Abstract
Introduction: Neoadjuvant endocrine treatment (NET) is a low-toxicity approach to achieve operability in locally advanced breast cancer, and to facilitate breast conservation in early breast cancer, particular in patients with highly estrogen receptor (ER) positive and HER2-negative disease. Here, we report the results obtained by neoadjuvant letrozole in patients with early breast cancer in a phase-II design.
Material and methods: A total of 119 postmenopausal women with ER-positive, HER2-negative operable breast cancer were assigned to four months of neoadjuvant letrozole before definitive surgery. Sentinel node or diagnostic fine needle aspiration cytology procedure was performed prior to treatment and the women were assessed prior, at two months, and before surgery with clinical examination, mammography and ultrasonography. Surgical specimens were examined for pathological response. Primary outcome was pathological and clinical response.
Results: The per protocol population consisted of 112 patients. Clinical response was evaluated in 109 patients and pathological response in 108. Overall a mean decrease in tumor size was 15% (p ≤ .0001). One patient had complete pathological response and 55% of patients had partial pathological response. ER at 100%, ductal subtype, tumor size below 2 cm and lymph node–negative status was significantly associated with a better response to NET and malignancy grade 3 with a poorer response to NET. One patient progressed during treatment and received neoadjuvant chemotherapy. Eight patients received adjuvant chemotherapy due to lack of response.
Conclusion: Neoadjuvant aromatase inhibitor therapy is an acceptable strategy in selected postmenopausal patients with ER-rich and HER2-negative early breast cancer with ductal histology and should be considered when chemotherapy either isn’t indicated or feasible.
Introduction
Neoadjuvant endocrine treatment (NET) is an low-toxicity approach to achieve operability in locally advanced breast cancer, particular in patients with highly estrogen receptor (ER) positive and HER2-negative disease [Citation1]. NET is furthermore increasingly used in patients with earlier-stage operable breast cancer for down staging to allow a less mutilating surgery; and as an research tool to obtain prognostic and predictive information using tumor response [Citation2]. Third generations aromatase inhibitors, such as letrozole, are in postmenopausal women preferred over receptor modulators such as tamoxifen due to higher response rates [Citation3–5]. Pathological complete response (pCR) has been the most commonly used endpoint in neoadjuvant trials, but a low pCR rate in ER-positive breast cancer has together with variable defined pCR criteria made the use of pCR challenging in patients with ER-positive breast cancer [Citation2]. A pCR following neoadjuvant chemotherapy (NCT) is associated with decreased mortality, but has not been validated as a surrogate endpoint for event-free or overall survival [Citation6]. Primary surgery continues to be the standard in patients with ER-positive and HER2-negative breast cancer and change in standard practice towards increasing use of NET will demand definitive survival data from phase-III trials comparing NET to adjuvant treatment or to NCT.
The Danish Breast Cancer Cooperative Group (DBCG) [Citation7] set up a phase-III trial comparing adjuvant letrozole for five years with neoadjuvant letrozole for four months combined with adjuvant letrozole to a total of five years following stable disease or response and combined with adjuvant chemotherapy following progressive disease. The original phase-III design was abandoned due to slow accrual and the trial was converted to a single arm phase-II trial. Here, we report the pathological and clinical results obtained by four months of neoadjuvant letrozole.
Material and methods
Study design
Initiated in 2009 the study was designed as a randomized phase-III study, at nine institutions in Denmark. In brief, at time of study initiation the primary study objective was to assess if letrozole was superior to surgery as primary therapy for early-stage ER-positive breast cancer in postmenopausal women. Eligible patients were randomized to definitive surgery followed by adjuvant letrozole for five years against letrozole for four months before definitive surgery followed by adjuvant letrozole to a total of five years in patients with responsive or stationary tumors. Patients with tumor progression should be considered for adjuvant chemotherapy.
On 10th October 2010, key changes were made due to poor accrual and the modified design allowed recruitment to neoadjuvant letrozole for four months in a phase-II study without randomization.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave written informed consent. The study and later amendment was approved by the National Committee on Health Research Ethics. The trial is registered on the ClinicalTrials.gov website NCT00908531.
Patients
Postmenopausal women with histological confirmed, invasive ER-positive, HER2-negative, operable breast cancer were eligible for the study. Eligible patients meet the following criteria: tumor size ≥1 cm, age ≥ 60 years, Eastern Cooperative Oncology Group score 0–2 and Charlson comorbidity index 0–2. Patients with prior cytotoxic treatment including aromatase inhibitors and patients with prior malignant disease were excluded. Patients was registered in the DBCG database and updated prospectively.
Treatment
Patients were treated with neoadjuvant letrozole 2.5 mg daily for four months. Treatment was discontinued if disease progression was suspected on ultrasonography, in case of severe toxicity, or if the patient withdrew consent.
Assessment
Patients underwent tumor evaluation upon study entry, after two months, and prior to surgery consisting of breast palpation, mammography and ultrasonography. Blood samples and core biopsies from the tumor along with the sentinel node (SN) procedure or diagnostic fine needle aspiration cytology (FNAC) from axillary lymph nodes were obtained before initiation of trial medication.
Surgery
After the neoadjuvant period, patients underwent mastectomy or breast conserving surgery. In case of lymph node involvement diagnosed prior to NET by SN or FNAC or in cases with progressive disease, patients were reassessed with SN prior to surgery. Patients who were initial lymph node positive underwent axillary lymph node dissection (ALND) irrespective of SN status prior to surgery and likewise in all cases with positive SN prior to surgery regardless of initial axillary status.
Endpoints
Following the amendment clinical response and pathological response, the original secondary endpoints, became the primary endpoints. Clinical response was assessed by ultrasonography and according to RECIST 1.1 defined as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [Citation8] and furthermore evaluated on a continues scale of relative tumor reduction from baseline until surgery. Pathological response was defined as loss of tumor cells ≥30% according to a modified Miller–Payne scale used by DBCG [Citation9]. On the modified scale response grade 1 equals no invasive tumor cells present; pathological complete response (pCR). Grade 2 more than 90% loss of tumor cells and grade 3 between 30% and 90% reduction in tumor cells are considered partial response. Grade 4 is defined as less than 30% loss of tumor cells and is considered no response.
Biomarkers was assessed centrally using international standards [Citation10–12]. The percentage of ER-positive cells by nuclear staining was registered and ER-positive status was defined as nuclear staining ≥10%. HER2-positive status was defined as HER2 3 + staining or HER2 gene amplification (ratio gen/cen ≥2) by FISH. In cases with multiple testing, the assessment with the highest count of ER, progesterone receptor (PgR) and Ki67 was used. In cases lacking central review or central review failed due to lack of tumor tissue, local assessment was used in the analysis.
Statistical analyses
Each factor was analyzed by univariate logistic regression to evaluate the association between the variable and response to NET. Factors were included in univariate models both categorical and continuously to investigate the functional form. Unknowns were included in separate categories. Odds ratio (OR) was estimated with a 95% confidence interval, using the category with highest number of patients as reference group, except for PgR to align it with ER. Multivariate analyzes including all characteristics were applied to assess the adjusted odd ratios. Association with outcome were tested with Pearson’s Chi-squared test, unknowns were excluded. Difference in relative tumor change was tested with the Wilcoxon Rank Sum test. Tumor change during NET was tested with a paired t-test. The distribution of ER, PgR and Ki67 did not meet the assumption of normality, and due to their heavy-tailed distribution the sign test was chosen to test for changes during NET. Level of significance was set to 5%. No new power calculations were made for the altered primary endpoints when the study was converted to a single arm study. All analyses performed with SAS Enterprise Guide version 7.11 (Cary, NC, USA).
Results
Between July 2009 and November 2012, a total of 119 patients were registered to receive letrozole, hereof 64 patients in the phase-III and 55 in the phase-II part. Two patients withdrew consent and two were tested HER2-positive after randomization, but before study initiation, thus 115 patients constituted the intention-to-treat population. An additional two patients were after initiation of letrozole diagnosed with a HER2-positive tumor and one was diagnosed with primary lung cancer and discontinued letrozole early and were excluded from the per-protocol population (n = 112), supplementary figure A. Patient’s basic characteristics are summarized in .
Table 1. Patients- and tumor characteristics of 112 Danish early Breast Cancer patients treated with neoadjuvant letrozole between 2009 and 2012.
In total, 111 (99%) patients completed four months of neoadjuvant letrozole as planned, one patient discontinued letrozole following progressive disease at the two months checkup.
Clinical response
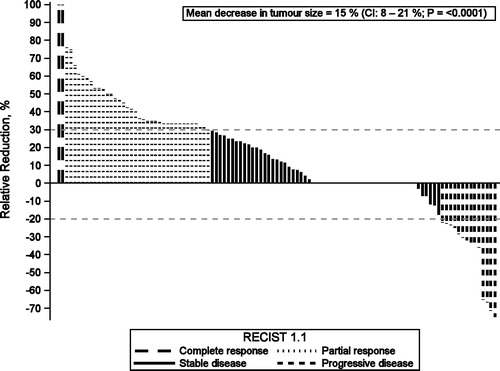
Clinical response to letrozole was available for 109 patients. Two had CR (2%), 36 had PR (33%), 57 had SD (52%) and 14 had PD (13%). Concerning changes in tumor size 63 (58%) had a 1 to 75 mm reduction in tumor size (mean 8.1 mm), 20 (18%) had an increase of 1–20 mm (mean 6.7 mm), and 26 (24%) had no change. Correspondingly, the relative changes for the 63 patients were a mean decrease in tumor size of 35% (2–100%). Twenty had a mean increase of 31% (3–75%). Overall, neoadjuvant letrozole lead to a mean decrease in tumor size of 15% (CI: 8–21%; p ≤ .0001) .
Figure 1. Relative reduction (%) in tumor size for each of the 109 patients with records of clinical outcome (one bar per patient). No change (n = 26) visualized by a straight line, negative values symbolizes growth (n = 20) and positive values tumor reduction (n = 63). Groups represent clinical response according to RECIST 1.1; Complete response (n = 2), Partial response (n = 36), Stable disease (n = 57) and Progressive disease (n = 14).
Pathological response
Pathological tumor response was available for 108 patients. One patient (0.9%) had pathological complete response (grade 1), seven (6%) had minimal residual disease (grade 2), 52 (48%) had moderate residual disease (grade 3), and 48 patients (44%) had no response (grade 4).
Fourteen patients were clinical node positive (n = 14) verified by FNAC, and 37 of the 97 who had a SN biopsy prior to NET were classified as SN positive, while 60 were classified as SN negative. One patient was not staged prior to treatment (). Of the 51 node-positive patients, 22 underwent a second SN prior to surgery and hereof 20 continued to ALND, 27 underwent ALND only and two were neither restaged nor underwent ALND. Of the 51 patients with initial lymph node involvement, 35 were verified as lymph node positive at time of surgery.
Of the 20 patients with progressive disease, 12 were initial lymph node positive and are included above. The remaining eight were initially node negative, five were restaged prior to surgery with SN, and were all still node negative and three were not restaged nor underwent ALDN. SN was removed prior to NET and none of 14 clinical node positive patients obtained a pCR in the axillary lymph nodes.
Biomarker response
ER status (n = 110) changed from mean 96 (10–100) to mean 93 (1–100), mean difference 3%, p = .01. PgR status (n = 51) changed from mean 56 (0–100) to 17 (0–100), mean difference 39%, p ≤ .0001. Ki67 index (n = 87) changed from mean 14% (0–90%) to 8% (1–95%), mean difference 6%, p ≤ .01. Two patients (1.8%) were reclassified as HER2-positive after the neoadjuvant period.
Factors associated with clinical and pathological response
The ORs for clinical response and pathological response and their association to tumor size, histological tumor type, grade, axillary status, Ki67, ER and PgR are summarized in . ER positivity at 100% was significantly associated with tumor reduction, however, not to pathological response. Tumors smaller than 20 mm had a better response both clinically and pathological than tumors above 20 mm. Ductal tumors had a significantly better pathological response to treatment than the other invasive subtypes, and node negative patients had a better clinical response. Malignancy grade 3 was associated with poorer response to NET than malignancy grade 1 and 2. None of the other tested variables were statistically significantly associated with neither clinical nor pathological outcome. When explanatory factors were tested as continuously they did not provide the model with a significant better fit and when tested in multivariate analyzes odds ratios were not significantly altered (data not shown). Neither changes in biomarkers nor clinical response did significantly associate with pathological response ().
Table 2. Univariate analyses for factors associated with clinical and pathological outcome after neoadjuvant treatment with letrozole in early breast cancer patients.
Table 3. Association between biomarker change and clinical tumor change to pathological outcome after neoadjuvant treatment with letrozole in early breast cancer patients.
Association between clinical and pathological response
Assessment of both clinical and pathological outcome was done in 106 patients. Thirty-nine patients (37%) had both clinical and pathological response. Nineteen (18%) had pathological response, but no reduction in the tumor size evaluated by ultrasonography. Twenty-four (23%) had ultrasonic regression, but no pathological response, and 24 patients (23%) had neither clinical nor pathological response.
Twenty of the 106 (19%) patient had an increase in tumor size on ultrasonography, 10 of them had pathological response. OR for pathological response if growth seen on ultrasonography is 0.62 (CI: 0.22–1.70), and for no change the OR is 0.4 (CI: 0.15–1.05) p = .16. Corresponding OR for clinical response according to RECIST 1.1; OR for pathological response if PD is 0.29 (0.08–1.04) and for SD 0.56 (0.24–1.32), p = .14 ().
Adjuvant treatment
In the adjuvant setting, eight (7%) patients received adjuvant chemotherapy and four of these resumed endocrine treatment after completion of chemotherapy. Adjuvant chemotherapy was justified by absence of a pathological response combined with clinically stabile or progressive disease (seven patients) or a mixed response with clinical but no pathological response (one patient). Endocrine treatment was continued without chemotherapy in 103 (92%) patients and one patient did not receive any adjuvant systemic treatment. In one patient, adjuvant tamoxifen was initiated following progression during NET and neoadjuvant chemotherapy. This patient developed bone metastasis within one year of the initial diagnosis. Adjuvant treatment regimens are shown in supplementary figure A and B according to clinical and pathological response.
Discussion
Our study shows that neoadjuvant aromatase inhibitor therapy in selected postmenopausal patients with ER-rich and HER2-negative early breast cancer leads to a modest clinical and pathological result in around half of the patients. Overall, only a 15% decrease in mean tumor size was achieved. One single patient (<1%) achieved a complete pathological response and overall 55% had partial pathological response. On the other hand, only one patient experienced progressive disease at the two months check up and went on to receive neoadjuvant chemotherapy; however it is important to note that the progression did not lead to the cancer being inoperable, due to locally advanced disease or dissemination. We confirm the heterogeneity in response to NET described by others, and in particular that preoperative finding of a 100% ER-positive tumor was associated with clinical response to NET [Citation6,Citation13]. Patients with ductal tumors achieved a better pathological response as compared to patients with other histological types. A lobular histology have previously been shown to predict not only a poorer response to NET but also to neoadjuvant chemotherapy [Citation14,Citation15]. Node-negative patients achieved a better response than node-positive patients and patients with a tumor with malignancy grade 3 achieved a reduced pathological response compared to malignancy grade 1 and 2. Overall, reduced tumor size detected by ultrasonography correlated well with pathological response, whereas tumor growth poorly predicted pathological response. A possible explanation is that an inflammatory response to NET can be mistaken as tumor growth.
The strengths of our study include prospectively planned diagnostic procedures, treatment, and follow-up according to national guidelines of a nationwide cooperative group. All endpoints were pre-planned.
This study has several potential limitations. As the study changed from a randomized phase-III study to a single arm phase-II study with no control group confounding issues may have been introduced. Tumor response and pCR were secondary endpoints in the original phase-III trial and became the primary endpoints when the trial was converted to a single arm phase-II study. Eight patients received chemotherapy following a less favorable outcome from NET, but we are unable to evaluate the possible benefits. Although, most patients with ER-positive and HER2-negative breast cancer do not benefit from chemotherapy, patients with endocrine non-responsive disease may potentially behave differently. Another limitation is the small sample size of this study resulting in limited power, especially when dividing patients into subgroups.
Aromatase inhibitors are the treatment option of choice; however, the optimal duration of treatment is yet to be determined. Letrozole for four months was used in this study and while the most substantial response is obtained during the first four months continuation beyond four months may result in further tumor shrinkage [Citation5,Citation16–18]. Arguably, NET for a pragmatic individualized timeframe will to a large extend maximize treatment benefit in responders but also increase the risk of clinical important treatment failure.
In conclusion, in postmenopausal patients with early breast cancer letrozole given in four months prior to surgery seems to lead to a limited clinical and pathological response but could be considered as a neoadjuvant treatment modality in selected cases where chemotherapy either is not indicated or feasible.
Signe_Skriver_et_al_Supplementary_material.zip
Download Zip (271.8 KB)Disclosure statement
One author has reported potential conflict of interest via manuscript central – Ann S Knoop: Lilly Eli (Fee), Pfizer (advisory board + teaching fee), Roche – advisory board and research grant).
None of the other authors have conflict of interests.
Additional information
Funding
References
- Horobin JM, Preece PE, Dewar JA, et al. Long-term follow-up of elderly patients with locoregional breast cancer treated with tamoxifen only. Br J Surg. 1991;78:213–217.
- Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–1516.
- Gnant M, Thomssen C, Harbeck N. St. Gallen/Vienna 2015: a brief summary of the consensus discussion. Breast Care. 2015;10:124–130.
- Dixon JM, Jackson J, Renshaw L, et al. Neoadjuvant tamoxifen and aromatase inhibitors: comparisons and clinical outcomes. J Steroid Biochem Mol Biol. 2003;86:295–299.
- Charehbili A, Fontein DBY, Kroep JR, et al. Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: a systematic review. Cancer Treat. Rev. 2014;40:86–92.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172.
- Christiansen P, Ejlertsen B, Jensen M-B, et al. Danish Breast Cancer Cooperative Group. Clin Epidemiol. 2016;8:445.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990. 2009;45:228–247.
- Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast Edinb Scotl. 2003;12:320–327.
- Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103:1656–1664.
- Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol 2010;28:2784–2795.
- Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3997–4013.
- Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:2342–2349.
- Delpech Y, Coutant C, Hsu L, et al. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br J Cancer. 2013;108:285–291.
- Loibl S, Volz C, Mau C, et al. Response and prognosis after neoadjuvant chemotherapy in 1,051 patients with infiltrating lobular breast carcinoma. Breast Cancer Res Treat. 2014; 144:153–162.
- Krainick-Strobel UE, Lichtenegger W, Wallwiener D, et al. Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer. 2008;8:62.
- Dixon JM, Renshaw L, Macaskill EJ, et al. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat. 2009;113:145–151.
- Carpenter R, Doughty JC, Cordiner C, et al. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat. 2014;144:569–576.
