and HC samples. At least 15% of the population is expected to not express pre-HP2 [Citation35]. Our genotyping confirmed the non-specificity of the zonulin kit manufactured by Cusabio, as all pre-HP2 non-producers (genotype HP 1.1) had detectable levels of zonulin. As a next step, we performed an additional ELISA employing a monoclonal antibody to pre-HP2 (kindly provided by Bio-Rad, France). Our results showed that IBS samples displayed significantly lower circulating pre-HP2 levels compared to HC; importantly, pre-HP2 ELISA matched haptoglobin genotyping. Whether these lower pre-HP2 levels in IBS are related to the pathophysiology of IBS or not, requires further investigation. No correlation with IBS-specific symptoms was found (not shown).
Zonulin was identified in 2000, by Wang and colleagues [Citation36], where they provided the amino acid sequence GGVLVQPG as zonulin’s NH2-terminal amino acid sequence. Later, Tripathi and colleagues [Citation21] proposed that zonulin is pre-HP2, even though that sequence was not found in pre-HP2, which was attributed to either high mutation rate of the zonulin genes or to a sequencing error in Wang’s publication [Citation36]. Despite these findings, the GGVLVQPG sequence was still utilized to generate the antibody of the zonulin ELISA kit manufactured by Immundiagnostik. It is also the sequence of zonulin’s antagonist, the peptide larazotide acetate, also known as AT-1001 [Citation37] and formerly known as FZI/0 [Citation38]. A BLAST search for the amino acid GGVLVQPG generates100-top sequences with significant alignments, 23 of which are heavy chain immunoglobulins, none of whose sequences are zonulin or pre-HP2 or HP related proteins.
More recently, Ajamian and colleagues [Citation25] pointed out the inability of both Cusabio and Immundiagnostik kits in detecting zonulin as their findings did not match haptoglobin phenotyping. According to their report, the Cusabio kit detects complement factor C3 and haptoglobin; moreover, the kit failed to detect recombinant zonulin. Another study [Citation26] reported that zonulin (unknown manufacture) failed to distinguish between IBS, organic diseases, functional dyspepsia, and healthy populations. Our data confirmed the non-specificity of the Cusabio ELISA kit indicated by Ajamian and colleagues [Citation25], as verified by HP genotyping.
As a control group, we also measured zonulin and pre-HP2 in serum samples from patients with CeD, before and after a gluten-free diet. CeD samples displayed much higher values for pre-HP2 compared to HC and IBS. Zonulin values were significantly higher than in HC yet similar to IBS. Both zonulin and pre-HP2 presented lower concentrations in this disease-control group one year after the gluten-free diet. Nevertheless, the values suggest that additional antigens also trigger zonulin/pre-HP2 release as they were continuously detectable within the range as displayed before the gluten-free diet. For instance, even though pre-HP2 lowered following a gluten-free diet, they were still high.
The association between colonic zonulin and increased permeability in our study may seem intriguing since zonulin has been suggested to be operative in the small intestine only [Citation14]. A conference abstract by Barbaro and colleagues [Citation39] reported higher serum zonulin levels (undefined ELISA kit, most likely the Cusabio kit, as these authors, recently published a follow-up paper [Citation40] using this kit) in patients with CeD, non-celiac gluten sensitivity and IBS-D and also higher colonic zonulin mRNA in IBS-D patients compared to HC.
One important limitation of our study is the lack of in vivo small and large intestinal permeability which could help to understand the contribution of zonulin or pre-HP2 to IBS, if any. Studies have demonstrated no effect of zonulin or its synthetic antagonist AT-1001 (or larazotide acetate) on colonic mucosa of rabbits [Citation41], mice [Citation20] and rhesus monkeys [Citation18]. However, zonulin release after gliadin challenge has been demonstrated in the human colonic epithelial cell line (Caco-2) [Citation42]. To our knowledge, there is no data supporting that zonulin or pre-HP2, either does or does not open TJ in the human colon. The proposed mechanism by which zonulin disablesTJ is dependent on the epidermal growth factor receptor (EGFR) and the protease-activated receptor (PAR2) [Citation43]. These receptors have also shown to be expressed apically in the colonic mucosa [Citation44]. Activation of disassembling TJ signaling pathway may not exclusively come from the apical side as both EGFR and PAR2 are present at the basolateral side of enterocytes [Citation45].
Zonulin’s use as a potential biomarker of intestinal permeability should not be recommended due to the lack of specificity of antibodies commercially available now. Based on our data, the zonulin kit manufactured by Cusabio does not measure zonulin, as previously shown [Citation25]. The pre-HP2 monoclonal antibody developed by Bio-Rad appears to be efficient and consistent, showing agreement with genotyping of HP genes, however, this antibody is not commercially available yet. As highlighted by others [Citation24,Citation25], the results of studies using the kits manufactured by Immundiagnostik and Cusabio must be viewed with caution, including our study on intestinal permeability in the elderly [Citation34]. As aforementioned, the NH2-terminal sequence of the human zonulin published by Wang and colleagues [Citation36] is the same sequence that was used to raise the zonulin antibody present in the Immundiagnostik kit, and to produce the peptide AT-1001 or larazotide acetate, a drug that has gone through several clinical trials already. Interestingly, a randomized study with 24 subjects has shown a very limited effect in the main endpoint results, intestinal permeability measured by lactulose‐to‐mannitol ratio [Citation37]. In the other studies with 86 and 184 subjects, respectively [Citation46,Citation47], larazotide acetate failed to protect from gluten challenge-induced increased permeability.
Two very recent studies [Citation40,Citation48] used circulating zonulin levels (measured by Cusabio ELISA kit) as a marker of intestinal permeability, and despite the robustness of these studies, none of them quoted the recent papers raising serious concerns on zonulin ELISA kits [Citation24,Citation25]. Our hope is that the present study helps researchers to avoid the use of these kits as a tool to measure intestinal permeability in future studies.
In conclusion, our study indicates that colonic permeability in IBS has no correlation with circulating zonulin or pre-HP2 levels, and thus, we do not recognize these molecules as circulating markers of colonic or intestinal permeability. Furthermore, it confirms earlier report concerning the inability of the Cusabio zonulin kit in measuring the proposed target protein and as such, its doubtfulness as a biomarker of intestinal permeability, as pointed out by others [Citation24–26], highlights that the results of studies using these kits must be viewed with caution.
Acknowledgments
We thank all patients for their participation in this study. We also thank Martin E. Winberg and Lena Svensson at Linköping University for their service in the Ussing laboratory and assistance with ELISA analyses. We thank Pascale Galea, who organized the transfer of the monoclonal antibody anti-pre-HP2 developed and kindly donated by Bio-Rad.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Oświęcimska J, Szymlak A, Roczniak W, et al. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62(1):17–30.
- Barbara G. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am J Gastroenterol. 2006;101(6):1295–1298.
- Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19(7):545–552.
- Del Valle-Pinero AY, Van Deventer HE, Fourie NH, et al. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin Chim Acta. 2013;418:97–101.
- Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101(6):1288–1294.
- Gecse K, Roka R, Sera T, et al. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion. 2012;85(1):40–46.
- Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201.
- Quigley E. Gut permeability in irritable bowel syndrome: more leaks add to slightly inflamed bowel syndrome conspiracy theory. Gastroenterology. 2009;137(2):728–730.
- Turcotte JF, Kao D, Mah SJ, et al. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest Endosc. 2013;77(4):624–630.
- Bednarska O, Walter SA, Casado-Bedmar M, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology. 2017;153(4):948–960.
- Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20(11–12):1317–1322.
- Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47(6):804–811.
- Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4(4):e1251384.
- Fasano A. Intestinal zonulin: open sesame! Gut. 2001;49(2):159–162.
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659.
- Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555–1557.
- Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355(9214):1518–1519.
- El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123(5):1607–1615.
- Smecuol E, Sugai E, Niveloni S, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol. 2005;3(4):335–341.
- Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55(10):1512–1520.
- Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106(39):16799–16804.
- Koch W, Latz W, Eichinger M, et al. Haptoglobin gene subtyping by restriction enzyme analysis. Clin Chem. 2003;49(11):1937–1940.
- Vanuytsel T, Vermeire S, Cleynen I. The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers. 2013;1(5):e27321.
- Scheffler L, Crane A, Heyne H, et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol. 2018;9:22.
- Ajamian M, Steer D, Rosella G, et al. Serum zonulin as a marker of intestinal mucosal barrier function: may not be what it seems. PLoS One. 2019;14(1):e0210728.
- Talley NJ, Holtmann GJ, Jones M, et al. Zonulin in serum as a biomarker fails to identify the IBS, functional dyspepsia and non-coeliac wheat sensitivity. Gut. 2020;69(9):1–3.
- Witt ST, Bednarska O, Keita AV, et al. Interactions between gut permeability and brain structure and function in health and irritable bowel syndrome. Neuroimage Clin. 2019;21:101602.
- Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491.
- Keita AV, Gullberg E, Ericson AC, et al. Characterization of antigen and bacterial transport in the follicle-associated epithelium of human ileum. Lab Invest. 2006;86(5):504–516.
- Koch W, Latz W, Eichinger M, et al. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem. 2002;48(9):1377–1382.
- Flanagan JJ, Arjomandi A, Delanoy ML, et al. Development of monoclonal antibodies to pre-haptoglobin 2 and their use in an enzyme-linked immunosorbent assay (ELISA). J Immunol Methods. 2014;406:34–42.
- Duerksen DR, Wilhelm-Boyles C, Veitch R, et al. A comparison of antibody testing, permeability testing, and zonulin levels with small-bowel biopsy in celiac disease patients on a gluten-free diet. Dig Dis Sci. 2010;55(4):1026–1031.
- Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann NY Acad Sci. 2000;915:214–222.
- Ganda Mall JP, Ostlund-Lagerstrom L, Lindqvist CM, et al. Are self-reported gastrointestinal symptoms among older adults associated with increased intestinal permeability and psychological distress? BMC Geriatr. 2018;18(1):75.
- Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42(10):1589–1600.
- Wang W, Uzzau S, Goldblum SE, et al. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(24):4435–4440.
- Paterson BM, Lammers KM, Arrieta MC, et al. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26(5):757–766.
- Clemente MG, De Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52(2):218–223.
- Barbaro MR, Cremon C, Caio G, et al. 247 zonulin serum levels are increased in non-celiac gluten sensitivity and irritable bowel syndrome with diarrhea. Gastroenterology. 2015;148(4):S56.
- Barbaro MR, Cremon C, Morselli-Labate AM, et al. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut. 2020;69(11):1966–1974.
- Fasano A, Uzzau S, Fiore C, et al. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology. 1997;112(3):839–846.
- Drago S, El Asmar R, Di Pierro M, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41(4):408–419.
- Cenac N, Chin AC, Garcia-Villar R, et al. PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways. J Physiol. 2004;558(3):913–925.
- Pontarollo G, Mann A, Brandao I, et al. Protease-activated receptor signaling in intestinal permeability regulation. FEBS J. 2020;287(4):645–658.
- Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2(9):416–422.
- Leffler DA, Kelly CP, Abdallah HZ, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012;107(10):1554–1562.
- Kelly CP, Green PH, Murray JA, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37(2):252–262.
- Tajik N, Frech M, Schulz O, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11(1):1995.

