ABSTRACT
Background
Opioid-related deaths have risen dramatically in rural communities. Prior studies highlight few medication treatment providers for opioid use disorder in rural communities, though literature has yet to examine rural-specific treatment barriers.
Objectives
We conducted a systematic review to highlight the state of knowledge around rural medication treatment for opioid use disorder, identify consumer- and provider-focused treatment barriers, and discuss rural-specific implications.
Methods
We systematically reviewed the literature using PsycINFO, Web of Science, and PubMed databases (January 2018). Articles meeting inclusion criteria involved rural samples or urban/rural comparisons targeting outpatient medication treatment for opioid use disorder, and were conducted in the U.S. to minimize healthcare differences. Our analysis categorized consumer- and/or provider-focused barriers, and coded barriers as related to treatment availability, accessibility, and/or acceptability.
Results
Eighteen articles met inclusion, 15 which addressed consumer-focused barriers, while seven articles reported provider-focused barriers. Availability barriers were most commonly reported across consumer (n = 10) and provider (n = 5) studies, and included the lack of clinics/providers, backup, and resources. Acceptability barriers, described in three consumer and five provider studies, identified negative provider attitudes about addiction treatment, and providers’ perceptions of treatment as unsatisfactory for rural patients. Finally, accessibility barriers related to travel and cost were detailed in four consumer-focused studies whereas two provider-focused studies identified time constraints.
Conclusions
Our findings consistently identified a lack of medication providers and rural-specific implementation challenges. This review highlights a lack of rural-focused studies involving consumer participants, treatment outcomes, or barriers impacting underserved populations. There is a need for innovative treatment delivery for opioid use disorder in rural communities and interventions targeting provider attitudes.
Background
Between 2015 and 2016, the synthetic opioid death rate doubled in the United States, and from 2010 to 2016, heroin-related deaths increased 400% (Citation1). These concerning trends mirror rises in overdose-related deaths in other countries like Australia and Canada (Citation2,Citation3). Within the United States, these issues are particularly pronounced in rural communities, where overdose-related deaths increased most dramatically, and by 2015 surpassed the urban overdose-death rate (Citation4). As a result, the White House declared a Public Health Emergency (Citation5), identifying a critical need for expanded availability of medication treatment for opioid use disorder (OUD) in rural communities (Citation6). Similar messages have been put forth across the international literature, documenting the need to expand access to medication treatment and emergency opioid-overdose reversal medications, particularly in rural areas (Citation7–Citation10).
Medication treatment, the gold-standard approach for treating OUD (Citation11–Citation13), uses medications (buprenorphine, methadone, or naltrexone) alongside concurrent psychosocial treatment (Citation14,Citation15). However, the availability and implementation of medication treatment in rural U.S. communities is limited (Citation16). This treatment gap is consistent with research demonstrating the limited availability of other evidence-based treatments in the rural U.S., including mental and behavioral health treatments (Citation17) and services sought in specialty hospital settings (Citation18).
In addition to the limited availability of medication treatment in rural communities, prior research reveals rural residents are more likely (than urban) to experience certain opioid-related use patterns and consequences. These include deaths from multiple drugs and prescription drugs, injection of heroin or prescription opioids, and the use of opioids for pain or in combination with other pain medications (Citation19–Citation26). Furthermore, the age-adjusted opioid-overdose death rates have steadily risen in rural areas for the past twenty years and data reveal that rural residents are less likely than urban residents to be administered naloxone during an opioid overdose in US emergency departments (Citation27). Additionally, from 2007 to 2015, drug-overdose death rates were higher in rural than in urban counties, though this pattern recently reversed direction (Citation28), with the steepest rise from 2016 to 2017 occurring among African Americans (Citation29). In addition, the literature consistently identifies other sociodemographic risk factors related to medication treatment access for OUD that overlap with rural status, potentially exacerbating this rural-specific risk. These studies highlight greater barriers to medication treatment among non-Hispanic Whites and younger adults (Citation16), two groups who have experienced rising overdose-related deaths and increased treatment seeking for OUD. Indeed, young adults (ages 18–34) represented 50% of U.S. treatment admissions in 2012, twice the rate in 2002 (Citation30), while Non-Hispanic Whites have had the greatest rise in opioid-related overdose deaths (Citation30,Citation31).
These same sociodemographic risk factors are connected to opioid use characteristics, such as injection, and may partially explain why rural residents are more likely to inject opioids. Persons of Non-Hispanic White background that use opioids inject roughly twice as often as people of Black/African American background (Citation32,Citation33). Research examining antecedents of injection opioid use, often in predominantly Non-Hispanic White samples, highlights limited awareness about injection risks (Citation34,Citation35) and co-occurring mental health problems (Citation26,Citation36,Citation37) as determinants of injection. Additionally, many rural residents experience income-related struggles and health insurance gaps (i.e., uninsured or publicly insured), both of which have been connected with greater risk for opioid-related consequences (Citation19,Citation20,Citation30,Citation31). Given these factors, it is not surprising that rural U.S. communities have demonstrated heightened vulnerability for “diseases of despair” (Citation38), including opioid-related deaths and other mortality-related conditions such as suicide, liver disease, accidental poisonings and chronic illnesses (Citation39).
As a whole, rural residents in the United States are an at-risk, yet understudied group affected by the opioid epidemic. To date, no research has comprehensively assessed the state of medication treatment for OUD in the rural context. In this review we identify consumer- and provider-focused barriers related to the availability, accessibility, and acceptability of medication treatment in rural settings. Our aims are to: highlight the state of knowledge around medication treatment for rural OUD; identify consumer- and provider-focused barriers to medication treatment for rural OUD; and demonstrate rural-specific policy, practice, and research implications.
Methods
Search strategy
We conducted a comprehensive, systematic review of published, peer-reviewed literature using PubMed, psycINFO, and Web of Science databases. No date restrictions were imposed aside from the search end date of January 2018. This study did not include human subjects and therefore was not subject to an ethics board review. All searches included the following terms: an urbanicity-focused key word (rural or rurality or urbanicity) in combination with keywords related to drug type (opioid or opiate or heroin or fentanyl or oxycodone or oxycontin) and treatment type (methadone or buprenorphine or naltrexone or vivitrol or naloxone or subutex or suboxone or narcan or “medication assisted treatment” or “medication-assisted treatment” or “opioid-replacement” or “opioid replacement” or treatment or therapy). We focused on outpatient medication treatment due to the National Institute on Drug Abuse (NIDA) initiative to expand the availability of treatment (Citation7). We defined outpatient medication treatment as methadone maintenance treatment (MMT), buprenorphine maintenance treatment (BMT), or other forms of medication treatment with varied medications including methadone, buprenorphine, buprenorphine-naloxone, or naltrexone.
The following six criteria were used to select articles for inclusion: 1) articles were only eligible if they involved study data collected in the United StatesFootnote1; 2) study findings related to the treatment of people with primary OUD; 3) the study identified their sample or setting as rural or conducted urban/rural comparisons; 4) the article presented empirical data, either quantitative or qualitative; 5) the study was published in a peer-reviewed, English-language journal; and 6) the study included an examination of barriers to medication treatment.
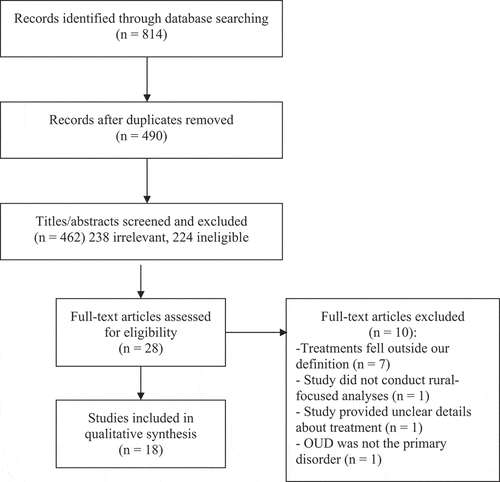
Our search yielded 490 unique results (see for PRISMA diagram). Articles were deemed irrelevant and excluded if a title and abstract review indicated that they were clearly not related to OUD or medication treatment for OUD (n = 238), or if they utilized a non-North American sample. After a full-text screen, several articles were also determined to be ineligible (n = 244). The most common reasons for ineligibility were: 1) a focus on general disparities in substance use treatment, rather than medication treatment for OUD specifically, 2) providing descriptive information or commentary only, rather than making urban/rural comparisons, or 3) a focus on rates of opioid use, risk factors for opioid use, or opioid use outcomes among rural and urban populations, without direct information about rural-specific barriers of medication treatment for OUD. Of note, many articles were excluded for multiple reasons. The titles and abstracts of these articles were screened by the first and third authors. Twenty-eight full-text articles were retained and examined. The first and second authors independently assessed each full-text article for inclusion. In the event of a split decision, those two authors met to achieve consensus. We reviewed reference lists of all articles meeting inclusion criteria and recent reviews of barriers to medication treatment for OUD experienced by specific populations (e.g., pregnant women) living in rural areas, as well as more general (i.e., non-opioid focused) reviews of substance use treatment in rural areas; however, no additional articles were identified through this strategy. Ten articles were excluded upon full-text review, resulting in eighteen articles addressing barriers to medication treatment in rural settings.
Analytic strategy
To assist with qualitative synthesis of articles meeting review criteria, data were extracted from each article and entered into a data extraction sheet with pre-defined data fields. Specifically, the data extraction sheet specified fields for type of barrier experienced (consumer, provider), medication treatment type (buprenorphine, methadone, etc.), sampling strategy/data source, sample size, sample demographics (i.e., race, gender, age), treatment outcomes, study time frame and follow-up period, regional setting, definition of rural/rurality (e.g. RUCC codes, UIC codes), and findings. Data were entered by the first and third authors and checked by the first and second authors. An overview of the studies addressing consumer-focused barriers for rural medication treatment are presented in , whereas an overview of the studies addressing provider-focused barriers for rural medication treatment are presented in .
Table 1. Consumer-focused barriers to medication treatment (N = 15 articles).
Table 2. Provider-focused barriers to medication treatment (N = 7 articles).
Following qualitative data entry, the first and second authors coded and tabulated articles based on several pre-defined criteria (see ). To help identify gaps in the literature and to create a more comprehensive understanding of strategies that can be used to increase access to care, we further reviewed each study to identify specific barriers to rural medication treatment. Barriers to medication treatment were categorized as being related to availability, accessibility, or acceptability, following an established classification system (Citation57) for understanding barriers to behavioral health care, which has been widely applied to the rural context (Citation58,Citation59). For the purposes of this review, we defined barriers related to availability as those representing the presence or absence of providers who are adequately qualified to provide outpatient medication treatment and/or the presence or absence of physical spaces/settings (e.g., clinics) where treatment is provided. Barriers related to accessibility represented practical challenges to accessing medication treatment, such as an inability to pay, lack of adequate insurance coverage or no insurance coverage, transportation challenges, childcare challenges, limited time and/or competing priorities, and an inability to miss work. Barriers related to acceptability included the stigma of obtaining medication treatment, previous negative treatment experiences, concerns around the lack of anonymity in rural settings, beliefs that treatment will not work or is not needed, and feelings of disconnect from and/or mistrust of distant, urban providers.
Table 3. Coding tabulations among the 18 included articles.
Results
Summary of overall search
Eighteen studies published between 2004 and 2018 met inclusion criteria for this systematic review. Eleven studies addressed consumer-focused barriers alone (Citation16,Citation40,Citation42–Citation44,Citation48–Citation53); three studies addressed provider-focused barriers alone (Citation54–Citation56); and four studies addressed both consumer- and provider-focused barriers (Citation41,Citation45–Citation47). This resulted in 15 studies addressing consumer-focused barriers and seven studies addressing provider-focused barriers.
Coding and tabulations from the overall search
Coding tabulations for all included studies are detailed in , whereas tabulation totals and frequencies for article characteristics are displayed in . These characteristics include data type, focus of barriers, medication treatment type, definition of rurality, census bureau region for data, consumer-focused barrier domains, provider-focused barrier domains, method, and medication treatment outcomes. Coding definitions for each characteristic are provided within table data.
Table 4. Tabulation totals and frequencies among the 18 included articles.
Overview of studies targeting consumer-focused barriers
We identified 15 studies addressing consumer-focused barriers to rural medication treatment (Citation16,Citation40–Citation53). For a summary of key findings, see . These articles were published from 2004 to 2018. Of these, ten focused on availability barriers, four focused on accessibility barriers, and three focused on acceptability barriers.
Table 5. Barrier domains focused on consumers and providers.
Consumer-focused availability barriers
Ten consumer-focused articles addressed availability barriers (Citation42,Citation44–Citation51,Citation53). Nine of these articles examined the availability of medication treatment providers in rural areas. Two articles identified a relative lack of or a smaller increase in opioid specialty clinics (Citation42,Citation44) in rural (compared to urban) areas, and four articles documented a relative lack of waivered buprenorphine practitioners (Citation42,Citation46,Citation49,Citation53). Two articles found a relative lack of either medication treatment clinics or practitioners in rural areas (Citation42,Citation44). Finally, one study highlighted a relative lack of available buprenorphine treatment at federally qualified health centers (FQHCs) in rural areas (Citation45). As illustration, one study (Citation46) revealed that rural counties represented all of the counties lacking either BMT or MMT in the state of Washington.
Four studies highlighted changes over time in medication treatment availability in the rural U.S. (Citation42,Citation48,Citation50,Citation51), all of which found improvements in availability. Dick and colleagues (2015) found an overall increase in the availability of opioid specialty clinics, waivered buprenorphine practitioners, or either option, throughout the U.S. between 2002 and 2011. However, rural counties experienced the smallest increase (Citation42). Similarly, Stein and colleagues (2015) found an increase nationally in buprenorphine treatment facilities regardless of urbanicity, however this increase was again smallest in rural settings. Of note, this study did identify the greatest increase of 100 patient-waivered providers in rural settings (Citation51). Two studies found a relationship between increases in medication treatment availability and increases in treatment utilization. In their 2012 study, Stein and colleagues found that, among a publicly insured (i.e., Medicaid) population, rural residents experienced the greatest increase in treatment (2007–2009, Citation50). Meyer and colleagues’ (2012) study among a sample of rural pregnant women also identified increases in availability and service utilization of medication treatment, as well as improved birth outcomes over time (Citation48).
Two articles documented availability barriers for concurrent psychiatric services among rural medication treatment populations (Citation46,Citation53). Wingrove & Bazemore’s (2016) study found that in rural areas, a high number of waivered physicians had a specialty in family medicine. In contrast, psychiatrists and internal medicine specialists more commonly practiced in urban settings (Citation53). Kvamme and colleagues (2013) found among a sample of waivered buprenorphine physicians that relatively fewer addiction medicine specialists and psychiatrists practiced in rural (compared to urban) areas (Citation46).
Finally, one study of availability barriers examined whether physician training opportunities increased the presence of medication treatment providers (Citation47). This was the only included article examining an intervention to address consumer-focused barriers. Findings showed that at eight months following training, nearly 60% of the physicians who completed training had received waivers to prescribe buprenorphine and 35% of the physicians were actively delivering buprenorphine treatment.
Consumer-focused accessibility barriers
Four consumer-focused articles addressed accessibility barriers (Citation16,Citation40,Citation41,Citation43). The most common accessibility barrier considered among included articles was travel hardships when seeking care from distant providers (Citation16,Citation40,Citation43). Two articles found rural consumers traveled further distances to medication treatment clinics (Citation16,Citation43), and a third article identified longer travel times to medication treatment clinics among pregnant rural OUD patients (Citation40). Rosenblum and colleagues’ (2011) study of 23,141 methadone patients enrolled in 84 opioid treatment programs across the United States demonstrated that rural consumers were more likely than their urban peers to commute across state lines to access medication treatment. Findings from this study also identified travel hardships as exacerbated among those of Non-Hispanic White background, compared to persons of Hispanic, African American, and other racial/ethnic backgrounds. Furthermore, travel hardships were also more common among consumers aged 18–29 years compared to consumers 44–81 years old (Citation16). One article also highlighted the cost of BMT as a barrier for consumers with OUD. In this study, providers perceived that the cost of BMT would be a deterrent to their rural consumers when seeking treatment (Citation41).
Consumer-focused acceptability barriers
Three consumer-focused articles addressed acceptability barriers for rural medication treatment (Citation41,Citation50,Citation52). DeFlavio and colleagues (2015) collected primary data from family medicine physicians in New England using mixed methods surveys. In the quantitative portion, physicians who did not deliver medication treatment, specifically BMT, were more likely to perceive their patients (consumers) with OUD as potentially dissatisfied with BMT (Citation41). Two other studies (Citation50,Citation52) identified differences in clinical decision making for rural consumers (compared to urban). Stein and colleagues (2012) showed rural consumers as less likely to be offered medication treatment, even when seen in the same clinic as urban peers. In contrast, rural consumers were more likely to be offered drug-free treatment (no medication treatment). In the same study, rural consumers (compared to urban) were more likely to receive BMT, office-based BMT, and less likely to receive MMT (Citation50). Similarly, Unger and colleagues (2010) demonstrated that rural women (compared to urban) reported lower rates of prior methadone treatment or any medication treatment, though rural women were more likely to report prior buprenorphine treatment and recent buprenorphine use. Also, when rural women were provided medication treatment, they were prescribed higher doses of both methadone and buprenorphine (Citation52). While not directly examined in study data, these differences may have been informed by providers’ views regarding the appropriateness or fit of medication treatment in general, or the appropriateness of specific types of medication treatment for rural consumers.
Overview of studies targeting provider-focused barriers
We identified seven articles (Citation41,Citation45–Citation47,Citation54–Citation56) addressing provider-focused barriers for rural medication treatment. These articles were published between 2004 and 2018. Of these articles, five examined availability barriers and five examined acceptability barriers, with two articles focusing on accessibility barriers. For a summary of key findings, see .
Provider-focused availability barriers
Five articles examined provider-focused availability barriers (Citation41,Citation46,Citation54–Citation56). The most commonly cited availability barriers were the lack of qualified personnel to support medication treatment, including a shortage of other rural providers (Citation56), a lack of consultants or specialty backup (Citation54,Citation56), and an inadequate number of trained staff (Citation41) or proximal mental health services (Citation56). Quest and colleagues (2012) found support, including other available colleagues providing medication treatment, online resources regarding behavioral health treatment, and clinical support systems, were potential facilitators of rural medication treatment. The lack of infrastructure including physical space shortages was cited as an availability barrier (Citation41). Kvamme and colleagues (2013) found that rural communities relied on family medicine and safety net (e.g., community health centers, FQHCs) settings to provide buprenorphine treatment, whereas waivered physicians in urban locations were more commonly in private practice (Citation46). Counter to Kvamme and colleagues’ (2013) findings, Hutchinson and colleagues (2014) did not find physicians relying on rural health clinics to differ in their medication treatment prescribing patterns. Of note, these studies involved different methodological approaches, which may account for discrepant findings.
Provider-focused accessibility barriers
Two articles addressed provider-focused accessibility barriers (Citation41,Citation54). In a national sample of 1,124 waivered rural physicians (Citation54) and a regional sample of 108 rural family physicians (Citation41), providers cited time constraints as a barrier to delivering medication treatment. In both studies (Citation41,Citation54), time constraint concerns were more common among non-prescribers. However, time constraint was also one of the most commonly cited barriers among prescribing physicians in the national sample (Citation54). Though only highlighted in two studies, these findings indicate time constraints might function as a barrier to medication treatment service delivery across provider type.
Provider-focused acceptability barriers
Five articles identified provider-focused acceptability barriers to rural medication treatment (Citation41,Citation45,Citation47,Citation54,Citation56). The most commonly identified acceptability barrier was a lack of confidence, belief, or interest in providing medication treatment (Citation41,Citation45,Citation47,Citation54). Two articles cited providers’ concerns about the potential risk of audits or ability to follow Drug Enforcement Agency (DEA) regulations (Citation41,Citation54). Furthermore, these two studies highlighted providers’ mistrust of people with substance use disorders (SUDs), concerns about attracting patients with SUDs, or other negative attitudes about consumers (e.g., believing medication treatment patients were unmotivated) (Citation41,Citation54). Other identified acceptability barriers included the challenge of treating patients with SUDs, who often have complex needs (Citation41), resistance from practice partners (Citation54), and perceptions that patients did not need medication treatment services. Despite results from national (Citation54) and multi-state (Citation41) samples suggesting physicians’ attitudes about medication treatment and patients with SUDs represent acceptability barriers, Quest and colleagues (2012) found that among waivered rural physicians, all identified BMT as efficacious and nearly all recommended other rural providers earn waivers (Citation56). Of note, these studies selected vastly different samples, highlighting that acceptability barriers are likely less problematic among subsets of providers already motivated to deliver medication treatment services.
Discussion
Summary of findings and gaps in the literature
This is the first systematic review to examine the scientific literature on rural medication treatment for OUD. Furthermore, it is the first review identifying rural-specific barriers to medication treatment. This review identified 18 articles that met inclusion criteria. Fifteen articles addressed consumer-focused barriers, and seven articles addressed provider-focused barriers. This review is particularly important given that rural areas in the U.S. have been hard-hit with increased rates opioid-related overdose deaths over the past several years, yet barriers to medication treatment are complex and poorly understood.
The most consistently identified consumer-focused barrier is the relative lack of available medication treatment services in rural communities. This finding aligns with prior literature demonstrating a lack of rural health and mental health services/providers (Citation17,Citation18). Although two articles found some evidence that this rural medication treatment disparity may be shrinking, our review clearly shows that rural areas within the U.S. continue to have a very limited supply of medication treatment options. Findings also indicate that travel burden is the most salient consumer-focused accessibility barrier to medication treatment, with articles revealing rural consumers face greater distances and increased travel times to access treatment. This finding is directly related to the lack of medication treatment providers in rural settings, which requires rural consumers to travel to distant, usually urban settings, for care. Last, our findings identify consumer-focused acceptability barriers regarding the appropriateness of medication treatment for rural consumers. These studies indicate that providers may not view medication treatment as an acceptable option for their rural consumers when considering accessibility barriers. As a result, providers may offer medication treatment less frequently to rural consumers, even when they are seen in the same clinic as urban consumers.
With regard to provider-focused barriers, articles included in this review consistently highlight availability and acceptability barriers. The most commonly cited availability barriers include a lack of supplemental workforce onsite to support medication treatment and a lack of available, adequately trained providers to deliver concurrent services (i.e., mental health treatment). Providers report the lack of backup, specialty care services left them feeling underprepared to delve into OUD and medication treatment services. It may be that these barriers also negatively influence providers’ views regarding the acceptability of delivering medication treatment. The most common acceptability barrier highlights provider concerns about being audited, failing to meet DEA regulations for medication treatment, and viewing OUD patient care as overly complex. Further complicating treatment acceptability, some studies demonstrate provider barriers related to negative attitudes about OUD patients and medication treatment, concerns about the risk for diversion, and a mistrust of patients dealing with SUDs. Some studies also highlighted an overall lack of belief in the efficacy of medication treatment. Accessibility barriers were less commonly reported among providers, though two articles identify the lack of time as a potential barrier to delivering rural medication treatment. As a whole, providers raised issues around limited resources, that when combined with negative attitudes and concerns regarding persons with SUDs and medication treatment as an approach, provides some insight into why so few providers deliver treatment in rural settings.
Implications for policy and practice
Our findings provide rural-specific implications for policy and practice needed to address barriers to medication treatment. The barriers we identify at the consumer-focused and provider-focused levels when taken together, demonstrate significant challenges to expanding rural medication treatment in the United States. Rural consumers have few options available, most of which require travel and cost, resulting in many people unable to access medication treatment. Meanwhile, our findings show rural providers in non-specialty settings (e.g., primary care, family medicine) feel underprepared to deliver treatment due to a lack of necessary supports and resources, and existing demands to provide treatment to their remaining (non-OUD) patient population. As a result, providers may find delivering medication treatment in rural settings as unappealing. Given consumer and provider barriers, this review shows that innovative treatment approaches are needed to improve rural consumers’ access to medication treatment for OUD.
Based on the reviewed studies, we can make specific recommendations to address barriers to treatment in rural settings. First, to improve the availability of rural medication treatment, a rapid expansion of telemedicine to treat rural patients with OUD may offer particular promise (Citation61,Citation62). This approach can help rural consumers lacking local medication treatment connect with providers they otherwise would not be able to access. It is important, however that policymakers, at both the local and national level, advocate for softening existing regulations under the Ryan Haight Act (Citation63), requiring in-person medical evaluations during the initial visit. Currently, this rule can be satisfied by conducting evaluations with prescribing or non-prescribing professionals if the patient is seen in a DEA-registered facility (Citation64). However, many rural consumers are unable to travel to DEA-registered facilities. Legislation that defines travel burden as a special circumstance allowed exemption from in-person evaluations would prove invaluable to many rural consumers. While these innovative approaches may help expand medication access, it is important that future research examines whether rural consumers relying on public healthcare or seeking medication treatment at rural clinics, encounter insurance or facility capacity barriers that hinder their access to treatment.
Second, we encourage the use of low-cost psychosocial approaches to complement medication treatments for rural consumers. Options include the use of technology-assisted psychosocial treatments (Citation65,Citation66) that can deliver evidence-based services remotely, as well as low-cost staffing options, such as peer support specialists (Citation67), to provide psychosocial services that do not require specialized training. Both of these options may facilitate medication treatment expansion among providers, many of whom cite the lack of available mental health services as a barrier. These options may also benefit the consumer by providing more co-located services, thereby mitigating additional travel.
Third, we recommend clinical directors, health departments, and policymakers work together to subsidize transportation costs through mileage reimbursement, non-emergency medical transportation, and ride sharing options as available (Citation68–Citation70). This is essential for patients in MMT, as well as those in the early stages of BMT, when clinic visits occur multiple days a week (or daily). Fourth, we recommend U.S. policymakers advance legislation that allows for dispensing of medication treatment at pharmacies. Approaches from countries experiencing similar opioid epidemics (Australia, Canada, and United Kingdom) allow accredited pharmacies to dispense medications once the consumer has a prescription or has been titrated to a stabilized dose by a specialty physician (Citation71). These efforts would help address accessibility barriers among rural consumers, which as our findings demonstrate, often live long distances from providers.
Last, our findings highlight the potential value of interventions targeting provider beliefs and views about medication treatment. We recommend that healthcare settings incentivize providers to complete comprehensive training efforts (Citation72) that may help address concerns about delivering medication treatment. These training efforts should be targeted to eligible rural providers not currently waivered, as well as those with waivers that are not actively delivering services.
The above recommendations involve coordination between a range of stakeholders (academic, healthcare systems, policymakers, community advocates). As a result, planning may benefit from developing community advisory boards (Citation73). These partnerships can help stakeholders identify appropriate federal, regional, and foundation funding opportunities to support medication treatment expansion in rural areas.
Limitations and implications for research
This systematic review identifies a number of limitations in the literature regarding barriers to rural medication treatment in the United States across sample selection, treatment focus, and research design. Regarding sample selection, we did not find a single study identifying or reporting acceptability barriers from the rural consumers’ perspective. Given that rural residents experience high rates of stigma about treatment and view formal treatment providers as unlikely to help with behavioral health problems (Citation74,Citation75), it is important to consider whether these issues may challenge the uptake of medication treatment. Additionally, it is difficult to interpret the validity of knowledge regarding rural consumer acceptability barriers, and troubling that rural consumers’ voices and perspectives have been overlooked in designs to date. First, we recommend future mixed methods research collect primary data directly from rural consumers with OUD to better understand their views on barriers to medication treatment. Second, the literature has yet to explore differential barriers among at-risk and underserved groups, aside from pregnant women. Future research should examine the rural treatment needs of racial minorities and in rural settings where there is a sizable representation of African American and Latinx populations, respectively. Third, there is a need for research in the Midwest and Southern regions of the U.S., the former of which has seen a rapid spike in overdose deaths (Citation76). We suggest future rural-focused investigations in overlooked regions (Citation77) that when combined with more comprehensive data (Citation78) can help provide information to aid rural treatment planning decisions in those jurisdictions.
These data demonstrate two important medication treatment gaps in the rural literature. We did not identify any rural-specific studies addressing barriers to naltrexone treatment. As naltrexone requires less infrastructure (Citation79), it is essential to gather more empirical information in the rural context. In addition, we did not identify a single rural study that analyzed methadone treatment alone, and the respective barriers to treatment. While this is not surprising given regulations around facilities delivering methadone and their propensity to be placed in urban areas, this unfortunately renders the literature on barriers to methadone treatment for rural consumers scant.
Our review also highlights limitations in research designs. None of the included studies examined medication treatment outcomes, though one study focused on birth outcomes among pregnant consumers. Future research is needed to examine whether barriers have a negative influence on medication treatment outcomes (e.g., dropout, relapse). We identified few studies using qualitative methods, none of which involved consumer-focused barriers. Furthermore, our data did not disentangle provider-focused barriers at the engagement level (i.e., obtaining a waiver to deliver medication treatment) compared to the implementation level (i.e., the number of consumers delivered medication treatment by the provider). It is possible barriers may have a differential function depending on which level is being analyzed, and that knowledge could help tailor training efforts to the audience (providers considering waivers, waivered providers that aren’t delivering services). Last, we did not include unpublished studies in this review. Although there may be additional, unpublished research in this area, we focused on documenting the current state of empirical knowledge via peer-reviewed published studies, to ensure that data yet to undergo critique did not potentially impact the reliability of our findings.
Conclusions
This is the first study to review barriers to medication treatment for OUD in rural settings. This systematic review provides information about the state of knowledge and offers rural-specific policy, practice, and research recommendations to address barriers to treatment among consumers seeking services and providers delivering care. This review offers findings and implications of significance regarding the opioid epidemic in the U.S., specific to the needs of rural communities that have demonstrated particular vulnerability to opioid-related consequences. We suggest clinical directors, health departments, policymakers, and other researchers consider our findings as they plan efforts to expand medication treatment in rural settings.
Disclosure statement
The authors report no relevant disclosures
Notes
1. We decided to streamline our search to the United States considering the different approach to healthcare with neighboring (e.g., Canada) and distant (e.g., Australia) countries (Citation80) experiencing similar opioid epidemics. We determined this difference would greatly influence the frequency and type of barriers to medication treatment, and complicate the study’s implications.
References
- Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999–2015. Hyattsville, MD: National Center for Health Statistics; 2017. NCHS data brief, no 273.
- Roxburgh A, Hall WD, Dobbins T, Gisev N, Burns L, Pearson S, Degenhardt L. Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug Alcohol Depend. 2017;179:291–98. doi:10.1016/j.drugalcdep.2017.07.018.
- Belzak L, Halverson J. Evidence synthesis – the opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can. 2018;38:224–33. doi:10.24095/hpcdp.38.6.02.
- Mack KA, Jones CM, Ballesteros MF. Illicit drug use, illicit drug use disorders, and drug overdose deaths in metropolitan and nonmetropolitan areas—United States. Am J Transplant. 2017;17:3241–52. doi:10.1111/ajt.14555.
- Department of Health and Human Services. HHS acting secretary declares public health emergency to address national opioid crisis. 2017. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html [last accessed 14 July 2019].
- Lister JJ. The opioid crisis is at its worse in rural areas: can telemedicine help? The conversation. 2017. https://theconversation.com/the-opioid-crisis-is-at-its-worst-in-rural-areas-can-telemedicine-help-86598 [last accessed 14 July 2019].
- National Institute on Drug Abuse (NIDA). 2016–2020 Strategic Plan: advancing Addiction Science. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Service; 2015.
- Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71:359–60. doi:10.1001/jamapsychiatry.2013.4450.
- Sigmon SC. The untapped potential of office-based buprenorphine treatment. JAMA Psychiatry. 2015;72:395–96. doi:10.1001/jamapsychiatry.2014.2421.
- Wood P, Opie C, Tucci J, Franklin R, Anderson K. “A lot of people call it liquid handcuffs”– barriers and enablers to opioid replacement therapy in a rural area. J Subst Use. 2019;24:150–55. doi:10.1080/14659891.2018.1523968.
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28:321–29. doi:10.1016/j.jsat.2005.02.007.
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;CD002207.
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370:2063–66. doi:10.1056/NEJMp1402780.
- Substance Abuse and Mental Health Services Administration (SAMHSA). Medication-assisted treatment. 2019. https://www.samhsa.gov/medication-assisted-treatment [last accessed 14 July 2019].
- Salamina G, Diecidue R, Vigna-Taglianti F, Jarre P, Schifano P, Bargagli AM, Davoli M, Amato L, Perucci CA, Faggiano F, et al. Effectiveness of therapies for heroin addiction in retaining patients in treatment: results from the VEdeTTE study. Subst Use Misuse. 2010;45:2076–92.
- Rosenblum A, Cleland CM, Fong C, Kayman DJ, Tempalski B, Parrino M. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi:10.1155/2011/948789.
- Ellis AR, Konrad TR, Thomas KC, Morrissey JP. County-level estimates of mental health professional supply in the United States. Psychiatr Serv. 2009;60:1315–22. doi:10.1176/ps.2009.60.10.1315.
- Kaufman BG, Thomas SR, Randolph RK, Perry JR, Thompson KW. The rising rate of rural hospital closures. J Rural Health. 2016;32:35–43. doi:10.1111/jrh.12128.
- Dunn KE, Barrett FS, Yepez-Laubach C, Meyer AC, Hruska BJ, Petrush K, Berman S, Sigmon SC, Fingerhood M, Bigelow GE. Opioid overdose experience, risk behaviors, and knowledge in drug users from a rural versus an urban setting. J Subst Abuse Treat. 2016;71:1–7. doi:10.1016/j.jsat.2016.08.006.
- Havens JR, Young AM, Havens CE. Nonmedical prescription drug use in a nationally representative sample of adolescents: evidence of greater use among rural adolescents. Arch Pediatr Adolesc Med. 2011;165:250–55. doi:10.1001/archpediatrics.2010.217.
- Prunuske JP, Hill CA, Hager KD, Lemieux AM, Swanoski MT, Anderson GW, Nawal Lutfiyya M. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: a population-based study using 2010 NAMCS data. BMC Health Serv Res. 2014;14:563. doi:10.1186/s12913-014-0563-8.
- Sorg MH. Patterns and trends of drug abuse in Maine: 2011 and early 2012. Epidemiologic trends in drug abuse. Proceedings of the Community Epidemiology Work Group Volume II; 2012; Bethesda, MD, National Institute of Drug Abuse, 157–68.
- Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94–100. doi:10.1016/j.pain.2013.09.009.
- Wang KH, Becker WC, Fiellin DA. Prevalence and correlates for nonmedical use of prescription opioids among urban and rural residents. Drug Alcohol Depend. 2013;127:156–62. doi:10.1016/j.drugalcdep.2012.06.027.
- Wunsch MJ, Nakamoto K, Behonick G, Massello W. Opioid deaths in rural Virginia: a description of the high prevalence of accidental fatalities involving prescribed medications. Am J Addict. 2009;18:5–14. doi:10.1080/10550490802544938.
- Young AM, Havens JR, Leukefeld CG. Route of administration for illicit prescription opioids: a comparison of rural and urban drug users. Harm Reduct J. 2010;7:24. doi:10.1186/1477-7517-7-24.
- Frank JW, Levy C, Calcaterra SL, Hoppe JA, Binswanger IA. Naloxone administration in US emergency departments, 2000–2011. J Med Toxicol. 2016;12:148–56. doi:10.1007/s13181-015-0525-5.
- Hedegaard H, Miniño AM, Warner M. Urban–rural differences in drug overdose death rates, by sex, age, and type of drugs involved, 2017. Hyattsville, MD: National Center for Health Statistics; 2019. NCHS Data Brief, no 345.
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Weekly Rep. 2019;67:1419–27.
- Substance Abuse and Mental Health Services Administration (SAMHSA). Results from the 2012 national survey on drug use and health: summary of national findings. Rockville, MD; 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13-4795.
- Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF, Hasin DS. Changes in US lifetime heroin use and heroin use disorder: prevalence from the 2001–2002 to 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:445–55. doi:10.1001/jamapsychiatry.2017.0113.
- Armstrong GL. Injection drug users in the United States, 1979–2002. Arch Intern Med. 2007;167:166–73. doi:10.1001/archinte.167.2.166.
- Stoltman JJ, Woodcock EA, Lister JJ, Greenwald MK, Lundahl LH. Exploration of the telescoping effect among not-in-treatment, intensive heroin-using research volunteers. Drug Alcohol Depend. 2015;148:217–20. doi:10.1016/j.drugalcdep.2015.01.010.
- Brener L, Spooner C, Treloar C. Preventing transitions to injecting amongst young people: what is the role of needle and syringe programmes? Int J Drug Policy. 2010;21:160–64. doi:10.1016/j.drugpo.2009.03.003.
- Des Jarlais D, Bramson H, Wong C, Gostnell K, Cepeda J, Arasteh K, Hagan H. Racial/ethnic disparities in HIV infection among people who inject drugs: an international systematic review and meta-analysis. Addiction. 2012;107:2087–95. doi:10.1111/add.2012.107.issue-12.
- Roy E, Arruda N, Bruenau J, Jutras-Aswad D. Epidemiology of injection drug use: new trends and prominent issues. Can J Psychiatry. 2016;61:136–44. doi:10.1177/0706743716632503.
- Wenger L, Lopez A, Kral A, Bluthenthal R. Moral ambivalence and the decision to initiate others into injection drug use: a qualitative study in two California cities. Int J Drug Policy. 2016;37:42–51. doi:10.1016/j.drugpo.2016.07.008.
- Case A, Deaton A. Mortality and morbidity in the 21st century. Brookings Pap Econ Act. 2017;397–476. doi:10.1353/eca.2017.0005.
- Stein EM, Gennuso KP, Ugboaja DC, Remington PL. The epidemic of despair among White Americans: trends in the leading causes of premature death, 1999–2015. Am J Public Health. 2017;107:1541–47. doi:10.2105/AJPH.2017.303941.
- Brown JD, Goodin AJ, Talbert JC. Rural and Appalachian disparities in neonatal abstinence syndrome incidence and access to opioid abuse treatment. J Rural Health. 2018;34:6–13. doi:10.1111/jrh.12251.
- DeFlavio JR, Rolin SA, Nordstrom BR, Kazal LA Jr. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:1–11.
- Dick AW, Pacula RL, Gordon AJ, Sorbero M, Burns RM, Leslie DL, Stein BD. Increasing potential access to opioid agonist treatment in U.S. treatment shortage areas. Health Aff (Millwood). 2015;34:1028–34. doi:10.1377/hlthaff.2014.1205.
- Heil SH, Sigmon SC, Jones HE, Wagner M. Comparison of characteristics of opioid-using pregnant women in rural and urban settings. Am J Drug Alcohol Abuse. 2008;34:463–71. doi:10.1080/00952990802122358.
- Hirchak KA, Murphy SM. Assessing differences in the availability of opioid addiction therapy options: rural versus urban and American Indian reservation versus nonreservation. J Rural Health. 2017;33:102–09. doi:10.1111/jrh.2017.33.issue-1.
- Jones EB. Medication‐assisted opioid treatment prescribers in Federally Qualified Health Centers: capacity lags in rural areas. J Rural Health. 2018;34:14–22. doi:10.1111/jrh.12260.
- Kvamme E, Catlin M, Banta-Green C, Roll J, Rosenblatt R. Who prescribes buprenorphine for rural patients? The impact of specialty, location and practice type in Washington State. J Subst Abuse Treat. 2013;44:355–60. doi:10.1016/j.jsat.2012.07.006.
- McCarty D, Rieckmann T, Green C, Gallon S, Knudsen J. Training rural practitioners to use buprenorphine: using The Change Book to facilitate technology transfer. J Subst Abuse Treat. 2004;26:203–08. doi:10.1016/S0740-5472(03)00247-2.
- Meyer M, Benvenuto A, Howard D, Johnston A, Plante D, Metayer J, Mandell T. Development of a substance abuse program for opioid-dependent nonurban pregnant women improves outcome. J Addict Med. 2012;6:124–30. doi:10.1097/ADM.0b013e3182541933.
- Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13:23–26. doi:10.1370/afm.1735.
- Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J, Farmer C. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: recent service utilization trends in the use of buprenorphine and methadone. Drug Alcohol Depend. 2012;123:72–78. doi:10.1016/j.drugalcdep.2011.10.016.
- Stein BD, Pacula RL, Gordon AJ, Burns RM, Leslie DL, Sorbero MJ, Bauhoff S, Mandell TW, Dick AW. Where is buprenorphine dispensed to treat opioid use disorders? The role of private offices, opioid treatment programs, and substance abuse treatment facilities in urban and rural counties. Milbank Q. 2015;93:561–83. doi:10.1111/milq.2015.93.issue-3.
- Unger AS, Martin PR, Kaltenbach K, Stine SM, Heil SH, Jones HE, Arria AM, Coyle MG, Selby P, Fischer G. Clinical characteristics of central European and North American samples of pregnant women screened for opioid agonist treatment. Eur Addict Res. 2010;16:99–107. doi:10.1159/000284683.
- Wingrove P, Park B, Bazemore A. Rural opioid use disorder treatment depends on family physicians. Am Fam Physician. 2016;94:546.
- Andrilla CH, Coulthard C, Larson EH. Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Ann Fam Med. 2017;15:359–62. doi:10.1370/afm.2099.
- Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Ann Fam Med. 2014;12:128–33. doi:10.1370/afm.1595.
- Quest TL, Merrill JO, Roll J, Saxon AJ, Rosenblatt RA. Buprenorphine therapy for opioid addiction in rural Washington: the experience of the early adopters. J Opioid Manag. 2012;8:29–38. doi:10.5055/jom.2012.0093.
- Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care. 1981;19:127–40. doi:10.1097/00005650-198102000-00001.
- Human J, Wasem C. Rural mental health in America. Am Psychol. 1991;46:232–39. doi:10.1037/0003-066X.46.3.232.
- Mohatt DF, Bradley MM, Adams SJ. Rural mental health: challenges and opportunities caring for the country. In: Cummings NA, O’Donohue WT, Cucciare MA, editors. Universal healthcare: readings for mental health professionals. Reno: Context Press; 2005. p. 127–50.
- US Census Bureau. Census Bureau regions and divisions with state FIPS codes. 2008.
- Eibl JK, Gauthier G, Pellegrini D, Daiter J, Varenbut M, Hogenbirk JC, Marsh DC. The effectiveness of telemedicine-delivered opioid agonist therapy in a supervised clinical setting. Drug Alcohol Depend. 2017;176:133–38. doi:10.1016/j.drugalcdep.2017.01.048.
- Lin LA, Casteel D, Shigekawa E, Weyrich MS, Roby DH, McMenamin SB. Telemedicine-delivered treatment interventions for substance use disorders: a systematic review. J Subst Abuse Treat. 2019;101:38–49. doi:10.1016/j.jsat.2019.03.007.
- Knopf A. Ryan Haight Act stands in way of buprenorphine telehealth. Alcohol Drug Abuse Weekly. 2018;29:7–8.
- Rubin R. Using telemedicine to treat opioid use disorder in rural areas. JAMA. 2019;322:1029–31. doi:10.1001/jama.2019.12574.
- Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood). 2014;33:194–99. doi:10.1377/hlthaff.2013.0992.
- Marsch LA, Guarino H, Acosta M, Aponte-Melendez Y, Cleland C, Grabinski M, Brady R, Edwards J. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. J Subst Abuse Treat. 2014;46:43–51. doi:10.1016/j.jsat.2013.08.012.
- Powell KG, Treitler P, Peterson NA, Borys S, Hallcom D. Promoting opioid overdose prevention and recovery: an exploratory study of an innovative intervention model to address opioid abuse. Int J Drug Policy. 2019;64:21–29. doi:10.1016/j.drugpo.2018.12.004.
- Nelson RE, Hicken B, West A, Rupper R. The effect of increased travel reimbursement rates on health care utilization in the VA. J Rural Health. 2012;28:192–201. doi:10.1111/jrh.2012.28.issue-2.
- Chaiyachati KH, Hubbard RA, Yeager A, Mugo B, Lopez S, Asch E, Shi C, Shea JA, Rosin R, Grande D. Association of rideshare-based transportation services and missed primary care appointments: a clinical trial. JAMA Intern Med. 2018;178:383–89. doi:10.1001/jamainternmed.2017.8336.
- Powers BW, Rinefort S, Jain SH. Nonemergency medical transportation: delivering care in the era of Lyft and Uber. JAMA. 2016;316:921–22. doi:10.1001/jama.2016.9970.
- Calcaterra SL, Bach P, Chadi A, Chadi N, Kimmel SD, Morford KL, Roy P, Samet JH. Methadone matters: what the United States can learn from the global effort to treat opioid addiction. J Gen Intern Med. 2019;34:1039–42. doi:10.1007/s11606-018-4801-3.
- Molfenter T, Knudsen HK, Brown R, Jacobson N, Horst J, Van Etten M, Kim J-S, Haram E, Collier E, Starr S, et al. Test of a workforce development intervention to expand opioid use disorder treatment pharmacotherapy prescribers: protocol for a cluster randomized trial. Implement Sci. 2017;12:135.
- Palombi LC, Vargo J, Bennett L, Hendler J, Coughlin P, Winter G, LaRue A. A community partnership to respond to the heroin and opioid abuse epidemic. J Rural Health. 2017;33:110–13. doi:10.1111/jrh.2017.33.issue-1.
- Stewart H, Jameson JP, Curtin L. The relationship between stigma and self-reported willingness to use mental health services among rural and urban older adults. Psychol Serv. 2015;12:141–48. doi:10.1037/a0038651.
- Wrigley S, Jackson H, Judd F, Komiti A. Role of stigma and attitudes toward help-seeking from a general practitioner for mental health problems in a rural town. Aust N Z J Psychiatry. 2005;39:514–21. doi:10.1080/j.1440-1614.2005.01612.x.
- Centers for Disease Control and Prevention/National Center for Health Statistics. Drug overdose mortality by state. 2019. https://www.cdc.gov/nchs/pressroom/sosmap/drug_poisoning_mortality/drug_poisoning.htm [last accessed 22 October 2019].
- Lister JJ, Ellis JD, Yoon, M. Opioid prescribing and opioid-overdose deaths in Michigan: urban-rural comparisons and changes across 2013–2017. Addict Behav Rep. 2019. doi:10.1016/j.abrep.2019.100234.
- Knudsen HK. The supply of physicians waivered to prescribe buprenorphine for opioid use disorders in the United States: a state-level analysis. J Stud Alcohol Drugs. 2015;24:644–54. doi:10.15288/jsad.2015.76.644.
- Substance Abuse and Mental Health Services Administration (SAMHSA). Naltrexone. 2016. https://www.samhsa.gov/medication-assisted-treatment/treatment/naltrexone [last accessed 14 July 2019].
- Schoen C, Osborn R, Squires D, Doty MM. Access, affordability, and insurance complexity are often worse in the United States compared to ten other countries. Health Aff (Millwood). 2013;32:2205–15. doi:10.1377/hlthaff.2013.0879.