ABSTRACT
Brain tumour patients with mild language disturbances are typically underdiagnosed due to lack of sensitive tests leading to negative effects in daily communicative and social life. We aim to develop a Dutch standardised test-battery, the Diagnostic Instrument for Mild Aphasia (DIMA) to detect characteristics of mild aphasia at the main linguistic levels phonology, semantics and (morpho-)syntax in production and comprehension. We designed 4 DIMA subtests: 1) repetition (words, non-words, compounds and sentences), 2) semantic odd-picture-out (objects and actions), 3) sentence completion and 4) sentence judgment (accuracy and reaction time). A normative study was carried out in a healthy Dutch-speaking population (N = 211) divided into groups of gender, age and education. Clinical application of DIMA was demonstrated in two brain tumour patients (glioma and meningioma). Standard language tests were also administered: object naming, verbal fluency (category and letter), and Token Test. Performance was at ceiling on all sub-tests, except semantic odd-picture-out actions, with an effect of age and education on most subtests. Clinical application DIMA: repetition was impaired in both cases. Reaction time in the sentence judgment test (phonology and syntax) was impaired (not accuracy) in one patient. Standard language tests: category fluency was impaired in both cases and object naming in one patient. The Token Test was not able to detect language disturbances in both cases. DIMA seems to be sensitive to capture mild aphasic deficits. DIMA is expected to be of great potential for standard assessment of language functions in patients with also other neurological diseases than brain tumours.
Introduction
A mild language disorder as a consequence of neurological diseases has negative effects on daily communication (Cruice et al., Citation2006). In the long term, the impact on communicative abilities might be as large as in severe and moderate aphasia. Difficulties are reported with word finding, rapid conversational techniques, storytelling, writing, reading and expression of nuances/subtleties in meaning (Fox et al., Citation2009; Harmon et al., Citation2019). Often, these limitations in language use do not become apparent until the moment patients return to their socio-professional life when full participation in daily situations is necessary. This can lead to frustration and lack of self-confidence in communication at both the professional and social level (Hickin et al., Citation2015). Also, concomitant cognitive deficits, such as problems in attention and executive functions may be present (Barker et al., Citation2017; Hunting-Pompon et al., Citation2011).
The mild aphasia patient group is relatively “invisible” in health care because mild aphasic symptoms are not always easy to recognize by the clinician. During anamnesis, elaborate conversation requiring complex answers is often necessary to get a clear picture of the communicative gaps. This difficulty in the diagnosis of a mild form of aphasia was already raised in 1980 at a round table panel discussion (Darley et al., Citation1980). “High-level” language abilities in patients with, for instance, academic professions could not be well objectified and/or treated with “standard” tools. At present, patients are still underdiagnosed, which was pointed out in a survey among a large group of speech-language pathologists across the United States (Mozeiko & Pascariello, Citation2020). They argued that the lack of adequate assessment tools was due to a prioritization bias towards more severe aphasic patients groups in standard health care They propose to shift the current practice more towards designing assessment tools for mild aphasic population given the greater chances of this group to return to work.
It is a definite fact that brain tumour patients such as low-grade gliomas frequently suffer from mild language deficits due to slow tumour growth rate (Duffau, Citation2014). Classical language test batteries such as the Aachen Aphasia Test (Graetz & Willmes, Citation1991) and tests such as the (shortened) Token Test (Renzi De & Faglioni, Citation1978) or the Boston Naming Test (BNT) (Kaplan et al., Citation2001), usually administered in stroke patients, appeared not always sensitive enough to detect the language disorders, presented during the anamnesis (Satoer, Visch-Brink et al., Citation2014; Satoer et al., Citation2012). However, spontaneous speech analyses are very sensitive to reveal abnormalities in glioma patients compared to healthy speakers, such as a larger number of incomplete sentences and shorter utterances (Satoer et al., Citation2018)
Also in stroke patients, language complaints with ceiling performance on standard aphasia tests have been reported, such as the Comprehensive Aphasia Test (CAT) (Swinburn et al., Citation2005), or the Boston Diagnostic Aphasia Examination (Goodglass et al., Citation2001) (Cavanaugh & Haley, Citation2020; Darley et al., Citation1980; Hickin et al., Citation2015). Discourse analyses are presented as good alternatives to capture mild language disturbances in comparison to standard aphasia tests (Pritchard et al., Citation2018). However, although sensitive, detailed spontaneous speech and discourse analyses are very time-consuming in daily practice.
Consequently, there is a need for a new tool to detect mild aphasia. Such an assessment should reflect the complaints of patients in daily communication and might be directed to complex argument structures, compound sentences (Murray et al., Citation1998), discourse elements (Obermeyer & Edmond, Citation2018) or tests with the addition of time constraints (e.g. speeded naming) (Moritz-Gasser et al., Citation2012).
In 2015, we published the Dutch Linguistic Intraoperative Protocol (DuLIP) for patients with a low-grade glioma (Witte De et al., Citation2015). The focus of this test battery concerned intraoperative language testing in awake brain tumour surgery with tests for production and comprehension at the main linguistic levels such as phonology, semantics and (morpho-)syntax. As an awake procedure can take up to 2 hours and patients’ language performance can deteriorate due to tumour resection, DuLIP comprises many tests with various levels of complexity. For an assessment of (pre- and postoperative) mild deviations in language use of brain tumour patients, but also in other neurological patients, a shortened version is needed with the focus on complex tests to detect mild aphasic disorders.
We designed the Diagnostic Instrument for Mild Aphasia (DIMA), based on clinical experience and earlier research with DuLIP. The most complex subtests and items were selected (and/or revised) at the main linguistic levels. A seamless integration of these linguistic levels is required for adequate communicative abilities. Another important characteristic is the addition of time constraints in several tests.
The aim of this paper is to present the design of the first Dutch test to detect mild aphasia, the DIMA, and its standardisation in healthy population. Clinical application is illustrated in two case studies.
Participants
We included 211 healthy participantsFootnote1 from the Netherlands and Belgium divided by gender (male and female), handedness (right, left, and ambidexterity), age (18–54 and 55–85 years) and education (≤12 and >12 years). A cut-off of 55 years old is often quoted as the age at which cognitive decline may begin (Ronnlund et al., Citation2005), and a cut-off of 12 years of education in the Netherlands and Belgium coincides with the standard period of primary and secondary education. The demographics of the healthy participants are summarized in . The inclusion criteria consisted of the following: (1) native speakers of Dutch; (2) no (history of) cardiovascular, neurological, psychiatric, developmental language and/or speech disorders; (3) normal or corrected vision; (4) normal or corrected hearing; (5) no toxic substance abuse (i.e. no drug or alcohol abuse); (6) no excessive use of sleep medication and (7) no use of psychopharmaca. Additionally, participants aged ≥75 years needed to have a score of at least 24/30 on the Mini Mental State Examination (MMSE) to screen for cognitive decline (e.g. Alzheimer’s disease) as the cut-off score for impairment is 23 (Crum et al., Citation1993). Participants were informed about the goals of the project and gave a written informed consent. The study was approved by the ethical committee of the Vrije Universiteit Brussel and the Medical Ethical Committee of the Erasmus MC Rotterdam, which waived the need for written informed consent from the patients because of the retrospective nature of the study and the (emotional) burden that would result from contacting the patients or their relatives to obtain consent.
Table 1. Demographic characteristics of the 211 healthy participants.
Table 2. Demographic characteristics of the 233 healthy participants (E-Prime).
Materials and methods
Description of tests
The test selection was based on different locations of eloquent cortical and subcortical language areas of the brain (Witte De et al., Citation2015). DIMA consists of four subtests: verbal repetition (words, compounds, non-words, and sentences), odd-picture-out (objects, actions), sentence completion and sentence judgment (phonology, semantics, and syntax) (see ). The Dutch databases CELEX (Kerkman et al., Citation1993), SUBTLEX-NL (Keuleers et al., Citation2010) and Positiewoordenboek (Weijters, Citation1983) were used to check the linguistic variables such as frequency, imageability (Van Loon-Vervoorn, Citation1989), word length and word form (see linguistic variables per test). To ensure that unreliable items are eliminated before standardisation, each item was tested on 110 healthy subjects. Items that did not meet the 90% threshold of correctness (i.e. at least 90% of the healthy controls are able to answer correctly) were deleted.
Table 3. Task description and examples of the DIMA.
Phonology: repetition
The repetition subtests (PHON A-D) are included to assess the phonological input and output route. In comparison with DuLIP, we added compound words and non-words as they are considered as phonologically more complex than single and existing words, respectively (Eiesland & Lind, Citation2012). Repetition tests are important to detect phonological distortion processes near the Arcuate Fasciculus, a white matter tract connecting Broca’s areas to Wernicke’s area (Catani & Mesulam, Citation2008). The quality of phonological processing in aphasic stroke patients appeared to be crucial for prognostics in recovery and repetition of phonological complex words appeared to be very sensitive in comparison to naming (El Hachioui et al., Citation2013; Sierpowska et al., Citation2017). We therefore decided to emphasize repetition subtests.
In each test, participants are instructed to repeat a list of words or sentences: ten three syllabic words (PHON A), ten three to six syllabic compound words (PHON B), ten three to four syllabic non-words (non-existing words) (PHON C) and ten sentences (PHON D). All word repetition tests include items with variable syllable stress patterns (e.g. gorilla – gorilla and paraplu – umbrella), consonant clusters (e.g. constructie – construction) and/or phonemic similarities (e.g. domino – domino) to increase the degree of articulatory and phonological complexity (Gierut, Citation2007).
The sentence repetition test includes items that differ in length (4–11 words) (e.g. Harry rent zich rot – Harry is running fast, De Griek ontdekte vier nietjes in de band van zijn fiets – The Greek discovered four staples in the tire of his bike). Just as within the word level, the test items comprise consonant clusters (e.g. De prikkelbare panter besprong haar prooi – The irritable panther jumped its prey) and/or phonemic similarities (e.g. In welke la leg jij de lepel? – In which drawer do you put the spoon?).
The answers are scored as correct (1 point) when the patient repeats the items correctly within four seconds without substantial hesitations, self-corrections and/or perseverations.
Semantics: naming with semantic odd-picture-out
To assess word retrieval and semantic judgment, two naming tests with a semantic odd-picture-out (SEM A, objects and SEM B, actions) are included. In comparison with DuLIP, actions were added. Action naming is reported to be more linguistically complex than object naming (Rofes & Miceli, Citation2014).
Semantic judgment in combination with naming is related to frontotemporal regions at the cortical level and the white matter tracts Inferior Frontal Occipital Fasciculus and the Inferior Longitudinal Fasciculus (Moritz-Gasser et al., Citation2013). Naming objects is correlated with the anterior temporal region, while naming actions is correlated with the parietal areas and the posterior temporal cortex (Pisoni et al., Citation2018; Rofes et al., Citation2017).
Participants are instructed to name the picture that does not semantically fit in a series of three black and white drawings representing objects (nouns) (Snodgrass & Vanderwart, Citation1980), animals (nouns) or actions (verbs) (Rofes et al., Citation2012). Two of the three pictures belong to the same semantic category. Every four seconds, a set of three pictures (5 x 3 objects and 5 × 3 actions) is presented in a PowerPoint presentation on a laptop screen. The test is presented within a four-second time frame (originating from the direct electrical stimulation paradigm in awake surgery (Duffau et al., Citation2002)).
The answers are scored as correct (1 point) when the participant names the odd object or action correctly within four seconds without substantial hesitations, self-corrections and/or perseverations.
(Morpho-)syntax: sentence completion
To assess “spontaneous speech in context”, a sentence completion test (SYN A) is included. The test evaluates mainly the syntax but also the semantics and phonology of semi-spontaneous speech. Sentence completion is relevant to assess for lesions near the Supplementary Motor Area, temporo-parietal regions, the insula, the Frontal Aslant Tract and the Inferior Longitudinal Fasciculus (Bello et al., Citation2007; Dick et al., Citation2019; Fontaine et al., Citation2002).
Participants are instructed to complete 10 sentences, which are auditorily presented, with one word (test items 1–4) or a constituent (test item 5–10), in a meaningful way. The sentence frames, with obligatory and less obligatory parts of speech, elicit a specific syntactic response but allow a diversity of answers in an ascending degree of difficulty (e.g. Hij viel van … = He fell from …, Om 5 uur … – At 5 o’clock …).
Answers are scored correctly (1 point) when the participant answers with a syntactic (and also semantic and phonological) correct phrase in a fluent way and initiates the response within approximately four seconds.
Sentence judgment: phonology, semantics, syntax
The different linguistic levels, phonology, semantics and syntax, are combined in a sentence judgment test and programmed in E-prime (Psychology Software Tools, Citation2012) (30 items in total). In comparison to DuLIP, all linguistic levels are combined, and incorrect and correct sentences are presented visually at random (30 items), with a time pressure by asking to respond as quickly as possible with a maximum time set at 10 seconds per item, but ultimately measured in milliseconds.Footnote2 Linguistic processing speed appeared to be of crucial importance to detect mild language disturbances (Moritz-Gasser et al., Citation2012; Ras et al., Citation2020). Visual sentence presentation places a demand on the visuo-orthographical route and is related to the supramarginal gyrus and the Inferior Longitudinal Fasciculus (Fernandez Coello et al., Citation2013).
The phonological sentence judgment test (PHON E) is included to assess phonological awareness and phonological decoding (10 items in total) and is associated with the phonological network located near the middle posterior superior temporal sulcus (Hickok, Citation2012). The incorrect sentences contain non-words (e.g. De wikker gaat naar parot – the wikker goes to parot). The sentences were based on anomalous sentences of a phonological aphasia treatment program (Visch-Brink & Van Waard, Citation2018).
The semantic sentence judgment test (SEM C) is included to assess semantic awareness (10 items in total) and is related to temporal regions and the white matter tract Inferior Frontal Occipital Fasciculus (Almairac et al., Citation2015). The incorrect sentences violate semantic restrictions (e.g. De sigaar verveelde zich – the cigar was bored). The sentences were based on anomalous sentences of a lexical semantic treatment program BOX, developed for aphasic patients (Visch-Brink, Citation2019).
The (morpho)syntactic sentence judgment test (SYN B) is included to assess grammatical awareness and is related to frontotemporal regions (Hagoort, Citation2003; Indefrey et al., Citation2001). The incorrect sentences violate grammatical restrictions, including word order and verb inflection rules (e.g. Hij gaat geschilderd op de muur – He goes painted on the wall). Answers are scored correctly (1 point) when the participant answers correctly and within ten seconds.
Test administration
The first two authors (DS and EDW) trained groups of bachelor and master-level students in clinical linguistics (the Netherlands and Flanders) to administer the DIMA. The subtests are always administered in identical order. Practice items are included to ensure that participants understand the test. Visual stimuli are presented in a PowerPoint presentation or using E-prime software (laptop screen). The duration of test administration is 15–20 minutes. Participants are tested in a quiet environment. The answers are audio-recorded and transcribed in orthographic script. The accuracy of the scoring of the tests is checked by one of the first two authors and is based on qualitative terms agreed on a priori. Uncertainties regarding the correctness of test items are discussed in the group (DS, EDW, PM, EVB), leading to a consensus.
Statistics
As cut-off values for the different subtests for different groups of participants need to be defined, we opted for a categorical analysis of background variables using SPSS 24 (IBM Corp, Citation2016). Factorial ANOVAs were used to examine the main effects of gender, age, education and nationality on the test scores, as well as all two-way interaction effects.Footnote3 For age and education, the binary classification presented in was maintained. This classification was also guided by methodological considerations because it allows for sufficient individuals per subgroup of participants. The number of correct answers and reaction times, both treated as linear variables, were used as outcome variables.
After having analysed which background variables significantly had an impact on the test scores, a number of descriptive statistics per subgroup were performed, which were defined on the basis of these variables (i.e. age and educationFootnote4) as well as the total sample. Next to the mean, median and range, two cut-off values that can be used for clinical practice are reported. These are determined in agreement with current clinical practice: for clinically (mild) impaired, the score coincided with the 7th percentile (equal to standard deviation 1.5) and for pathologically (severe) impaired the 2nd percentile (equal to standard deviation 2.0). Percentiles are calculated using SPSS’s standard weighted average formula (HAVERAGE command).
Results
Normative study
Ceiling effects were observed for all subtests, with median values for the total sample (see Appendices A–C) being either perfect scores (i.e. 100%: 5/5 or 10/10) or almost perfect scores (i.e. 90%: 9/10). The overall subtest performance was well with means ranging between 4.11/5 (i.e. 82.2%, semantic odd-picture out, actions – SEM B) and 9.73/10 (i.e. 97.3%, repetition words – PHON A), with SEM B standing out as the most difficult test (<90% correct). The scores were not normally distributed, which has to be taken into account in the interpretation of the results.Footnote5 For example, significant differences between group means can in fact be rather small in terms of absolute values (see Appendices A–D).
Factorial ANOVAs showed that age has a significant impact on all the subtest scores (repetition non-words – PHON C; repetition sentences – PHON D; semantic odd-picture out, objects – SEM A; semantic odd-picture out, actions – SEM B: p < .001; Sentence completion SYN A: p < .01; and repetition compounds – PHON B: p < .05) and Total Reaction Times (all linguistic levels) – RTs (p < .001). The older group had lower scores and longer RTs. Also, education had a significant impact on the scores and RTs, albeit to a smaller extent than age. The 12+ education group scored higher on repetition compounds – PHON B, repetition non-words – PHON C (p < .01), repetition sentences – PHON D (p < .05), semantic odd-picture out, actions – SEM B and sentence completion – SYN A (p < .05), all RTs per linguistic level (SYN B RT, PHON E RT: p < .001; SEM C RT: p < .01) and syntactic accuracy – SYN B ACC (p < .01) and phonological accuracy – PhonE ACC (p < .05). For word repetition compounds – PHON B, a significant interaction effect of education and gender is observed, with education significantly impacting scores positively only for the female participants. Gender only had a significant main effect (p < .05) on syntactic accuracy – SYN B ACC. A significant interaction effect of gender and age was found for this subtest as well. Male participants scored higher than female participants, most notably in the age group 55 + .
Descriptive statistics and cut-off points for four subgroups (based on age and education) are provided in Appendices A–D. For some of the groups, the number of participants was too low to calculate the 2nd percentile cut-off (i.e. both 55+ groups for E-Prime tests, with 33 and 37 subjects respectively) and cut-off values are sensitive to outliers (especially the 2nd percentile), since most groups contain around 50 participants or fewer. The reported statistics confirm that education and age play a significant role for most of the subtests.
Case studies
We present two case studies, each with a different type of brain tumour in which clinical application of the DIMA is demonstrated. Both patients also received a selection of “classical” language and cognitive tests. Because mild language disturbances frequently occur due to slow tumour growth, the DIMA was administered in a low-grade glioma patient, before and after operation. A meningioma patient at one year after surgery was selected as cognitive impairments are known to exist in this patient group (Meskal et al., Citation2016).
Brain tumour patient: F.E
F.E., a right-handed 35-year-old male, with >12 years of education, working as a research assistant was admitted to the department of neurosurgery at the Erasmus MC Rotterdam with a left frontoparietal glioma near the arcuate fasciculus (see ). He suffered from problems aimed at word finding and pronunciation. He was elected for awake surgery (see ) because of tumour localization in language eloquent areas. We administered the DIMA, classical language tests including the shortened Token Test (TT) (Renzi De & Faglioni, Citation1978), Boston Naming Test (BNT) (Kaplan et al., Citation2001) and verbal fluency (category – CF and letter – LF) (Luteijn & Barelds, Citation2004; Schmand et al., Citation2008) and tests for verbal memory (Hopkins Verbal Learning Test), attention (Trail Making Test A) and concept-shifting (Trail Making Test B) (Benedict et al., Citation1998; Lezak et al., Citation2012). The language tests were used to determine the baseline language level and to select DuLIP intraoperative tests. A follow-up test protocol was administered around three months after surgery. shows the test-results.
Table 4. Test-scores F.E pre- and postoperatively.
Figure 1. (a-f) Pre- and postoperative Diffusion Tensor Images (DTI) and Magnetic Resonance Imaging (MRI) with reconstruction of the arcuate fasciculus (AF) in patient F.E.
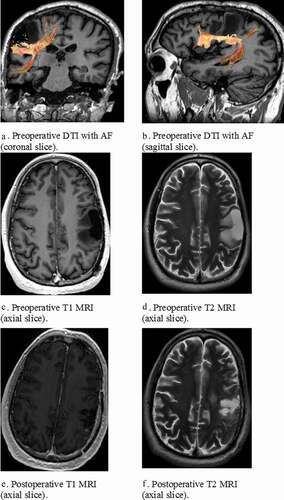
Patient F.E. was performed within the normal range on the classical language tests BNT, TT, CF (animals) and LF (z-score >-1.5). A mild deviation was observed in CF (professions) (z-score <-1.5). However, he was impaired in DIMA repetition due to hesitations, self-corrections, substitutions, false starts and deletion of words (e.g. compound: feestverlichting --> feestver [uhm] feestverlichting, non-word: frodusloka --> frorusloka, sentences: De Griek ontdekte vier nietjes in de band van zijn fiets --> De griek ontdekte vier nietjes in __ zijn band ___ ___ ___) (total score), 29/40 <2 percentiles) also seen in the total DIMA score (48/60, <2 percentiles). Mild impairments were detected in phonological and syntactic sentence judgment with respect to reaction time (<7 percentiles), but not accuracy (>7 percentiles). As for the other cognitive tests, verbal learning was intact at all subtests (direct recall, delayed recall and recognition), and Trail Making Tests A and B were intact (z-score >-1.5), whereas the interference score Trail Making Test BA was mildly impaired (z-score <-1.5).
Three months after surgery, F.E. was tested at the outpatient clinic. Unfortunately, due to time constraints, not all tests could be administered. Patient F.E. was still performed within the normal range on the classical language tests BNT and TT (>7 percentiles). He remained impaired in repetition (total score, 27/40 <2 percentiles) and in the total DIMA score (46/60, <2 percentiles). Verbal learning direct recall became mildly deviant (z <- 1.5). Trail Making A and B remained stable, andTrail Making BA was improved (z-score < −1.5). No clinical (mild) or pathological (severe) deterioration was observed (difference z-score 1.5/2 resp.). shows the test results.
Brain tumour patient: D.E
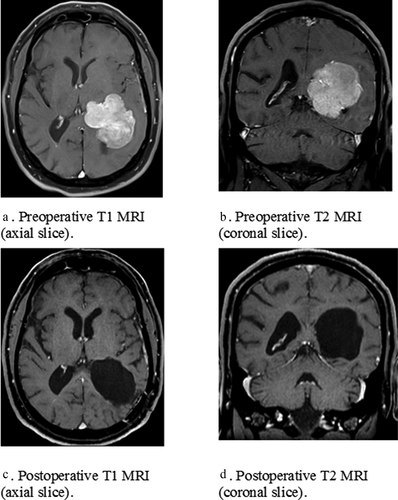
Patient D.E., an ambidextrous, 60-year-old male, with 12 years of education working as a trucker, entered the department of neurosurgery at the Erasmus MC Rotterdam with a left parieto-occipital meningioma (see ). He was elected for surgery due to persistent headaches and problems with spontaneous speech, word-finding, writing, and memory. Immediately postoperatively, language problems deteriorated in addition to the development of ideomotor apraxia and he was referred to clinical rehabilitation for several weeks. One year later, his language problems were still present. A language test battery was administered including the DIMA (except sentence judgment due to time constraints) and classical language tests: the shortened Token Test (Renzi De & Faglioni, Citation1978), verbal fluency (category – CF and letter – LF) (Luteijn & Barelds, Citation2004; Schmand et al., Citation2008) in addition to DuLIP object naming (Witte De et al., Citation2015). A short cognitive screening was also administered: short-term memory (WAIS digit span forward and backward), attention (TMT A) and concept-shifting (TMT B) (Lezak et al., Citation2012; Wechsler, Citation2008). shows the test results.
Table 5. Test-scores D.E.
Patient D.E. was performed within the normal range on classical language tests: TT, LF (>7 percentiles) but not on DuLIP object naming (<7 percentiles) and CF (z-score < −2.00). He was impaired in 2 phonological DIMA subtests (repetition non-words + sentences, <2 percentiles) due to hesitations, phonemic paraphasias, substitutions, false starts and deletion of a word (e.g. non-words: frodusloka --> flo fro dus jo la da, sentences: De piepende wielen hielden hem uit zijn slaap --> De pietende piepend_ wielers …). Other DIMA subtests, semantic odd-picture-out and sentence completion, were intact (>7e percentile). In the cognitive screening, D.E. showed impaired test-scores on all subtests of TMT (A, B, and BA, z < −2.00): attention and concept-shifting. Working memory (WAIS digit span) was intact (>7 percentile).
Discussion
For the first time, a Dutch test was developed and standardised to detect mild aphasia. A normative study was carried out in a large group of native Dutch-speaking healthy adults. Clinical application was demonstrated in two patients with a brain tumour pathology. One patient was treated with awake surgery and the other demonstrated persistent language complaints a year after classical brain tumour surgery (not awake). In both cases, subtests of the DIMA appeared to be sensitive in addition to some classical language tests. Our preliminary analyses indicate that DIMA has the potential to become a valuable addition to the standard clinical procedure concerning language assessment in individuals suffering from mild language disorders.
Normative data of DIMA
Ceiling effects were found for all tests, except for the semantic odd-picture-out actions (SEM B) as a mean score of less than 90% percentage correct was reached in healthy participants. Errors in this test consisted mainly of slow reactions exceeding the four seconds time constraint per slide. Slow reactions can be due to the underlying three-dimensional requirements of this test: it concerns verb retrieval, semantic judgment as well as visual (image) perception. It has already been demonstrated that the retrieval of verbs is more difficult than the retrieval of nouns, as argument structure and theta roles are implicitly activated (Bastiaanse & Van Zonneveld, Citation2004). This process in combination with semantic judgment may be too demanding to be executed within four seconds. We therefore conclude that this subtest is not suitable within the DIMA in its current form and needs to be revised (possibly without or with a longer a time constraint).
As expected, test performance differed significantly by age and (partly by) education: the higher the age, the lower the scores and the higher the education, the higher the scores. These effects are in agreement with our earlier DuLIP results and various other normative studies (Lezak et al., Citation2012; Witte De et al., Citation2015).
Clinical application of DIMA
The normative data were used to compare and to interpret the DIMA scores of individuals with potential language problems. Subtests of the DIMA, in particular, the repetition subtests (phonological level), appeared to be more sensitive than the BNT, the Token Test and Letter Fluency in a patient with a glioma. Moreover, for the first time, language disturbances were described in a patient with a meningioma, also evidenced with DIMA repetition subtests, in addition to object naming and category fluency, requiring semantic processing and word-finding. The relevance of phonology and its prognostic relation to the quality of verbal communication at the long run were already demonstrated in aphasic patients after stroke (El Hachioui et al., Citation2013). Therefore, it is important to monitor subtle changes in phonological production (see also (Sierpowska et al., Citation2017) as an indicator for the overall quality of language processing.
Although not sensitive in our cases, sentence completion could be helpful in the assessment of spontaneous speech (in context) where mainly not only syntactic but also semantic and phonological processing is addressed. Sentence completion tests are useful for recognizing dynamic aphasia, that is, intact performance on naming, repetition and comprehension, but disturbed spontaneous speech (Robinson et al., Citation2005; Satoer, Kloet et al., Citation2014), a common syndrome in patients with tumours near the medial frontal lobe.
Performance on the semantic odd-picture-out was not disturbed in our clinical cases. However, it is a complex test as it concerns a combination of word-finding and semantic judgment. Neither case had a lesion near the Inferior Frontal Occipital Fascicle, which is known to be involved in semantic processing (Moritz-Gasser et al., Citation2013; Sarubbo et al., Citation2015). The use of time constraints to the semantic odd-picture-out test and to sentence judgment aims to reflect quick communicative reactions in daily life and it also appeared to be relevant in relation to return to work (Moritz-Gasser et al., Citation2012). In this line of reasoning, the lack of time constraints in the Boston Naming Test could clarify normal performance in patient F.E., whereas the timed (4 seconds paradigm) DuLIP object naming test applied in D.E. was disturbed. Several studies have demonstrated the sensitivity of naming speed compared to accuracy (Moritz-Gasser et al., Citation2012; Ras et al., Citation2020).
The only “standard” language test that was impaired in both cases is category fluency, which was not unexpected (Rinehardt et al., Citation2014; Satoer et al., Citation2016). The sensitivity of this test can be explained by its multidimensional background depending on language (lexical retrieval), semantic memory, attention and executive functioning (switching and clustering) (Van den Berg et al., Citation2017). Therefore, we suggest to also administer a cognitive screening in order to disentangle non-verbal cognitive disturbances from language impairments. In our cases, impairments in attention, concept-shifting and verbal memory were found in conjunction with language deficits. In particular, F.E. showed impaired reaction time in sentence judgment (phonology and syntax), whereas a speeded attentive test Trail Making A was intact. D.E. was impaired in Trail Making, whereas a semantic test under time constraints (SEM A and B) was intact. This suggests an isolated slowness in the language domain in F.E. with the opposite scenario for D.E., which is isolated slowness in a non-language domain. It is important to reveal the underlying cognitive/linguistic defects to design a patient-tailored rehabilitation program.
To summarize, based on our case illustrations, we suggest to administer DIMA along with (speeded) object naming, verbal fluency and cognitive tests when language complaints are present, while intact scores are observed on a standard aphasia test, such as the Token Test.
Limitations of the study
Due to the small subgroups based on age and education, it was not possible to compute cut-off scores at the 2nd and 7th percentiles for all subtests (see Appendices C and D). If the cut-off score is absent, we advise the clinician to select the cut-off scores based on the total group (e.g. if PHON D, group 3, 2nd percentile would be needed, then PHON D Total group 2nd percentile should be chosen).
Clinical application was only done illustratively in two case studies. It is to be explored whether the DIMA subtests are sensitive at the group level in different neurological aetiologies. We used basic statistical analyses to establish norms and evaluate the influence of age, education and gender. For subsequent analyses, involving longitudinal data and a larger clinical sample, we will apply linear mixed models and potentially use Bayesian regression to more efficiently establish norms, building on the results of the present study (Voncken et al., Citation2021). Recently, in the field of mild cognitive impairment (MCI), two brief cognitive tools were developed and appeared to highly discriminate between MCI and matched healthy controls (Jørgensen et al., Citation2020).
Future directions
There is an urgent need felt by speech-language therapists for a new test for mild aphasia. Therefore, we are currently validating (a digital version of) the DIMA in a larger clinical population in the Netherlands and also internationally (see French DIMA adaptation of Clément et al. submitted). A more extensive neurolinguistic and cognitive test battery will allow us to identify specific profiles of impairments (language or more diffuse cognitive deficits). These behavioural data could be related to lesion location as obtained using neuroimaging techniques to improve our knowledge about anatomo-clinical correlations and sensitivity of the DIMA.
Additional tests for mild aphasia need to be developed, such as tests targeting discourse, reading and writing (e.g. summarizing). Also, with regard to the reported word-finding complaints, we suggest a sensitive speeded naming test, as reaction time (in ms) appears to be an important element to detect mild aphasia (Moritz-Gasser et al., Citation2012; Ras et al., Citation2020) and a subjective tool to detect mild language complaints.
Conclusion
In this study, a new Dutch test battery to detect mild aphasia was designed and standardised in a group of 221 healthy participants. Language production and comprehension deficits can be captured with DIMA at the most important linguistic levels (i.e. phonology, semantics and (morpho-)syntax). In brain tumour patients, the DIMA appeared to be sensitive to capture mild deficits in addition to some classic language tests. The DIMA is expected to be of added value in the standard language assessment in patients with neurological diseases.
Acknowledgments
We are grateful to students from the University of Groningen (the Netherlands) and Vrije Universiteit Brussels (Belgium), in particular: Pauline Cuperus, Mayon Bulthuis, Fleur Versteeg, Bram de Beer, Charlotte Abspoel and Saskia Mooijman. We are grateful to healthcare organization Zinn Zorg Haren for recruiting healthy test 75+ years participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1 The E-prime test (sentence judgment) has been administered in 233 healthy participants.
2 We defined 10 seconds as a maximal threshold based on a pilot study in healthy controls (6 and 8 seconds was too fast in our control group).
3 Handedness was not included as a variable because of the limited number of left-handed and ambidextrous participants.
4 Although nationality had a significant effect on the scores of a number of subtests (see Results section), we decided not to use it as a grouping variable, since, arguably, methodological rather than substantial reasons can explain this finding (i.e. potential differences in training, rating and selection procedures).
5 In spite of the violation of the normality assumption, we opted for parametric tests (i.e. factorial ANOVAs) since the sample size is sufficiently large to rely on the central limit theorem to assume normality of the sampling distribution (Field, Citation2009) and because these tests allow interaction effects to be modelled.
References
- Almairac, F., Herbet, G., Moritz-Gasser, S., de Champfleur, N. M., & Duffau, H. (2015). The left inferior fronto-occipital fasciculus subserves language semantics: A multilevel lesion study. Brain Structure & Function, 220(4), 1983–1995. https://doi.org/10.1007/s00429-014-0773-1
- Barker, M. S., Young, B., & Robinson, G. A. (2017). Cohesive and coherent connected speech deficits in mild stroke. Brain and Language, 168, 23–36. https://doi.org/10.1016/j.bandl.2017.01.004
- Bastiaanse, R., & Van Zonneveld, R. (2004). Broca’s aphasia, verbs and the mental lexicon. Brain and Language, 90(1–3), 198–202. https://doi.org/10.1016/S0093-934X(03)00432-2
- Bello, L., Gallucci, M., Fava, M., Carrabba, G., Giussani, C., Acerbi, F., Baratta, P., Songa, V., Conte, V., Branca, V., Stocchetti, N., Papagno, C., & Gaini, S. M. (2007). Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery, 60(1), 67–80; discussion 80–62. https://doi.org/10.1227/01.NEU.0000249206.58601.DE
- Benedict, R., Schretlen, D. L. G., & Brandt, J. (1998). The Hopkins verbal learning test-revised: Normative data and analysis of interform and test–retest reliability. Clinical Neuropsychologist, 12(1), 43–55. https://doi.org/10.1076/clin.12.1.43.1726
- Catani, M., & Mesulam, M. (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex, 44(8), 953–961. https://doi.org/10.1016/j.cortex.2008.04.002
- Cavanaugh, R., & Haley, K. L. (2020). Subjective communication difficulties in very mild aphasia. American Journal Of Speech-language Pathology / American Speech-Language-Hearing Association, 29(1S), 437–448. https://doi.org/10.1044/2019_AJSLP-CAC48-18-0222
- Cruice, M., Worrall, L., & Hickson, L. (2006). Perspectives of quality of life by people with aphasia and their family: Suggestions for successful living. Topics in Stroke Rehabilitation, 13(1), 14–24. https://doi.org/10.1310/4JW5-7VG8-G6X3-1QVJ
- Crum, R. M., Anthony, J. C., Bassett, S. S., & Folstein, M. F. (1993). Population-based norms for the mini-mental state examination by age and educational level. JAMA: The Journal of the American Medical Association, 269(18), 2386–2391. https://doi.org/10.1001/jama.1993.03500180078038
- Darley, F. L. H. N., Holland, A., & Linebaugh, C. (1980). Techniques in treating mild or high-level aphasic impairment. In Clinical Aphasiology panel (eds). Clinical aphasiology conference 1980 (pp. 338–345). Minneapolis: BRK Publishers.
- Dick, A. S., Garic, D., Graziano, P., & Tremblay, P. (2019). The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex, 111, 148–163. https://doi.org/10.1016/j.cortex.2018.10.015
- Duffau, H. (2014). The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex, 58, 325–337. https://doi.org/10.1016/j.cortex.2013.08.005
- Duffau, H., Capelle, L., Sichez, N., Denvil, D., Lopes, M., Sichez, J. P., Bitar, A., & Fohanno, D. (2002). Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain, 125(Pt 1), 199–214. https://doi.org/10.1093/brain/awf016
- Eiesland, E. A., & Lind, M. (2012). Compound nouns in spoken language production by speakers with aphasia compared to neurologically healthy speakers: An exploratory study. Clinical Linguistics & Phonetics, 26(3), 232–254. https://doi.org/10.3109/02699206.2011.607376
- El Hachioui, H., Lingsma, H. F., Van de Sandt-Koenderman, M. W., Dippel, D. W., Koudstaal, P. J., & Visch-Brink, E. G. (2013). Long-term prognosis of aphasia after stroke. Journal of Neurology, Neurosurgery, and Psychiatry, 84(3), 310–315. https://doi.org/10.1136/jnnp-2012-302596
- Fernandez Coello, A., Moritz-Gasser, S., Martino, J., Martinoni, M., Matsuda, R., & Duffau, H. (2013). Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. Journal of Neurosurgery, 119(6), 1380–1394. https://doi.org/10.3171/2013.6.JNS122470
- Field, A. (2009). Discovering statistics using SPSS (3rd ed.). Sage publications.
- Fontaine, D., Capelle, L., & Duffau, H. (2002). Somatotopy of the supplementary motor area: Evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery, 50(2), 297–303; discussion 303–295. https://doi.org/10.1097/00006123-200202000-00011
- Fox, S., Armstrong, E., & Boles, L. (2009). Conversational treatment in mild aphasia: A case study. Aphasiology, 23(7–8), 951–964. https://doi.org/10.1080/02687030802669526
- Gierut, J. A. (2007). Phonological complexity and language learnability. American Journal Of Speech-language Pathology / American Speech-Language-Hearing Association, 16(1), 6–17. https://doi.org/10.1044/1058-0360(2007/003)
- Goodglass, H., Kaplan, E., & Barresi, B. (2001). Boston diagnostic aphasia examination (3rd ed.). (BDAE-3) Pro-Ed.
- Graetz, S. D. B. P., & Willmes, K. (1991). Akense afasie test: Dutch. Swets & Zeitlinger.
- Hagoort, P. (2003). How the brain solves the binding problem for language: A neurocomputational model of syntactic processing. Neuroimage, 20(Suppl 1), S18–29. https://doi.org/10.1016/j.neuroimage.2003.09.013
- Harmon, T. G., Jacks, A., Haley, K. L., & Bailliard, A. (2019). Dual-task effects on story retell for participants With moderate, mild, or no aphasia: Quantitative and qualitative findings. Journal of Speech, Language, and Hearing Research : JSLHR, 62(6), 1890–1905. https://doi.org/10.1044/2019_JSLHR-L-18-0399
- Hickin, J., Mehta, B., & Dipper, L. (2015). To the sentence and beyond: A single case therapy report for mild aphasia. Aphasiology, 29(9), 1038–1061. https://doi.org/10.1080/02687038.2015.1010474
- Hickok, G. (2012). The cortical organization of speech processing: Feedback control and predictive coding the context of a dual-stream model. Journal of Communication Disorders, 45(6), 393–402. https://doi.org/10.1016/j.jcomdis.2012.06.004
- Hunting-Pompon, R., Kendall, D., & Bacon Moore, A. (2011). Examining attention and cognitive processing in participants with self-reported mild anomia. Aphasiology, 25(6–7), 800–812. https://doi.org/10.1080/02687038.2010.542562
- IBM Corp. (2016). IBM SPSS statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.
- Indefrey, P., Hagoort, P., Herzog, H., Seitz, R. J., & Brown, C. M. (2001). Syntactic processing in left prefrontal cortex is independent of lexical meaning. Neuroimage, 14(3), 546–555. https://doi.org/10.1006/nimg.2001.0867
- Jørgensen, K., Nielsen, T. R., Nielsen, A., Waldorff, F. B., & Waldemar, G. (2020). Validation of the brief assessment of impaired cognition and the brief assessment of impaired cognition questionnaire for identification of mild cognitive impairment in a memory clinic setting. International Journal of Geriatric Psychiatry, 35(8), 907–915. https://doi.org/10.1002/gps.5312
- Kaplan, E., Goodglass, H., & Weintraub, S. (2001). Boston naming test. Lippincott, Williams and Wilkins.
- Kerkman, H. B. R. H., Piepenbrock, R., & Van Rijn, H. (1993). The CELEX lexical database (CD-ROM). Centre for Lexical Information, Max Planck Institute for Psycholinguistics. https://www.worldcat.org/title/celex-lexical-database/oclc/81643377
- Keuleers, E., Brysbaert, M., & New, B. (2010). SUBTLEX-NL: A new measure for Dutch word frequency based on film subtitles. Behavior Research Methods, 42(3), 643–650. https://doi.org/10.3758/BRM.42.3.643
- Lezak, M., Howieson, D., Bigler, D., & Tranel, D. (2012). Neuropsychological assessment. Oxford University Press.
- Luteijn, F., & Barelds, D. P. F. (2004). Groninger Intelligentie Test 2 (GIT-2). Pearson.
- Meskal, I., Gehring, K., Rutten, G. J., & Sitskoorn, M. M. (2016). Cognitive functioning in meningioma patients: A systematic review. Journal of Neuro-oncology, 128(2), 195–205. https://doi.org/10.1007/s11060-016-2115-z
- Moritz-Gasser, S., Herbet, G., & Duffau, H. (2013). Mapping the connectivity underlying multimodal (verbal and non-verbal) semantic processing: A brain electrostimulation study. Neuropsychologia, 51(10), 1814–1822. https://doi.org/10.1016/j.neuropsychologia.2013.06.007
- Moritz-Gasser, S., Herbet, G., Maldonado, I. L., & Duffau, H. (2012). Lexical access speed is significantly correlated with the return to professional activities after awake surgery for low-grade gliomas. Journal of Neuro-oncology, 107(3), 633–641. https://doi.org/10.1007/s11060-011-0789-9
- Mozeiko, J., & Pascariello, A. (2020). How are SLPs managing services for people with mild aphasia? Journal of Communication Disorders, 84, 105983. https://doi.org/10.1016/j.jcomdis.2020.105983
- Murray, L. L., Holland, A. L., & Beeson, P. M. (1998). Spoken language of individuals with mild fluent aphasia under focused and divided-attention conditions. Journal of Speech, Language, and Hearing Research, 41(1), 213–227.
- Obermeyer, J. A., & Edmonds, L. A. (2018). Attentive reading with constrained summarization adapted to address written discourse in people with mild aphasia. American Journal of Speech-Language Pathology, 27(1S), 392–405.
- Pisoni, A., Mattavelli, G., Casarotti, A., Comi, A., Riva, M., Bello, L., & Papagno, C. (2018). Object-action dissociation: A voxel-based lesion-symptom mapping study on 102 patients after glioma removal. NeuroImage: Clinical, 18, 986–995. https://doi.org/10.1016/j.nicl.2018.03.022
- Pritchard, M., Hilari, K., Cocks, N., & Dipper, L. (2018). Psychometric properties of discourse measures in aphasia: acceptability, reliability, and validity. International Journal of Language & Communication Disorders, 53(6), 1078–1093.
- Psychology Software Tools. (2012). E-prime 2.0 (2.0.8.22 ed.). htto://www.pstnet.com
- Ras, P., Satoer, D., Rutten, G., Vincent, A., & Visch-Brink, E. (2020). Een sensitieve snelle benoemtest voor woordvindproblemen bij patiënten met een laaggradig glioom. English title: A sensitive speeded naming test for word finding difficulties in LGG patients. Stem -, Taal- En Spraakpathologie, 25, 15–29. https://doi.org/10.21827/32.8310/2020-15
- Renzi de, E., & Faglioni, P. (1978). Normative data and screening power of a shortened version of the Token Test. Cortex, 14(1), 41–49. https://doi.org/10.1016/S0010-9452(78)80006-9
- Rinehardt, E., Eichstaedt, K., Schinka, J. A., Loewenstein, D. A., Mattingly, M., Fils, J., Duara, R., & Schoenberg, M. R. (2014). Verbal fluency patterns in mild cognitive impairment and Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 38(1–2), 1–9. https://doi.org/10.1159/000355558
- Robinson, G., Shallice, T., & Cipolotti, L. (2005). A failure of high level verbal response selection in progressive dynamic aphasia. Cognitive Neuropsychology, 22(6), 661–694. https://doi.org/10.1080/02643290442000239
- Rofes, A., De Witte, E., Marien, P., & Bastiaanse, R. (2012). The verb in sentence context test (VISC). Rijksuniversiteit Groningen. http://eprints-phd.biblio.unitn.it/1572/1/151008Rofes_phdthesis.pdf
- Rofes, A., & Miceli, G. (2014). Language mapping with verbs and sentences in awake surgery: A review. Neuropsychology Review, 24(2), 185–199. https://doi.org/10.1007/s11065-014-9258-5
- Rofes, A., Spena, G., Talacchi, A., Santini, B., Miozzo, A., & Miceli, G. (2017). Mapping nouns and finite verbs in left hemisphere tumors: A direct electrical stimulation study. Neurocase, 23(2), 105–113. https://doi.org/10.1080/13554794.2017.1307418
- Ronnlund, M., Nyberg, L., Backman, L., & Nilsson, L. G. (2005). Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging, 20(1), 3–18. https://doi.org/10.1037/0882-7974.20.1.3
- Sarubbo, S., De Benedictis, A., Merler, S., Mandonnet, E., Balbi, S., Granieri, E., & Duffau, H. (2015). Towards a functional atlas of human white matter. Human Brain Mapping, 36(8), 3117–3136.
- Satoer, D., Kloet, A., Vincent, A., Dirven, C., & Visch-Brink, E. (2014). Dynamic aphasia following low-grade glioma surgery near the supplementary motor area: A selective spontaneous speech deficit. Neurocase, 20(6), 704–716. https://doi.org/10.1080/13554794.2013.841954
- Satoer, D., Vincent, A., Ruhaak, L., Smits, M., Dirven, C., & Visch-Brink, E. (2018). Spontaneous speech in patients with gliomas in eloquent areas: Evaluation until 1 year after surgery. Clinical Neurology and Neurosurgery, 167, 112–116. https://doi.org/10.1016/j.clineuro.2018.02.018
- Satoer, D., Visch-Brink, E., Dirven, C., & Vincent, A. (2016). Glioma surgery in eloquent areas: Can we preserve cognition? Acta Neurochirurgica (Wien), 158(1), 35–50. https://doi.org/10.1007/s00701-015-2601-7
- Satoer, D., Visch-Brink, E., Smits, M., Kloet, A., Looman, C., Dirven, C., & Vincent, A. (2014). Long-term evaluation of cognition after glioma surgery in eloquent areas. Journal of Neuro-oncology, 116(1), 153–160. https://doi.org/10.1007/s11060-013-1275-3
- Satoer, D., Vork, J., Visch-Brink, E., Smits, M., Dirven, C., & Vincent, A. (2012). Cognitive functioning early after surgery of gliomas in eloquent areas. Journal of Neurosurgery, 117(5), 831–838. https://doi.org/10.3171/2012.7.JNS12263
- Schmand, B., Groenink, S. C., & Van den Dungen, M. (2008). [Letter fluency: Psychometric properties and Dutch normative data] Letter fluency: Psychometrische eigenschappen en Nederlandse normen. Tijdschrift Voor Gerontologie En Geriatrie, 39(2), 64–76. https://doi.org/10.1007/BF03078128
- Sierpowska, J., Gabarros, A., Fernandez-Coello, A., Camins, A., Castaner, S., Juncadella, M., Moris, J., & Rodriguez-Fornells, A. (2017). Words are not enough: Nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. Journal of Neurosurgery, 126(2), 435–445. https://doi.org/10.3171/2016.2.JNS151592
- Snodgrass, J. G., & Vanderwart, M. (1980). A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology. Human Learning and Memory, 6(2), 174–215. https://doi.org/10.1037/0278-7393.6.2.174
- Swinburn, K., Porter, G., & Howard, D. (2005). Comprehensive Aphasia Test (CAT). Psychology Press.
- Van den Berg, E., Jiskoot, L. C., Grosveld, M. J. H., Van Swieten, J. C., & Papma, J. M. (2017). Qualitative assessment of verbal fluency performance in frontotemporal dementia. Dementia and Geriatric Cognitive Disorders, 44(1–2), 35–44. https://doi.org/10.1159/000477538
- Van Loon-Vervoorn, W. A. (1989). Eigenschappen van basiswoorden. Swets and Zeitlinger.
- Visch-Brink, E. (2019). BOX. Lexicaal Semantische Afasietherapie. Bohn Stafleu van Loghum.
- Visch-Brink, E., & Van Waard, D.-R. M. (2018). Fonologische Afasietherapie FIKS. Bohn Stafleu van Loghum.
- Voncken, L., Kneib, T., Albers, C. J., Umlauf, N., & Timmerman, M. E. (2021). Bayesian Gaussian distributional regression models for more efficient norm estimation. The British Journal of Mathematical and Statistical Psychology, 74(1), 99–117. https://doi.org/10.1111/bmsp.12206
- Wechsler, D. (2008). Wechsler adult intelligence scale - (4th ed.). Pearson.
- Weijters, T. (1983). Positiewoordenboek van de Nederlandse Taal. Swets and Zeitlinger.
- Witte De, E., Satoer, D., Robert, E., Colle, H., Verheyen, S., Visch-Brink, E., & Marien, P. (2015). The Dutch linguistic intraoperative protocol: A valid linguistic approach to awake brain surgery. Brain and Language, 140, 35–48. https://doi.org/10.1016/j.bandl.2014.10.011