Abstract
Abstract: The mushroom body is required for a variety of behaviors of Drosophila melanogaster. Different types of intrinsic and extrinsic mushroom body neurons might underlie its functional diversity. There have been many GAL4 driver lines identified that prominently label the mushroom body intrinsic neurons, which are known as “Kenyon cells.” Under one constant experimental condition, we analyzed and compared the the expression patterns of 25 GAL4 drivers labeling the mushroom body. As an internet resource, we established a digital catalog indexing representative confocal data of them. Further more, we counted the number of GAL4-positive Kenyon cells in each line. We found that approximately 2,000 Kenyon cells can be genetically labeled in total. Three major Kenyon cell subtypes, the γ, α′/β′, and α/β neurons, respectively, contribute to 33, 18, and 49% of 2,000 Kenyon cells. Taken together, this study lays groundwork for functional dissection of the mushroom body.
INTRODUCTION
“For approaching the enormous complexity of the insect brain one may choose first to study the sensory and motor periphery in the hope to finally work one's way up to the central processing stages of the brain or, alternatively, one may parachute in the midst of the jungle, experimentally altering the brain and try to understand the concomitant changes in behavior”
The mushroom body (MB) is a major landmark in the “jungle” of the Drosophila brain (Fahrbach, Citation2006; Heisenberg, Citation2003), consisting of thousands of intrinsic and extrinsic neurons. Kenyon cells, the second-order olfactory interneurons in Drosophila, constitute the majority of the intrinsic neurons. Their cell bodies form a pair of quadruple clusters at the dorsal posterior cortex. Their extensive dendritic arborizations contribute to the globular structure beneath the cell bodies, called the calyx, in which the collaterals of the olfactory projection neurons terminate (Stocker et al., Citation1990). The axon bundle of the Kenyon cells further project anteriorly through the pedunculus to the lobes. The lobes of the MB are considered as the main output site of Kenyon cells, but also receive many inputs from extrinsic neurons (Ito et al., Citation1998; Tanaka et al., Citation2008; Johard et al., Citation2008).
Various behavioral and physiological functions that the MB is known to support range from olfactory learning to decision making under uncertain conditions. Its structural heterogeneity may anatomically reflect the organization of circuits that are required to achieve an array of distinct behavioral functions in one brain structure.
Drosophila Kenyon cells are roughly classified into three subtypes by their projections in the lobes: the γ, α′/β′, and α/β lobe neurons in order of birth (Crittenden et al., Citation1998; Jefferis et al., Citation2002). The γ-neurons project only to the medial lobe, while the α′/β′ and α/β neurons bifurcate at the anterior end of the pedunculus to project to the medial and vertical lobes (Crittenden et al., Citation1998; Ito et al., Citation1998). Recent studies revealed that α′/β′ and α/β lobes harbor further subdivisions (Strausfeld et al., Citation2003; Tanaka et al., Citation2008; Yang et al., Citation1995). In addition to their morphological distinction, these subtypes are differentiated with respect to their gene expression, neurotransmitter systems, connectivity to extrinsic neurons, and behavioral functions (Keene & Waddell, Citation2007; Strausfeld et al., Citation2003; Tanaka et al., Citation2008).
Compared to the other insect species with large numbers of Kenyon cells (Leitch & Laurent, Citation1996; Neder, Citation1959; Witthöft, Citation1967), the advantages of studying the Drosophila MB are its smaller size with a conserved, layered cellular organization (Mobbs, Citation1984; Rybak & Menzel, Citation1993; Sjöholm et al., Citation2006; Strausfeld, Citation2002; Strausfeld et al., Citation2003) and its amenability to genetic manipulation (Venken & Bellen, Citation2005). This allows counting, rather than only estimating, the total number of Kenyon cells, and indeed, various studies have reported total numbers based on direct counting or extrapolation (Balling et al., Citation1987; Hinke, Citation1961; Ito & Hotta, Citation1992; Lee et al., Citation1999; Mader, Citation2004; Technau & Heisenberg, Citation1982; Technau, Citation1984). However, the average numbers in these studies range approximately from 1,000 to 2,500. Although the precise number is required to construct quantitative network models of the Drosophila MB and to assess the integrity of the genetic manipulation of Kenyon cells, no study has seriously addressed this discrepancy.
Laboratory members at the Biocenter in Würzburg have often witnessed Martin Heisenberg's extraordinary interest in determining the total number of Kenyon cells. His “favorite” number was 2,500, which was published in the early days of his laboratory (e.g., Technau & Heisenberg, Citation1982). Martin asserted that counting the fiber number in cross-sections of the posterior part of the pedunculus from electron micrographs resulted in the least errors, because 1) the fiber number represents the cell number, since Kenyon cell axons do not bifurcate at that level, and 2) the majority of thin and round fibers represent Kenyon cells.
We decided to reexamine the total number of Kenyon cells by using the GAL4/UAS system, since the cell type can be readily discerned. An array of GAL4 drivers have been identified with prominent expression in the entire or specific subsets of the MB (MB-GAL4; ). The specificity of GAL4 expression inside and outside the MB is critical, especially for the interpretation of functional manipulation when using MB-GAL4s (Ito et al., Citation2003). Here, we show the expression patterns of 25 different MB-GAL4s in the brain and the subesophageal ganglion under one constant experimental condition. We present a catalog that indexes the confocal micrographs of all analyzed drivers. By counting GAL4-positive Kenyon cells, we here endeavor to reveal the total number of Kenyon cells. Further, we demonstrate the numerical composition of the Kenyon cell subtypes.
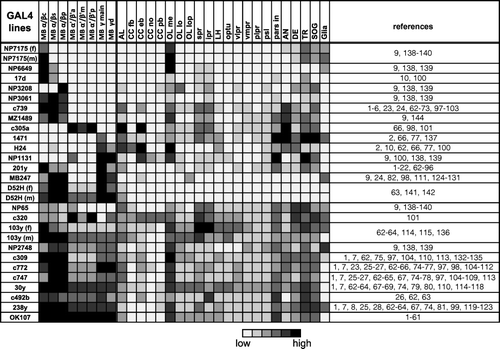
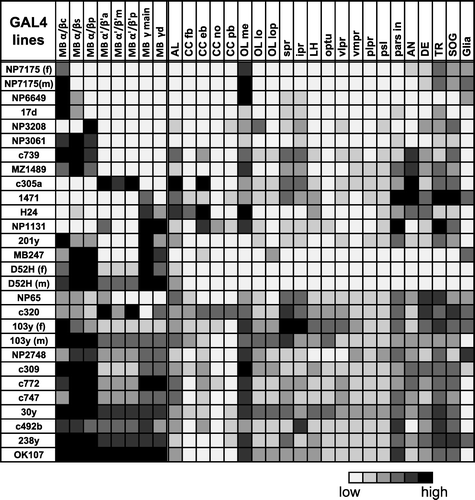
Figure 1 Expression pattern of 25 GAL4 lines. Summary of the expression levels of 25 MB-GAL4s in various brain areas defined by anti-Synapsin immunostaining. Gray scale indicates subjectively evaluated signal intensity. Note that a higher level of fluorescent signals in the certain brain area can result from larger population of GAL4 expressing cells and/or stronger GAL4 expression in each cell. MB, mushroom body; c, core subdivision: s, surface subdivision; p, posterior subdivision; a, anterior subdivision; m, middle subdivision; p, posterior subdivision; d, dorsal subdivision; AL, antennal lobe; CC, central complex; fb, fan-shaped body; eb, ellipsoid body; no, noduli; pb, protocerebral bridge; OL, optic lobe; me, medulla; lo, lobula; lop, lobula plate; spr, superior protocerebrum; ipr, inferior protocerebrum; LH, lateral horn; optu, optic tubercle; vlpr, ventrolateral protocerebrum; plpr, posteriorlateral protocerebrum; vmpr, ventromedial protocerebrum; psl, posterior slope; pars in, pars intercerebralis; AN, antennal nerve; DE, deutocerebrum; TR, tritocerebrum; SOG, subesophageal ganglion. References where these GAL4 drivers have been used are listed in Supplementary .
MATERIALS AND METHODS
Fly Strains
Fly stocks were grown on standard Drosophila medium (cornmeal, agar, molasses, yeast, and nipagin) under a constant light-dark cycle (14/10 hours) at 25°C and 60% relative humidity. Twenty-five GAL4 strains were selected from the literature (). These drivers have been used for behavioral, developmental, physiological, and/or anatomical studies of Kenyon cells (Supplemental ). To visualize the GAL4 expressing cells, males of these GAL4 lines were crossed with females of a reporter strain carrying multiple copies of UAS-mCD8::GFP (G3). No specific labeling was detected in the absence of a GAL4 driver (data not shown). For the GAL4 drivers on the X chromosome (D52H, NP3208, NP65, and NP7175), females of each driver were crossed with G3 males to detect the expression in F1 males. For the double drivers, one driver strain was crossed with another driver, and then F1 males heterozygous for two GAL4 insertions were crossed with G3 females. The genotypes of F2 progenies were determined with characteristic expression of each GAL4 line.
Immunohistochemistry
We examined flies between 5 and 10 days after eclosion and analyzed at least 2 males and 3 females for each cross. The brains were dissected in Ringer's solution, fixed in phosphate-buffered saline (PBS) containing 2% formaldehyde and 0.3% Triton X-100 (PBT; Sigma, St. Louis, Missouri, USA) for 1 hour at room temperature and subsequently rinsed with PBT three times (3x 10 minutes). After being blocked with PBT containing 3% normal goat serum (Sigma) for 1 hour at room temperature, the brains were incubated with the primary antibodies in PBT at 4°C overnight. The GAL4-expressing cells and overall neuropils were stained with the rabbit polyclonal antibody against green fluorescent protein (GFP) (1:1000; Molecular Probes, Eugene, Oregon, USA; A6455) and the mouse monoclonal antibody against Synapsin, a presynaptic protein (3C11; 1:20) (Klagges et al., Citation1996), respectively. The brains were washed with PBT for 20 minutes three times and incubated with secondary antibodies in the blocking solution at 4°C overnight. The employed secondary antibodies were Alexa Fluor488–conjugated goat antirabbit (1:1000 or 1:500; Molecular Probes) and Cy3-conjugated (1:250) goat antimouse IgG. Finally, the brains were rinsed with PBT (3×20 minutes + 1×1 hour) and mounted in Vectashield (Vector, Burlingame, California, USA). For the quantitative analysis of the cell numbers, we stained the brains of different genotypes in the same tube. Their genotypes were post-hoc identified according to their expression patterns.
Confocal Microscopy
Frontal optical sections of whole-mount brains were taken with confocal microscopy (Leica SP1, Leica, Wetzlar, Germany). Image stacks were collected at 1.5-µm intervals with a 20X objective lens to cover an entire brain. For cell counting, we collected confocal stacks at 0.8-µm intervals with a 40X objective lens. The posterior region of the MB was zoomed at such a magnification that all the GAL4-expressing cell bodies are in a frame. The posterior MBs in the left and right hemispheres were separately scanned and analyzed. For the quantitative analysis, brains were scanned with comparable intensity and offset. Images of the confocal stacks were analyzed with the open-source software (Image-J; National Institutes of Health, Bethesda, Maryland, USA). To avoid overlooking diffuse and/or weakly labeled structures, all pictures were repeatedly examined by different experimenters.
Cell Counting
Confocal stacks of the magnified cell-body region were first subjected to automatic marking of nuclei by a combination of ImageJ plug-ins: “Particle analyzer” and “Cell counter”. Particle analyzer, which was customized to recognize the circles (nuclei) by intensity thresholding at multiple different levels, automatically detected the nuclei of GAL4-expressing cells in each confocal slice. According to the coordinate information, the centers of the nuclei of the labeled cells were marked by “Cell counter.” Subsequently, all the stacks were reexamined manually to correct the errors in the automatic detection. Labeled, but not Kenyon, cells or unlabeled Kenyon cells surrounded by labeled cells were discriminated by their size, shape, location, fine neurites to the calyx, and reporter signals from the cytosol and membrane. Typically, one nucleus spanning different confocal sections was automatically marked twice or three times in the close vicinity. By manually checking duplicated markers in neighboring slices, we subtracted these redundant counts from the final number. Experimenters counted the numbers of Kenyon cells without information on genotypes. There was no statistical difference between experimenters, which was verified by counting the same samples (data not shown). The numbers of labeled Kenyon cells were statistically compared by using multiple pair-wise comparisons (t-test, followed by Bonferroni correction).
RESULTS
Collection of the Expression of MB-GAL4 Drivers
The interpretation of genetic manipulation using MB-GAL4 crucially depends on the expression pattern inside and outside of the MBs. We, thus, systematically reexamined the GAL4 expression of many published MB-GAL4 drivers under one constant experimental condition. We selected 25 MB-GAL4 drivers from the literature and evaluated their expression patterns with confocal microscopy (; Supplemental ).
As a reporter gene, we chose multiple copies of UAS-mCD8::GFP inserted in different genomic loci (G3). Since CD8 is a membrane protein, GFP is targeted predominantly to the plasma membrane of GAL4-expressing cells. After immunostaining, we frequently noticed that these multiple copies of the reporter construct enabled visualization of weakly labeled cells that had been overlooked in previous reports. Moreover, G3 is advantageous for counting the number of labeled cells, since the nuclei of the GAL4-expressing cells are clearly outlined (e.g., A).
A previous study showed that different reporter genes, different staining conditions, and the insertion site of a reporter gene may influence the results (Ito et al., Citation2003). G3 can minimize such insertion-specific biases, since there are multiple different insertions of UAS-mCD8::GFP.
The expression patterns of MB-GAL4s in the brain and subesophageal ganglion (SOG) are summarized in and Supplemental . By relative signal intensity, we subjectively ranked the degree to which fibers were resolved in various neuropils. Therefore, these ranks are neither to indicate the absolute intensities nor to compare the expression levels between different GAL4 drivers, but are rather to compare the signal intensities within a sample. MB-GAL4s vary, to a great extent, in terms of the specificity within the MB and the expression outside the MB (; supplemental ). In the following sections, we describe the expression pattern of these drivers. Based on the expression pattern within the MB of female samples, we categorized them into five groups: MB-GAL4s labeling the α/β lobes, α′/β′ lobes, γ lobes, multiple lobes, and all of the lobes. Except for three lines (NP7175, D52H, and 103y), expression pattern was very similar in males and females.
As an Internet resource, we indexed representative confocal data of all analyzed driver strains (“der Pilz”; http://mushroombody.net). This web-based database is publicly available. Thus, readers are encouraged to download and scrutinize the raw data by themselves, rather than solely relying on the subjectively ranked expression and brief description here.
Drivers Labeling the α/β Neurons
GAL4 expression in NP7175, NP6649, 17d, NP3208, NP3061, c739 and MZ1489 in the MB was restricted to the α/β neurons. NP7175, NP6649, 17d, and NP3208 labeled limited subdivisions (A–2D). GAL4-positive Kenyon cells in NP7175, 17d, and NP6649 preferentially innervated the core of the α/β lobes (α/βc). Given the variable occupancy, the α/βc lobes are likely to consist of multiple populations with concentric organization (; see also Tanaka et al., Citation2008). Within the MB, NP3208 has its expression specifically in the posterior division of the α/β lobes (α/βp), as previously described (Tanaka et al., Citation2008). The α/βp neurons have dendritic arbors specifically in the accessory calyx, where the projection neurons do not directly terminate (Tanaka et al., Citation2008). In NP7175, reporter signals inside and outside the MB differed between the sexes (Compare A and A). With differences in expression levels among subdivisions, c739, MZ1489, and NP3061 seemed to drive GAL4 expression in most, if not all, subdivisions of the α/β lobes (E–2G).
Figure 2 Drivers labeling the α/β neurons. Stereopairs show reconstructions of MB-GAL4s preferentially labeling the α/β neurons. The applied color illustrates the depth (see scale bar [25 µm] for the color code). Because of the space limitation, labels of the neuropils have been omitted from all figures. See also original confocal stacks for detail (http://mushroombody.net). Expression pattern was indistinguishable between males and females, unless stated. (A) Female NP7175 exclusively innervated the α/βc lobes in the MB. It also labeled the subesophageal ganglion, the cells in the medulla, and the processes in the tritocerebrum. See A for the expression pattern in the male. (B) NP6649 strongly labeled the α/βc, while the expression in the α/βs was weak. As in NP7175, the cells in the medulla were strongly labeled. It also has expression in the dorsal giant interneuron (DGI) (Ito et al., Citation1997). (C) As in NP6649, the α/βc were strongly labeled in 17d, while the expression in the α/βs was weak. In addition, weak labeling was occasionally detected in the pars intercerebralis, cells located around the subesophageal ganglion, DGI, and large neurons located around the calyx and protocerebral bridge. One very large neuron located ventrally to the calyx projects anteriorly and terminates in the superior and inferior protocerebrum. Collaterals of this neuron may also project to the deutocerebrum. Similar neurons were labeled by NP3208, c739, MZ1489, NP65, and c492b. Especially in MZ1489, their huge cell bodies are clearly visible (see G). (D) NP3208 specifically innervated the α/βp. The accessory calyx is visible as a protrusion originating from the dorsal calyx. The paired giant neurons located ventral to the calyx (see also the legend of C) and processes in the subesophageal ganglion were labeled. Further faint signals were detected in many neuropils, including the optic lobes. (E) NP3061 labeled all over the α/β lobes. Occasionally, barely detectable signals were in the pars intercerebralis, DGI, tritocerebrum, and subesophageal ganglion. NP3061 contained the least expression outside the MB in all the MB-GAL4s analyzed here. (F) c739 strongly innervated the entire α/β lobes. Outside the MB, it labeled elements in a wide range of neuropils, including local interneurons in the antennal lobe, the antennal nerve, ellipsoid body, a cluster of neurons projecting from/to the optic lobes, and many other cells throughout the brain (see also ). (G) MZ1489 innervated the entire α/β lobe, though the signal in the α/βc was less intense. Outside of the MB, it labeled a wide range of neuropils, including the pars intercerebralis, many cells in the medulla, antennal nerve, paired giant neurons located ventral to the calyx (see also the legend of C), and many other cells throughout the brain (see also ).
![Figure 2 Drivers labeling the α/β neurons. Stereopairs show reconstructions of MB-GAL4s preferentially labeling the α/β neurons. The applied color illustrates the depth (see scale bar [25 µm] for the color code). Because of the space limitation, labels of the neuropils have been omitted from all figures. See also original confocal stacks for detail (http://mushroombody.net). Expression pattern was indistinguishable between males and females, unless stated. (A) Female NP7175 exclusively innervated the α/βc lobes in the MB. It also labeled the subesophageal ganglion, the cells in the medulla, and the processes in the tritocerebrum. See Figure 7A for the expression pattern in the male. (B) NP6649 strongly labeled the α/βc, while the expression in the α/βs was weak. As in NP7175, the cells in the medulla were strongly labeled. It also has expression in the dorsal giant interneuron (DGI) (Ito et al., Citation1997). (C) As in NP6649, the α/βc were strongly labeled in 17d, while the expression in the α/βs was weak. In addition, weak labeling was occasionally detected in the pars intercerebralis, cells located around the subesophageal ganglion, DGI, and large neurons located around the calyx and protocerebral bridge. One very large neuron located ventrally to the calyx projects anteriorly and terminates in the superior and inferior protocerebrum. Collaterals of this neuron may also project to the deutocerebrum. Similar neurons were labeled by NP3208, c739, MZ1489, NP65, and c492b. Especially in MZ1489, their huge cell bodies are clearly visible (see G). (D) NP3208 specifically innervated the α/βp. The accessory calyx is visible as a protrusion originating from the dorsal calyx. The paired giant neurons located ventral to the calyx (see also the legend of C) and processes in the subesophageal ganglion were labeled. Further faint signals were detected in many neuropils, including the optic lobes. (E) NP3061 labeled all over the α/β lobes. Occasionally, barely detectable signals were in the pars intercerebralis, DGI, tritocerebrum, and subesophageal ganglion. NP3061 contained the least expression outside the MB in all the MB-GAL4s analyzed here. (F) c739 strongly innervated the entire α/β lobes. Outside the MB, it labeled elements in a wide range of neuropils, including local interneurons in the antennal lobe, the antennal nerve, ellipsoid body, a cluster of neurons projecting from/to the optic lobes, and many other cells throughout the brain (see also Figure 1). (G) MZ1489 innervated the entire α/β lobe, though the signal in the α/βc was less intense. Outside of the MB, it labeled a wide range of neuropils, including the pars intercerebralis, many cells in the medulla, antennal nerve, paired giant neurons located ventral to the calyx (see also the legend of C), and many other cells throughout the brain (see also Figure 1).](/cms/asset/d7d55611-706d-442a-9bef-20e421ac840d/ineg_a_347339_f0002_b.jpg)
Driver Labeling the α′/β′ Neurons
Among the MB-GAL4s we analyzed, only c305a had selective expression in the α'/β' neurons within the MB ( and ). The expression was detected in all subdivisions in the α′/β′ lobes. NP65 and NP1131 labeled the specific subtype of the α′/β′ lobe (i.e., the α′/β′a neurons), but had additional expressions in other subsets (A and 5E). Therefore, we categorized NP65 to the MB-GAL4s labeling multiple lobes, although the expression within the MB had been reported to be restricted in the α′/β′a neurons (Tanaka et al., Citation2008). Similarly, NP2748 has been described as a line specific for the α′/β′ neurons (Tanaka et al., Citation2008), but we observed reporter signals also in the α/β and γ neurons. These differences in expression are presumably due to the employed reporter genes.
Figure 3 Driver labeling the α′/β′ neurons. Stereopair shows a reconstruction of c305a preferentially labeling the entire α′/β′ lobes. The applied color illustrates the depth (see scale bar [25 µm] for the color code). See also original confocal stacks for detail (http://mushroombody.net). Outside the MB, it labeled broad neuropils, including the local interneurons in the antennal lobe, antennal nerve, ellipsoid body, large paired neurons originating from the subesophageal ganglion, and many other cells throughout the brain (see also ).
![Figure 3 Driver labeling the α′/β′ neurons. Stereopair shows a reconstruction of c305a preferentially labeling the entire α′/β′ lobes. The applied color illustrates the depth (see scale bar [25 µm] for the color code). See also original confocal stacks for detail (http://mushroombody.net). Outside the MB, it labeled broad neuropils, including the local interneurons in the antennal lobe, antennal nerve, ellipsoid body, large paired neurons originating from the subesophageal ganglion, and many other cells throughout the brain (see also Figure 1).](/cms/asset/9b3129d3-7112-47ba-9239-1e55ae2f3867/ineg_a_347339_f0003_b.jpg)
Drivers Labeling the γ-Neurons
H24 and 1471 preferentially marked the γ lobe within the MB (). With G3, H24 additionally labeled the α/β neurons weakly. Also, 1471 had selective expression in the γ neurons of the MB, as originally reported (Isabel et al., Citation2004), although occasionally it seems to drive additional expression in the α/β neurons (Keleman et al., Citation2007). Further, 1471 seemed not to include all of the γ neurons, since the reporter expression in the dorsomedial tip of the γ lobe was conspicuously weak (C and 4D). To distinguish this dorsal subdivision from of the rest of the γ lobe (main part), we named it “γd.” Does this subdivision belong to the γ neurons? We magnified the corresponding region of the γd in MB247, c320, and c305a (E–G). MB247 labeled this part, but not the α′/β′ lobes (E). c320 had innervation there, but not in the rest of the γ lobe (F). c305a labeled the entire α′/β′ lobes, but not the γd (G). These results indicate that the γd is supplied by a subpopulation of the γ neurons, but not the α′/β′ neurons.
Figure 4 Drivers labeling the γ neurons. (A, B) Stereopairs show reconstructions of MB-GAL4s preferentially labeling the γ lobe. The applied color illustrates the depth (see scale bar [25 µm] for the color code). See also original confocal stacks for detail (http://mushroombody.net). (A) In the MB, expression of 1471 was restricted in the γ neurons. Expression in the γd neurons was very weak, if any. It labeled a broad range of neuropils outside of the MB, including the pars intercerebralis, antennal lobe, antennal nerve, and sensory nerves. Large paired neurons located ventrally to the SOG and projecting to the deutocerebrum and/or tritocerebrum were also labeled. Similar neurons are also found in NP65, H24, and 201y. (B) H24 strongly labeled the γ neurons, with extremely weak additional expression in the α/β neurons. Outside the MB, local interneurons in the antennal lobe, the medulla, ellipsoid body, deutocerebrum, and large paired neurons located ventrally to the subesophageal ganglion showed the reporter signals. (C–G) Frontal views of the projections of three consecutive confocal slices, including the γd lobe. In the left panels, the GAL4-positive processes (green) are shown with counterstaining of neuropils (anti-Synapsin; magenta). The right panels show only the reporter channel of the same stacks (black). In these sections, the γ lobe occupies the most dorsal part, while the β lobe occupies the most ventral part. The β′ lobe typically lies in between them. Arrows indicate the medial end of the γd subdivision. 1471 labeled the main part of the γ lobe, but not the γd subdivision (C), whereas the expression both in the γd subdivision and the main part of the γ lobe was seen in H24 and MB247 (D and E). (F) c320 labeled the γd subdivision without labeling the main γ lobe. (G) c305a specifically labeled the α′/β′ lobe, but not the γd subdivision. Scale bar=25 µm for C–G.
![Figure 4 Drivers labeling the γ neurons. (A, B) Stereopairs show reconstructions of MB-GAL4s preferentially labeling the γ lobe. The applied color illustrates the depth (see scale bar [25 µm] for the color code). See also original confocal stacks for detail (http://mushroombody.net). (A) In the MB, expression of 1471 was restricted in the γ neurons. Expression in the γd neurons was very weak, if any. It labeled a broad range of neuropils outside of the MB, including the pars intercerebralis, antennal lobe, antennal nerve, and sensory nerves. Large paired neurons located ventrally to the SOG and projecting to the deutocerebrum and/or tritocerebrum were also labeled. Similar neurons are also found in NP65, H24, and 201y. (B) H24 strongly labeled the γ neurons, with extremely weak additional expression in the α/β neurons. Outside the MB, local interneurons in the antennal lobe, the medulla, ellipsoid body, deutocerebrum, and large paired neurons located ventrally to the subesophageal ganglion showed the reporter signals. (C–G) Frontal views of the projections of three consecutive confocal slices, including the γd lobe. In the left panels, the GAL4-positive processes (green) are shown with counterstaining of neuropils (anti-Synapsin; magenta). The right panels show only the reporter channel of the same stacks (black). In these sections, the γ lobe occupies the most dorsal part, while the β lobe occupies the most ventral part. The β′ lobe typically lies in between them. Arrows indicate the medial end of the γd subdivision. 1471 labeled the main part of the γ lobe, but not the γd subdivision (C), whereas the expression both in the γd subdivision and the main part of the γ lobe was seen in H24 and MB247 (D and E). (F) c320 labeled the γd subdivision without labeling the main γ lobe. (G) c305a specifically labeled the α′/β′ lobe, but not the γd subdivision. Scale bar=25 µm for C–G.](/cms/asset/b9906eb9-dc17-4143-ad90-c2430c8bf352/ineg_a_347339_f0004_b.jpg)
Drivers Labeling Multiple Types of Kenyon Cells
In NP1131, 201y, MB247, D52H (female), NP65, and c320, there was expression in many, but not all, the MB lobes ( and 5). NP1131 selectively labeled the γ and α′/β′a neurons in the MB (A). Also, 201y had expression in the γ and α/βc neurons (B). MB247 and D52H (female) strongly marked the α/β and γ neurons. MB247 and D52H (female) exhibited an unusually low level of background expression (C and D). For both lines, there was very little, if any, expression in α′/β′ neurons. In D52H, the expression showed a sexual difference in the MB. The males of D52H additionally strongly expressed the reporter in the α′/β′ lobes (compare D and B). NP65 and c320 labeled subsets of the α/β and α′/β′ neurons (E and 5F). In NP2748 and female 103y, reporter signals were detectable in the all lobes, although very weak in some subdivisions ( and 5).
Figure 5 Drivers labeling multiple types of Kenyon cells. Stereopairs show reconstructions of MB-GAL4s preferentially labeling the multiple types of Kenyon cells. The applied color illustrates the depth (see scale bar [25 µm] for the color code). Some lines exhibited detectable expression in all types of Kenyon cells, but were categorized into this group, because expression in certain subdivisions was remarkably weak. See also original confocal stacks for detail (http://mushroombody.net). (A) NP1131 labeled the α′/β′a and γ lobes. Additional expression was seen in the ellipsoid body, subesophageal ganglion, pars intercerebralis, large interneurons connecting the optic lobe and the central brain, and other cells distributed in the brain (see also ). (B) 201y labeled the α/βc and γ neurons. Outside the MB, additional expression was seen in the several glomeruli in the antenna lobe, pars intercerebralis, large paired neurons located ventral to the subesophageal ganglion, DGI, and other neurons. (C) MB247 strongly labeled the α/β and γ neurons with very low background expression. Expression in the α/βc was weaker than in the other α/β subdivisions. Additional expression was detected in the cells in the lobula plate and surface glia. (D) Female D52H strongly labeled the α/β and γ neurons with very low background expression. Expression in the α/βc was weaker than in the other α/β subdivisions. See B for the expression pattern in the male. (E) NP65 labeled the α/βs, α/βc, and α′/β′a neurons. We observed reporter signals also in the antennal lobe, deutocerebrum, tritocerebrum, DGI, large paired neurons located ventral to the calyx (see also the legend of C), two large neurons projecting medially to the subesophageal ganglion, and many cells throughout the central brain. (F) In c320, the α/β, α′/β′, and γd lobes were labeled (F). Expression in the α′/β′m neurons was weaker than the rest of α′/β′ subdivisions. Outside the MB, it labeled the majority of neuropils. Among them, relatively strong expressions were observed in surface glia, the protocerebral bridge, subesophageal ganglion, optic lobes, and superior protocerebrum (see also ). (G) Female 103y labeled the α/β neurons strongly. Outside the MB, it labeled the optic lobes, inferior and superior protocerebrum, cells on the subesophageal ganglion, surface glia, and many cells in the posterior cortex. See C for expression pattern in the male. (H) In NP2748, weak reporter signals were observed in all of the lobes. It also labeled the medulla, pars intercerebralis, DGI, surface glia, and many other cells.
![Figure 5 Drivers labeling multiple types of Kenyon cells. Stereopairs show reconstructions of MB-GAL4s preferentially labeling the multiple types of Kenyon cells. The applied color illustrates the depth (see scale bar [25 µm] for the color code). Some lines exhibited detectable expression in all types of Kenyon cells, but were categorized into this group, because expression in certain subdivisions was remarkably weak. See also original confocal stacks for detail (http://mushroombody.net). (A) NP1131 labeled the α′/β′a and γ lobes. Additional expression was seen in the ellipsoid body, subesophageal ganglion, pars intercerebralis, large interneurons connecting the optic lobe and the central brain, and other cells distributed in the brain (see also Figure 1). (B) 201y labeled the α/βc and γ neurons. Outside the MB, additional expression was seen in the several glomeruli in the antenna lobe, pars intercerebralis, large paired neurons located ventral to the subesophageal ganglion, DGI, and other neurons. (C) MB247 strongly labeled the α/β and γ neurons with very low background expression. Expression in the α/βc was weaker than in the other α/β subdivisions. Additional expression was detected in the cells in the lobula plate and surface glia. (D) Female D52H strongly labeled the α/β and γ neurons with very low background expression. Expression in the α/βc was weaker than in the other α/β subdivisions. See Figure 7B for the expression pattern in the male. (E) NP65 labeled the α/βs, α/βc, and α′/β′a neurons. We observed reporter signals also in the antennal lobe, deutocerebrum, tritocerebrum, DGI, large paired neurons located ventral to the calyx (see also the legend of Figure 2C), two large neurons projecting medially to the subesophageal ganglion, and many cells throughout the central brain. (F) In c320, the α/β, α′/β′, and γd lobes were labeled (Figure 3F). Expression in the α′/β′m neurons was weaker than the rest of α′/β′ subdivisions. Outside the MB, it labeled the majority of neuropils. Among them, relatively strong expressions were observed in surface glia, the protocerebral bridge, subesophageal ganglion, optic lobes, and superior protocerebrum (see also Figure 1). (G) Female 103y labeled the α/β neurons strongly. Outside the MB, it labeled the optic lobes, inferior and superior protocerebrum, cells on the subesophageal ganglion, surface glia, and many cells in the posterior cortex. See Figure 7C for expression pattern in the male. (H) In NP2748, weak reporter signals were observed in all of the lobes. It also labeled the medulla, pars intercerebralis, DGI, surface glia, and many other cells.](/cms/asset/b7381959-8a07-4e99-802c-4115fa48c907/ineg_a_347339_f0005_b.jpg)
GAL4 Drivers Labeling All Lobe Systems
c309, c772, c747, 30y, c492b, 238y, and OK107 labeled the α/β, α′/β′, and γ lobes. OK107 covered all the subdivisions of all the lobes, whereas, in the other drivers, the expression in one or more subdivisions of a lobe system was weak or undetectable. For instance, the expression of c309 was strong in α/β and γ lobes, but weak in the α′/β′ lobes (A). All drivers in this group had significant GAL4 expression outside the MB ( and 6). Therefore, functional manipulation of all lobe systems in the MB might require either a combination of selective GAL4 drivers with little additional expression or different MB-GAL4s covering all lobe systems with nonoverlapping expressions outside the MB. Based on the expression in the lobes, it is possible that some of these drivers (e.g., OK107) genetically label all Kenyon cells.
Figure 6 GAL4 strains labeling all of the lobes. Stereopairs show reconstructions of MB-GAL4s labeling all of the lobes. The applied color illustrates the depth (see scale bar [25 µm] for the color code). Since most of the lines in this category exhibited expression in many neuropils, we describe only strong signals here (see also ). See also original confocal stacks for detail (http://mushroombody.net). (A) In the MB, c309 strongly labeled the α/β and γ neurons. We also observed very weak expression in the α′/β′ neurons. Outside the MB, the majority of the labeled neuropils included the antennal lobe, tritocerebrum, subesophageal ganglion, and optic lobes (see also ). Additionally, many different sensory nerves and the cervical connectives were stained. (B) c772 preferentially labeled the α/βp, α/βs, and γ neurons with weaker expression in the α/βc and α′/β′ neurons. It also labeled the optic lobes, ventrolateral protocerebrum, local interneurons in the antennal lobe, and many cells on the subesophageal ganglion. (C) As in c772, c747 labeled the α/βp, α/βs, and γ neurons strongly and the α/βc and α′/β′ neurons weakly. Outside the MB, we observed reporter signals in the optic lobes, antennal nerve, local interneurons in the antennal lobe, pars intercerebralis, and many cells on the subesophageal ganglion. (D) In 30y, all the MB subdivisions were innervated. It also labeled the optic lobes, antennal lobe, pars intercerebralis, and many cells surrounding the subesophageal ganglion. It also labeled a cluster of small neurons that are located dorsolateral to the optic tubercle and that project first ventrally and extend laterally toward the dorsolateral edge of the ventrolateral protocerebrum. Similar neurons were observed in 238y. (E) c492b labeled all types of Kenyon cells, although expression in the γd neurons was relatively weak. Compared to other lines in this category, expressions outside the MB were less pronounced. We observed reporter signals in the pars intercerebralis, local inter neurons in the antennal lobe, deutocerebrum, subesophageal ganglion, and large paired neurons located ventral to the calyx (see also the legend of C). (F) All types of the Kenyon cells were strongly labeled by 238y. Outside the MB, this line labeled the optic lobe, superior protocerebrum, pars intercerebralis, and many neurons surrounding the entire subesophageal ganglion. (G) In OK107, all the subdivisions of the MB were strongly and uniformly labeled. Outside the MB, we observed strong reporter signals in the optic lobe, antennal lobe, pars intercerebralis, and cells on the subesophageal ganglion.
![Figure 6 GAL4 strains labeling all of the lobes. Stereopairs show reconstructions of MB-GAL4s labeling all of the lobes. The applied color illustrates the depth (see scale bar [25 µm] for the color code). Since most of the lines in this category exhibited expression in many neuropils, we describe only strong signals here (see also Figure 1). See also original confocal stacks for detail (http://mushroombody.net). (A) In the MB, c309 strongly labeled the α/β and γ neurons. We also observed very weak expression in the α′/β′ neurons. Outside the MB, the majority of the labeled neuropils included the antennal lobe, tritocerebrum, subesophageal ganglion, and optic lobes (see also Figure 1). Additionally, many different sensory nerves and the cervical connectives were stained. (B) c772 preferentially labeled the α/βp, α/βs, and γ neurons with weaker expression in the α/βc and α′/β′ neurons. It also labeled the optic lobes, ventrolateral protocerebrum, local interneurons in the antennal lobe, and many cells on the subesophageal ganglion. (C) As in c772, c747 labeled the α/βp, α/βs, and γ neurons strongly and the α/βc and α′/β′ neurons weakly. Outside the MB, we observed reporter signals in the optic lobes, antennal nerve, local interneurons in the antennal lobe, pars intercerebralis, and many cells on the subesophageal ganglion. (D) In 30y, all the MB subdivisions were innervated. It also labeled the optic lobes, antennal lobe, pars intercerebralis, and many cells surrounding the subesophageal ganglion. It also labeled a cluster of small neurons that are located dorsolateral to the optic tubercle and that project first ventrally and extend laterally toward the dorsolateral edge of the ventrolateral protocerebrum. Similar neurons were observed in 238y. (E) c492b labeled all types of Kenyon cells, although expression in the γd neurons was relatively weak. Compared to other lines in this category, expressions outside the MB were less pronounced. We observed reporter signals in the pars intercerebralis, local inter neurons in the antennal lobe, deutocerebrum, subesophageal ganglion, and large paired neurons located ventral to the calyx (see also the legend of Figure 2C). (F) All types of the Kenyon cells were strongly labeled by 238y. Outside the MB, this line labeled the optic lobe, superior protocerebrum, pars intercerebralis, and many neurons surrounding the entire subesophageal ganglion. (G) In OK107, all the subdivisions of the MB were strongly and uniformly labeled. Outside the MB, we observed strong reporter signals in the optic lobe, antennal lobe, pars intercerebralis, and cells on the subesophageal ganglion.](/cms/asset/5e34e8bb-2796-4b14-bcc8-6d369daf65b3/ineg_a_347339_f0006_b.jpg)
Three MB-GAL4s, NP7175, D52H, and 103y, showed clear sexual differences in their expression patterns ( and 7). For NP7175 and D52H, a cause of the difference in reporter expression might be because the GAL4 insertions are on the X chromosome. Compared to the female, male 103y more strongly labeled the α′/β′ and γ lobes (G and 7C).
Figure 7 GAL4 strains with sex-dependent difference. Stereopairs show reconstructions of MB-GAL4s with sex-specific reporter expression. The applied color illustrates the depth (see scale bar [25 µm] for the color code). See also original confocal stacks for detail (http://mushroombody.net). (A) Compared to the females, male NP7175 labeled slightly broader α/βc ( A). In addition to the background expression seen in the female, surface glia was strongly labeled. (B) In contrast to the female, male D52H additionally labeled the α′/β′ neurons. Moreover, the innervation of one glomerulus by the olfactory receptor neurons was more pronounced in the male. Otherwise, background expression was unusually low as in females. (C) Male 103y labeled all subtypes of Kenyon cells, whereas in the female, the expression in the α′/β′ and γ lobes was very faint (see G). In addition, expression in surface glia and dense terminals in the superior medial protocerebrum were less pronounced in males. Outside the MB, it labeled the processes in the medulla and lobula, middle superior lateral protocerebrum, local interneurons in the antennal lobe, and many neurons supplying the subesophageal ganglion and tritocerebrum.
![Figure 7 GAL4 strains with sex-dependent difference. Stereopairs show reconstructions of MB-GAL4s with sex-specific reporter expression. The applied color illustrates the depth (see scale bar [25 µm] for the color code). See also original confocal stacks for detail (http://mushroombody.net). (A) Compared to the females, male NP7175 labeled slightly broader α/βc (Figure 2 A). In addition to the background expression seen in the female, surface glia was strongly labeled. (B) In contrast to the female, male D52H additionally labeled the α′/β′ neurons. Moreover, the innervation of one glomerulus by the olfactory receptor neurons was more pronounced in the male. Otherwise, background expression was unusually low as in females. (C) Male 103y labeled all subtypes of Kenyon cells, whereas in the female, the expression in the α′/β′ and γ lobes was very faint (see Figure 5G). In addition, expression in surface glia and dense terminals in the superior medial protocerebrum were less pronounced in males. Outside the MB, it labeled the processes in the medulla and lobula, middle superior lateral protocerebrum, local interneurons in the antennal lobe, and many neurons supplying the subesophageal ganglion and tritocerebrum.](/cms/asset/e3f60927-f8be-45e2-82a0-e3c066b8955f/ineg_a_347339_f0007_b.jpg)
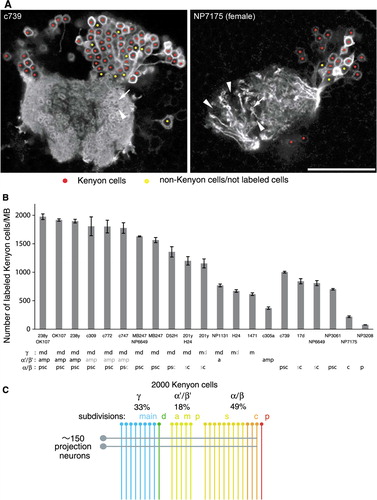
Figure 8 The number of genetically labeled Kenyon cells. (A) Single confocal sections through the calyx and cell-body cluster of Kenyon cells as representative pictures of the counting procedure (c739 and NP7175). Nuclei of Kenyon cells are marked with red, while non-Kenyon cells, unlabeled cells, or spaces between cells are marked with yellow. The arrow and arrowhead indicate microglomeruli innervated and not innervated by labeled Kenyon cells, respectively. Scale bar=25 µm. (B) The numbers of genetically labeled Kenyon cells in different MB-GAL4 drivers of the females. Labeled Kenyon cell subtypes are indicated below (see also ). The label in gray shows weak expression. See the legend of for abbreviations. N=5–8. Error bars represent standard error of the mean. (C) Model of the numerical composition of the Drosophila MB. The γ, α′/β′, and α/β neurons, respectively, contribute to 33, 18, and 49% of∼2,000 Kenyon cells. One circle and line represents∼75 cells. Approximately 150 iACT projections neurons terminate on the Kenyon cells, except for the α/βp neurons (rightmost line).
The Number of Genetically Labeled Kenyon Cells
The total number of Kenyon cells in the Drosophila MB was reported to range from 800 to 2,900 (Balling et al., Citation1987; Hinke, Citation1961; Ito & Hotta, Citation1992; Lee et al., Citation1999; Mader, Citation2004; Nikolaï et al., Citation2003; Technau & Heisenberg, Citation1982; Technau, Citation1984). The variability mainly depends on the method of counting and estimation. MB-GAL4s with expression in all lobe systems () prompted us to address how many Kenyon cells of the Drosophila MB can be marked by the genetic method.
Consistent with their expression in the lobes, the largest numbers of Kenyon cells were stained in OK107 and 238y: on average, 1,917 and 1,898 cells, respectively (B). These numbers were, however, smaller than those in some reports (Balling et al., Citation1987; Hinke, Citation1961; Technau & Heisenberg, Citation1982; Technau, Citation1984). To examine the highest possible number of genetically labeled Kenyon cells, we generated the flies carrying both OK107 and 238y (OK107/238y) and counted the labeled cells. If these single drivers incompletely labeled Kenyon cells with different patterns, the number of the GAL4-positive cells in OK107/238y could exceed that of each single driver. In OK107/238y, we counted 1,975 labeled Kenyon cells, on average, but not significantly higher than OK107 or 238y alone (P>0.05; n≥7; B).
Likewise, the numbers of GAL4-expressing Kenyon cells were counted in MB-GAL4s with expression in specific subdivisions of the lobes (B). Interestingly, the expression pattern in the lobe does not always predict the number of the labeled neurons. For example, the expression patterns of NP3061 and c739 were similar in the α/β lobes (, 2E, and 2F), whereas the number of GAL4-expressing Kenyon cells of NP3061 was significantly less than that of c739 (P<0.001; n≥5; B). Similarly, the combination of MB247/NP6649 only slightly increased the number of labeled cells, compared to MB247 alone, indicating that weaker expression in the α/βc of MB247 was sufficiently strong for cell counting. The MB-GAL4 that labels the smallest population in the analyzed lines was NP3208. The expression was detected in 76 cells, on average, and all of them projected to the α/βp (Tanaka et al., Citation2008) ( and 2E).
Based on these results, we next examined the total number of genetically labeled Kenyon cells by summing up the numbers of MB-GAL4s labeling specific lobes. If the marked cell population in OK107/238y did not represent the entire Kenyon cells, the sum could exceed 1,975. Since we counted, on average, 1,002, 370, and 671 cells for c739 (α/β), c305a (α′/β′), and H24 (γ), respectively, the sum of these lines was 2,043 cells, which matches the number in OK107/238y with∼3% of a mean difference (). The sum of MB247/NP6649 (α/β and γ; 1,630 cells) and c305a (α′/β′; 370 cells) also matched the number in OK107/238y (2,000 vs. 1,975 cells). Taken together, we conclude the average total number of Kenyon cells of the MB that can be genetically labeled is 2,000.
DISCUSSION
The Number of Drosophila Kenyon Cells
How many Kenyon cells does adult Drosophila have? With genetic labeling and confocal microscopy, we reached a number of 2,000 Kenyon cells per hemisphere (B). This is roughly, on average, 500 cells fewer than previous reports, where the numbers of cell bodies (2,380–2,640) and axon fibers (2,140–2,990) were estimated and counted, respectively (Balling et al., Citation1987; Hinke, Citation1961; Technau & Heisenberg, Citation1982; Technau, Citation1984).
We demonstrated that any combinations of MB-GAL4s with complementary or overlapping patterns covering the entire lobe systems failed to exceed 2,000 (B). This is consistent with the number of cell bodies in one MB neuroblast clone, using elav-GAL4 or OK107 (i.e.,∼500 cells) (Lee et al., Citation1999)). It is still possible to assume that MB-GAL4s systematically fail to label 500 Kenyon cells. One parsimonious explanation for the discrepancy could be a retardation of Kenyon cell proliferation induced by overexpression of mCD8::GFP. This possibility can be clarified by counting the number of Kenyon cell fibers at the posterior pedunculus. Indeed, we observed that the fiber number in OK107/G3 was smaller than that in the wild-type Canton-S on electron micrographs (A.B.F. and Y.A., unpublished observation). In any event, this study suggests that approximately 2,000 Kenyon cells might be the limit that can be labeled by the GAL4/UAS system.
Alternatively, the lack of cell type–specific labeling in the previous studies could obscure the total numbers. Indeed, MB-APL, one of the non-Kenyon-cell intrinsic neurons, and GABA-ergic neuron(s) have thin parallel fibers projecting through the pedunculus as Kenyon cells do (Tanaka et al., Citation2008; Yasuyama et al., Citation2002). In our confocal micrographs, we counted at least 45 fibers at the caudal pedunculus derived from a single MB-APL (K.G. and Y.A., unpublished observation). Without specific labeling, such thin fibers, but not of Kenyon cells, might potentially lead to an overestimation of the total fiber number. Lack of specific labeling might have obscured previous cell-body counting (Hinke, Citation1961; G. Technau & Heisenberg, Citation1982). Although Kenyon cell nuclei look distinct from those of surrounding cells without cell type–specific labeling, such a border turned out to be not always reliable after genetic labeling of Kenyon cell nuclei (data not shown).
It is noteworthy that there also exist studies that have estimated significantly smaller numbers of Kenyon cells than this one (Ito & Hotta, Citation1992; Mader, Citation2004). Mader, in his diploma thesis, analyzed 19 different MB-GAL4s by expressing nls-lacZ. His tallies were systematically fewer than ours (e.g., 825 nuclei in MB247; ). This difference might be due to a mere technical pitfall. For instance, there could be a significant overlap of multiple Kenyon cell nuclei due to the usage of the 20X objective lens, leading to lower Z resolution in confocal microscopy. Based on the number of Kenyon cells that incorporated BrdU in defined time periods, another study estimated that MB neuroblasts give rise to 800–1,200 until adulthood (Ito & Hotta, Citation1992). Although we have no clear explanation, the deviation might, again, be technical, such as inefficient BrdU incorporation.
Considering the production of 2,000 neurons in the adult, using the typical cell-division scheme of the neuroblast, the cell cycle of MB neuroblasts must be unusually short—within an hour (2,000 cells during∼200 hours of MB neuroblast proliferation). Thus, one or more of the assumptions of the neuroblast division might not be applied to the Kenyon cell proliferation. For instance, the ganglion mother cells from the MB neuroblasts might exceptionally divide more than once, not as in many other neurons in insects.
Seminal papers from the Heisenberg group showed that the structure and the fiber number of the MB undergo significant changes with many developmental and environmental factors, such as different wild-type strains (Balling et al., Citation1987; Heisenberg et al., Citation1995; Technau, Citation1984). Thus, direct comparison of these different counting methods under a constant experimental condition could, at least partially, settle the dispute on the total number of Drosophila Kenyon cells.
The total Kenyon cell number may have important implications when it comes to devising a quantitative network model of the Drosophila MB. For example, it might be important to reconsider the connectivity of the 2,000 genetically labeled Kenyon cells with the projection neurons at the calyx. Approximately 150 iACT projection neurons are the major source of afferents to the calyx and were calculated to supply in the calyx∼1,000 presynaptic boutons, which comprises the cores of microglomeruli (Jefferis et al., Citation2007; Stocker et al., Citation1990; Turner et al., Citation2008). Based on the electron microscopic data (Yasuyama et al., Citation2002), Turner et al. estimated 30 active zones, on average, at a single bouton (Turner et al., Citation2008). Since each Kenyon cell has dendritic arbors on five microglomeruli, on average (Zhu et al., Citation2003), each projection neuron bouton could be presynaptic to 10 different Kenyon cell claws (=2000 cells×5 terminals/1,000 microglomeruli). Given that several postsynaptic sites can be assigned to a single presynaptic release site (Prokop & Meinertzhagen, 2005; Yasuyama et al., Citation2002), each Kenyon cell claw, on average, may have more than six postsynaptic sites (=multiple postsynapses [≥2]×30 active zones in a projection neuron bouton/10 Kenyon cell claw). These numbers, however, should be experimentally verified, such as by the high-resolution analysis of Kenyon cell dendrites and the reconstruction of microglomeruli with electron microscopy.
Composition of the MB
In addition to the total number of Kenyon cells, this study reveals, for the first time, the proportion of genetically labeled Kenyon cells contributing to subdivisions. This opens new questions that are of direct functional relevance. What is the minimal set of MB subdivisions to which reproducible genetic manipulation can be applied? Can all lobe systems similarly receive odor information represented in the calyx? More generally, how is the Drosophila MB composed of different Kenyon cell subtypes?
According to the numbers of Kenyon cells contributing to subdivisions, we estimate that the γ neurons, α′/β′ neurons, and α/β neurons occupy 33 (671 cells), 18 (370 cells), and 49% (1,002 cells) of the total labeled Kenyon cells (). This composition fits well with the volumetric ratio of the cell bodies of MB neuroblast clones: 35, 23, and 42%, respectively (Lee et al., Citation1999). The lack of the γ neurons born before the induction of neuroblast clones might be compensated by the larger cell bodies of early-born Kenyon cells (Maurange et al, Citation2008). Interestingly, the cell number of each subtype does not seem to reflect the volume of each lobe, implying heterogeneous synaptic terminals of the subtypes.
Subtype-specific MB-GAL4s allowed us to estimate the smallest population of Kenyon cells; the α/βc and α/βp neurons in the α/β lobes have at least 203 (10%; NP7175) and 76 (4%; NP3208) cells, respectively. Given that the α/βp neurons exceptionally arborize in the accessory calyx, the α/βc lobes might contain the least Kenyon cells that project to the main calyx. For the γd and α′/β′ neurons, we could not calculate the number of each subtype due to the lack of a specific driver line. Yet, we estimated the numbers of the γd and α′/β′a neurons to be approximately 55 and 98, respectively (H24 – 1471 and NP1131 – H24 for the γd and α′/β′a neurons, respectively).
It may be important to consider whether each subdivision can represent all odors by contacting all the terminals of the projection neurons in the calyx. To completely cover 1,000 presynaptic boutons with five dendritic terminals of each Kenyon cell, at least 200 Kenyon cells are theoretically required (=1,000 microglomeruli/5 terminals). Therefore, 203 of the α/βc neurons could receive odor information from all microglomeruli, but at least two of the three subtypes in the α′/β′ neurons (370 cells in total) could not. If we assume that the projection neurons innervating the same antennal lobe glomerulus convey identical information, only 10 Kenyon cells, in principle, could sufficiently receive the entire odor repertoire, given∼50 glomeruli in the antennal lobe (50 different terminals=5 claws×10 Kenyon cells). However, in practice, it seems unlikely that even 200 cells cover all microglomeruli in the calyx, since each subtype has spatial preference of dendritic terminals (Jefferis et al., Citation2007; Lin et al., Citation2007; Tanaka et al., Citation2004). Consistently, we also found microglomeruli not innervated in MB-GAL4s that label 1,000 Kenyon cells (e.g., arrows and arrowheads in the calyx of c739 in A). Therefore, we suppose that each subtype of Kenyon cells can have its unique, yet overlapping, odor space.
Application of MB-GAL4s
According to the expression pattern in the lobes, c739 and NP3061 have very similar, if not identical, patterns in the lobes (). However, the numbers of labeled Kenyon cells are significantly different (ca. 300 cells more in c739; B). This indicates that expression pattern in the lobes does not always predict the number of genetically labeled neurons; genetic subpopulations of Kenyon cells do not always correlate the morphological subdivisions of the MB. Since only a few Kenyon cells (∼6%) represent each odor (Turner et al., Citation2008; Wang et al., Citation2004), the small difference in the labeled population may have a significant functional consequence. Indeed, Akalal et al. (Citation2006) found the differential phenotypes of 17d and c739 on the memories of different odor combinations (Akalal et al., Citation2006), although both label the α/β neurons (). Therefore, outcome of genetic manipulation may vary with subtle difference in the population of labeled cells in the target lobes.
Our comprehensive analysis revealed that most MB-GAL4s have significant expression outside the MB. If the spatial specificity is absolutely required, combinatorial methods, such as “split GAL4” (Luan et al., Citation2006) or the GAL80 enhancer-trap system (Suster et al., Citation2004), should be applied to confine the effector-reporter expression to the MB.
In addition to the spatial specificity, the expression of MB-GAL4s analyzed here changes during development. For instance, c739 is expressed broadly in the γ neurons during the larval stages (Kurusu et al., Citation2002). Therefore, additional temporal control is favorable to circumvent such a “developmental effect” and to restrict the transgene expression to the adult (e.g., GeneSwitch or the GAL80[ts] system) (McGuire et al., Citation2003; Osterwalder et al., Citation2001; Roman et al., Citation2001).
SUPPL. FIG. 1. EXPRESSION PATTERN OF 25 GAL4 LINES AND REFERENCE LIST
Summary of the expression levels of 25 MB-GAL4s in various brain areas defined by anti-Synapsin immunostaining. Gray scale indicates subjectively evaluated signal intensity. Note that higher level of fluorescent signals in the certain brain area can result from larger population of GAL4 expressing cells and/or stronger GAL4 expression in each cell. Abbreviations: MB: mushroom body; c: core subdivision: s: surface subdivision; p: posterior subdivision; a: anterior subdivision; m: middle subdivision; p: posterior subdivision; d: dorsal subdivision; AL: antennal lobe; CC: central complex; fb: fan-shaped body; eb: ellipsoid body; no: noduli; pb: protocerebral bridge; OL: optic lobe; me: medulla; lo: lobula; lop: lobula plate; spr: superior protocerebrum; ipr: inferior protocerebrum; LH: lateral horn; optu: optic tubercle; vlpr: ventrolateral protocerebrum; plpr: posteriorlateral protocerebrum; vmpr: ventromedial protocerebrum; psl: posterior slope; pars in: pars intercerebralis; AN: antennal nerve; DE: deutocerebrum; TR: tritocerebrum; SOG: subesophageal ganglion. References: Original articles were sought using Flybase (http://flybase.org), and we manually tried to complement recent articles in addition. 1: (McGuire, Le, & Davis, Citation2001); 2: (Peng, Xi, Zhang, Zhang, & Guo, Citation2007); 3: (Ramaekers et al., Citation2005); 4: (Masuda-Nakagawa, Tanaka, & O'Kane, Citation2005); 5: (Wu et al., Citation2007); 6: (Fushima & Tsujimura, Citation2007); 7: (Connolly et al., Citation1996); 8: (Kurusu et al., Citation2002); 9: (Tanaka, Tanimoto, & Ito, Citation2008); 10: (Manoli et al., Citation2005); 11: (Awasaki & Ito, Citation2004); 12: (Boyle, Nighorn, & Thomas, Citation2006); 13: (H. Gu & O'Dowd, Citation2006); 14: (Nicolai, Lasbleiz, & Dura, Citation2003); 15: (J. Wang et al., Citation2004); 16: (J. Wang, Zugates, Liang, Lee, & Lee, Citation2002); 17: (Watts, Hoopfer, & Luo, Citation2003); 18: (Z. Yang, Edenberg, & Davis, Citation2005); 19: (Zheng et al., Citation2003); 20: (Zheng et al., Citation2006); 21: (Zhu, Chiang, & Lee, Citation2003); 22: (Schuldiner et al., Citation2008); 23: (X. Liu, Krause, & Davis, Citation2007); 24: (Grillenzoni, Flandre, Lasbleiz, & Dura, Citation2007); 25: (DeZazzo et al., Citation2000); 26: (Kitamoto, Citation2002); 27: (Suh et al., Citation2004); 28: (Y. Wang, Mamiya, Chiang, & Zhong, Citation2008); 29: (Orihara-Ono et al., Citation2005); 30: (Adachi et al., Citation2003); 31: (Agrawal et al., Citation2005); 32: (Billuart, Winter, Maresh, Zhao, & Luo, Citation2001); 33: (G. Gu, Yang, Mitchell, & O'Tousa, Citation2004); 34: (Kim, Lee, Seong, & Han, Citation2003); 35: (Kobayashi et al., Citation2006); 36: (Komiyama, Sweeney, Schuldiner, Garcia, & Luo, Citation2007); 37: (T. Lee, Lee, & Luo, Citation1999); 38: (Lin, Lai, Chin, Chen, & Chiang, Citation2007); 39: (Z. Liu, Steward, & Luo, Citation2000); 40: (Martini, Roman, Meuser, Mardon, & Davis, Citation2000); 41: (Ng & Luo, Citation2004); 42: (Ng et al., Citation2002); 43: (Niimi, Clements, Gehring, & Callaerts, Citation2002); 44: (Pan, Zhang, Woodruff, & Broadie, Citation2004); 45: (Pascual & Preat, Citation2001); 46: (Pascual, Huang, & Preat, Citation2005); 47: (Plaza et al., Citation2001); 48: (Scott, Lee, & Luo, Citation2001); 49: (Su & O'Dowd, Citation2003); 50: (Y. Wang et al., Citation2004); 51: (J. Wang, Lee, Lin, & Lee, Citation2006); 52: (Whited, Robichaux, Yang, & Garrity, Citation2007); 53: (Wojtowicz, Flanagan, Millard, Zipursky, & Clemens, Citation2004); 54: (Zelhof & Hardy, Citation2004); 55: (Zhu et al., Citation2006); 56: (Zhu, Perez, Pan, & Lee, Citation2005); 57: (Nishimura, Sakoda, & Yoshikawa, Citation2008); 58: (Chen et al., Citation2008); 59: (Johard et al., Citation2008); 60: (Campusano, Su, Jiang, Sicaeros, & O'Dowd, Citation2007); 61: (Martin, Rogers, Chagneau, & Brulet, Citation2007); 62: (W. J. Joiner, Crocker, White, & Sehgal, Citation2006); 63: (Tettamanti et al., Citation1997); 64: (Zars, Wolf, Davis, & Heisenberg, Citation2000); 65: (Kraft, Levine, & Restifo, Citation1998); 66: (Keleman, Kruttner, Alenius, & Dickson, Citation2007); 67: (M. Y. Yang, Armstrong, Vilinsky, Strausfeld, & Kaiser, Citation1995); 68: (Mery, Belay, So, Sokolowski, & Kawecki, Citation2007); 69: (Kaun, Hendel, Gerber, & Sokolowski, Citation2007); 70: (Cheng et al., Citation2001); 71: (Moreau-Fauvarque, Taillebourg, Boissoneau, Mesnard, & Dura, Citation1998); 72: (O'Dell, Armstrong, Yang, & Kaiser, Citation1995); 73: (Kido & Ito, Citation2002); 74: (Zars, Fischer, Schulz, & Heisenberg, Citation2000); 75: (Rodan, Kiger, & Heberlein, Citation2002); 76: (McGuire, Le, Osborn, Matsumoto, & Davis, Citation2003); 77: (Acevedo, Froudarakis, Kanellopoulos, & Skoulakis, Citation2007); 78: (Rodan et al., Citation2002); 79: (M. A. Joiner & Griffith, Citation2000); 80: (Mehren & Griffith, Citation2004); 81: (Y. Wang et al., Citation2003); 82: (Shi et al., Citation2007); 83: (Chang, Shi, & Min, Citation2003); 84: (Diegelmann, Fiala, Leibold, Spall, & Buchner, Citation2002); 85: (Enerly, Larsson, & Lambertsson, Citation2003); 86: (Goldstein, Jan, & Luo, Citation2005); 87: (Honjo & Furukubo-Tokunaga, Citation2005); 88: (Koushika, Lisbin, & White, Citation1996); 89: (T. Lee, Marticke, Sung, Robinow, & Luo, Citation2000); 90: (Luo, Lee, Nardine, Null, & Reuter, Citation1999); 91: (Michel, Kraft, & Restifo, Citation2004); 92: (Soller et al., Citation2006); 93: (Verkhusha et al., Citation2001); 94: (Walker et al., Citation2006); 95: (Watts, Schuldiner, Perrino, Larsen, & Luo, Citation2004); 96: (Peng & Guo, Citation2007); 97: (Manseau et al., Citation1997); 98: (Krashes & Waddell, Citation2008); 99: (Yeh, Gustafson, & Boulianne, Citation1995); 100: (Akalal et al., Citation2006); 101: (Krashes, Keene, Leung, Armstrong, & Waddell, Citation2007); 102: (Casares, Calleja, & Sanchez-Herrero, Citation1996); 103: (Parker, Fessler, Nelson, & Fessler, Citation1995); 104: (Waddell, Armstrong, Kitamoto, Kaiser, & Quinn, Citation2000); 105: (Astle, Kozlova, & Thummel, Citation2003); 106: (Broughton, Kitamoto, & Greenspan, Citation2004); 107: (Grammenoudi, Kosmidis, & Skoulakis, Citation2006); 108: (Presente, Boyles, Serway, de Belle, & Andres, Citation2004); 109: (Reeve et al., Citation2005); 110: (Pitman, McGill, Keegan, & Allada, Citation2006); 111: (Schwaerzel, Heisenberg, & Zars, Citation2002); 112: (Sakai & Kitamoto, Citation2006); 113: (Dubnau, Grady, Kitamoto, & Tully, Citation2001); 114: (Gatti, Ferveur, & Martin, Citation2000); 115: (Belgacem & Martin, Citation2002); 116: (Amrein & Axel, Citation1997); 117: (McBride et al., Citation1999); 118: (Rosay, Armstrong, Wang, & Kaiser, Citation2001); 119: (Comas, Petit, & Preat, Citation2004); 120: (Kammermeier et al., Citation2001); 121: (Noveen, Daniel, & Hartenstein, Citation2000); 122: (Schneeberger & Raabe, Citation2003); 123: (Yu, Baird, Tsien, & Davis, Citation2003); 124: (Schwaerzel et al., Citation2003); 125: (Acevedo, Froudarakis, Tsiorva, & Skoulakis, Citation2007); 126: (Zhang, Guo, Peng, Xi, & Guo, Citation2007); 127: (Schulz, Chromey, Lu, Zhao, & Olson, Citation1996); 128: (Xi et al., Citation2008); 129: (Thum, Jenett, Ito, Heisenberg, & Tanimoto, Citation2007); 130: (Kim, Lee, & Han, Citation2007); 131: (Baker, Beckingham, & Armstrong, Citation2007); 132: (H. Lee, Stultz, & Hursh, Citation2007); 133: (Koushika, Soller, & White, Citation2000); 134: (Nitz, van Swinderen, Tononi, & Greenspan, Citation2002); 135: (Villella, Ferri, Krystal, & Hall, Citation2005); 136: (Garcia-Lopez et al., Citation2008); 137: (Isabel, Pascual, & Preat, Citation2004); 138: (Yoshihara & Ito, Citation2000); 139: (Hayashi et al., Citation2002); 140: (Tanaka, Awasaki, Shimada, & Ito, Citation2004); 141: (Qiu & Davis, Citation1993); 142: dunce promoter GAL4, courtesy of R. Davis via T. Zars. See reference 63 and 141 for the dunce promoter; 143: (Wilson & Laurent, Citation2005); 144: (Ito, Urban, & Technau, Citation1995)
Acknowledgements
The authors thank B. Mühlbauer for excellent antibody staining; M. Braun, S. Konrad, and S. Pünzeler for electron microscopy and counting Kenyon cells; Bloomington Stock Center, K. Ito, S. Waddell, and T. Zars for fly stocks; and the Max-Planck Society for hosting the Internet resource of this study. The authors are also grateful to M. Heisenberg, K. Ito, M. Kurusu, F. Leiss, L. Luo, I. A. Meinertzhagen, F.-W. Schürmann, N. K. Tanaka, and G. M. Technau for discussion and/or critical reading of the manuscript. Special thanks go to M. Heisenberg for his persistent and extraordinary passion for studying the Drosophila mushroom body, including the number of Kenyon cells. As a tribute to his contribution, we dedicate this paper to his emeritus. Y.A. and S.B. received a doctoral fellowship from Deutscher Akademischer Austausch Dienst and Boehringer-Ingelheim Fonds, respectively. This work was supported by the Emmy-Noether Program from Deutsche Forschungsgemeinschaft (H.T.) and Max-Planck-Gesellschaft (H.T.).
References
- Akalal D. B., Wilson C. F., Zong L., Tanaka N. K., Ito K., Davis R. L. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem 2006; 13(5)659–668
- Balling A., Technau G. M., Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet 1987; 4(2–3)65–73
- Crittenden J. R., Skoulakis E. M., Han K. A., Kalderon D., Davis R. L. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem 1998; 5(1–2)38–51
- Fahrbach S. E. Structure of the mushroom bodies of the insect brain. Annu Rev Entomol 2006; 51: 209–232
- Heisenberg M. Mutants of brain structure and function: what is the significance of the mushroom bodies for behavior?. Basic Life Sci 1980; 16: 373–390
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci 2003; 4(4)266–275
- Heisenberg M., Heusipp M., Wanke C. Structural plasticity in the Drosophila brain. J Neurosci 1995; 15(3 Pt 1)1951–1960
- Hinke W. Das relative postembryonale Wachstum der Hirnteile von Culex pipiens, Drosophila melanogaster und Drosophila-Mutanten. [The relative postembryonic growth of brain regions in Culex pipens, Drosophila melanogaster and Drosphila Mutants]. Z Morph Ökol Tiere 1961; 50: 81–118
- Isabel G., Pascual A., Preat T. Exclusive consolidated memory phases in Drosophila. Science 2004; 304(5673)1024–1027
- Ito K., Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol 1992; 149(1)134–148
- Ito K., Okada R., Tanaka N. K., Awasaki T. Cautionary observations on preparing and interpreting brain images using molecular biology-based staining techniques. Microsc Res Tech 2003; 62(2)170–186
- Ito K., Sass H., Urban J., Hofbauer A., Schneuwly S. GAL4-responsive UAS-tau as a tool for studying the anatomy and development of the Drosophila central nervous system. Cell Tissue Res 1997; 290(1)1–10
- Ito K., Suzuki K., Estes P., Ramaswami M., Yamamoto D., Strausfeld N. J. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn Mem 1998; 5(1–2)52–77
- Jefferis G. S., Marin E. C., Watts R. J., Luo L. Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies. Curr Opin Neurobiol 2002; 12(1)80–86
- Jefferis G. S., Potter C. J., Chan A. M., Marin E. C., Rohlfing T., Maurer C. R., Jr, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 2007; 128(6)1187–1203
- Johard H. A., Enell L. E., Gustafsson E., Trifilieff P., Veenstra J. A., Nassel D. R. Intrinsic neurons of Drosophila mushroom bodies express short neuropeptide F: relations to extrinsic neurons expressing different neurotransmitters. J Comp Neurol 2008; 507(4)1479–1496
- Keene A. C., Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci 2007; 8(5)341–354
- Keleman K., Kruttner S., Alenius M., Dickson B. J. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat Neurosci 2007; 10(12)1587–1593
- Klagges B. R., Heimbeck G., Godenschwege T. A., Hofbauer A., Pflugfelder G. O., Reifegerste R., et al. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J Neurosci 1996; 16(10)3154–3165
- Kurusu M., Awasaki T., Masuda-Nakagawa L. M., Kawauchi H., Ito K., Furukubo-Tokunaga K. Embryonic and larval development of the Drosophila mushroom bodies: concentric layer subdivisions and the role of fasciclin II. Development 2002; 129(2)409–419
- Lee T., Lee A., Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 1999; 126(18)4065–4076
- Leitch B., Laurent G. GABAergic synapses in the antennal lobe and mushroom body of the locust olfactory system. J Comp Neurol 1996; 372(4)487–514
- Lin H. H., Lai J. S., Chin A. L., Chen Y. C., Chiang A. S. A map of olfactory representation in the Drosophila mushroom body. Cell 2007; 128(6)1205–1217
- Luan H., Peabody N. C., Vinson C. R., White B. H. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 2006; 52(3)425–436
- Mader, M. T. 2004. Analyse von Expressionsmustern in den Pilzkörpern von Drosophila melanogaster [Analysis of expression pattern in the mushroom body of Drosophila melanogaster] [diploma thesis]. University of Würzburg, WürzburgGermany.
- Maurange C., Cheng L., Gould A. P. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell 2008; 133(5)891–902
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 2003; 302(5651)1765–1768
- Mobbs P. G. Neural networks in the mushroom bodies of the honeybee. J Insect Physiol 1984; 30(1)43–58
- Neder R. Allometrisches Wachstum von Hirnteilen bei drei verschieden großen Schabenarten. [Allometric growth of brain regions in three different large cockroaches]. Zool Jahrb Abt Anat Ontogenie Tiere 1959; 4: 411–464
- Nicolaï M., Lasbleiz C., Dura J. M. Gain-of-function screen identifies a role of the Src64 oncogene in Drosophila mushroom body development. J Neurobiol 2003; 57(3)291–302
- Osterwalder T., Yoon K. S., White B. H., Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A 2001; 98(22)12596–12601
- Prokop A., Meinertzhagen I. A. Development and structure of synaptic contacts in Drosophila. Semin Cell Dev Biol 2006; 17(1)20–30
- Roman G., Endo K., Zong L., Davis R. L. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A 2001; 98(22)12602–12607
- Rybak J., Menzel R. Anatomy of the mushroom bodies in the honey bee brain: the neuronal connections of the alpha-lobe. J Comp Neurol 1993; 334(3)444–465
- Sjöholm M., Sinakevitch I., Strausfeld N. J., Ignell R., Hansson B. S. Functional division of intrinsic neurons in the mushroom bodies of male Spodoptera littoralis revealed by antibodies against aspartate, taurine, FMRF-amide, Mas-allatotropin, and DC0. Arthropod Struct Dev 2006; 35(3)153–168
- Stocker R. F., Lienhard M. C., Borst A., Fischbach K. F. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res 1990; 262(1)9–34
- Strausfeld N. J. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. J Comp Neurol 2002; 450(1)4–33
- Strausfeld N. J., Sinakevitch I., Vilinsky I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc Res Tech 2003; 62(2)151–169
- Suster M. L., Seugnet L., Bate M., Sokolowski M. B. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer trap. Genesis 2004; 39(4)240–245
- Tanaka N. K., Awasaki T., Shimada T., Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol 2004; 14(6)449–457
- Tanaka N. K., Tanimoto H., Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol 2008; 508(5)711–755
- Technau G., Heisenberg M. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature 1982; 295(5848)405–407
- Technau G. M. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex, and experience. J Neurogenet 1984; 1(2)113–126
- Turner G. C., Bazhenov M., Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol 2008; 99(2)734–746
- Venken K. J., Bellen H. J. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet 2005; 6(3)167–178
- Wang Y., Guo H. F., Pologruto T. A., Hannan F., Hakker I., Svoboda K., et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci 2004; 24(29)6507–6514
- Witthöft W. Absolute Anzahl und Verteilung der Zellen im Hirn der Honigbiene. [Absolute number and distribution of cells in the honey bee brain]. Zoomorphology 1967; 61(1)160–184
- Yang M. Y., Armstrong J. D., Vilinsky I., Strausfeld N. J., Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron 1995; 15(1)45–54
- Yasuyama K., Meinertzhagen I. A., Schürmann F. W. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol 2002; 445(3)211–226
- Zhu S., Chiang A. S., Lee T. Development of the Drosophila mushroom bodies: elaboration, remodeling, and spatial organization of dendrites in the calyx. Development 2003; 130(12)2603–2610
- Acevedo S. F., Froudarakis E. I., Kanellopoulos A., Skoulakis E. M. Protection from premature habituation requires functional mushroom bodies in Drosophila. Learn Mem 2007; 14(5)376–384
- Acevedo S. F., Froudarakis E. I., Tsiorva A. A., Skoulakis E. M. Distinct neuronal circuits mediate experience-dependent, non-associative osmotactic responses in Drosophila. Mol Cell Neurosci 2007; 34(3)378–389
- Adachi Y., Hauck B., Clements J., Kawauchi H., Kurusu M., Totani Y., et al. Conserved cis-regulatory modules mediate complex neural expression patterns of the eyeless gene in the Drosophila brain. Mech Dev 2003; 120(10)1113–1126
- Agrawal N., Pallos J., Slepko N., Apostol B. L., Bodai L., Chang L. W., et al. Identification of combinatorial drug regimens for treatment of Huntington's disease using Drosophila. Proc Natl Acad Sci U S A 2005; 102(10)3777–3781
- Akalal D. B., Wilson C. F., Zong L., Tanaka N. K., Ito K., Davis R. L. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem 2006; 13(5)659–668
- Amrein H., Axel R. Genes expressed in neurons of adult male Drosophila. Cell 1997; 88(4)459–469
- Astle J., Kozlova T., Thummel C. S. Essential roles for the Dhr78 orphan nuclear receptor during molting of the Drosophila tracheal system. Insect Biochem Mol Biol 2003; 33(12)1201–1209
- Awasaki T., Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol 2004; 14(8)668–677
- Baker D. A., Beckingham K. M., Armstrong J. D. Functional dissection of the neural substrates for gravitaxic maze behavior in Drosophila melanogaster. J Comp Neurol 2007; 501(5)756–764
- Belgacem Y. H., Martin J. R. Neuroendocrine control of a sexually dimorphic behavior by a few neurons of the pars intercerebralis in Drosophila. Proc Natl Acad Sci U S A 2002; 99(23)15154–15158
- Billuart P., Winter C. G., Maresh A., Zhao X., Luo L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell 2001; 107(2)195–207
- Boyle M., Nighorn A., Thomas J. B. Drosophila Eph receptor guides specific axon branches of mushroom body neurons. Development 2006; 133(9)1845–1854
- Broughton S. J., Kitamoto T., Greenspan R. J. Excitatory and inhibitory switches for courtship in the brain of Drosophila melanogaster. Curr Biol 2004; 14(7)538–547
- Campusano J. M., Su H., Jiang S. A., Sicaeros B., O'Dowd D. K. nAChR-mediated calcium responses and plasticity in Drosophila Kenyon cells. Dev Neurobiol 2007; 67(11)1520–1532
- Casares F., Calleja M., Sanchez-Herrero E. Functional similarity in appendage specification by the Ultrabithorax and abdominal-A Drosophila HOX genes. EMBO J 1996; 15(15)3934–3942
- Chang K. T., Shi Y. J., Min K. T. The Drosophila homolog of Down's syndrome critical region 1 gene regulates learning: implications for mental retardation. Proc Natl Acad Sci U S A 2003; 100(26)15794–15799
- Chen G., Li W., Zhang Q. S., Regulski M., Sinha N., Barditch J., et al. Identification of synaptic targets of Drosophila pumilio. PLoS Comput Biol 2008; 4(2)1000026
- Cheng Y., Endo K., Wu K., Rodan A. R., Heberlein U., Davis R. L. Drosophila fasciclinII is required for the formation of odor memories and for normal sensitivity to alcohol. Cell 2001; 105(6)757–768
- Comas D., Petit F., Preat T. Drosophila long-term memory formation involves regulation of cathepsin activity. Nature 2004; 430(6998)460–463
- Connolly J. B., Roberts I. J., Armstrong J. D., Kaiser K., Forte M., Tully T., et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science 1996; 274(5295)2104–2107
- DeZazzo J., Sandstrom D., de Belle S., Velinzon K., Smith P., Grady L., et al. nalyot, a mutation of the Drosophila myb-related Adf1 transcription factor, disrupts synapse formation and olfactory memory. Neuron 2000; 27(1)145–158
- Diegelmann S., Fiala A., Leibold C., Spall T., Buchner E. Transgenic flies expressing the fluorescence calcium sensor Cameleon 2.1 under UAS control. Genesis 2002; 34(1-2)95–98
- Dubnau J., Grady L., Kitamoto T., Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature 2001; 411(6836)476–480
- Enerly E., Larsson J., Lambertsson A. Silencing the Drosophila ribosomal protein L14 gene using targeted RNA interference causes distinct somatic anomalies. Gene 2003; 320: 41–48
- Fushima K., Tsujimura H. Precise control of fasciclin II expression is required for adult mushroom body development in Drosophila. Dev Growth Differ 2007; 49(3)215–227
- Garcia-Lopez A., Monferrer L., Garcia-Alcover I., Vicente-Crespo M., Alvarez-Abril M. C., Artero R. D. Genetic and chemical modifiers of a CUG toxicity model in Drosophila. PLoS ONE 2008; 3(2)1595
- Gatti S., Ferveur J. F., Martin J. R. Genetic identification of neurons controlling a sexually dimorphic behaviour. Curr Biol 2000; 10(11)667–670
- Goldstein A. Y., Jan Y. N., Luo L. Function and regulation of Tumbleweed (RacGAP50C) in neuroblast proliferation and neuronal morphogenesis. Proc Natl Acad Sci U S A 2005; 102(10)3834–3839
- Grammenoudi S., Kosmidis S., Skoulakis E. M. Cell type-specific processing of human Tau proteins in Drosophila. FEBS Lett 2006; 580(19)4602–4606
- Grillenzoni N., Flandre A., Lasbleiz C., Dura J. M. Respective roles of the DRL receptor and its ligand WNT5 in Drosophila mushroom body development. Development 2007; 134(17)3089–3097
- Gu G., Yang J., Mitchell K. A., O'Tousa J. E. Drosophila ninaB and ninaD act outside of retina to produce rhodopsin chromophore. J Biol Chem 2004; 279(18)18608–18613
- Gu H., O'Dowd D. K. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci 2006; 26(1)265–272
- Hayashi S., Ito K., Sado Y., Taniguchi M., Akimoto A., Takeuchi H., et al. GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis 2002; 34(1-2)58–61
- Honjo K., Furukubo-Tokunaga K. Induction of cAMP response element-binding protein-dependent medium-term memory by appetitive gustatory reinforcement in Drosophila larvae. J Neurosci 2005; 25(35)7905–7913
- Isabel G., Pascual A., Preat T. Exclusive consolidated memory phases in Drosophila. Science 2004; 304(5673)1024–1027
- Ito K., Urban J., Technau G. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Dev Genes Evol 1995; 204(5)284–307
- Johard H. A., Enell L. E., Gustafsson E., Trifilieff P., Veenstra J. A., Nässel D. R. Intrinsic neurons of Drosophila mushroom bodies express short neuropeptide F: relations to extrinsic neurons expressing different neurotransmitters. J Comp Neurol 2008; 507(4)1479–1496
- Joiner M. A., Griffith L. C. Visual input regulates circuit configuration in courtship conditioning of Drosophila melanogaster. Learn Mem 2000; 7(1)32–42
- Joiner W. J., Crocker A., White B. H., Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature 2006; 441(7094)757–760
- Kammermeier L., Leemans R., Hirth F., Flister S., Wenger U., Walldorf U., et al. Differential expression and function of the Drosophila Pax6 genes eyeless and twin of eyeless in embryonic central nervous system development. Mech Dev 2001; 103(1-2)71–78
- Kaun K. R., Hendel T., Gerber B., Sokolowski M. B. Natural variation in Drosophila larval reward learning and memory due to a cGMP-dependent protein kinase. Learn Mem 2007; 14(5)342–349
- Keleman K., Kruttner S., Alenius M., Dickson B. J. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat Neurosci 2007; 10(12)1587–1593
- Kido A., Ito K. Mushroom bodies are not required for courtship behavior by normal and sexually mosaic Drosophila. J Neurobiol 2002; 52(4)302–311
- Kim Y. C., Lee H. G., Han K. A. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci 2007; 27(29)7640–7647
- Kim Y. C., Lee H. G., Seong C. S., Han K. A. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr Patterns 2003; 3(2)237–245
- Kitamoto T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc Natl Acad Sci U S A 2002; 99(20)13232–13237
- Kobayashi M., Michaut L., Ino A., Honjo K., Nakajima T., Maruyama Y., et al. Differential microarray analysis of Drosophila mushroom body transcripts using chemical ablation. Proc Natl Acad Sci U S A 2006; 103(39)14417–14422
- Komiyama T., Sweeney L. B., Schuldiner O., Garcia K. C., Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell 2007; 128(2)399–410
- Koushika S. P., Lisbin M. J., White K. ELAV, a Drosophila neuron-specific protein, mediates the generation of an alternatively spliced neural protein isoform. Curr Biol 1996; 6(12)1634–1641
- Koushika S. P., Soller M., White K. The neuron-enriched splicing pattern of Drosophila erect wing is dependent on the presence of ELAV protein. Mol Cell Biol 2000; 20(5)1836–1845
- Kraft R., Levine R. B., Restifo L. L. The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J Neurosci 1998; 18(21)8886–8899
- Krashes M. J., Keene A. C., Leung B., Armstrong J. D., Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 2007; 53(1)103–115
- Krashes M. J., Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci 2008; 28(12)3103–3113
- Kurusu M., Awasaki T., Masuda-Nakagawa L. M., Kawauchi H., Ito K., Furukubo-Tokunaga K. Embryonic and larval development of the Drosophila mushroom bodies: concentric layer subdivisions and the role of fasciclin II. Development 2002; 129(2)409–419
- Lee H., Stultz B. G., Hursh D. A. The Zic family member, odd-paired, regulates the Drosophila BMP, decapentaplegic, during adult head development. Development 2007; 134(7)1301–1310
- Lee T., Lee A., Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 1999; 126(18)4065–4076
- Lee T., Marticke S., Sung C., Robinow S., Luo L. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 2000; 28(3)807–818
- Lin H. H., Lai J. S., Chin A. L., Chen Y. C., Chiang A. S. A map of olfactory representation in the Drosophila mushroom body. Cell 2007; 128(6)1205–1217
- Liu X., Krause W. C., Davis R. L. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron 2007; 56(6)1090–1102
- Liu Z., Steward R., Luo L. Drosophila Lis1 is required for neuroblast proliferation, dendritic elaboration and axonal transport. Nat Cell Biol 2000; 2(11)776–783
- Luo L., Lee T., Nardine T., Null B., Reuter J. Using the MARCM system to positively mark mosaic clones in Drosophila. Dros Inf Serv 1999; 82: 102–105
- Manoli D. S., Foss M., Villella A., Taylor B. J., Hall J. C., Baker B. S. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 2005; 436(7049)395–400
- Manseau L., Baradaran A., Brower D., Budhu A., Elefant F., Phan H., et al. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev Dyn 1997; 209(3)310–322
- Martin J. R., Rogers K. L., Chagneau C., Brulet P. In vivo bioluminescence imaging of Ca signalling in the brain of Drosophila. PLoS ONE 2007; 2(3)275
- Martini S. R., Roman G., Meuser S., Mardon G., Davis R. L. The retinal determination gene, dachshund, is required for mushroom body cell differentiation. Development 2000; 127(12)2663–2672
- Masuda-Nakagawa L. M., Tanaka N. K., O'Kane C. J. Stereotypic and random patterns of connectivity in the larval mushroom body calyx of Drosophila. Proc Natl Acad Sci U S A 2005; 102(52)19027–19032
- McBride S. M., Giuliani G., Choi C., Krause P., Correale D., Watson K., et al. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 1999; 24(4)967–977
- McGuire S. E., Le P. T., Davis R. L. The role of Drosophila mushroom body signaling in olfactory memory. Science 2001; 293(5533)1330–1333
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 2003; 302(5651)1765–1768
- Mehren J. E., Griffith L. C. Calcium-independent calcium/calmodulin-dependent protein kinase II in the adult Drosophila CNS enhances the training of pheromonal cues. J Neurosci 2004; 24(47)10584–10593
- Mery F., Belay A. T., So A. K., Sokolowski M. B., Kawecki T. J. Natural polymorphism affecting learning and memory in Drosophila. Proc Natl Acad Sci U S A 2007; 104(32)13051–13055
- Michel C. I., Kraft R., Restifo L. L. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci 2004; 24(25)5798–5809
- Moreau-Fauvarque C., Taillebourg E., Boissoneau E., Mesnard J., Dura J. M. The receptor tyrosine kinase gene linotte is required for neuronal pathway selection in the Drosophila mushroom bodies. Mech Dev 1998; 78(1-2)47–61
- Ng J., Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 2004; 44(5)779–793
- Ng J., Nardine T., Harms M., Tzu J., Goldstein A., Sun Y., et al. Rac GTPases control axon growth, guidance and branching. Nature 2002; 416(6879)442–447
- Nicolai M., Lasbleiz C., Dura J. M. Gain-of-function screen identifies a role of the Src64 oncogene in Drosophila mushroom body development. J Neurobiol 2003; 57(3)291–302
- Niimi T., Clements J., Gehring W. J., Callaerts P. Dominant-negative form of the Pax6 homolog eyeless for tissue-specific loss-of-function studies in the developing eye and brain in Drosophila. Genesis 2002; 34(1-2)74–75
- Nishimura I., Sakoda J. Y., Yoshikawa K. Drosophila MAGE controls neural precursor proliferation in postembryonic neurogenesis. Neuroscience 2008; 154(2)572–581
- Nitz D. A., van Swinderen B., Tononi G., Greenspan R. J. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol 2002; 12(22)1934–1940
- Noveen A., Daniel A., Hartenstein V. Early development of the Drosophila mushroom body: the roles of eyeless and dachshund. Development 2000; 127(16)3475–3488
- O'Dell K. M., Armstrong J. D., Yang M. Y., Kaiser K. Functional dissection of the Drosophila mushroom bodies by selective feminization of genetically defined subcompartments. Neuron 1995; 15(1)55–61
- Orihara-Ono M., Suzuki E., Saito M., Yoda Y., Aigaki T., Hama C. The slender lobes gene, identified by retarded mushroom body development, is required for proper nucleolar organization in Drosophila. Dev Biol 2005; 281(1)121–133
- Pan L., Zhang Y. Q., Woodruff E., Broadie K. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol 2004; 14(20)1863–1870
- Parker C. G., Fessler L. I., Nelson R. E., Fessler J. H. Drosophila UDP-glucose:glycoprotein glucosyltransferase: sequence and characterization of an enzyme that distinguishes between denatured and native proteins. EMBO J 1995; 14(7)1294–1303
- Pascual A., Huang K. L., Preat T. Conditional UAS-targeted repression in Drosophila. Nucleic Acids Res 2005; 33(1)7
- Pascual A., Preat T. Localization of long-term memory within the Drosophila mushroom body. Science 2001; 294(5544)1115–1117
- Peng Y., Guo A. Novel stimulus-induced calcium efflux in Drosophila mushroom bodies. Eur J Neurosci 2007; 25(7)2034–2044
- Peng Y., Xi W., Zhang W., Zhang K., Guo A. Experience improves feature extraction in Drosophila. J Neurosci 2007; 27(19)5139–5145
- Pitman J. L., McGill J. J., Keegan K. P., Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 2006; 441(7094)753–756
- Plaza S., Prince F., Jaeger J., Kloter U., Flister S., Benassayag C., et al. Molecular basis for the inhibition of Drosophila eye development by Antennapedia. EMBO J 2001; 20(4)802–811
- Presente A., Boyles R. S., Serway C. N., de Belle J. S., Andres A. J. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci U S A 2004; 101(6)1764–1768
- Qiu Y., Davis R. L. Genetic dissection of the learning/memory gene dunce of Drosophila melanogaster. Genes Dev 1993; 7(7B)1447–1458
- Ramaekers A., Magnenat E., Marin E. C., Gendre N., Jefferis G. S., Luo L., et al. Glomerular Maps without Cellular Redundancy at Successive Levels of the Drosophila Larval Olfactory Circuit. Curr Biol 2005; 15(11)982–992
- Reeve S. P., Bassetto L., Genova G. K., Kleyner Y., Leyssen M., Jackson F. R., et al. The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Curr Biol 2005; 15(12)1156–1163
- Rodan A. R., Kiger J. A., Jr, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in . Drosophila. J Neurosci 2002; 22(21)9490–9501
- Rosay P., Armstrong J. D., Wang Z., Kaiser K. Synchronized neural activity in the Drosophila memory centers and its modulation by amnesiac. Neuron 2001; 30(3)759–770
- Sakai T., Kitamoto T. Differential roles of two major brain structures, mushroom bodies and central complex, for Drosophila male courtship behavior. J Neurobiol 2006; 66(8)821–834
- Schneeberger D., Raabe T. Mbt, a Drosophila PAK protein, combines with Cdc42 to regulate photoreceptor cell morphogenesis. Development 2003; 130(3)427–437
- Schuldiner O., Berdnik D., Levy J. M., Wu J. S., Luginbuhl D., Gontang A. C., et al. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell 2008; 14(2)227–238
- Schulz R. A., Chromey C., Lu M. F., Zhao B., Olson E. N. Expression of the D-MEF2 transcription in the Drosophila brain suggests a role in neuronal cell differentiation. Oncogene 1996; 12(8)1827–1831
- Schwaerzel M., Heisenberg M., Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron 2002; 35(5)951–960
- Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 2003; 23(33)10495–10502
- Scott E. K., Lee T., Luo L. enok encodes a Drosophila putative histone acetyltransferase required for mushroom body neuroblast proliferation. Curr Biol 2001; 11(2)99–104
- Shi L., Lin S., Grinberg Y., Beck Y., Grozinger C. M., Robinson G. E., et al. Roles of Drosophila Krüppel-homolog 1 in neuronal morphogenesis. Dev Neurobiol 2007; 67(12)1614–1626
- Soller M., Haussmann I. U., Hollmann M., Choffat Y., White K., Kubli E., et al. Sex-peptide-regulated female sexual behavior requires a subset of ascending ventral nerve cord neurons. Curr Biol 2006; 16(18)1771–1782
- Su H., O'Dowd D. K. Fast synaptic currents in Drosophila mushroom body Kenyon cells are mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J Neurosci 2003; 23(27)9246–9253
- Suh G. S., Wong A. M., Hergarden A. C., Wang J. W., Simon A. F., Benzer S., et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 2004; 431(7010)854–859
- Tanaka N. K., Awasaki T., Shimada T., Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol 2004; 14(6)449–457
- Tanaka N. K., Tanimoto H., Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol 2008; 508(5)711–755
- Tettamanti M., Armstrong J. D., Endo K., Yang M. Y., Furukubo-Tokunaga K., Kaiser K., et al. Early development of the Drosophila mushroom bodies, brain centres for associative learning and memory. Development Genes and Evolution 1997; 207(4)242–252
- Thum A. S., Jenett A., Ito K., Heisenberg M., Tanimoto H. Multiple memory traces for olfactory reward learning in Drosophila. J Neurosci 2007; 27(41)11132–11138
- Verkhusha V. V., Otsuna H., Awasaki T., Oda H., Tsukita S., Ito K. An enhanced mutant of red fluorescent protein DsRed for double labeling and developmental timer of neural fiber bundle formation. J Biol Chem 2001; 276(32)29621–29624
- Villella A., Ferri S. L., Krystal J. D., Hall J. C. Functional analysis of fruitless gene expression by transgenic manipulations of Drosophila courtship. Proc Natl Acad Sci U S A 2005; 102(46)16550–16557
- Waddell S., Armstrong J. D., Kitamoto T., Kaiser K., Quinn W. G. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 2000; 103(5)805–813
- Walker J. A., Tchoudakova A. V., McKenney P. T., Brill S., Wu D., Cowley G. S., et al. Reduced growth of Drosophila neurofibromatosis 1 mutants reflects a non-cell-autonomous requirement for GTPase-Activating Protein activity in larval neurons. Genes Dev 2006; 20(23)3311–3323
- Wang J., Lee C. H., Lin S., Lee T. Steroid hormone-dependent transformation of polyhomeotic mutant neurons in the Drosophila brain. Development 2006; 133(7)1231–1240
- Wang J., Ma X., Yang J. S., Zheng X., Zugates C. T., Lee C. H., et al. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron 2004; 43(5)663–672
- Wang J., Zugates C. T., Liang I. H., Lee C. H., Lee T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron 2002; 33(4)559–571
- Wang Y., Chiang A. S., Xia S., Kitamoto T., Tully T., Zhong Y. Blockade of neurotransmission in Drosophila mushroom bodies impairs odor attraction, but not repulsion. Curr Biol 2003; 13(21)1900–1904
- Wang Y., Guo H. F., Pologruto T. A., Hannan F., Hakker I., Svoboda K., et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci 2004; 24(29)6507–6514
- Wang Y., Mamiya A., Chiang A. S., Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci 2008; 28(17)4368–4376
- Watts R. J., Hoopfer E. D., Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron 2003; 38(6)871–885
- Watts R. J., Schuldiner O., Perrino J., Larsen C., Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol 2004; 14(8)678–684
- Whited J. L., Robichaux M. B., Yang J. C., Garrity P. A. Ptpmeg is required for the proper establishment and maintenance of axon projections in the central brain of Drosophila. Development 2007; 134(1)43–53
- Wilson R. I., Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci 2005; 25(40)9069–9079
- Wojtowicz W. M., Flanagan J. J., Millard S. S., Zipursky S. L., Clemens J. C. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell 2004; 118(5)619–633
- Wu C. L., Xia S., Fu T. F., Wang H., Chen Y. H., Leong D., et al. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci 2007; 10(12)1578–1586
- Xi W., Peng Y., Guo J., Ye Y., Zhang K., Yu F., et al. Mushroom bodies modulate salience-based selective fixation behavior in Drosophila. Eur J Neurosci 2008; 27(6)1441–1451
- Yang M. Y., Armstrong J. D., Vilinsky I., Strausfeld N. J., Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron 1995; 15(1)45–54
- Yang Z., Edenberg H. J., Davis R. L. Isolation of mRNA from specific tissues of Drosophila by mRNA tagging. Nucleic Acids Res 2005; 33(17)148
- Yeh E., Gustafson K., Boulianne G. L. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci U S A 1995; 92(15)7036–7040
- Yoshihara M., Ito K. Improved Gal4 screening kit for large-scale generation of enhancer-trap strains. Dros Inf Serv 2000; 83: 199–202
- Yu D., Baird G. S., Tsien R. Y., Davis R. L. Detection of calcium transients in Drosophila mushroom body neurons with camgaroo reporters. J Neurosci 2003; 23(1)64–72
- Zars T., Fischer M., Schulz R., Heisenberg M. Localization of a short-term memory in Drosophila. Science 2000; 288(5466)672–675
- Zars T., Wolf R., Davis R., Heisenberg M. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learn Mem 2000; 7(1)18–31
- Zelhof A. C., Hardy R. W. WASp is required for the correct temporal morphogenesis of rhabdomere microvilli. J Cell Biol 2004; 164(3)417–426
- Zhang K., Guo J. Z., Peng Y., Xi W., Guo A. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science 2007; 316(5833)1901–1904
- Zheng X., Wang J., Haerry T. E., Wu A. Y., Martin J., O'Connor M. B., et al. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 2003; 112(3)303–315
- Zheng X., Zugates C. T., Lu Z., Shi L., Bai J. M., Lee T. Baboon/dSmad2 TGF-beta signaling is required during late larval stage for development of adult-specific neurons. EMBO J 2006; 25(3)615–627
- Zhu S., Chiang A. S., Lee T. Development of the Drosophila mushroom bodies: elaboration, remodeling and spatial organization of dendrites in the calyx. Development 2003; 130(12)2603–2610
- Zhu S., Lin S., Kao C. F., Awasaki T., Chiang A. S., Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell 2006; 127(2)409–422
- Zhu S., Perez R., Pan M., Lee T. Requirement of Cul3 for axonal arborization and dendritic elaboration in Drosophila mushroom body neurons. J Neurosci 2005; 25(16)4189–4197