?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Controlling the magnetic properties of a nanoparticle efficiently via its particle size to achieve optimized heat under alternating magnetic field is the central point for magnetic hyperthermia-mediated cancer therapy (MHCT). Here, we have shown the successful use of stevioside (a natural plant-based glycoside) as a promising biosurfactant to control the magnetic properties of Fe3O4 nanoparticles by controlling the particle size. The biocompatibility and cellular uptake efficiency by rat C6 glioma cells and calorimetric magnetic hyperthermia profile of the nanoparticles were further examined. Our finding suggests superior properties of stevioside-coated magnetite nanoparticles in comparison to polysorbate-80 and oleic acid coated nanomagnets as far as particle size reduction, biocompatibility, hyperthermic effect, and cellular uptake by the glioblastoma cancer cells are concerned. The stevioside-coated nanomagnets exhibiting the maximum temperature rise were further investigated as heating agents in in vitro magnetic hyperthermia experiments (405 kHz, 168 Oe), showing their efficacy to induce cell death of rat C6 glioma cells after 30 min at a target temperature T = 43 °C.
Introduction
Magnetic nanoparticles (MNPs) have been extensively used for various biomedical applications such as hyperthermia, in vivo targeted drug delivery and MRI contrast agents [Citation1–4]. The superparamagnetic behavior of the nanomagnets, where magnetization relaxes very fast due to thermal fluctuations has been used for these techniques. However, the uncoated nanoparticles have a tendency to agglomerate owning to the various types of interparticle attractive forces, namely van der Waals forces, dipole-dipole interactions, electrostatic forces and Brownian effects [Citation5].This agglomeration may slow down the fluctuation of the magnetization and turn the nano-magnets ineffective for magnetic hyperthermia-mediated cancer therapy (MHCT). To overcome this situation, a huge amount of effort has been devoted to modify or functionalize the surface of the nanoparticles using appropriate biocompatible coating materials [Citation6]. One such modification includes carbohydrate coating onto the surface of the particles [Citation6–10]. Such surface modifications of the particles not only reduce the cytotoxicity of the nanoparticles but also aid in tuning the geometrical properties (size and shape) of the particles when added during nanoparticle synthesis. The geometrical properties of the nanoparticles, anisotropy energy and particle-particle interactions are shown to influence the magnetic property significantly and hence the hyperthermic effect of MNPs [Citation11–13]. Numerous studies have demonstrated the effect of surfactant layer addition onto the nanoparticles surface along with the reaction temperature and concentration of the precursor molecules to tune the particle size, shape and dispersity of the particles.
Recent studies report ex-situ use of a new biosurfactant – Stevioside (STE), in conjugation with soy protein isolate for efficient stabilization of nanosuspensions [Citation14,Citation15]. Stevioside is the most abundant constituent of steviol glycosides (ent-kaurene type diterpene) isolated from the leaves of Stevia rebaudiana Bertoni and widely used as the non-caloric natural sweeteners. It is an amphiphilic moiety consisting of a hydrophilic part made up of glucosyl and sophorosyl residues and hydrophobic part made up of diterpenoid or steviol backbone. Stevioside requires attention as a new generation biosurfactant due to its extensive biological properties like antihyperglycemic, immunomodulatory effects and antitumor action [Citation16–18]. Therefore, surface modification of magnetite nanoparticles with stevioside may provide dual targeting of cancer cells, namely with magnetite nanoparticles based cancer therapy and antitumor effect of the stevioside coating onto the particles [Citation18–20]. On the other hand, oleic acid (OA) and polysorbate-80 (P-80) are the commonly used surfactant molecules to stabilize and generate monodispersive (uniform sized nanoparticles with narrower particle size distribution) magnetic nanoparticle systems [Citation21–23]. Oleic acid is an anionic naturally occurring moiety while polysorbate-80 is a non-ionic synthetic molecule. The chemical structures of stevioside (STE), polysorbate-80 (P-80) and oleic acid (OA) are given in .
The primary objective of this study was to synthesize in situ stevioside coated magnetite nanoparticles without any conjugation with protein isolates by traditional hydrothermal synthesis route for use as hyperthermia agents. The effect of other routinely used surfactant moieties (OA and P-80) on the particle size and magnetic property was further investigated and compared with that of the STE-coated MNPs. The nano-magnets synthesized were then investigated for their heat generating capabilities to potentiate the role of stevioside-coated magnetic nanoparticles for use in MHCT.
Experimental section
Materials
All the chemicals and reagents used were of analytical grade purchased from Sigma-Aldrich, St. Louis, Missouri, USA unless specified and were used directly without any further purifications or modifications. The water utilized in all experiments was purified using a Milli-Q Plus 185 water purification system (Millipore, Bedford, MA) with a resistivity higher than 18 MΩ·cm. Stevioside (80% purity) was purchased from TCI chemicals, Tokyo, Japan. Rat C6 glioma cell line was procured from NCCS, Pune, India. The cell culture media Dulbecco modified eagle’s medium (DMEM), fetal bovine serum (FBS), trypsin-EDTA, antibiotic-antimycotic solution (penicillin, amphotericin B and streptomycin) and MTT (3–(4,5-dimethythiazol-2yl)-2,5-diphenyl-tetrazolium bromide) powder were purchased from Himedia, Mumbai, India.
Synthesis of magnetite nanoparticles
Magnetite nanoparticles (Fe3O4) were synthesized according to the protocol suggested by Song et al. 2009 [Citation24]. Similarly, for the synthesis of coated MNPs with oleic acid (OA), polysorbate-80 (P-80) and stevioside (STE), the same above protocol was followed with slight variations. To generate OA- and P-80-coated Fe3O4 nanoparticles, 0.5 ml of OA and P-80 was added, respectively after the addition of 2.5 ml ammonium hydroxide into the solution followed by 10 min air stirring. For the generation of STE-coated Fe3O4 nanoparticles, depending upon the concentration of STE coating required onto the magnetite nanoparticles, 0.5 g or 1.0 g STE was stirred in 11.5 ml water on a magnetic stirrer for 2 h followed by the addition of ferrous salt. The aqueous solution of STE was pre-cooled at 4 °C before use. Next, the same protocol as for the synthesis of bare Fe3O4 nanoparticles was followed.
Characterization of magnetite nanoparticles
The crystalline structure and the approximate size of the particles were determined by Bruker D8 Advanced X-ray diffraction (XRD) system, Billerica, Massachusetts, USA using Cu Kα radiation source from 20 to 80° (2θ) with an increment of 0.02° min−1. The particle size and size distribution profile of the nanosystems were investigated using a JEOL JEM 2100 transmission electron microscope (TEM) Akishima, Tokyo, Japan at an acceleration voltage of 200 KV. Dynamic light scattering (DLS) measurements of the synthesized magnetite nanoparticles were measured by Malvern Zetasizer Nano-ZS, Malvern, Worcestershire, USA to determine the hydrodynamic size of the particles. The surface charge of the MNPs was also evaluated using Malvern Zetasizer Nano-ZS. Measurements were carried out for diluted MNPs suspensions in triplicates.
Fourier transform infrared (FTIR) spectroscopy measurements were performed with Agilent Technologies Cary 600 series spectrometer, Santa Clara, California, USA using KBr pressed discs. A Quantum Design Dynacool PPMS, California, USA magnetometer was used to determine the magnetic properties of the nanoparticles.
Cell culture
Rat C6 glioma cells and mouse fibroblasts NIH3T3 cells were grown on DMEM medium with 10% heat-inactivated fetal bovine serum (FBS) and 100× antibiotic-antimycotic solution (10,000 U penicillin, 25 μg Amphotericin B and 10 mg Streptomycin per mL in 0.9% normal saline). Cell cultures were performed at 37 °C in a humidified atmosphere with 5% CO2. Cells were grown in 75 cm2 cell culture flasks with 10 ml of medium that was changed every second day until a sufficient number of cells were obtained. Cell density and viability were determined through staining with trypan blue and counting cells using a hemocytometer with 0.9 mm3 counting chamber.
The biocompatibility of the synthesized nano-magnets was evaluated on rat Glioma C6 cells and of STE-Fe3O4 (0.5g) MNPs was evaluated against a normal murine fibroblasts cell line (NIH3T3) by the conventional MTT assay (data not shown), using the Equation (1):
Where At is the average absorbance of test wells and Ac is the average absorbance of control wells. All experiments were run in triplicates.
Cellular uptake and persistence study
Prussian blue staining assay was performed to validate the cellular uptake of the magnetite nanoparticles by the C6 glioma cells [Citation25]. The cells were pre-incubated with MNPs concentration equivalent to 350 μg Fe for 6 and 12 h, respectively, fixed in ice-cold methanol (5 min), stained with an equal volume of aqueous solutions of 20% hydrochloric acid and 10% potassium ferrocyanide trihydrate for 20 min and then counterstained with 0.2% neutral red for 2 min at room temperature. The preparations were then washed with distilled water, air dried, and mounted on glass slides. Microscopic observations and photography were performed in Zeiss Axio Vert.A1 inverted microscope, Oberkochen, Germany under bright light illumination. The quantitative estimation of amount of iron internalized in the cells (normalized to the number of cells/well) was also done using ICP-AES for all the nanosystems generated in the study.
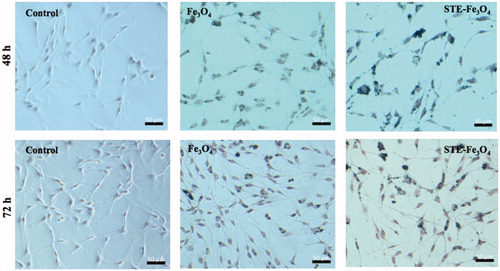
The nanoformulations synthesized in the study were further analyzed for their capability to be retained inside the rat glioma C6 cells for 48 and 72 h post-incubation with the MNPs by Prussian blue staining [Citation26]. The cells pre-incubated with 10 μg/mL of bare and STE-coated MNPs (0.5 g) for 24 h were washed twice with PBS and subsequently fresh media was added into each well. The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2 for 48 and 72 h, respectively. Next, the Prussian blue staining was done according to the method described above to check the retention of nanoparticles inside the cells.
Sub-cellular localization study
The STE-Fe3O4 nanoparticles were conjugated to FITC (Himedia) to generate STE-Fe3O4-FITC nanoparticles for the fluorescent study. The STE-Fe3O4-FITC MNPs were synthesized by freshly incubating 1 mg STE-Fe3O4 nanoparticles in 250 μl of 1 mg/mL stock solution of FITC in DMSO for 24 h at room temperature. Following incubation, the nanoparticles were magnetically separated and washed twice with water to remove any unbound FITC.
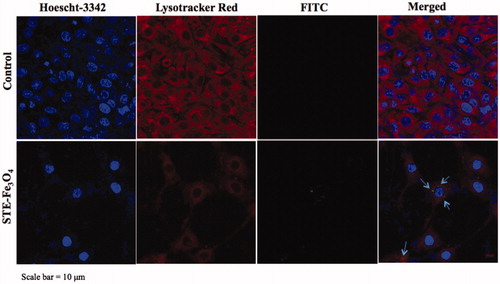
To determine the sub-cellular localization of STE-Fe3O4-FITC nanoparticles in glioma cells, confocal microscopy was done using Zeiss LSM880 confocal microscope (Carl Zeiss, Thornwood, New York). Cells were grown to confluency on 0.01% poly-l-lysine coated coverslips in 6-well tissue culture plates. After pre-incubation with 50 μg/mL of nanoparticles for 24 h, cells were fixed with 4% paraformaldehyde for 10 min at room temperature and then were labeled with 50 nM LysoTracker Red DND-99 (Invitrogen, Carlsbad, California, USA) for 20 min at room temperature to label endocytic compartments of the cells. Following washing with PBS, the cell nuclei were stained with 2 μg/mL Hoescht-3342 for 5 min. The cells were washed again and the coverslips were then mounted and viewed under fluorescent microscope at 63 X magnification.
Hyperthermia measurements
Calorimetric measurements
The magnetic hyperthermia efficiency of the MNPs was tested using the DM2 applicator equipped DM100 system (nB nanoscale Biomagnetics, Zaragoza, Spain). The nanoparticle dispersions prepared in water (1 mg/mL) were subjected to an alternating current (AC) magnetic field (H) at f = 405 kHz with field amplitude of 168 Oe to determine their SAR values. A fluoro-optic thermometer fiber probe was used to probe the temperature every 0.2 s after switching on the magnetic field. The experiments were run in triplicates and each run time was set to 20 min, unless specified. Next, to determine the effect of frequency on the heating efficiency of the nanosystems; the MNP dispersions were subjected to a constant AC magnetic field of strength 168 Oe at four different fixed frequencies (101 kHz, 234 kHz, 359 kHz and 405 kHz). The effect of nanoparticle concentration on the thermal behavior was also evaluated by subjecting the MNP solution to an AC magnetic field of strength 168 Oe at f = 405 kHz for 10 min, at different concentrations varying from 0.5 to 10 mg/mL. The SAR values (W/g MNP) of the nanosystems were calculated according to the following equation [Citation27]:
where C is the specific heat of the solution which is assumed to be equal to that of pure water (Cw = 4.185 J g−1 K−1), m is the concentration of the MNP tested in the solution, ΔTmax represents the maximum temperature rise achieved and τ represents the relaxation time of the particles calculated from the complete T-vs-t curve fit to the exponential trend characteristic of isoperibol conditions. The fitted T-vs-t graphs are shown in Supplementary file (Figure S5, Figure S6 and Table 4). For all the measurements, the magnetic field was switched on after a min of thermal stabilization. The SAR values obtained were further normalized with the amount of Fe present in the nanosystems. The iron estimation was done using ICP-MS system (Agilent Technologies 7700 Series).
In vitro measurements
For in-vitro hyperthermia experiments, C6 cells were grown up to confluent state in sample vials (same as that used for calorimetric measurements) [Citation28]. Cells were cultured overnight at 37 °C with 100 μg/ml of STE-coated MNPs. After 24 h of incubation, the cells were washed with PBS and 500 μL of fresh culture media was added to each vial. The sample vials were then placed in DM2 system working at f = 405 kHz and field amplitude of 168 Oe with a constant target-temperature program feedback at 43 °C set point for 30 min [Citation29]. The MHCT controls were established as follows: (a) cells only; (b) cells treated with MNP but without AMF; (c) cells without MNPs but treated to AMF for 30 min. To observe the long-term effects of the hyperthermia treatment, the cell viabilities of the treated cells were calculated immediately after the treatment and after 4 h of re-culture using the previously mentioned MTT assay.
Results and discussion
Effect of stevioside coating on magnetic nanoparticle size and agglomeration property
The nanoparticles synthesized were identified as Fe3O4 (magnetite) by X-ray diffraction (XRD) [Citation24]. No traces of other iron oxide phases were found. XRD spectra in reveals the nanocrystal nature of the nanoparticles synthesized. The relative intensity and position of all peaks match well with standard Fe3O4 powder diffraction data, indicating that each sample is Fe3O4 crystal. Peak broadening in coated nanoparticle samples indicates the reduction in particle size of the magnetite nanoparticles as a result of surfactant addition [Citation30]. As seen in , nanoparticles coated with STE (0.5 g or 1 g) show maximum peak broadening which further validates the role of STE as a potential biosurfactant for magnetic nanosystems.
Figure 2. The XRD diffraction pattern of bare Fe3O4, OA-Fe3O4, P-80-Fe3O4 and STE-Fe3O4 (1.0 g and 0.5 g).
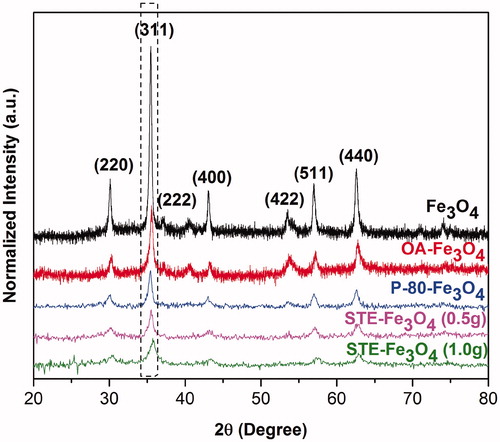
The average crystal size deduced from the broadening of the (311) reflection peak in the XRD pattern using Debye Scherrer formula was similar to the mean particle size obtained from TEM images (Supplementary Table 1).
FTIR analysis confirms successful coating of P-80, OA and STE onto the bare MNPs (Figure S1). The dynamic light scattering results of the bare and differently coated magnetite nanoparticles suggest that STE-coated nanosystems have the smallest hydrodynamic size as compared with other nanosystems synthesized in the study (). The hydrodynamic diameter for bare Fe3O4 nanoparticles obtained was 447.56 ± 15.97 nm, for P-80-Fe3O4 nanoparticles was 191.5 ± 5.72 nm, for OA-Fe3O4 nanoparticles was 270.4 ± 7.25 nm, for STE-Fe3O4 nanoparticles (0.5 g) was 56.83 ± 10.76 nm and for STE-Fe3O4 nanoparticles (1.0 g) was 49.77 ± 6.98 nm. The results substantiate the role of stevioside as a biosurfactant as it is found to be most effective in preventing the nanoparticles from aggregating than the routinely used surfactant moieties [Citation31–33]. The surface of all the MNPs was found to be negatively charged and similar in magnitude (Figure S2).
Figure 3. (A) Hydrodynamic size measurements of MNPs. (B–F) TEM image analysis and size distribution histograms of (B) Fe3O4, (C) P-80-Fe3O4, (D) OA-Fe3O4, (E) STE-Fe3O4 (0.5 g) and (F) STE-Fe3O4 (1.0 g). Dm represents the mean diameter and σ represents the standard deviation of the nanoparticles.
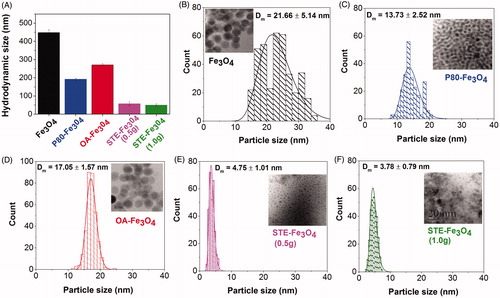
shows a representative TEM image of the bare magnetite nanoparticles and nanoparticles coated with STE, OA and P-80 generated by hydrothermal route. The mean diameter of bare Fe3O4 nanoparticles was 21.66 ± 5.14 nm, 13.70 ± 2.52 nm for P-80-coated Fe3O4 nanoparticles, 17.05 ± 1.57 nm for OA-coated Fe3O4 nanoparticles, 4.75 ± 1.01 nm for STE (0.5g)-coated Fe3O4 nanoparticles and 3.78 ± 0.79 nm for STE (1.0g)-coated Fe3O4 nanoparticles, as calculated by Digital Micrograph software, Thermo Fisher Scientific, Waltham, Massachusetts, USA. The effect of surfactant addition on the particle size of the nanoparticles is evident from a shift in the lognormal distribution curve for the nanoparticles towards the smaller size as a consequence of surfactant addition. Moreover, in-situ biosurfactant (stevioside) addition is also shown to promote uniform nucleation and subsequent ageing of the MNPs as observed by narrow particle size distribution exhibited by the STE-coated nanoparticles () as compared to other systems generated in the study (). In turn, such uniform nanoparticle systems would further have enhanced biomedical applications in cancer therapy [Citation34]. Also, in addition to the reduction in size and narrow size distribution, stevioside coating onto the surface layer of magnetite nanoparticles also acts as a matrix in which the nanoparticles have been embedded. This feature could further impact superior drug-loading capacity to stevioside-coated nanoparticles in this matrix as compared to other systems [Citation35].
In , the hysteresis loops of the coated and uncoated magnetite nanoparticles measured at 5 K and 300 K are presented. As shown in the graph, at room temperature (300 K), no hysteresis is seen for P-80, OA and STE-coated nanosystems, which indicates the superparamagnetic behavior of these systems. However, at 5 K, hysteresis can be observed for all the nanosystems, STE-coated Fe3O4 (1g) nanoparticles exhibit the lowest coercivity (Hc) in comparison to the all other samples. shows the coercivity as a function of particle size, which suggests that with decreasing particle size coercivity decreases as a function of particle size because of reduction of anisotropy energy [Citation36]. The hyperthermic measurements performed are under AC magnetic field, where as the coercivity measured for these samples are from DC magnetic measurements. Hence, it is far from easy to deduce a straightforward relation between measured coercivity and observed hyperthermia effect. However, our observed coercivity values re-confirm the reduction of particle size for STE-coated nanosystems in comparison to uncoated, P-80 and OA-coated nanosystems [Citation37].
Figure 4. Magnetic measurements of iron oxide nanoparticles. (A) Magnetization versus magnetic field measured at 300 K and 5 K, (B) Coercivity as a function of particle size at 300 K and 5 K.
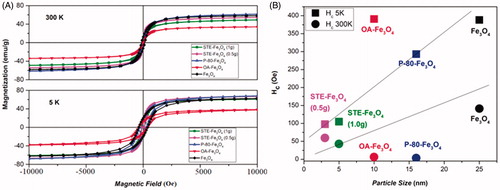
Overall, the X-ray diffraction pattern and TEM imaging demonstrate the potential of stevioside as a biosurfactant which is capable of significantly altering the size of nanoparticles with respect to other surfactants under identical conditions. The size of MNPs was found to decrease with increase in STE concentration () and is suggestive of capability to tune the particle size of nanosystems by only varying the biosurfactant concentration. The TEM micrographs () represent STE as a matrix in which the MNPs are embedded which further increases their interparticle distance, thus reducing the dipolar interactions that would exist between the nanoparticles. Therefore, it would help particles in aligning quickly with the external magnetic field leading to lower coercivity and reduced time gap in switching between their magnetic states. The matrix-like systems could further aid in entrapping certain chemotherapeutic drugs, resulting in more stable and efficient delivery to the cancer cells. Overall, these findings suggest the potential use of stevioside as a surfactant moiety to tune the particle size and the properties of nanosystems for use in cancer therapy in the near future.
Role of stevioside coating on magnetic nanoparticles internalization
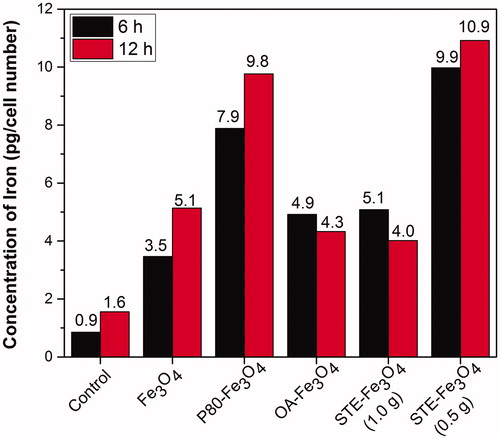
confirms the intracellular uptake of iron oxide nanoparticles described above by using a classical method of stain. To this end, C6 cells pre-incubated for 6 and 12 h, respectively with MNPs corresponding to 350 μg of Fe were stained with Prussian blue and visualized. It can be seen that most of the lysosomes of the C6 cells incubated with magnetite nanoparticles were stained in blue, whereas no stain was observed in the cytoplasm of the control C6 cells without magnetite nanoparticles under identical conditions. As the incubation time increased to 12 h, the Prussian blue staining was found to be gradually accumulated into the C6 glioma cells, as the cell population increased with the incubation time. As seen, STE-Fe3O4 (0.5 g) nanoparticles showed higher uptake into the C6 cells in comparison to other MNPs and were also found to be compatible with the cells, as the cell morphology remained unchanged upon 12 h incubation with the nanoparticles. From these results, it is clear that the stevioside coating as a biosurfactant supports the internalization of magnetic nanoparticles inside glioma cancer (C6) cells. This was further confirmed by quantitative estimation of iron uptake by the C6 glioma cells using ICP-AES (). The STE-Fe3O4 (0.5 g) nanoparticles exhibited most enhanced uptake in comparison to other nanosystems generated in the study within 6 h of incubation with the glioma cells. Also, the study by Chithrani et al. 2006 [Citation38], suggests that gold nanoparticle aggregate size of around 50 nm has cellular uptake half-life of about 1.90 h and with further increase in aggregate size, the uptake-half-life time increases. In accordance with this study, as shown in , the stevioside coating onto the surface layer of MNPs resulted in reduction of hydrodynamic size of the particles to 50 nm as compared to around 400 nm size obtained for the uncoated MNPs . Therefore, overall, it suggests that the reduced aggregate size of the MNPs because of stevioside coating, favors the internalization of STE-coated MNPs into the glioma cells resulting in their enhanced cellular uptake.
Figure 5. Prussian blue staining of C6 glioma cells indicating cellular uptake by C6 glioma cells at (A) 6 and (B) 12 h, respectively for control, bare and coated nanosystems (Scale bar = 100 μm).
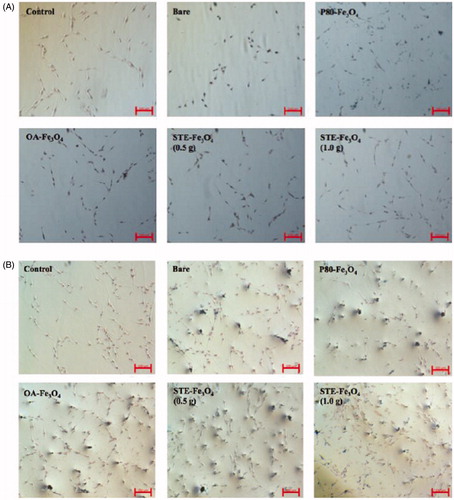
Figure 6. Quantitative analysis of MNP uptake by C6 glioma cells by ICP-AES after 6 and 12 h of treatment with the nanoparticles.
The sub-cellular localization of STE-coated nanoparticles (0.5 g) in endocytic compartments of the glioma cells was further verified by confocal microscopy (). Further, Prussian blue staining was done after 48 and 72 h post-incubation with bare and STE-coated MNPs for 24 h also demonstrates successful retention of MNPs in the glioma cells with higher cellular persistence exhibited by STE-coated MNPs upto 72 h after MNP removal (). It is suggestive of the capability of the MNPs to be available inside the cells for a sufficient period (upto at least 72 h) during which further treatment strategies can be employed for cancer therapy. Overall, the stronger interaction between the STE-coated magnetite nanoparticles and C6 cells might be the result of the carbohydrate nature of the biosurfactant used. There are various other studies in the literature also that report enhanced uptake of carbohydrate-coated nanomaterials in the cells [Citation39,Citation40]. In addition, this is the first time that stevioside-coated nanoformulations have been checked directly inside the cancer cells under a visible light microscope using complementary techniques like Prussian blue staining, to confirm the internalization process.
Figure 7. Confocal microscopy of C6 glioma cells to determine sub-cellular localization of nanoparticles in C6 cells (Scale bar = 10 μm). In the figure, nuclei are stained with Hoescht-3342, lysosomes with Lysotracker Red, and MNPs are represented by FITC channel. Arrows indicate sub-cellular localisation of FITC-conjugated MNPs in cells.
Stevioside as a potential anti-cancer hyperthermia agent
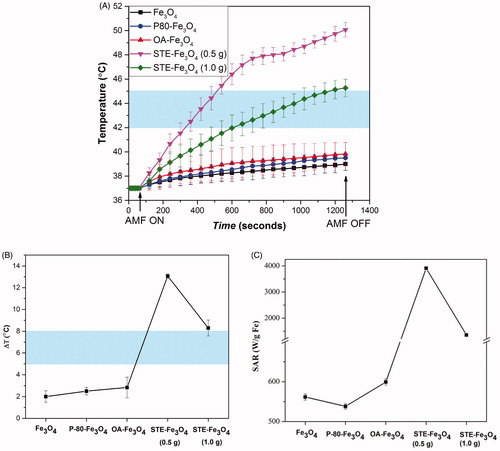
represents the calorimetric characterization of all the nanosystems generated. As shown in , only STE-coated MNPs showed significant temperature rise (9–13 °C) achieving hyperthermia range temperature. While bare and other coated MNPs (P-80 and OA) showed temperature rise of only up to 4 °C (as depicted in ). The SAR values obtained for stevioside-coated nanoparticles (3913.55 and 1355.36 W/g Fe for STE-Fe3O4 (0.5 g) and STE-Fe3O4 (1.0 g), respectively) were significantly higher than those for other nanosystems at f = 405 kHz and H = 168 Oe (). Various other studies have also reported that SAR values for superparamagnetic nanomaterials with low-particle concentration and small crystallite sizes subjected to alternating magnetic fields up to 225.72 Oe typically lie between 10 and 100 W/g MNP [Citation41]. Hence, proving the potential role of stevioside in enhancing the hyperthermia efficiency of the coated nanosystems. Moreover, with the increase in stevioside concentration the SAR value was found to decrease. The coating material is known to interact with the surface atoms of the magnetic core forming a magnetically disordered layer, which results in the reduction of the total magnetic phase content and thus, the heating rates of MNPs [Citation6,Citation42,Citation43]. The STE-MNPs despite of being ultra-small in size (around 5 nm) were found to exhibit significant heat generation in comparison to other nanosystems generated in our study. It could be possible that these STE-coated nanoparticles aggregate together to produce cluster-like formations, which results in such enhanced hyperthermia [Citation44,Citation45].
Figure 9. (A) The temperature change for Fe3O4 nanoparticles coated with P-80, OA, STE and bare Fe3O4 nanoparticles at a concentration of 1 mg/mL on application of AMF of strength 168 Gauss at f = 405 kHz for 20 min. (B) Total temperature change achieved for the nanosystems achieved and their corresponding SAR values (C). The shaded region represents the desired hyperthermia temperature window for the therapeutic effect.
As only STE-coated MNPs were capable of achieving desirable hyperthermia temperature range window at the maximum frequency available (f = 405 kHz), frequency and concentration (0.5 – 10 mg/mL) dependence on the heating profile was further evaluated for these nanosystems at the available fixed frequencies mentioned before.
A fall in heating capability of the nanosystems (13–0.9 °C for STE-Fe3O4 (0.5g) and 9.4–2.3 °C for STE-Fe3O4 (1.0 g) MNPs) was observed with decrease in frequency (405–101 kHz) (). Various other studies have also reported increase in SAR values for magnetic nanosystems with increasing frequency of the alternating field applied [Citation46].
Figure 10. Effect of frequency of STE-Fe3O4 MNPs (0.5 g) (A) and STE- Fe3O4 MNPs (1.0 g), (B) and effect of MNP concentration on STE- Fe3O4 MNPs (0.5 g), (C) and STE- Fe3O4 MNPs (1.0 g), (D) on thermal behavior of the nanosystems at f = 405 kHz and 168 Oe. The values written on curve C and D represent the total degree rise in temperature for different concentrations tested. The shaded region represents the desired hyperthermia temperature window for therapeutic effect.
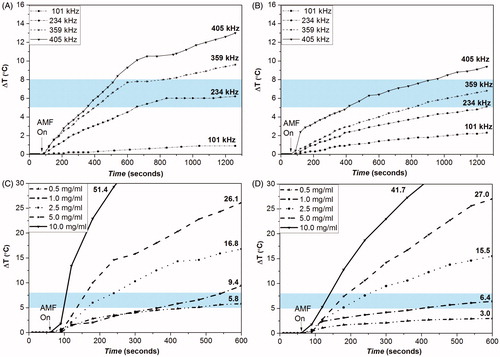
shows the influence of MNP concentration on variation in temperature rise in an alternating magnetic field of strength 168 Oe at f = 405 kHz. The total temperature rise of the STE-coated MNPs was observed to increase with the increasing concentration of the nanoparticles. The rise in temperature of the highest concentrated dispersions (10 mg/ml) for both STE-Fe3O4 (0.5 g) and STE-Fe3O4 (1.0 g) was found to be 51.4 and 41.7 °C, respectively, within 10 min of measurement. The temperature rise required for cancer hyperthermia was achieved within 2 min of switching on the magnetic field with concentration ranging from 2.5 mg/mL to 10 mg/mL for both STE-coated nanosystems. Such rapid rise in temperature is advantageous for cancer hyperthermia therapy. The SAR values of STE-coated nanosystems with increasing particle concentration is also reported in supplementary file (Table 3).
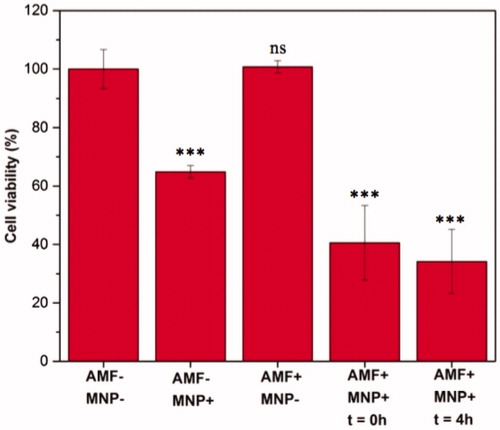
The in vitro hyperthermia results showed that the cell viability examined immediately after the AMF exposure (168 Oe at f = 405 kHz) decreases down to 40% in the presence of MNPs as compared to control samples (). Moreover, reseeding the cells and analyzing the viability after 4 h showed further decrease in cell viability (34%) reflecting the extent of cell damage induced by magnetic hyperthermia on various cellular processes [Citation47]. The control cells kept their viabilities up to 100%, ensuring that neither only MNPs nor AMF alone had an effect on cell viability. Overall, the STE-coated MNPs exhibited remarkable heating effect, which suggests their great potential for use in localized hyperthermia treatment of tumors like GBM, as shown in the study.
Conclusions
Here, we have demonstrated the potential of stevioside as a new biosurfactant for hyperthermia application of magnetic nanoparticles. Our results showed that coating the nanoparticles with the biosurfactant not only improved the cellular uptake of the nano-magnets in glioma cells but also enhanced its retention time as compared to the bare nanoparticles. Not only this, the stevioside coating further exhibited significant improvement in the calorimetric hyperthermia activity, through particle size reduction, which is essential for magnetic hyperthermia-mediated cancer therapy as demonstrated in in vitro experiments conducted on rat C6 glioma cells.
Supplemental Material
Download PDF (1 MB)Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Hergt R, Dutz S, Müller R, et al. Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. J Phys Condens Matter. 2006;18:S2919–S2934.
- Laurent S, Dutz S, Häfeli UO, et al. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8–23.
- Yu MK, Jeong YY, Park J, et al. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed. 2008;47:5362–5365.
- Li K, Shen M, Zheng L, et al. Magnetic resonance imaging of glioma with novel APTS-coated superparamagnetic iron oxide nanoparticles. Nanoscale Res Lett. 2014;9:304.
- Lim EWC, Feng R. Agglomeration of magnetic nanoparticles. J Chem Phys. 2012;136:124109
- Grüttner C, Müller K, Teller J, et al. Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. Int J Hyperth. 2013;29:777–789.
- Cherian AK, Rana AC, Jain SK. Self-assembled carbohydrate-stabilized ceramic nanoparticles for the parenteral delivery of insulin. Drug Dev Ind Pharm. 2000;26:459–463.
- Kennedy DC, Orts-Gil G, Lai CH, et al. Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake. J Nanobiotechnology. 2014;12:59.
- Bojarova P, Kren V. Sugared biomaterial binding lectins: achievements and perspectives. Biomater Sci. 2016;4:1142–1160.
- Muñoz-Bonilla A, Marcelo G, Casado C, et al. Preparation of glycopolymer-coated magnetite nanoparticles for hyperthermia treatment. J Polym Sci A Polym Chem. 2012;50:5087–5096.
- Wadehra N, Gupta R, Prakash B, et al. Biocompatible ferrite nanoparticles for hyperthermia: effect of polydispersity, anisotropy energy and inter-particle interaction. Mater Res Express. 2017;4:5–7.
- Kolhatkar AG, Jamison AC, Litvinov D, et al. Tuning the magnetic properties of nanoparticles. Int J Mol Sci. 2013;14:15977–16009.
- Dennis CL, Ivkov R. Physics of heat generation using magnetic nanoparticles for hyperthermia. Int J Hyperthermia. 2013;29:715–729.
- Wan ZL, Wang LY, Yang XQ, et al. Controlled formation and stabilization of nanosized colloidal suspensions by combination of soy protein and biosurfactant stevioside as stabilizers. Food Hydrocoll. 2016;52:317–328.
- Wan ZL, Wang LY, Wang JM, et al. Synergistic interfacial properties of soy protein-stevioside mixtures: relationship to emulsion stability. Food Hydrocoll. 2014;39:127–135.
- Barwal I, Sood A, Sharma M, et al. Development of stevioside Pluronic-F-68 copolymer based PLA-nanoparticles as an antidiabetic nanomedicine. Colloids Surf B Biointerfaces. 2013;101:510–516.
- Bunprajun T, Yimlamai T, Soodvilai S, et al. Stevioside enhances satellite cell activation by inhibiting of NF-κB signaling pathway in regenerating muscle after cardiotoxin-induced injury. J Agric Food Chem. 2012;60:2844–2851.
- Yasukawa K, Kitanaka S, Seo S. Inhibitory effect of stevioside on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Biol Pharm Bull. 2002;25:1488–1490.
- Paul S, Sengupta S, Bandyopadhyay TK, et al. Stevioside induced ROS-mediated apoptosis through mitochondrial pathway in human breast cancer cell line MCF-7. Nutr Cancer. 2012;64:1087–1094.
- Ren HP, Yin XY, Yu HY, et al. Stevioside induced cytotoxicity in colon cancer cells via reactive oxygen species and mitogen-activated protein kinase signaling pathways-mediated apoptosis. Oncol Lett. 2017;13:2337–2343.
- Teng X, Yang H. Effects of surfactants and synthetic conditions on the sizes and self-assembly of monodispersive iron oxide nanoparticles. J Mater Chem. 2004;14:774–779.
- Frey NA, Peng S, Cheng K, et al. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem Soc Rev. 2009;38:2532–2542.
- Mourdikoudis S, Liz-Marzan LM. Oleylamine in nanoparticle synthesis. Chem Mater. 2013;25:1465–1476.
- Ge S, Shi X, Sun K, et al. A facile hydrothermal synthesis of iron oxide nanoparticles with tunable magnetic properties. J Phys Chem C. 2009;113:13593–13599.
- Oh Y, Lee N, Kang HW, et al. In vitro study on apoptotic cell death by effective magnetic hyperthermia with chitosan-coated MnFe2O4. Nanotechnology. 2016;27:115101.
- Calero M, Chiappi M, Lazaro-Carrillo A, et al. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnology. 2015;13:16.
- Andreu I, Natividad E. Accuracy of available methods for quantifying the heat power generation of nanoparticles for magnetic hyperthermia. Int J Hyper. 2013;29:739–751.
- Yao X, Niu X, Ma K, et al. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small. 2017;13:1–11.
- Ramirez-Nuñez AL, Jimenez-Garcia LF, Goya GF, et al. In vitro magnetic hyperthermia using polyphenol-coated Fe3O4@γ- Fe2O3 nanoparticles from Cinnamomun verum and Vanilla planifolia: the concert of green synthesis and therapeutic possibilities. Nanotechnology. 2018;29:074001.
- Wulandari IO, Mardila VT, Santjojo DJHD, et al. Preparation and characterization of chitosan-coated Fe3O4 nanoparticles using ex-situ co-precipitation method and tripolyphosphate/sulphate as dual crosslinkers. IOP Conf Ser Mater Sci Eng. 2018;299:012064.
- Lv Y, Yang Y, Fang J, et al. Size dependent magnetic hyperthermia of octahedral Fe3O4 nanoparticles. RSC Adv. 2015;5:76764–76771.
- Kim DK, Mikhaylova M, Zhang Y, et al. Protective coating of superparamagnetic iron oxide nanoparticles. Chem Mater. 2003;15:1617–1627.
- Issa B, Obaidat IM, Albiss BA, et al. Magnetic nanoparticles: surface effects and properties related to biomedicine applications. IJMS. 2013;14:21266–21305.
- Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev. 2012;41:2575–2589.
- Hazra C, Kundu D, Chatterjee A, et al. Poly(methyl methacrylate) (core)-biosurfactant (shell) nanoparticles: size controlled sub-100 nm synthesis, characterization, antibacterial activity, cytotoxicity and sustained drug release behavior. Colloids Surf A Physicochem Eng Asp. 2014;449:96–113.
- Smolensky ED, Park HYE, Zhou Y, et al. Scaling laws at the nano size: the effect of particle size and shape on the magnetism and relaxivity of iron oxide nanoparticle contrast agents. J Mater Chem B. 2013;1:2818–2828.
- Blanco-Gutierrez V, Saez-Puche R, Torralvo-Fernandez MJ. Superparamagnetism and interparticle interactions in ZnFe2O4 nanocrystals. J Mater Chem. 2012;22:2992–3003.
- Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett . 2006;6:662–668.
- Kang B, Opatz T, Landfester K, et al. Carbohydrate nanocarriers in biomedical applications: functionalization and construction. Chem Soc Rev. 2015;44:8301.
- Salatin S, Khosroushahi AY. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J Cell Mol Med. 2017;21:1668–1686.
- Araújo-Neto RP, Silva-Freitas EL, Carvalho JF, et al. Monodisperse sodium oleate coated magnetite high susceptibility nanoparticles for hyperthermia applications. J Magn Magn Mater. 2014;364:72–79.
- Theodorou IG, Ruenraroengsak P, Gow A, et al. Effect of pulmonary surfactant on the dissolution, stability and uptake of zinc oxide nanowires by human respiratory epithelial cells. Nanotoxicology. 2016;10:1351–1362.
- Soares PIP, Lochte F, Echeverria C, et al. Thermal and magnetic properties of iron oxide colloids: influence of surfactants. Nanotechnology. 2015;26:425704.
- Hugounenq P, Levy M, Alloyeau D, et al. Iron oxide monocrystalline nanoflowers for highly efficient magnetic hyperthermia. J Phys Chem C . 2012;116:15702–15712.
- Wang W, Tang B, Benzhi J, et al. Size-controlled synthesis of water-dispersible superparamagnetic Fe3O4 nanoclusters and their magnetic responsiveness. RSC Adv. 2015;5:75292–75299.
- Cheraghipour E, Javadpour S. Cationic albumin-conjugated magnetite nanoparticles, novel candidate for hyperthermia cancer therapy. Int J Hyperth. 2013;29:511–519.
- Goya GF, Asín L, Ibarra MR. Cell death induced by ac magnetic fields and magnetic nanoparticles: current state and perspectives. Int J Hyperth. 2013;29:810–818.