Abstract
The bacterial flagellum is a motility structure and represents one of the most sophisticated nanomachines in the biosphere. Here, we review the current knowledge on the flagellum, its architecture with respect to differences between Gram-negative and Gram-positive bacteria and other species-specific variations (e.g. the flagellar filament protein, Flagellin). We further focus on the mechanism by which the two nucleotide-binding proteins FlhF and FlhG ensure the correct reproduction of flagella place and number (the flagellation pattern). We will finish the review with an overview of current biotechnological applications, and a perspective of how understanding flagella can contribute to developing modules for synthetic approaches.
Introduction
The bacterial flagellum – a nanomachine with many faces
Many bacteria move by rotating a rigid, helical organelle, the flagellum (Berg & Anderson, Citation1973). The flagellum represents one of the tiniest complex motors in the biosphere and enables bacteria to move through liquids (swimming) (Chevance & Hughes, Citation2008) and highly viscous environments or surfaces (swarming) (Kearns, Citation2010; McCarter, Citation2004). Bacterial motility relies on chemosensory systems, which allow bacteria to act on and respond to environmental changes by a well-studied process named chemotaxis (Sourjik & Wingreen, Citation2012).
However, flagella have a variety of further functions beyond their essential role as motility structures. They seem to contribute to biofilm formation, which seems the common mode of bacterial growth in the environment (Aguilar, Vlamakis, Losick, & Kolter, Citation2007). This fact has long been overlooked as most of the laboratory strains are domesticated and have lost their ability to form biofilms (Aguilar et al., Citation2007). Mature biofilms contain non-motile, but flagellated cells that are embedded in an extracellular matrix (EPS) are composed of polysaccharides, proteins, and DNA (Branda, Vik, Friedman, & Kolter, Citation2005). In a number of organisms (e.g. Escherichia, Bacillus, Pseudomonas) mutations in flagellar genes affect biofilm formation implicating that flagella are a prerequisite for biofilms (e.g. (Barken et al., Citation2008; Kobayashi, Citation2007; Pratt & Kolter, Citation1998; Wood, Gonzalez Barrios, Herzberg, & Lee, Citation2006). Recent work investigating the physiological heterogeneity of E. coli macrocolonies proposes an architectural role of flagella, which seems not related to motility (Serra, Richter, Klauck, Mika, & Hengge, Citation2013). Flagella also represent important virulence factors of pathogenic bacteria although this function appears rather moon lightening. Intuitively, directed motility should be relevant for the pathogen to reach its primary site of infection, and establish the first step of an infection (Moens & Vanderleyden, Citation1996; Ottemann & Miller, Citation1997). On the other side, invasion of various host cells by bacterial pathogens is an ‘old threat’, which is handled by the innate immune system. The latter engages an array of germline-encoded pattern-recognition receptors to detect invariant microbial motifs at the frontline of defense against infection. Extracellular toll-like receptors (TLRs, i.e. TLR5) and components of the intracellular inflammasome complexes (e.g. NLRC4) detect flagellar proteins (e.g. the highly abundant flagellar protein, Flagellin) (e.g. (Lu & Sun, Citation2012). Besides its diverse biological functions, the flagellum represents an amazing biological structure, which has been an inspiring blueprint for a variety of biotechnological innovations (see below).
Architecture of the bacterial flagellum
Flagella are approximately 20 nm in diameter and about 5–20 nm in size (e.g. Doetsch & Sjoblad, Citation1980). The flagellum consists of approximately 30 proteins and its architecture can be divided into: the basal body and the exterior hook, hook-filament junction, and filament structures (Chevance & Hughes, Citation2008; Macnab, Citation2003) (Figure (a)). In the following paragraphs, we will shortly summarize what is known for the individual substructures:
Figure 1. The bacterial flagellum. (a) Flagellar architecture and protein composition of Gram-negative (left) and Gram-positive (right) bacteria. (b) Domain architecture of Flagellin. The variable domains that are inserted into the conserved D1 domain (i.e. D1-N and D1-C in yellow and cyan, respectively) are shown in green. The conserved D0 that comprises N- and C-terminus of Flagellin is shown in red and blue, respectively. (c) Models of the Flagellin structures from S. typhimurium (pdb: 1UCU), Sphingomonas (based on pdb: 2ZBI), and Bacillus subtilis (this work). The color code is as in Figure (b). (d) Cartoon of the flagellar filament with the different Flagellins from Figure (c). The color code is as in Figure (b).
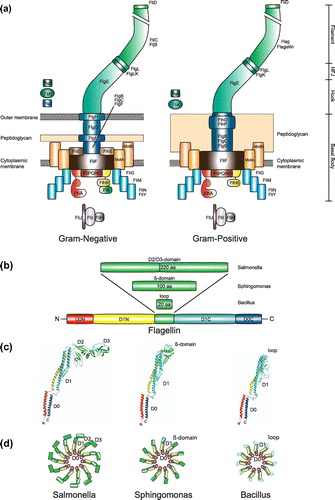
Basal body
The basal body spans from the inner to the outer cytoplasmic membrane in Gram-negative bacteria. In Gram-positive bacteria, the basal body extends from the inner membrane to the peptidoglycan layer (cell wall) (Figure (a)). The conserved MS-ring consists of approximately 26 copies of FliF (Suzuki, Yonekura, & Namba, Citation2004) and is connected to the stator proteins MotA and MotB via the C-ring (Thomas, Francis, Xu, & DeRosier, Citation2006). The stator proteins MotA and MotB (12 copies of each (Stolz & Berg, Citation1991) form a proton channel through the membrane. How the proton motif force (PMF) is used to provide energy for torque generation is poorly understood. The C-ring is located at the cytoplasmic face of the MS-ring and is composed of three proteins: FliM, FliN, and FliG. The C-ring is important for transmitting chemosensory signals into torque generation. FliG achieves connection between C-ring and stators. Changes in the C-ring/stator interface severely impact torque generation (Vartanian, Paz, Fortgang, Abramson, & Dahlquist, Citation2012). Several models predict between 26 and 34 molecules of FliG, about 34 molecules of FliM, and about 100 molecules of FliN in a single flagellum (Kawamoto et al., Citation2013). The diameter of the C-ring is therefore assumed to be approximately 40–45 nm (Thomas et al., Citation2006; Vartanian et al., Citation2012). The number of FliM might vary the motor switching between counterclockwise and clockwise (Lele, Branch, Nathan, & Berg, Citation2012). FliG and FliM seem to be well conserved, while deviations are found for FliN. In B. subtilis, the protein FliY is partly homologous to FliN, but contains an additional N-terminal CheC domain not found in canonical FliN proteins (Bischoff & Ordal, Citation1992). The largest variations between Gram-negative and Gram-positive species seem to occur in the rod architecture, which seems to be reasoned by the different envelope architectures (Figure (a)). In Gram-negatives, the rod consists of a central core part and two rings, the P-, and L-ring, which connect the basal body to peptidoglycan layer and outer membrane, respectively. In S. typhimurium, the rod is built up by FlgB, FlgC, FlgF, FlgG, FlgH, and the rod-adaptor protein FliE (reviewed in: (Macnab, Citation2003)). Several copies of FlgH and FlgL form the L-ring and P-ring, respectively. In Gram-positive bacteria, the architecture of the rod is unclear. The rod proteins FlgB, FlgC, and FlgG have been found in purified basal bodies and outer P- and L-rings are absent (Figure (a)). The rod has a diameter of approximately 10 nm and a length of about 20 nm from the center of the MS-ring (Kubori et al., Citation1997).
Hook
The hook is approximately 55 nm in length and acts as universal joint connecting rod and filament (Berg & Anderson, Citation1973; Samatey et al., Citation2004; Silverman & Simon, Citation1974). In S. typhimurium and E. coli, it is composed of the structural protein FlgE; and the cap protein FlgD is required for assembly (Figure (a)). Two proteins, FlhB and FliK, seem to control the hook length and change export sensitivity upon hook completion (Erhardt, Singer, Wee, Keener, & Hughes, Citation2011). In B. subtilis, however, three proteins FlhO, FlhP, and FlgE are partially homologous to FlgE from S. typhimurium (Courtney, Cozy, & Kearns, Citation2012). FlgE is the main hook protein in B. subtilis. FlgD, FliK, FlhO, and FlhP are also required for proper hook assembly in B. subtilis (Courtney et al., Citation2012). With 71 nm, the hook in B. subtilis is about one-third larger than in S. typhimurium (Kubori et al., Citation1997).
Hook-filament junction
To increase the flexibility and strengthen the connection of hook and filament, the hook-filament junction proteins, FlgK and FlgL, form two zones on the distal end of the hook (Macnab, Citation2003). The cap-protein FliD that later mediates the assembly of Flagellin monomers into the filament, is also associated to FlgL in the beginning of filament formation.
Filament
The filament is the largest part of the bacterial flagellum and consists of more than 20.000 copies of a single protein named Flagellin (also: FliC, FljB, Flagellin, Hag; Figure (a)). Flagellin assembles in a helical pattern of 11 subunits per turn (Yonekura, Maki-Yonekura, & Namba, Citation2003) with the assistance of the pentameric filament-cap protein FliD (Yonekura, Maki-Yonekura, & Namba, Citation2001). Crystal structures of Flagellin from S. typhimurium revealed that the protein is composed of three distinct domains: D0, D1, and D2/3 (Samatey et al., Citation2001) (Figure (b)–(d)). The D0 and D1 domains, which comprise the N- and C-termini of Flagellin, respectively, each consist of elongated α-helices and act together with the D1 domain as scaffolds that stabilize the filament structure (Yonekura et al., Citation2003). The D2/3 domains are inserted into the D1 domain and locate at the outer surface of the filament (Yonekura, Maki-Yonekura, & Namba, Citation2003). While the D0 and D1 domains are highly conserved among different bacterial species, other domains (e.g. the β-domain in Sphingomonas sp. (Maruyama, Momma, Mikami, Hashimoto, & Murata, Citation2008) can replace the D2/3 domain (Figures (b) and (c)). In Bacillus and relatives, these domains are replaced by a short loop of about 20 amino acids (Figure (b) and (c)). Therefore, it seems likely that different bacteria for adaption reasons have modified the variable domain of Flagellin, and with that the surface of their flagellar filament.
Flagellar type-III secretion system
Flagellar biosynthesis relies on secretion of proteins that build the different extracellular structures (Macnab, Citation2004; Minamino, Citation2013). Export of flagellar proteins is handled by a flagellum-specific type-III secretion system (fT3SS). The fT3SS is evolutionarily related to the virulence-associated type-III secretion systems (vT3SS, injectisome) (Buttner, Citation2012; Cornelis, Citation2006). Recent studies that the fT3SS is the ancestor of the vT3SS, which makes the injectisome a diversification for host cell adaption of pathogens (Abby & Rocha, Citation2012). The fT3SS locates within the basal body and is primarily energized by PMF (Minamino & Namba, Citation2008; Paul, Erhardt, Hirano, Blair, & Hughes, Citation2008). Six integral membrane proteins FlhA, FlhB, FliP, FliQ, FliR, and FliO form the pore complex in the inner membrane (Macnab, Citation2004). FlhA coordinates the delivery of late flagella-building blocks (e.g. hook-filament junctions; filament-cap; Flagellin) to the fT3SS (Bange et al., Citation2010; Kinoshita, Hara, Imada, Namba, & Minamino, Citation2013; Minamino et al., Citation2012). FlhB is integral to coordinate the export sequence by switching specificity of the fT3SS from early to late export substrates (Ferris et al., Citation2005). This export switch is triggered by an autocleavage of FlhB (Ferris et al., Citation2005; Zarivach et al., Citation2008), which seems connected to FliK controlled completion of the hook structure (Ferris & Minamino, Citation2006). Although essential for the fT3SS, almost nothing is known on the function of the remaining transmembrane proteins (Macnab, Citation2004). At the cytoplasmic face of the fT3SS, three proteins are associated: FliI, FliH, and FliJ (also: ATPase complex). FliI is a hexameric ATPase (Imada, Minamino, Tahara, & Namba, Citation2007), which seems to play roles in initiating and coordinating the secretion process (Stafford et al., Citation2007; Thomas, Stafford, & Hughes, Citation2004). FliH represents a negative regulator of FliI (Minamino & MacNab, Citation2000). FliJ interacts with the cytosolic domain of FlhA, but can also bind the FliH/FliI complex (Bange et al., Citation2010; Ibuki et al., Citation2013). It plays an important role in coordinating substrate delivery to the fT3SS (and vT3SS) by a yet poorly understood mechanism (Bange et al., Citation2010; Evans & Hughes, Citation2009; Evans, Stafford, Ahmed, Fraser, & Hughes, Citation2006). Taken together, the ATPase complex is closely connected to the pore complex of the fT3SS, not only by direct interaction through FliJ but also in functional terms. However, a precise mechanism for the molecular action of the ATPase complex remains rather mysterious.
Diversity of flagella place and number – the flagellation pattern
Already early in microbial research, it has been noticed that bacterial species differ in their number and localization of flagella (see: Leifson, Citation1960). We will refer to flagella place and number as the ‘flagellation pattern’. Typical flagellation patterns and some representative bacterial species are given in Figure (a). Unusual are the endoflagella of spirochetes, which reside in the periplasm (Figure (a)). Although the architecture of the flagellum seems rather conserved (see above), some contain a flagellar sheath, which can be found in some members of the polar and lophotrichous types (e.g. Vibrio cholera and Helicobacter pylori, respectively; Figure (a)). However, composition and function of the sheath, which appears to be continuous with the outer cell membrane, remain rather enigmatic. No sheath structure has been so far described for peritrichous-flagellated bacteria. Some bacteria (e.g. Azo-/Rhodospirillum and Vibrio) contain dual flagellation systems and are able to change their flagellation pattern depending on environmental conditions (McCarter, Citation2004). For example, Vibrio parahaemolyticus continuously produces one polar flagellum sufficient for swimming in liquid media. However, swarming over surfaces or swimming in highly viscous media induces peritrichous flagella. Taken together, even though evolution developed an enormous number of different bacterial species, which conquered almost every environmental niche thinkable, most bacteria rely on one of these few described flagellation patterns.
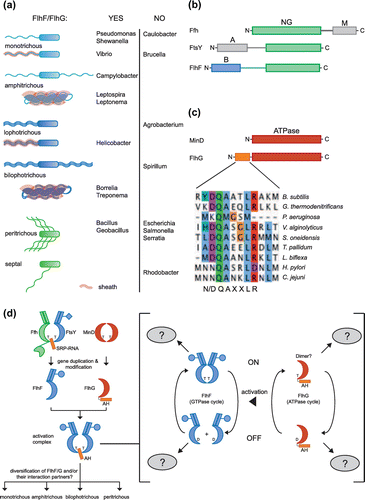
Figure 2. Bacterial flagellation patterns. (a) Polar flagellation patterns are colored in blue, and lateral patterns are marked in green. Sheathed flagella are shadowed in red. The listed organisms are grouped according to the occurrence and absence of FlhF and FlhG. (b) Domain architecture of SRP-GTPases. The conserved NG-domain is in green. The B-domain of FlhF is in blue. M- and A- domains of Ffh and FtsY, respectively, are in grey. (c) Domain architecture of MinD and FlhG. The N-terminus of FlhG contains the conserved ‘DQAxxLR’ motif. (d) Molecular evolution and diversification of the regulatory circuit of FlhF and FlhG. ‘T’ and ‘D’ stand for ATP/GTP and ADP/GDP, respectively. For further description see text.
FlhF and FlhG-dependent surveillance of the flagellation pattern
Maintaining the correct number and placement of flagella during cell division is essential and might require a tight regulation of the flagellation pattern. However, the molecular mechanisms are far from being understood. However, FlhF and FlhG (also: FleN, YlxH, MinD2) play fundamental roles in this process (reviewed in: (Bulyha, Hot, Huntley, & Sogaard-Andersen, Citation2011; Kazmierczak & Hendrixson, Citation2013)). There are a variety of functional studies in many different organisms available, which for reasons of clarity have been summarized in Table . Interestingly, both proteins – although conserved among the different bacterial species – are essential to establishing polar (for example, Vibrio sp.), lophothrichous (for example, Helicobacter) as well as peritrichous flagellation (for example, Bacillus sp.) (Figure (a), Table ). We note that FlhF and FlhG also are present in genomes of endoflagella-containing species, although no functional studies are available so far. Overall, both proteins are found in ~30% of all bacterial genomes from all clades (except the α-proteobacteria, e.g. Caulobacter) as direct genomic neighbors, with flhG as the adjacent downstream gene of flhF (Bange et al., Citation2011). However, the molecular mechanisms by which FlhF and FlhG are able to regulate these different flagellation patterns are unclear.
Table 1. Roles of FlhF and FlhG in different bacterial species.
The signal recognition particle (SRP)-type GTPase FlhF
FlhF and FlhG belong to the group of SIMIBI class (after: Signal recognition particle, MinD and BioD) of nucleotide-binding proteins (Leipe, Wolf, Koonin, & Aravind, Citation2002). FlhF together with Ffh (SRP54 in archaea, eukaryotes) and FtsY (SRP in eukaryotes) belongs to the SIMIBI subgroup of the signal recognition particle (SRP)-type GTPases. SRP-GTPases share the conserved NG domain (Bange, Petzold, Wild, Parlitz, & Sinning, Citation2007; Freymann, Keenan, Stroud, & Walter, Citation1997; Montoya, Svensson, Luirink, & Sinning, Citation1997) (Figure (b)). All three SRP-GTPases contain additional domains, which reflect their specific functional roles (Figure (b)). A GTP-dependent heterodimer of Ffh and FtsY regulates the co-translational targeting/insertion of membrane proteins by mediating the transfer of a ribosome-nascent chain complex onto a vacant translocation channel in the membrane (Akopian, Shen, Zhang, & Shan, Citation2013; Grudnik, Bange, & Sinning, Citation2009). Ffh contains a methionine-rich domain (M-domain) at its C-terminus, which serves to signal peptide recognition and SRP-RNA binding (Batey, Rambo, Lucast, Rha, & Doudna, Citation2000; Hainzl, Huang, Merilainen, Brannstrom, & Sauer-Eriksson, Citation2011; Janda et al., Citation2010). In E. coli, FtsY contains an acidic and natively unfolded domain at its N-terminus (A-domain; Stjepanovic et al., Citation2011), which mediates the interaction with anionic membrane phospholipids (de Leeuw et al., Citation2000). However, length and charge of the A-domain differ among the different bacterial species (Grudnik et al., Citation2009). Similarly to FtsY, FlhF also contains a natively unfolded but rather basic domain at its N-terminus (B-domain, (Bange, Petzold, Wild, Parlitz, & Sinning, Citation2007). We note that also the B-domain of FlhF varies among the different bacterial species. Although functionally important (Green et al., Citation2009), no molecular role for the B-domain of FlhF has yet been defined. FlhF forms a GTP-dependent homodimer (Bange et al., Citation2007; Shen, Chern, Silva, & Ronald, Citation2001), which exhibits a similar structural architecture as the heterodimer of Ffh and FtsY (Bange et al., Citation2007). However, the cellular function of the FlhF homodimer is far from being understood.
The MinD/ParA-type ATPase FlhG
In silico analysis revealed that FlhG is a close homologue of the MinD ATPase, which hallmarks another SIMIBI subgroup (Leipe et al., Citation2002). MinD serves the correct positioning of the cell division plane during bacterial cell division (Lutkenhaus, Citation2007). In E. coli, MinD together with MinC and MinE undergoes a coupled cell-to-cell pole oscillation, which provides the lowest concentration of the cell division inhibitor MinC exactly at midcell (Lutkenhaus, Citation2008). Oscillation starts with the ATP-dependent membrane association of MinD dimers in close vicinity to one pole. Recruitment of MinC by MinD drives cell division inhibition at place by destabilizing FtsZ ring formation (Gregory, Becker, & Pogliano, Citation2008; Shen & Lutkenhaus, Citation2010). MinE triggers ATP hydrolysis of MinD, which drives disassembly of the MinCD complex initiating another wave of the oscillation pattern (Park et al., Citation2011; Park, Wu, Lovell, & Lutkenhaus, Citation2012). However, oscillation of the Min complex has only been observed in E. coli and close relatives. In Bacillus subtilis, the Min system seems to regulate cell division by a rather static arrangement. Also, the composition differs, because MinE is absent and functionally replaced by DivIVA (Marston, Thomaides, Edwards, Sharpe, & Errington, Citation1998). Sequence alignments suggest that FlhG is a bona fide MinD-type ATPase carrying an additional N-terminus extension (Bange et al., Citation2011). This extension varies in length from around 5 – 30 amino acids among the different bacterial species. It includes a conserved motif (DQAxxLR, x is any amino acid, Figure (b)) in a variety of bacterial species except Pseudomonas sp. and close relatives (Schniederberend, Abdurachim, Murray, & Kazmierczak, Citation2013).
Molecular evolution of regulatory circuits forming flagellation patterns
Recent structural and computational work showed that FlhF and FlhG emerged from the SRP-GTPase Ffh and the MinD ATPase, respectively (Bange et al., Citation2011; Leipe et al., Citation2002). Both proteins constitute a novel regulatory module, which originated from two well-established and conserved pathways (i.e. SRP- and Min-system; (Bange et al., Citation2011; Bange & Sinning, Citation2013)). Only modest modifications were necessary during the course of molecular evolution: (i) FlhF changed into a homodimeric SRP-GTPase with the newly acquired B-domain at its N-terminus and (ii) FlhG acquired an N-terminal extension with the conserved DQAxxLR motif (Figure (b) and (c)). This conserved motif is necessary and sufficient to stimulate GTP hydrolysis in FlhF through the conserved glutamine (Bange et al., Citation2011). In this regard, FlhG represents a functional homologue of the SRP-RNA, which is required to stimulate GTP hydrolysis with the Ffh/FtsY SRP-GTPase heterodimer (Voigts-Hoffmann et al., Citation2013). Both, FlhG and SRP-RNA use strikingly similar mechanisms to co-catalytically stimulate the FlhF homodimer and the Ffh/FtsY heterodimer, respectively (Bange & Sinning, Citation2013). The FlhF GTPase can be viewed as a molecular switch with two distinct states: (i) the GTP-bound ‘ON’ state and (ii) the GDP-bound ‘OFF’ state (Figure (d), right). Both states might allow FlhF to interact with different partner/effectors to fulfill its precise function within the context of the cell. However, no interaction partner of FlhF (other than FlhG) is known to date. Transition from ‘ON’ to ‘OFF’ state is regulated by FlhG, which stimulates GTP hydrolysis in FlhF (Bange et al., Citation2011). Assuming FlhG a functional ATPase, a molecular switch with ATP-bound ‘ON’ and ADP-bound ‘OFF’ state might also be true for this protein (Figure (d), right). The interception of both switches would allow the integration of a multitude of cellular signals. However, also for FlhG no interaction partners are known. Another fascinating question arises when thinking about how FlhF and FlhG can establish/maintain different flagellar patterns in different bacterial species (Figure (d), left). Although only little is known on the molecular determinants of pattern formation, we speculate that this diversification is not only found in the subtle variations of FlhF and FlhG among different species, but especially in the way these proteins are embedded in the regulatory network of the cell. Interaction partners of FlhF and FlhG play a key role in interpreting the inputs and outputs of that switch and in transferring this information into a flagellation pattern. We therefore predict that the interaction partners of FlhF and FlhG must at least partially differ or deviate between the different patterns, and might therefore be pattern specific.
The flagellum as a hub for synthetic approaches
Cell appendages like the archaeal or bacterial flagella (archaella), pili, or injectisomes have a long history as subjects of biotechnological approaches (Van Gerven, Waksman, & Remaut, Citation2011). Their properties as covalently or non-covalently linked protein subunits in long repetitive assemblies, as well as the capability to secrete peptides and proteins or to transport DNA have led to the development of many different applications in e.g. DNA transfer and uptake, protein transport and delivery, charge transport, protein and peptide display, and filaments as therapeutic targets.
The flagellar-type-III secretion system (fT3SS)
The fT3SS can be converted into an export machinery for peptides and proteins unrelated to the flagellum (e.g. enolase, green fluorescent protein (GFP)). For this reason, the genes encoding Flagellin and the filament-cap protein, FliD, were deleted in E. coli, and the export regulatory 173-bp untranslated DNA fragment upstream fliC as well as a transcriptional terminator from fliC were fused to the gene of interest resulting in a strain that was capable to efficiently secrete polypeptides up to 434 residues in the surrounding growth medium at concentration levels ranging from 1 to 15 mg/l (Majander et al., Citation2005). In a second approach, N-terminal FliC hybrid proteins e.g. N-terminal FliC combined with the eukaryotic GFP (238 residues) were generated to enable export up to >50% of total secreted protein (Majander et al., Citation2005). This method elegantly allows the production of a variety of recombinant proteins at high concentration and purity.
Flagellar filament as a versatile scaffold and nanopolymer
The flagellar filament is a long and tubular polymer that can extend to over 20,000 copies of Flagellin. By modifying the surface exposed and less conserved variable D3 region of the major filament subunit FliC, several peptide or protein copies (up to 20,000) can be displayed in close arrangements. This massive peptide and protein scaffold has been used in the development of recombinant filaments for vaccination in combination with the intrinsic feature of the Salmonella flagellum as a potent immunogen (Newton, Jacob, & Stocker, Citation1989). Also, filament subunits were modified to scaffold protein functions, e.g. biomineralization of bone protein-derived peptides. By tuning the surface characteristics of the flagellar filament, nanofibers of defined properties can be generated, e.g. for the morphology-controlled synthesis of silica nanotubes by flagellar biotemplates (Dong et al., 2012). Additionally, the filament-capping protein FliD can be modified in its variable regions to convert the filament in a multihybrid display system in which up to three foreign peptides can be integrated (Majander, Korhonen, & Westerlund-Wikstrom, Citation2005). Comparable to phage display systems, where random peptides are displayed on the virion surface, similar random peptide display systems have been developed for high-throughput screening of random peptide libraries e.g. for nickel bisorption (Dong et al., Citation2006). Furthermore, random epitopes were exposed on the filament surface to explore protein–protein interactions (Lu et al., Citation1995). In terms of the length of displayable peptides, the tolerated size might be approximately 60 aa (Georgiou et al., Citation1997). However, successful display of a 302 aa YadA adhesin Flagellin fusion was reported (Westerlund-Wikstrom et al., Citation1997). A first step towards self-assembling three-dimensional scaffolds has been done by engineering filaments to form higher level bundle assemblies by inducing self- or dis-assembly of Cys-loop Flagellin-modified filaments through oxidizing or reducing conditions (Kumara, Srividya, Muralidharan, & Tripp, Citation2006). Taken together, the flagellar filament represents a versatile scaffold for many biotechnological applications.
FlhF and FlhG as blueprint for synthetic ‘navigation’ modules
An important challenge in synthetic biology is the design of modules, which allow a defined positioning of structures, such as membrane-bound enzymatic cascades, within cells. These synthetic modules should be simple to allow easy implementation into the host or even into future synthetic cells. Therefore, it would be great to establish simple and autonomous parts that allow the recruitment of synthetic components to cellular membranes. When considering synthetic membrane positioning and polarization, FlhF and FlhG may be the promising candidates to target proteins or their complexes in a controllable pattern and stoichiometry. Nature employs FlhF and FlhG to define a variety of different flagellar patterns. Understanding the underlying molecular mechanisms might offer the basis for synthetic ‘navigation systems’. In a wide future perspective, these navigation systems might then serve as standardized systems for the positioning of complexes or biological nanomachines other then the flagellum itself.
Acknowledgments
The LOEWE program of the state of Hesse/Germany supported this work (to G.B.). J.S. is a fellow of the Fonds der Chemischen Industrie (FCI). The authors thank M. Rakwalska-Bange, Milena Stephan, and Felix Dempwolff for critical reading of the manuscript. We apologize to all authors whose work could not be cited due to space restrictions.
The authors declare no financial conflict of interest.
References
- Abby, S. S., & Rocha, E. P. (2012). The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLoS Genetics, 8(9), e1002983.10.1371/journal.pgen.1002983
- Aguilar, C., Vlamakis, H., Losick, R., & Kolter, R. (2007). Thinking about Bacillus subtilis as a multicellular organism. Current Opinion in Microbiology, 10, 638–643.10.1016/j.mib.2007.09.006
- Akopian, D., Shen, K., Zhang, X., & Shan, S. O. (2013). Signal recognition particle: An essential protein-targeting machine. Annual Review of Biochemistry, 82, 693–721.10.1146/annurev-biochem-072711-164732
- van Amsterdam, K., & van der Ende, A. (2004). Helicobacter pylori HP1034 (ylxH) is required for motility. Helicobacter, 9, 387–395.10.1111/hel.2004.9.issue-5
- Balaban, M., & Hendrixson, D. R. (2011). Polar flagellar biosynthesis and a regulator of flagellar number influence spatial parameters of cell division in Campylobacter jejuni. PLoS Pathogens, 7, e1002420.10.1371/journal.ppat.1002420
- Balaban, M., Joslin, S.N., & Hendrixson, D. R. (2009). FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. Journal of Bacteriology, 191, 6602–6611.10.1128/JB.00884-09
- Bange, G., Kummerer, N., Engel, C., Bozkurt, G., Wild, K., & Sinning, I. (2010). FlhA provides the adaptor for coordinated delivery of late flagella building blocks to the type III secretion system. Proceedings of the National Academy of Sciences, 107, 11295–11300.10.1073/pnas.1001383107
- Bange, G., Kümmerer, N., Grudnik, P., Lindner, R., Petzold, G., & Kressler, D. (2011). Structural basis for the molecular evolution of SRP-GTPase activation by protein. Nature Structural & Molecular Biology, 18, 1376–1380.10.1038/nsmb.2141
- Bange, G., Petzold, G., Wild, K., Parlitz, R. O., & Sinning, I. (2007). The crystal structure of the third signal-recognition particle GTPase FlhF reveals a homodimer with bound GTP. Proceedings of the National Academy of Sciences, 104, 13621–13625.10.1073/pnas.0702570104
- Bange, G., & Sinning, I. (2013). SIMIBI twins in protein targeting and localization. Nature Structural & Molecular Biology, 20, 776–780.10.1038/nsmb.2605
- Barken, K. B., Pamp, S. J., Yang, L., Gjermansen, M., Bertrand, J. J., Klausen, M., … Tolker-Nielsen, T. (2008). Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environmental Microbiology, 10, 2331–2343.10.1111/emi.2008.10.issue-9
- Batey, R. T., Rambo, R. P., Lucast, L., Rha, B., & Doudna, J. A. (2000). Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science, 287, 1232–1239.10.1126/science.287.5456.1232
- Berg, H. C., & Anderson, R. A. (1973). Bacteria swim by rotating their flagellar filaments. Nature, 245, 380–382.10.1038/245380a0
- Bischoff, D. S., & Ordal, G. W. (1992). Identification and characterization of FliY, a novel component of the Bacillus subtilis flagellar switch complex. Molecular Microbiology, 6, 2715–2723.10.1111/mmi.1992.6.issue-18
- Branda, S. S., Vik, S., Friedman, L., & Kolter, R. (2005). Biofilms: The matrix revisited. Trends in Microbiology, 13, 20–26.10.1016/j.tim.2004.11.006
- Bulyha, I., Hot, E., Huntley, S., & Sogaard-Andersen, L. (2011). GTPases in bacterial cell polarity and signalling. Current Opinion in Microbiology, 14, 726–733.10.1016/j.mib.2011.09.001
- Buttner, D. (2012). Protein export according to schedule: Architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiology and Molecular Biology Reviews, 76, 262–310.10.1128/MMBR.05017-11
- Chevance, F. F., & Hughes, K. T. (2008). Coordinating assembly of a bacterial macromolecular machine. Nature Reviews Microbiology, 6, 455–465.10.1038/nrmicro1887
- Cornelis, G. R. (2006). The type III secretion injectisome. Nature Reviews Microbiology, 4, 811–825.10.1038/nrmicro1526
- Correa, N. E., Peng, F., & Klose, K. E. (2005). Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. Journal of Bacteriology, 187, 6324–6332.10.1128/JB.187.18.6324-6332.2005
- Courtney, C. R., Cozy, L. M., & Kearns, D. B. (2012). Molecular characterization of the flagellar hook in Bacillus subtilis. Journal of Bacteriology, 194, 4619–4629.10.1128/JB.00444-12
- de Leeuw, E., te Kaat, K., Moser, C., Menestrina, G., Demel, R., de Kruijff, B., … Sinning, I. (2000). Anionic phospholipids are involved in membrane association of FtsY and stimulate its GTPase activity. The EMBO Journal, 19, 531–541.10.1093/emboj/19.4.531
- Doetsch, R. N., & Sjoblad, R. D. (1980). Flagellar structure and function in eubacteria. Annual Review of Microbiology, 34, 69–108.10.1146/annurev.mi.34.100180.000441
- Dong, J., Liu, C., Zhang, J., Xin, Z. T., Yang, G., Gao, B., … Xue, Y. N. (2006). Selection of novel nickel-binding peptides from flagella displayed secondary peptide library. Chemical Biology Drug Design, 68, 107–112.10.1111/jpp.2006.68.issue-2
- Erhardt, M., Singer, H. M., Wee, D. H., Keener, J. P., & Hughes, K. T. (2011). An infrequent molecular ruler controls flagellar hook length in Salmonella enterica. The EMBO Journal, 30, 2948–2961.10.1038/emboj.2011.185
- Evans, L. D., & Hughes, C. (2009). Selective binding of virulence type III export chaperones by FliJ escort orthologues InvI and YscO. FEMS Microbiology Letters, 293, 292–297.10.1111/fml.2009.293.issue-2
- Evans, L. D., Stafford, G. P., Ahmed, S., Fraser, G. M., & Hughes, C. (2006). An escort mechanism for cycling of export chaperones during flagellum assembly. Proceedings of the National Academy of Sciences, 103, 17474–17479.10.1073/pnas.0605197103
- Ferris, H. U., & Minamino, T. (2006). Flipping the switch: Bringing order to flagellar assembly. Trends in Microbiology, 14, 519–526.10.1016/j.tim.2006.10.006
- Ferris, H. U., Furukawa, Y., Minamino, T., Kroetz, M. B., Kihara, M., Namba, K., & Macnab, R. M. (2005). FlhB regulates ordered export of flagellar components via autocleavage mechanism. Journal of Biological Chemistry, 280, 41236–41242.10.1074/jbc.M509438200
- Freymann, D. M., Keenan, R. J., Stroud, R. M., & Walter, P. (1997). Structure of the conserved GTPase domain of the signal recognition particle. Nature, 385, 361–364.10.1038/385361a0
- Georgiou, G., Stathopoulos, C., Daugherty, P. S., Nayak, A. R., Iverson, B. L., & Curtiss 3rd, R. (1997). Display of heterologous proteins on the surface of microorganisms: From the screening of combinatorial libraries to live recombinant vaccines. Nature Biotechnology, 15, 29–34.10.1038/nbt0197-29
- Green, J. C., Kahramanoglou, C., Rahman, A., Pender, A. M., Charbonnel, N., & Fraser, G. M. (2009). Recruitment of the earliest component of the bacterial flagellum to the old cell division pole by a membrane-associated signal recognition particle family GTP-binding protein. Journal of Molecular Biology, 391, 679–690.10.1016/j.jmb.2009.05.075
- Gregory, J. A., Becker, E. C., & Pogliano, K. (2008). Bacillus subtilis MinC destabilizes FtsZ-rings at new cell poles and contributes to the timing of cell division. Genes & Development, 22, 3475–3488.10.1101/gad.1732408
- Grudnik, P., Bange, G., & Sinning, I. (2009). Protein targeting by the signal recognition particle. Biological Chemistry, 390, 775–782.
- Guttenplan, S. B., Shaw, S., & Kearns, D. B. (2013). The cell biology of peritrichous flagella in Bacillus subtilis. Molecular Microbiology, 87, 211–229.10.1111/mmi.2013.87.issue-1
- Hainzl, T., Huang, S., Meriläinen, G., Brännström, K., & Sauer-Eriksson, A. E. (2011). Structural basis of signal-sequence recognition by the signal recognition particle. Nature Structural & Molecular Biology, 18, 389–391.10.1038/nsmb.1994
- Ibuki, T., Uchida, Y., Hironaka, Y., Namba, K., Imada, K., & Minamino, T. (2013). Interaction between FliJ and FlhA, components of the bacterial flagellar type III export apparatus. Journal of Bacteriology, 195, 466–473.10.1128/JB.01711-12
- Imada, K., Minamino, T., Tahara, A., & Namba, K. (2007). Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits. Proceedings of the National Academy of Sciences, 104, 485–490.10.1073/pnas.0608090104
- Janda, C. Y., Li, J., Oubridge, C., Hernández, H., Robinson, C. V., & Nagai, K. (2010). Recognition of a signal peptide by the signal recognition particle. Nature, 465, 507–510.10.1038/nature08870
- Kawamoto, A., Morimoto, Y. V., Miyata, T., Minamino, T., Hughes, K. T., Kato, T., & Namba, K. (2013). Common and distinct structural features of Salmonella injectisome and flagellar basal body. Scientific Reports, 3, 1–6.
- Kazmierczak, B. I., & Hendrixson, D. R. (2013). Spatial and numerical regulation of flagellar biosynthesis in polarly flagellated bacteria. Molecular Microbiology, 88, 655–663.10.1111/mmi.2013.88.issue-4
- Kearns, D. B. (2010). A field guide to bacterial swarming motility. Nature Reviews Microbiology, 8, 634–644.10.1038/nrmicro2405
- Kinoshita, M., Hara, N., Imada, K., Namba, K., & Minamino, T. (2013). Interactions of bacterial flagellar chaperone-substrate complexes with FlhA contribute to co-ordinating assembly of the flagellar filament. Molecular Microbiology, 90, 1249–1261.10.1111/mmi.2013.90.issue-6
- Kobayashi, K. (2007). Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Molecular Microbiology, 66, 395–409.10.1111/mmi.2007.66.issue-2
- Kubori, T., Okumura, M., Kobayashi, N., Nakamura, D., Iwakura, M., & Aizawa, S. I. (1997). Purification and characterization of the flagellar hook-basal body complex of Bacillus subtilis. Molecular Microbiology, 24, 399–410.10.1046/j.1365-2958.1997.3341714.x
- Kumara, M. T., Srividya, N., Muralidharan, S., & Tripp, B. C. (2006). Bioengineered flagella protein nanotubes with cysteine loops: Self-assembly and manipulation in an optical trap. Nano Letters, 6, 2121–2129.10.1021/nl060598u
- Kusumoto, A., Kamisaka, K., Yakushi, T., Terashima, H., Shinohara, A., & Homma, M. (2006). Regulation of polar flagellar number by the flhF and flhG genes in Vibrio alginolyticus. Journal of Biochemistry, 139, 113–121.10.1093/jb/mvj010
- Kusumoto, A., Shinohara, A., Terashima, H., Kojima, S., Yakushi, T., & Homma, M. (2008). Collaboration of FlhF and FlhG to regulate polar-flagella number and localization in Vibrio alginolyticus. Microbiology, 154, 1390–1399.10.1099/mic.0.2007/012641-0
- Leifson, E. (1960). Atlas of bacterial flagellation. Record number: 19601101988.
- Leipe, D. D., Wolf, Y. I., Koonin, E. V., & Aravind, L. (2002). Classification and evolution of P-loop GTPases and related ATPases. Journal of Molecular Biology, 317, 41–72.10.1006/jmbi.2001.5378
- Lele, P. P., Branch, R. W., Nathan, V. S., & Berg, H. C. (2012). Mechanism for adaptive remodeling of the bacterial flagellar switch. Proceedings of the National Academy of Sciences, 109, 20018–20022.10.1073/pnas.1212327109
- Lu, J., & Sun, P. D. (2012). The structure of the TLR5-flagellin complex: A new mode of pathogen detection, conserved receptor dimerization for signaling. Science Signalling, 5, e11.
- Lu, Z., Murray, K. S., Cleave, V., LaVallie, E. R., Stahl, M. L., & McCoy, J. M. (1995). Expression of thioredoxin random peptide libraries on the Escherichia coli cell surface as functional fusions to flagellin: A system designed for exploring protein–protein interactions. Bio/Technology , 13, 366–372.10.1038/nbt0495-366
- Lutkenhaus, J. (2007). Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annual Review of Biochemistry, 76, 539–562.10.1146/annurev.biochem.75.103004.142652
- Lutkenhaus, J. (2008). Min oscillation in bacteria. Advances in Experimental Medicine and Biology, 641, 49–61.
- Macnab, R. M. (2003). How bacteria assemble flagella. Annual Review of Microbiology, 57, 77–100.10.1146/annurev.micro.57.030502.090832
- Macnab, R. M. (2004). Type III flagellar protein export and flagellar assembly. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research, 1694, 207–217.10.1016/j.bbamcr.2004.04.005
- Majander, K., Anton, L., Antikainen, J., Lang, H., Brummer, M., Korhonen, T. K., & Westerlund-Wikstrom, B. (2005). Extracellular secretion of polypeptides using a modified Escherichia coli flagellar secretion apparatus. Nature Biotechnology, 23, 475–481.10.1038/nbt1077
- Majander, K., Korhonen, T. K., & Westerlund-Wikstrom, B. (2005). Simultaneous display of multiple foreign peptides in the FliD capping and FliC filament proteins of the Escherichia coli flagellum. Applied and Environmental Microbiology, 71, 4263–4268.10.1128/AEM.71.8.4263-4268.2005
- Marston, A. L., Thomaides, H. B., Edwards, D. H., Sharpe, M. E., & Errington, J. (1998). Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes & Development, 12, 3419–3430.10.1101/gad.12.21.3419
- Maruyama, Y., Momma, M., Mikami, B., Hashimoto, W., & Murata, K. (2008). Crystal structure of a novel bacterial cell-surface flagellin binding to a polysaccharide. Biochemistry, 47, 1393–1402.10.1021/bi701872x
- McCarter, L. L. (2004). Dual flagellar systems enable motility under different circumstances. Journal of Molecular Microbiology and Biotechnology, 7, 18–29.10.1159/000077866
- Minamino, T. (2013). Protein export through the bacterial flagellar type III export pathway. Biochimca et Biophysica Acta.
- Minamino, T., Kinoshita, M., Hara, N., Takeuchi, S., Hida, A., Koya, S., … Namba, K. (2012). Interaction of a bacterial flagellar chaperone FlgN with FlhA is required for efficient export of its cognate substrates. Molecular Microbiology, 83, 775–788.10.1111/j.1365-2958.2011.07964.x
- Minamino, T., & MacNab, R. M. (2000). FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Molecular Microbiology, 37, 1494–1503.10.1046/j.1365-2958.2000.02106.x
- Minamino, T., & Namba, K. (2008). Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature, 451, 485–488.10.1038/nature06449
- Moens, S., & Vanderleyden, J. (1996). Functions of bacterial flagella. Critical Reviews in Microbiology, 22, 67–100.10.3109/10408419609106456
- Montoya, G., Svensson, C., Luirink, J., & Sinning, I. (1997). Crystal structure of the NG domain from the signal-recognition particle receptor FtsY. Nature, 385, 365–368.10.1038/385365a0
- Murray, T. S., & Kazmierczak, B. I. (2006). FlhF is required for swimming and swarming in Pseudomonas aeruginosa. Journal of Bacteriology, 188, 6995–7004.10.1128/JB.00790-06
- Newton, S. M., Jacob, C. O., & Stocker, B. A. (1989). Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science, 244, 70–72.10.1126/science.2468182
- Ottemann, K. M., & Miller, J. F. (1997). Roles for motility in bacterial-host interactions. Molecular Microbiology, 24, 1109–1117.10.1046/j.1365-2958.1997.4281787.x
- Pandza, S., Baetens, M., Park, C. H., Au, T., Keyhan, M., & Matin, A. (2000). The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Molecular Microbiology, 36, 414–423.10.1046/j.1365-2958.2000.01859.x
- Park, K. T., Wu, W., Battaile, K. P., Lovell, S., Holyoak, T., & Lutkenhaus, J. (2011). The Min oscillator uses MinD-dependent conformational changes in MinE to spatially regulate cytokinesis. Cell, 146, 396–407.10.1016/j.cell.2011.06.042
- Park, K. T., Wu, W., Lovell, S., & Lutkenhaus, J. (2012). Mechanism of the asymmetric activation of the MinD ATPase by MinE. Molecular Microbiology, 85, 271–281.10.1111/mmi.2012.85.issue-2
- Paul, K., Erhardt, M., Hirano, T., Blair, D. F., & Hughes, K. T. (2008). Energy source of flagellar type III secretion. Nature, 451, 489–492.10.1038/nature06497
- Pratt, L. A., & Kolter, R. (1998). Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Molecular Microbiology, 30, 285–293.10.1046/j.1365-2958.1998.01061.x
- Samatey, F. A., Imada, K., Nagashima, S., Vonderviszt, F., Kumasaka, T., Yamamoto, M., & Namba, K. (2001). Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature, 410, 331–337.10.1038/35066504
- Samatey, F. A., Matsunami, H., Imada, K., Nagashima, S., Shaikh, T. R., Thomas, D., … Namba, K. (2004). Structure of the bacterial flagellar hook and implication for the molecular universal joint mechanism. Nature, 431, 1062–1068.10.1038/nature02997
- Schniederberend, M., Abdurachim, K., Murray, T. S., & Kazmierczak, B. I. (2013). The GTPase activity of FlhF is dispensable for flagellar localization, but not motility, in Pseudomonas aeruginosa. Journal of Bacteriology, 195, 1051–1060.10.1128/JB.02013-12
- Serra, D. O., Richter, A. M., Klauck, G., Mika, F., & Hengge, R. (2013). Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. MBio, 4, e00103–00113.
- Shen, B., & Lutkenhaus, J. (2010). Examination of the interaction between FtsZ and MinCN in E. coli suggests how MinC disrupts Z rings. Molecular Microbiology, 75, 1285–1298.10.1111/mmi.2010.75.issue-5
- Shen, Y., Chern, M., Silva, F. G., & Ronald, P. (2001). Isolation of a Xanthomonas oryzae pv. oryzae flagellar operon region and molecular characterization of flhF. Molecular Plant-Microbe Interactions, 14, 204–213.10.1094/MPMI.2001.14.2.204
- Silverman, M., & Simon, M. (1974). Flagellar rotation and the mechanism of bacterial motility. Nature, 249, 73–74.10.1038/249073a0
- Sourjik, V., & Wingreen, N. S. (2012). Responding to chemical gradients: Bacterial chemotaxis. Current Opinion in Cell Biology, 24, 262–268.10.1016/j.ceb.2011.11.008
- Stafford, G. P., Evans, L. D., Krumscheid, R., Dhillon, P., Fraser, G. M., & Hughes, C. (2007). Sorting of early and late flagellar subunits after docking at the membrane ATPase of the type III export pathway. Journal of Molecular Biology, 374, 877–882.10.1016/j.jmb.2007.09.080
- Stjepanovic, G., Kapp, K., Bange, G., Graf, C., Parlitz, R., Wild, K., … Sinning, I. (2011). Lipids trigger a conformational switch that regulates signal recognition particle (SRP)-mediated protein targeting. Journal of Biological Chemistry, 286, 23489–23497.10.1074/jbc.M110.212340
- Stolz, B., & Berg, H. C. (1991). Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. Journal of Bacteriology, 173, 7033–7037.
- Suzuki, H., Yonekura, K., & Namba, K. (2004). Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. Journal of Molecular Biology, 337, 105–113.10.1016/j.jmb.2004.01.034
- Thomas, J., Stafford, G. P., & Hughes, C. (2004). Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export. Proceedings of the National Academy of Sciences, 101, 3945–3950.10.1073/pnas.0307223101
- Thomas, D. R., Francis, N. R., Xu, C., & DeRosier, D. J. (2006). The three-dimensional structure of the flagellar rotor from a clockwise-locked mutant of Salmonella enterica serovar Typhimurium. Journal of Bacteriology, 188, 7039–7048.10.1128/JB.00552-06
- Van Gerven, N., Waksman, G., & Remaut, H. (2011). Pili and flagella biology, structure, and biotechnological applications. Progress in Molecular Biology and Translational Science, 103, 21–72.10.1016/B978-0-12-415906-8.00005-4
- Vartanian, A. S., Paz, A., Fortgang, E. A., Abramson, J., & Dahlquist, F. W. (2012). Structure of flagellar motor proteins in complex allows for insights into motor structure and switching. Journal of Biological Chemistry, 287, 35779–35783.10.1074/jbc.C112.378380
- von Moltke, J., Ayres, J. S., Kofoed, E.M., Chavarría-Smith, J., & Vance, R. E. (2013). Recognition of bacteria by inflammasomes. Annual Review of Immunology, 31, 73–106.10.1146/annurev-immunol-032712-095944
- Voigts-Hoffmann, F., Schmitz, N., Shen, K., Shan, S. O., Ataide, S. F., & Ban, N. (2013). The structural basis of FtsY recruitment and GTPase activation by SRP RNA. Molecular Cell, 52, 643–654.10.1016/j.molcel.2013.10.005
- Westerlund-Wikstrom, B., Tanskanen, J., Virkola, R., Hacker, J., Lindberg, M., Skurnik, M., & Korhonen, T. K. (1997). Functional expression of adhesive peptides as fusions to Escherichia coli flagellin. Protein Engineering Design and Selection, 10, 1319–1326.10.1093/protein/10.11.1319
- Wood, T. K., González Barrios, A. F., Herzberg, M., & Lee, J. (2006). Motility influences biofilm architecture in Escherichia coli. Applied Microbiology and Biotechnology, 72, 361–367.10.1007/s00253-005-0263-8
- Yonekura, K., Maki-Yonekura, S., & Namba, K. (2001). Structure analysis of the flagellar cap-filament complex by electron cryomicroscopy and single-particle image analysis. Journal of Structural Biology, 133, 246–253.10.1006/jsbi.2000.4345
- Yonekura, K., Maki-Yonekura, S., & Namba, K. (2003). Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature, 424, 643–650.10.1038/nature01830
- Zarivach, R., Deng, W., Vuckovic, M., Felise, H. B., Nguyen, H. V., Miller, S., … Strynadka, N. C. (2008). Structural analysis of the essential self-cleaving type III secretion proteins EscU and SpaS. Nature, 453, 124–127.10.1038/nature06832

