ABSTRACT
Selenite is a form of selenium (Se) commonly found in Se-excessive soils. To regulate the Se content in plants in high-Se areas, a potted soil experiment was performed on oilseed rape (Brassica napus L.) to evaluate the effects of varied amounts of sulfur (S) on the biomass, accumulation and distribution of Se in B. napus under the conditions of different amounts of Se in the soil. The results showed that the seedlings of B. napus were more sensitive to Se than the mature plants were. The addition of S significantly alleviated the growth inhibition in seedlings and facilitated the growth of mature plants under higher Se (15 mg kg−1) conditions. S treatment significantly decreased soil pH within the range of 0.22–0.60. An appropriate moderate amount (150 mg kg−1) of S exerted the strongest inhibition on Se concentration and accumulation in B. napus at the seedling stage, but a higher amount (300 mg kg−1) of S led to a more significant decrease in the mature plants under higher Se conditions, with the maximum reduction in various parts of B. napus reaching 51.3–60.9% and 42.5–53.4%, respectively. The application of S only affected the uptake of Se, and not the translocation of Se; the accumulation of Se in B. napus follows the sequence of pod ≈ stem > rapeseed > root, and the distribution ratio is approximately 1.00:0.97:0.69:0.49. Overall, the application of S alleviated the inhibitory effect on growth caused by excessive Se by reducing the Se concentration in B. napus and facilitating its growth, suggesting that S treatment is a suitable and highly cost-effective method to regulate the content of Se in B. napus.
1. Introduction
Selenium (Se) is considered an essential micronutrient for animals and human beings because it is required for the synthesis of important antioxidant proteins such as cytosolic glutathione peroxidase (Combs et al. Citation2011). The enzyme protects mammalian systems from oxidative stress and is related to the prevention of chronic diseases and cancer (Beckett and Arthur Citation2005). It was reported that the average dietary uptake of Se was only 26–32 μg per day by Chinese adults (Chen et al. Citation2002), while the recommended dietary allowance (RDA) of Se for an adult human is 55 μg per day based on the maximization of glutathione (GSH-Px) enzyme activity (Navarro-Alarcon and Cabrera-Vique Citation2008). The prevention and treatment of diseases caused by Se deficiency through Se-supplemented food have attracted more and more attention (Duffield-Lillico et al. Citation2002). Plants are usually rich in organic Se, which is much more effective in improving human immunity than inorganic Se additives in food are, so Se-rich plants are the preferred choice and main source of Se supplementation (Rayman Citation2008).
Selenium levels in soil generally reflect its presence in food and the Se levels in human populations (Navarro-Alarcon and Cabrera-Vique Citation2008; Durán et al. Citation2013; Yasin et al. Citation2015). In China, approximately 2/3 of the land by area is Se-deficient, which often results in human Se deficiency (Cao et al. Citation2001). However, China is also a major source of Se in the world. Several Se-rich regions have been identified in Hubei, Shaanxi, Yunnan, Sichuan, Hunan and Guangzhou, with the average soil concentration of Se ranging from 10 to 40 mg kg−1 (Luo et al. Citation2004; Zhu et al. Citation2008). Enshi Municipality is the only location in the world with proven independent Se deposits and by far the most selenium ore reserves, and has been granted the title ‘world selenium capital’ by The 14th International Meeting on Trace Elements in Man and Animals (TEMA14). It is viable to offset regional deficiencies of Se with Se-rich plant products from Se-rich regions (Williams et al. Citation2009). The development of Se-rich products is a possible avenue of economic development in these regions. However, soils containing more than 3.0 mg kg−1 total Se can be defined as excessive for human nutrition (Hawkesford and Zhao Citation2007). The range of Se intake between Se deficiency and toxicity in humans is rather narrow (Navarro-Alarcon and López-Martınez Citation2000), and a daily uptake above 400 μg will be in excess of human requirements (Kieliszek and Błażejak Citation2013), leading to the loss of fingernails and hair, general fatigue and other symptoms, and even death in severe cases (Aldosary et al. Citation2012). In addition, it also causes damages to plants at a high concentration (Feng et al. Citation2013). Therefore, it is important to take measures to produce safe Se-rich products without affecting their yield, and support the economic development of such regions.
Oilseed rape (Brassica napus L.) produces seeds with high contents of oil and proteins for human and animal nutrition, as well as non-edible uses (Girondé et al. Citation2014). Oilseed rape is a cruciferous crop with a high capacity for Se bioconcentration, and is widely cultivated in Se-rich regions. Numerous studies have shown that the application of Se in soil can promote plant growth, and increase the activities of antioxidant enzymes (Cartes et al. Citation2011; Chu et al. Citation2013). The effects of sulfur (S) application on Se uptake have been widely studied, but most studies were carried out with the application of selenate (Shinmachi et al. Citation2010; Stroud et al. Citation2010; Bañuelos and Dhillon Citation2011). Selenate can be taken up via sulfate transporters in plants due to the chemical similarity between selenate and sulfate (Sors et al. Citation2005). This mechanism has been thoroughly described (Zhu et al. Citation2009). Nevertheless, selenite is a predominant form of Se available to plants, especially in anaerobic soils (Zhang et al. Citation2014), and the relationship between S and selenite on B. napus is not well understood currently. In contrast to selenate, selenite is less toxic, and more resistant to leaching, with a longer lasting effect and lower environmental risk; thus, it is generally used in agricultural production as an Se fertilizer source (Huang et al. Citation2013).
The aim of the present study was to investigate the sensitivity of B. napus to Se in different growth stages, and the regulatory effects of S on the concentration and distribution ratio of Se in various parts of B. napus with the soil amended with varied amounts of selenite.
2. Materials and methods
2.1 Soil sample description
The soil used in the experiment was Aquepts taken from Mahu village in Wuhan, Hubei province, China. Its basic physical and chemical properties were listed in . The high-quality, high-yield oilseed rape (Brassica napus L. cv. xiangnongyou 571) (Liao and Guan Citation2001) was chosen as the test material.
Table 1. Physical and chemical properties of the soil tested.
2.2 Experimental design
The air-dried and sieved soil (8 kg) was placed into a pot with a 25 cm diameter and a 27 cm height. Sulfur was used as the S source at rates of 0, 150 and 300 mg kg−1. The Se source was sodium selenite (Na2SeO3), which was applied at the rates of 0, 5 and 15 mg kg−1. A factorial design was carried out and each treatment was replicated 4 times. The amounts of macro elements (g kg−1 soil) were fertilized with nitrogen (N) 0.3, phosphorus pentoxide (P2O5) 0.2 and potassium oxide (K2O) 0.25 supplied in the form of carbamide (CO(NH2)2), potassium phosphate monobasic (KH2PO4) and potassium chloride (KCl), respectively, and the microelements supplied were 0.025 mg ethylenediaminetetraacetic acid-ferric sodium salt (Fe-EDTA), 1.81 mg manganese chloride (MnCl2·4H2O), 0.08 mg copper sulfate (CuSO4·5H2O), 0.22 mg zinc sulfate (ZnSO4·7H2O) and 2.86 mg boric acid (H3BO3) per kg soil. After the soil was sufficiently stirred and balanced for 1 week, it became semi-moist and was evenly pounded. The B. napus seeds underwent surface sterilization for 15 minutes with a 0.5% solution of sodium hypochlorite (NaClO), followed by washing and soaking in deionized water, and then germination with 0.5 mmol L−1 calcium chloride (CaCl2) solution in a dark environment at 25°C. Twenty uniform seeds were sown directly in the soil and covered with a small amount of fine soil on the surface of the pots to prevent evaporation. One week later, the seedlings were thinned to four plants per pot. The pots were watered daily to 70% of field capacity and re-randomized weekly. Three plants and one plant were harvested after 30 d and 210 d growth, respectively. Soil samples were randomly taken from 5 points with a 3 cm diameter punch at the time of plant sampling and then mixed.
2.3 Sample preparation and analysis
The shoots of B. napus were taken during seedling stage, while the roots, stems, pod and seed were taken during maturity stage. After collection, they were oven dried for 72 h at 65°C, weighed, and then ground to a fine powder through a 0.42-mm nylon sieve for the analysis of Se. The total Se content of B. napus was determined by digestion with 10 mL of nitric acid and perchloric acid (HNO3–HClO4) (4:1) at 180°C until the occurrence of white smoke, followed by the addition of 10 mL of 6 mol L−1 hydrochloric acid (HCl) until the emergence of white smoke again. Then, the mixture was cooled and filtered at a set volume. Finally, the Se concentration was measured with atomic fluorescence spectrometry (HG-AFS-8220, Beijing Titan Instruments Co., China) (Zhang et al. Citation2012).
Soil samples were air-dried, triturated with a wooden roller to pass through a 1-mm sieve, and stored at room temperature after homogenization. The soil pH and the concentrations of available nutrients were measured according to the Soil Physicochemical Analysis Handbook (Bao Citation2000). Soil organic carbon (C) was determined using the wet dichromate digestion method (Walkley and Black Citation1934). Soil pH was determined potentiometrically in 1:2.5 soil/distilled water suspensions after shaking. The alkaline hydrolysis diffusion method was used to determine the soil available N. The available phosphorus (P) was determined using Olsen’s method. The available potassium (K) was extracted with 1 mol L−1 ammonium acetate (NH4OAc) and determined using flame photometry (FP6410, INESA, China). The total Se concentration of soil was measured under the same conditions for plant Se concentration measurement, except that HNO3–HClO4 (3:2) was used for digestion.
2.4 Statistical analysis
The data were statistically analyzed using the analysis of variance (ANOVA) procedure provided by the Statistical Product and Service Solutions (SPSS) 17.0 software. The main effects of factors and their interactions were assessed by Fisher (F) statistics, and the mean values of each treatment underwent multiple comparisons using the least significant difference (LSD) test at p < 0.05.
3. Results
3.1 Effects of S and Se on biomass of B. napus
The application of both S and Se resulted in a slight stimulation of growth at lower levels and inhibition at higher levels in seedlings of B. napus. The application of 15 mg kg−1 Se significantly (p < 0.05) inhibited the growth of seedlings under the zero-S treatment condition, but the significant inhibitory effect disappeared with S supply (). This indicated that the addition of S significantly reversed the inhibitory effect of excessive Se on the growth of seedlings.
Table 2. Biomass of oilseed rape (Brassica napus L.) under different sulfur (S) and selenium (Se) treatments in soil culture. Values are means ± standard error (SE; dry matter basis).
In contrast to the seedling stage, without added Se, the application of S caused a noticeable stimulation, with the biomass in various parts of B. napus increasing from 5.3 to 13.2%. The effects of Se on growth of B. napus at the maturity stage were similar to those at the seedling stage, but the inhibitory effects of 15 mg kg−1 Se on biomass of various parts showed no significant difference (). However, the addition of S caused a significant increase in the biomass of stem and grain over the no-S treatment under the conditions of high Se treatment (15 mg kg−1).
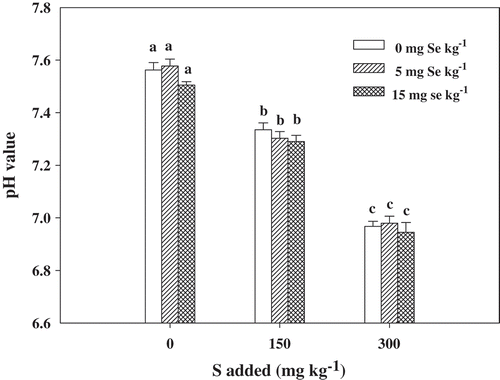
3.2 Effects of S and Se on soil pH
It is widely known that the application of S can change the soil pH, but the soil pH is highly related to the uptake of Se by plants (Zhao et al. Citation2010), so the soil pH was also measured in the present study. As shown in , the addition of S significantly (p < 0.05) decreased the soil pH, regardless of the Se level. When treated with 150 mg kg−1 S and 300 mg kg−1 S, the soil pH decreased from 7.58 to 7.30 and 6.98 in the range of 0.22–0.28 and 0.56–0.60 units at the maturity stage, respectively.
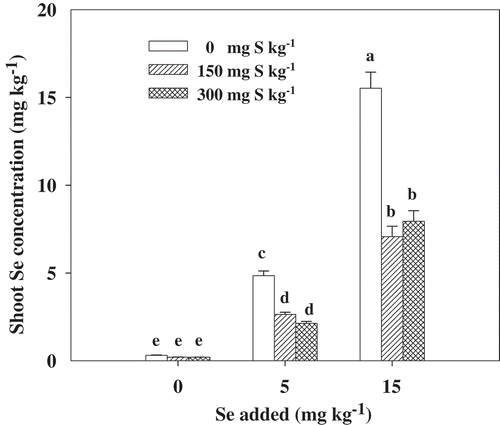
3.3 Effects of S and Se on the se concentration of B. napus at seedling stage
There was a significantly positive relationship between the Se concentration in the shoots of B. napus and the applied Se amount. The addition of S significantly (p < 0.05) reduced the Se concentration in the shoots, showing a decrease of 45.4 and 56.1% for the treatment with 5 mg kg−1 Se, and 48.2 and 45.9% for the treatment with 15 mg kg−1 Se, respectively (). The results indicated that the inhibitory effects of two S treatments on the uptake of Se by B. napus were nearly equal, regardless of the level of Se.
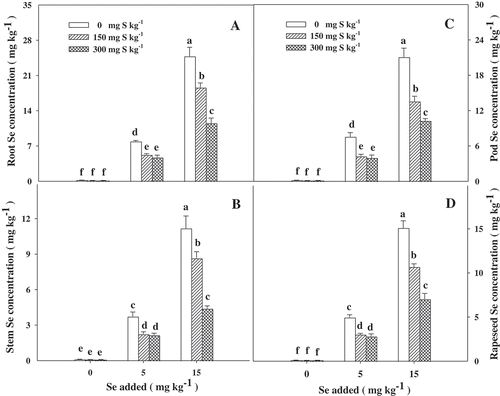
3.4 Effects of S and Se on the Se concentration of B. napus at maturity stage
The concentration of Se in various parts of B. napus significantly increased with increasing Se in the soil. The data in A–D showed that the addition of 150 mg kg−1 and 300 mg kg−1 S significantly (p < 0.05) reduced the Se concentrations in various parts of B. napus for the treatment with 5 mg kg−1 Se, with a decrease ranging between 33.8–44.1% and 40.2–47.5%, respectively, but there was no significant difference between the two S treatments. Under a higher Se treatment (15 mg kg−1), the 300 mg kg−1 S treatment had a more significant (p < 0.05) effect than the 150 mg kg−1 S treatment did in reducing the Se concentration in various parts of B. napus (A–D), with the decrease ranging from 25.0–35.7% to 51.3–60.9%. The addition of S caused the biggest reduction in the pod Se concentration of B. napus and the smallest reduction in the root among all the tested parts. The Se concentration in B. napus followed the sequence of root > pod > seed > stem, and it was not affected by either S rates or Se rates.
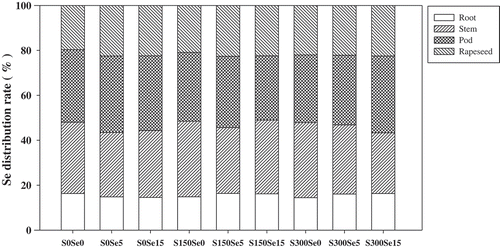
3.5 Effects of S and Se on Se accumulation and distribution in B. napus
Similar to the effects of S on Se concentrations in various parts of B. napus, the two S treatments significantly (p < 0.05) decreased the Se accumulation in various parts of B. napus. The addition of 300 mg kg−1 S also showed a more significant (p < 0.05) effect than the 150 mg kg−1 S treatment did in reducing the Se accumulation in various parts of B. napus, ranging from 9.8–30.2% to 42.5–53.4% (). The Se accumulation in B. napus followed the sequence of pod ≈ stem > seed > root (), and the distribution ratio was approximately 1:0.97:0.69:0.49, which was not affected by the addition of S (). As shown in and , the accumulation and distribution ratio in B. napus was relatively stable, and not strongly related to the application of Se, indicating that the application of S has no effect on the translocation of Se from roots to the other parts of B. napus.
Table 3. Selenium (Se) accumulation in various parts of Brassica napus L. grown at the maturity stage with different concentrations of sulfur (S) and Se. The values are means ± standard error (SE; n = 4).
4. Discussion
Numerous studies have demonstrated that Se positively regulates plant growth at a low concentration, while it is toxic to crops at a concentration exceeding the upper limit of their Se tolerance (Hladun et al. Citation2011; Schiavon et al. Citation2012). A moderate amount of Se application increased silique number and seed number per silique and improved the nutritional quality of B. napus (Seppänen et al. Citation2010; Nezami and Bybordi Citation2012). Sharma et al. (Citation2010) reported that Se caused a slight stimulation of growth at lower levels (2 mg kg−1) in rape seedlings, which is similar to the results observed at the seedling stage in the present study. Reports have shown that excessive Se gives rise to the robust accumulation of reactive oxygen species (ROS) in plants, although the actual role of Se in plants has not yet been resolved (Feng et al. Citation2013). The addition of S significantly alleviated the growth inhibition in seedlings and facilitated the biomass of stem and grain in mature plants under the higher Se (15 mg kg−1) condition. This is mainly because not only did S significantly reduce the Se concentration in B. napus but also the S requirements increased with the growth of B. napus (Girondé et al. Citation2014). Therefore, the threshold of Se concentration for the inhibition of growth depends on the plant age and the S concentration in the medium.
We found that the Se concentration was the highest in the root, followed by pod, seed and stem, consistent with a previous study on wheat (Triticum aestivum L.), which reported the Se concentration followed a descending order from roots to grain and stem (Dhillon and Dhillon Citation2009). This indicates that there are differences between various parts of B. napus in the biconcentration capacity of Se. It was demonstrated in a hydroponic experiment that selenite and sulfate had no antagonistic effects on Se uptake (Zhang et al. Citation2006; Li et al. Citation2008), but in a field experiment, Lee et al. (Citation2011b) reported that the Se concentration in the grain of wheat grown in naturally high Se areas decreased at least 53.3% by using 80 kg S ha−1. Cartes et al. (Citation2006) also found that the Se concentration in the shoots of ryegrass (Lolium perenne cv. Aries) can decrease at least 33.0% by using 100 mg S kg−1 with soil amended with 2 mg kg−1 selenite. These results were consistent with the present results in that the application of S reduced the concentrations of Se in various parts of B. napus in the range of 25.0–60.9%. Although the uptake mechanism of selenite in plants is not well understood, numerous studies have demonstrated that the uptake of selenite by plants is closely related to pH (Zhao et al. Citation2010; Zhang et al. Citation2014). Selenite exists as selenious acid (H2SeO3), hydrogen selenite radical (HSeO3−) and selenite radical (SeO32−) at different pH values (Zhang et al. Citation2010; Zhao et al. Citation2010). Zhao et al. (Citation2010) demonstrated that HSeO3− was taken up by plant roots through active absorption, while H2SeO3 and SeO32− were absorbed by plant roots through passive diffusion. The proportions of these Se aqueous species vary greatly with pH (Zhang et al. Citation2006, Citation2010; Zhao et al. Citation2010). Although we did not detect the forms of selenite present in soil solution, the reasons for the decrease of Se concentration by S supply can be explained by several previous studies. The selenite adsorption capacity of the soils was strongly dependent on soil pH and decreased with an increase of the pH between 5 and 9 (Lee et al. Citation2011a). The application of S fertilizer significantly decreased the pH of soils and increased the microorganism activity, which increased the amount and strength of selenite fixed in the soils (Darcheville et al. Citation2008). Reduced pH also benefits iron activity. Selenite has a very high affinity for iron and manganese oxides in soil, which formed oxides with extremely low solubility and thus strongly reduced their effectiveness (Wang et al. Citation2012; Huang et al. Citation2015). Yanaia et al. (Citation2015) reported that the soluble Se content had significant positive correlation with soil pH, and the total Se content in combination with soil pH was the main determining factor of the soluble Se content of agricultural soils. Liu et al. (Citation2015) also reported that S can promote inefficient Se fractions in soil and inhibit their conversion into efficient species by lowering soil pH. Li et al. (Citation2010) found that, with the addition of selenite, selenate was detected in soil solution, indicating that selenite might be oxidized to selenate in its rhizosphere, thus creating competition between selenate and sulfate.
B. napus is a member of the Brassicaceae family, which is a secondary accumulator of Se that typically contains up to 350 mg Se kg−1 when grown in soils contaminated with moderate levels of Se (Terry et al. Citation2000). In this study, with the soil amended with the highest amount of Se (15 mg kg−1), the concentrations of Se in all parts of B. napus were below 100 mg kg−1 ( and ), far from reaching the saturation point. The addition of S only reduced the amount of Se absorbed by the roots, and it was not involved in the root-to-aboveground translocation and distribution of Se in B. napus supplied with selenite, which might be the main reason why different S and Se rates did not affect the distribution rate of Se in B. napus.
Compared with other crops such as cereals, oilseed rape is particularly sensitive to S deficiency due to its high demand for S (Girondé et al. Citation2014). Yield, oil and protein concentrations in seeds have been shown to increase with S fertilization (Malhi et al. Citation2007). Therefore, using S fertilizer has a broad application prospect for increasing crop yields, and enhancing the safety of agricultural production in Se-contaminated regions.
Additional information
Funding
References
- Aldosary BM, Sutter ME, Schwartz M, Morgan BW 2012: Case series of selenium toxicity from a nutritional supplement. Clini. Toxicol., 50, 57–64. doi:10.3109/15563650.2011.641560
- Bañuelos G, Dhillon K 2011: Developing a sustainable phytomanagement strategy for excessive selenium in western United States and India. Int. J. Phytoremediat., 13, 208–228. doi:10.1080/15226514.2011.568544
- Bao SD 2000: Soil Agrochemical Analysis. China Agriculture Press, Beijing (in Chinese).
- Beckett GJ, Arthur JR 2005: Selenium and endocrine systems. J. Endocrinol., 184, 455–465. doi:10.1677/joe.1.05971
- Cao Z, Wang X, Yao D, Zhang X, Wong M 2001: Selenium geochemistry of paddy soils in Yangtze River Delta. Environ. Int., 26, 335–339. doi:10.1016/S0160-4120(01)00009-5
- Cartes P, Gianfreda L, Paredes C, Mora M 2011: Selenium uptake and its antioxidant role in ryegrass cultivars as affected by selenite seed pelletization. J. Soil Sci. Plant Nutr., 11, 1–14. doi:10.4067/S0718-95162011000400001
- Cartes P, Shene C, Mora ML 2006: Selenium distribution in ryegrass and its antioxidant role as affected by sulfur fertilization. Plant Soil, 285, 187–195. doi:10.1007/s11104-006-9004-8
- Chen L, Yang F, Xu J, Hu Q, Zhang Y, Pan G 2002: Determination of selenium concentration of rice in China and effect of fertilization of selenite and selenate on selenium content of rice. J. Agric. Food Chem., 50, 5128–5130. doi:10.1021/jf0201374
- Chu J, Yao X, Yue Z, Li J, Zhao J 2013: The effects of selenium on physiological traits, grain selenium content and yield of winter wheat at different development stages. Biol. Trace Elem. Res., 151, 434–440. doi:10.1007/s12011-012-9575-6
- Combs GF, Jackson MI, Watts JC, et al. 2011: Differential responses to selenomethionine supplementation by sex and genotype in healthy adults. Brit. J. Nutr., 107, 1514–1525. doi:10.1017/S0007114511004715
- Darcheville O, Février L, Haichar FZ, Berge O, Martin-Garin A, Renault P 2008: Aqueous, solid and gaseous partitioning of selenium in an oxic sandy soil under different microbiological states. J. Environ. Radioactiv., 99, 981–992. doi:10.1016/j.jenvrad.2007.11.006
- Dhillon SK, Dhillon KS 2009: Phytoremediation of selenium-contaminated soils: the efficiency of different cropping systems. Soil Use Manage., 25, 441–453. doi:10.1111/j.1475-2743.2009.00217.x
- Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC 2002: Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the nutritional prevention of cancer trial. Cancer Epidemiol. Biomarkers Prev., 11, 630–639.
- Durán P, Acuña JJ, Jorquera MA, Azcónc P, Boriea F, Cornejoa P, Mora ML 2013: Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: A preliminary study as a potential Se biofortification strategy. J. Cereal Sci., 57, 275–280. doi:10.1016/j.jcs.2012.11.012
- Feng R, Wei C, Tu S 2013: The roles of selenium in protecting plants against abioticstresses. Environ. Exp. Bot., 87, 58–68. doi:10.1016/j.envexpbot.2012.09.002
- Girondé A, Dubousset L, Trouverie J, Etienne P, Avice J 2014: The impact of sulfate restriction on seed yield and quality of winter oilseed rape depends on the ability to remobilize sulfate from vegetative tissues to reproductive organs. Front. Plant Sci., 5, 695.
- Hawkesford MJ, Zhao FJ 2007: Strategies for increasing the selenium content of wheat. J. Cereal Sci., 46, 282–292. doi:10.1016/j.jcs.2007.02.006
- Hladun KR, Parker DR, Trumble JT 2011: Selenium accumulation in the floral tissues of two Brassicaceae species and its impact on floral traits and plant performance. Environ. Exp. Bot., 74, 90–97. doi:10.1016/j.envexpbot.2011.05.003
- Huang Q, Du W, Wang Q, Zhang J, Jiang R, Li H 2013: Uptake and accumulation of Se by rice in different soils supplied with selenite or selenate. Acta Sci. Circ., 33, 1423–1429 (in Chinese).
- Huang Q, Yu Y, Wang Q, Luo Z, Jiang R, Li H 2015: Uptake kinetics and translocation of selenite and selenate as affected by iron plaque on root surfaces of rice seedlings. Planta, 241, 907–916. doi:10.1007/s00425-014-2227-7
- Kieliszek M, Blazejak S 2013: Selenium: Significance, and outlook for supplementation. Nutrition, 29, 713–718. doi:10.1016/j.nut.2012.11.012
- Lee S, Doolittle JJ, Woodard HJ 2011a: Selenite adsorption and desorption in selected South Dakota soils as a function of pH and other oxyanions. Soil Sci., 176, 73–79. doi:10.1097/SS.0b013e31820a0ff6
- Lee S, Woodard HJ, Doolittle JJ 2011b: Effect of phosphate and sulfate fertilizers on selenium uptake by wheat (Triticum aestivum). Soil Sci. Plant Nutr., 57, 696–704. doi:10.1080/00380768.2011.623282
- Li HF, Lombi E, Stroud JL, McGrath SP, Zhao FJ 2010: Selenium speciation in soil and rice: influence of water management and Se fertilization. J. Agric. Food Chem., 58, 11837–11843. doi:10.1021/jf1026185
- Li H-F, McGrath SP, Zhao F-J 2008: Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol., 178, 92–102. doi:10.1111/j.1469-8137.2007.02343.x
- Liu X, Zhao Z, Duan B, Hu C, Zhao X, Guo Z 2015: Effect of applied sulphur on the uptake by wheat of selenium applied as selenite. Plant Soil, 386, 35–45. doi:10.1007/s11104-014-2229-z
- Liao G, Guan C 2001: The simulation analysis of relationship between seeding date and growth-development characters and seed yield formation of Xiangnongyou571. J. Hunan Agric. Univ., 27, 17–20 (in Chinese).
- Luo K, Xu L, Tan J, Wang D, Xiang L 2004: Selenium source in the selenosis area of the Daba region, South Qinling Mountain, China. Environ. Geol., 45, 426–432. doi:10.1007/s00254-003-0893-z
- Malhi SS, Gan Y, Raney JP 2007: Yield, seed quality, and sulfur uptake of Brassica oilseed crops in response to sulfur fertilization. Agron. J., 99, 570–577. doi:10.2134/agronj2006.0269
- Navarro-Alarcon M, Cabrera-Vique C 2008: Selenium in food and the human body: a review. Sci. Total Environ., 400, 115–141. doi:10.1016/j.scitotenv.2008.06.024
- Navarro-Alarcon M, López-Martınez M 2000: Essentiality of selenium in the human body: relationship with different diseases. Sci. Total Environ., 249, 347–371. doi:10.1016/S0048-9697(99)00526-4
- Nezami MT, Bybordi A 2012: The effect of different amounts of selenium on yield and yield components of two canola cultivars. J. Food Agric. Environ., 10, 603–606.
- Rayman MP 2008: Food-chain selenium and human health: emphasis on intake. Brit. J. Nutr., 100, 254–268.
- Schiavon M, Pittarello M, Pilon-Smits EAH, Wirtz M, Hell R, Malagoli M 2012: Selenate and molybdate alter sulfate transport and assimilation in Brassica juncea L. Czern.: implications for phytoremediation. Environ. Exp. Bot., 75, 41–51. doi:10.1016/j.envexpbot.2011.08.016
- Seppänen MM, Kontturi J, Heras IL, Madrid Y, Cámara C, Hartikainen H 2010: Agronomic biofortification of Brassica with selenium—enrichment of SeMet and its identification in Brassica seeds and meal. Plant Soil, 337, 273–283. doi:10.1007/s11104-010-0523-y
- Sharma S, Bansal A, Dhillon SK, Dhillon KS 2010: Comparative effects of selenate and selenite on growth and biochemical composition of rapeseed (Brassica napus L.). Plant Soil, 329, 339–348. doi:10.1007/s11104-009-0162-3
- Shinmachi F, Buchner P, Stroud JL, Parmar S, Zhao FJ, McGrath SP, Hawkesford MJ 2010: Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol., 153, 327–336. doi:10.1104/pp.110.153759
- Sors TG, Ellis DR, Salt DE 2005: Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res., 86, 373–389. doi:10.1007/s11120-005-5222-9
- Stroud JL, Zhao FJ, Buchner P, Shinmachi F, McGrath SP, Abecassis J, Hawkesford MJ, Shewry PR 2010: Impacts of sulphur nutrition on selenium and molybdenum concentrations in wheat grain. J. Cereal Sci., 52, 111–113. doi:10.1016/j.jcs.2010.03.011
- Terry N, Zayed A, De Souza M, Tarun A 2000: Selenium in higher plants. Annu. Rev. Plant Biol., 51, 401–432. doi:10.1146/annurev.arplant.51.1.401
- Walkley A, Black IA 1934: An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci., 37, 29–38. doi:10.1097/00010694-193401000-00003
- Wang S, Liang D, Wang D, Wei W, Fu D, Lin Z 2012: Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci. Total Environ., 427–428, 159–164. doi:10.1016/j.scitotenv.2012.03.091
- Williams PN, Lombi E, Sun GX, et al. 2009: Selenium characterization in the global rice supply chain. Environ. Sci. Technol., 43, 6024–6030. doi:10.1021/es900671m
- Yanaia J, Mizuharab S, Yamadaa H 2015: Soluble selenium content of agricultural soils in Japan and its determining factors with reference to soil type, land use and region. Soil Sci. Plant Nutr., 61, 312–318. doi:10.1080/00380768.2014.997147
- Yasin M, Mehdawi AFE, Jahn CE, Anwar A, Turner MFS, Faisal M, Pilon-Smits EAH 2015: Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant Soil, 386, 385–394. doi:10.1007/s11104-014-2270-y
- Zhang L, Hu B, Li W, Che R, Deng K, Li H, Yu F, Ling H, Li Y, Chu C 2014: OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol., 201, 1183–1191. doi:10.1111/nph.12596
- Zhang L, Shi W, Wang X 2006: Difference in selenite absorption between high- and low-selenium rice cultivars and its mechanism. Plant Soil, 282, 183–193. doi:10.1007/s11104-005-5706-6
- Zhang L, Yu F, Shi W, Li Y, Miao Y 2010: Physiological characteristics of selenite uptake by maize roots in response to different pH levels. J. Plant Nutr. Soil Sci., 173, 412–422. doi:10.1002/jpln.200900260
- Zhang M, Hu C, Zhao X, Tan Q, Sun X, Li N 2012: Impact of molybdenum on Chinese cabbage response to selenium in solution culture. Soil Sci. Plant Nutr., 58, 595–603. doi:10.1080/00380768.2012.723603
- Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF 2010: Involvement of silicon influx transporter OsNIP2; 1 in selenite uptake in rice. Plant Physiol., 153, 1871–1877. doi:10.1104/pp.110.157867
- Zhu J, Wang N, Li S, Li L, Su H, Liu C 2008: Distribution and transport of selenium in Yutangba, China: impact of human activities. Sci. Total Environ., 392, 252–261. doi:10.1016/j.scitotenv.2007.12.019
- Zhu Y-G, Pilon-Smits EAH, Zhao F-J, Williams PN, Meharg AA 2009: Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci., 14, 436–442. doi:10.1016/j.tplants.2009.06.006