 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In transabdominal histotripsy, ultrasound pulses are focused into the body to noninvasively destroy soft tissues via cavitation. However, the ability to focus is limited by phase aberration, or decorrelation of the ultrasound pulses due to spatial variation in the speed of sound throughout heterogeneous tissue. Phase aberration shifts, broadens, and weakens the focus, thereby reducing the safety and efficacy of histotripsy therapy. This paper reviews and discusses aberration effects in histotripsy and in related therapeutic ultrasound techniques (e.g., high intensity focused ultrasound), with an emphasis on aberration by soft tissues. Methods for aberration correction are reviewed and can be classified into two groups: model-based methods, which use segmented images of the tissue as input to an acoustic propagation model to predict and compensate phase differences, and signal-based methods, which use a receive-capable therapy array to detect phase differences by sensing acoustic signals backpropagating from the focus. The relative advantages and disadvantages of both groups of methods are discussed. Importantly, model-based methods can correct focal shift, while signal-based methods can restore substantial focal pressure, suggesting that both methods should be combined in a 2-step approach. Aberration correction will be critical to improving histotripsy treatments and expanding the histotripsy treatment envelope to enable non-invasive, non-thermal histotripsy therapy for more patients.
1. Introduction
Histotripsy is a therapeutic ultrasound (TU) technique that uses cavitation to mechanically ablate soft tissues [Citation1]. As originally conceived, repeated microsecond-duration pulses from a strongly focused ultrasound source produce a large localized rarefactional pressure in tissue, generating a cloud of inertial cavitation bubbles that expand and contract to rupture cells [Citation2]. Spontaneous cavitation occurs when a sound field directly exceeds the intrinsic threshold for cavitation bubble nucleation [Citation2] or indirectly through shock-scattering where reflected acoustic waves undergo a phase inversion adding to trailing incoming cycles in the pulse [Citation3]. Boiling histotripsy is a variant producing similar tissue disruption effects but through a different mechanism using much longer millisecond duration tone bursts to initiate boiling [Citation4]. Positive amplitude shock fronts from highly nonlinear millisecond-duration acoustic tone bursts rapidly heat tissue to create a millimeter-sized vapor cavity. Subsequent shock fronts interact with the cavity to mechanically disintegrate adjacent cells [Citation5]. Histotripsy as a noninvasive, non-thermal tissue ablation technique has been investigated pre-clinically and/or clinically for many applications, including therapy in the brain, heart, liver, kidney, and prostate [Citation6–22]. Notably, histotripsy has recently begun multi-center clinical trials for the ablation of liver tumors in the United States (NCT04572633) and Europe (NCT04573881).
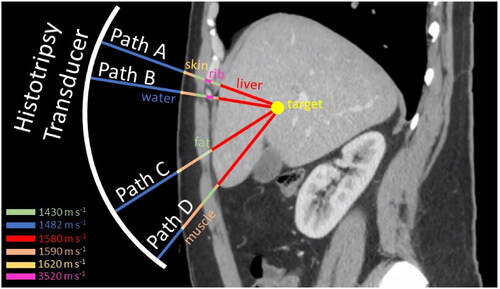
Histotripsy, like other therapeutic ultrasound techniques, relies on focusing the ultrasound beam to the target. However, heterogenous intervening tissues can introduce variations in the speed of sound along the beam path, causing the converging wavefront to accumulate phase errors that compromise constructive interference at the focus [Citation23–26]. Also, tissue boundaries cause obliquely incident sound waves to refract, shifting the focus of the ultrasound beam away from its intended location. These two effects are collectively called phase aberration in this paper. Phase aberration weakens, broadens, and shifts the beam focus, limiting the efficacy and safety of therapeutic ultrasound treatments [Citation27–31]. In histotripsy, aberration reduces the focal pressure amplitude and thus hinders the formation of bubbles for therapy. Specifically, lower focal pressures produce smaller bubble clouds or boiling bubbles and thus smaller lesions [Citation32,Citation33]. In cases of severe amplitude reduction, bubble (and lesion) formation can be prevented altogether [Citation14]. Aberration also diverts energy from the focus to intervening tissues, elevating the risk of heating when highly absorptive tissues (i.e., ribs) are present. Finally, refraction can shift the focus by several millimeters, causing therapy mis-targeting [Citation34]. The effects of aberration are moderate when the beam path is mostly homogeneous (e.g., when focusing on a shallow soft tissue target) but can be substantial when the beam path is strongly heterogeneous (e.g., when focusing through thick perinephric, visceral, breast or abdominal fat or through bony tissues like skull and ribs), as illustrated in . Therefore, the ultimate consequence of aberration is to limit the range of feasible treatment locations for histotripsy in the body (the treatment ‘envelope’) and restrict the population of patients who can receive treatment.
Figure 1. Heterogeneous tissue paths. Ultrasound waves from different portions of the histotripsy transducer aperture take heterogeneous paths to the target due to differences in the speed of sound among water and different tissues.
For this reason, efforts to correct aberration in therapeutic ultrasound and histotripsy have increased substantially in recent years. Aberration can be corrected by subdividing the aperture of the therapy transducer into an array of smaller transducer elements (a ‘phased array’) and adjusting the transmission phase of each element to compensate spatially varying phase differences, which are determined using one of two groups of methods [Citation35–37]. The first group maps the speed of sound distribution in tissue and feeds this map into a numerical acoustic propagation model to predict the phase errors accumulated along the path to the target. These methods are called ‘model-based methods’ in this paper. The second group, here called ‘signal-based methods,’ use the therapy array to sense the acoustic signals that backpropagate from the focus and thereby measure the phase differences between elements. Model-based methods and signal-based methods have distinct advantages and disadvantages, which will be discussed in the following sections.
This review synthesizes research on aberration effects and correction methods for histotripsy. Histotripsy shares similar aberration effects with other therapeutic ultrasound modalities. Therefore, much of the cited work and discussion in this review is derived from or applicable to other therapeutic modalities (e.g., high intensity focused ultrasound). Also, this review centers primarily on soft tissue aberration, which has historically received less attention than aberration by skull and ribs. Reviews specific to transcranial or transcostal aberration correction for therapeutic ultrasound can be found in [Citation38–40]. Finally, the Discussion section of this review outlines potential strategies and future work to support the translation of histotripsy aberration correction to the clinic, with the goal of improving histotripsy and enabling treatment of patients with diverse body habitus.
2. Soft tissue phase aberration of histotripsy
Early in its development, histotripsy (produced by inertial cavitation from short acoustic pulses) was considered ‘resilient’ to aberration. This idea of resiliency arose because histotripsy was shown to generate precise lesions in the presence of strong aberrations from skull and ribs [Citation41–43]. Diffraction through ribs can distort and split the ultrasound focus into multiple ‘grating’ lobes for long or continuous wave exposures as in High Intensity Focused Ultrasound (HIFU) [Citation44–48] producing collateral damage (necrosis outside of the main focal lobe) [Citation49]. Histotripsy using very short pulses greatly reduces the amplitude of grating lobes [Citation50]. The threshold mechanism for histotripsy from short pulses restricts the generation of cavitation (and thus any damage) to only occur where the pressure exceeds a critical amplitude (>28 MPa) [Citation2,Citation51]. Therefore, histotripsy lesions can be created without collateral damage by setting the transducer power output such that only the main focal lobe exceeds the cavitation threshold while any secondary lobes remain below the cavitation threshold [Citation32,Citation41]. For all these reasons and the demonstrated ability to safely generate lesions through bone, aberration correction appeared to be less critical for histotripsy than for other TU modalities. Aberration from soft tissue was considered even less critical. The differences in the speed of sound among soft tissues are relatively modest (<10%) [Citation52]. Additionally, histotripsy uses relatively low ultrasound frequencies (usually ≤1 MHz) [Citation1,Citation53]. Arrival time variations from soft tissue were assumed to be much shorter than an acoustic cycle and thus unlikely to cause significant aberration effects.
However, more recent work indicates that soft tissues can cause severe de-focusing of histotripsy and other TU modalities despite low therapeutic frequencies. Soft tissues of the breast, abdomen, and pelvis can induce large arrival time variations that attenuate, distort, and shift the TU focus [Citation54–61]. Clinical studies of HIFU in the kidney reported that treatments failed in some patients despite low obstruction from bone [Citation62]. The failed treatments were later attributed to de-focusing by thick layers of abdominal fat [Citation60,Citation61,Citation63]. Similarly, Khoklova et al. found that boiling histotripsy treatments in porcine liver failed in some subjects due to aberration induced by the abdominal wall [Citation14]. This substantial soft tissue aberration effect has been attributed to the large aperture and strong curvature (f-number ≤1.5) of TU transducers [Citation34,Citation64]. The large aperture focuses the ultrasound beam through a large extent of the body. Over this extent, the composition and spatial distribution of intervening tissues can vary substantially, increasing variation in the sound path and thus arrival time across the aperture [Citation64]. The strong curvature creates large angles of incidence, increasing refractive aberrations and inducing large shifts in the focus point of the ultrasound beam [Citation34].
Histotripsy transducers frequently have very large aperture and strong curvature (f-number ≤1) and therefore may be even more susceptible to focal pressure loss and focal shift from soft tissue aberration than other TU modalities [Citation65,Citation66]. Yeats et al. used computational simulations with human data to investigate transabdominal aberration effects for the large aperture, low f-number histotripsy transducer geometry currently in clinical use for liver tumor ablation [Citation67]. It was found that propagation through the human abdomen led to average arrival time differences (ATD) across the aperture of about 0.65 µs (0.5 cycles at the 750-kHz central frequency). A representative example of the arrival time variation across the aperture is shown in . In this example, the maximum ATD across the aperture exceeded 1.3 µs (1 cycle at the central frequency). The resulting destructive interference caused an average focal pressure amplitude loss of 50% and severely distorted the focal shape, as shown in . Propagation through abdominal layers also produced a large average focal shift of ∼6 mm (primarily along the axial direction), in agreement with observations in vivo [Citation42]. These findings indicate that aberration correction could substantially improve the focal pressure amplitude and targeting accuracy for transabdominal histotripsy treatments.
Figure 2. Soft tissue histotripsy phase aberration. (a) a histotripsy transducer (blue) was simulated focusing through the human abdomen at a target (yellow) in the liver. (b) Simulated arrival time differences to the target projected onto the transducer aperture. Portions blocked by rib and bowel/lung are shown in gray and black, respectively. (c) Axial profiles of the focal pressure amplitude, with (orange) and without (blue) aberration correction. (d), (e) 12-mm × 12-mm transverse cross sections of the focal pressure amplitude, without (d) and with (e) aberration correction (this figure is adapted from Yeats et al. [Citation67]).
![Figure 2. Soft tissue histotripsy phase aberration. (a) a histotripsy transducer (blue) was simulated focusing through the human abdomen at a target (yellow) in the liver. (b) Simulated arrival time differences to the target projected onto the transducer aperture. Portions blocked by rib and bowel/lung are shown in gray and black, respectively. (c) Axial profiles of the focal pressure amplitude, with (orange) and without (blue) aberration correction. (d), (e) 12-mm × 12-mm transverse cross sections of the focal pressure amplitude, without (d) and with (e) aberration correction (this figure is adapted from Yeats et al. [Citation67]).](/cms/asset/ba334717-9dea-4c24-8dbe-db43091774f1/ihyt_a_2266594_f0002_c.jpg)
Yeats et al. also found that focal shift and focal pressure amplitude loss arise from distinct components of abdominal wall heterogeneity [Citation67]. Specifically, large scale layering of acoustic media (e.g., water bath over tissue) governs the focal shift, while irregular layer morphologies (e.g., fluctuations in fat thickness) dominate the pressure loss. Division of the transabdominal beam path into layers creates gross transitions in the speed of sound along the transducer axis. For example, at the water to tissue interface, the speed of sound increases from ∼1480 m s−1 (water) to ∼1560 m s−1 (tissue). Sound waves from inner regions of the aperture are incident at small angles to the interface and thus maintain their direction of propagation. Sound waves from outer regions of the aperture intersect the interface with large angles of incidence and thus refract toward the transducer axis, following Snell’s law. Due to this refraction, outer waves propagate farther at higher sound speed (in tissue) than inner waves. Therefore, trailing portions of the outer (spherical) waves intersect leading portions of the inner waves to interfere maximally at a point preceding the transducer geometric center, shifting the focus closer to the transducer. The resulting differences in propagation time to the geometric center have axial symmetry across the aperture. Such symmetric propagation time differences do not cause destructive interference but rather allow sound waves to interfere constructively at a point away from the geometric center (closer to the transducer in this case), shifting the focus location but causing negligible pressure loss. These observations agree with previous TU studies. Fan and Hynynen simulated transabdominal HIFU and modeled the abdomen as parallel, flat layers [Citation68] and later as curved layers of constant thickness [Citation69]. In both cases, the focus was shifted pre-focally, but pressure loss was negligible. Pressure loss, unlike focal shift, arises from irregular variations in the thickness of tissue layers. These thickness fluctuations lead to random, asymmetric arrival time differences that do not permit convergence at any off-focus location but rather produce destructive interference and amplitude loss. Liu et al. updated Fan and Hynynen’s model of the abdominal wall to include tissue layers of irregularly varying thickness [Citation70]. The updated model showed a large pressure amplitude loss in addition to the focal shift. The relative independence of focal shift and focal pressure loss in transabdominal aberration may permit a two-part correction approach, in which one part aims to correct focal shift, while another part corrects for pressure loss. The two-step approach is addressed in the Discussion section.
3. Aberration reduction methods
Aberration correction for histotripsy requires a phased array of many elements (typically >100) and phase detection strategies based on modeling acoustic propagation or receiving ultrasound signals on the array elements. These requirements increase the complexity of the histotripsy system and treatment. To avoid this added complexity, some early work sought not to correct but to reduce aberration by modifying the frequency content of the therapeutic ultrasound beam [Citation71–73]. Lower frequency waves are less susceptible to decorrelation in heterogeneous tissue than higher frequencies and thus maintain greater coherence in the presence of aberration [Citation74]. However, higher frequencies are desirable because they produce a smaller focus and thus enable selective spatial targeting of the therapy. White et al. [Citation72] and later Lin et al. [Citation73] proposed to combine these properties by specially designing the therapy beam. White et al. took advantage of nonlinear propagation effects to convert the energy of the therapy beam from the fundamental frequency (272 kHz) to the second harmonic (544 kHz) at a point between the nearfield and the focus [Citation72]. As a result, the therapy beam propagated through the aberrating region (ex vivo human skull) at low (272 kHz) frequency and then focused in the target region at higher frequency (544 kHz). The focus of this nonlinearly propagated beam showed reduced aberration effects (improved focal symmetry) compared to simple linear propagation of a 544-kHz source. In another approach developed specifically for histotripsy, Lin et al. combined low and high frequencies by direct linear summation of two confocal sources, each transmitting at subtherapeutic amplitude such that only the summed amplitude at the focus exceeded the cavitation threshold [Citation73]. The idea was to deliver one portion of the beam energy at a lower frequency and thus mitigate attenuation from absorption and aberration. The other portion was delivered at higher frequency to restrict the focus size and thus maintain spatial selectivity of the histotripsy therapy. Proof-of-concept was demonstrated for these ‘aberration reduction’ techniques by simulation and by experiments in vitro [Citation72,Citation73]. However, these techniques only modestly improve the focal pressure (White et al. achieved a maximum transcranial focusing gain of 60% [Citation72]) and in general do not correct the focal shift.
4. Model-based aberration correction methods
In model-based aberration correction, tissues are imaged (e.g., CT, MRI) and assigned appropriate acoustic properties to create a 3-D map of the acoustic beam path. This map is input to a numerical model of acoustic propagation to predict phase errors from aberration. The acoustic propagation model can range from complex (e.g., full-wave simulations accounting for diffraction, scattering, and absorption) to simple (e.g., ray tracing), typically requiring a compromise between accuracy and computational efficiency. The model-based approach has been applied to minimize energy deposition at the ribs during transcostal HIFU [Citation75–81] and to correct phase aberration through the skull for both HIFU and histotripsy [Citation82–90]. In transcranial aberration correction, the rigid skull bone can be repeatably registered to a pre-operative image (i.e., CT) obtained several days before treatment. Abdominal tissues, however, readily shift and deform during patient repositioning or respiratory motion, thereby altering the tissue path and invalidating the phase estimates obtained from the numerical model. Due to these challenges, model-based correction methods incorporating aberration from soft tissues have remained largely theoretical [Citation70,Citation91].
To overcome complications from tissue deformation during repositioning, one approach is to integrate the histotripsy array with a mechanically co-registered point-of-care imaging modality to map the soft tissue conformation at the time of therapy and thus preserve spatial information. Systems integrated with point-of-care MRI have been developed for HIFU treatment of the uterus [Citation92–94] and breast [Citation95,Citation96]. Model-based AC using point-of-care MR images has been demonstrated in breast phantoms [Citation64,Citation97]. This approach has not yet been applied for soft tissue AC in the lower abdomen or pelvis. However, Wagner et al. recently developed a transabdominal histotripsy system with integrated point-of-care Cone Beam Computed Tomography (CBCT) imaging for automated therapy targeting [Citation98]. The CBCT images map the tissue path at the time of treatment and could thus be input to a numerical propagation model for aberration correction. Implementation of this approach in a clinically relevant timeframe would require [Citation1] rapid (automatic) segmentation of the 3-D images into different tissue types and [Citation2] a rapid, computationally efficient numerical propagation model. Rapid segmentation could likely be accomplished using machine learning, which has significantly advanced automated medical image labeling in recent years [Citation99–102]. Rapid propagation modeling could be accomplished by a simple ray tracing or hybrid angular spectrum (HAS) approach [Citation87,Citation103–105]. A ray tracing approach for modeling transabdominal propagation is illustrated in . Ray tracing models have been applied to rapidly correct for refractive aberrations and eliminate focal shift for transabdominal ultrasound imaging [Citation106–108]. Thus, model-based AC with point-of-care imaging is likely feasible for transabdominal histotripsy.
Figure 3. The ray tracing method described by Liu et al. to correct phase errors when focusing through the human abdomen [Citation70]. (a) abdominal magnetic resonance image of a patient receiving therapeutic ultrasound treatment for a uterine fibroid. (b) Segmentation of the patient image into water (W), fat (F), muscle (M), uterus (U), and fibroid (Fi). (c) ray tracing method to determine phase correction. Step 1: Project rays from the transducer assuming a homogeneous (h) medium to find the normal velocity at the fibroid surface. Step 2: Project the conjugate velocity
back to a transducer through the heterogeneous path to find
Step 3: Extract the corrected phase as
(This figure is adapted from Liu et al. [Citation70]).
![Figure 3. The ray tracing method described by Liu et al. to correct phase errors when focusing through the human abdomen [Citation70]. (a) abdominal magnetic resonance image of a patient receiving therapeutic ultrasound treatment for a uterine fibroid. (b) Segmentation of the patient image into water (W), fat (F), muscle (M), uterus (U), and fibroid (Fi). (c) ray tracing method to determine phase correction. Step 1: Project rays from the transducer assuming a homogeneous (h) medium to find the normal velocity va at the fibroid surface. Step 2: Project the conjugate velocity va* back to a transducer through the heterogeneous path to find vb. Step 3: Extract the corrected phase as arg(vb*). (This figure is adapted from Liu et al. [Citation70]).](/cms/asset/c5e65c05-37e7-43ee-9724-265df3c6de88/ihyt_a_2266594_f0003_c.jpg)
Another significant challenge to model-based AC in the abdomen is tissue motion from respiration and cardiac activity. Respiratory and cardiac motion cyclically alter the location of the target tissue with respect to the therapy array and the conformation of the tissue layers in the beam path. The target displacement can be compensated by tracking tissue motion and appropriately adjusting the array position with a mechanical positioner [Citation109]. The changes in layer conformation, however, slightly alter the refracted beam path and therefore cause the magnitude of the focal shift to fluctuate. Compensation of these fluctuations would require real-time updates to the model-based phase correction or pre-computation of the correction over the entire respiratory cycle. Both strategies would be difficult to implement in practice. Nonetheless, the fluctuations caused by respiration are likely much smaller than the average magnitude of the focal shift and thus unlikely to strongly affect the targeting error.
Despite their challenges, model-based methods will be critical to histotripsy aberration correction. Importantly, model-based AC methods can correct for focal location shift, while signal-based AC methods cannot. Signal-based methods can only access phase information from the focus [Citation110,Citation111]. If the focus has been shifted by refraction, the measured phase differences can improve coherence at the shifted focus but cannot re-focus on the target [Citation112]. Model-based AC methods have access to the speed of sound distribution throughout the entire tissue path and can therefore predict and correct the geometric wavefront distortions and refractive aberrations that shift the focus away from the intended target [Citation113]. Furthermore, model-based methods can correct focal shift in soft tissues using only a coarse resolution of the speed of sound distribution. Dong et al. used a full-wave acoustic model based on automatically segmented MR images to predict the location of the HIFU focus for transmission through ex vivo multilayer porcine tissue [Citation114]. The authors found that a coarse image segmentation, in which each tissue layer was modeled as a homogenous, isotropic material with a single speed of sound, performed similarly to a fine image segmentation, in which the speed of sound was varied at the resolution limit of the MR image. This result agrees well with previous observations that the gross geometry of the speed of sound distribution (e.g., tissue layers) governs the focal shift [Citation34]. Additionally, for soft tissue, the accuracy of the model-based focal point correction is not highly sensitive to the value of sound speed assigned to each tissue layer [Citation103,Citation107]. In a study on abdominal US imaging, model-based AC corrected the focal position to within 1 mm of the target, even after introducing errors of ±50 m/s in the sound speed assigned to fat [Citation107]. Therefore, model-based AC is a feasible and necessary step to correct focal point error for transabdominal histotripsy.
Importantly, while model-based aberration correction methods are effective for correcting focal shift, they may not substantially increase pressure amplitude at the focus. Unlike the symmetric phase errors that govern focal shift, the asymmetric phase errors that decrease pressure amplitude must be known with high accuracy to maximize coherence at the focus. These asymmetric phase errors result from fine variations in the thickness of tissue layers and from tissue microstructure (e.g., fat striations in muscle) [Citation115,Citation116]. Medical images have limited spatial resolution (often > λ/2 at typical histotripsy frequencies) and soft tissue resolution (ability to distinguish tissues beyond major types like muscle, fat, etc.), which limit the resolution of fine tissue features. Additionally, published values for acoustic properties vary substantially, and it is possible that this variance is intrinsic [Citation117]. Sound speed estimates derived from CT Hounsfield units are prone to system-specific bias [Citation118]. Therefore, a model-based approach is unlikely to predict phase with the accuracy necessary to maximize coherence at typical histotripsy wavelengths. For this reason, focal shift correction with a model-based AC method must be complemented with a signal-based AC method to restore focal pressure amplitude. Signal-based AC methods will be discussed in the following section.
5. Signal-based aberration correction methods
Signal-based aberration correction methods aim to determine phase errors by receiving ultrasound signals from the focus. Unlike model-based methods, signal-based methods make direct acoustic measurements and thus avoid errors from modeling and co-registration. Also, acoustic data collection can be very fast and potentially interleaved with therapy emissions to update the aberration correction for focusing at different locations within a large treatment volume. Signal-based methods were first developed for aberration correction of ultrasound imaging [Citation110,Citation119–122] and were extended to therapeutic ultrasound and histotripsy following the introduction of receive-capable therapeutic arrays [Citation35,Citation123–125]. The ability to receive signal on each array channel facilitates the determination of the element-to-element phase differences at the focus. Signal-based methods can be divided into two categories based on the type of signals used for correction. These are ultrasound speckle signals and signals from bubbles (contrast agents or cavitation). Correction methods based on each signal type are discussed below.
5.1. Ultrasound speckle signals
The ideal signal for aberration correction is a point source or beacon [Citation119,Citation121]. Beacon signals have high spatial coherence and deterministic phase and thus enable accurate determination of the element-to-element phase differences from just one signal acquisition [Citation119]. However, beacons are rarely available endogenously in biological tissue. The most common signal source is scattering of the ultrasound beam by sub-wavelength reflectors within tissues (“speckle”) [Citation126]. Speckle signals originating from the beam focus can be used for aberration correction but have limited spatial coherence and stochastic phase. Consequently, the speckle signals on spatially distant array elements have low covariance, hindering the determination of the element-to-element phase differences [Citation127]. The spatial coherence of speckle depends on the focal spot size and thus decreases with acoustic frequency [Citation128]. Therefore, aberration correction based on speckle signals is especially challenging for low-frequency modalities like histotripsy.
Thomas et al. addressed the challenge of low speckle coherence for histotripsy by taking advantage of nonlinear effects at the focus and by iteratively estimating the phase differences [Citation129]. The large pressure amplitudes employed in histotripsy produce strong nonlinear effects including the generation of harmonic frequencies at the focus [Citation130]. The resulting harmonic echoes have a smaller focal spot size than the fundamental frequency and thus increase the spatial coherence of speckle [Citation131]. To increase the signal-to-noise ratio of the harmonic echoes, Thomas et al. employed a pulse-inversion scheme, transmitting two pulses of opposite polarity with a 1.5-MHz boiling histotripsy array and receiving the amplified second harmonic (3-MHz) on the therapy elements [Citation129]. Then, the spatial coherence at the focus was improved iteratively by estimating the phase differences from the received speckle, re-transmitting with phase correction, and repeating, as illustrated in . Iterative correction progressively decreased the focal spot size and improved the covariance of the received signals until converging at an optimal phase correction, restoring the focal pressure amplitude and focusing quality to un-aberrated levels when focusing through an abdominal wall phantom in vitro. The technique was later applied in vivo and successfully reduced the acoustic power required for therapy in the pig liver by up to 45% [Citation132].
Figure 4. Iterative phase estimation using ultrasound speckle signals. Left side: color-coded maps of the detected arrival time for each element of a boiling histotripsy array focusing through in vivo porcine abdomen. Right side: the speckle signal received by each array element. The dashed line marks the time window corresponding to the focus. Iterations 0, 2, and 8 (final) are shown (this figure is adapted from Thomas et al. [Citation132]).
![Figure 4. Iterative phase estimation using ultrasound speckle signals. Left side: color-coded maps of the detected arrival time for each element of a boiling histotripsy array focusing through in vivo porcine abdomen. Right side: the speckle signal received by each array element. The dashed line marks the time window corresponding to the focus. Iterations 0, 2, and 8 (final) are shown (this figure is adapted from Thomas et al. [Citation132]).](/cms/asset/44967392-994b-42d5-a676-12d2daa44722/ihyt_a_2266594_f0004_c.jpg)
For effective convergence of the iterative estimation technique, every signal acquisition must represent the same group of sub-wavelength reflectors. This criterion is easily satisfied in a static environment, allowing efficient convergence in vitro. In [Citation129], effective correction was achieved in vitro after 7.6 iterations on average, requiring ∼7.6 s total. However, convergence is complicated in vivo by tissue motion. Movement from respiration cyclically alters the position of the focus in tissue and thus varies the distribution of sub-wavelength reflectors contributing to the speckle signal. To converge in the presence of tissue motion, each iteration must acquire speckle signals from the same point in the breath cycle (i.e., the same group of reflectors). In the in vivo study, speckle signals were acquired over a duration >1 breath cycle at each iteration to track and identify the signals corresponding to the same group of reflectors, slowing the iteration rate to below the respiration rate [Citation132]. For each focus location tested in the liver, the iterative estimation converged in ∼1 min on average. This convergence time is clinically acceptable if the correction procedure is required at only a few different focus locations but may become unacceptably long if phase estimation is required at many locations (e.g., over a large treatment volume).
To perform speckle-based aberration correction without iteration, Herbert et al. indirectly estimated the aberration phases via measurements of the focusing quality, building on earlier work from ultrasound imaging [Citation120,Citation133]. Instead of directly comparing signals between elements to estimate phase differences, Herbert measured the focusing quality as a function of different orthogonal combinations of element phase in transmission and then inverted the relationship to solve for aberration phase errors using a linear best fit. Each orthogonal combination of element phase was encoded into one pulse transmission of the therapy array. (A total of pulses were required, where
is the number of array elements). Focusing quality was measured noninvasively by receiving speckle signals from the focus and detecting spatial shifts of the tissue due to the acoustic radiation force, which is proportional to the focal pressure amplitude [Citation134,Citation135]. Using this technique, Herbert et al. demonstrated reduction of the element-to-element phase differences and improved focusing through an aberrating phantom in vitro. However, the technique has significant challenges that limit its applicability to histotripsy in vivo. First, in vivo tissue clutter can reduce the signal-to-noise ratio of speckle signals received from the focus [Citation136,Citation137] and may therefore hinder the measurement of acoustic radiation force displacements. Second, the displacements from the acoustic radiation force are very small (<0.1 mm) and difficult to distinguish from tissue motion. For in vivo implementation, tissue motion would have to be removed from signals by applying a high-pass filter across pulses or compensated by gating each phase encoded pulse with the respiratory and/or cardiac cycles. For a typical histotripsy array with >100 elements, the latter approach would quickly become intractable (given that
emissions are required).
5.2. Signals from bubbles
Due to the challenges of aberration correction with speckle signals, researchers have proposed to place a beacon signal at the focus by introducing a bubble [Citation138–141]. Signals from bubbles (scattering and emissions) approximate point sources and thus allow phase measurement without iteration and by direction comparison of the received signals across elements [Citation119]. Bubbles can be introduced via intravascular injection (e.g., contrast agents like microbubbles or droplets) or by using the histotripsy array to generate inertial cavitation at the focus.
In the first method, chemically stabilized gas bubbles or bubble-forming droplets are injected, arrive at the target tissue through the bloodstream, and are then insonified by low (subtherapeutic) amplitude pulses from the transducer [Citation142–145]. If the bubbles are small compared to the ultrasound wavelength, the backscattered waves from the bubbles appear omnidirectional to the array and can thus act as an effective beacon [Citation146]. Notably, Haworth et al. formed bubbles at the focus by vaporizing injected liquid droplets with ultrasound pulses and then received backscatter from the bubbles to correct for phase differences induced by ex vivo human skull [Citation147]. Similarly, phase aberration correction with microbubble contrast agents has been applied to improve transcranial ultrasound imaging of cerebral vasculature [Citation148–153]. One advantage of using droplets or contrast agents is that beacon-like echoes can be obtained using low amplitudes (does not require large focal pressure amplitude at the focus) [Citation154]. The primary disadvantage of these techniques for histotripsy is that they require an otherwise unnecessary injection.
Beacon signals for aberration correction can also be introduced noninvasively by generating cavitation at the focus. Inertial cavitation bubbles emit acoustic signals corresponding to their initial nucleation (formation), oscillation, and eventual collapse (termination) [Citation155–157]. These acoustic emissions can be received to serve as beacon signals for AC. Notably, the nucleation and collapse of histotripsy bubble clouds produce highly broadband shockwave signals that propagate spherically out from the focus and thus approximate an ideal point source [Citation158,Citation159]. Pernot et al. first proposed to correct aberration with acoustic cavitation emissions and demonstrated proof-of-concept for an US imaging transducer focusing through an aberrating phantom [Citation138]. The cavitation-based correction restored focusing quality to non-aberrated levels, as shown in . Continuing this work, Gateau et al. used a therapeutic US transducer to generate cavitation and receive the resulting emissions for transcranial phase correction [Citation160]. The cavitation-based correction closely matched the AC performance of a hydrophone (the laboratory gold standard), increasing the focal pressure to 95% of the hydrophone-corrected amplitude on average with a standard deviation over 60 trials of 1%. This method was later applied specifically to histotripsy for aberration correction through ex vivo human skull [Citation90] and porcine abdominal wall [Citation161,Citation162].
Figure 5. Focusing quality improvement using cavitation-based aberration correction. Transverse cross-section of the focal pressure amplitude from an ultrasound imaging array after propagation through water only (a) and an intervening rubber aberrator without correction (b) and with cavitation-based aberration correction (c) (this figure is from Pernot et al. [Citation138]).
![Figure 5. Focusing quality improvement using cavitation-based aberration correction. Transverse cross-section of the focal pressure amplitude from an ultrasound imaging array after propagation through water only (a) and an intervening rubber aberrator without correction (b) and with cavitation-based aberration correction (c) (this figure is from Pernot et al. [Citation138]).](/cms/asset/2af68eec-f0c7-4d60-9c00-469968516d95/ihyt_a_2266594_f0005_b.jpg)
The study in [Citation162] compared the AC performance of the signals from nucleation and collapse. When using the nucleation signal from a single bubble cloud to compute the correction, the focal pressure amplitude varied substantially (between 49 and 99% of the hydrophone-corrected amplitude). The focal pressure amplitude obtained using the collapse signal, however, was highly consistent (range: 95 to 99% of the hydrophone-corrected amplitude). This difference in performance resulted from the distinct spatiotemporal properties of the nucleation and collapse signals. During nucleation, each bubble forming the cavitation cloud expands and emits a shockwave, producing a cluster of overlapping received signals, as illustrated in . Consequently, individual shockwaves cannot always be resolved, introducing ambiguity to the phase measurement. At collapse, the entire bubble cloud involutes and terminates concertedly, producing a single shockwave, as illustrated in (or 2 – 3 well-separated shockwaves, if different parts of a spatially disparate bubble cloud collapse at slightly different times) [Citation163,Citation164]. This singular shockwave (or sparse set of shockwaves) is easily resolved and permits unambiguous determination of the phase differences.
Figure 6. Shockwaves from inertial cavitation. (a) The cavitation cloud nucleation emits a cluster of overlapping shockwaves. (b) The cavitation cloud collapse often emits a single shockwave. Top: shadowgraph images, bottom: hydrophone recordings at 12 cm from the shockwave origin. Red arrows denote shockwaves. The purple arrow denotes the histotripsy wavefront, propagating upwards in these images (this figure is adapted from Yeats et al. [Citation162]).
![Figure 6. Shockwaves from inertial cavitation. (a) The cavitation cloud nucleation emits a cluster of overlapping shockwaves. (b) The cavitation cloud collapse often emits a single shockwave. Top: shadowgraph images, bottom: hydrophone recordings at 12 cm from the shockwave origin. Red arrows denote shockwaves. The purple arrow denotes the histotripsy wavefront, propagating upwards in these images (this figure is adapted from Yeats et al. [Citation162]).](/cms/asset/abe796ea-a2df-4b33-89df-b2ead0ce3fa1/ihyt_a_2266594_f0006_c.jpg)
These observations suggest that the collapse signal could enable fast and robust aberration correction for the treatment of large volumes with transabdominal histotripsy. In [Citation162], phase correction using the collapse signal was accurate, used few acquisitions, and was computed in ∼200 ms. Furthermore, because no iteration is required, the collapse-based correction method does not require respiratory gating. Therefore, the collapse signal could be used to update the correction as the beam is re-focused at different locations throughout the volume or to pre-compute the corrections without significantly extending procedure times.
One limitation of cavitation-based AC is the requirement for sufficient focal pressure amplitude to generate cavitation emissions. For therapy targets with substantial insertion loss from absorption and aberration (e.g., deep or rib-obstructed targets), the histotripsy array may not have sufficient power to reach the intrinsic threshold amplitude for generating cavitation at the focus. In these scenarios, cavitation could potentially be generated with pressures below the intrinsic threshold by pulsing at high repetition rates (>20 kHz) over extended periods (∼100 s) [Citation165]. Alternatively, speckle-based AC could be used to obtain a correction at one focus location in the treatment volume and generate cavitation. The correction could then be applied at adjacent focus locations and continually updated using the collapse signal.
Like other signal-based AC methods, cavitation-based AC methods cannot correct focal shift, because the signals used for correction originate from the aberrated focus location. Furthermore, signal-based AC methods can moderately increase focal shift. With cavitation-based methods, the cavitation shockwave can originate slightly off-focus and encodes this spatial deviation into the received signal phase. Consequently, the corrected beam re-focuses at the origin of the shockwave. Similarly, iterative speckle-based techniques re-focus on the strongest sub-wavelength reflector, which can also lie slightly off-focus. Cavitation-based correction in [Citation162] and speckle-based correction in [Citation132] both displaced the focus by up to 1 mm in liver tissue and tissue-mimicking phantoms, respectively. This displacement is much smaller than the focal shift caused by aberration (∼6 mm through abdominal wall [Citation67]) but should be considered when planning treatment. Thomas et al. estimated the spatial deviation due to speckle-based AC by finding the location of maximum delay-and-sum beamformed signal energy via gradient-descent optimization [Citation132]. The phase differences caused by the spatial deviation were then removed from the phase correction to reduce the targeting error. A similar approach could also be applied to cavitation-based AC.
6. Discussion
Aberration correction improves the efficacy, efficiency, and accuracy of histotripsy treatments by increasing the focal pressure amplitude, restoring focal gain, and reducing targeting error. These focusing improvements permit the generation of larger bubble clouds or boiling bubbles at the target while minimizing energy delivery to intervening tissues. This ability would not only benefit current treatments but could also expand the feasible treatment range to include deeper and more obstructed targets, thereby enabling treatment of more patients.
Aberration hinders both intrinsic threshold histotripsy (generation of cavitation by short pulses with a large amplitude rarefactional phase) and histotripsy methods that rely on the alignment of the compressional phase (boiling histotripsy and shock scattering histotripsy). However, aberration may be severe for the different histotripsy approaches due to distinct reasons. Boiling histotripsy and shock scattering histotripsy rely on the alignment of the narrow, positive phase shock fronts that form due to nonlinear distortion and the generation of high frequency harmonics. Constructive interference of the high frequency shock fronts requires more precise alignment than a linear waveform at the fundamental frequency. Khokhlova et al. focused a boiling histotripsy array through porcine abdominal wall and measured the shock fronts from individual elements as well as the entire array without aberration correction. As illustrated in , the arrival time differences from transabdominal propagation precluded alignment of the shock fronts from individual elements, preventing formation of a sufficiently high amplitude shock front from the entire array and impeding the initiation of therapy until aberration correction was applied. Intrinsic threshold histotripsy relies on the alignment of the negative (rarefactional) phase of the pressure waveform. The negative phase largely retains its shape and width (even broadening slightly) despite strong nonlinear distortion of the waveform at the focus. Therefore, the negative phase maintains a level of constructive interference approaching that of a linearly propagating waveform at the fundamental frequency. However, intrinsic threshold histotripsy typically requires transducers with larger aperture and lower f-number than boiling histotripsy and shock scattering histotripsy. As discussed earlier in this paper, these features can increase the magnitude of the phase errors and thereby increase destructive interference. Therefore, aberration correction will be critical to the improvement of all histotripsy methods.
Figure 7. Alignment of the positive phase of the pressure waveform for boiling histotripsy. (a) Focal pressure waveforms were measured from a 12-element boiling histotripsy array focusing through an excised porcine abdomen using a fiber optic hydrophone (FOPH). (b) pressure waveforms from individual elements (top) and the entire array (bottom) in the free field (water only). (c) pressure waveforms from individual elements (top) and the entire array (bottom) without (dark blue) and with (green) aberration correction. (this figure is from Khokhlova et al. [Citation14]).
![Figure 7. Alignment of the positive phase of the pressure waveform for boiling histotripsy. (a) Focal pressure waveforms were measured from a 12-element boiling histotripsy array focusing through an excised porcine abdomen using a fiber optic hydrophone (FOPH). (b) pressure waveforms from individual elements (top) and the entire array (bottom) in the free field (water only). (c) pressure waveforms from individual elements (top) and the entire array (bottom) without (dark blue) and with (green) aberration correction. (this figure is from Khokhlova et al. [Citation14]).](/cms/asset/e5d2f61b-c9f9-4d60-bd7a-b3d1250f55b3/ihyt_a_2266594_f0007_c.jpg)
As described in preceding sections, aberration correction methods for histotripsy can be categorized as model-based (using numerical acoustic propagation models) or signal-based (using acoustic signals received by the therapy array). These methods have opposite strengths and limitations. Model-based AC methods are effective at correcting the focal shift but may not substantially increase the focal pressure amplitude. Signal-based methods can recover substantial focal pressure amplitude but cannot correct the focal shift. Therefore, both methods would ideally be combined in a 2-step approach, where the first step applies model-based AC to correct focal shift, and the second step applies signal-based AC to restore substantial focal pressure. (The focal shift is corrected first, because otherwise the signal-based AC procedure would maximize focal pressure at the shifted focus location). The 2-step AC approach was proposed and demonstrated by Gateau et al. for transcranial HIFU [Citation160] and applied by Lu et al. to transcranial histotripsy [Citation90]. In [Citation90], the first step numerically modeled acoustic propagation with ray tracing based on a registered CT image to reduce focal shift (∼1 mm without correction) through ex vivo human skull and place the focus within 0.3 mm of the target. (This first step also moderately increased the focal pressure). Then, the second step used the therapy array to generate cavitation at the corrected focus location and estimated the phase from the received bubble emissions to restore substantial focal pressure. (The 2-step approach increased the focal pressure to ∼90% of the hydrophone-corrected pressure amplitude on average, compared to ∼50% after the first step and ∼21% with no correction).
For transabdominal histotripsy, the first step (modeling-based AC) has additional challenges. First, soft tissue is non-rigid and is thus challenging to register to a pre-operative image. Therefore, a model-based AC procedure for transabdominal histotripsy would ideally image at the time of treatment (the point-of-care) and rapidly compute the phase correction with a fast numerical propagation model (e.g., ray tracing). For soft tissue, the focal point correction requires only a low-resolution map of the tissue acoustic properties. Therefore, point-of-care modalities like cone beam CT [Citation98] or 3D ultrasound imaging [Citation166,Citation167] may be sufficient. Second, abdominal tissues oscillate with respiratory and cardiac motion, causing the focal shift to vary periodically. These fluctuations should be measured in future studies. If the fluctuations cause significant targeting error, then the model-based correction will need to be pre-computed for the entire respiratory cycle or updated in real-time, increasing the requirements for computational efficiency.
The second step of 2-step AC for transabdominal histotripsy could use acoustic signals from speckle (scatter by sub-wavelength reflectors) or from bubbles (cavitation emissions). An advantage of speckle-based AC is that the reflection signals used for correction can be produced with low (subtherapeutic) focal pressure amplitudes. Cavitation-based AC, on the other hand, requires sufficient focal pressure amplitude to generate cavitation. Therefore, for target locations with substantial insertion loss from absorption and aberration, the focal pressure amplitude may be below the threshold for generating cavitation with a short (1-2 cycle) pulse. In these situations, the cavitation threshold could potentially be lowered by increasing the number of cycles [Citation165]. Another potential limitation for cavitation-based AC is that the production of cavitation will also likely result in at least a limited amount of tissue damage. Thus, it is essential that the targeting procedure is adequate to guarantee this cavitation only occurs within the intended treatment volume. A disadvantage of speckle-based AC is the requirement for iterative phase estimation using the same group of sub-wavelength reflectors. In the presence of respiratory motion, this requirement entails gating the phase estimation to the breath cycle and thus extends the correction time. A correction procedure of ∼1 min is acceptable for a single focus location but would significantly prolong the total treatment time if correction is required at multiple focus locations (e.g., for treating a large tumor). Cavitation-based AC, on the other hand, does not require iteration and could therefore reduce the time needed to correct aberration at multiple locations.
For a tissue volume, the number of locations requiring phase estimation is determined by the size of the isoplanatic patch, a volumetric region over which the estimated phase correction can be applied without loss of focusing quality [Citation168]. The size of the isoplanatic patch depends on the heterogeneity of the tissue path, the transducer geometry, and the acoustic frequency. For a 3.75-MHz, small aperture 13-mm diameter source, the correlation length (analogous to the isoplanatic patch) through human abdominal wall was measured as 5–7 mm, implying that a given phase correction would have decreased efficacy if applied >5 mm from the location of phase estimation [Citation115]. Similar measurements are needed for the large apertures and low frequencies used for therapy. These values will help determine the spatial frequency required for phase estimation in transabdominal histotripsy and thereby inform the development of signal-based AC strategies.
Aberration correction increases the cost and complexity of the transabdominal histotripsy system and treatment. One source of additional cost is the requirement for a phased array with multiple elements. To minimize this cost for a given aperture size, future studies should determine the minimum number of elements required for effective transabdominal phase correction. This minimum number of elements (or maximum element size) depends on the spatial frequency of the arrival time variation over the aperture and could be determined from acoustic simulations with human anatomical data (see ). In addition to reducing cost, future research should also justify the extra cost of implementing AC by demonstrating the resulting improvement in histotripsy treatment with in vivo animal studies and simulations with human data. It will be particularly important to investigate the effect of aberration correction on the treatment envelope (range of feasible treatment locations) for transabdominal histotripsy in the human liver and other organs. Expansion of the treatment envelope with aberration correction is expected but should be quantified to demonstrate the clinical value of aberration correction: specifically, its potential to enable effective treatment at more locations and thus provide non-thermal, noninvasive histotripsy therapy to more patients.
7. Summary
Soft tissues of the abdomen can cause substantial aberration of histotripsy and other therapeutic ultrasound acoustic fields despite low ultrasound frequencies. Aberration weakens, broadens, and shifts the focus of the histotripsy beam. Aberration correction methods developed for histotripsy and therapeutic ultrasound can be divided into two main groups: model-based methods, which use anatomical images paired with a numerical acoustic propagation model to predict and correct phase errors, and signal-based methods, which use acoustic signals from the focus for phase correction. Model-based methods are well-suited for correcting focal shift, while signal-based methods are most effective at increasing the focal pressure amplitude. Both methods can be combined in a 2-step approach to both correct focal shift and restore focal pressure amplitude. Future work is needed to determine the maximum element size required for effective aberration correction and the minimum spatial frequency at which phase correction should be repeated when treating a tissue volume with transabdominal histotripsy. This information will aid the design of devices and strategies for aberration correction of transabdominal histotripsy.
Acknowledgement
The authors would like to thank Hadrien Padilla for his help assembling and Zhen Xu for her thoughtful feedback.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Khokhlova VA, Fowlkes JB, Roberts WW, et al. Histotripsy methods in mechanical disintegration of tissue: towards clinical applications. Int J Hyperthermia. 2015;31(2):145–162. doi: 10.3109/02656736.2015.1007538.
- Maxwell AD, Cain CA, Hall TL, et al. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med Biol. 2013;39(3):449–465. doi: 10.1016/j.ultrasmedbio.2012.09.004.
- Maxwell AD, Wang TY, Cain CA, et al. Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoust Soc Am. 2011;30(4):1888–1898. doi: 10.1121/1.3625239.
- Khokhlova TD, Canney MS, Khokhlova VA, et al. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am. 2011;130(5):3498–3510. doi: 10.1121/1.3626152.
- Pahk KJ, Gélat P, Kim H, et al. Bubble dynamics in boiling histotripsy. Ultrasound Med Biol. 2018;44(12):2673–2696. doi: 10.1016/j.ultrasmedbio.2018.07.025.
- Lu N, Gupta D, Daou BJ, et al. Transcranial magnetic resonance-guided histotripsy for brain surgery: pre-clinical investigation. Ultrasound Med Biol. 2022;48(1):98–110. doi: 10.1016/j.ultrasmedbio.2021.09.008.
- Sukovich JR, Cain CA, Pandey AS, et al. In vivo histotripsy brain treatment. J Neurosurg. 2018;131(4):1–8. doi: 10.3171/2018.4.JNS172652.
- Hendricks-Wenger A, Weber P, Simon A, et al. Histotripsy for the treatment of cholangiocarcinoma liver tumors: in vivo feasibility and ex vivo dosimetry study. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2953–2964. doi: 10.1109/TUFFC.2021.3073563.
- Schade GR, Wang Y-N, D'Andrea S, et al. Boiling histotripsy ablation of renal cell carcinoma in the eker rat promotes a systemic inflammatory response. Ultrasound Med Biol. 2019;45(1):137–147. doi: 10.1016/j.ultrasmedbio.2018.09.006.
- Xu Z, Owens G, Gordon D, et al. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121(6):742–749. doi: 10.1161/CIRCULATIONAHA.109.889071.
- Vlaisavljevich E, Owens G, Lundt J, et al. Non-Invasive liver ablation using histotripsy: preclinical safety study in an in vivo porcine model. Ultrasound Med Biol. 2017;43(6):1237–1251. doi: 10.1016/j.ultrasmedbio.2017.01.016.
- Owens GE, Miller RM, Owens ST, et al. Intermediate-term effects of intracardiac communications created noninvasively by therapeutic ultrasound (histotripsy) in a porcine model. Pediatr Cardiol. 2012;33(1):83–89. doi: 10.1007/s00246-011-0094-6.
- Heo J, Joung C, Pahk K, et al. Investigation of the long-term healing response of the liver to boiling histotripsy treatment in vivo. Sci Rep. 2022;12(1):14462. doi: 10.1038/s41598-022-18544-7.
- Khokhlova TD, Schade GR, Wang YN, et al. Pilot in vivo studies on transcutaneous boiling histotripsy in porcine liver and kidney. Sci Rep. 2019;9(1):20176. doi: 10.1038/s41598-019-56658-7.
- Vlaisavljevich E, Kim Y, Allen S, et al. Image-Guided Non-Invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med Biol. 2013;39(8):1398–1409. doi: 10.1016/j.ultrasmedbio.2013.02.005.
- Roberts WW, Hall TL, Ives K, et al. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175(2):734–738. doi: 10.1016/S0022-5347(05)00141-2.
- Hall TL, Kieran K, Ives K, et al. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. J Endourol. 2007;21(10):1159–1166. doi: 10.1089/end.2007.9915.
- Schade GR, Maxwell AD, Khokhlova T, et al. Boiling histotripsy of the kidney: preliminary studies and predictors of treatment effectiveness. J Acoust Soc Am. 2014;136(4_Supplement):2251–2251. doi: 10.1121/1.4900125.
- Dubinsky TJ, Khokhlova TD, Khokhlova V, et al. Histotripsy: the next generation of high-intensity focused ultrasound for focal prostate cancer therapy. J Ultrasound Med. 2020;39(6):1057–1067. doi: 10.1002/jum.15191.
- Hall TL, Hempel CR, Wojno K, et al. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009;74(4):932–937. doi: 10.1016/j.urology.2009.03.049.
- Schuster TG, Wei JT, Hendlin K, et al. Histotripsy treatment of benign prostatic enlargement using the vortx Rx system: initial human safety and efficacy outcomes. Urology. 2018;114:184–187. doi: 10.1016/j.urology.2017.12.033.
- Vidal-Jove J, Serres X, Vlaisavljevich E, et al. First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study. Int J Hyperthermia. 2022;39(1):1115–1123. doi: 10.1080/02656736.2022.2112309.
- O'Donnell M, Flax SW. Phase aberration measurements in medical ultrasound: human studies. Ultrason Imaging. 1988;10(1):1–11. doi: 10.1177/016173468801000101.
- Mallart R, Fink M. Sound speed fluctuations in medical ultrasound imaging comparison between different correction algorithms. In: Ermert H, Harjes HP, editors. Acoustical imaging. [Internet]. Boston, MA: Springer US; 1992. p. 213–218. doi: 10.1007/978-1-4615-3370-2_34.
- Sumino Y, Waag RC. Measurements of ultrasonic pulse arrival time differences produced by abdominal wall specimens. J Acoust Soc Am. 1991;90(6):2924–2930. doi: 10.1121/1.401766.
- Mast TD, Hinkelman LM, Orr MJ, et al. Simulation of ultrasonic pulse propagation through the abdominal wall. J Acoust Soc Am. 1997;102(2 Pt 1):1177–1190. doi: 10.1121/1.421015.
- Thomas JL, Fink MA. Ultrasonic beam focusing through tissue inhomogeneities with a time reversal mirror: application to transskull therapy. IEEE Trans Ultrason Ferroelect Freq Contr. 1996;3(6):1122–1129. doi: 10.1109/58.542055.
- Kyriakou A, Neufeld E, Werner B, et al. Full-wave acoustic and thermal modeling of transcranial ultrasound propagation and investigation of skull-induced aberration correction techniques: a feasibility study. J Ther Ultrasound. 2015;3(1):11. doi: 10.1186/s40349-015-0032-9.
- Kaye EA, Chen J, Pauly KB. Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn Reson Med. 2011;65(3):738–743. doi: 10.1002/mrm.22662.
- Peek AT, Hunter C, Kreider W, et al. Bilayer aberration-inducing gel phantom for high intensity focused ultrasound applications. J Acoust Soc Am. 2020;148(6):3569–3580. doi: 10.1121/10.0002877.
- Zhen-Bo L, Ting-Bo F, Dong Z, et al. Influence of the abdominal wall on the nonlinear propagation of focused therapeutic ultrasound. Chinese Phys B. 2009;18(11):4932–4937. doi: 10.1088/1674-1056/18/11/052.
- Lin K, Kim Y, Maxwell AD, et al. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61(2):251–265. doi: 10.1109/TUFFC.2014.6722611.
- Pahk KJ, de Andrade MO, Kim H, et al. The effects of the size of a boiling bubble on lesion production in boiling histotripsy. J Phys: conf Ser. 2019;1184(1):012007. doi: 10.1088/1742-6596/1184/1/012007.
- Li D, Shen G, Bai J, et al. Focus shift and phase correction in soft tissues during focused ultrasound surgery. IEEE Trans Biomed Eng. 2011;58(6):1621–1628.
- Wang H, Ebbini ES, O'Donnell M, et al. Phase aberration correction and motion compensation for ultrasonic hyperthermia phased arrays: experimental results. IEEE Trans Ultrason, Ferroelect, Freq Contr. 1994;41(1):34–43. doi: 10.1109/58.265818.
- Hynynen K, Jones RM. Image-guided ultrasound phased arrays are a disruptive technology for non-invasive therapy. Phys Med Biol. 2016;61(17):R206–R248. doi: 10.1088/0031-9155/61/17/R206.
- Pernot M, Aubry JF, Tanter M, et al. High power phased array prototype for clinical high intensity focused ultrasound: applications to transcostal and transcranial therapy. In: 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2007. p. 234–7. doi: 10.1109/IEMBS.2007.4352266.
- Tanter M, Pernot M, Aubry JF, et al. Compensating for bone interfaces and respiratory motion in high-intensity focused ultrasound. Int J Hyperthermia. 2007;23(2):141–151. doi: 10.1080/02656730701209996.
- Kyriakou A, Neufeld E, Werner B, et al. A review of numerical and experimental compensation techniques for skull-induced phase aberrations in transcranial focused ultrasound. Int J Hyperthermia. 2014;30(1):36–46. doi: 10.3109/02656736.2013.861519.
- de Senneville BD, Moonen C, Ries M. MRI-Guided HIFU methods for the ablation of liver and renal cancers. In: Escoffre JM, Bouakaz A, editors. Therapeutic ultrasound. [Internet]. Cham: Springer International Publishing; 2016 p. 43–63.
- Kim Y, Wang TY, Xu Z, et al. Lesion generation through ribs using histotripsy therapy without aberration correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(11):2334–2343. doi: 10.1109/TUFFC.2011.2091.
- Kim Y, Vlaisavljevich E, Owens GE, et al. In vivotranscostal histotripsy therapy without aberration correction. Phys Med Biol. 2014;59(11):2553–2568. doi: 10.1088/0031-9155/59/11/2553.
- Sukovich JR, Xu Z, Kim Y, et al. Targeted lesion generation through the skull without aberration correction using histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63(5):671–682. doi: 10.1109/TUFFC.2016.2531504.
- Gao J, Cochran S, Huang Z. Ultrasound beam distortion and pressure reduction in transcostal focused ultrasound surgery. Appl Acoust. 2014;76:337–345. doi: 10.1016/j.apacoust.2013.06.003.
- Li JL, Liu XZ, Zhang D, et al. Influence of ribs on the nonlinear sound field of therapeutic ultrasound. Ultrasound Med Biol. 2007;33(9):1413–1420. doi: 10.1016/j.ultrasmedbio.2007.05.001.
- Khokhlova VA, Bobkova SM, Gavrilov LR. Focus splitting associated with propagation of focused ultrasound through the rib cage. Acoust Phys. 2010;56(5):665–674. doi: 10.1134/S106377101005012X.
- Lin J, Liu X, Gong X, et al. Computational study on the propagation of strongly focused nonlinear ultrasound in tissue with rib-like structures. J Acoust Soc Am. 2013;134(2):1702–1714. doi: 10.1121/1.4812897.
- Lorton O, Guillemin PC, M'Rad Y, et al. A novel concept of a phased-array HIFU transducer optimized for MR-guided hepatic ablation: embodiment and first in-vivo studies. Front Oncol. 2022;12:899440. doi: 10.3389/fonc.2022.899440.
- Bobkova S, Gavrilov L, Khokhlova V, et al. Focusing of High-Intensity ultrasound through the rib cage using a therapeutic random phased array. Ultrasound Med Biol. 2010;36(6):888–906. doi: 10.1016/j.ultrasmedbio.2010.03.007.
- Bardsley BG, Christensen DA. Beam patterns from pulsed ultrasonic transducers using linear systems theory. J Acoust Soc Am. 1981;69(1):25–30. doi: 10.1121/1.385346.
- Vlaisavljevich E, Maxwell A, Warnez M, et al. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61(2):341–352. doi: 10.1109/TUFFC.2014.6722618.
- Duck FA. Chapter 4 - Acoustic properties of tissue at ultrasonic frequencies. In Duck FA, editor. Physical properties of tissues [Internet]. London: Academic Press; 1990. p. 73–135.
- Xu Z, Hall TL, Vlaisavljevich E, et al. Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int J Hyperthermia. 2021;38(1):561–575. doi: 10.1080/02656736.2021.1905189.
- Liu Z, Guo X, Tu J, et al. Variations in temperature distribution and tissue lesion formation induced by tissue inhomogeneity for therapeutic ultrasound. Ultrasound Med Biol. 2014;40(8):1857–1868. doi: 10.1016/j.ultrasmedbio.2014.02.004.
- Li K, Chen Y, Xu Y, et al. Tilting high-intensity focused ultrasound phased array to augment the focal steering range for treatment of uterine fibroids. Appl Acoust. 2020;166:107342. doi: 10.1016/j.apacoust.2020.107342.
- Farrer AI, Almquist S, Dillon CR, et al. Phase aberration simulation study of MRgFUS breast treatments. Med Phys. 2016;43(3):1374–1384. doi: 10.1118/1.4941013.
- Narumi R, Matsuki K, Azuma T, et al. Numerical estimation of HIFU focal error for breast cancer treatment. In 2013 IEEE International Ultrasonics Symposium (IUS). 2013. p. 926–9. doi: 10.1109/ULTSYM.2013.0238.
- Grisey A, Heidmann M, Letort V, et al. Influence of skin and subcutaneous tissue on High-Intensity focused ultrasound beam: experimental quantification and numerical modeling. Ultrasound Med Biol. 2016;42(10):2457–2465. doi: 10.1016/j.ultrasmedbio.2016.06.013.
- Okita K, Narumi R, Azuma T, et al. Effects of breast structure on high-intensity focused ultrasound focal error. J Ther Ultrasound. 2018;6(1):4. doi: 10.1186/s40349-018-0111-9.
- Ritchie R, Collin J, Coussios C, et al. Attenuation and De-focusing during High-Intensity focused ultrasound therapy through peri-nephric fat. Ultrasound Med Biol. 2013;39(10):1785–1793. doi: 10.1016/j.ultrasmedbio.2013.04.010.
- Suomi V, Jaros J, Treeby B, et al. Nonlinear 3-D simulation of high-intensity focused ultrasound therapy in the kidney. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Orlando, FL, USA: IEEE; 2016 p. 5648–51. doi: 10.1109/EMBC.2016.7592008.
- Ritchie RW, Leslie T, Phillips R, et al. Extracorporeal high intensity focused ultrasound for renal tumours: a 3‐year follow‐up. BJU Int. 2010;106(7):1004–1009. doi: 10.1111/j.1464-410X.2010.09289.x.
- Abbas AM, Coussios CC, Cleveland OR. Patient specific simulation of HIFU kidney tumour ablation In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2018. p. 5709–5712.
- Mougenot C, Tillander M, Koskela J, et al. High intensity focused ultrasound with large aperture transducers: a MRI based focal point correction for tissue heterogeneity. Med Phys. 2012;39(4):1936–1945. doi: 10.1118/1.3693051.
- Vlaisavljevich E, Gerhardson T, Hall T, et al. Effects of f-number on the histotripsy intrinsic threshold and cavitation bubble cloud behavior. Phys Med Biol. 2017;62(4):1269–1290. doi: 10.1088/1361-6560/aa54c7.
- Khokhlova T, Rosnitskiy P, Hunter C, et al. Dependence of inertial cavitation induced by high intensity focused ultrasound on transducer F-number and nonlinear waveform distortion. J Acoust Soc Am. 2018;144(3):1160–1169. doi: 10.1121/1.5052260.
- Yeats E, Gupta D, Xu Z, et al. Effects of phase aberration on transabdominal focusing for a large aperture, low f-number histotripsy transducer. Phys Med Biol. 2022;67(15):155004. doi: 10.1088/1361-6560/ac7d90.
- Fan X, Hynynen K. The effect of wave reflection and refraction at soft tissue interfaces during ultrasound hyperthermia treatments. J Acoust Soc Am. 1992;91(3):1727–1736. doi: 10.1121/1.402452.
- Fan X, Hynynen K. The effects of curved tissue layers on the power deposition patterns of therapeutic ultrasound beams. Med Phys. 1994;21(1):25–34. doi: 10.1118/1.597250.
- Liu HL, McDannold N, Hynynen K. Focal beam distortion and treatment planning in abdominal focused ultrasound surgery: abdominal focused ultrasound surgery. Med Phys. 2005;32(5):1270–1280. doi: 10.1118/1.1895525.
- Yin X, Hynynen K. A numerical study of transcranial focused ultrasound beam propagation at low frequency. Phys Med Biol. 2005;50(8):1821–1836. doi: 10.1088/0031-9155/50/8/013.
- White PJ, von Pattenberg P, Clement GT. A nonlinear method for high-intensity focused ultrasound (HIFU) aberration reduction. In: 2008 IEEE Ultrasonics Symposium. 2008. p. 2059–2061.
- Lin KW, Duryea AP, Kim Y, et al. Dual-beam histotripsy: a low-frequency pump enabling a high-frequency probe for precise lesion formation. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61(2):325–340. doi: 10.1109/TUFFC.2014.6722617.
- Christopher T. Finite amplitude distortion-based inhomogeneous pulse echo ultrasonic imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 1997;44(1):125–139. doi: 10.1109/58.585208.
- Gélat P, ter Haar G, Saffari N. A comparison of methods for focusing the field of a HIFU array transducer through human ribs. Phys Med Biol. 2014;59(12):3139–3171. doi: 10.1088/0031-9155/59/12/3139.
- Quesson B, Merle M, Köhler MO, et al. A method for MRI guidance of intercostal high intensity focused ultrasound ablation in the liver. Med Phys. 2010;37(6):2533–2540. doi: 10.1118/1.3413996.
- Cochard E, Prada C, Aubry JF, et al. Ultrasonic focusing through the ribs using the DORT method. Med Phys. 2009;36(8):3495–3503. doi: 10.1118/1.3159755.
- Ballard JR, Casper AJ, Wan Y, et al. Adaptive transthoracic refocusing of Dual-Mode ultrasound arrays. IEEE Trans Biomed Eng. 2010;57(1):93–102. doi: 10.1109/TBME.2009.2028150.
- Liu HL, Chang H, Chen WS, et al. Feasibility of transrib focused ultrasound thermal ablation for liver tumors using a spherically curved 2D array: a numerical study. Med Phys. 2007;34(9):3436–3448. doi: 10.1118/1.2759888.
- Aubry JF, Pernot M, Marquet F, et al. Transcostal high-intensity-focused ultrasound: ex vivo adaptive focusing feasibility study. Phys Med Biol. 2008;53(11):2937–2951. doi: 10.1088/0031-9155/53/11/012.
- Botros YY, Ebbini ES, Volakis JL. Two-step hybrid virtual array ray (VAR) technique for focusing through the rib cage. IEEE Trans Ultrason Ferroelectr Freq Control. 1998;45(4):989–1000. doi: 10.1109/58.710577.
- Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol. 2002;47(8):1219–1236. doi: 10.1088/0031-9155/47/8/301.
- Jones RM, Hynynen K. Comparison of analytical and numerical approaches for CT-based aberration correction in transcranial passive acoustic imaging. Phys Med Biol. 2016;61(1):23–36. doi: 10.1088/0031-9155/61/1/23.
- Pinton GF, Aubry JF, Tanter M. Direct phase projection and transcranial focusing of ultrasound for brain therapy. IEEE Trans Ultrason Ferroelectr Freq Control. 2012;59(6):1149–1159. doi: 10.1109/tuffc.2012.2305.
- Aubry JF, Tanter M, Pernot M, et al. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113(1):84–93. doi: 10.1121/1.1529663.
- Marquet F, Pernot M, Aubry JF, et al. Non-invasive transcranial ultrasound therapy based on a 3D CT scan: protocol validation andin vitroresults. Phys Med Biol. 2009;54(9):2597–2613. doi: 10.1088/0031-9155/54/9/001.
- Almquist S, Parker DL, Christensen DA. Rapid full-wave phase aberration correction method for transcranial high-intensity focused ultrasound therapies. J Ther Ultrasound. 2016;Dec 84(1):30. doi: 10.1186/s40349-016-0074-7.
- Jing Y, Meral FC, Clement GT. Time-reversal transcranial ultrasound beam focusing using a k-space method. Phys Med Biol. 2012;57(4):901–917. doi: 10.1088/0031-9155/57/4/901.
- Miller GW, Eames M, Snell J, et al. Ultrashort echo-time MRI versus CT for skull aberration correction in MR-guided transcranial focused ultrasound: in vitro comparison on human calvaria. Med Phys. 2015;42(5):2223–2233. doi: 10.1118/1.4916656.
- Lu N, Hall TL, Sukovich JR, et al. Two-step aberration correction: application to transcranial histotripsy. Phys Med Biol. 2022;67(12):125009. doi: 10.1088/1361-6560/ac72ed.
- Haqshenas SR, Gélat P, van ’t Wout E, et al. A fast full-wave solver for calculating ultrasound propagation in the body. Ultrasonics. 2021;110:106240. doi: 10.1016/j.ultras.2020.106240.
- McDannold N, Tempany CM, Fennessy FM, et al. Uterine leiomyomas: MR imaging–based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240(1):263–272. doi: 10.1148/radiol.2401050717.
- deSouza NM, Gedroyc W, Rivens I, et al. Tissue specific considerations in implementing high intensity focussed ultrasound under magnetic resonance imaging guidance. Front Oncol. 2022;12:1037959. doi: 10.3389/fonc.2022.1037959.
- Hyvärinen M, Huang Y, David E, et al. Comparison of computer simulations and clinical treatment results of magnetic resonance-guided focused ultrasound surgery (MRgFUS) of uterine fibroids. Med Phys. 2022;49(4):2101–2119. doi: 10.1002/mp.15263.
- Payne A, Merrill R, Minalga E, et al. A Breast-Specific MR guided focused ultrasound platform and treatment protocol: first-in-human technical evaluation. IEEE Trans Biomed Eng. 2021;Mar68(3):893–904. doi: 10.1109/TBME.2020.3016206.
- Deckers R, Merckel LG, Denis De Senneville B, et al. Performance analysis of a dedicated breast MR-HIFU system for tumor ablation in breast cancer patients. Phys Med Biol. 2015;60(14):5527–5542. doi: 10.1088/0031-9155/60/14/5527.
- Dillon CR, Farrer A, McLean H, et al. Experimental assessment of phase aberration correction for breast MRgFUS therapy. Int J Hyperthermia. 2018;34(6):731–743. doi: 10.1080/02656736.2017.1422029.
- Wagner MG, Periyasamy S, Kutlu AZ, et al. An X-ray C-arm guided automatic targeting system for histotripsy. IEEE Trans Biomed Eng. 2023;70(2):592–602. doi: 10.1109/TBME.2022.3198600.
- Weston AD, Korfiatis P, Kline TL, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology. 2019;290(3):669–679. doi: 10.1148/radiol.2018181432.
- Lenchik L, Heacock L, Weaver AA, et al. Automated segmentation of tissues using CT and MRI: a systematic review. Acad Radiol. 2019;26(12):1695–1706. doi: 10.1016/j.acra.2019.07.006.
- Chen Y, Ruan D, Xiao J, et al. Fully automated multiorgan segmentation in abdominal magnetic resonance imaging with deep neural networks. Med Phys. 2020;47(10):4971–4982. doi: 10.1002/mp.14429.
- Chowdhary CL, Acharjya DP. Segmentation and feature extraction in medical imaging: a systematic review. Int Conf Comput Intell Data Sci. 2020;167:26–36. doi: 10.1016/j.procs.2020.03.179.
- Wang T, Jing Y. Transcranial ultrasound imaging with speed of sound-based phase correction: a numerical study. Phys Med Biol. 2013;58(19):6663–6681. doi: 10.1088/0031-9155/58/19/6663.
- Leung SA, Moore D, Webb TD, et al. Transcranial focused ultrasound phase correction using the hybrid angular spectrum method. Sci Rep. 2021;11(1):6532. doi: 10.1038/s41598-021-85535-5.
- Bancel T, Houdouin A, Annic P, et al. Comparison between ray-tracing and full-wave simulation for transcranial ultrasound focusing on a clinical system using the transfer matrix formalism. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(7):2554–2565. doi: 10.1109/TUFFC.2021.3063055.
- Yasuda J, Yoshikawa H, Tanaka H. Phase aberration correction for focused ultrasound transmission by refraction compensation. Jpn J Appl Phys. 2019;58(SG):SGGE22. doi: 10.7567/1347-4065/ab19aa.
- van Hal VHJ, Muller JW, van Sambeek MRHM, et al. An aberration correction approach for single and dual aperture ultrasound imaging of the abdomen. Ultrasonics. 2023;131:106936. doi: 10.1016/j.ultras.2023.106936.
- Qu X, Azuma T, Lin H, et al. Phase aberration correction by multi-stencils fast marching method using sound speed image in ultrasound computed tomography. In 2016. p. 979018. doi: 10.1117/12.2216689.
- Thomas GPL, Khokhlova TD, Khokhlova VA. Partial respiratory motion compensation for abdominal extracorporeal boiling histotripsy treatments with a robotic arm. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2861–2870. doi: 10.1109/TUFFC.2021.3075938.
- Liu D, Waag RC. Time‐shift compensation of ultrasonic pulse focus degradation using least‐mean‐square error estimates of arrival time. J Acoust Soc Am. 1994;95(1):542–555. doi: 10.1121/1.408348.
- Rachlin D. Direct estimation of aberrating delays in pulse‐echo imaging systems. J Acoust Soc Am. 1990;88(1):191–198. doi: 10.1121/1.399940.
- Jaeger M, Robinson E, Akarçay HG, et al. Full correction for spatially distributed speed-of-sound in echo ultrasound based on measuring aberration delays via transmit beam steering. Phys Med Biol. 2015;60(11):4497–4515. doi: 10.1088/0031-9155/60/11/4497.
- Ali R, Dahl JJ. Distributed phase aberration correction techniques based on local sound speed estimates. In 2018 IEEE International Ultrasonics Symposium (IUS) [Internet]. Kobe: IEEE; 2018 [cited 2022 Feb 21]. p. 1–4. Available from: https://ieeexplore.ieee.org/document/8580139/. doi: 10.1109/ULTSYM.2018.8580139.
- Dong Z, Zheng H, Zhu Q, et al. Focus prediction of high-intensity focused ultrasound (HIFU) in biological tissues based on magnetic resonance images. Appl Acoust. 2022;197:108927. doi: 10.1016/j.apacoust.2022.108927.
- Hinkelman LM, Mast TD, Metlay LA, et al. The effect of abdominal wall morphology on ultrasonic pulse distortion. Part I. Measurements. J Acoust Soc Am. 1998;104(6):3635–3649. doi: 10.1121/1.423946.
- Mast TD, Hinkelman LM, Orr MJ, et al. The effect of abdominal wall morphology on ultrasonic pulse distortion. Part II. Simulations. J Acoust Soc Am. 1998;104(6):3651–3664. doi: 10.1121/1.423947.
- Goss SA, Johnston RL, Dunn F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J Acoust Soc Am. 1978;64(2):423–457. doi: 10.1121/1.382016.
- Oh JH, Choi SP, Wee JH, et al. Inter-scanner variability in hounsfield unit measured by CT of the brain and effect on gray-to-white matter ratio. Am J Emerg Med. 2019;37(4):680–684. doi: 10.1016/j.ajem.2018.07.016.
- Flax SW, O'Donnell M. Phase-aberration correction using signals from point reflectors and diffuse scatterers: basic principles. IEEE Trans Ultrason Ferroelectr Freq Control. 1988;35(6):758–767. doi: 10.1109/58.9333.
- Nock L, Trahey GE, Smith SW. Phase aberration correction in medical ultrasound using speckle brightness as a quality factor. J Acoust Soc Am. 1989;85(5):1819–1833. doi: 10.1121/1.397889.
- Fink M. Time reversal of ultrasonic fields. I. Basic principles. IEEE Trans Ultrason Ferroelectr Freq Control. 1992;39(5):555–566. doi: 10.1109/58.156174.
- Li Y, Robinson D, Carpenter D. Phase aberration correction using near-field signal redundancy. II. Experimental results. IEEE Trans Ultrason Ferroelectr Freq Control. 1997;44(2):372–379. doi: 10.1109/58.585121.
- Hynynen K, Clement GT, McDannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52(1):100–107. doi: 10.1002/mrm.20118.
- Daum DR, Smith NB, King R, et al. In vivo demonstration of noninvasive thermal surgery of the liver and kidney using an ultrasonic phased array. Ultrasound Med Biol. 1999;25(7):1087–1098. doi: 10.1016/s0301-5629(99)00053-8.
- McGough RJ, Kessler ML, Ebbini ES, et al. Treatment planning for hyperthermia with ultrasound phased arrays. IEEE Trans Ultrason, Ferroelect, Freq Contr. 1996;43(6):1074–1084. doi: 10.1109/58.542051.
- Montaldo G, Tanter M, Fink M. Time reversal of speckle noise. Phys Rev Lett. 2011;106(5):054301. doi: 10.1103/PhysRevLett.106.054301.
- Mallart R, Fink M. Adaptive focusing in scattering media through sound‐speed inhomogeneities: the van cittert zernike approach and focusing criterion. J Acoust Soc Am. 1994;96(6):3721–3732. doi: 10.1121/1.410562.
- Mallart R, Fink M. The van cittert–zernike theorem in pulse echo measurements. J Acoust Soc Am. 1991;90(5):2718–2727. doi: 10.1121/1.401867.
- Thomas GPL, Khokhlova TD, Bawiec CR, et al. Phase-Aberration correction for HIFU therapy using a multielement array and backscattering of nonlinear pulses. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(4):1040–1050. doi: 10.1109/TUFFC.2020.3030890.
- Bawiec CR, Rosnitskiy PB, Peek AT, et al. Inertial cavitation behaviors induced by nonlinear focused ultrasound pulses. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68(9):2884–2895. doi: 10.1109/TUFFC.2021.3073347.
- Zhang B, Pinton GF, Nightingale KR. On the relationship between spatial coherence and in situ pressure for abdominal imaging. Ultrasound Med Biol. 2021;47(8):2310–2320. doi: 10.1016/j.ultrasmedbio.2021.03.008.
- Thomas GPL, Khokhlova TD, Sapozhnikov OA, et al. In vivo aberration correction for transcutaneous HIFU therapy using a multi-element array. IEEE Trans Ultrason Ferroelectr Freq Control. 2022;69(10):2955–2964.
- Urban M, Bernal M, Greenleaf J. Phase aberration correction using ultrasound radiation force and vibrometry optimization. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(6):1142–1153. doi: 10.1109/tuffc.2007.368.
- Torr GR. The acoustic radiation force. Am J Phys. 1984;52(5):402–408. doi: 10.1119/1.13625.
- Nightingale K, Bentley R, Trahey G. Observations of tissue response to acoustic radiation force: opportunities for imaging. Ultrason Imaging. 2002;24(3):129–138. doi: 10.1177/016173460202400301.
- Pinton GF, Trahey GE, Dahl JJ. Sources of image degradation in fundamental and harmonic ultrasound imaging using nonlinear, full-wave simulations. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(4):754–765. doi: 10.1109/TUFFC.2011.1868.
- Soulioti DE, Santibanez F, Pinton G. Deconstruction and reconstruction of image-degrading effects in the human abdomen using Fullwave: phase aberration, multiple reverberation, and trailing reverberation. 2021 [cited 2022 Mar 1]; Available from: https://arxiv.org/abs/2106.13890.
- Pernot M, Montaldo G, Tanter M, et al. “Ultrasonic stars” for time-reversal focusing using induced cavitation bubbles. Appl Phys Lett. 2006;88(3):034102.
- Kripfgans OD, Fowlkes JB, Woydt M, et al. In vivo droplet vaporization for occlusion therapy and phase aberration correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49(6):726–738. doi: 10.1109/tuffc.2002.1009331.
- Couture O, Aubry JF, Tanter M, et al. Time-reversal focusing of therapeutic ultrasound on targeted microbubbles. Appl Phys Lett. 2009;94(17):173901.
- Seo J, Choi JJ, Fowlkes JB, et al. Aberration correction by nonlinear beam mixing: generation of a pseudo point sound source. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52(11):1970–1980. doi: 10.1109/tuffc.2005.1561666.
- Rojas JD, Dayton PA. Optimizing acoustic activation of phase change contrast agents with the activation pressure matching method: a review. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64(1):264–272. doi: 10.1109/TUFFC.2016.2616304.
- Lindsey BD, Nicoletto HA, Bennett ER, et al. 3-D transcranial ultrasound imaging with bilateral phase aberration correction of multiple isoplanatic patches: a pilot human study with microbubble contrast enhancement. Ultrasound Med Biol. 2014;40(1):90–101. doi: 10.1016/j.ultrasmedbio.2013.09.006.
- Sheeran PS, Dayton PA. Phase-Change contrast agents for imaging and therapy. Curr Pharm Des. 2012;18(15):2152–2165. doi: 10.2174/138161212800099883.
- Sheeran PS, Wong VP, Luois S, et al. Decafluorobutane as a phase-change contrast agent for Low-Energy extravascular ultrasonic imaging. Ultrasound Med Biol. 2011;37(9):1518–1530. doi: 10.1016/j.ultrasmedbio.2011.05.021.
- Psychoudakis D, Fowlkes JB, Volakis JL, et al. Potential of microbubbles for use as point targets in phase aberration correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(12):1639–1648. doi: 10.1109/tuffc.2004.1386681.
- Haworth KJ, Fowlkes JB, Carson PL, et al. Towards aberration correction of transcranial ultrasound using acoustic droplet vaporization. Ultrasound Med Biol. 2008;34(3):435–445. doi: 10.1016/j.ultrasmedbio.2007.08.004.
- Demené C, Deffieux T, Pernot M, et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases doppler and ultrasound sensitivity. IEEE Trans Med Imaging. 2015;34(11):2271–2285. doi: 10.1109/TMI.2015.2428634.
- Soulioti DE, Espíndola D, Dayton PA, et al. Super-resolution imaging through the human skull. IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67(1):25–36. doi: 10.1109/TUFFC.2019.2937733.
- O'Reilly MA, Hynynen K. A super-resolution ultrasound method for brain vascular mapping. Med Phys. 2013;40(11):110701. doi: 10.1118/1.4823762.
- Jones RM, Huang Y, Meng Y, et al. Echo-Focusing in transcranial focused ultrasound thalamotomy for essential tremor: a feasibility study. Mov Disord. 2020;35(12):2327–2333. doi: 10.1002/mds.28226.
- Robin J, Demené C, Heiles B, et al. In vivo adaptive focusing for clinical contrast-enhanced transcranial ultrasound imaging in human. Phys Med Biol. 2023;68(2):025019. doi: 10.1088/1361-6560/acabfb.
- Ivancevich NM, Pinton GF, Nicoletto HA, et al. Real-time 3-D contrast-enhanced transcranial ultrasound and aberration correction. Ultrasound Med Biol. 2008;34(9):1387–1395. doi: 10.1016/j.ultrasmedbio.2008.01.015.
- Fabiilli ML, Haworth KJ, Fakhri NH, et al. The role of inertial cavitation in acoustic droplet vaporization. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56(5):1006–1017. doi: 10.1109/TUFFC.2009.1132.
- Leighton TG. The acoustic bubble. San Diego, United States: academic Press; 1994.
- Zhong P, Cioanta I, Cocks FH, et al. Inertial cavitation and associated acoustic emission produced during electrohydraulic shock wave lithotripsy. J Acoust Soc Am. 1997;101(5 Pt 1):2940–2950. doi: 10.1121/1.418522.
- Johnston K, Tapia-Siles C, Gerold B, et al. Periodic shock-emission from acoustically driven cavitation clouds: a source of the subharmonic signal. Ultrasonics. 2014;54(8):2151–2158. doi: 10.1016/j.ultras.2014.06.011.
- Sukovich JR, Macoskey JJ, Lundt JE, et al. Real-Time transcranial histotripsy treatment localization and mapping using acoustic cavitation emission feedback. IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67(6):1178–1191. doi: 10.1109/TUFFC.2020.2967586.
- Lauterborn W, Vogel A. Shock wave emission by laser generated bubbles. In: Delale CF, editor. Bubble dynamics and shock waves [internet]. Berlin, Heidelberg: springer Berlin Heidelberg; 2013. p. 67–103. Available from: doi: 10.1007/978-3-642-34297-4_3.
- Gateau J, Marsac L, Pernot M, et al. Transcranial ultrasonic therapy based on time reversal of acoustically induced cavitation bubble signature. IEEE Trans Biomed Eng. 2010;57(1):134–144. doi: 10.1109/TBME.2009.2031816.
- Macoskey JJ, Hall TL, Sukovich JR, et al. Soft-tissue aberration correction for histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2018;65(11):2073–2085. doi: 10.1109/TUFFC.2018.2872727.
- Yeats E, Lu N, Sukovich JR, et al. Soft tissue aberration correction for histotripsy using acoustic emissions from cavitation cloud nucleation and collapse. Ultrasound Med Biol. 2023;49(5):1182–1193. doi: 10.1016/j.ultrasmedbio.2023.01.004.
- Mørch KA. On the collapse of cavity clusters in flow cavitation. In: Lauterborn W, editor. Cavitation and inhomogeneities in underwater acoustics. Berlin, Heidelberg: Springer Berlin Heidelberg; 1980. p. 95–100.
- Wang YC, Brennen CE. Shock wave development in the collapse of a cloud of bubbles. In: cavitation and multiphase flow [Internet]. New York, NY: American Society of Mechanical Engineers; 1994. p. 15–19. Fluids Engineering Division; vol. 194).
- Xu Z, Fowlkes JB, Rothman ED, et al. Controlled ultrasound tissue erosion: the role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am. 2005;117(1):424–435. doi: 10.1121/1.1828551.
- Cerrolaza JJ, Safdar N, Biggs E, et al. Renal segmentation from 3D ultrasound via fuzzy appearance models and patient-specific alpha shapes. IEEE Trans Med Imaging. 2016;35(11):2393–2402. doi: 10.1109/TMI.2016.2572641.
- Marsousi M, Plataniotis KN, Stergiopoulos S. An automated approach for kidney segmentation in Three-Dimensional ultrasound images. IEEE J Biomed Health Inform. 2017;21(4):1079–1094. doi: 10.1109/JBHI.2016.2580040.
- Fellgett PB, Linfoot EH, Redman RO. On the assessment of optical images. Philos Trans R Soc Lond Ser Math Phys Sci. 1997;247(931):369–407.

