Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease. The standard first-line treatment for PDAC is gemcitabine chemotherapy which, unfortunately, offers only limited chance of a lasting cure. This review further evaluates the hypothesis that the effectiveness of gemcitabine can be improved by combining it with evidence-based complementary measures. Previously, supported by clinical trial data, we suggested that a number of dietary factors and nutraceuticals can be integrated with gemcitabine therapy. Here, we evaluate a further 10 agents for which no clinical trials have (yet) been carried out but there are promising data from in vivo and/or in vitro studies including experiments involving combined treatments with gemcitabine. Two groups of complementary agents are considered: Dietary factors (resveratrol, epigallocatechin gallate, vitamin B9, capsaicin, quercetin and sulforaphane) and nutraceutical agents (artemisinin, garcinol, thymoquinone and emodin). In addition, we identified seven promising agents for which there is currently only basic (mostly in vitro) data. Finally, as a special case of combination therapy, we highlighted synergistic drug combinations involving gemcitabine with “repurposed” aspirin or metformin. We conclude overall that integrated management of PDAC currently is likely to produce the best outcome for patients and for this a wide range of complementary measures is available.
1. Introduction
Pancreatic cancer, the most common form of which is “pancreatic ductal adenocarcinoma” (PDAC), is one of the hardest to treat cancers both because of the difficulty of early diagnosis and the limited effectiveness of the available therapies (Citation1–3). With a five-year survival rate of only some 6%, the associated mortality rates are regional (deaths per 100,000 people) being ca. 7.2 (Europe), 6.5 (North America) and 1.2 (East Africa) (Citation4). PDAC is age-related; its incidence has been rising steeply for a number of years, and it is expected to become the most common cause of cancer-related deaths in the USA by 2030 (Citation5). PDAC arises in the pancreatic ducts which perform the gland’s main exocrine functions. The liver is often the first major organ to be metastasized due to the proximity of the hepatic portal vein (Citation6). In fact, liver failure is frequently the first sign and the main cause of death from PDAC. In the context of integrated management, therefore, treatments should ideally protect also the liver.
The most common first-line treatment for PDAC is gemcitabine chemotherapy (Citation7, Citation8). Gemcitabine is an “antimetabolite” pro-drug which becomes active once phosphorylated into diphosphate or triphosphate inside cells at very specific phases of the mitotic cycle. Thus, it works most effectively on fast-dividing cells, hence cancer cells. The basic mechanism of cell death is DNA damage (Citation9). Apart from its inherent limited effectiveness and the common undesirable side effects of the treatment, use of gemcitabine suffers from the eventual onset of resistance to the drug. In these respects, therefore, any adjustment to gemcitabine chemotherapy that will increase its sensitivity (and hence lower its dosage of use) and lengthen its period of effectiveness (e.g., overcoming resistance) would be welcome. Our hypothesis is that these are possible by combining the gemcitabine chemotherapy with evidence-based complementary agents. Indeed, use of complementary agents in cancer treatment generally is increasingly, being favored by patients as well as by oncologists (Citation10–14).
In the first instance, an association between dietary/nutritional lifestyle and PDAC is apparent epidemiologically from the fact that the PDAC incidence and mortality rates are much higher in relatively developed countries with diets rich in fatty, oily or sweet foods (Citation4). Whilst our understanding of this association has come a long way over the years, we know much less about how dietary and nutraceutical agents, as well as lifestyle factors, could associate with PDAC during treatment (Citation15). In our previous review, we advanced the hypothesis that the best outcome for PDAC currently would be obtained by integration of clinical medicine with evidence-based natural complementary agents and lifestyle factors (Citation16). Of the former, 9 (six dietary and three nutraceutical compounds) were chosen based upon evidence from clinical trials as the essential criterion, as well as meta-analyses and in vivo and in vitro experiments. In addition, however, there is a range of natural agents that did not meet all of those strict criteria but may do so in the future as more evidence is gathered. Here, we evaluate this second group of “emerging” agents incorporating both dietary factors and nutraceuticals. For all these, there is both in vivo and/or in vitro evidence including combination treatments. in vivo animal models include molecularly appropriate transgenic models wherever possible. As regards in vitro experiments, the data used come mainly from human cells.
Central to our approach, again, is the epigenetic nature of cancer, including PDAC, which means that genes and their products can be regulated significantly including by dietary and lifestyle factors (Citation16–20). Indeed, overall, ca. 40% of cancers are due to modifiable, hence reversible factors (www.cancer.org/latest-news/more-than-4-in-10-cancers-and-cancer-deaths-linked-to-modifiable-risk-factors.html). Not surprisingly, therefore, the link between nutrition and cancer has been questioned for over a century (Citation21). Since pancreas is a strongly hormonal (both endocrine and exocrine) organ, PDAC would be expected to be particularly sensitive to the body’s biochemistry and chemical balance (e.g., Refs. (Citation22–25)).
2. Dietary Factors
In our previous review we considered dietary factors in 3 different categories (Citation16). General/background conditioners included acidity, glycaemic index and cholesterol. As multifactorial foodstuffs, we covered red and processed meat, fish, fruit and vegetables, dairy, honey and coffee. Finally, as specific dietary agents the following satisfied our full criteria: Vitamins A, C, D, and E, curcumin and genistein. Here, we have accepted for further consideration six specific dietary factors for which significant in vivo and in vitro data were available. The emphasis again is on the possible potentiating effect of these agents on gemcitabine chemotherapy. Such dietary agents can also be taken as supplements.
2.1. Resveratrol
Resveratrol is a natural phytoalexin that is produced in plants as a defensive response against fungal infections and other environmental stressors. It is particularly abundant in the skin of red grapes, blueberries, raspberries, mulberries and nuts (). Resveratrol has been shown to inhibit proliferation and invasiveness of PDAC cells by reducing “nuclear factor κ-light-chain-enhancer of activated B cells” (NF-κB) expression, downregulating lipid metabolism and activating pro-apoptotic caspase-3 (Citation25–27). Furthermore, markers of epithelial-mesenchymal transition (EMT), an early event in invasiveness, were suppressed (Citation28). A synthetic, more stable derivative of resveratrol (triacetyl resveratrol) also selectively induced apoptosis in PDAC cells in vitro without affecting normal pancreatic ductal cells and this occurred via downregulation of hedgehog signaling (Citation29). Combined treatment of PDAC cells with gemcitabine and resveratrol (i) increased apoptosis highly significantly and (ii) reduced gemcitabine resistance in vitro and this involved adenosine monophosphate kinase (AMPK) (Citation30, Citation31). Also, in vitro, the inhibition of colony formation by gemcitabine was enhanced by resveratrol addition, again, involving an enhanced pro-apoptotic effect () (Citation27). Consistent with this, in an in vivo orthotopic mouse model of PDAC, the anti-tumorigenic effect of gemcitabine was potentiated by combination with resveratrol () (Citation32). These results were confirmed by Gupta et al. and Xu, Q. et al. (Citation33, Citation34). Importantly, also, resveratrol suppressed both the “basal” stemness of PDAC cells and that promoted by gemcitabine () (Citation27). In addition, pterostilbene, a natural analogue derived from resveratrol, enhanced gemcitabine sensitivity in part by inhibiting expression of the multi-drug resistance gene, MDR1 (Citation35).
Figure 1. Effects of a dietary compound on PDAC: Resveratrol. For all parts of the figure (A to C), treatments were as follows: control (Citation1), resveratrol (Citation2), gemcitabine (Citation3) and their combination (Citation4). A. In vitro effects on growth (quantified as colony number) of treating two different human PDAC cell lines (MiaPaCa-2/gray bars and Panc-1/black bars) with resveratrol, gemcitabine and their combination. For both cell lines, the inhibition of growth was significantly greater for the combination than gemcitabine alone (P < 0.01). From Zhou, C et al. (Citation27). B. In vivo growth of MiaPaCa-2 cells in an orthotopic xenograft model of PDAC. The inhibition of growth was significantly greater for the combination than gemcitabine alone (P < 0.001). Modified from Harikumar et al. (Citation32). C. Assessment of stemness in PDAC cells obtained from tissue sections of KPC mice. Stemness was quantified as the Sox2-positive area, expressed as a percentage of total. Resveratrol significantly reduced the effect of gemcitabine in inducing stemness (P < 0.001), bringing it to control level. Dotted horizontal line denotes the median control level, as reference. From Zhou, C. et al. (Citation27).
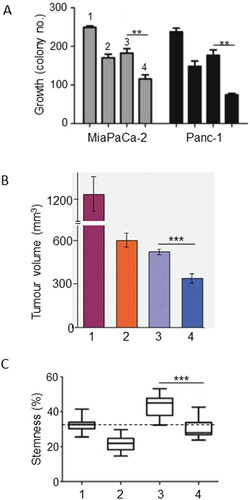
Table 1. Dietary and nutraceutical agents with emerging anti-PDAC effects.
Interestingly, addition of resveratrol to the combined application of gemcitabine and capsaicin restored the effectiveness of chemotherapy and increased radiosensitivity in in vivo (xenograft) models of human PDAC (Citation36, Citation37). These studies would raise the possibility of extending the integrated management to triple (or more) combinations without incurring antagonism, as we suggested earlier (Citation16). Finally, resveratrol reduced the undesirable side effects of chemotherapy on heart and liver by increasing the activity of antioxidant enzymes (Citation38).
In conclusion, resveratrol has excellent properties both by itself as an anti-PDAC agent and for possible integration with gemcitabine chemotherapy in the future.
2.2. Epigallocatechin Gallate
Epigallocatechin gallate (EGCG) is abundant in teas, especially green tea, and fruits, especially berries (). Epidemiological studies have shown beneficial effects of green tea on cancers of lung, breast, esophageal, stomach, liver and prostate (Citation39, Citation40). As regards PDAC, however, such studies have given somewhat inconsistent results. Most meta-analyses concluded (i) that green tea consumption was not associated with PDAC risk and (ii) that high consumption was associated with slightly lower risk (Citation41). This seemed more pronounced among Chinese populations (Citation42, Citation43). Abe et al. recently evaluated the available epidemiological evidence for green tea consumption and showed that whilst PDAC risk was not affected, several other cancers were associated significantly with reduced risk (Citation44). These included, importantly, liver cancer. Possible reasons for the discrepancy between the epidemiological data could include (i) the ranges of green tea consumption and (ii) compounding lifestyle factors especially smoking. Importantly, however, no adverse effect has been reported. Furthermore, green tea consumption could be beneficial indirectly by reducing the impact of diabetes, obesity and inflammation including pancreatitis (Citation40, Citation45–47).
There is a wealth of in vitro evidence showing that green tea and one of its most active ingredients, EGCG, inhibit development and progression of cancer, including PDAC (Citation48, Citation49). As regards “combination therapies,” an initial in vitro study suggested significant synergistic effects of gemcitabine with EGCG (over a range of concentrations). This involved increased expression of “signal transducer and activator of transcription 3” (STAT3) target genes and enhanced apoptosis () (Citation50). More recently, EGCG was able to enhance the gemcitabine-induced reduction in insulin-like growth factor receptor and Akt/protein kinase B signaling, and thus reduce cell migration and invasion in vitro () (Citation51). in vivo also (xenograft mouse model of PDAC), intraperitoneal EGCG downregulated glycolysis and potentiated the effect of gemcitabine, together reducing tumor weight by an additional ca. 30% () (Citation52).
Figure 2. Effects of a dietary compound on PDAC: Epigallocatechin gallate. A. Apoptotic responses of Panc-1 cells to treatment with increasing concentrations of epigallocatechin gallate (EGCG) and combination with a fixed dose of gemcitabine (GEM). The histobars denote the following: GEM alone (Citation1), GEM + EGCG (2-4, increasing concentrations of EGCG). Data represent mean ± SD. There was a statistically significant difference between the effects of all the combinations compared with GEM alone (P < 0.05). From Tang et al. (Citation50). B. Effects of GEM, EGCG and their combination on the invasiveness on two pancreatic cancer cell lines, Panc-1 (black histobars) and MiaPaCa-2 (gray histobars). Data represent mean ± SD. Effects of the treatments are expressed as a percentage of control (Cont). For both cell lines, the effect of the combination was significantly greater than GEM alone (P < 0.01). From Wei, R. et al. (Citation51). C. Effect of GEM, EGCG and their combination on tumorigenesis in a subcutaneous xenograft model of PDAC (KPC cells). “Cont” denotes control data from untreated animals. Data represent mean ± SD. The decrease in tumor weight induced by the combination was greater than GEM alone (P < 0.05). From Wei, R. et al. (Citation52).
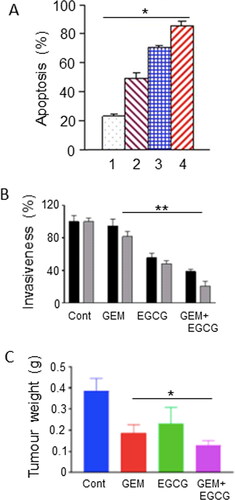
In conclusion, the evidence for EGCG as an anti-PDAC agent is overall positive, albeit limited. The evidence is stronger for liver cancer. Hence, this agent would be worth considering for possible integration with gemcitabine chemotherapy in the future.
2.3. Vitamin B9
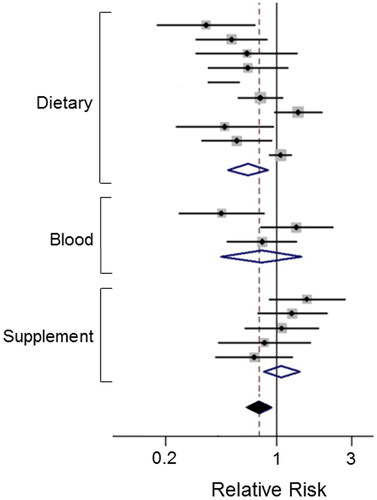
Vitamin B9 (folate) is one of eight B vitamins. It is abundant in foods such as citrus fruits, green leafy vegetables and legumes () (Citation53). Taken as folic acid, it is converted to folate by the body. Folate deficiency has been shown to induce chromosomal breaks and mutations in tumor suppressor genes, hence its supplementation proved beneficial against PDAC (Citation54). The level of reduced risk from increased folate intake varied between studies, but importantly, no adverse effect has been reported (Citation53, Citation55). Lin, H. et al. found in a meta-analysis that a high intake of folate, especially as part of diet, reduced the relative risk of PDAC by as much as 34% () (Citation53). This conclusion was supported by Yallew et al., also reporting a significant inverse association between folate intake and PDAC (Citation56). Studies have suggested that folate supplementation may be most beneficial when taken with certain folate-rich foods, e.g., spinach and asparagus (Citation56). Although vitamin B9 has not been tested in combination with gemcitabine systemically, it is one of the four components of the FOLFIRINOX regime, which is given to cases advanced (metastatic) PDAC. So, it can be considered acceptable for integrated management of PDAC.
Figure 3. Effects of a dietary compound on PDAC: Vitamin B9. Meta-analysis of studies examining the effects of folate levels (assessed from dietary intake, blood levels and supplements) on pancreatic cancer risk. Diamonds indicate the average and the spread of the data. White diamonds relate to the individual data sets. Black diamond indicates overall significantly reduced relative risk, despite the noticeable variability across the three sets of studies. Modified from Lin, H. et al. (Citation53), where further details and primary data can be found.
In conclusion, we consider vitamin B9 to be an effective complement to gemcitabine chemotherapy of PDAC. We should note, however, that B vitamins are sometimes taken as a “complex” of the various forms, but this should best be avoided, since there is some evidence that B12 may promote cancer including PDAC (Citation57). The latter is consistent with dietary sources of B12 (animal products such as meat, dairy etc.) also being undesirable (Citation16).
2.4. Capsaicin
Capsaicin is the active ingredient of hot red peppers () (Citation58). It has anticancer properties through its ability to induce cell cycle arrest and cause apoptosis (Citation58). Capsaicin reduced the growth of PDAC xenografts in mice by promoting apoptosis mediated by caspase-3 and caspase-8 (Citation59). Additionally, capsaicin downregulated PI3 kinase signaling, leading to an increase in cell cycle arrest. Through the inhibition of the mitochondrial electron transport chain, capsaicin could generate reactive oxygen species (ROS) and increase DNA damage, without affecting healthy pancreatic cells (Citation60). There is no study on the possible effects of combining capsaicin with gemcitabine. However, in cases where gemcitabine monotherapy was ineffective, supplementation with capsaicin and resveratrol restored the full effectiveness of gemcitabine in vivo () (Citation37). This effect occurred through apoptosis. If, however, gemcitabine was at its full effectiveness, no increase following treatment with resveratrol and capsaicin was observed. Combinations of resveratrol and capsaicin were also able to sensitize some PDAC cell lines to radiotherapy, leading to further reduction in tumor volume in vivo () (Citation36). Capsaicin may also benefit patients through pain relief and reduction of inflammation (Citation61).
Figure 4. Effects of a dietary compound on PDAC: Capsaicin. A. Effects on tumorigenesis of treatment with gemcitabine coupled with capsaicin (CAPS) and resveratrol (RES) combined in a xenograft (Capan-2) model of pancreatic cancer. Tumorigenesis was quantified as tumor weight expressed relative to control. The treatments were as follows: control (Citation1), high-dose GEM (Citation2), high-dose GEM coupled with CAPS and RES combination (Citation3), low-dose GEM (Citation4), and low-dose GEM coupled with CAPS and RES combination (Citation5). There was no added effect of treatment with high-dose GEM combined with CAPS + RES, suggesting a saturating effect of the chemotherapy (3 vs. 2). Although low-dose GEM had no effect on tumorigenesis (Citation4), combination with CAPS + RES produced a marked inhibitory effect (5 vs. 4, statistics not specified). Dotted horizontal line indicates null effect. Modified from Vendrely et al. (Citation37), where further details can be found. B. Tumorigenesis in a xenograft (Capan-2) model of pancreatic cancer. The treatments were as follows: control (Citation1), 2 Gy radiotherapy (Citation2), and radiotherapy with CAPS + RES (Citation3). Data are presented as mean ± SEM. The effect of the radiotherapy in suppressing tumorigenesis was potentiated significantly by combination with CAPS + RES (3 vs. 2; P < 0.05). From Vendrely et al. (Citation36), where further details can be found.
In conclusion, capsaicin has excellent properties for possible integration into management of PDAC, both directly and as regards side effects of the treatment.
2.5. Quercetin
Quercetin (also known as sophoretin or meletin) belongs to the flavonoid group of polyphenols and found naturally in apples, grapes, red raspberry and onions (). Although by itself it is not readily bioavailable, its various metabolites (present in systemic circulation after consumption) demonstrate significant biological (antioxidant and anti-inflammatory) activity. In vitro, quercetin suppressed a range of PDAC cell behaviors by regulating a variety of signaling pathways. Cell viability was suppressed dose-dependently () (Citation62, Citation64). Apoptotic cell death was increased significantly () (e. g., Ref. (Citation63)). The pro-apoptotic/anti-tumor effect of quercetin was confirmed in frozen tissue sections of human PDAC xenografts and shown to involve miR-let-7c as an intermediary (Citation65). Importantly, also, in human primary PDAC cells, quercetin upregulated miR-200b-3p expression and inhibited Notch signaling, resulting in inhibition of stemness and self-renewal (Citation66). Parallel to these effects, quercetin strongly downregulated the expression of a range of EMT markers () (Citation28, Citation64). Consistent with these effects, taken together, cellular invasiveness was significantly decreased, and this was dose dependent () (Citation64).
Figure 5. Effects of a dietary compound on PDAC: Quercetin. A. Cell viability of MiaPaCa-2 cells following treatment with increasing concentrations of quercetin (QUER) and gemcitabine (GEM). Cells were pretreated with quercetin at various concentrations for 24 h, and then treated with a fixed concentration of gemcitabine for 72 h. The effects of the combinations with 25 and 50 µM QUER were inferred to be significantly greater than GEM alone (P < 0.05). From Lan et al. (Citation62). B. PANC-1 cell apoptosis. Effects of QUER and GEM, and their combination, on compared with control (Cont). Apoptosis was defined as the number of apoptotic cells expressed as a percentage of control. Modified from Lee et al. (Citation63). C. Effects of treatment with increasing QUER concentrations on mRNA expression of EMT markers (E-cadherin, N-cadherin, and Vimentin) in PATU-8988 cells. Quercetin treatment resulted in dose-dependent reductions in expression of all three mRNAs. In all conditions and concentrations, treatment with quercetin was significant compared with the control (P < 0.05). From Yu et al. (Citation64). D. Invasiveness (quantified arbitrarily as the number of invaded cells per field of view) in PATU-8988 cells treated with increasing concentrations of QUER. For all concentrations, treatment with quercetin produced a significantly greater reduction in invasiveness compared with the control (P < 0.05). From Yu et al. (Citation64). E. Apoptosis (measured by annexin-V FITC) in gemcitabine-resistant MiaPaCa-2 cells. Cells were treated with QUER, GEM or their combination, compared with untreated Cont. The effect of the combination was markedly greater than gemcitabine alone (statistics not performed). From Lan et al. (Citation62). F. Tumor volume in a xenograft model of human primary PDAC cells. AsanPaCa cells were implanted into chick embryos and left untreated (Cont) or treated with QUER. In a parallel experiment, the cells were processed so as to silence miR-let-7c (miR2), or its negative control (miR1). MiR2 and QUER treatment decreased tumor volume significantly compared to the relative controls (P < 0.01). From Nwaeburu et al. (Citation65).
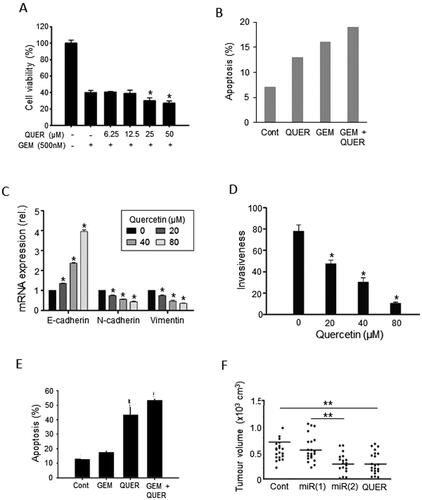
There is also some evidence from human PDAC cells in vitro that quercetin potentiates the effectiveness of gemcitabine in inhibiting cell viability and promoting apoptosis (). Also, on two human PDAC cell lines, Serri et al. used biodegradable nanoparticles and showed, again, that quercetin could enhance the impact of gemcitabine on cell viability by 15–20% (Citation67). Consistent with these results, Lan et al. showed that quercetin had a remarkable pro-apoptotic effect on a gemcitabine-resistant variant of Mia-Paca-2 cells () (Citation62). This was extended recently to several other gemcitabine-resistant PDAC as well as hepatocarcinoma cell lines by Liu ZJ et al. (Citation68). This effect was revealed to involve the “receptor for advanced glycation end products” (RAGE) (Citation62). In a further study, a natural isoform of quercetin, quercetin-3-O-glucoside, exhibited synergy with low concentrations of gemcitabine on human PDAC CFPAC-1 and SNU-213 cells in suppressing transverse migration induced by basic fibroblast growth factor (bFGF) (Citation69).
In vivo, also, quercetin caused a significant reduction of tumor volume in a xenograft model of PDAC, again involving let-7c () (Citation65). However, less work has been done to test the possible effectiveness of combining quercetin with gemcitabine in vivo. In the one available study, Angst et al. showed in an orthotopic model of PDAC that quercetin maintained its anti-proliferative and pro-apoptotic effects and reduced tumor weight (Citation70). In combination with gemcitabine, quercetin had a noticeably (ca. two-fold) greater effect on proliferative activity. In contrast, the additional effects of quercetin combined with gemcitabine on apoptosis and tumor weight were modest (ca. 10%). However, these effects did not reach significance, probably due to the limited number of animals used (n = 6) and the inherent variability of in vivo testing especially with combinations of agents (Citation70).
In conclusion, the evidence is consistent that quercetin suppresses PDAC and potentiates the effect of gemcitabine in vitro. In vivo, also, quercetin produces noticeable anti-PDAC effects, but more work is required to substantiate the evidence for the added benefit of combination with gemcitabine.
2.6. Sulforaphane
Cruciferous vegetables, named as such for their cross-shaped flowers, are rich in sulforaphane (other active ingredients include indole-3-carbinol and 3,3′-diindolylmethane). Sulforaphane-rich foods include broccoli, cabbage, cauliflower, brussels sprouts, collard greens and kale () (Citation71). Appari et al. showed that three servings of cruciferous vegetables per day was associated with a 50% decrease in PDAC risk (Citation72). A more extensive meta-analysis of four cohort and five case-control studies also found that a high intake of cruciferous vegetables decreased PDAC risk significantly by some 20% (Citation71). This was extended to a clinical trial (https://clinicaltrials.gov/ct2/show/NCT01879878). Thus, Lozanovski et al. reported that PDAC patients consuming sulforaphanes (freeze-dried broccoli) whilst undergoing palliative chemotherapy experienced improved survival albeit for limited time () (Citation73). A range of anticancer modes of action has been associated with sulforaphane (Citation76). In one study, indole-3-carbinol reduced the assembly of prostate cancer cells into organoids (Citation77). This finding could be significant to PDAC due to some similarities with prostate cancer, especially hormone (insulin) sensitivity. Both are also cancers of affluence and express similar stem cell markers (Citation78, Citation79). Sulforaphane suppressed the genetic damage of carcinogens, induced apoptosis and inhibited proliferation of PDAC cells, in culture as well as in vivo (Citation80). Specifically, combining sulforaphane with “tumor necrosis factor-related apoptosis inducing ligand” (TRAIL) was able to reduce resistance to the drug (Citation81). Furthermore, using a xenograft model of PDAC, synergy between sulforaphane and gemcitabine has been demonstrated in suppressing tumorigenesis and this involved suppression of Notch signaling and cancer stem cells (CSCs) () (Citation74). Finally, combining sulforaphane with radiotherapy suppressed PDAC cell viability by increasing cell cycle arrest () (Citation75). Interestingly, attempts are being made to develop stable synthetic variants of sulforaphane as anticancer drugs (https://evgen.com/).
Figure 6. Effects of a dietary compound on PDAC: Sulforaphane. A. Kaplan-Meier analysis of cumulative survival of PDAC patients (undergoing palliative chemotherapy). Patients were treated with a sulforaphane preparation (n = 29, solid line) compared with placebo (n = 11, dotted line). Plus signs indicate patients who were lost to follow-up. The shaded areas denote 95% confidence intervals (dark gray for treatment, light gray for placebo). From Lozanovski et al. (Citation73). B. Effects of 72-hour pretreatment of pancreatic CSC-like cells with sulforaphane (SFN), gemcitabine (GEM) and their combination on volume of tumors formed by subsequent injection (subcutaneous) into nude mice. Time indicated is from the day of inoculation. Control mice (Cont) were injected with untreated cells. Gemcitabine delayed tumorigenesis and its effect was potentiated by co-treatment with sulforaphane, suppressing tumorigenesis completely during the experimental period. Sulforaphane alone was also effective in completely suppressing the tumorigenesis. The data points for SFN and SFN + GEM (circles on the horizontal axis) were indistinguishable. From Kallifatidis et al. (Citation74), where further details can be found. C. Effect of combining sulforaphane (SFN) with two doses of irradiation, 2 and 6 Grays (Gy), on clonogenic viability (“survival”), relative to control (100%). Four different cell lines were used (AsPC-1, BxPC-3, MiaPaCa-2 and Panc-1), each indicated as a histobar. The effects of the combination were clearly greater than radiotherapy alone (formal statistics not performed). Modified from Naumann et al. (Citation75).
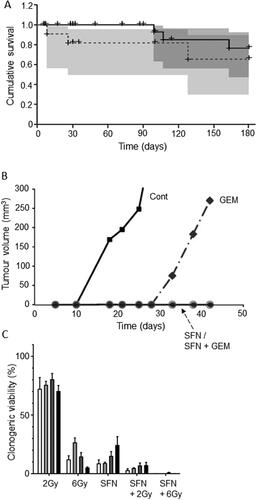
In conclusion, the available evidence for sulforaphane is promising as regards its potential both as a preventative agent by itself as well as in combination with chemotherapy, including gemcitabine.
3. Nutraceutical Agents
Nutraceuticals are defined generally as natural substances that are not a part of normal diet but can be formulated and consumed as extracts or supplements. We have identified four nutraceutical compounds for which there are promising therapeutic effects (in vitro and/or in vivo) against PDAC and in some cases the associated organs, especially the liver (Citation82). These are listed in alongside their chemical formulae, main modes of action/cellular effects and natural sources.
3.1. Artemisinin
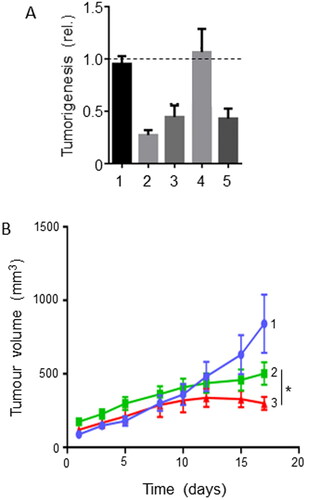
This compound is isolated from Artemisia annua, sweet wormwood, a herb commonly employed in traditional Chinese medicine against malaria (Citation83). Artemisinin and its derivatives (e.g., dihydroartemisinin) produced a variety of in vitro and in vivo effects against PDAC, including inhibition of growth and induction of apoptosis (Citation84–86). These effects have been associated with the upregulation of several miRNAs (Citation83). It has also been suggested that artemisinin generates ROS to induce oxidative stress and thereby cell death in PDAC cell lines (Citation87). Additionally, dihydroartemisinin increased T-cell proliferation and activity, which could promote an anticancer immune response (Citation88). Importantly, dihydroartemisnin increased the sensitivity of PDAC in vitro and in vivo to therapies including gemcitabine and TRAIL (Citation85, Citation89). In a xenograft model of PDAC, dihydroartemisinin significantly potentiated the effect of gemcitabine in supressing tumorigenesis by some 25% () (Citation85). Histochemical analyses of the tissues from the in vivo experiments showed that the enhancing effect of dihydroartemisinin involved suppression of proliferation and increased apoptosis () (Citation85). Additionally, dihydroartemisinin was able to sensitize liver cancer cells to gemcitabine in vivo, thereby further reducing tumor burden significantly (Citation90).
Figure 7. Effects of a nutraceutical compound on PDAC: Artemisinin. A. Effects of dihydroartemisinin (Citation2), gemcitabine (Citation3) and their combination (Citation4) on volume of pancreatic cancer tumors; compared with control (Citation1). BxPC-3 tumors were xenografted in nude mice. Data are presented as means ± standard deviation. The effect of the combination was significantly greater than gemcitabine alone (P < 0.05). B & C. Effects of treatments on status of proliferation (B) and apoptosis (C) determined from tissue sections taken from the induced tumors in (A). Treatments were as follows: gemcitabine (GEM), dihydroartemisinin (DHA) and their combination (GEM + DHA), compared with control (Cont). Data are presented as means ± standard deviation. For both parameters, the effect of the combination was greater than the effect of gemcitabine alone (P < 0.05). From Wang, SJ. et al. (Citation85).
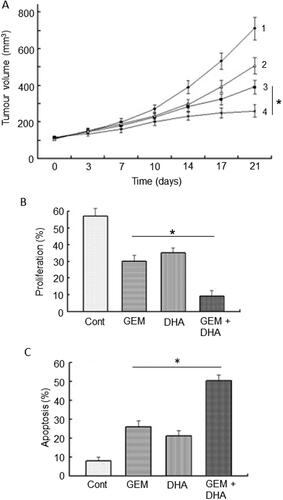
In conclusion, artemisinin and its derivatives can produce anti-PDAC effects and potentiate the effectiveness of gemcitabine. In addition, it may support liver function (). Altogether, therefore, it has great promise for integration into chemotherapy of PDAC.
3.2. Garcinol
This is a derivative isolated from Garcinia indica, a plant of the mangosteen family, commonly known as “kokum”. It is a potent inhibitor of histone acetyltransferases, a modulator of gene expression, both in vitro and in vivo. Garcinol could produce a variety of anti-PDAC effects, promoting apoptosis by increasing activities of caspase-9 and caspase-3 In Vitro (Citation91). By modulating the expression of miRNAs involved in chemoresistance, garcinol also allowed gemcitabine to become significantly more effective in suppressing proliferation and promoting apoptosis In Vitro (Citation92). Importantly, garcinol could also suppress the self-renewal and tumor-forming ability of CSCs (Citation93). Alone or in combination with gemcitabine, garcinol suppressed tumor growth in a transgenic (KPC) mouse model of PDAC () (Citation94). Furthermore, quantifying the stage of PDAC as pancreatic intraepithelial neoplasia (PanIN), combined treatment resulted in disease remaining in earlier stages. Thus, compared with gemcitabine treatment alone, PanIN lesions of all grades were consistently fewer in the combination treatment () (Citation94).
Figure 8. Effects of a nutraceutical compound on PDAC: Garcinol. A. Genetically engineered pancreatic cancer mouse model (KPC). Effects of treatment with garcinol (GAR), gemcitabine (GEM) and their combination on tumorigenesis over five weeks, quantified as the percentage of tumors which increased in size (determined by MRI). The combination produced about a four-fold greater effect than gemcitabine alone (statistics not available). B. Treatments as in (A), showing effects on tumor progression, quantified as the number of observed PanIN1 and PanIN3 lesions (light and dark bars, respectively, emphasized as 1 and 3 in the control data). Data are presented as a percentage of the PanIN1 level in control (Cont). Compared with gemcitabine alone, the combination produced consistently greater effects on both PanIN lesions, but noticeably more on PanIN3. However, significance was not reached, probably due to the limited number of animals used in the study (n = 8 in each group) and the inherent variability of the pathology. Redrawn from data given in Saadat et al. (Citation94).
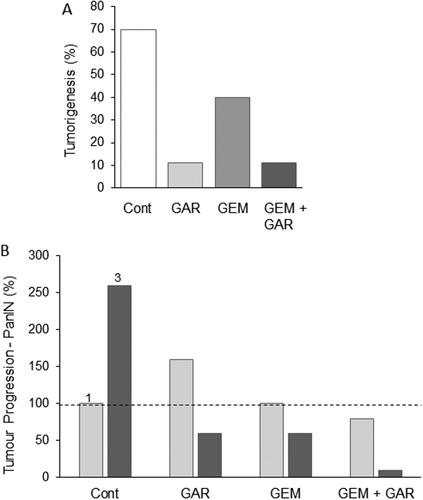
In conclusion, the available evidence suggests consistently that garcinol has significant effects against PDAC in vitro and in vivo and would seem ready to be tested in gemcitabine combination treatments.
3.3. Thymoquinone
This is an active ingredient isolated from the black seed, Nigella sativa (). It has been investigated for its anti-oxidant and anti-inflammatory activities against PDAC, both in vitro and in vivo, and was found to be effective without significant side effects (Citation95). Thymoquinone also has anticancer including anti-metastatic properties (Citation96). Mahmoud & Abdelrazek reviewed these properties as well as the hepatoprotective effects of thymoquinone (Citation97). Specifically, in PDAC, thymoquinone downregulated the expression of the anti-adhesive protein MUC4, thereby leading to decreased cell motility (Citation98). Several studies have suggested a synergistic effect of thymoquinone in combination with gemcitabine. When these agents were combined in vitro, cell viability fell by more than 50% compared with monotherapy () (Citation99). In a xenograft model of PDAC, combination of thymoquinone with gemcitabine produced a significantly greater effect (by some 80%) on tumor weight compared with gemcitabine alone () (Citation99). This effect involved potentiation of caspase (3 and 9) activation, leading to apoptosis (Citation100). This pro-apoptotic effect appeared to be specific to PDAC cells (Citation101). Thymoquinone-induced histone acetylation was proposed to be the basis of its synergy with gemcitabine (Citation102).
Figure 9. Effects of a nutraceutical compound on PDAC: Thymoquinone. A. Effects of gemcitabine (Citation1), thymoquinone (Citation2) and their combination (Citation3) on the viability of PANC-1 cells. All treatments produced dose-dependent reduction in viability. The effects of the combination were markedly greater than gemcitabine alone at all concentrations tested. Calculations of the “combination index” confirmed significant synergy. From Pandita et al. (Citation99), where further details can be found. B. Effects of thymoquinone (TMQ), gemcitabine (GEM) and their combination (GEM + TMQ), compared with control (Cont), in mice bearing orthotopically induced PANC-1 tumors. Data show effects on both tumor weight (lefthand axis, dark bars) and associated caspase-3 activity (righthand axis, light bars). In both cases, data are shown relative to the respective control level at 100%. For both parameters, the effects of the combination were significantly greater than treatment with gemcitabine alone (P < 0.01 for both). Replotted from data given in Mu et al. (Citation100).
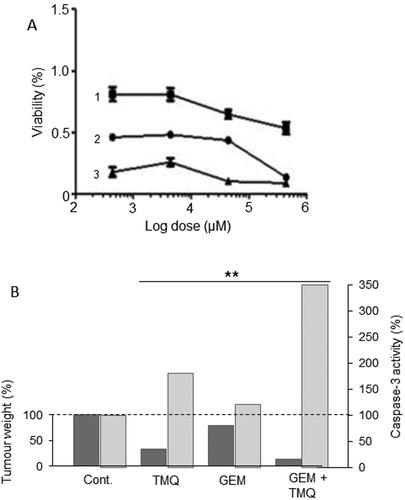
In conclusion, from the available in vivo combination evidence, supported by in vitro data, we conclude that thymoquinone has excellent potential to be incorporated into an integrated management regimen for PDAC.
3.4. Emodin
This is a purgative resin from rhubarb (Polygonum cuspidatum), buckthorn and Japanese knotweed (Fallopia japonica) (). By increasing activation of tumor suppressor genes via DNA demethylation, emodin suppressed PDAC cell proliferation (Citation103–105). In combined treatments in vitro, emodin “sensitized” a drug-resistant cell line to gemcitabine () (Citation105). In an orthotopic xenograft model of PDAC, emodin promoted the effect of gemcitabine in suppressing tumorigenesis () (Citation106). The effects of emodin involved increased apoptosis and inhibited angiogenesis with possible transcriptional control via NF-κB (Citation106–109). Finally, in a mouse model of PDAC, emodin dose-dependently decreased metastasis to liver by up to ca. 50% whilst “normalising” miR1271 expression and inhibiting EMT (Citation107). In vitro and in vivo, liver cancer itself benefited from emodin treatment which also enhanced the effectiveness of a biological (sorafenib) therapy (Citation110). Finally, also in an in vivo mouse model, cachexia could be reversed by long-term treatment with emodin and this occurred through decreased hypoxia inducible factor (HIF) - 1α signaling (Citation111).
Figure 10. Effects of a nutraceutical compound on PDAC: Emodin. A. Bxpc-3 cells and their gemcitabine-resistant versions (Bxpc-3/Gem) (black and white bars, respectively). Effects of treatment of cells with emodin (EMO), gemcitabine (GEM) and their combination (GEM + EMO) on apoptosis. Data are plotted as a percentage of the control level for Bxpc-3 cells (at 100%). The effect of the combination was significantly greater than gemcitabine alone, but on the resistant cell line only (P < 0.05). The GEM-resistance of the Bxpc-3/Gem cell line was confirmed by significant lack of apoptotic response (P < 0.05). Replotted from data given in Zhang, W. et al. (Citation104). B. Orthotopically induced pancreatic cancer in mice, treated with GEM, EMO and their combination. Tumor weights (in grams) were determined and compared with tumor-bearing control animals treated with saline only (Cont). The effect of the combination was significantly greater than gemcitabine alone (P < 0.05). Modified from Lin, SZ. et al. (Citation106), where further details can be found.
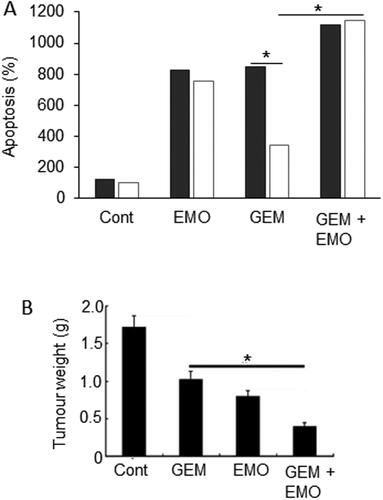
In conclusion, emodin can produce beneficial effects against PDAC, as well as liver cancer, by itself and can potentiate the effectiveness of gemcitabine in vitro and in vivo. Thus, it could be considered for clinical application.
4. Further Emerging Potential Complementary Agents
We should note that there are a number of other “agents” (dietary and nutraceutical) with anti-PDAC properties but for which evidence is mainly from In Vitro experiments and no In Vivo combination data are available. Such emerging agents are noted here with the expectation that the evidence in their favor could become stronger in time. Some examples of these emerging agents include the following.
4.1. Lycopene
This is the red pigment that gives red and pink fruit, such as tomatoes, watermelons, pink grapefruit and guava their characteristic color. A meta-analysis of 18 eligible studies concluded that lycopene intake and PDAC risk were inversely correlated (Citation112). This conclusion was supported later by the mechanistic demonstration that lycopene induces apoptosis in PDAC cells (Citation113). Thus, the overall evidence for the anti-PDAC role of lycopene is significant. However, it has never been tested in combination therapies. Lycopene can also protect against acute pancreatitis and diabetes (e.g., Refs. Citation114, Citation115). Accordingly, lycopene may currently be used in a preventative setting. Although it is not known if it would potentiate gemcitabine chemotherapy of PDAC, it could reduce or even prevent the side effects of chemotherapy due to its antioxidant and anti-inflammatory properties (Citation116, Citation117).
4.2. Zinc
This is an essential mineral found naturally in high concentrations in oysters, crab, beans and pumpkin seeds. It is frequently mentioned as an anticancer agent but the evidence for its possible anti-PDAC effect is quite contradictory (Citation118–120). This is probably due to the multi-faceted role of this metal as a structural component of many proteins and a co-factor in enzymatic reactions. Overall, a meta-analysis concluded that high consumption of zinc was associated with a decreased risk of PDAC (Citation121). Consistent with this, more recent evidence has concluded that zinc dyshomeostasis (caused by the dysfunction of zinc transporters) can contribute to the initiation and/or progression of various cancers, including PDAC (Citation122). Accordingly, the zinc ionophore, clioquinol, was suggested to be efficacious against PDAC (Citation123). Furthermore, “Bhasma” (incinerated processed zinc widely used in Ayurveda for various ailments) could also be effective against PDAC (Citation124). Finally, zinc could protect against cachexia in PDAC (Citation125). While there is no evidence for possible synergy between chemotherapy and zinc, it has been deemed acceptable for treatment of liver disease (Citation126, Citation127).
4.3. Selenium
Selenium is a trace element that plants accumulate from soil and convert to organic forms. Thus, it is naturally present in many foods, especially Brazil nuts, seafood, pasta and eggs. Han X et al. originally reported anti-PDAC effects of selenium (Citation128, Citation129). A meta-analysis and a later nested case-control study found no adverse effect on PDAC but a negative association was apparent for patients with body mass index (BMI) higher than 25 (Citation130, Citation131). Beneficial effects and no adverse effects have also been reported against diabetes (Citation132–134). However, high-level consumption could promote insulin resistance and should be avoided (Citation135).
4.4. Magnesium
Magnesium-rich foods include dark-green leafy vegetables, seeds, wholegrains, fish and nuts. A recent study revealed significantly lower levels of magnesium in urine of PDAC patients compared to healthy controls (Citation136). Consistent with this, a prospective cohort study showed that magnesium supplementation may help prevent PDAC (Citation137). Mechanistic evidence suggested (i) that Mg2+-permeant ion channels associate with PDAC (Citation138) and (ii) that the Mg2+ transporter protein SLC41A1 could be a viable anti-PDAC target (Citation139). Indirectly, also, magnesium may reduce the risk of PDAC through its well-established negative relationship with diabetes (Citation140, Citation141).
4.5. Plumbagin
This is a derivative of naphthalene obtained from the roots of the “chitrak” that has been used in Ayuverdic medicine for more than 2,500 years. A bioinformatics study revealed several “mainstream” cancer signaling mechanisms whereby plumbagin could affect survival, apoptosis and metabolism in PDAC cells (Citation142). Indeed, experimentally, plumbagin has been shown to inhibit the growth of PDAC cells (including CSCs) and induce autophagy in vitro and in vivo and may target the tumor suppressor p53 (Citation142–145). Most recently, Pandey et al. showed that plumbagin induced apoptosis in PDAC cells both in monolayers and three-dimensional tumor spheroids (Citation146). This effect involved production of ROS and cleavage of caspases 3 and 9. Work is ongoing to develop nanoparticles of plumbagin as an anti-angiogenic drug (Citation147).
4.6. Milk Thistle
This has been used as a traditional herbal remedy for almost 2000 years. Its active ingredient, silymarin (major constituent, silibinin/silybin) has been shown to inhibit proliferative activity and angiogenesis in models of PDAC (Citation148). Silibinin also promoted viability and promoted apoptosis and autophagy in human PDAC cells (Citation149). These effects were mediated by increased “stress-activated protein kinase” (JNK/SAPK) signaling. Used by itself, silibinin could suppress tumor growth and cachexia in in vivo mouse models of PDAC (Citation150). Silibinin is also well known for its “hepatoprotective” property and for promoting liver regeneration (Citation151). Indeed, a randomized phase two clinical trial on childhood acute lymphoblastic leukemia (ALL) undergoing chemotherapy employed in combination silymarin (milk thistle extract) with the aim of reducing liver toxicity and a positive effect was reported (https://clinicaltrials.gov/ct2/show/NCT00055718) (Citation151).
4.7. Berberine
This is an isoquinoline alkaloid extracted from a variety of natural herbs (Citation152). Its molecular targets include upregulation of tumor suppressor genes, activation of AMPK and downregulation of matrix metalloproteinase production, leading to decreased cellular proliferation and invasion (Citation153). In vitro, berberine induced apoptosis and suppressed the CSC population in PDAC cell lines even more effectively than gemcitabine (Citation154, Citation155). Another study showed that berberine inhibited PDAC cell viability by dysregulating cellular energetics by targeting citrate metabolism (Citation156). In vivo, also, berberine could reduce tumor growth in mouse models of PDAC (Citation157). In addition, berberine demonstrated insulin-regulating properties and could suppress PDAC also indirectly (Citation158). Consistent with this, it could potentiate the therapeutic effect of metformin (see section 5.2) (Citation159).
4.8. Ginseng
Panax ginseng (“ginseng”) is a perennial plant that grows in East Asia. It is a traditional medicinal herb with a unique family of active saponin ingredients called “ginsenosides”. It is available in various forms (e.g., fresh, white, steamed, acid-processed and fermented) leading to a range ginsenoside compositions with diverse pharmacological properties (Citation160). Yun & Choi found in an early case-control study concluded ginseng consumption would reduce the risk of PDAC and liver cancer (Citation161, Citation162). Experimentally, extracts of ginseng (leaves, flowers and roots) and some nanoparticle preparations have been tested against various human PDAC cell lines and shown to inhibit cell viability, proliferation and angiogenesis whilst promoting apoptosis (e.g., Refs. Citation163–165). Some anti-PDAC effects have also been reported in vivo (Citation166, Citation167). Furthermore, ginseng could delay the development of type 1 diabetes and pancreatitis, both PDAC risk factors, in rats (Citation168, Citation169). Finally, ginseng has also been reported to enhance the effectiveness of in vitro gemcitabine (and some other chemotherapeutic agents) on PDAC cells as well as liver, lung and prostate cancers (Citation170–172).
5. Drug Combinations
Drug combinations refer to more than one drug being taken together. Although this topic is outside the immediate scope of the current review, as another “combination,” we would like to highlight it here for awareness and possible benefit to patients (Citation173). In fact, use of drug combinations is common in cancer therapy and is already applied to PDAC in the form of FOLFIRINOX. A novel approach is including in the combination a drug that has been developed and prescribed for conditions other than cancer (a practice known as “repurposing”). We highlight here two examples of such drug combinations.
5.1. Aspirin
This is a natural agent used commonly against pain and inflammation. Its general health benefits including anticancer properties have been highlighted over many years (Citation174, Citation175). However, caution has also been expressed as regards its possible detrimental side effects on uncontrolled high blood pressure, bleeding disorders, asthma and stomach ulcers (Citation176, Citation177). As regards PDAC, evidence suggests that high and medium usage (one or more 75 mg tablet a day) may reduce the risk by as much as 50%, the risk of those who stop taking it subsequently increasing (Citation178, Citation179). This is through its inhibition of the COX-2 enzyme which indeed is upregulated in PDAC and may promote the disease by enhancing inflammation, a risk factor for PDAC (Citation180). A negative association between aspirin use and PDAC risk was demonstrated from a meta-analysis () (Citation181, Citation183). The association was stronger with increased frequency and duration of aspirin consumption (Citation178, Citation181, Citation184). The apparent time-dependent correlation could be due to the fact that development of PDAC begins long before its diagnosis so, to derive benefit, consumption of aspirin should start well before occurrence (Citation185).
Figure 11. Drug combination: effects of combining aspirin with chemotherapy. A. Forest plot showing improved relative risk of pancreatic cancer resulting from taking aspirin. Diamond indicates the average and the spread of the data. From Qiao et al. (Citation181), where further details and primary data can be found. B. Effects of aspirin (ASP), gemcitabine (GEM) and their combination (GEM + ASP) on volume of tumor induced by orthotopic inoculation of PANC-1 cells in immunodeficient mice. There was a clear trend in the data, the combination producing a significant effect compared to the control (P < 0.05). Although a marked difference between the effects of GEM + ASP and GEM alone was apparent, this did not reach significance, possibly because of the limited number of experimental animals involved (n = 6). C. Data from the same experiment as in (B) showing survival fraction for tumor-bearing mice treated with ASP (Citation2), GEM (Citation3) and their combination (Citation4); control data are indicated as (Citation1). The combination treatment significantly improved survival compared with GEM alone (P < 0.05). B and C, from Zhang, Y. et al. (Citation182).
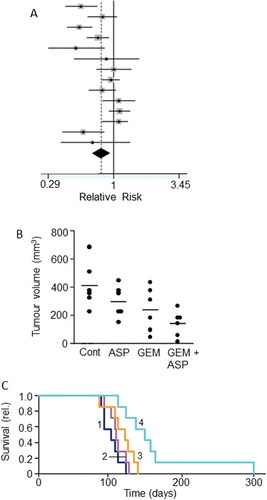
In terms of “combination therapy,” aspirin proved an effective adjuvant to gemcitabine in the treatment of PDAC in an in vivo transgenic mouse model (Citation186). This study also showed that the combined treatment prolonged survival by ca. 30% over gemcitabine treatment alone. In an extensive, combined in vitro and in vivo study, including cells taken from PDAC patients, aspirin (at clinical doses) enhanced the effectiveness of gemcitabine in suppressing tumorigenesis () (Citation182). The effect involved induction of apoptosis, reduction of cell viability and expression of proteins involved in inflammation and stem cell signaling (Citation182). This study also showed that the aspirin + gemcitabine combination significantly prolonged the survival of mice, by some 40%, compared with gemcitabine alone (). Importantly, also, aspirin sensitized cells that were resistant to gemcitabine (Citation182, Citation187). In a more recent phase 1b clinical trial, aspirin has been included as a part of an immunotherapy/vaccination regime against PDAC (Ref: CHUV-DO-0017_PC-PEPDC_2017) (Citation188).
In conclusion, aspirin can be taken safely in a preventative setting and can also be used in combination with gemcitabine chemotherapy to improve treatment efficacy.
5.2. Metformin
Metformin is the most common oral drug prescribed for type II diabetes (Citation189). Epidemiological evidence suggests that diabetics treated with metformin are less likely to develop PDAC (Citation190). Indeed, meta-analyses revealed that metformin treatment increased survival in diabetic PDAC patients as well as presumed non-diabetics (Citation190, Citation191).
By inhibiting oxidative phosphorylation, metformin reduces levels of the energy rich compound adenosine triphosphate (ATP) in cells, which in turn downregulates signaling mechanisms involved in cellular survival and proliferation (Citation192). Consistent with this, a study on a transgenic mouse model found that metformin reduced PDAC incidence (quantified by PanIN pathology) by up to 50% (Citation193). Importantly, in in vivo mouse models of PDAC, metformin reduced CSC proliferation as well as infiltration and activation of tumor-associated macrophages which contribute to desmoplasia (Citation194). Thus, metformin could induce stromal depletion and enhance penetration of a nanoparticulate form of gemcitabine (Citation195).
There is also increasing evidence that metformin can enhance the effectiveness of gemcitabine (Citation196, Citation197). Li, X. et al. performed a meta-analysis of rather disparate data and concluded that patients taking metformin (with or without chemotherapy) had improved overall survival compared with a control group not taking metformin () (Citation198). Subgroup analysis showed that the effect of metformin correlated with tumor stage and was associated with improved survival in patients who had surgery or locally advanced cancer, but not in patients with metastatic disease (Citation198).
Figure 12. Drug combination: effects of adding metformin to chemotherapy. A. Forest plot demonstrating hazard ratios associated with use of metformin for pancreatic cancer patients. The average (indicated by the diamond) revealed significantly reduced risk (P = 0.01). Modified from Li, X. et al. (Citation198), where further details and primary data can be found. B. Effects of gemcitabine (GEM), metformin (MET) and their combination on tumor weight, compared with untreated controls (Cont), in a gemcitabine-resistant pancreatic cancer (BxG30) mouse xenograft model. Data are plotted relative to the control level at 100%. There was a clear trend, the combination producing a significant effect compared to the control (P < 0.05). Although a noticeable difference between the effects of GEM + MET and GEM alone was apparent, this did not reach significance. Replotted from data given in Suzuki et al. (Citation199). C. Kaplan-Meier survival analysis of KPC mice treated with control/sterile water (Citation1), MET (Citation2), GEM (Citation3) and GEM + MET (Citation4). There was a significant difference between the effects of the combination and GEM alone (P < 0.05), confirming the beneficial effect of the combined treatment. Modified from Qian et al. (Citation200). D. Effects combining metformin (MET) with irradiation (RAD) or RAD + GEM on clonogenic survival of MiaPaCa-2 cells. Survival was quantified as a percentage of total. Adding MET to both RAD or RAD + GEM produced a significant further decrease in survival (P < 0.05 for both comparisons). Modified from Fasih et al. (Citation201).
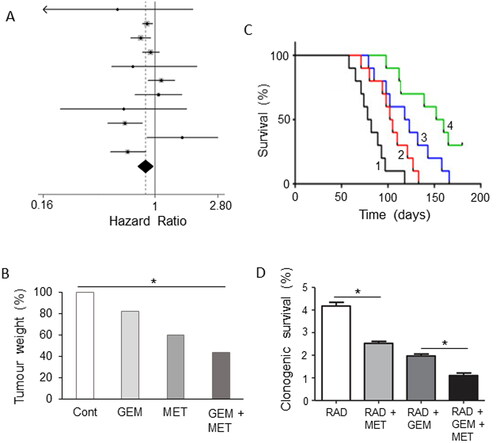
The apparent improvement of effectiveness of chemotherapy by metformin is also supported by in vitro experiments involving several human PDAC cell lines (Citation202, Citation203). In another study, even resistance to gemcitabine was reversed by metformin, albeit in vitro (Citation204). Only one in vitro study reported inhibition of the pro-apoptotic effect of gemcitabine (Citation205). However, this study involved (i) murine cell lines and (ii) the concentration of metformin used (20 mM) was rather high (cf. Ref. Citation192). Also, in an in vivo mouse xenograft model of PDAC, metformin demonstrated antitumor activity against a drug-resistant tumor (Citation199). Combination of gemcitabine with metformin generated a noticeably further reduction in tumor weight, compared with the effect of gemcitabine alone () (Citation199). Furthermore, in xenograft and genetically engineered mouse models of PDAC, metformin enhanced significantly the effect of gemcitabine in improving survival () (Citation200). This enhancement effect was suggested to involve inhibition of CSCs and suppression of angiogenesis (Citation200, Citation206).
Synergistic effects of combining metformin with radiation (plus gemcitabine in some cases) were observed in PDAC cells in vitro () (Citation201). This “radiosensitization” effect was thought to involve AMPK (Citation201, Citation202).
Several clinical trials are evaluating the anti-PDAC potential of metformin by itself and in combined administration with chemotherapy (gemcitabine and nab-paclitaxel) and radiotherapy. The trials that have been concluded so far were those on late-stage or gemcitabine-resistant disease and, unfortunately, did not find significant improvement by adding metformin (Citation207, Citation208). Other trials are still to report.
In conclusion, there is promising preclinical evidence that metformin can be added safely as an adjuvant to chemotherapy of PDAC. Currently, this would appear more promising for early-stage disease.
6. Future Perspectives and Conclusion
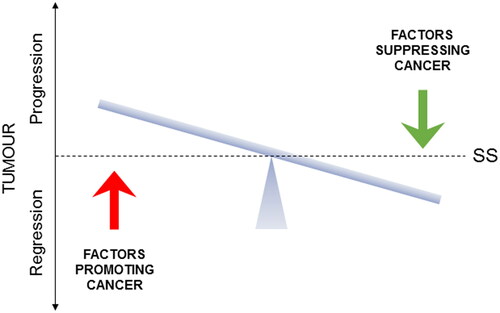
In our previous study, supported by clinical trial data, we identified six dietary and three nutraceutical agents for integration with gemcitabine chemotherapy of PDAC (Citation16). Here, based upon more limited available evidence, we evaluated another 10 agents (six dietary and four nutraceutical) which, in due course, may be added to the kind of integrative scheme we presented earlier. In fact, remembering the direness of PDAC, these 10 agents could even be introduced into integrated management now since, although the evidence in their favor is limited, there is no associated adverse effect. In addition to the incorporation of such agents, one should remember that integrative lifestyle factors should also be considered for completeness. These would include limited alcohol intake, no smoking, and regular exercise and avoiding obesity (Citation16). In overall conclusion, cancer-causing processes can be suppressed, and current treatment methods can be potentiated significantly by integrating with complementary factors to the extent that it may ultimately be possible to live with cancer chronically () (Citation209). In fact, any improvement to life expectancy would be hugely beneficial to PDAC patients as research continues intensely in this field and new clinical therapies as well as novel integration regimes can be expected.
Figure 13. A schematic representation of the homeostatic balance of cancer. The figure illustrates that cancers are under the dynamic influence of opposite factors that promote or suppress the cancer. Depending on their balance, cancer will progress or regress. The horizontal bar indicates steady-state (SS), representing either a cancer-free or a benign state. The ultimate promise of integrated management of cancer is the possibility of living with cancer chronically as long as the balance is kept tilting away from “progression.” Adapted from Djamgoz and Plant (Citation209).
In further evaluating the kinds of dietary and nutraceutical agents covered here, a number of issues should be born in mind. These were covered in some detail by Jentzsch et al. (Citation16). Some key issues are the quality of the agents and the possible personalized nature of their effects. Future research should aim at ascertaining such issues as well as determining the molecular mechanisms/modes of action of the emerging agents and, most importantly, extending the evaluations to clinical trials.
In overall conclusion, both existing and emerging evidence strongly suggests that introduction of complementary agents and lifestyle factors into mainstream treatment of PDAC can produce significant beneficial effects, as regards both disease status and its side effects (Citation16, Citation210).
| Abbreviations | ||
| AMPK | = | Adenosine Monophosphate Kinase |
| ASP | = | Aspirin |
| CAPS | = | Capsaicin |
| CSC | = | Cancer Stem Cell |
| DHA | = | Dihydroartemisinin |
| EGCG | = | Epigallocatechin Gallate |
| EMO | = | Emodin |
| EMT | = | Epithelial-Mesenchymal Transition |
| FOLFIRINOX | = | Folinic acid + Fluorouracil + Irinotecan + Oxaliplatin |
| GAR | = | Garcinol |
| GEM | = | Gemcitabine |
| MET | = | Metformin |
| NF-κB | = | Nuclear Factor κ-light-chain-enhancer of activated B cells |
| PDAC | = | Pancreatic Ductal Adenocarcinoma |
| QUER | = | Quercetin |
| RAD | = | Radiation |
| RES | = | Resveratrol |
| ROS | = | Reactive Oxygen Species |
| SFN | = | Sulforaphane |
| TRAIL | = | Tumor Necrosis Factor-Related Apoptosis Inducing Ligand |
| TMQ | = | Thymoquinone |
Acknowledgments
This study was funded by The Sunflower Jam charity and is dedicated to the memory of Jon Lord. We thank Ms Vicky Lord and Ms Jacky Paice and, also, the College of Medicine, Dr Michael Dixon in particular, for encouragement. Our research – neuroscience solutions to cancer – overall is supported by the Pro Cancer Research Fund (PCRF).
Conflict of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. doi:https://doi.org/10.1016/S0140-6736(20)30974-0
- Ferrone CR, Ryan DP. Pancreatic cancer: a time to change. Ann Surg. 2020;271(6):1003–4. doi:https://doi.org/10.1097/SLA.0000000000003910
- Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV. Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol. 2020;12(2):173–81. doi:https://doi.org/10.4251/wjgo.v12.i2.173
- Ushio J, Kanno A, Ikeda E, Ando K, Nagai H, Miwata T, Kawasaki Y, Tada Y, Yokoyama K, Numao N, et al. Pancreatic ductal adenocarcinoma: epidemiology and risk factors. Diagnostics. 2021;11(3):562. doi:https://doi.org/10.3390/diagnostics11030562
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi:https://doi.org/10.1158/0008-5472.CAN-14-0155
- Lu F, Poruk KE, Weiss MJ. Surgery for oligometastasis of pancreatic cancer. Chin J Cancer Res. 2015;27(4):358–67.
- Raufi AG, Manji GA, Chabot JA, Bates SE. Neoadjuvant treatment for pancreatic cancer. Semin Oncol. 2019;46(1):19–27. doi:https://doi.org/10.1053/j.seminoncol.2018.12.002
- El Hassouni B, Li Petri G, Liu DSK, et al. Pharmacogenetics of treatments for pancreatic cancer. Expert Opin Drug Metab Toxicol. 2019;15(6):437–47. doi:https://doi.org/10.1080/17425255.2019.1620731
- Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17 (Suppl 5):v7–v12. doi:https://doi.org/10.1093/annonc/mdj941
- Marasini B, Sahu RP. Natural anti-cancer agents: implications in gemcitabine-resistant pancreatic cancer treatment. Mini Rev Med Chem. 2017;17(11):920–7.
- Lin SR, Chang CH, Hsu CF, et al. Natural compounds as potential adjuvants to cancer therapy: preclinical evidence. Br J Pharmacol. 2020;177(6):1409–23. doi:https://doi.org/10.1111/bph.14816
- Drozdoff L, Klein E, Kiechle M, Paepke D. Use of biologically-based complementary medicine in breast and gynecological cancer patients during systemic therapy. BMC Complement Altern Med. 2018;18(1):259. doi:https://doi.org/10.1186/s12906-018-2325-3
- Shalom-Sharabi I, Frenkel M, Caspi O, et al. Integrative oncology in supportive cancer care in Israel. Integr Cancer Ther. 2018;17(3):697–706. doi:https://doi.org/10.1177/1534735418764839
- Keene MR, Heslop IM, Sabesan SS, Glass BD. Complementary and alternative medicine use in cancer: a systematic review. Complement Ther Clin Pract. 2019;35:33–47. doi:https://doi.org/10.1016/j.ctcp.2019.01.004
- Mittelman SD. The role of diet in cancer prevention and chemotherapy efficacy. Annu Rev Nutr. 2020;40:273–97. doi:https://doi.org/10.1146/annurev-nutr-013120-041149
- Jentzsch V, Davis JAA, Djamgoz MBA. Pancreatic cancer (PDAC): introduction of evidence-based complementary measures into integrative clinical management. Cancers. 2020;12(11):3096. doi:https://doi.org/10.3390/cancers12113096
- Supic G, Jagodic M, Magic Z. Epigenetics: a new link between nutrition and cancer. Nutr Cancer. 2013;65(6):781–92. doi:https://doi.org/10.1080/01635581.2013.805794
- Tiffon C. The impact of nutrition and environmental epigenetics on human health and disease. IJMS. 2018;19(11):3425. doi:https://doi.org/10.3390/ijms19113425
- Hernando-Requejo O, García de Quinto H, Rubio Rodríguez MC. Nutrition as an epigenetic factor in develops of cancer. Nutr Hosp. 2019;36:53–7. doi:https://doi.org/10.20960/nh.02810
- Nasir A, Bullo MMH, Ahmed Z, et al. Nutrigenomics: epigenetics and cancer prevention: a comprehensive review. Crit Rev Food Sci Nutr. 2020;60(8):1375–87. doi:https://doi.org/10.1080/10408398.2019.1571480
- Campbell TC. The past, present, and future of nutrition and cancer: part 1—was a nutritional association acknowledged a century ago?Nutr Cancer. 2017;69(5):811–7. doi:https://doi.org/10.1080/01635581.2017.1317823
- Azimi H, Khakshur AA, Abdollahi M, Rahimi R. Potential new pharmacological agents derived from medicinal plants for the treatment of pancreatic cancer. Pancreas. 2015;44(1):11–5. doi:https://doi.org/10.1097/MPA.0000000000000175
- Lu PY, Shu L, Shen SS, Chen XJ, Zhang XY. Dietary patterns and pancreatic cancer risk: a meta-analysis. Nutrients. 2017;9(1):38. doi:https://doi.org/10.3390/nu9010038
- Weisbeck A, Jansen RJ. Nutrients and the pancreas: an epigenetic perspective. Nutrients. 2017;9(3):283. doi:https://doi.org/10.3390/nu9030283
- Lohse I, Wildermuth E, Brothers SP. Naturally occurring compounds as pancreatic cancer therapeutics. Oncotarget. 2018;9(83):35448–57. doi:https://doi.org/10.18632/oncotarget.26234
- Ko J-H, Sethi G, Um J-Y, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The role of resveratrol in cancer therapy. IJMS. 2017;18(12):2589. doi:https://doi.org/10.3390/ijms18122589
- Zhou C, Qian W, Ma J, et al. Resveratrol enhances the chemotherapeutic response and reverses the stemness induced by gemcitabine in pancreatic cancer cells via targeting SREBP1. Cell Prolif. 2019;52(1):e12514. doi:https://doi.org/10.1111/cpr.12514
- Hoca M, Becer E, Kabadayı H, Yücecan S, Vatansever HS. The effect of resveratrol and quercetin on epithelial-mesenchymal transition in pancreatic cancer stem cell. Nutr Cancer. 2020;72(7):1231–42. doi:https://doi.org/10.1080/01635581.2019.1670853
- Fu J, Shrivastava A, Shrivastava SK, Srivastava RK, Shankar S. Triacetyl resveratrol upregulates miRNA‑200 and suppresses the Shh pathway in pancreatic cancer: a potential therapeutic agent. Int J Oncol. 2019;54(4):1306–16. doi:https://doi.org/10.3892/ijo.2019.4700
- Cheng L, Yan B, Chen K, et al. Resveratrol-induced downregulation of NAF-1 enhances the sensitivity of pancreatic cancer cells to gemcitabine via the ROS/Nrf2 signaling pathways. Oxid Med Cell Longev. 2018;2018:9482018. doi:https://doi.org/10.1155/2018/9482018
- Jiang Z, Chen X, Chen K, Sun L, Gao L, Zhou C, Lei M, Duan W, Wang Z, Ma Q, et al. YAP inhibition by resveratrol via activation of AMPK enhances the sensitivity of pancreatic cancer cells to gemcitabine. Nutrients. 2016;8(10):546. doi:https://doi.org/10.3390/nu8100546
- Harikumar KB, Kunnumakkara AB, Sethi G, et al. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic. Int J Cancer. 2010;127(2):257–68.
- Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215:150–60. doi:https://doi.org/10.1111/j.1749-6632.2010.05852.x
- Xu Q, Zong L, Chen X, Jiang Z, Nan L, Li J, Duan W, Lei J, Zhang L, Ma J, et al. Resveratrol in the treatment of pancreatic cancer. Ann NY Acad Sci. 2015;1348(1):10–9. doi:https://doi.org/10.1111/nyas.12837
- Hsu YH, Chen SY, Wang SY, Lin JA, Yen GC. Pterostilbene enhances cytotoxicity and chemosensitivity in human pancreatic cancer cells. Biomolecules. 2020;10(5):709. doi:https://doi.org/10.3390/biom10050709
- Vendrely V, Amintas S, Noel C, et al. Combination treatment of resveratrol and capsaicin radiosensitizes pancreatic tumor cells by unbalancing DNA repair response to radiotherapy towards cell death. Cancer Lett. 2019;451:1–10. doi:https://doi.org/10.1016/j.canlet.2019.02.038
- Vendrely V, Peuchant E, Buscail E, et al. Resveratrol and capsaicin used together as food complements reduce tumor growth and rescue full efficiency of low dose gemcitabine in a pancreatic cancer model. Cancer Lett. 2017;390:91–102. doi:https://doi.org/10.1016/j.canlet.2017.01.002
- Xiao Q, Zhu W, Feng W, et al. A Review of resveratrol as a potent chemoprotective and synergistic agent in cancer chemotherapy. Front Pharmacol. 2019;9:1534.
- Filippini T, Malavolti M, Borrelli F, et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2020;3(3):CD005004.
- Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea catechins. IJMS. 2020;21(5):1744. doi:https://doi.org/10.3390/ijms21051744
- Chang B, Sang L, Wang Y, Tong J, Wang BY. Consumption of tea and risk for pancreatic cancer: a meta-analysis of published epidemiological studies. Nutr Cancer. 2014;66(7):1109–23. doi:https://doi.org/10.1080/01635581.2014.951730
- Wang J, Zhang W, Sun L, et al. Green tea drinking and risk of pancreatic cancer: a large-scale, population-based case-control study in urban Shanghai. Cancer Epidemiol. 2012;36(6):e354–e358. doi:https://doi.org/10.1016/j.canep.2012.08.004
- Zeng JL, Li ZH, Wang ZC, Zhang HL. Green tea consumption and risk of pancreatic cancer: a meta-analysis. Nutrients. 2014;6(11):4640–50. doi:https://doi.org/10.3390/nu6114640
- Abe SK, Inoue M. Green tea and cancer and cardiometabolic diseases: a review of the current epidemiological evidence. Eur J Clin Nutr. 2020. doi:https://doi.org/10.1038/s41430-020-00710-7
- Khan N, Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2018;11(1):39. doi:https://doi.org/10.3390/nu11010039
- Lin Y, Shi D, Su B, et al. The effect of green tea supplementation on obesity: a systematic review and dose–response meta‐analysis of randomized controlled trials. Phytother Res. 2020;34(10):2459–2470. doi:https://doi.org/10.1002/ptr.66971–1.
- Babu BI, Malleo G, Genovese T, et al. Green tea polyphenols ameliorate pancreatic injury in cerulein-induced murine acute pancreatitis. Pancreas. 2009;38(8):954–67. doi:https://doi.org/10.1097/MPA.0b013e3181b28d11
- Bimonte S, Leongito M, Barbieri A, et al. Inhibitory effect of (-)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect Agent Cancer. 2015;10:22.
- Li L, Leung PS. Use of herbal medicines and natural products: an alternative approach to overcoming the apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol. 2014;53:224–36. doi:https://doi.org/10.1016/j.biocel.2014.05.021
- Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131(1):30–40. doi:https://doi.org/10.1002/ijc.26323
- Wei R, Penso NEC, Hackman RM, Wang Y, Mackenzie GG. Epigallocatechin-3-gallate (EGCG) suppresses pancreatic cancer cell growth, invasion, and migration partly through the inhibition of akt pathway and epithelial-mesenchymal transition: enhanced efficacy when combined with gemcitabine. Nutrients. 2019a;11(8):1856. doi:https://doi.org/10.3390/nu11081856
- Wei R, Hackman RM, Wang Y, Mackenzie GG. Targeting glycolysis with epigallocatechin-3-gallate enhances the efficacy of chemotherapeutics in pancreatic cancer cells and xenografts. Cancers (Basel). 2019b;11(10):1496. doi:https://doi.org/10.3390/cancers11101496
- Lin HL, An QZ, Wang QZ, Liu CX. Folate intake and pancreatic cancer risk: an overall and dose-response meta-analysis. Public Health. 2013;127(7):607–13. doi:https://doi.org/10.1016/j.puhe.2013.04.008
- Gong Z, Holly EA, Bracci PM. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control. 2009;20(8):1317–25. doi:https://doi.org/10.1007/s10552-009-9352-9
- Park JY, Bueno-de-Mesquita HB, Ferrari P, Weiderpass E, Batlle J, Tjønneland A, Kyro C, Rebours V, Boutron-Ruault M-C, Mancini FR, et al. Dietary folate intake and pancreatic cancer risk: results from the European prospective investigation into cancer and nutrition. Int J Cancer. 2019;144(7):1511–21. doi:https://doi.org/10.1002/ijc.31830
- Yallew W, Bamlet WR, Oberg AL, Anderson KE, Olson JE, Sinha R, Petersen GM, Stolzenberg-Solomon RZ, Jansen RJ. Association between alcohol consumption, folate intake, and risk of pancreatic cancer: a case-control study. Nutrients. 2017;9(5):0448. doi:https://doi.org/10.3390/nu9050448
- Arendt JFH, Sørensen HT, Horsfall LJ, Petersen I. Elevated vitamin B12 levels and cancer risk in uk primary care: a THIN Database Cohort Study. Cancer Epidemiol Biomarkers Prev. 2019;28(4):814–21. doi:https://doi.org/10.1158/1055-9965.EPI-17-1136
- Lin CH, Lu WC, Wang CW, Chan YC, Chen MK. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement Altern Med. 2013;13:46. doi:https://doi.org/10.1186/1472-6882-13-46
- Zhang JH, Lai FJ, Chen H, Luo J, Zhang R-Y, Bu H-Q, Wang Z-H, Lin H-H, Lin S-Z. Involvement of the phosphoinositide 3-kinase/Akt pathway in apoptosis induced by capsaicin in the human pancreatic cancer cell line PANC-1. Oncol Lett. 2013;5(1):43–8. doi:https://doi.org/10.3892/ol.2012.991
- Pramanik KC, Boreddy SR, Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS One. 2011;6(5):e20151. doi:https://doi.org/10.1371/journal.pone.0020151
- Boreddy SR, Srivastava SK. Pancreatic cancer chemoprevention by phytochemicals. Cancer Lett. 2013;334(1):86–94. doi:https://doi.org/10.1016/j.canlet.2012.10.020
- Lan CY, Chen SY, Kuo CW, Lu CC, Yen GC. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food Drug Anal. 2019;27(4):887–96. doi:https://doi.org/10.1016/j.jfda.2019.07.001
- Lee JH, Lee HB, Jung GO, et al. Effect of quercetin on apoptosis of PANC-1 cells. J Korean Surg Soc. 2013;85(6):249–60. doi:https://doi.org/10.4174/jkss.2013.85.6.249
- Yu D, Ye T, Xiang Y, Shi Z, Zhang J, Lou B, Zhang F, Chen B, Zhou M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. Onco Targets Ther. 2017;10:4719–29. doi:https://doi.org/10.2147/OTT.S136840
- Nwaeburu CC, Bauer N, Zhao Z, et al. Up-regulation of microRNA let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl. Oncotarget. 2016; 7:58367–80. doi:https://doi.org/10.18632/oncotarget.11122
- Nwaeburu CC, Abukiwan A, Zhao Z, Herr I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol Cancer. 2017;16(1):23. doi:https://doi.org/10.1186/s12943-017-0589-8
- Serri C, Quagliariello V, Iaffaioli RV, et al. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: a preliminary in vitro study. J Cell Physiol. 2019;234(4):4959–69. doi:https://doi.org/10.1002/jcp.27297
- Liu ZJ, Xu W, Han J, et al. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anticancer Drugs. 2020;31(7):684–92. doi:https://doi.org/10.1097/CAD.0000000000000933
- Lee J, Lee J, Kim SJ, Kim JH. Quercetin-3-O-glucoside suppresses pancreatic cancer cell migration induced by tumor-deteriorated growth factors in vitro. Oncol Rep. 2016;35(4):2473–9. doi:https://doi.org/10.3892/or.2016.4598
- Angst E, Park JL, Moro A, et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas. 2013;42(2):223–9. doi:https://doi.org/10.1097/MPA.0b013e318264ccae
- Li LY, Luo Y, Lu MD, et al. Cruciferous vegetable consumption and the risk of pancreatic cancer: a meta-analysis. World J Surg Oncol. 2015;13:44. doi:https://doi.org/10.1186/s12957-015-0454-4
- Appari M, Babu KR, Kaczorowski A, Gross W, Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int J Oncol. 2014;45(4):1391–400. doi:https://doi.org/10.3892/ijo.2014.2539
- Lozanovski VJ, Polychronidis G, Gross W, et al. Broccoli sprout supplementation in patients with advanced pancreatic cancer is difficult despite positive effects: results from the POUDER pilot study. Invest New Drugs. 2020;38:776–84. doi:https://doi.org/10.1007/s10637-019-00826-z
- Kallifatidis G, Labsch S, Rausch V, et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19(1):188–95. doi:https://doi.org/10.1038/mt.2010.216
- Naumann P, Liermann J, Fortunato F, et al. Sulforaphane enhances irradiation effects in terms of perturbed cell cycle progression and increased DNA damage in pancreatic cancer cells. PLoS One. 2017;12(7):e0180940. doi:https://doi.org/10.1371/journal.pone.0180940
- Kamal MM, Akter S, Lin CN, Nazzal S. Sulforaphane as an anticancer molecule: mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch Pharm Res. 2020;43(4):371–84. doi:https://doi.org/10.1007/s12272-020-01225-2
- Lee Y-R, Chen M, Lee JD, Zhang J, Lin S-Y, Fu T-M, Chen H, Ishikawa T, Chiang S-Y, Katon J, et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science. 2019;364(6441):eaau0159. doi:https://doi.org/10.1126/science.aau0159
- Pernar CH, Ebot EM, Wilson KM, Mucci LA. The epidemiology of prostate cancer. Cold Spring Harb Perspect Med. 2018;8(12):a030361. doi:https://doi.org/10.1101/cshperspect.a030361
- Di Sebastiano KM, Pinthus JH, Duivenvoorden WCM, Mourtzakis M. Glucose impairments and insulin resistance in prostate cancer: the role of obesity, nutrition and exercise. Obes Rev. 2018;19(7):1008–16. doi:https://doi.org/10.1111/obr.12674
- Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem. 2013;373(1-2):217–27. doi:https://doi.org/10.1007/s11010-012-1493-6
- Kallifatidis G, Rausch V, Baumann B, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58(7):949–63. doi:https://doi.org/10.1136/gut.2008.149039
- Li Y, Go VL, Sarkar FH. The role of nutraceuticals in pancreatic cancer prevention and therapy: targeting cellular signaling, microRNAs, and epigenome. Pancreas. 2015;44(1):1–10. doi:https://doi.org/10.1097/MPA.0000000000000257
- Li Y, Wang Y, Kong R, et al. Dihydroartemisinin suppresses pancreatic cancer cells via a microRNA-mRNA regulatory network. Oncotarget. 2016;7(38):62460–73. doi:https://doi.org/10.18632/oncotarget.11517
- Chen H, Sun B, Pan S, Jiang H, Sun X. Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anticancer Drugs. 2009;20(2):131–40. doi:https://doi.org/10.1097/CAD.0b013e3283212ade
- Wang SJ, Gao Y, Chen H, et al. Dihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett. 2010;293(1):99–108. doi:https://doi.org/10.1016/j.canlet.2010.01.001
- Jia G, Kong R, Ma Z-B, Han B, Wang Y-W, Pan S-H, Li Y-H, Sun B. The activation of c-Jun NH2-terminal kinase is required for dihydroartemisinin-induced autophagy in pancreatic cancer cells. J Exp Clin Cancer Res. 2014;33(1):8. doi:https://doi.org/10.1186/1756-9966-33-8
- Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol. 2012;2012:247597. doi:https://doi.org/10.1155/2012/247597
- Zhou ZH, Chen FX, Xu WR, et al. Enhancement effect of dihydroartemisinin on human γδ T cell proliferation and killing pancreatic cancer cells. Int Immunopharmacol. 2013;17(3):850–7. doi:https://doi.org/10.1016/j.intimp.2013.09.015
- Kong R, Jia G, Cheng ZX, et al. Dihydroartemisinin enhances Apo2L/TRAIL-mediated apoptosis in pancreatic cancer cells via ROS-mediated up-regulation of death receptor 5. PLoS One. 2012;7(5):e37222. doi:https://doi.org/10.1371/journal.pone.0037222
- Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res. 2008;14(17):5519–30. doi:https://doi.org/10.1158/1078-0432.CCR-08-0197
- Parasramka MA, Gupta SV. Synergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cells. J Oncol. 2012;2012:1–8. doi:https://doi.org/10.1155/2012/709739
- Parasramka MA, Ali S, Banerjee S, Deryavoush T, Sarkar FH, Gupta S. Garcinol sensitizes human pancreatic adenocarcinoma cells to gemcitabine in association with microRNA signatures. Mol Nutr Food Res. 2013;57(2):235–48. doi:https://doi.org/10.1002/mnfr.201200297
- Huang CC, Lin CM, Huang YJ, et al. Garcinol downregulates Notch1 signaling via modulating miR-200c and suppresses oncogenic properties of PANC-1 cancer stem-like cells. Biotechnol Appl Biochem. 2017;64(2):165–73. doi:https://doi.org/10.1002/bab.1446
- Saadat N, Akhtar S, Goja A, et al. Dietary garcinol arrests pancreatic cancer in p53 and K-ras conditional mutant mouse model. Nutr Cancer. 2018;70(7):1075–87. doi:https://doi.org/10.1080/01635581.2018.1502327
- Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J Pharmacopuncture. 2017;20(3):179–93. doi:https://doi.org/10.3831/KPI.2017.20.021
- Rauf A, Imran M, Khan IA, et al. Anticancer potential of quercetin: a comprehensive review. Phytother Res. 2018;32(11):2109–30. doi:https://doi.org/10.1002/ptr.6155
- Mahmoud YK, Abdelrazek HMA. Cancer: thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed Pharmacother. 2019;115:108783. doi:https://doi.org/10.1016/j.biopha.2019.108783
- Torres MP, Ponnusamy MP, Chakraborty S, et al. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9(5):1419–31. doi:https://doi.org/10.1158/1535-7163.MCT-10-0075
- Pandita A, Kumar B, Manvati S, et al. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS One. 2014;9(9):e107154. doi:https://doi.org/10.1371/journal.pone.0107154
- Mu GG, Zhang LL, Li HY, Liao Y, Yu HG. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig Dis Sci. 2015;60(4):1067–80. doi:https://doi.org/10.1007/s10620-014-3394-x
- Khan MA, Tania M, Fu S, Fu J. Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8(31):51907–19. doi:https://doi.org/10.18632/oncotarget.17206
- Relles D, Chipitsyna GI, Gong Q, Yeo CJ, Arafat HA. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv Prev Med. 2016;2016:1–9. doi:https://doi.org/10.1155/2016/1407840
- Pan FP, Zhou HK, Bu HQ, et al. Emodin enhances the demethylation by 5-Aza-CdR of pancreatic cancer cell tumor-suppressor genes P16, RASSF1A and ppENK. Oncol Rep. 2016;35(4):1941–9. doi:https://doi.org/10.3892/or.2016.4554
- Zhang W, Chen H, Liu DL, et al. Emodin sensitizes the gemcitabine-resistant cell line Bxpc-3/Gem to gemcitabine via downregulation of NF-κB and its regulated targets. Int J Oncol. 2013;42(4):1189–96. doi:https://doi.org/10.3892/ijo.2013.1839
- Zhang H, Chen L, Bu HQ, et al. Effects of emodin on the demethylation of tumor-suppressor genes in pancreatic cancer PANC-1 cells. Oncol Rep. 2015;33(6):3015–23. doi:https://doi.org/10.3892/or.2015.3914
- Lin SZ, Wei WT, Chen H, et al. Antitumor activity of emodin against pancreatic cancer depends on its dual role: promotion of apoptosis and suppression of angiogenesis. PLoS One. 2012;7(8):e42146. doi:https://doi.org/10.1371/journal.pone.0042146
- Li N, Wang C, Zhang P, You S. Emodin inhibits pancreatic cancer EMT and invasion by up‑regulating microRNA‑1271. Mol Med Rep. 2018;18(3):3366–74.
- Guo HC, Bu HQ, Luo J, et al. Emodin potentiates the antitumor effects of gemcitabine in PANC-1 pancreatic cancer xenograft model in vivo via inhibition of inhibitors of apoptosis. Int J Oncol. 2012;40(6):1849–57.
- Wang ZH, Chen H, Guo HC, et al. Enhanced antitumor efficacy by the combination of emodin and gemcitabine against human pancreatic cancer cells via downregulation of the expression of XIAP in vitro and in vivo. Int J Oncol. 2011;39(5):1123–31.
- Kim Y-S, Lee Y-M, Oh T-I, Shin D, Kim G-H, Kan S-Y, Kang H, Kim J, Kim B, Yim W, et al. Emodin sensitizes hepatocellular carcinoma cells to the anti-cancer effect of sorafenib through suppression of cholesterol metabolism. IJMS. 2018;19(10):3127. doi:https://doi.org/10.3390/ijms19103127
- Hu L, Cui R, Liu H, Wang F. Emodin and rhein decrease levels of hypoxia-inducible factor-1α in human pancreatic cancer cells and attenuate cancer cachexia in athymic mice carrying these cells. Oncotarget. 2017;8(50):88008–20. doi:https://doi.org/10.18632/oncotarget.21330
- Huang X, Gao Y, Zhi X, Ta N, Jiang H, Zheng J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: evidence from epidemiologic studies. Sci Rep. 2016;6:38936. doi:https://doi.org/10.1038/srep38936
- Jeong Y, Lim JW, Kim H. Lycopene inhibits reactive oxygen species-mediated NF-κB signaling and induces apoptosis in pancreatic cancer cells. Nutrients. 2019;11(4):762. doi:https://doi.org/10.3390/nu11040762
- Lv JC, Wang G, Pan SH, Bai XW, Sun B. Lycopene protects pancreatic acinar cells against severe acute pancreatitis by abating the oxidative stress through JNK pathway. Free Radic Res. 2015;49(2):151–63. doi:https://doi.org/10.3109/10715762.2014.988150
- Zhu R, Chen B, Bai Y, et al. Lycopene in protection against obesity and diabetes: a mechanistic review. Pharmacol Res. 2020;159:104966. doi:https://doi.org/10.1016/j.phrs.2020.104966
- Sahin K, Sahin N, Kucuk O. Lycopene and chemotherapy toxicity. Nutr Cancer. 2010;62(7):988–95. doi:https://doi.org/10.1080/01635581.2010.509838
- van Steenwijk HP, Bast A, de Boer A. The role of circulating lycopene in low-grade chronic inflammation: a systematic review of the literature. Molecules. 2020;25(19):4378. doi:https://doi.org/10.3390/molecules25194378
- Hoang BX, Han B, Shaw DG, Nimni M. Zinc as a possible preventive and therapeutic agent in pancreatic, prostate, and breast cancer. Eur J Cancer Prev. 2016;25(5):457–61. doi:https://doi.org/10.1097/CEJ.0000000000000194
- Costello LC, Franklin RB. Decreased zinc in the development and progression of malignancy: an important common relationship and potential for prevention and treatment of carcinomas. Expert Opin Ther Targets. 2017;21(1):51–66. doi:https://doi.org/10.1080/14728222.2017.1265506
- Jin H, Liu P, Wu Y, et al. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109(9):2946–56. doi:https://doi.org/10.1111/cas.13737
- Li L, Gai X. The association between dietary zinc intake and risk of pancreatic cancer: a meta-analysis. Biosci Rep. 2017;37(3):BSR20170155.
- Wang J, Zhao H, Xu Z, Cheng X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol Med. 2020;17(3):612–25. doi:https://doi.org/10.20892/j.issn.2095-3941.2020.0106
- Costello LC, Franklin RB. Zinc: the wonder drug for the treatment of carcinomas. Acta Sci Cancer Biol. 2020;4(5):33–9.
- Chandran S, Patgiri B, Bedarkar P, Mathat D. Anticancer activity of Yashada Bhasma (bioactive nanoparticles of zinc): a human pancreatic cancer cell line study. Ayu. 2019;40(1):58–63. doi:https://doi.org/10.4103/ayu.AYU_239_17
- Shakri AR, Zhong TJ, Ma W, Coker C, Kim S, Calluori S, Scholze H, Szabolcs M, Caffrey T, Grandgenett PM, et al. Upregulation of ZIP14 and altered zinc homeostasis in muscles in pancreatic cancer cachexia. Cancers (Basel). 2019;12(1):3. doi:https://doi.org/10.3390/cancers12010003
- Mohammad K, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27(1):8–20. doi:https://doi.org/10.1177/0884533611433534
- Katayama K. Zinc and protein metabolism in chronic liver diseases. Nutr Res. 2020;74:1–9. doi:https://doi.org/10.1016/j.nutres.2019.11.009
- Han X, Li J, Brasky TM, et al. Antioxidant intake and pancreatic cancer risk: the VITamins And Lifestyle (VITAL) study. Cancer. 2013;119(7):1314–20. doi:https://doi.org/10.1002/cncr.27936
- Alexander MS, Cullen JJ. Treating pancreatic cancer: more antioxidants more problems?Expert Rev Gastroenterol Hepatol. 2018;12(9):849–51. doi:https://doi.org/10.1080/17474124.2018.1494572
- Wang L, Wang J, Liu X, et al. Association between selenium intake and the risk of pancreatic cancer: a meta-analysis of observational studies. Biosci Rep. 2016;36(5):e00395.
- Chatterjee S, Combs GF, Jr, Chattopadhyay A, Stolzenberg-Solomon R. Serum selenium and pancreatic cancer: a prospective study in the Prostate, Lung, Colorectal and Ovarian Cancer Trial cohort. Cancer Causes Control. 2019;30(5):457–64. doi:https://doi.org/10.1007/s10552-019-01147-5
- Jacobs ET, Lance P, Mandarino LJ, et al. Selenium supplementation and insulin resistance in a randomized, clinical trial. BMJ Open Diabetes Res Care. 2019;7(1):e000613. doi:https://doi.org/10.1136/bmjdrc-2018-000613
- Ashrafizadeh M, Ahmadi Z, Samarghandian S, et al. MicroRNA-mediated regulation of Nrf2 signaling pathway: implications in disease therapy and protection against oxidative stress. Life Sci. 2020;244:117329. doi:https://doi.org/10.1016/j.lfs.2020.117329
- Hannon BA, Fairfield WD, Adams B, et al. Use and abuse of dietary supplements in persons with diabetes. Nutr Diabetes. 2020;10(1):14.
- Behar A, Dennouni-Medjati N, Harek Y, et al. Selenium overexposure induces insulin resistance: in silico study. Diabetes Metab Syndr. 2020;14(6):1651–7. doi:https://doi.org/10.1016/j.dsx.2020.08.005
- Schilling K, Larner F, Saad A, et al. Urine metallomics signature as an indicator of pancreatic cancer. Metallomics. 2020;12(5):752–7. doi:https://doi.org/10.1039/d0mt00061b
- Dibaba D, Xun P, Yokota K, White E, He K. Magnesium intake and incidence of pancreatic cancer: the VITamins and Lifestyle study. Br J Cancer. 2015;113(11):1615–21. doi:https://doi.org/10.1038/bjc.2015.382
- Rybarczyk P, Vanlaeys A, Brassart B, Dhennin-Duthille I, Chatelain D, Sevestre H, Ouadid-Ahidouch H, Gautier M. The transient receptor potential melastatin 7 channel regulates pancreatic cancer cell invasion through the Hsp90α/uPA/MMP2 pathway. Neoplasia. 2017;19(4):288–300. doi:https://doi.org/10.1016/j.neo.2017.01.004
- Xie J, Cheng CS, Zhu XY, et al. Magnesium transporter protein solute carrier family 41 member 1 suppresses human pancreatic ductal adenocarcinoma through magnesium-dependent Akt/mTOR inhibition and bax-associated mitochondrial apoptosis. Aging (Albany NY). 2019;11(9):2681–98. doi:https://doi.org/10.18632/aging.101940
- Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR, de Andrade M, Oberg AL, Rabe KG, Anderson KE, Olson JE, Sinha R, et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J Gastrointest Canc. 2013;44(2):152–61. doi:https://doi.org/10.1007/s12029-012-9441-y
- Hruby A, Guasch-Ferré M, Bhupathiraju SN, Manson JE, Willett WC, McKeown NM, Hu FB. Magnesium intake, quality of carbohydrates, and risk of type 2 diabetes: results from three U.S. cohorts. Dia Care. 2017;40(12):1695–702. doi:https://doi.org/10.2337/dc17-1143
- Pan Q, Zhou R, Su M, Li R. The effects of plumbagin on pancreatic cancer: a mechanistic network pharmacology approach. Med Sci Monit. 2019;25:4648–54. doi:https://doi.org/10.12659/MSM.917240
- Hafeez BB, Jamal MS, Fischer JW, Mustafa A, Verma AK. Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-κB signaling pathways. Int J Cancer. 2012;131(9):2175–86. doi:https://doi.org/10.1002/ijc.27478
- Wang F, Wang Q, Zhou ZW, et al. Plumbagin induces cell cycle arrest and autophagy and suppresses epithelial to mesenchymal transition involving PI3K/Akt/mTOR-mediated pathway in human pancreatic cancer cells. Drug Des Devel Ther. 2015;9:537–60.
- Subramaniam D, Kaushik G, Dandawate P, Anant S. Targeting cancer stem cells for chemoprevention of pancreatic cancer. Curr Med Chem. 2018;25(22):2585–94. doi:https://doi.org/10.2174/0929867324666170127095832
- Pandey K, Tripathi SK, Panda M, Biswal BK. Prooxidative activity of plumbagin induces apoptosis in human pancreatic ductal adenocarcinoma cells via intrinsic apoptotic pathway. Toxicol in Vitro. 2020;65:104788. doi:https://doi.org/10.1016/j.tiv.2020.104788
- Duraipandy N, Dharunya G, Lakra R, Korapatti PS, Syamala Kiran M. Fabrication of plumbagin on silver nanoframework for tunable redox modulation: implications for therapeutic angiogenesis. J Cell Physiol. 2019;234(8):13110–27. doi:https://doi.org/10.1002/jcp.27981
- Nambiar D, Prajapati V, Agarwal R, Singh RP. In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer Lett. 2013;334(1):109–17. doi:https://doi.org/10.1016/j.canlet.2012.09.004
- Zhang X, Jiang J, Chen Z, Cao M. Silibinin inhibited autophagy and mitochondrial apoptosis in pancreatic carcinoma by activating JNK/SAPK signaling. Pathol Res Pract. 2019;215(9):152530. doi:https://doi.org/10.1016/j.prp.2019.152530
- Shukla SK, Dasgupta A, Mehla K, et al. Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget. 2015;6(38):41146–61. doi:https://doi.org/10.18632/oncotarget.5843
- Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144–9. doi:https://doi.org/10.4254/wjh.v6.i3.144
- Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah SA, Khalil AT. Plant-derived anticancer agents: a green anticancer approach. Asian Pac J Trop Biomed. 2017;7(12):1129–50. doi:https://doi.org/10.1016/j.apjtb.2017.10.016
- Guo P, Cai C, Wu X, et al. An insight into the molecular mechanism of berberine towards multiple cancer types through systems pharmacology. Front Pharmacol. 2019;10:857.
- Park SH, Sung JH, Chung N. Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Mol Cell Biochem. 2014;394(1-2):209–15. doi:https://doi.org/10.1007/s11010-014-2096-1
- Park SH, Sung JH, Kim EJ, Chung N. Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Braz J Med Biol Res. 2015;48(2):111–9. doi:https://doi.org/10.1590/1414-431X20144293
- Liu J, Liu P, Xu T, et al. Berberine induces autophagic cell death in acute lymphoblastic leukemia by inactivating AKT/mTORC1 signaling. Drug Des Devel Ther. 2020;14:1813–23. doi:https://doi.org/10.2147/DDDT.S239247
- Kisfalvi K, Sinnett-Smith J, Young SH, Eibl G, Rozengurt E. Berberine inhibits the growth of human pancreatic cancer cells in vitro and in vivo. Gastroenterology. 2012;142(5):S-907. doi:https://doi.org/10.1016/S0016-5085(12)63521-5
- Akula SM, Candido S, Libra M, et al. Abilities of berberine and chemically modified berberines to interact with metformin and inhibit proliferation of pancreatic cancer cells. Adv Biol Regul. 2019;73:100633. doi:https://doi.org/10.1016/j.jbior.2019.04.003
- Abrams SL, Follo MY, Steelman LS, et al. Abilities of berberine and chemically modified berberines to inhibit proliferation of pancreatic cancer cells. Adv Biol Regul. 2019;71:172–82. doi:https://doi.org/10.1016/j.jbior.2018.10.003
- Piao XM, Huo Y, Kang JP, Mathiyalagan R, Zhang H, Yang DU, Kim M, Yang DC, Kang SC, Wang YP. Diversity of ginsenoside profiles produced by various processing technologies. Molecules. 2020;25(19):4390. doi:https://doi.org/10.3390/molecules25194390
- Yun TK, Choi SY. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prev. 1995;4(4):401–8.
- Elshafay A, Tinh NX, Salman S, et al. Ginsenoside Rk1 bioactivity: a systematic review. PeerJ. 2017;5:e3993. doi:https://doi.org/10.7717/peerj.3993
- Wang L, Xu J, Yan Y, Liu H, Li F. Synthesis of gold nanoparticles from leaf Panax notoginseng and its anticancer activity in pancreatic cancer PANC-1 cell lines. Artif Cells Nanomed Biotechnol. 2019;47(1):1216–23. doi:https://doi.org/10.1080/21691401.2019.1593852
- Cheung SS, Tai J, Hasman D, Ou D, Warnock GL. Inhibition of human pancreatic cancer cell proliferation by Devil’s club oplopanax horridus and its polyacetylene bioactive compound. Nutr Cancer. 2015;67(6):954–64. doi:https://doi.org/10.1080/01635581.2015.1055367
- Qian M, Yi L, Song-Lin L, Jie Y, Ping-Hu Z, Qiang W. Chemical profiles and anticancer effects of saponin fractions of different polarity from the leaves of Panax notoginseng. Chin J Nat Med. 2014;12(1):30–7. doi:https://doi.org/10.1016/S1875-5364(14)60006-6
- Hao M, Wang W, Zhao Y, Zhang R, Wang H. Pharmacokinetics and tissue distribution of 25-hydroxyprotopanaxadiol, an anti-cancer compound isolated from Panax ginseng, in athymic mice bearing xenografts of human pancreatic tumors. Eur J Drug Metab Pharmacokinet. 2011;35(3-4):109–13. doi:https://doi.org/10.1007/s13318-010-0022-9
- Wang P, Zhang L, Yao J, Shi Y, Li P, Ding K. An arabinogalactan from flowers of Panax notoginseng inhibits angiogenesis by BMP2/Smad/Id1 signaling. Carbohydr Polym. 2015;121:328–35. doi:https://doi.org/10.1016/j.carbpol.2014.11.073
- Ju C, Jeon SM, Jun HS, Moon CK. Diol-ginsenosides from Korean Red Ginseng delay the development of type 1 diabetes in diabetes-prone biobreeding rats. J Ginseng Res. 2020;44(4):619–26. doi:https://doi.org/10.1016/j.jgr.2019.06.001
- Lee S, Park J-M, Jeong M, Han Y-M, Go EJ, Ko WJ, Cho JY, Kwon CI, Hahm KB. Korean red ginseng ameliorated experimental pancreatitis through the inhibition of hydrogen sulfide in mice. Pancreatology. 2016;16(3):326–36. doi:https://doi.org/10.1016/j.pan.2016.02.012
- Chian S, Zhao Y, Xu M, Yu X, Ke X, Gao R, Yin L. Ginsenoside Rd reverses cisplatin resistance in non-small-cell lung cancer A549 cells by downregulating the nuclear factor erythroid 2-related factor 2 pathway. Anticancer Drugs. 2019;30(8):838–45. doi:https://doi.org/10.1097/CAD.0000000000000781
- Wang SH, Wang YC, Nie YL, et al. Antiproliferative activity of the Chinese medicinal compound, delisheng, compared with rg3 and gemcitabine in HepG2 cells. Indian J Pharm Sci. 2013;75(5):578–84.
- Wang W, Wang H, Rayburn ER, Zhao Y, Hill DL, Zhang R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. Br J Cancer. 2008;98(4):792–802. doi:https://doi.org/10.1038/sj.bjc.6604227
- Miller AL, Garcia PL, Yoon KJ. Developing effective combination therapy for pancreatic cancer: an overview. Pharmacol Res. 2020;155:104740. doi:https://doi.org/10.1016/j.phrs.2020.104740
- Elwood PC, Pickering JE, Morgan G, Galante J, Weightman AL, Morris D, Longley M, Mason M, Adams R, Dolwani S, et al. Systematic review update of observational studies further supports aspirin role in cancer treatment: time to share evidence and decision-making with patients?PLoS One. 2018;13(9):e0203957. doi:https://doi.org/10.1371/journal.pone.0203957
- Hua H, Zhang H, Kong Q, Wang J, Jiang Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med Res Rev. 2019;39(1):114–45. doi:https://doi.org/10.1002/med.21514
- Costa AC, Reina-Couto M, Albino-Teixeira A, Sousa T. Aspirin and blood pressure: effects when used alone or in combination with antihypertensive drugs. Rev Port Cardiol. 2017;36(7-8):551–67. doi:https://doi.org/10.1016/j.repc.2017.05.008
- García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PLoS One. 2016;11(8):e0160046. doi:https://doi.org/10.1371/journal.pone.0160046
- Risch HA, Lu L, Streicher SA, Wang J, Zhang W, Ni Q, Kidd MS, Yu H, Gao Y-T. Aspirin use and reduced risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(1):68–74. doi:https://doi.org/10.1158/1055-9965.EPI-16-0508
- Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. 2020;31(5):558–68. doi:https://doi.org/10.1016/j.annonc.2020.02.012
- Mitrugno A, Sylman JL, Ngo ATP, Pang J, Sears RC, Williams CD, McCarty OJT. Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: implications for the oncoprotein c-MYC. Am J Physiol Cell Physiol. 2017;312(2):C176–C189. doi:https://doi.org/10.1152/ajpcell.00196.2016
- Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, Lu Z. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer. 2018;18(1):288. doi:https://doi.org/10.1186/s12885-018-4156-5
- Zhang Y, Liu L, Fan P, et al. Aspirin counteracts cancer stem cell features, desmoplasia and gemcitabine resistance in pancreatic cancer. Oncotarget. 2015;6(12):9999–10015. doi:https://doi.org/10.18632/oncotarget.3171
- Choi JH, Lee SH, Huh G, Chun JW, You MS, Paik WH, Ryu JK, Kim Y-T. The association between use of statin or aspirin and pancreatic ductal adenocarcinoma: a nested case-control study in a Korean nationwide cohort. Cancer Med. 2019;8(17):7419–30. doi:https://doi.org/10.1002/cam4.2617
- Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi:https://doi.org/10.1016/S0140-6736(10)62110-1
- Zhang YP, Wan YD, Sun YL, Li J, Zhu RT. Aspirin might reduce the incidence of pancreatic cancer: a meta-analysis of observational studies. Sci Rep. 2015;5(1):15460. doi:https://doi.org/10.1038/srep15460
- Plassmeier L, Knoop R, Waldmann J, Kesselring R, Buchholz M, Fichtner-Feigl S, Bartsch DK, Fendrich V. Aspirin prolongs survival and reduces the number of Foxp3+ regulatory T cells in a genetically engineered mouse model of pancreatic cancer. Langenbecks Arch Surg. 2013;398(7):989–96. doi:https://doi.org/10.1007/s00423-013-1105-2
- Ou YQ, Zhu W, Li Y, et al. Aspirin inhibits proliferation of gemcitabine-resistant human pancreatic cancer cells and augments gemcitabine-induced cytotoxicity. Acta Pharmacol Sin. 2010;31(1):73–80. doi:https://doi.org/10.1038/aps.2009.172
- Bassani-Sternberg M, Digklia A, Huber F, et al. A Phase Ib study of the combination of personalized autologous dendritic cell vaccine, aspirin, and standard of care adjuvant chemotherapy followed by nivolumab for resected pancreatic adenocarcinoma: a proof of antigen discovery feasibility in three patients. Front Immunol. 2019;10:1832.
- Gou S, Cui P, Li X, et al. Low concentrations of metformin selectively inhibit CD133+ cell proliferation in pancreatic cancer and have anticancer action. PLoS One. 2013;8(5):e63969. doi:https://doi.org/10.1371/journal.pone.0063969
- Zhou PT, Li B, Liu FR, et al. Metformin is associated with survival benefit in pancreatic cancer patients with diabetes: a systematic review and meta-analysis. Oncotarget. 2017;8(15):25242–50. doi:https://doi.org/10.18632/oncotarget.15692
- Dong YW, Shi YQ, He LW, Cui XY, Su PZ. Effects of metformin on survival outcomes of pancreatic cancer: a meta-analysis. Oncotarget. 2017;8(33):55478–88. doi:https://doi.org/10.18632/oncotarget.18233
- Griss T, Vincent EE, Egnatchik R, et al. Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol. 2015;13(12):e1002309. doi:https://doi.org/10.1371/journal.pbio.1002309
- Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and mTOR signaling. Transl Oncol. 2013;6(6):649–59. doi:https://doi.org/10.1593/tlo.13556
- Incio J, Suboj P, Chin SM, et al. Metformin reduces desmoplasia in pancreatic cancer by reprogramming stellate cells and tumor-associated macrophages. PLoS One. 2015;10(12):e0141392. doi:https://doi.org/10.1371/journal.pone.0141392
- Han H, Hou Y, Chen X, et al. Metformin-induced stromal depletion to enhance the penetration of gemcitabine-loaded magnetic nanoparticles for pancreatic cancer targeted therapy. J Am Chem Soc. 2020;142(10):4944–54. doi:https://doi.org/10.1021/jacs.0c00650
- Peng M, Darko KO, Tao T, et al. Combination of metformin with chemotherapeutic drugs via different molecular mechanisms. Cancer Treat Rev. 2017;54:24–33. doi:https://doi.org/10.1016/j.ctrv.2017.01.005
- Zhou HY, Yao XM, Chen XD, et al. Mechanism of metformin enhancing the sensitivity of human pancreatic cancer cells to gem-citabine by regulating the PI3K/Akt/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(23):10283–9.
- Li X, Li T, Liu Z, Gou S, Wang C. The effect of metformin on survival of patients with pancreatic cancer: a meta-analysis. Sci Rep. 2017;7(1):5825. doi:https://doi.org/10.1038/s41598-017-06207-x
- Suzuki K, Takeuchi O, Suzuki Y, Kitagawa Y. Mechanisms of metformin’s anti‑tumor activity against gemcitabine‑resistant pancreatic adenocarcinoma. Int J Oncol. 2019;54(2):764–72.
- Qian W, Li J, Chen K, et al. Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer. Life Sci. 2018;208:253–61. doi:https://doi.org/10.1016/j.lfs.2018.07.046
- Fasih A, Elbaz HA, Hüttemann M, Konski AA, Zielske SP. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res. 2014;182(1):50–9. doi:https://doi.org/10.1667/RR13568.1
- Shi Y, He Z, Jia Z, Xu C. Inhibitory effect of metformin combined with gemcitabine on pancreatic cancer cells in vitro and in vivo. Mol Med Rep. 2016;14(4):2921–8. doi:https://doi.org/10.3892/mmr.2016.5592
- Candido S, Abrams SL, Steelman L, et al. Metformin influences drug sensitivity in pancreatic cancer cells. Adv Biol Regul. 2018;68:13–30. doi:https://doi.org/10.1016/j.jbior.2018.02.002
- Baron B, Wang Y, Maehara S, et al. Resistance to gemcitabine in the pancreatic cancer cell line KLM1-R reversed by metformin action. Anticancer Res. 2015;35(4):1941–9.
- Zechner D, Albert AC, Bürtin F, Vollmar B. Metformin inhibits gemcitabine induced apoptosis in pancreatic cancer cell lines. J Cancer. 2017;8(10):1744–9. doi:https://doi.org/10.7150/jca.17972
- Chai X, Chu H, Yang X, et al. Metformin increases sensitivity of pancreatic cancer cells to gemcitabine by reducing CD133+ cell populations and suppressing ERK/P70S6K signaling. Sci Rep. 2015;5:14404. doi:https://doi.org/10.1038/srep14404
- Braghiroli MI, de Celis Ferrari AC, Pfiffer TE, et al. Phase II trial of metformin and paclitaxel for patients with gemcitabine-refractory advanced adenocarcinoma of the pancreas. Ecancermedicalscience. 2015;9:563. doi:https://doi.org/10.3332/ecancer.2015.563
- Reni M, Dugnani E, Cereda S, et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase II trial. Clin Cancer Res. 2016;22(5):1076–85. doi:https://doi.org/10.1158/1078-0432.CCR-15-1722
- Djamgoz M, Plant J. 2014. Beat cancer: how to regain control of your health and your life. London: Vermilion (Ebury Publishing).
- Zhang D, Wu J, Liu S, Zhang X, Zhang B. Network meta-analysis of Chinese herbal injections combined with the chemotherapy for the treatment of pancreatic cancer. Medicine (Baltimore). 2017;96(21):e7005. doi:https://doi.org/10.1097/MD.0000000000007005
- Ladas EJ, Kroll DJ, Oberlies NH, et al. A randomized, controlled, double-blind, pilot study of milk thistle for the treatment of hepatotoxicity in childhood acute lymphoblastic leukemia (ALL). Cancer. 2010;116(2):506–13. doi:https://doi.org/10.1002/cncr.24723

