ABSTRACT
Objectives: A substantial body of recent research has aimed to better understand the clinical sequelae of military trauma through the application of advanced brain imaging procedures in Veteran populations. The primary objective of this review was to highlight a portion of these recent studies to demonstrate how imaging tools can be used to understand military-associated brain injury. Methods: We focus here on the phenomenon of mild traumatic brain injury (mTBI) given its high prevalence in the Veteran population and current recognition of the need to better understand the clinical implications of this trauma. This is intended to provide readers with an initial exposure to the field of neuroimaging of mTBI with a brief introduction to the concept of traumatic brain injury, followed by a summary of the major imaging techniques that have been applied to the study of mTBI. Results: Taken together, the collection of studies reviewed demonstrates a clear role for neuroimaging towards understanding the various neural consequences of mTBI as well as the clinical complications of such brain changes. Conclusions: This information must be considered in the larger context of research into mTBI, including the potentially unique nature of blast exposure and the long-term consequences of mTBI.
KEYWORDS:
Introduction
There has been a substantial growth in research using neuroimaging to elucidate consequences of military-associated brain trauma. The focus of this review is on recent imaging studies in the US military and Veteran population with deployment-related mild traumatic brain injury (mTBI or concussion). Specifically, this review provides an update of brain imaging work since a 2009 introduction to imaging techniques by Van Boven and colleagues [Citation1]. We attempt to highlight representative work from many fields of neuroimaging with an application to mTBI. However, this is not a comprehensive review (although our aim was to include the majority of studies in each field since 2009), and we do not fully evaluate all studies included here on their merit. Thus, each of the various studies reported has strengths, weaknesses, and idiosyncrasies that make them difficult to compare directly, and a detailed comparative assessment across the range of studies is beyond the scope of this review. The reader is therefore encouraged to examine the primary sources discussed for full comprehension of the work. We instead note some of the primary features to consider in the application of brain imaging to the study of mTBI and then review recent work in this domain. The vast majority of the work reviewed is in the military context, and we clarify any cases when discussing non-military work. We intend this review to be accessible to readers with little background in military trauma, as well as individuals with limited background in brain imaging. We therefore first summarize concepts related to traumatic brain injury (TBI) in general and in the military population, and next follow this with brief summaries of the various brain imaging domains used in the study of TBI. Finally, we conclude with considerations of the study of military mTBI and discuss directions for future work.
Diagnostic challenges of mTBI
Much of the research on the effects of mTBI on the brain relies heavily on diagnosis of the injury in order to assign the participant to a research group. There are a number of factors that can make this diagnosis of mTBI difficult. First, it is logistically difficult to image Servicemembers near the time of a military-related head injury, and so most imaging studies take place after the symptoms of the injury have resolved (7–10 days after the event, in most cases [Citation2]). Thus, researchers must either rely on retrospective self-reports of the event and the symptoms sustained at the time, or assess post-concussive syndrome (PCS). Self-reports can be unreliable, and there is even evidence that the psychological trauma of the event may additionally impact memory [Citation3]. Meanwhile, symptoms of PCS are non-specific, and can often be equally attributed to other common military-related conditions such as posttraumatic stress disorder (PTSD) [Citation4]. Further, some Veterans are motivated to attribute their persistent symptoms to a brain injury rather than a mental health disorder. Even when diagnosis of concussion takes place at the time of the injury, there can be factors that contribute to subjectivity in the diagnosis. Physicians are forced to rely heavily on the self-report of the soldier as to the nature and severity of their symptoms. Many factors, including the desire to serve with their comrades or to avoid appearing weak, will cause the injured individual to ‘down-play’ their symptoms, avoid seeking care altogether, or make efforts to memorize answers to common cognitive tests so that they will appear uninjured. Further, the symptoms at the time of the injury are transient and dynamic, and thus may be missed by neuropsychological tests designed to diagnose TBI, such as the Military Acute Concussion Evaluation [Citation5] or the Automated Neuropsychological Assessment Metrics [Citation6], perhaps even depending on when they are administered to the soldier. Finally, even when a diagnosis was made at the time of the event, it may not be available in medical records for investigators to access, so the researchers are still left to rely on retrospective reports. Taken together, it is clear why diagnosing or even defining this condition is challenging. These complications are especially relevant for research studies of military cohorts and such limitations of this research should be considered throughout this review.
Considerations for the study of military TBI
Traumatic brain injury is a broad etiological and pathobiological concept and there are certain aspects of this condition that require unique consideration as a clinical entity. Thus, we present information here that would be helpful in assessing any given neuroimaging study of TBI. One important consideration for the study of TBI is the vast heterogeneity of injuries. Due to the unique nature of the events that result in any given TBI (e.g. ‘degree’ of exposure, how hard a hit was, the direction of the exposure, or where the impact occurred on the head), the injuries themselves are expected to vary from individual to individual to a much larger extent than brain disorders arising from stereotyped biological processes such as sporadic neurodegenerative disease (e.g. Alzheimer’s disease). Another consideration is the length of time since the traumatic exposure. It is difficult in military contexts to study injury in a relatively acute phase and this is rarely achieved. An exception is the work of Mac Donald and colleagues [Citation7–Citation9], described later in this review. Rather, it is common for trauma to be examined months to years post-exposure and differences in timing across studies and participants will likely have an important impact on the clinical presentation and ability to detect the injury. As such, detection of group differences requires large, well-characterized cohorts, which are technically and financially challenging to acquire. In addition, traditional methods for group-based analysis of brain imaging data (e.g. registration to a template brain and comparison of similar anatomic locations) may not be the most sensitive for this type of population, as individuals may be better conceptualized as a set of case studies as opposed to a homogeneous group.
In the context of military TBI, there are a number of unique factors that may further complicate an individual’s response to TBI, including the fact that most Veterans experience multiple TBIs throughout their lifetime, often of various etiologies and severities, and these may not be captured in the clinical scales assessing TBI history. For this and other reasons, the question of how best to assess and characterize these injuries is one that is evolving as new knowledge is gained. Currently the predominant schema is to categorize TBIs into mild, moderate and severe [Citation10], based on several factors including the depth of coma (typically assessed by Glasgow Coma Scale(GCS), especially in civilian TBI) and consideration of the duration of symptoms arising at the time of the injury. According to many guidelines for TBI diagnosis, including the VA/DoD guidelines [Citation11], mild cases of TBI have no visible injury on traditional clinical imaging (). Most TBIs, in both military and civilian contexts, are mild in severity, and recovery of the initially presented symptoms is expected. In these cases, generally no emergency intervention is required. Mild TBI will be the focus of this review, as mTBIs account for 84% of military brain injuries [Citation12] and have more recently been recognized as an important clinical entity. It should be noted that the definitions described below are not universally utilized, and differences in the definition of TBI severity across studies likely contribute to differences in conclusions.
Table I. Guidelines for TBI diagnosis.
Another feature of military-related TBI are the highly comorbid mental health issues, notably PTSD and depression. There is considerable discussion, especially regarding the long-term functional and cognitive outcomes of mTBI, as to what extent outcomes due to these comorbid conditions may be misattributed to mTBI. Careful characterization and attention to demographic differences across groups and in statistical comparisons are necessary to completely address research questions while considering these factors. With the implementation of the comprehensive TBI evaluation (CTBIE) in 2007 [Citation13], retrospective accounts of TBI have become more available to researchers, and have thus become the predominant method of assessing military TBI in research. However, caveats of this method should be noted, including potential memory disruptions from the psychological trauma surrounding the event that may ultimately affect the categorization of the TBI’s severity [Citation14].
What is a ‘mild’ TBI?
‘Mild’ TBI is, in many ways, an almost distinct entity from moderate to severe TBI, where damage to the brain is typically prominent and therefore brain imaging is an important component of clinical management [Citation15]. Abnormal neuroimaging after moderate or severe TBI may include skull fracture or intracranial hemorrhage, typically detected on computed tomography (CT). These findings are often clinically indicative of the need for emergency intervention in the intensive care unit (ICU), which may involve restoration of respiratory function, neurosurgical intervention, and monitoring of intracranial pressure [Citation15]. Recovery from severe TBI takes significant time and individuals typically retain some form of deficit and are at increased risk of mortality [Citation15]. None of these characteristics are typically present for mTBI. Rather, mTBI is commonly defined as individuals who have had brain trauma resulting in mild deficits on the GCS [Citation16] (score ranging from 13 to 15, when available), loss of consciousness (LOC) of less than or equal to 30 minutes, post-traumatic amnesia (PTA) of less than or equal to 24 hours, and/or alteration of mental status less than or equal to 24 hours (). Clinically, if the symptoms following a head injury indicate that it is a mild TBI, neuroimaging may not be involved in diagnosing the injury. Although there is a general belief that individuals with mild symptomology are primarily ‘free’ from significant abnormalities on conventional clinical imaging, research in civilian mTBI demonstrates that injury can often be detected even in this mild range with intraparenchymal lesions found in 50% of individuals on CT and 75% on acute 3T MRI [Citation17]. Hemorrhagic traumatic axonal injury was detected in 4.5% on CT and 47% on MRI, non-hemorrhagic traumatic axonal injury was found in 1% on MRI and cerebral contusions were found in 36% on CT and 58% by MRI. However, these detectible lesions were not linked to cognitive deficits in this population suggesting that other more quantitative imaging metrics may be necessary to predict long-term outcomes from mTBI [Citation17,Citation18]. Research in blast-related mTBI demonstrate similar findings on 3T MRI, with a recent report showing a high incidence of white matter hyperintensities on T2-weighted imaging as well as pituitary abnormalities in individuals with blast-related mild TBI [Citation19], although it is currently unknown if or how this may relate to cognition. Overall, it is important to consider that although not necessarily clinically actionable, it is likely that overt brain tissue damage is prevalent in individuals with clinically defined mTBI and such findings are variable based on the technology (e.g. CT v MRI).
Prior reviews
Several prior reviews have been written on TBI and mTBI in the civilian and Veteran fields extending from the peri-2009 period and before [Citation20–Citation30]. It is recommended that these prior sources complement this work. However, a substantive body of research has been produced specifically regarding mTBI in the expanding Veteran population in recent years, and summarizing this work is the unique goal of this current review.
Neuroimaging techniques
We have adopted an imaging-centric approach to this review, with sections organized based on imaging modality and limited to those modalities that have been used in recent studies of military mTBI. Although certain techniques show clear promise, there is currently no obviously dominant procedure for the identification and assessment of mTBI and, therefore, continued exploration across the various existing imaging domains will be necessary to determine the relative value of each procedure. From a neuroscience perspective, each technique is typically utilized to probe a particular aspect of the brain biology, and therefore this organization also allows for a consideration of such neural properties as well. For this reason, modalities are organized into ‘structural’ and ‘functional,’ and a note about potential biological processes that can be assessed with the technique are included in the description.
The biological processes following mTBI have been reviewed in detail elsewhere [Citation31]. Briefly, forces from physical trauma, including acceleration, deceleration, or blast pressure waves, are transmitted to the brain. Certain areas of the brain may be particularly vulnerable to these forces, including long white matter pathways and areas of tissue boundaries. At the cellular level, these forces disrupt membrane integrity, allowing a brief period of unfettered diffusion between the intracellular and extracellular space. This results in the loss of the electrochemical gradient, which must be restored by the cell before proper function can resume. The influx of ions from the extracellular space, especially calcium, results in both immediate release of excitatory neurotransmitter and a longer-term biochemical cascade, both of which can be toxic to cells. High concentrations of calcium in the cell can result in impaired mitochondrial function, exacerbating and prolonging the dysfunctional energy needs. While these changes occur at a much smaller spatial scale than can be assessed by in vivo imaging techniques, many aspects of this biological process are expected to result in macroscopic changes that are visible in modern imaging techniques. However, it must be emphasized that, while these biological processes serve as motivation for examining brain injuries using imaging techniques, the changes discovered with imaging may arise from entirely different, even currently unknown, processes.
Structural imaging. Brain morphometry
Structural imaging refers to a set of procedures that provide anatomic contrast (). While these images are used clinically to find abnormalities (including those discussed in the section on mTBI above), quantitative tools can be used to measure more subtle alterations in the amount of brain tissue in different regions of the brain in individuals or groups of individuals ( and ). In neurodegenerative conditions such as Alzheimer’s disease, reduction in the amount of grey matter tissue measured by quantitative structural imaging (e.g. the measurement of ‘cortical thickness’ [Citation32]) is found in areas that exhibit profound histopathology. Thus, to some degree such procedures can be used as a proxy for degenerative changes, yet it should be noted that such procedures cannot clearly differentiate among various potential mechanisms of cortical thinning in different conditions. In mTBI, a common observation is thinner cortex (or less grey matter volume), and we may expect this to be an assessment of cell death or loss of dendritic branches but such histological correlates have not yet been examined and thus such interpretations should be cautioned. For example, rodent models of blast injuries have also identified shrunken neurons that may manifest in human studies as thinner cortical structures [Citation33].
Figure 1. Structural brain imaging refers to a set of procedures for measuring a variety of morphometric properties of the brain. Alterations in brain structure in individuals or groups of individuals may be linked to pathologic processes. Different types of imaging contrasts measure various aspects of brain structure including (A) T1-weighted (typically used for differentiation of gray and white matter and cortical surface modeling), (B) T2-weighted (typically used to detect tissue pathology with increased fluid content), and (C) Fluid attenuated inversion recovery (FLAIR; typically used to measure pathology including stroke). The pial and white matter surfaces are outlined in yellow and green, respectively, capturing the thickness of the cortex. Aberrations in each scan can represent neural abnormalities such as white matter lesions (red arrow) or hemosiderins (orange arrow).
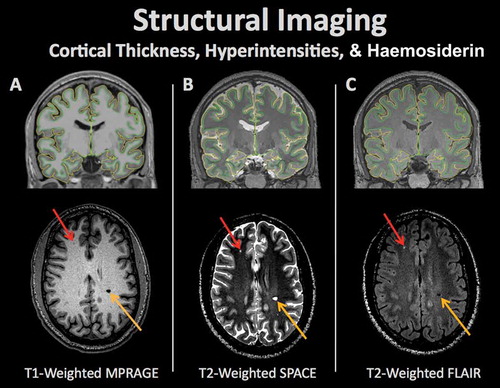
Figure 2. Cortical thickness in structural imaging is a measurement of the distance from the grey-white matter boundary (green outline) to the edge of the cortex, or pial surface (yellow outline). Cortical thickness can change with normal aging as well as pathology. An example of a single subject is shown on the right with yellow regions demonstrating the areas with the highest cortical thickness and thinner regions of the cortex in red.
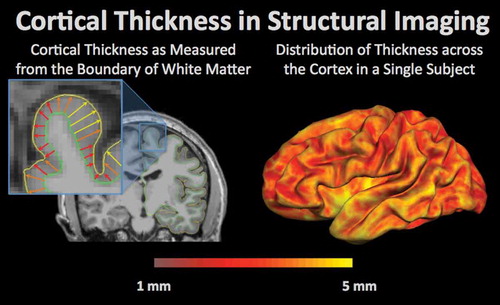
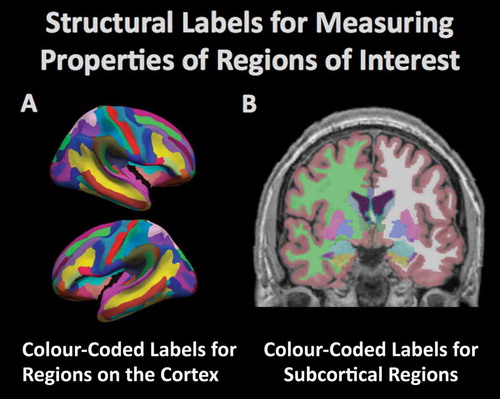
Figure 3. Computational procedures can be used to model the brain and to measure the structural properties of brain tissue within defined regions. The figure demonstrates the automated (A) parcellation of the cerebral cortex based on gyral anatomy and (B) segmentation of subcortical regions by the FreeSurfer software suite (freesurfer.net). The amount of tissue in the different regions is typically compared between groups to determine regional vulnerability to degenerative processes.
Using structural imaging procedures, Lindemer and colleagues [Citation34] found that mTBI exacerbated the effects of PTSD on reducing cortical thickness. Michael et al. [Citation35] and Tate et al. [Citation36] found reduced regional cortical thickness in symptomatic mild-to-moderate blast-TBI in military Veterans. More recently, Tate and colleagues [Citation37] examined additional morphometric parameters and found increased volume of subcortical structures in a symptomatic mTBI group but also reduced surface area of the left thalamus. Morphometric measures did not differ between the mTBI group and a pure PTSD group. Overall, these studies provide evidence for an association between mTBI and brain structure. Further, results from Lindemer et al. [Citation34] and others suggest a potential interaction of PTSD and mTBI, however, this requires further study.
Diffusion-weighted imaging (tissue microstructure)
Diffusion-weighted imaging refers to a set of procedures including diffusion tensor imaging (DTI) that use the diffusion of water within brain tissue to probe microstructural tissue properties (). Additionally, due to the fact that neural fiber membranes typically restrict the directions that water can diffuse, diffusion imaging can be used to map anatomical properties of cerebral white matter fascicles. In the context of TBI, altered diffusion properties within white matter may be indicative of demyelination and axonal degeneration, which would allow more ‘free’ diffusion as well as potential ‘leakage’ of water through less intact myelin structures. This is often quantified through fractional anisotropy (FA), and lower FA is generally associated with tissue injury. Animal models have demonstrated both increases in the permeability of axons [Citation38] and demyelination [Citation39] associated with brain injury. As such, diffusion imaging is an imaging domain that may be more sensitive to the subtle effects of mTBI, which has been associated with axonal injury post-mortem [Citation40]. As noted for brain morphometry however, the extent to which diffusion imaging can be mapped to a pathologic mechanism is yet to be determined for mTBI. Diffusion imaging has been applied in several studies of TBI in both military and civilian contexts [Citation41–Citation43]. A recent literature review of approximately 100 studies over the decade up to 2011 that were not specific to military/Veteran TBI concluded that diffusion imaging effectively differentiates individuals with TBI from those without [Citation44]. However, the reliability and sensitivity of these procedures are yet to be determined and little, if any, work has directly compared the utility of diffusion procedures to other classes of brain imaging. Regardless, several published studies suggest that diffusion imaging may contribute to our understanding of mTBI.
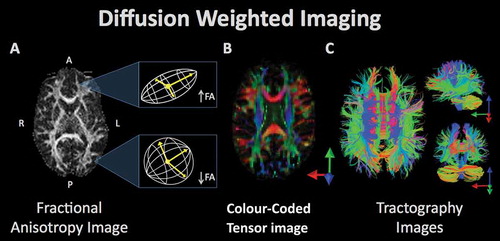
Figure 4. Diffusion weighted imaging refers to a set of magnetic resonance imaging procedures that exploit water diffusion in tissue as the basis of image contrast. Diffusion imaging measures various properties of the behavior of water in the brain such as fractional anisotropy (FA), radial diffusivity, etc. These measurements can be used to quantify various aspects of tissue microstructure, which can be compared between groups, as well as used in visualization of the anatomy of prominent white matter fascicles and other anatomical properties. (A) The white matter tracts of a single subject’s brain, reconstructed from the FA values where high FA represents regions of strict, directed water diffusion (along the borders of white matter bundles) and low FA indicates regions with isotropic water diffusion. (B) The white matter tracts from FA values are color-coded to indicate the direction of water diffusion in order to illustrate the arrangement and organization of bundles of white matter. (C) Visualization of diffusion tensor imaging (DTI) data in the axial (left), sagittal (top right), and coronal plane (bottom right). Fiber bundle directionality is indicated by color (red = right to left/left to right (e.g. corpus callosum), green = posterior to anterior/anterior to posterior (e.g. cingulum bundle), blue = inferior to superior/superior to inferior (e.g. corticospinal tracts)).
Levin and colleagues [Citation45] examined the utility of diffusion imaging to detect white matter abnormalities linked to mild to moderate blast-related TBI in a sample of 37 Iraq or Afghanistan Veterans and Servicemembers. Using tractography and region-of-interest procedures, the investigators were not able to detect abnormalities due to mild to moderate blast TBI or associations with symptom measures. However, the authors did report associations between diffusion measures and neuropsychological performance. These findings demonstrate that tissue variation is linked to cognitive status, yet a lack of findings linked to blast-TBI may suggest that novel methodology is required to detect abnormalities in individuals with non-stereotyped pathology.
Davenport and colleagues [Citation46] examined the potential regional heterogeneity (spatial inconsistencies across individuals) in white matter abnormalities in 25 Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) military Servicemembers with and 33 without blast-related mTBI. They found that effects in blast-exposed individuals were diffuse and global, and were not detectable by using standard spatial matching procedures. Moreover, individuals exposed to more than one blast mTBI had a greater number of abnormalities compared to single blast injury. This study demonstrated that effects of mTBI may be spatially variable and therefore require unique data processing and quantification procedures. Since Davenport et al.’s original publication, several other studies investigating white matter in blast-related mTBI have found similar results, suggesting that the injury is associated with diffuse white matter abnormalities, particularly in those who experienced a LOC [Citation47–Citation49]. Additionally, these diffuse white matter disruptions have been associated with both neurocognitive [Citation47,Citation49] and behavioral [Citation48] outcomes in blast-related mTBI, with more recent work suggesting that the associated cognitive impairment of these global disruptions is also heterogeneous [Citation50]. This heterogeneity may be to blame for studies that did not find differences in DTI imaging associated with military mTBI, some of which found that other contributing factors, such as cognitive impairment [Citation51] or co-occurring PTSD [Citation52] (but see [Citation47,Citation48]) could account for variability among individuals with mTBI.
Mac Donald and colleagues [Citation9] examined US military personnel with clinical diagnosis of blast-related mTBI that were evacuated from theater to Landstuhl Regional Medical Center in Germany for evaluation within 90 days of the injury. This provides a unique window into the semi-acute effects of blast-associated injury, as most studies of military mTBI examine individuals at much longer post-injury intervals. They found abnormalities on diffusion imaging that were attributed to traumatic axonal injury in Veterans with mTBI. Effects were distributed throughout the brain, but most prominent in the middle cerebellar peduncles, cingulum, and orbitofrontal white matter and these abnormalities were persistent across time. The authors also noted that it was not clear to what degree the primary blast overpressure injury and blunt injury from blast debris contributed to the effects, given the rarity of isolated primary blast exposure in the sample. However, the authors did confirm cerebellar white matter abnormalities in a follow-up paper investigating a small sample of primary blast mTBI individuals, suggesting that primary blast exposure may have specific disruptions to the cerebellum [Citation8]. This group has recently published a 5-year follow-up in this cohort where 74% of Veterans with blast-related mTBI were found to have imaging abnormalities [Citation53]. The authors note that, rather than the appearance of these relatively uncomplicated mTBIs resolving with time, the deficits appear to be more prevalent. Because of differences in data acquisition and processing across the studies, it is not clear if this should be interpreted as evidence of progressive changes brought on by brain injury or a cautionary note on the limitations of diffusion imaging in robust diagnosis of mTBI.
Functional imaging. Task-based functional brain imaging
Functional brain imaging refers to a class of dynamic brain imaging procedures that measure time-dependent properties of the brain either directly or indirectly linked to neurophysiological processes (–). Task-based functional brain imaging uses dynamic procedures such as the blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) technique to identify brain regions that are time-locked to the performance of a task. In fMRI, regional changes in blood oxygenation resulting from vascular coupling with neuronal activity are used as an indirect marker of that neural activity. One of the benefits of task-based fMRI is that it is noninvasive and can be performed over repeated sessions. Additionally, researchers can measure task performance simultaneously with specific brain functions. As such, fMRI has been used to investigate brain function correlated with cognitive performance after brain injury in Veteran (and civilian) populations. For example, in a study investigating response inhibition, Fischer and colleagues [Citation54] used the Stop Signal Task (SST) during fMRI. The TBI group (which included mild-to-moderate TBI) had reduced activation with correct inhibition responses compared to the control participants in brain regions supporting inhibitory control. Failure to inhibit was associated with greater activity in the military TBI group in temporal, caudate, and cerebellar regions. This was the opposite effect of civilian TBI, which was also included in this study, and the authors attribute this difference to blast vs. blunt etiology. In a group of military Veterans, Scheibel and colleagues [Citation55] found that blast-related mTBI was associated with enhanced activation during a stimulus-response compatibility task in anterior cingulate, medial frontal, and visual cortical regions. This activation pattern was more extensive after statistically controlling for reaction time and symptoms of PTSD and depression. There was also a negative relationship between symptoms of PTSD and activation within posterior brain regions. These results provide evidence for increased task-related activation (generally interpreted as recruitment of more neural resources to accomplish the same task) following mild, blast-related TBI and additional changes associated with emotional symptoms. Limitations of this study include not matching for combat exposure and different recruitment strategies so that the control group was largely a community-based sample, while many TBI subjects were seeking services [Citation56]. Nonetheless, together these studies raise the possibility that mTBI is associated with altered brain activity that may include compensatory neural activation. However, it is currently unknown whether enhanced activation reflects compensatory processes, or rather may be associated with other, unknown processes in the injured brain.
Figure 5. Task-related fMRI refers to the use of MRI to measure regional brain responses to cognitive and/or behavioral stimulation. (A) In the fMRI paradigm, an experimental task is performed at known times during imaging, and the resulting expected brain response (measured by MRI as the ‘hemodynamic response’ due to the phenomenon of neurovascular coupling) can be statistically analyzed to determine regions of the brain supporting the operations used to perform the task. (B) Activation patterns associated with behavioral fluctuations during a sustained attention task in 145 veterans from the TRACTS cohort (image courtesy of Dr. Michael Esterman, VA Boston Healthcare System).
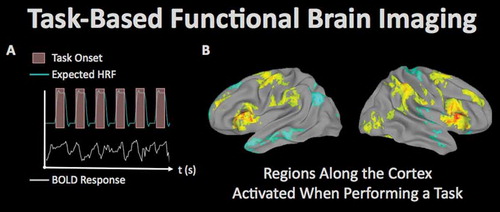
Figure 6. Functional connectivity refers to procedures used to examine covariance in regional brain activity. Correlated activity across brain regions is interpreted to indicate shared demands for a cognitive operation between regions, and potentially direct communication between regions. The image demonstrates a ‘seed’ region in the posterior cingulate (top) in which the fMRI signal (based on the blood oxygenation level-dependent mechanism of contrast) is quantified across time (blue waveform) and correlated with other regions throughout the brain. This analysis highlights functional connectivity among a set of regions referred to as the ‘default mode network,’ which has been demonstrated to be compromised across a range of conditions including blast exposure [Citation73]. The red-yellow regions demonstrate positive correlation to the seed in which the fMRI signal is quantified across time (green waveform) and plotted over the fMRI signal from the seed (middle). The positive correlation between these two time-series is plotted in yellow (middle right). The blue-light blue regions demonstrate anticorrelation to the seed in which the fMRI signal is quantified across time (red waveform) and plotted over the fMRI signal from the seed. The anticorrelation between these two time-series is plotted in light blue (bottom right).
![Figure 6. Functional connectivity refers to procedures used to examine covariance in regional brain activity. Correlated activity across brain regions is interpreted to indicate shared demands for a cognitive operation between regions, and potentially direct communication between regions. The image demonstrates a ‘seed’ region in the posterior cingulate (top) in which the fMRI signal (based on the blood oxygenation level-dependent mechanism of contrast) is quantified across time (blue waveform) and correlated with other regions throughout the brain. This analysis highlights functional connectivity among a set of regions referred to as the ‘default mode network,’ which has been demonstrated to be compromised across a range of conditions including blast exposure [Citation73]. The red-yellow regions demonstrate positive correlation to the seed in which the fMRI signal is quantified across time (green waveform) and plotted over the fMRI signal from the seed (middle). The positive correlation between these two time-series is plotted in yellow (middle right). The blue-light blue regions demonstrate anticorrelation to the seed in which the fMRI signal is quantified across time (red waveform) and plotted over the fMRI signal from the seed. The anticorrelation between these two time-series is plotted in light blue (bottom right).](/cms/asset/b148e5d3-6750-4a08-add5-abef6f4acd58/ibij_a_1327672_f0006_oc.jpg)
Figure 7. Electrophysiological activity in the brain can be evaluated using electroencephalography (EEG) and magnetoencephalography (MEG). These techniques measure changes in the electric and magnetic fields, respectively, which are presumed to originate in the brain sources and rapidly propagate toward the participants’ scalp, affording high temporal resolution of the recordings. Event-related data can elucidate neural processing during performance on a task, discerning differences typically lasting between 50 and 500 ms. Top-left shows time-courses of the average electrophysiological response evoked by stimuli from two conditions (40 trials per condition) at FC1 and P5 EEG sensors positioned on the scalp (by convention negative voltages are plotted up). The scalp topography at the time-points (indicated by white arrows), when the between-condition differences were maximal at each of these electrodes, is shown in top-right (the data from 64 EEG sensors, locations of FC1 and P5 sensors indicated by the black dot). The fronto-central effect shown in blue (the time-course displayed in blue is more negative) and the left-posterior effect shown in red (the time-course displayed in yellow is more negative) peaked approximately 50 ms apart and were likely generated by distinct neural sources. Models can be estimated that localize EEG and MEG data, recorded at the scalp, to the neural sources. Bottom-left shows time-courses of the average evoked electrophysiological activity (60 trials per condition) estimated at the cortical regions shown in bottom-right. The time-course in the experimental condition shown in yellow peaked a few dozens of ms earlier in the left temporal cortex (TC) than in the left prefrontal cortex (PFC).
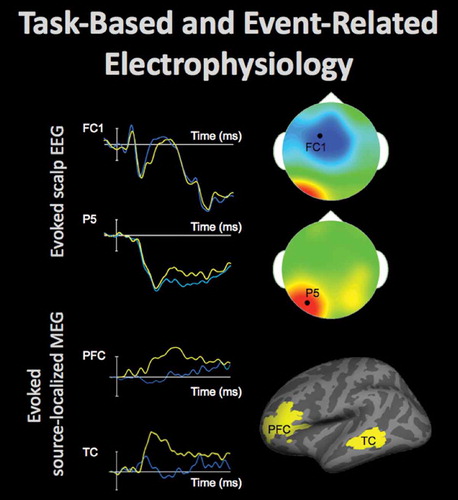
Figure 8. High temporal resolution of EEG/MEG, combined with an acceptable spatial resolution (on the order of 10 mm) of the source-localized activity estimates, afford dynamic information on connectivity in the large-scale functional neural networks, which is complementary to the fMRI data. Amplitude fluctuations in the band-limited electrophysiological oscillations (e.g. in the 15–30 Hz beta band) can be measured in regions of interest (ROIs), such as areas in the inferior-parietal cortex (IPC) and temporal cortex (TC) shown in top-left, by taking an absolute value of the Hilbert-transformed data, as shown in top-right (oscillating activity time-course is shown in blue, and the Hilbert-transformed amplitude-envelope is shown in red). Then, functional connectivity between the ROIs can be evaluated by computing a correlation between the amplitude-envelope time-courses, as shown for the TC and IPC envelopes in bottom-left (yellow dots show the corresponding time-point data). The functional connectivity between ROIs may be dynamic as is evident from the values of the correlation between the ROI envelope time-courses, which may vary over time, as shown in bottom-right for IPC (red) and TC (blue) time-courses: for approx. 10 seconds the correlation was close to zero, but for the next approx. 10 seconds-long time-interval the correlation increased to 0.61.
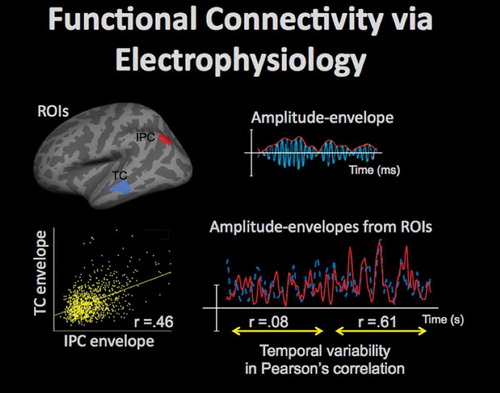
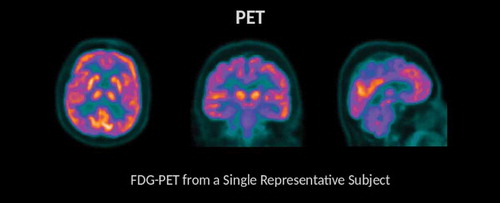
Figure 9. FDG-PET is a procedure that provides information about energy utilization in the brain. This is achieved through the use of an injected radiolabeled analog of glucose (FDG). Given that glucose is a primary energy substrate in the brain, FDG accumulation within brain areas is a marker of metabolic activity and this measure of energetics is regionally diminished in a variety of clinical conditions. Brighter colors are found in regions with greater tracer uptake (and therefore greater metabolic activity).
Functional connectivity
Functional connectivity refers to a set of neuroimage analysis procedures to quantify regionally correlated brain signal dynamics. Distant brain regions within a common network engage simultaneously to perform cognitive operations, thus, when interregional correlations are measured, for example, using fMRI, it can be interpreted as network communication (). Alterations in functional connectivity (changes in the measured correlated signal between brain regions) may therefore indicate some sort of damage to or dysregulation of neural network systems. However, it should be noted that, because fMRI is an indirect measure of neural activity, changes in fMRI might be equally related to neural function or the biological processes that mediate the translation of neural function into the hemodynamic response. In mTBI, there is evidence that some of these underlying processes may be affected [Citation57,Citation58], and this should be considered when interpreting fMRI findings. Functional connectivity is sometimes also referred to as ‘resting state fMRI’ because the analysis does not require a cognitive task be performed by the subject during the measurement, so for practical reasons, the subject is often simply instructed to rest.
Vakhtin and colleagues [Citation59] examined resting state networks in 13 Veterans with blast-related mTBI who had PCS in comparison to 50 healthy controls with no history of TBI. They found differences in the spatial extent of networks and temporal dynamics within certain regions, primarily in the default mode network (DMN). Moreover, they found that the mTBI group had reduced functional connectivity between several networks, primarily in the attentional/motor domains. However, other networks may equally contribute to cognitive dysfunction in blast-related mTBI. Gilmore and colleagues [Citation60] examined visual network connectivity in Veterans with blast-related mTBI. The authors reported that blast mTBI severity was associated with connectivity to various nodes within the visual network, which were further predictive of performance on executive function tasks outside the scanner.
Two studies in particular examined the role of time since injury. Han and colleagues [Citation7], utilizing a longitudinal approach, examined U.S. military personnel with blast-related mTBI and a matched blast-exposed cohort within 90 days of injury. The investigators reported altered brain network properties (between-module connectivity or ‘participation coefficient,’ a graph theory technique) in the individuals with TBI, which appeared to recover with time. Using an individualized subject approach, the investigators were able to classify approximately 65% of the TBI cohort tested as having ‘abnormal’ connectivity (with less sensitivity in a replication cohort). Nathan and colleagues [Citation61] examined Veterans who had been evacuated from Iraq or Afghanistan with mTBI and compared them to US Military active-duty controls. They assessed DMN connectivity through a goodness-of-fit metric, finding that the DMN had a lower goodness-of-fit score in the mTBI group compared to controls. Cross-sectionally, this score decreased with increasing time since injury, suggesting progressive dysfunction.
Recent work has examined the role of comorbid mental health conditions in Veterans with mTBI using functional connectivity measures. For example, Spielberg and colleagues [Citation62] found that mTBI modified the role of PTSD on brain connectivity patterns. Similarly, Newsome and colleagues [Citation63] examined the role of PTSD in the functional connectivity of blast-TBI Veterans and deployed, demographically matched controls. They found that many of the group differences were ultimately better attributed to PTSD, although connectivity differences in the globus pallidus remained, even after accounting for PTSD.
Electroencephalography (EEG) and event related potentials (ERP)
EEG is a functional brain imaging modality that measures electrical activity of the brain with very fine temporal resolution (ability to resolve millisecond timing events) yet somewhat limited spatial resolution (ability to localize effects in the brain on the order of centimeters). Functionally, information from EEG is typically extracted in the form of event-related potentials, or ERPs, which represent an index of the neural response to a cognitive or sensory event (typically triggered by a specific task paradigm or stimulus) ( and ). In addition to the biological factors mentioned above that may affect neural response to a stimulus, there is evidence from animal studies that neurons exhibit altered electrical excitability after mTBI [Citation64,Citation65], a feature of neural activity that may be particularly well-addressed by EEG or MEG (discussed below), which probe the electrical activity of neurons.
EEG is an early technique used in the study of combat-associated concussion, demonstrated by a study by Trudeau and colleagues published in 1998, which showed effects of remote blast history on EEG markers in combat veterans and offered some early insights into the overlapping nature of PTSD and post-concussive conditions [Citation66]. This study included World War II, Korean conflict, Vietnam War, Cold War minefield duty, and Desert Storm Veterans. Since 2009 several studies have applied EEG in this context. Sponheim and colleagues used EEG to examine neural communication in individuals with blast-related mTBI using a novel time- and frequency- based procedure to quantify EEG phase synchronization [Citation67]. Shu and colleagues examined combat Veterans with mTBI with and without concomitant PTSD [Citation68]. They tested participants with the Reading the Mind in the Eyes Test of empathy and emotional appraisal of facial features to extract task based ERPs. The investigators found that mTBI individuals with PTSD exhibited larger ERPs in response to emotional faces and that this response in posterior cortical regions (posterior cingulate/precuneus) was linked to the degree of PTSD symptoms. These results contribute to an understanding of how affective stimuli are processed in Veterans with mTBI and comorbid PTSD. In a more recent study [Citation69], the investigators used the Stop Task of inhibitory control and found larger ERPs to inhibitory processing in the comorbid mTBI and PTSD participants localized to anterior cingulate cortex. The authors suggested that such abnormalities could be directly related to the control of thought processes in Veterans with mTBI and PTSD. Franke and colleagues examined the spectral power of the EEG signal during rest (a task-less paradigm similar to what is done in many fMRI connectivity studies) in Veterans with a range of mTBI and PTSD exposure and severity [Citation70]. Individuals with PTSD had reduced low frequency power localized to right temporal and parietal cortex. This was in contrast to individuals with blast-related mTBI who had greater low frequency power localized to prefrontal and right temporal areas. An interesting potential interpretation from this work is that the distinct profiles of neurophysiological deficits linked to mTBI compared to PTSD can be differentiated using spectral EEG procedures.
Magnetoencephalography (MEG)
MEG is a functional brain imaging procedure that employs extremely sensitive detectors to measure disruptions of magnetic fields resulting from electrical activity in the brain. High temporal resolution measurements (millisecond scale) are possible, allowing a more direct assessment of neural impulses than achievable from the fMRI techniques described above, which are dependent on the slower neurovascular response. While the spatial resolution of MEG is coarser than the MRI techniques described above, it can be considerably higher than that of EEG (although the procedures are measuring different aspects of neural signals). MEG has shown promise in detecting abnormalities due to TBI in both military and civilian contexts [Citation71]. For example, Huang and colleagues [Citation72] examined MEG correlates of TBI in 45 Veterans with mTBI (due to both blast and non-blast etiologies) and 10 Veterans with moderate TBI. The MEG method was able to identify abnormal brain activity in 87% of the mTBI group through abnormal slow-wave (1–4 Hz) generation that was correlated with PCS scores in individuals with TBI. Another MEG study by Luo and colleagues [Citation73] demonstrated reduced MEG signal complexity in Veterans with TBI and abnormal brain signals were related to neuropsychological measures including motor responses, visual perception, and memory.
Fluorodeoxyglucose positron emission tomography (FDG-PET)
Regional brain metabolism (assessed as glucose uptake) can be measured using FDG-PET (). In this technique, FDG, a radioactive sugar similar to glucose, is injected into the participant. Unlike glucose, FDG accumulates in cells after being metabolized. Thus, brain metabolic properties can be assessed by measuring regional signal produced from radiation emission, which is proportional to the regional glucose utilization. By and large, hypometabolism (that is, less FDG uptake) has been found in individuals with mTBI, which is consistent with the understanding of concussion as leading to an energy crisis [Citation31]. Peskind and colleagues [Citation74] used FDG-PET to measure brain function in 12 Iraq war Veterans who reported one or more blast-related mTBIs. They found that Veterans with mTBI had decreased cerebellar, vermis, pons, and medial temporal lobe metabolism relative to control participants, suggesting that brain hypometabolism may contribute to impairments in individuals with military mTBI. This work shows overlap in the cerebellum but some differential effects in hypometabolim in comparison to a civilian cohort of boxers who underwent FDG imaging [Citation75], potentially suggesting particular vulnerability of the cerebellum to a range of brain trauma exposures. In another recent study of 14 Veterans with a history of blast mTBI or blast exposure, the mTBI group exhibited significantly lower metabolic activity across several brain regions including the amygdala, hippocampus, and parahippocampal gyrus compared to a control group of 11 Veterans without blast exposure or mTBI [Citation76]. These regions of hypometabolism were apparent in wakefulness as well as during rapid eye movement (REM) sleep, potentially suggesting a link to chronic sleep disturbances. The reader is referred to a recent review discussing the technical and theoretical considerations of the use of FDG-PET in the study of TBI [Citation77].
Multimodal imaging
Given the difficulties in isolating a specific neural signature of mTBI using individual imaging domains, there is some suggestion that the combined strengths of two or more imaging domains may provide a robust multimodal marker of mTBI and may be better able to determine system-level deficits involving multiple neural properties (e.g. structural and functional perturbations). To date, most of the studies that have utilized multimodal approaches have compared their findings to DTI.
Petrie and colleagues [Citation78] examined 34 Iraq and Afghanistan Veterans with a history of combined blast/impact-related mTBI and 18 Veterans without such a history. They combined FDG-PET and DTI and found that Veterans with mTBI had disruptions to the white matter microstructure in several tracts, as well as reduced cerebral glucose metabolism in parietal, somatosensory, and visual cortices. However, this study was unable to demonstrate whether the white matter microstructure had any direct relationship with PET imaging findings.
Huang and colleagues [Citation79] integrated DTI with neuromagnetic signals obtained from MEG to determine whether previously reported pathological low-frequency signal (delta waves 1–4 Hz) in individuals with TBI (civilian- or military-related) were attributable to neurons exhibiting axonal injury (inferred through diffusion anisotropy measures). They examined 10 individuals with TBI who showed minimal abnormalities on conventional imaging. The investigators demonstrated that the combined imaging modalities were more sensitive to subtle injury than either modality in isolation and that abnormal neural slow waves in individuals with TBI measured by MEG may be linked to regions of fiber deafferentiation.
As noted above, Sponheim and colleagues [Citation67] found altered phase synchrony in individuals with mTBI. This work additionally investigated the utility of combined DTI and EEG. They found EEG phase synchrony alterations in frontal regions linked to blast-related mTBI. These were associated with the structural integrity of white matter tracts of the frontal lobe and were further linked to combat-stress symptoms. However, cognitive function did not appear impaired. Effects were independent of PTSD and depression suggesting that mTBI may contribute to a ‘disconnection’ syndrome.
Matthews and colleagues [Citation80] examined 11 individuals with history of blast exposure and comorbid major depressive disorder using DTI and fMRI. They found abnormal activity in amygdala circuitry during performance of a fear-matching task in blast-exposed individuals with major depression relative to individuals without depression. Abnormal activity was linked to variation in white matter microstructure assessed by diffusion imaging. This work demonstrates that the complicated comorbidities common in Veteran populations may be intimately tied to mTBI-associated brain alterations, and highlights the importance of the dense characterization of large-scale Veteran populations in examining the multi-faceted nature of military trauma. It is important for future work to continue to use multimodal approaches in mTBI to both understand the interplay between mTBI and the common comorbidities affecting Veterans as well as to determine the utility of such applications in biomarker quantification.
Additional imaging modalities with promise for applications to mTBI
Outside the study of military-related TBI, other neuroimaging techniques have been utilized and have demonstrated utility in the study of brain injury in other contexts, such as sports concussion or moderate to severe TBI. These include spectroscopic imaging [Citation81], susceptibility-weighted imaging [Citation82], arterial spin labeling [Citation83], and PET imaging with other tracers, such as those designed to detect signs of neurodegeneration [Citation84,Citation85]. While these techniques have not been commonly used in military mTBI, they are available for use and may well contribute to our understanding of the effects of military-related mTBI on brain health.
Recent considerations in the study of mTBI
Given the findings reviewed above, it is clear that neuroimaging will play an important role in the future of research into understanding mTBI. Although we have touched on many important considerations for this work in this review, we briefly discuss more recent classes of questions that have emerged from the study of military populations and mTBI as well as some future directions for this research in the remaining sections.
Is blast exposure/injury a special case of mTBI?
Much has been written in recent years about the potentially unique phenomenon of blast-induced brain injury [Citation86]. Exposure to explosive munitions is one distinct category of trauma that is highly prevalent and somewhat unique to the military population. In fact, blast exposure is the most common form of TBI linked to recent conflicts [Citation87]. Blunt trauma is frequently a component of a blast event, and so the degree to which primary blast alone contributes to brain changes measured by neuroimaging in the absence of secondary blunt trauma is still unknown. Few Veteran studies have examined the effects of isolated blast. An FDG-PET study by Mendez and colleagues [Citation88] found greater hypometabolism in a cohort of 12 Veterans with blast-related mTBI compared to 12 Veterans with blunt-force mTBI. Results from DTI suggests that blast exposures, without accompanying symptoms of concussion may produce changes in neural health [Citation89–Citation91], and functional imaging suggests that this may even be specific to blast exposure at close range (self-report of <10 meters from blast) [Citation92].
Animal models of TBI may shed light on the differences between contributions of blast and blunt etiologies, as they can provide unique insight into cellular mechanisms and biochemical effects of TBIs. However, animal models of brain injury are difficult to translate to humans. First, there are substantial differences between the anatomy of model organisms (primarily rats, although mice and swine are also common) and humans, and these differences will have a large effect on how external physical forces propagate through brain tissue. While physiology is more similar, it is still imperfectly matched, notably with regards to time scales (e.g. [Citation93]). Given the role of symptom duration in assessing injury severity in humans, and our understanding of TBIs as time-varying biochemical processes [Citation94,Citation95], this is a significant hurdle to the development and validation of experimental brain injury procedures to model specific types of brain injury. This may, at least in part, account for why TBI treatments that work well in animal studies have had limited success in humans [Citation96]. To gain insight into human injury from animal models, an animal model that produces an analogous injury is vital, and the fact that different strains of mice react differently to experimental brain injury [Citation97,Citation98] underscores the difficulty of matching animal injuries to those of humans. Additionally, while the physics of producing blunt trauma is relatively straightforward, modeling the external forces (let alone the effects on tissue) for blast is complex, and not all models work well (see [Citation99] for review). Thus, direct comparisons of blunt and blast-only brain injuries are difficult, even in experimental brain injury. Despite these difficulties, across animal studies, we see that one of the components of blast injuries that separate them from blunt injuries is the contribution from vascular effects [Citation100], which are more pronounced in blast injuries. Indeed, one of the potential mechanisms of injury is transmission of the pressure wave from the thorax through major blood vessels to the brain [Citation101]. Additionally, cavitation, where blood gasses change permeability with rapidly changing pressure and form bubbles which then collapse with great force, is a unique aspect of blast-related injury [Citation102]. One study modeled blast-only and ‘complex’ injuries in rats by changing whether the rats were directly in front of the blast tube (changing the degree to which the head moved) and found that the blast-only model was not associated with neuronal death, however, both model types were associated with glial injuries, and the blast-only setup, especially without body protection, was more highly associated with systemic neuroendocrine and neurotrophic factors in serum [Citation103]. However, most biochemical components of brain injury are present in both etiologies, although perhaps to different extents. For a review of animal models of TBI (blast and blunt, see [Citation104]).
Long term consequences of mTBI
An active area of research aims to determine whether an early-life TBI alters brain aging trajectories, which, in turn, may increase the likelihood of the development of late-life dementia or specific neurodegenerative conditions such as Alzheimer’s disease. This has been the topic of several recent discussions in the literature [Citation105,Citation106] with a range of epidemiological and clinical studies suggesting that TBI increases later life cognitive risk to some degree [Citation107–Citation109]. Brain imaging has demonstrated the potential modification of aging trajectories, at least cross-sectionally, in Veterans with blast exposure compared to unexposed Veterans [Citation91,Citation110] and for Veterans with symptomatic blast-related mTBI compared to pre-deployed military personnel [Citation91]. Moreover, recent imaging work demonstrates that genetic risk for Alzheimer’s disease and exposure to TBI have a potentially interactive effect on cortical thickness [Citation111], which may suggest some enhanced risk for degenerative processes that have long term consequences. Despite the limitations noted in the field about the lack of confirmable records of past TBI, particularly in older military cohorts, and the fidelity of self-reports, we emphasize here that understanding how TBI influences brain aging and the risk for future neurodegenerative disease is of utmost importance. The alternative is an epidemic of dementia care from an aging military cohort from Operations Enduring Freedom, Iraqi Freedom, and New Dawn (OEF/OIF/OND) that will begin to approach the age range where early dementia is typical within the next 15–20 years. As such, not preparing for this possibility may result in a major medical and societal burden.
Conclusions: The future of research into neural mechanisms of mTBI
Although much has been uncovered since the application of sophisticated brain imaging to the study of mTBI, a vast amount remains to be learned. Towards this end, important major efforts are underway to further elucidate the causes of TBI-associated symptoms and disability. Through enhanced understanding of mechanisms there is hope that more effective therapeutics will ultimately be devised. It is imperative that research proceeds to develop large, well-characterized cohorts of Veterans and military service members that can be followed over time to chart and understand the long-term effects of both physical and psychological trauma incurred prior to and during military deployment. In order to illuminate specific noninvasive neuroimaging metrics that are sensitive to identify the instance of mTBI and determine its long-term course, it is essential to also capture specific factors of the brain injury itself [Citation112]. A number of research studies are currently ongoing to meet these goals. Additionally, efforts are being made to make data freely available to the research community, which will increase the likelihood of important findings from this work and further our understanding of combat-associated TBI. The Rehabilitation Research and Development service of VA funded the Translational Research Center for TBI and Stress Disorders (TRACTS) in 2009, and the center has now collected detailed biological, neuropsychology, psychiatric, lifetime trauma and multimodal neuroimaging data for over 500 OEF/OIF/OND Veterans, with more than half having returned for longitudinal evaluation [Citation113]. Requests for data from the TRACTS longitudinal cohort study can be made through visiting the TRACTS website (http://www.boston.va.gov/research/Translational_Research_Center_for_TBI_and_Stress_Disorders_TRACTS.asp) or through email to the study Director, Dr. Regina McGlinchey ([email protected]). The Chronic Effects of Neurotrauma Consortium (CENC, https://cenc.rti.org), a joint venture by VA and DOD was funded in 2013 [Citation114,Citation115], and is acquiring brain imaging and other characterization data from several US sites in an effort to examine the long-term effects of combat and other military-related mTBI. The Alzheimer’s Disease Neuroimaging Initiative- Department of Defense (ADNI-DOD; http://adni.loni.usc.edu/study-design/collaborative-studies/dod-adni) is an adjunct project to the large-scale initial ADNI projects that aims to understand how military trauma in early life results in late life brain and other changes. Data are accessible through their website. In addition to these studies, invaluable supporting information about the pathological basis of imaging findings will be available through complementary efforts such as the VA Biorepository Brain Bank (http://www.research.va.gov/programs/specimen_biobanking.cfm). Moving into the future, the continued efforts of large-scale longitudinal studies that leverage many imaging modalities combined with thorough characterization of both the brain injury history and the biological and psychological context in which they occurred demonstrate much promise to propel the field forward. These advances will promote the understanding of the impact of mTBI on Veteran health and options for future treatment.
Declaration of interest
The authors report no declarations of interest. This research was supported by the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury National Network Research Center (B9254-C) and the National Institutes of Mental Health (NIMH) training grant (T32MH019836-01) awarded to Terence Keane, Ph.D., National Center for PTSD at VA Boston Healthcare System supporting DRM.
Acknowledgements
The authors thank Ms. Kimberly Stephens and Drs. Michael Esterman, Victoria Poole, and Tatiana Sitnikova for assistance in figure generation for this review.
References
- Van Boven RW, Harrington GS, Hackney DB, Ebel A, Gauger G, Bremner JD, D’Esposito M, Detre JA, Haacke EM, Jack CR, Jr., et al. Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. J Rehabil Res Dev. 2009;46(6):717–57.
- Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995.
- Alosco ML, Aslan M, Du M, Ko J, Grande L, Proctor SP, Concato J, Vasterling JJ. Consistency of recall for deployment-related traumatic brain injury. J Head Trauma Rehabil. 2016;31(5):360–8.
- Lagarde E, Salmi L-R, Holm LW, Contrand B, Masson Fß, Rib√©reau-Gayon Rg, Laborey M, Cassidy JD. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs postconcussion syndrome. JAMA Psychiatry. 2014;71(9):1032–40.
- French L, McCrea M, Baggett M. The military acute concussion evaluation (MACE). J Spec Oper Med. 2008;8(1):68–77.
- Ibarra S. Automated neuropsychological assessment metrics. Encyclopedia of clinical neuropsychology. Springer; 2011. p 325–7.
- Han K, Mac Donald CL, Johnson AM, Barnes Y, Wierzechowski L, Zonies D, Oh J, Flaherty S, Fang R, Raichle ME, et al. Disrupted modular organization of resting-state cortical functional connectivity in U.S. military personnel following concussive ‘mild’ blast-related traumatic brain injury. NeuroImage. 2014;84:76–96.
- Mac Donald C, Johnson A, Cooper D, Malone T, Sorrell J, Shimony J, Parsons M, Snyder A, Raichle M, Fang R, et al. Cerebellar White Matter Abnormalities following Primary Blast Injury in US Military Personnel. PLoS ONE. 2013;8(2):e55823.
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364(22):2091–100.
- Menon DK, Schwab K, Wright DW, Maas AI, Demographics, Clinical Assessment Working Group of the I, Interagency Initiative toward Common Data Elements for Research on Traumatic Brain I, Psychological H. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–40.
- Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev 2009;46(6): CP1.
- Farmer CM, Krull H, Concannon TW, Simmons M, Pillemer F, Ruder T, Parker AM, Purohit MP, Hiatt L, Batorsky B, et al. Understanding treatment of mild traumatic brain injury in the military health system; 2016.
- Belanger HG, Vanderploeg RD, Soble JR, Richardson M, Groer S. Validity of the Veterans Health Administration’s traumatic brain injury screen. Arch Phys Med Rehabil. 2012;93(7):1234–9.
- Nelson NW, Anderson CR, Thuras P, Kehle-Forbes SM, Arbisi PA, Erbes CR, Polusny MA. Factors associated with inconsistency in self-reported mild traumatic brain injury over time among military personnel in Iraq. Br J Psychiatry. 2015;206(3):237–44.
- Ling GS, Marshall SA. Management of traumatic brain injury in the intensive care unit. Neurol Clin. 2008;26(2):409–26, viii.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4.
- Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma. 2008;25(9):1049–56.
- Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25(4):241–55.
- Riedy G, Senseney JS, Liu W, Ollinger J, Sham E, Krapiva P, Patel JB, Smith A, Yeh PH, Graner J, et al. Findings from structural MR imaging in military traumatic brain injury. Radiology. 2016;279(1):207–15.
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte I, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):137–92.
- Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6(2):108–36.
- Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, LaConte SM. Neuroimaging after mild traumatic brain injury: review and meta-analysis. NeuroImage Clin. 2014;4:283–94.
- Graner J, Oakes TR, French LM, Riedy G. Functional MRI in the investigation of blast-related traumatic brain injury. Front Neurol. 2013;4:16.
- Hayes JP, Bigler ED, Verfaellie M. Traumatic brain injury as a disorder of brain connectivity. J Int Neuropsychol Soc. 2016;22(2):120–37.
- Duhaime AC, Gean AD, Haacke EM, Hicks R, Wintermark M, Mukherjee P, Brody D, Latour L, Riedy G, Common Data Elements Neuroimaging Working Group Members PWGM. Common data elements in radiologic imaging of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1661–6.
- Haacke EM, Duhaime AC, Gean AD, Riedy G, Wintermark M, Mukherjee P, Brody DL, DeGraba T, Duncan TD, Elovic E, et al. Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging. 2010;32(3):516–43.
- Kou Z, Wu Z, Tong KA, Holshouser B, Benson RR, Hu J, Haacke EM. The role of advanced MR imaging findings as biomarkers of traumatic brain injury. J Head Trauma Rehabil. 2010;25(4):267–82.
- McDonald BC, Saykin AJ, McAllister TW. Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav. 2012;6(2):193–207.
- Brenner LA. Neuropsychological and neuroimaging findings in traumatic brain injury and post-traumatic stress disorder. Dialogues Clin Neurosci. 2011;13(3):311–23.
- Hunter JV, Wilde EA, Tong KA, Holshouser BA. Emerging imaging tools for use with traumatic brain injury research. J Neurotrauma. 2012;29(4):654–71.
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30(1):33–48.
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510.
- Pun PB, Kan EM, Salim A, Li Z, Ng KC, Moochhala SM, Ling E-A, Tan MH, Lu J. Low level primary blast injury in rodent brain. Traumat Injur Nerv Syst. 2011:29.
- Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin. 2013;2:601–11.
- Michael AP, Stout J, Roskos PT, Bolzenius J, Gfeller J, Mogul D, Bucholz R. Evaluation of cortical thickness after traumatic brain injury in military veterans. J Neurotrauma. 2015;32(22):1751–8.
- Tate DF, York GE, Reid MW, Cooper DB, Jones L, Robin DA, Kennedy JE, Lewis J. Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav. 2014;8(1):102–9.
- Tate DF, Wade BS, Velez CS, Drennon AM, Bolzenius J, Gutman BA, Thompson PM, Lewis JD, Wilde EA, Bigler ED, et al. Volumetric and shape analyses of subcortical structures in United States service members with mild traumatic brain injury. J Neurol. 2016;263(10):2065–79.
- Stone JR, Okonkwo DO, Dialo AO, Rubin DG, Mutlu LK, Povlishock JT, Helm GA. Impaired axonal transport and altered axolemmal permeability occur in distinct populations of damaged axons following traumatic brain injury. Exp Neurol. 2004;190(1):59–69.
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma Acute Care Surg. 2001;50(4):695–706.
- Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc 2004;10(5):794–806.
- Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28(2):189–201.
- Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, Gold T, Shifteh K, Ardekani BA, Branch CA. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252(3):816–24.
- Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, Bello JA, Branch CA. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25(11):1335–42.
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. 2013;34(11):2064–74.
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, Radaideh M, Wu T, Yallampalli R, Chu Z, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma. 2010;27(4):683–94.
- Davenport ND, Lim KO, Armstrong MT, Sponheim SR. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. NeuroImage. 2012;59(3):2017–24.
- Hayes JP, Miller DR, Lafleche G, Salat DH, Verfaellie M. The nature of white matter abnormalities in blast-related mild traumatic brain injury. NeuroImage Clin. 2015;8(0):148–56.
- Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Human Brain Mapp. 2016;37(1):220–9.
- Jorge RE, Acion L, White T, Tordesillas-Gutierrez D, Pierson R, Crespo-Facorro B, Magnotta VA. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry. 2012;169(12):1284–91.
- Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with overall cognitive status in blast-related mTBI. Brain Imag Behav. 2016:1–10.
- Sorg SF, Delano-Wood L, Luc N, Schiehser DM, Hanson KL, Nation DA, Lanni E, Jak AJ, Lu K, Meloy MJ, et al. White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J Head Trauma Rehabil. 2013;29(1):21–32.
- Davenport ND, Lim KO, Sponheim SR. White matter abnormalities associated with military PTSD in the context of blast TBI. Hum Brain Mapp. 2015;36(3):1053–64.
- Mac Donald CL, Barber J, Andre J, Evans N, Panks C, Sun S, Zalewski K, Sanders RE, Temkin N. 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. NeuroImage Clin. 2017;14:371–8.
- Fischer BL, Parsons M, Durgerian S, Reece C, Mourany L, Lowe MJ, Beall EB, Koenig KA, Jones SE, Newsome MR, et al. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J Neurotrauma. 2014;31(2):169–79.
- Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, Levin HS. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J Int Neuropsychol Soc. 2012;18(1):89–100.
- Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, Levin HS. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J Int Neuropsychol Soc. 2012;18(01):89–100.
- Kallakuri S, Desai A, Feng K, Tummala S, Saif T, Chen C, Zhang L, Cavanaugh JM, King AI. Neuronal injury and glial changes are hallmarks of open field blast exposure in swine frontal lobe. PloS ONE. 2017;12(1):e0169239.
- Tsai Y-D, Liliang P-C, Cho C-L, Chen J-S, Lu K, Liang C-L, Wang KW. Delayed neurovascular inflammation after mild traumatic brain injury in rats. Brain Inj. 2013;27(3):361–5.
- Vakhtin AA, Calhoun VD, Jung RE, Prestopnik JL, Taylor PA, Ford CC. Changes in intrinsic functional brain networks following blast-induced mild traumatic brain injury. Brain Inj. 2013;27(11):1304–10.
- Gilmore CS, Camchong J, Davenport ND, Nelson NW, Kardon RH, Lim KO, Sponheim SR. Deficits in visual system functional connectivity after blast-related mild TBI are associated with injury severity and executive dysfunction. Brain Behav. 2016;6(5):e00454.
- Nathan DE, Oakes TR, Yeh PH, French LM, Harper JF, Liu W, Wolfowitz RD, Wang BQ, Graner JL, Riedy G. Exploring variations in functional connectivity of the resting state default mode network in mild traumatic brain injury. Brain Connect. 2015;5(2):102–14.
- Spielberg JM, McGlinchey RE, Milberg WP, Salat DH. Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry. 2015;78(3):210–6.
- Newsome MR, Mayer AR, Lin X, Troyanskaya M, Jackson GR, Scheibel RS, Walder A, Sathiyaraj A, Wilde EA, Mukhi S, et al. Chronic effects of blast-related tbi on subcortical functional connectivity in veterans. J Int Neuropsychol Soc. 2016;22(6):631–42.
- Baalman KL, Cotton RJ, Rasband SN, Rasband MN. Blast wave exposure impairs memory and decreases axon initial segment length. J Neurotrauma. 2013;30(9):741–51.
- Greer JE, Hnell A, McGinn MJ, Povlishock JT. Mild traumatic brain injury in the mouse induces axotomy primarily within the axon initial segment. Acta Neuropathol. 2013;126(1):59–74.
- Trudeau DL, Anderson J, Hansen LM, Shagalov DN, Schmoller J, Nugent S, Barton S. Findings of mild traumatic brain injury in combat veterans with PTSD and a history of blast concussion. J Neuropsychiatry Clin Neurosci. 1998;10(3):308–13.
- Sponheim SR, McGuire KA, Kang SS, Davenport ND, Aviyente S, Bernat EM, Lim KO. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. NeuroImage. 2011;54 Suppl 1:S21–9.
- Shu IW, Onton JA, Prabhakar N, O’Connell RM, Simmons AN, Matthews SC. Combat veterans with PTSD after mild TBI exhibit greater ERPs from posterior-medial cortical areas while appraising facial features. J Affect Disord. 2014;155:234–40.
- Shu IW, Onton JA, O’Connell RM, Simmons AN, Matthews SC. Combat veterans with comorbid PTSD and mild TBI exhibit a greater inhibitory processing ERP from the dorsal anterior cingulate cortex. Psychiatry Res. 2014;224(1):58–66.
- Franke LM, Walker WC, Hoke KW, Wares JR. Distinction in EEG slow oscillations between chronic mild traumatic brain injury and PTSD. Int J Psychophysiol. 2016;106:21–9.
- Tarapore PE, Findlay AM, Lahue SC, Lee H, Honma SM, Mizuiri D, Luks TL, Manley GT, Nagarajan SS, Mukherjee P. Resting state magnetoencephalography functional connectivity in traumatic brain injury. J Neurosurg. 2013;118(6):1306–16.
- Huang MX, Nichols S, Robb A, Angeles A, Drake A, Holland M, Asmussen S, D’Andrea J, Chun W, Levy M, et al. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. NeuroImage. 2012;61(4):1067–82.
- Luo Q, Xu D, Roskos T, Stout J, Kull L, Cheng X, Whitson D, Boomgarden E, Gfeller J, Bucholz RD. Complexity analysis of resting state magnetoencephalography activity in traumatic brain injury patients. J Neurotrauma. 2013;30(20):1702–9.
- Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, Hart K, Yu CE, Raskind MA, Cook DG, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. NeuroImage. 2011;54 Suppl 1:S76–82.
- Provenzano FA, Jordan B, Tikofsky RS, Saxena C, Van Heertum RL, Ichise M. F-18 FDG PET imaging of chronic traumatic brain injury in boxers: a statistical parametric analysis. Nucl Med Commun. 2010;31(11):952–7.
- Stocker RP, Cieply MA, Paul B, Khan H, Henry L, Kontos AP, Germain A. Combat-related blast exposure and traumatic brain injury influence brain glucose metabolism during REM sleep in military veterans. NeuroImage. 2014;99:207–14.
- Byrnes KR, Wilson CM, Brabazon F, von Leden R, Jurgens JS, Oakes TR, Selwyn RG. FDG-PET imaging in mild traumatic brain injury: a critical review. Front Neuroenerget. 2014;5:13.
- Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, Hoff D, Hart K, Mayer C, Tarabochia M, et al. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma. 2014;31(5):425–36.
- Huang MX, Theilmann RJ, Robb A, Angeles A, Nichols S, Drake A, D’Andrea J, Levy M, Holland M, Song T, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma. 2009;26(8):1213–26.
- Matthews SC, Strigo IA, Simmons AN, O’Connell RM, Reinhardt LE, Moseley SA. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. NeuroImage. 2011;54 Suppl 1:S69–75.
- Lin A, Liao H, Merugumala S, Prabhu S, Meehan III W, Ross B. Metabolic imaging of mild traumatic brain injury. Brain Imag Behav. 2012;6(2):208–223.
- Liu J, Xia S, Hanks R, Wiseman N, Peng C, Zhou S, Haacke EM, Kou Z. Susceptibility weighted imaging and mapping of micro-hemorrhages and major deep veins after traumatic brain injury. J Neurotrauma. 2016;33(1):10–21.
- Andre JB. Arterial spin labeling magnetic resonance perfusion for traumatic brain injury: technical challenges and potentials. Top Magn Reson Imag. 2015;24(5):275–87.
- Catafau AM, Bullich S. Amyloid PET imaging: applications beyond Alzheimer’s disease. Clin Translat Imag. 2015;3(1):39–55.
- Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015;14(1):114–24.
- Elder GA, Mitsis EM, Ahlers ST, Cristian A. Blast-induced mild traumatic brain injury. Psychiatr Clin North Am. 2010;33(4):757–81.
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358(5):453–63.
- Mendez MF, Owens EM, Reza Berenji G, Peppers DC, Liang L-J, Licht EA. Mild traumatic brain injury from primary blast vs. blunt forces: post-concussion consequences and functional neuroimaging. NeuroRehabilitation. 2013;32(2):397–407.
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, Morey RA. White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil. 2015;30(1):E15–25.
- Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J Head Trauma Rehabil. 2013;28(1):1–12.
- Trotter BB, Robinson ME, Milberg WP, McGlinchey RE, Salat DH. Military blast exposure, ageing and white matter integrity. Brain. 2015;138(Pt 8):2278–92.
- Robinson ME, Lindemer ER, Fonda JR, Milberg WP, McGlinchey RE, Salat DH. Close-range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. Hum Brain Mapp. 2015;36(3):911–22.
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci. 2013;110(9):3507–12.
- Elsayed M, Agoston DV. Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder. Front Neurol. 2012;3:107.
- Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(04):S24.
- Marklund N, Bakshi A, Castelbuono DJ, Conte V, McIntosh TK. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharmaceut Des. 2006;12(13):1645–1680.
- Reid WM, Rolfe A, Register D, Levasseur JE, Churn SB, Sun D. Strain-related differences after experimental traumatic brain injury in rats. J Neurotrauma. 2010;27(7):1243–53.
- Tan AA, Quigley A, Smith DC, Hoane MR. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J Neurotrauma. 2009;26(4):539–48.
- Needham CE, Ritzel D, Rule GT, Wiri S, Young L. Blast testing issues and TBI: experimental models that lead to wrong conclusions. Front Neurol. 2015;6:72.
- Elder GA, Gama Sosa MA, De Gasperi R, Stone JR, Dickstein DL, Haghighi F, Hof PR, Ahlers ST. Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front Neurol. 2015;6:48.
- Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cerebr Blood Flow Metabol. 2010;30(2):255–266.
- Goeller J, Wardlaw A, Treichler D, O’Bruba J, Weiss G. Investigation of cavitation as a possible damage mechanism in blast-induced traumatic brain injury. J Neurotrauma. 2012;29(10):1970–81.
- Svetlov SI, Prima V, Glushakova O, Svetlov A, Kirk D, Gutierrez H, Serebruany V, Curley K, Wang KK, Hayes RL. Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to‚ Äúcomposite‚ Äù blast. Front Neurol. 2012;3:15.
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–42.
- Vincent AS, Roebuck-Spencer TM, Cernich A. Cognitive changes and dementia risk after traumatic brain injury: implications for aging military personnel. Alzheimers Dement. 2014;10(3 Suppl):S174–87.
- McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 Suppl):S242–53.
- Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. Prog Brain Res. 2007;161:303–16.
- Johnson VE, Stewart W. Traumatic brain injury: age at injury influences dementia risk after TBI. Nat Rev Neurol. 2015;11(3):128–30.
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490–7.
- Yeh PH, Guan Koay C, Wang B, Morissette J, Sham E, Senseney J, Joy D, Kubli A, Yeh CH, Eskay V, et al. compromised neurocircuitry in chronic blast-related mild traumatic brain injury. Hum Brain Mapp. 2016.
- Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, Reagan A, Salat DH, Wolf EJ, McGlinchey RE. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer Äôs disease. Brain. 2017:aww344.
- Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, Morra L, Clark A, Milberg WP, McGlinchey RE. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. J Head Trauma Rehabil. 2014;29(1):89–98.
- Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, Reagan A, Salat DH, Wolf EJ, McGlinchey RE, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017;140(3):813–25.
- Cifu DX, Dixon KJ. Chronic effects of neurotrauma consortium. Brain Inj. 2016;30(12):1397–8.
- Walker WC, Carne W, Franke LM, Nolen T, Dikmen SD, Cifu DX, Wilson K, Belanger HG, Williams R. The Chronic Effects of Neurotrauma Consortium (CENC) multi-centre observational study: description of study and characteristics of early participants. Brain Inj. 2016;30(12):1469–1480.

