ABSTRACT
Idiopathic intracranial hypertension (IIH) predominantly affects reproductive-aged females with obesity. However, the prevalence and impact of a healthy weight body mass index (BMI) at disease presentation is not known. This study aimed to evaluate the visual and headache outcomes stratified by the presenting BMI. This was a longitudinal prospective cohort study (IIH Life) based on a tertiary neuro-ophthalmology IIH service, University Hospitals Birmingham NHS Trust, United Kingdom, recruiting consecutive patients living with IIH between 2012 and 2021. Those with a presenting BMI were included. The outcome measures included visual outcomes of LogMAR visual acuity, Humphrey visual field perimetric mean deviation (PMD), optical coherence tomography (OCT) measurements, and headache outcomes of frequency, severity, and Headache Impact Test-6 score. Three hundred seventy-five people with IIH and a documented baseline BMI. About 3.7% of the entire cohort had a healthy weight BMI at IIH presentation and 15.5% BMI < 30 kg/m2. The baseline PMD was worse in patients without obesity; however, OCT papilloedema measures were similar. The presence of obesity was associated with a small but significant greater worsening in visual acuity but slower macular ganglion cell layer loss. There was no impact on PMD or papilloedema prognosis related to baseline obesity. The headache outcomes showed heterogeneity, with worse baseline headache frequency in patients with obesity. No BMI group was associated with worse headache outcomes. Patients with a healthy weight BMI or those without obesity at baseline make up a small proportion of IIH patients. BMI at presentation does not appear to influence long-term visual or headache outcomes.
Introduction
Idiopathic intracranial hypertension (IIH) predominantly affects reproductive-aged females with obesity.Citation1,Citation2 IIH is a disease of raised intracranial pressure, which can lead to chronic headaches, visual loss, and cognitive dysfunction.Citation3–8 Atypical IIH is a term given to people who meet the diagnostic criteria for IIH but do not fit the typical phenotype, including males or females living without obesity.Citation3,Citation9 Obesity is defined by the World Health Organisation as a body mass index (BMI) ≥30 kg/m2,Citation10 which is reduced to 27.5 kg/m2 in some ethnic groups due to increased cardiometabolic risk at a lower BMI.Citation11,Citation12
The adiposity associated with IIH is centripetalCitation13,Citation14 and is correlated with lumbar puncture opening pressure.Citation13 A two-fold increase in cardiovascular disease risk has been found in people living with IIH, in comparison to age-, sex- and BMI-matched controls.Citation2 Other metabolic complications are associated with IIH, such as diabetes mellitus, gestational diabetes, pre-eclampsia, and arterial hypertension.Citation2,Citation15,Citation16 In animal studies, both high-fat diet and IIH-associated inflammatory factor-exposed female rats showed increased CSF secretion.Citation17 The adipocytes in IIH appear to be metabolically primed for lipogenesisCitation18 with an additional insulin and leptin resistant phenotype in excess of that driven by obesity alone.Citation18,Citation19 IIH is also associated with hypertestosteronism in females, independent of obesity, and testosterone increases CSF secretion through the Na+/K+-ATPase pump in the choroid plexus.Citation20 There is also a debate in the literature about whether IIH is caused by problems with CSF secretion or drainage (including cerebral venous stenosis), or a combination of both, and multiple factors are likely to influence both these mechanisms.Citation21,Citation22
Currently, the only disease-modifying therapy for IIH is weight loss,Citation3,Citation23 since weight loss shown to be associated with disease remission.Citation24–26 In the IIH weight trial, those with a BMI ≥35 kg/m2 disease remission, defined as normalisation of intracranial pressure (ICP), may require up to 24% weight loss.Citation27
The presence of a healthy weight BMI on IIH and associated pathophysiology has previously been studied in a single retrospective study, which found better visual outcomes in those with a healthy weight BMI as compared to those with an increased BMI.Citation9 The lack of literature in this area may be because a healthy weight BMI at IIH diagnosis may be uncommon. The metabolic alterations related to obesity have been thought to be the main pathological drivers of IIH given the improvement associated with weight loss, what is not known is that, in the subset of IIH with a healthy weight BMI, there may be a different underlying pathogenesis. Therefore, in a rare condition, despite its rising prevalence, it would be difficult to adequately statistically power a trial to assess differing interventions in this subset.Citation9,Citation28,Citation29
This study is the first to prospectively assess the prognosis in IIH in a cohort of people with a healthy weight BMI. The hypothesis is that IIH patients without obesity would have different visual and headache outcomes compared to typically obese IIH counterparts.
Methods
The methodologyCitation5 of this prospective observational cohort study has been published and ethically approved by NHS National Research Ethics Committee (14/LO/1208), IIH LIFE study. All the patients included in this study attended a specialist IIH clinic (neuro-ophthalmology and neurology joint clinic) at a single tertiary neuroscience centre (University Hospitals Birmingham NHS Foundation Trust (UHB), United Kingdom). All the patients gave written informed consent to participate in this study. The consecutive data were collected between April 2012 and September 2021, for all visits for each patient.
Eligible patients included those who met either a definite or probable diagnosis for IIH based on the 2013 revised diagnostic criteria for IIHCitation30 and had a baseline BMI recorded.
The revised diagnostic criteria required for a definite diagnosis of IIH (adapted from Friedman et al.Citation31 include:
the presence of papilloedema,
a normal neurological examination (except sixth nerve palsy),
normal brain parenchyma on neuroimaging (no hydrocephalus, mass, structural lesion, or meningeal enhancement), with exclusion of venous sinus thrombosis,
normal CSF constituents, and
an elevated lumbar puncture opening pressure ≥25 cm CSF.Citation30
A probable IIH diagnosis could be made in a typical patient, where criteria A to D are met. Patients with secondary causes for raised intracranial pressure or IIH without papilloedema were excluded.Citation3,Citation31 Secondary causes excluded were cerebral venous sinus thrombosis, mass lesions, infective causes, and medical contributors such as profound anaemia and regular/recent use of tetracyclines. Appropriate imaging was performed in all patients either after first visit or at their previous hospital.
This study stratified patients according to their BMI status at their first visit (baseline) in the specialist clinic. Patients were initially categorised as having (≥30 kg/m2) or not having obesity (<30 kg/m2). Then, subsequently by World Health Organisation (WHO) BMI categories,Citation10: healthy weight BMI (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obesity class 1 (30.0–34.9 kg/m2) and obesity class 2, and above (≥35.0 kg/m2). As IIH has now been recognised as an obesity related disease by the National Institute of Health and Clinical Excellence in the UK, with regard to criteria for referral to a weight management speciality clinic obesity class 2 and above was used as the highest grouping.Citation12 BMI groups were not adjusted by ethnicity.
Visual outcomes were: LogMAR visual acuity (measured using Logarithm of the Minimum Angle of Resolution), Humphrey visual field perimetric mean deviation (PMD) (24–2 SITA standard) and optical coherence tomography (OCT, Heidelberg Spectralis™) measures of average global peripapillary retinal nerve fibre layer thickness (RNFL), automatically measured total retinal thickness (TRT) (by the software on the RNFL circle and extracted as TRT) and macular ganglion cell layer (GCL) volume (1, 2.22, 3.45 mm ETDRS plot with the volume measurement as given by the software which would include all nine sectors, by the pre-set macular volume and/or posterior pole methods). To ensure accuracy of segmentation of the retinal layers in moderate-to-severe papilloedema, manual segmentation of RNFL and TRT was performed in peripapillary scans and in cross-sectional slices of optic disc scans for the basement membrane (BM) and inner limiting layer (ILM), where appropriate. During the course of the study, the scanning protocol for GCL changed; however, excellent correlation has previously been published between the macular volume and posterior pole measures (Pearson correlation 0.98).Citation5
Headache outcomes were clinically assessed using the following indices: monthly headache days (days/month); monthly migraine-like headache days (days/month); headache severity (0–10 numerical rating scale, where 0 is no pain and 10 equates to the most severe); and headache disability using the Headache Impact Test-6 (HIT-6) score (score is between 36 and 78).
The methods for data collection and statistical analysis have previously been reported.Citation5 The statistical analysis was performed using R v4.1.0.Citation32 Continuous variables were reported as mean (standard deviation (SD)) and categorical variables as number (percentage). Lme4Citation33 used for regression modelling with continuous form of the dependent variables assumed and independent modelling. Statistical significance is indicated by 95% confidence intervals and is met when the intervals do not cross zero or overlap. Adjustments were made for multiple testing. LOESS (locally weighted scatterplot smoothing) graphs were created prior to regression analysis to ascertain the variables’ relationships and any trends.
The study was guided by patient and public involvement of IIHUK, a national patient charity (Registered Charity in England and Wales no 1,143,522 & Scotland SCO43294) that supports carers and patients living with IIH. They provided advice and guidance for the development of the IIH Life questionnaire.
Results
In this nine-year longitudinal cohort study, 375 people with IIH and a documented baseline BMI were prospectively recruited. A healthy weight BMI was uncommon at presentation representing 3.7% of the cohort (14/375). Patients with higher BMI categories were more common with 11.7% (44/375) classed as overweight, 20% (75/375) obesity class 1, and 64.5% (242/375) obesity class 2 and above (). Therefore, 15.5% (58/375) had a BMI <30 kg/m2, compared to 84.5% (317/375) with obesity. Patients with a healthy weight BMI had the lowest diagnostic CSF opening pressure ().
Table 1. Baseline table by baseline BMI.
Cerebrospinal fluid diversion surgery rates (during the course of follow-up for this study) were lower for those with a healthy weight BMI at presentation (7%) compared to patients with IIH and higher BMI, with 3.5% of overweight, 19% of obesity class 1, and 15% of obesity class 2+ (). However, this was not statistically significantly different between any group. Similar proportions of patients were treated with ICP lowering medications in all groups: 57% of healthy weight BMI, 61% of overweight, 63% obesity class 1 and 54% obesity class 2 + .
The duration of follow-up in this cohort seen within our specialist service was a median of 17 months (range 1–86, interquartile range 6–37) for those with at least one follow-up visit. This was analogous to the BMI groups: healthy weight BMI (21 (3–33, 6–25) months), overweight (14 (1–86, 7–37) months), obesity class 1 (19 (1–70, 6–40) months), and obesity class 2 and above (17 (1–80, 7–38) months).
Presence of obesity and visual outcomes
The presence of obesity did not, however, influence baseline visual acuity, papilloedema measured by OCT (RNFL and TRT), or macular ganglion cell layer volume (). A BMI <30 kg/m2 (normal or overweight BMI) at baseline visit was associated with worse baseline Humphrey visual field PMD than patients with obesity (−5.65 dB (95% CI: −7.51, −3.80) versus −3.20 dB (−3.98, −2.41)) ().
Table 2. Baseline estimates and trajectory from regression modelling for obesity BMI cut-off (30 kg/m2).
Visual outcomes over time, however, were affected differently (). Visual acuity had a worse trajectory in those with a BMI <30 kg/m2 compared to patients with obesity at baseline (, ). There was a small additional statistically significant worsening (increase) in acuity of 0.004 logunits/month (0.002, 0.0061) in the former group compared to the latter, although this would likely remain clinically imperceptible. The PMD trajectory did not differ between the groups with similar long-term outcomes ().
Figure 1. Longitudinal visual data from baseline visit for IIH patients categorised by whether BMI < 30 or ≥30 kg/m2, and LOESS smoothers added to show trends across the categories.
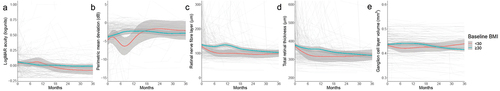
Papilloedema OCT trajectories were similar between the groups (); however, in the initial six months following the baseline visit, patients with a BMI <30 kg/m2 had a more rapid additional reduction in RNFL of 10.79 µm/month (−26.51, +4.92) and TRT of 5.90 µm/month (−36.29, +24.48) (). This was not statistically significant. The macular ganglion cell layer volume declined slightly quicker in patients without obesity (), with a statistically significant greater decline of 0.000559 mm3/month (−0.001068, −0.00005).
Presence of obesity and headache outcomes
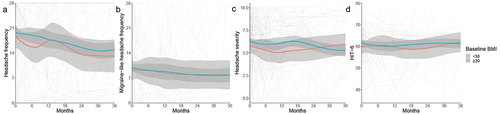
The headache and migraine-like headache frequencies were high in the IIH cohort at baseline (, ). The mean baseline frequency was lower in patients with BMI <30 kg/m2 (15.95 days/month (12.02, 19.87) versus 20.32 (18.63, 22.00) with obesity) (), and this was significantly lower in the non-obesity group by −4.37 days/month (95%CI: −8.64, −0.11)). The migraine-like headache frequency did not significantly differ between patients with or without obesity (9.16 (7.69, 10.63) and 8.41 (5.06, 11.77), respectively) (, ). Headache severity was moderate in both groups () and did not significantly differ at baseline or in trajectory (). Headaches had a substantial impact on the quality of life as measured by the HIT-6, with very similar scores and confidence intervals (baseline scores 61.57 (58.04, 65.09) and 61.27 (59.74, 62.80) for BMI <30 kg/m2 and ≥30 kg/m2, respectively) (, ). The trajectories were not significantly different between <30 kg/m2 and ≥30 kg/m2 groups for any of the headache outcomes.
Figure 2. Longitudinal headache data from baseline visit for IIH patients categorised by whether BMI <30 or ≥30 kg/m2, and LOESS smoothers added to show trends across the categories.
BMI groups and outcomes
PMD was significantly worse (more negative) at baseline in those patients with a healthy weight BMI (−9.0 (−12.8, −5.2)) compared to those with obesity class 1 or class 2+ (−2.8 (−4.4, −1.2) and −3.3 (−4.2, −2.4), respectively) (). It was also worse, but not significantly so, compared to the overweight group (−4.5 (−6.6, −2.4)) (, ). OCT TRT papilloedema measurement was significantly worse for overweight patients compared to those with healthy weight BMI, 80.54 µm (12.22, 148.86) higher, but not for either of the obesity groups (). Macular GCL at baseline in the healthy weight BMI group was 0.3998 mm3 (0.3556, 0.4441) () and was significantly higher for both overweight and obesity class 2+ categories compared to healthy weight BMI, 0.0508 (0.0012, 0.1004) and 0.0451 (0, 0.0903), respectively, but not obesity class 1 (). When compared to a healthy weight BMI, no other group significantly differed at baseline for visual acuity or OCT RNFL outcomes ().
Figure 3. Longitudinal visual data from baseline visit for IIH patients categorised baseline BMI group, and LOESS smoothers added to show trends across the categories.
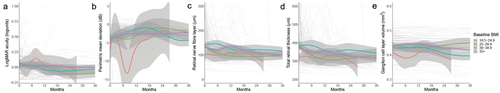
Table 3. Baseline estimates and trajectory for visual and headache outcomes by baseline BMI from regression modelling.
Trajectories for all visual outcomes did not differ compared to healthy weight BMI at baseline group (, ).
Higher headache frequency was reported on average in those with higher baseline BMIs (, ). There was no group significantly different from healthy weight BMI at baseline () due to wide confidence intervals. Similarly, migraine-like headache frequency, headache severity, and HIT6 did not differ by BMI group at baseline ().
Figure 4. Longitudinal headache data from baseline visit for IIH patients categorised by baseline BMI group, and LOESS smoothers added to show trends across the categories.
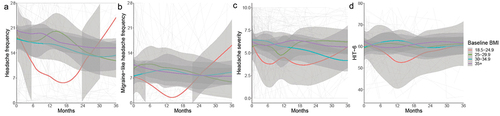
Trajectories for headache outcomes were similar for all outcomes (, ) apart from headache severity in the overweight group which had a worse trajectory by 0.15 units/month (0.02, 0.29) compared to healthy weight BMI patients.
Discussion
In this prospective longitudinal cohort of people living with IIH, a healthy weight BMI at presentation is uncommon. The visual outcomes are similar between BMI groups; however, the improvement in papilloedema was observed to be more rapid in patients without obesity. The headache frequency was higher in IIH patients with obesity as compared to those without, and over time, the headache outcomes were similar between BMI groups.
The prevalence of a healthy weight BMI in this study is similar to a previous large study of IIH.Citation9 In this cohort, those with a healthy weight BMI, including those without obesity, had worse visual field function at baseline, despite similar OCT papilloedema measures. This could potentially reflect a delay in diagnosis due to a lower suspicion of IIH in a person with a healthy weight BMI. The OCT imaging measures have been shown to be a more acute marker of disease activity,Citation5 as well as a better correlation to intracranial pressure than clinical symptoms.Citation34,Citation35 Thus, the comparable OCT measures, with worse PMD, may indicate a delayed presentation of the disease.Citation5 The trend seen in CSF diversion surgery rates may reflect that patients with a higher BMI are more likely to present in fulminant IIH and thus require surgery, although this requires further evaluation in other cohorts as was not statistically significant in this cohort. A statistically significant difference was seen in the visual acuity and macular ganglion cell layer volume trajectories between those with obesity and those without (). However, the clinical significance is uncertain due to the small magnitude of this variation which is likely not be perceivable by the patient or of clinical significance.
The trajectories of the headache outcomes were not significantly different between <30 kg/m2 and ≥30 kg/m2. This is consistent in that baseline BMI potentially and has a greater influence on visual outcomes than headache outcomes which may be influenced additionally by more significant factors as previous demonstrated in the literature.Citation5
Although IIH predominantly affects reproductive-aged women with obesity,Citation3,Citation5,Citation36,Citation37 there are atypical presentations.Citation9,Citation38 Defining patients by whether obesity was present at baseline visit is clinically useful for potential management of their metabolic syndrome, although this involves an arbitrary cut off at 30 kg/m2 which could miss some patients who could benefit. It is important for clinicians to note that baseline BMI did not impact the trajectory of visual or headache outcomes, while for some this could be reassuring; it is important to actively manage those who do not have obesity.
The only current disease-modifying therapy for the systemic manifestations of IIH is weight loss.Citation3,Citation5,Citation23,Citation26,Citation27 Its role in patients with a normal baseline BMI is not yet understood. This would be a useful future analysis as a recent analysis of IIH Life database found that a change in BMI was a prognostic marker for visual outcomes.Citation5 In other scenarios such as pregnancy, the role of weight loss is not clear.Citation16,Citation39
Although the outcomes based on BMI are similar for people living with IIH, it should not be concluded that the underlying pathogenesis is the same. IIH has been shown to be associated with hypertestosteronism independent of obesity,Citation20 with metabolic priming towards lipogenesis.Citation18,Citation19 In people with obesity, significant weight loss of 24% body weight is often required for disease remission.Citation25,Citation27,Citation40 Potential pathogenesis in lean IIH could be inferred from the phenotypically similar condition of polycystic ovarian syndrome (PCOS). In PCOS, one study reported that 42% of the patients had a healthy weight BMI (defined as BMI 18.5–23 kg/m2) with a significantly lower prevalence in lean PCOS of deranged lipid profiles, impaired glucose tolerance test, and insulin resistance.Citation41 The treatment for insulin resistance with metformin has been shown to lessen hyperandrogenism in PCOSCitation42 and has also been shown to potentially be of benefit in IIH.Citation43 A limitation of the IIH life database is that metformin use is not routinely documented, and therefore, no further analysis could be done. In IIH with a normal baseline BMI, there may be a role for dietary modifications and pharmacotherapy to avoid future weight gain as this has been shown to be of benefit in lean PCOS.Citation44 Anecdotally, clinicians have recommended weight loss in those with a normal BMI where there was a direct history of weight gain prior to IIH diagnosis, as long as not deemed detrimental to the patient’s health.Citation3 There is a clear need for further research into the metabolic profile of IIH in those living without obesity, as potentially, there could be a similar metabolic complication risk profile despite the lower body weight, and this could warrant an alternative management approach.
IIH with a healthy weight BMI, or without obesity, may more closely reflect another condition, spaceflight associated neuro-ocular syndrome (SANS) given the healthy weight BMIs observed in astronauts. SANS occurs during long duration spaceflight and risks include hyperopic refractive error shifts, acute visual loss, and cognitive changes. The OCT changes have been noted in SANS with some similarities to IIH, and an increase in the total retinal thickness of 20 µm is used as the earliest indication of optic disc oedema.Citation45,Citation46 Translational benefits for both conditions could be established if the underlying pathophysiology was determined.
The major limitation of this study was the relatively small sample size for healthy weight BMI patients. This was similar to a previous studyCitation9 and likely reflective of the rarity of atypical IIH in an already rare condition. The baseline BMI was used to categorise patients; however, lead-time bias may have been induced with some initial visits being elsewhere prior to tertiary neuro-ophthalmology referral. To minimise this, we created a surrogate marker of disease duration which was defined as the time from the diagnostic lumbar puncture to the first encounter at baseline visit, adjusted for it in the multivariate regression modelling. It is acknowledged that patient reported symptom onset is not recorded within the IIH Life, and this may predate diagnosis by an uncertain and variable duration. Visual function assessments are challenging for patients to perform with both intra-visit and inter-visit factors that are known to influence reliability.Citation4,Citation47,Citation48 Given this was a real-world clinical practice study, follow-up intervals were based on the decisions of the attending clinician at each visit and would be influenced by the person’s clinical factors such as disease state, papilloedema severity, and symptomatology. As a result, individuals whose disease was in remission would be more rapidly discharged or their care transferred back to local hospitals. Hence, missing follow-up data increased over the course of time. In addition, some people were lost to follow-up. Therefore, caution in the over interpretation of the long-term outcomes as seen in the LOESS smoother curve should be applied due to the reducing sample size over the course of the study. The diagnosis of IIH is based on the revised diagnostic criteria.Citation30 however, due to referral patterns, the cohort contains those with definite and those with probable diagnoses of IIH. It is often challenging to retrieve the initial diagnostic lumbar puncture opening pressure measurement. This analysis prompted and included many further inquiries to external hospital units for this piece of essential clinical data for the clinical record. The lumbar punctures are invasive and can be challenging.Citation49,Citation50 It is therefore important that guideline recommendations are adopted to ensure the accurate recording of the opening pressure and help reduce diagnostic uncertainty.Citation3,Citation48,Citation51
Conclusions
People with a normal BMI make up a minority of people diagnosed with IIH. They appear to have similar visual and headache outcomes to people living with IIH and obesity. While at the baseline visit, people without obesity had worse visual field function but similar papilloedema measures, compared to people living with obesity, and their long-term outcomes were similar. The underlying pathophysiology of those without obesity and in whom IIH develops is yet to be discovered.
Authors’ contributions
MT was involved in data acquisition, data analysis, interpretation of the data, drafting of the work, revisions, and final approval.
VH was involved in data analysis, interpretation of the data, manuscript revisions, and final approval.
SA was involved in interpretation of the data, manuscript revisions, and final approval.
JH was involved in interpretation of the data, manuscript revisions, and final approval.
SPM was involved in concept and designing this project, data acquisition, data analysis, interpretation of the data, manuscript revisions, and final approval.
AJS was involved in concept and designing this project, data acquisition, data analysis, interpretation of the data, manuscript revisions, and final approval.
Ethics statement
This research was ethically approved by NHS National Research Ethics Committee (14/LO/1208), IIH LIFE study, and complies with the Declaration of Helsinki. All patients gave written informed consent to participate in this study.
Acknowledgments
We would like to thank all the people living with IIH who contributed to this study and to IIHUK for their ongoing support of our research.
Disclosure statement
MT and VH report no conflicts. SA reports consultancy fees (Johnson & Johnson). JH reports speaker fees (Novo Nordisk). SPM reports consultancy fees (Invex Therapeutics; Velux Foundation); advisory board fees (AbbVie; Gensight; Invex therapeutics; Ocular Therapeutix) and speaker fees (Heidelberg engineering). AJS reports personal fees from Invex therapeutics during the conduct of the study as well as share option and shareholdings, speaker fees (Novartis; Allergan; Teva UK) and consulting fees (Allergan; Chiesi; Novartis; Lundbeck).
Data availability statement
Professor Sinclair takes full responsibility for the data, the analyses and interpretation, and the conduct of the research. She has full access to all the data and has the right to publish any and all data separate and apart from any sponsor. Proposals for data access should be made to the corresponding author. Reasonable scientifically sound proposals, from appropriately qualified research groups, will provide data beginning 12 months and ending 3 years after the publication of this article to researchers whose proposed use of the data is approved by the corresponding author. Requesters will need to sign a data access agreement, which will cover the terms and conditions of the release of data and will include publication requirements, authorship, acknowledgements, and obligations for the responsible use of data.
Additional information
Funding
References
- Yiangou A, Mollan SP, Sinclair AJ. Idiopathic intracranial hypertension: a step change in understanding the disease mechanisms. Nat Rev Neurol. 2023;19(12):769–785. doi:10.1038/s41582-023-00893-0.
- Adderley NJ, Subramanian A, Nirantharakumar K, et al. Association between idiopathic intracranial hypertension and risk of cardiovascular diseases in women in the United Kingdom. JAMA Neurol. 2019;76(9):1088–1098. doi:10.1001/jamaneurol.2019.1812.
- Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89(10):1088–1100. doi:10.1136/jnnp-2017-317440.
- Grech O, Clouter A, Mitchell JL, et al. Cognitive performance in idiopathic intracranial hypertension and relevance of intracranial pressure. Brain Commun. 2021;3:fcab202. doi:10.1093/braincomms/fcab202.
- Thaller M, Homer V, Hyder Y, et al. The idiopathic intracranial hypertension prospective cohort study: evaluation of prognostic factors and outcomes. J Neurol. 2023;270(2):851–863. doi:10.1007/s00415-022-11402-6.
- Adderley NJ, Subramanian A, Perrins M, Nirantharakumar K, Mollan SP, Sinclair AJ. Headache, opiate use, and prescribing trends in women with idiopathic intracranial hypertension: a population-based matched cohort study. Neurology. 2022;99:e1968–1978. doi:10.1212/WNL.0000000000201064.
- Mollan SP, Wakerley BR, Alimajstorovic Z, et al. Intracranial pressure directly predicts headache morbidity in idiopathic intracranial hypertension. J Headache Pain. 2021;22(1):118. doi:10.1186/s10194-021-01321-8.
- Hyder YF, Homer V, Thaller M, et al. Defining the phenotype and prognosis of people with idiopathic intracranial hypertension after cerebrospinal fluid diversion surgery. Am J Ophthalmol. 2023;250:70–81. doi:10.1016/j.ajo.2023.01.016.
- Bruce BB, Kedar S, Van Stavern GP, Corbett JJ, Newman NJ, Biousse V. Atypical idiopathic intracranial hypertension: normal BMI and older patients. Neurology. 2010;74(22):1827–1832. doi:10.1212/WNL.0b013e3181e0f838.
- World Health Organisation. Body mass index – BMI [Online]. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations. 2010. Accessed July 27, 2023.
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi:10.1016/S0140-6736(03)15268-3.
- National Institute for Health and Care Excellence. Obesity: identification, assessment and management clinical guideline [CG189]. 2014.
- Hornby C, Botfield H, O’Reilly MW, et al. Evaluating the fat distribution in idiopathic intracranial hypertension using dual-energy X-ray absorptiometry scanning. Neuroophthalmology. 2018;42:99–104. doi:10.1080/01658107.2017.1334218.
- Hornby C, Mollan SP, Botfield H, O’Reilly MW, Sinclair AJ. Metabolic concepts in idiopathic intracranial hypertension and their potential for therapeutic intervention. J Neuroophthalmol. 2018;38:522–530. doi:10.1097/WNO.0000000000000684.
- Fric R, Pripp AH, Eide PK. Cardiovascular risk factors in Chiari malformation and idiopathic intracranial hypertension. Brain Behav. 2017;7:e00677. doi:10.1002/brb3.677.
- Thaller M, Mytton J, Wakerley BR, Mollan SP, Sinclair AJ. Idiopathic intracranial hypertension: evaluation of births and fertility through the hospital episode statistics dataset. BJOG. 2022;129(12):2019–2027. doi:10.1111/1471-0528.17241.
- Alimajstorovic Z, Pascual-Baixauli E, Hawkes CA, et al. Cerebrospinal fluid dynamics modulation by diet and cytokines in rats. Fluids Barriers CNS. 2020;17(1):10. doi:10.1186/s12987-020-0168-z.
- Westgate CS, Botfield HF, Alimajstorovic Z, et al. Systemic and adipocyte transcriptional and metabolic dysregulation in idiopathic intracranial hypertension. JCI Insight. 2021;6:e145346. doi:10.1172/jci.insight.145346.
- Westgate CSJ, Markey K, Mitchell JL, et al. Increased systemic and adipose 11β-HSD1 activity in idiopathic intracranial hypertension. Eur J Endocrinol. 2022;187:323–333. doi:10.1530/EJE-22-0108.
- O’Reilly MW, Westgate CS, Hornby C, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight. 2019;4. doi:10.1172/jci.insight.125348.
- Thaller M, Sinclair AJ, Tsermoulas G, Mollan SP. Considerations from venous stenosis to metabolic underpinnings in idiopathic intracranial hypertension. Cephalalgia. 2023;43(6):3331024231184445. doi:10.1177/03331024231184445.
- Fargen KM, Coffman S, Torosian T, Brinjikji W, Nye BL, Hui F. “Idiopathic” intracranial hypertension: an update from neurointerventional research for clinicians. Cephalalgia. 2023;43(4):3331024231161323. doi:10.1177/03331024231161323.
- Mollan SP, Tahrani AA, Sinclair AJ. The potentially modifiable risk factor in idiopathic intracranial hypertension: body weight. Neurol Clin Pract. 2021;11:e504–e507. doi:10.1212/CPJ.0000000000001063.
- Thaller M, Tsermoulas G, Sun R, Mollan SP, Sinclair AJ. Negative impact of COVID-19 lockdown on papilloedema and idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2021;92(7):795–797. doi:10.1136/jnnp-2020-325519.
- Mollan SP, Mitchell JL, Ottridge RS, et al. Effectiveness of bariatric surgery vs community weight management intervention for the treatment of idiopathic intracranial hypertension: a randomized clinical trial. JAMA Neurol. 2021;78(6):678–686. doi:10.1001/jamaneurol.2021.0659.
- Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341(jul07 2):c2701. doi:10.1136/bmj.c2701.
- Mollan SP, Mitchell JL, Yiangou A, et al. Association of amount of weight lost after bariatric surgery with intracranial pressure in women with idiopathic intracranial hypertension. Neurology. 2022;99:e1090–e1099. doi:10.1212/WNL.0000000000200839.
- Wall M, Kupersmith MJ, Kieburtz KD, et al. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol. 2014;71(6):693–701. doi:10.1001/jamaneurol.2014.133.
- Sharma B, Seervi N, Sharma V, Panagariya A, Goel D. Clinical and radiological profile of 122 cases of idiopathic intracranial hypertension in a tertiary care centre of India: an observational study. Neurol India. 2022;70(2):704–709. doi:10.4103/0028-3886.344644.
- Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–1165. doi:10.1212/WNL.0b013e3182a55f17.
- Mollan SP, Chong YJ, Grech O, Sinclair AJ, Wakerley BR. Current perspectives on idiopathic intracranial hypertension without papilloedema. Life (Basel). 2021;11(6). doi:10.3390/life11060472.
- R Core Team. R: a language and environment for statistical computing [Online]. https://www.R-project.org/.
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi:10.18637/jss.v067.i01.
- Vijay V, Mollan SP, Mitchell JL, et al. Using optical coherence tomography as a surrogate of measurements of intracranial pressure in idiopathic intracranial hypertension. JAMA Ophthalmol. 2020;138(12):1264–1271. doi:10.1001/jamaophthalmol.2020.4242.
- Mitchell JL, Lyons HS, Walker JK, et al. The effect of exenatide on intracranial pressure in idiopathic intracranial hypertension: a randomised clinical trial. Brain. 2023;146:1821–1830. doi:10.1093/brain/awad003.
- Mollan SP, Hornby C, Mitchell J, Sinclair AJ. Evaluation and management of adult idiopathic intracranial hypertension. Pract Neurol. 2018;18(6):485–488. doi:10.1136/practneurol-2018-002009.
- Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ. The expanding burden of idiopathic intracranial hypertension. Eye (Lond). 2019;33(3):478–485. doi:10.1038/s41433-018-0238-5.
- Chen BS, Newman NJ, Biousse V. Atypical presentations of idiopathic intracranial hypertension. Taiwan J Ophthalmol. 2021;11(1):25–38. doi:10.4103/tjo.tjo_69_20.
- Thaller M, Wakerley BR, Abbott S, Tahrani AA, Mollan SP, Sinclair AJ. Managing idiopathic intracranial hypertension in pregnancy: practical advice. Pract Neurol. 2022;22(4):295–300. doi:10.1136/practneurol-2021-003152.
- Aguiar M, Frew E, Mollan SP, et al. The health economic evaluation of bariatric surgery versus a community weight management intervention analysis from the idiopathic intracranial hypertension weight trial (IIH:WT). Life (Basel). 2021;11(5):409. doi:10.3390/life11050409.
- Saxena P, Prakash A, Nigam A, Mishra A. Polycystic ovary syndrome: is obesity a sine qua non? A clinical, hormonal, and metabolic assessment in relation to body mass index. Indian J Endocrinol Metab. 2012;16:996–999. doi:10.4103/2230-8210.103011.
- Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. J Clin Endocrinol Metab. 1997;82:4075–4079. doi:10.1210/jc.82.12.4075.
- Glueck CJ, Golnik KC, Aregawi D, Goldenberg N, Sieve L, Wang P. Changes in weight, papilledema, headache, visual field, and life status in response to diet and metformin in women with idiopathic intracranial hypertension with and without concurrent polycystic ovary syndrome or hyperinsulinemia. Transl Res. 2006;148:215–222. doi:10.1016/j.trsl.2006.05.003.
- Toosy S, Sodi R, Pappachan JM. Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018;17(2):277–285. doi:10.1007/s40200-018-0371-5.
- Laurie SS, Lee SMC, Macias BR, et al. Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol. 2020;138(2):165–172. doi:10.1001/jamaophthalmol.2019.5261.
- Scott RA, Tarver WJ, Brunstetter TJ, Urquieta E. Optic nerve tortuosity on earth and in space. Aerosp Med Hum Perform. 2020;91:91–97. doi:10.3357/AMHP.5406.2020.
- Mollan SP, Bodoza S, Ni Mhealoid A, et al. Visual field pointwise analysis of the idiopathic intracranial hypertension weight trial (IIH:WT). Transl Vis Sci Technol. 2023;12:1. doi:10.1167/tvst.12.5.1.
- Mollan SP, Fraser CL, Digre KB, et al. Guidelines of the international headache society for controlled clinical trials in idiopathic intracranial hypertension. Cephalalgia. 2023;43(8):3331024231197118. doi:10.1177/03331024231197118.
- Mollan SP, Momin SNA, Khatkar PS, Grech O, Sinclair AJ, Tsermoulas G. A neuro-ophthalmologist’s guide to advances in intracranial pressure measurements. Eye Brain. 2023;15:113–124. doi:10.2147/EB.S404642.
- Scotton WJ, Mollan SP, Walters T, et al. Characterising the patient experience of diagnostic lumbar puncture in idiopathic intracranial hypertension: a cross-sectional online survey. BMJ Open. 2018;8(5):e020445. doi:10.1136/bmjopen-2017-020445.
- Hoffmann J, Mollan SP, Paemeleire K, Lampl C, Jensen RH, Sinclair AJ. European headache federation guideline on idiopathic intracranial hypertension. J Headache Pain. 2018;19(1):93. doi:10.1186/s10194-018-0919-2.

