Abstract
Purpose: Relapse remains an unsolved problem for previously radio-treated patients. Our purpose is to evaluate the role of radio-hyperthermia (RT-HT) in the retreatment of superficial recurrences.
Materials and methods: From 1998 to 2007, 51 patients affected by four histological types (breast recurrences (group A), melanoma recurrences (group B), head and neck recurrences (group C), and others (group D)) of 76 superficial lesions, were enrolled at Mauriziano Hospital at the Research Institute of Cancer Care Candiolo (IRCC) in Turin. All patients had previously undergone RT except 6 patients of group B. The total mean retreatment dose was 31.8 Gy (20–60 Gy), while the mean of HT sessions was 5 (1 to 8), temperature ranged from 38.5°C (T min) to 44°C (T max).
Results: Acute cutaneous toxicity was 77.6% G1, 22.4% G2, none for G3. Forty-five days later we observed: for group A 65.9% complete response (CR), 29.5% partial response (PR), 4.5% non-response (NR); for group B 33.3% CR, 25% PR and 41.7% NR; for group C 40% CR, 13.3% PR, 46.7% NR, for group D 60% CR and 40% NR. 18 months later group A presented 72.7% local control (LC), 20.5% stable disease (SD) and 6.8% non-control (NC), group B 50% LC, 16,7% SD and 33.3% NC, group C 33.3% LC, 40% SD and 26.7% NC, group D 40% LC and 60% NC. Early response, size of lesions ≤3 cm, T max ≥42°C and RT doses ≥40 Gy were predictive outcome factors.
Conclusions: We confirmed that radio-hyperthermia is useful in re-irradiation with a very high patient compliance.
Introduction
There has been an increasing necessity to perform a second course of radiotherapy on those patients who develop late recurrences or second primary tumours, within or close to the previous treatment portals. In order to make rational clinical choices precise knowledge on long-term recovery of occult radiation damage in various organs is required. The results of various studies Citation1–3 reveal that usually acutely responding tissues recover from radiation injuries within a few months, and therefore can tolerate another full course of radiation. Late toxicity, which is one of the aims of clinical studies, depends on the capacity of tissues to recover from occult radiation damage, and is probably related to stem-cell density reduction, too. Skin, mucosas, lung, and the spinal cord do recover from sub-clinical injury depending on the organ type, the size of the irradiation fields, the total dose used during the first course and, to a lesser extent, the interval between radiation courses Citation1–8. Furthermore, several studies have shown that local control rates of over 30% can be achieved with total curative doses but are ineffective with lower doses Citation8. Consequently, an effective dose in re-irradiation is necessary to lessen tissue damage. Previous studies have shown that hyperthermia is a useful way to improve local sensibility to common therapies, such as radiotherapy or chemotherapy. The reason is the demonstrated biological effects of hyperthermia on tissues Citation9–12. The mechanism of action is partly a direct effect on radio-resistant cells Citation13–14, and on an indirect effect through the inhibition of the DNA damage repair mechanism, due to ionizing radiation Citation15, angiogenesis Citation16, DNA replication in vivo, the protein accretion of nuclear matrix and other alterations in the chromatin structure Citation17–18. Moreover, radiosensitization could also increase tumour oxygenation as a result of hyperthermia-induced changes in tumour blood flow Citation19–20.
The most widely performed form of local hyperthermia consists of a single length microwave to obtain the prescribed tumour temperature between 42°C to 44°C Citation21. These effects are confirmed in several studies, even in phase III, demonstrating that adjuvant hyperthermia improves clinical responses to radiotherapy Citation9–11, Citation22–27. Moreover, the best time and way to approach combined treatment has to be considered, i.e. when the cost/benefit ratio is favourable to insert hyperthermia into routine work. The aim of our study is to evaluate, in a prospectic way, the feasibility and the efficacy, in terms of local control, of lower-middle re-irradiation doses associated with hyperthermia.
Material and methods
Patients and tumour characteristics
From October 1998 to October 2007, 51 patients affected by 76 histologically confirmed superficial recurrent or metastatic lesions were enrolled in a prospectic way at Mauriziano Hospital in Turin and at the Cancer Research Institute of Candiolo, which are the reference points for clinical hyperthermia in the Piedmont region.
The ineligibility criteria were: tumour >4 cm in depth from body surface; Karnofsky Index Score (KPS) <70; significant cardiovascular disease; haemorrhagic or thrombotic disorders; children (<12 years); acute dermatological disease; reduced thermal sensibility; pregnancy; large metallic implants; pacemaker and cardioverter defibrillators.
We treated a heterogeneous group of patients, mean age 64.2 years (range from 45 to 79) classified according to the type of tumour: breast recurrences on chest wall (Group A), melanoma recurrences (Group B), head and neck recurrences (Group C), and others, such as sarcomas and lymphoma (Group D).
All lesion sizes were measured by means of ultrasound scanner (US) and/or computer tomography (CT) scan and/or magnetic resonance imaging (MRI) obtaining the maximum diameter (D max in cm) and the depth (in cm).
Patients, tumours and treatment characteristics are reported in .
Table I. Patients and lesions characteristics.
Radiotherapy
All patients had been previously treated with a full postoperative or radical RT (except 6 patients of group B who had received surgery and chemotherapy).
The previous postoperative doses of RT ranged from 45 to 63 Gy, while radical doses ranged from 66.6 to 70.2 Gy. The aim of current combined treatment was curative for 27.6% of cases and palliative for the rest (72.4% of cases).
The radiation therapy was performed by CLINAC VARIAN (Palo Alto, CA) (2100 CD, 600CD) using electrons (6–12 MeV) or Megavoltage photons (6MV) or Röentgentherapy (200 KVp) by Therapax USA.
Total prescribed retreatment doses ranged from 20 to 60 Gy (mean dose 31.8 Gy). The choice of radiation doses depended on the previous RT treatment (dose, field and time from previous RT), the KPS and on patient compliance.
Conventional fractionation (1.8–2 Gy) was generally practised, while hypofractionation (2.5–5 Gy) was preferred for melanoma. The irradiation field encompassed 2–3 cm beyond the gross tumour volume (GTV). All the lesions treated were divided into 4 categories according to the RT doses received (<30 Gy, 30–39 Gy, 40–49 Gy, ≥50 Gy) so as to evaluate the dose-response relationship.
Hyperthermia
Our patients were treated with the Alba hyperthermia system, which is a superficial microwave HT system that operates at 434 MHz/45–75 W and has been already described in other studies Citation28.
The system is now endowed with a more powerful generator able to deliver a maximum power of 200 W. Other technological implements regarded some accessories: an ultrasound scanner (HS 2000, Honda, Japan) and an Oxford Optronix Laser Doppler Probe Oxylab (Oxford, UK), that were recently purchased by the centre but are now used only in selected cases to evaluate their utility in clinical practice. Moreover, an upgrading of the operating system and hardware, so as to efficiently acquire and record images into the patient database and to improve data manipulation and management were introduced.
The HT session was delivered twice a week on alternate days (Monday/Thursday or Tuesday/Friday) to reduce thermotolerance, as soon as possible after irradiation. An average temperature of 43°C was prescribed to the tumour site for 30 min, but 5 min more were required to reach the prescribed temperature, as demonstrated in our previous experiences Citation14,Citation20,Citation25,Citation27.
However, since it is not always possible to obtain a 30-min effective session, especially when coupling is difficult, we decided to double the treatment time in the future according to the quality assurance guidelines for ESHO protocols Citation28.
The mean of the HT sessions was 5 (range from 1 to 8).
The application was performed by using contact curved microstrip applicators (CCMAs) Citation29 which are curved and rigid applicators able to operate at 434 MHz whose main features are described in and displayed in .
Table II. Contact curved microstrip applicators (CCMAs) main features according to the Operative Manual of Alba Hyperthermia System.
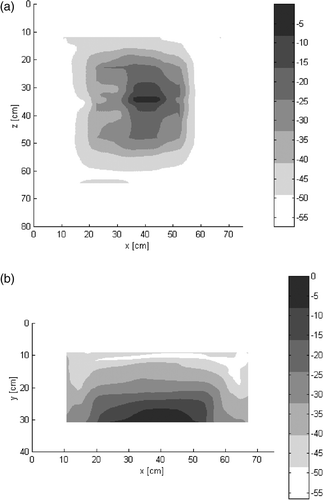
CCMAs are used in the oncological field and represent a rather effective device to practice superficial and (semi)deep hyperthermia Citation30–31. For this study, alpha, beta or gamma CCMA applicators were used depending on lesion size and depth. The most frequently used applicator was the beta type (80.3%), the alpha type was used in 18.4% of cases and the gamma type was used in just one patient. The depth penetration of these applicators has already been studied Citation28–29 but the 3-D specific absorption rate (SAR) distribution, recently evaluated, is reported for the beta type in .
Figure 2. Experimental normalized distribution of 3D SAR (in dB) of beta-type antenna, (a) on a parallel plane 1 cm under the bolus; (b) on a perpendicular plane. The numerical analysis was performed by BEST, a proprietary FDTD code. Dielectric parameters of various materials were: Bolus: ε (relative dielectric constant) = 78, σ (S/m) (electrical conductivity) = 0.04; Muscle: ε = 56.86, σ (S/m) = 0.8; Applicator substrate: ε = 2.17, σ (S/m) = 0.18.
During treatment, a skin cooling system, which is a water bolus that does not exceed 39°C, is used to refrigerate the tissues around the target site and produce a good coupling of impedance and low reflection with a very low energy loss. Moreover, it has been demonstrated that the thickness of the bolus can affect the geometry and depth of the SAR distribution Citation32. Experimental data demonstrates that the bolus thickness must be included in a range of 0.5 cm–2.5 cm when working at 434 MHz. So, before treatment, a preliminary ‘coupling session’ was performed, aimed at reducing the electromagnetic reflected power to almost zero.
On the basis of our current experience we plan to raise temperature bolus in future sessions when the superficial temperature is lower than prescribed.
The therapy consisted of the release of microwave power through the applicator and in the monitoring of temperatures in the target volume (with interstitial or surface Type-T probes), as well as many other parameters (direct power, reflected power, lost power).
The US was directly connected to the system in order to accurately visualize tumour location and allow for an appropriate choice of the MW applicator with the proper penetration depth and effective field size. Images were acquired and recorded into the database of each patient. By comparing the images related to different succeeding treatments in the same patient, the tumour evolution (necrotic area, dimension, etc.) was monitored.
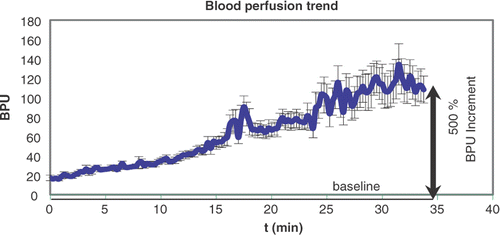
Increase in temperature enhances blood perfusion in the tissues by eliciting physiological responses: for this reason a local monitoring of perfusion during the hyperthermic session Citation33–34 gives useful information about the efficiency of heating the target volume. Subcutaneous monitoring has been performed as well, since the study of Acker et al. Citation35, in 1990.
Previous measurements obtained by inserting the Laser Doppler (LD) probe in catheters implanted into the tissue at different depths Citation20, showed that the increasing rate of blood perfusion depends on many parameters, such as temperature increase, basal local perfusion, the number of treatments, the occurrence of previous/concomitant radiotherapy and of concomitant chemotherapy.
After the preliminary self-test and after excluding any interference between the LD signal and the MWs emitted from the applicator, the LD signal was monitored by the LD probe and acquired before (baseline value) and throughout the treatment with sample rate =1 s and appeared on a monitor window in real time ().
Thermometry
Temperature was monitored through thermometry probes Oxford Optronix Ltd that were located on the skin surface and one of them was implanted on the centre of the lesion by US guide more frequently, another one was implanted only when the irregular anatomy of the lesion and of its position required it.
T min, T avg, and T max were respectively the means of minimum, average and maximum measured temperatures recorded by both the superficial and deep probes used during each treatment course. Actual measured temperatures detected ranged from 38.5°C (T min) to 44°C (T max) and are reported for each group in .
Table III. Hyperthermia treatment, main characteristics.
Deep thermometric measures were performed in the first HT session and were repeated only when a clinical change of dimension in the lesions was noted during therapies in order to study when the prescribed temperature was reached and to establish the parameters of the session. Our results reported the mean of all measures because of the fewer number of deep probes. The differences between temperatures recorded after implant of probes and the same temperatures recorded superficially ranged from −0.5 to −1.2°C which were lower for the deeper ones.
To evaluate temperature response rate we divided the various treatments into two groups <42°C of T max mean and ≥42°C.
Study endpoints
Patients were evaluated daily during the RT-HT treatment course, at the first follow-up 6 weeks after completion of therapy and at 3, 6, 12 and 18 months thereafter.
The endpoints for this study were to observe local response to combined RT-HT retreatment based on initial clinical response of lesions at first follow-up and definitive response after 18 months. We have also assessed acute and late toxicity (RTOG/EORTC scale) of treatment Citation36–37 according to the World Health Organization (WHO) Citation38. We also recorded the compliance to therapies by considering the number of times the patient requested we stop the treatment. The palliation of pain and other symptoms were clinically analysed too.
The parameters of clinical response were complete response (CR), i.e. the complete clinical disappearance of all measurable disease in the treated field, partial response (PR), i.e. a 50% or greater decrease in the product of two orthogonal dimensions of the measurable lesion or in the sum of the products of orthogonal diameters of multiple lesions, and lastly no response (NR). Stable disease (SD) was defined as <50% decrease in tumour size, and progression disease (PD) was a 25% or more increase in the size of one or more measurable lesions. In the long term follow up we considered local control (LC), non-control (NC) or progression (P).
Statistical methods
The statistical analysis in this study was done using statistical software (SPSS for Windows). The actuarial probability of local control was plotted from the time of initiation of treatment using the Kaplan-Meier method Citation39. We used Cox-regression test for univariate and multivariate analysis and BED calculation: E/α = nd (1 + d/α/β) Citation40 for retreatment.
Results
At our first follow up after 45 days, the residual acute toxicity and initial response were evaluated. The definitive results were reported at follow up 18 months later.
A summary of the results is reported in ; it shows a trend between early and definitive response, therefore precocious response could be a prognostic factor.
Table IV. Results per treatment groups.
The sessions were very well tolerated in most patients: in fact we observed only three definitive interruptions of treatment (3.9% of total treatment) with an unsatisfying response. One of these patients was a 56-year-old woman from group A who had stopped RT treatment after 5 fractions (10 Gy) but had continued with HT, because of the insertion of chemotherapy, due to systemic progression of the disease. Another patient was a 72-year-old man from Group C affected by neck nodal recurrence of laryngeal cancer: the treatment was interrupted at 26 Gy because of the onset of a cervical fistula and progression of disease. The third case was a 57-year-old man from group B who could not tolerate the HT-RT session for psychological problems after 6 Gy.
A temporary interruption of only a few minutes was required by 18.4% of treatments during an HT session. In these cases an uncomfortable position or more rarely a nuisance from the antenna (related to the increase of the power in some cases) was reported; no burning was referred. When the interruption occurred within the first 15 minutes, the treatment was entirely repeated; otherwise the HT session was completed. No relation was found between the temporary interruption and response to treatment.
As to thermal injuries, none of our patients experienced severe, acute reactions. All patients presented a good symptomatic response. Acute cutaneous toxicity for combined therapies was for all groups 77.6% G1, 22.4% G2; none showed G3 toxicity according to EORTC/RTOG scale.
During follow up, acute reaction gradually reduced, only 3.9% presented residual clinical evidence (i.e. telangiectasis, hyperchromia) at a second control, three months later.
We analysed the dose RT-response based on four dose RT levels as described in the Material and methods session and results are reported in . The major gain on local control for every group was at higher doses. Statistical evaluation of the dose-response correlation showed a total CR for the RT treatment <30 Gy was 35.7% (the best results regarded group A which presented 43.8% of CR), and total LC of 42.9% (56.3% for group A). As to treatment with doses between 30–39 Gy (39.5% of treatments) a total CR of 66.7% was found (with best results obtained by group A, 70% of CR) and a LC of 70% (78.9% in Group A). The B and C groups seemed to need higher doses (≥40 Gy) to reach CR ≥50%. Statistical consideration about temperature data showed no significant results considering T min and T avg, only T max could be related to early and late response. Consequently, the relation between the mean of T max and response was analysed: these data are reported in . The correlation between temperature ≥42°C and the response in particular for group A (which showed 76.5% of CR against 30% for T max <42°C) is evident. This group had a higher rate of temperature ≥42°C (probably because of the better coupling of the antennas). Moreover, these results were confirmed in every group, so they represent a prognostic factor.
Table V. Dose RT-response relationship per treatment groups.
Table VI. The mean of T max-response relationship per treatment groups.
Also, the size of lesions seems to represent a prognostic factor for local control. Correlation between type of treated lesion (A, B, C, D) or dimension of the lesion and short- and long-term follow up was studied. The lesions were divided into two groups by using two different cut-off values, 3 cm and 5 cm. No correlation was observed between lesion type and both short- or long-term follow up. On the contrary, a correlation of about 51% was found only when a cut-off of 3 cm was considered, in patients who had a lesion smaller than 3 cm and in those who presented a good response at short-term follow up.
As to population survival, which was not the endpoint of our study, at follow up after 18 months for every group, we observed no significant relation between response and overall survival.
Discussion
Most patients affected by a recurrent disease had an extremely poor prognosis, especially if they had received treatment previously. Few studies have analysed the association RT+ HT in re-irradiation. Our study shows how hyperthermia can become a useful support in difficult conditions especially when we are not certain of being able to administer the necessary effective dose. We observed the immediate efficacy of the combined treatment because most patients showed a precocious response; consequently, this approach could also be used in patients with a short life expectancy. Moreover our study confirms that hyperthermia treatment when performed with modern equipment is a safe method. The involved organ, the total dose, the fractionation, the field size, the eventual concomitant therapies, the time between the treatments, the performance status and the compliance of the patients has to be considered to evaluate the tolerance level.
Literature presents few clinical studies, one of the largest about head and neck re-irradiation presented by Lee et al. Citation41 included a total of 654 patients with recurrent nasopharyngeal carcinoma. It demonstrated that the extent of local recurrence was the most important prognostic factor, with 35% of local control rates for T1 compared with 11% for T3. Initial and retreatment doses were calculated in terms of biologically effective dose (BED) using α/β ratios of 10 Gy for tumour control and 2 or 3 Gy for late normal tissue damage.
Other studies of the head and neck area (approximately one hundred) Citation41–43 showed a benefit on local control between 11% and 59% with or without the association of brachytherapy, an overall survival rate from 8% to 24% and late complication from 9% to 69%.
The association of hyperthermia in head and neck cancer has not been studied enough yet. Some of the most important articles Citation10,Citation44 showed an increase of control rate with or without HT between 23% and 32% with an increase of survival from 14% to 53% in long-term follow-up.
The historical experience of re-irradiation on head and neck recurrences Citation45 showed a 36% of overall local control. At the Radiotherapy Division of Turin University from 1983 to 1991, 100 head and neck recurrent lesions (89 patients) were treated. 70 were submitted to RT-HT treatment, 26 to HT alone and 4 to chemo-hyperthermia. The overall response rate was of 32% and the 3-year local control was 22%. The best results were evidenced in the RT-HT group with 42% of CR and 48% PR, and 30% LC. Negative results were obtained when only hyperthermia (9% CR) was used. Considering the collateral effects we had 1% of G3 but 3 patients died of haemorrhagic complications.
In the present study, the patients did not develop serious acute events, probably because of the precise re-evaluation of the previous treatment planning, the calculation of RT dose based on BED analysis and lastly the multipoint effective temperature measurements.
In superficial treatment, skin and connective tissue, which had previously undergone radiation treatment, showed an increase in fibrous tissue and a relative radio-resistance due to lower oxygenation as well. Some results suggest a low retreatment tolerance (50–70% BEDt) for acute reactions Citation1,Citation2; however, less information is available on late radiation effects. Moreover, modern radiotherapy techniques aim at using higher doses to lower volumes so that re-irradiation doses can be lower: in this case radiosensibilization methods, such as hyperthermia are employed.
The efficacy of radiohyperthermia in superficial tumours is demonstrated in several articles. With the introduction of hyperthermia, absolute control rates increase in the order of 20–30%. Several phase III studies showed the results of hyperthermia: the improvement in response rate, local control and survival. Statistical significance was obtained especially for recurrent breast cancer, head and neck cancer, oesophageal cancer and melanoma Citation9–11, Citation22–26, Citation46–48.
The RTOG 8104 showed a total of 307 patients divided into two arms: radiation alone or radiation followed by hyperthermia. No significant differences were noted (CR 30 versus 32% of combined therapy), but there were subgroups in which there was a better response: head and neck or breast chest-wall recurrences and DM <3 cm lesions that were localized in the breast, trunk and extremities. Another recent promising study by Jones Citation22 showed an increase in response rate in HT arm of 23.8%.
For breast carcinoma several studies in favour of the association of RT-HT Citation11,Citation22,Citation44 showed a gain in the response rate between RT alone and RT-HT from 6% (for large recurrences) to 20%. There is less evidence that a combined treatment improves overall survival; however, this is not the endpoint of most studies.
Radiotherapy can be very effective in the treatment of breast recurrences: our previous results Citation8 on 19 patients (28 lesions treated) demonstrated an overall response rate (ORR) of about 96.4%, the best results are in the combined treatment (CR 58.8%) compared to radiotherapy (CR 50%) or HT (CR 45.5%) alone.
As to malignant melanoma, the randomized trial published by Overgaard Citation9 on 68 patients with 134 metastatic or recurrent lesions proved the benefit of combined therapy with an improvement of 27% on the local control rate. Our results Citation45 in relation to combined therapies practised from 1983 to 1994 in 48 patients affected by recurrent primary or metastatic malignant melanoma showed an overall response rate of 72%, with CR 36% and a LC 24% at 60 months. In head and neck melanoma recurrences 9 patients (15 lesions) were compared with a group of patients treated with RT alone with a 46.5% CR, 50% PR, and 46.5% NR, 34% LC at 24 months using combined RT-HT, while 37.5% CR, 50% PR and 25% LC at 24 months were observed with RT alone Citation45.
In particular, from the actual (and the previous) reports it was proved that local HT, in addition to low RT doses (mean 31.8 Gy) improves response rate and local control.
In rare cases (group D) the possibility of using hyperthermia could help in deciding whether or not to perform re-irradiation even in the absence of literature data.
Our observational considerations showed that HT-RT is an optimal palliative solution and is the convenient approach in all breast recurrences independently of doses (higher doses are preferred) but is less convenient in other tumours (especially head and neck) if the effective dose ≥40 Gy with conventional fractionation cannot be reached.
Our results confirm that it is very important to achieve high thermal doses, too. So it is evident that in all groups a significant number of patients received T max <42°C; moreover, the T-min, which presents a lack of correlation, is almost equal to our bolus temperature. So this means that a higher bolus temperature in future treatments is necessary. Another way to obtain better results could be by doubling treatment time, as has been indicated by several studies and by the ESHO guidelines Citation28.
The improved systems which can now monitor some basic physiological parameters (i.e. blood perfusion), store more parameters of clinical interest, perform accurate positioning of the applicators on the lesion by US imaging, could develop a better clinical management of the treated lesions. Nevertheless, a longer follow up is needed.
At the moment studies are in progress for non-invasive monitoring of tumour temperature by scanning the tissue after the preliminary injection of a thermo-sensitive contrast agent (gas bubbles): the procedure has been only tested in in vitro experimental systems Citation30.
Conclusion
This study confirms that RT-HT treatment is useful and safe. In fact, patients present a very high compliance to the treatment and the absence of G3 toxicity has been evidenced. The results of this study are better than those of our previous studies even if they are not randomized. This is probably because of the improvement of both RT and HT techniques. However, further improvement of these techniques, especially HT, is favourable. Therefore, we think that RT-HT combined treatment should be the first choice in the treatment of superficial recurrences.
Acknowledgements
This paper was presented in part at the 24th annual meeting of ESHO, Prague 2007 Citation49.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brown JM, Probert JC. Early and late radiation changes following a second course of irradiation. Radiology 1975; 115: 711–716
- Terry NHA, Tucker SL, Travis EL. Time course and loss of residual radiation damage in murine skin assessed by retreatment. Int J Radiat Oncol Biol Phys 1989; 55: 271–283
- Stewart FA. Re-treatment after a full-course radiotherapy: Is it a viable option?. Acta Oncol 1999; 38: 855–862
- Denekamp J. Residual radiation damage in mouse skin 5 to 8 months after irradiation. Radiology 1975; 115: 191–195
- Wondergem J, Haveman J. The effect of previous treatment on the response of mouse feet to irradiation and hyperthermia. Radiother Oncol 1987; 10: 253–261
- Simmonds RH, Hopewell JW, Robbins ME. Residual radiation-induced injuries in dermal tissue: Implication for retreatment. Br J Radiol 1989; 62: 915–920
- Van der Kogel AJ, Sissingh HA, Zoetelief J. Effect of X-rays and neutrons on repair and regeneration in the rat spinal cord. Int J Radiat Oncol Biol Phys 1982; 8: 2095–2097
- Sannazzari GL, Gabriele P, Orecchia R, Tseroni V. Results of hyperthermia, alone or combined with irradiation, in chest wall recurrences of breast cancer. Tumori 1989; 75: 284–288
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995; 345: 540–543
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1994; 28: 163–169
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, Van der Zee J, Van Putten WL, Van Rhoon GC, Van Dijk JD, Gonzalez Gonzalez D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Lepock JR. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int J Hyperthermia 2003; 19: 252–266
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys 1989; 16: 535–549
- Gabriele P, Orecchia R, Tseroni V, Ragona GL, Sannazzari GL. Hyperthermia alone in the treatment of recurrences of malignant tumors. Experience on 60 lesions. Cancer 1990; 66: 2191–2195
- Kampinga HH, Dynlacht JR, Dikomey E. Mechanism of radiosensitization by hyperthermia (>or =43°C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperthermia 2004; 20: 131–139, Review
- Roca C, Primo L, Valdembri D, Cividalli A, Declerck P, Carmeliet P, Gabriele P, Bussolino F. Hyperthermia inhibits angiogenesis by a plasminogen activator inhibitor-1 dependent mechanism. Cancer Research 2003; 63: 1500–1507
- Roti Roti JL, Turkel N. Heat-shock-induced changes in nuclear protein and cell killing in thermotolerant HeLa cells. Radiat Res 1994; 138: 286–290
- Warters RL. Hyperthermia blocks DNA processing at the nuclear matrix. Radiat Res 1988; 115: 258–272
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984; 44: 4721–4730
- Guiot C, Madon E, Allegro D, Pianta PG, Baiotto B, Gabriele P. Perfusion and thermal field during hyperthermia. Experimental measurements and modelling in recurrent breast cancer. Phys Med Biol 1998; 43: 2831–2843
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001; 17: 1–18, Review
- Jones EL, Oleson JR, Prosnitz LR, Salmuski TV, Vujaskovic Z, Yu D, Sanders LL, Dehirst MW. Randomized trial of hyperthermia and radiation for superficial tumours. J Clin Oncol 2005; 23: 3079–3085
- Amichetti M, Griaff C, Fellin G, Pani G, Bolner A, Maluta S, Valdagni R. Cisplatin, Hyperthermia and radiation (trimodal therapy) in patients with locally advanced head and neck tumors: A phase I-II study. Int J Radiat Oncol Biol Phys 1993; 26: 801–807
- Van der Zee J, Gonzalez Gonzalez D, Van Rhoon GC, Van Dijk JD, Van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Gabriele P, Amichetti R, Orecchia R, Valdagni R, Tseroni V. Radiation therapy and hyperthermia in advanced or recurrent parotid tumors. A bi-institutional phase I-II study. Cancer 1995; 75: 908–913
- Donato V, Zurlo A, Nappa M, Capua A, Banelli M, Martelli M, Gabriele P, Amichetti M, Biagini C. Multicentre experience with combined hyperthermia and radiation therapy in the treatment of superficially located non-Hodgkin's lymphomas. J Exp Clin Cancer Res 1997; 16: 87–90
- Sannazzari GL, Gabriele P, Orecchia R. Thermal dose and fractionation in clinical hyperthermia. Radiol Med (Torino) 1990; 79: 614–621
- Hand JW, Lagendijk JJW, Bach Andersen J, Bolomey JC. Quality assurance guidelines for ESHO protocols. Int J Hyperthermia 1989; 5: 421–428
- Marini P, Baiotto B, Gabriele P, Guiot C. Technological improvements in MW Hyperthermia system allowing a better control of power delivering and treatment efficiency. 11th ESHO Meeting 2001. Verona. 41
- De Bruijne M, Wielheesen DHM, Van Der Zee J, Chavannes N, Van Rhoon GC. Benefits of superficial hyperthermia treatment planning: Five case studies. Int J Hyperthermia 2007; 21: 417–429
- Guiot C, Cavalli R, Gaglioti P, Danelon D, Musacchio C, Trotta M, Todros T. Temperature monitoring using ultrasound contrast agents: In vitro investigation on thermal stability. Ultrasonics 2004; 42: 927–930
- Gelvich EA, Mazokhin VN, Troshin II. An attempt at quantitative specification of SAR distribution homogeneity. Int J Hyperthermia 1996; 12: 431
- Vaupel P, Kluge M, Ambroz MC. Laser Doppler flowmetry in subepidermal tumours and in normal skin of rats during localized ultrasound hyperthermia. Int J Hyperthermia 1988; 4: 307–321
- Song CW. Effect of local hyperthermia on blood flow and microenviroment: A review. Cancer Res 1984; 44: S4721–S4730
- Acker JC, Dewhirst MW, Honore GM, Samulski TV, Tucker JA, Oleson JR. Blood perfusion measurements in human tumours: Evaluation of Laser Doppler methods. Int J Hyperthermia 1990; 6: 287–304
- Cox JD, Stetz JA, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group RTOG and the European Organization for Research and Treatment of Cancer EORTC. Int J Radiat Oncol Biol Phys 1995; 31: 1341–1346
- Pavy JJ, Denekamp J, Letschert J, Littbrand B, Mornex F, Bernier J, Gonzales-Gonzales D, Horiot JC, Bolla M, Bartelink H. EORTC late effects working group. Late effects toxicity scoring: the SOMA scale. Int J Radiat Oncol Biol Phys 1995; 31: 1043–1047, Radiother Oncol 1995;35:11–15
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481
- Fowler J. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989; 62: 679
- Lee AWM, Foo W, Law SCK, et al. Total biological effect on late reactive tissue following reirradiation for recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2000; 46: 865–872
- De Crevoisier R, Bourhis J, Domenge C. Full-dose reirradiation for unresectable head and neck carcinoma: Experience at the Gustave Roussy Institute in a series of 169 patients. J Clin Oncol 1998; 16: 3556–3562
- Yan JH, Hu YM, Gu Z. Radiation therapy of recurrent nasopharyngeal carcinoma. Acta Radiol Oncol 1983; 22: 23–28
- Hiraoka M, Nishimura Y, Nagata Y, Mitsumori M, Okuno Y, Li PY, Abe M, Takahashi M, Masunaga S, Akuta K, et al. Site-specific phase I, II trials of hyperthermia at Kyoto University. Int J Hyperthermia 1994; 10: 403–410, Review
- Loco-regional recurrences of head and neck tumors. International workshop of otolaryngology, Turin, 5–7 March 1992, pp. 17–27, 141–149, 155–170
- Sakamoto T, Katoh H, Shimizu T, Yamashita I, Takemori S, Tazawa K, Fujimaki M. Clinical results of treatment of advanced esophageal carcinoma with hyperthermia in combination with chemoradiotherapy. Chest 1997; 112: 1487–1493
- Kitamura K, Kuwano H, Araki K, Egashira A, Kawaguchi H, Saeki H, Morita M, Ohno S, Sugimachi K. Clinicopathologic features of patients with oesophageal cancer obtaining a histological complete response for preoperative hyperthermo-chemo-radiotherapy. Int J Hyperthermia 1998; 14: 233–243
- Kuwano H, Sumiyoshi K, Watanabe M, Sadanaga N, Nozoe T, Yasuda M, Sugimachi K. Preoperative hyperthermia combined with chemotherapy and irradiation for the treatment of patients with esophageal carcinoma. Tumori 1995; 81: 18–22
- Gabriele P, Baiotto B, Giovannini V, Pacetti P, Guiot C. Local hyperthermia and radiation therapy in the treatment of recurrent pretreated superficial tumors. 24th annual meeting of the European Society for Hyperthermic Oncology. PragueCzech Republique 14–16 June, 2007; 13, Abstracts