Abstract
Background: The study of the intrinsic molecular subtypes of breast cancer has revealed differences among them in terms of prognosis and response to chemotherapy and endocrine therapy. However, the ability of intrinsic subtypes to predict benefit from adjuvant radiotherapy has only been examined in few studies.
Methods: Gene expression-based intrinsic subtyping was performed in 228 breast tumors collected from two independent post-mastectomy clinical trials (British Columbia and the Danish Breast Cancer Cooperative Group 82b trials), where pre-menopausal patients with node-positive disease were randomized to adjuvant radiotherapy or not. All patients received adjuvant chemotherapy and a subgroup of patients underwent ovarian ablation. Tumors were classified into intrinsic subtypes: Luminal A, Luminal B, HER2-enriched, Basal-like and Normal-like using the research-based PAM50 classifier.
Results: In the British Columbia study, patients treated with radiation had an overall significant lower incidence of locoregional recurrence compared to the controls. For Luminal A tumors the risk of loco-regional recurrence was low and was further lowered by adjuvant radiation. These findings were validated in the DBCG 82b study. The individual data from the two cohorts were merged, the hazard ratio (HR) for loco-regional recurrence associated with giving radiation was 0.34 (0.19 to 0.61) overall and 0.12 (0.03 to 0.52) for Luminal A tumors.
Conclusions: In both postmastectomy trials, patients with Luminal A tumors turned out to have a significant lower incidence of loco-regional recurrence when randomized to adjuvant radiotherapy, leaving no indication to omit postmastectomy adjuvant radiation in pre-menopausal high-risk patients with Luminal A tumors. It was not possible to evaluate the effect of radiotherapy among the other subtypes because of limited sample sizes.
Introduction
In the past two decades, there has been a growing focus on breast tumor heterogeneity, and genomic studies have defined five major intrinsic subtypes of importance: Luminal A, Luminal B, Basal-like, HER2-enriched, and Normal-like [Citation1,Citation2]. Intrinsic subtype was initially discovered by global-gene expression profiling, later a 50-gene profile (PAM50) was developed to be applied on formalin-fixed paraffin-embedded tumor tissue [Citation3] and developed as a qualitative assay that utilizes gene expression data, weighted together with clinical variables to generate a risk category and numerical score, to assess a patient's risk of distant recurrence of disease [Citation4].
The benefit of administering adjuvant radiation therapy (RT) in combination with adjuvant systemic chemotherapy was first demonstrated by two independent randomized trials: The British Columbia (BC) Randomized Radiation trial [Citation5] and the Danish Breast Cancer Group (DBCG) protocol 82b [Citation6]. After 10 years of follow up, women in the DBCG 82b trial assigned to chemotherapy plus RT had a 23% reduction in the rate of loco-regional recurrence (LRR) and a 9% reduction in mortality. A similar effect was demonstrated in the BC trial after 15 years of follow-up: patients treated with RT had a 33% reduction of LRR and a 29% reduction in mortality from breast cancer. These findings have had a profound impact on the indication of RT, and all high-risk patients, particularly those with node involvement more than four positive nodes, often receive adjuvant RT regardless of tumor characteristics and adjuvant systemic treatment. However, there is still a substantial portion of patients who will develop loco-regional relapse.
The study of the intrinsic molecular subtypes of breast cancer has revealed differences among them in terms of prognosis and response to chemotherapy and endocrine therapy [Citation7–13]. To a lesser extent, studies have tried to clarify if intrinsic subtypes may affect the effect of RT [Citation14–16].
Here, we aim to test if intrinsic subtypes have predictive impact on the effect of postmastectomy RT among young lymph-node–positive patients treated with systemic therapy. We first tested the intrinsic subtypes in the BC-trial and then validated our findings in a subset of patients from the DBCG 82b trial.
Patients and methods
Patient populations
A detailed description of the trials is found in Supplementary Table 1. The British Columbia (BC) trial enrolled 318 high-risk pre-menopausal patients from 1979 to 1986 [Citation5]. The inclusion criterion was pathological examined lymph-node-positive disease. All patients were treated with mastectomy and axillary dissection; adjuvant systemic treatment was cyclophosphamide-methotrexate and 5-flurouracil (CMF). The patients were randomized to postmastectomy RT or no RT. The dose of RT was 37.5 Gy (given in 16 fractions) through two tangential fields of the chest wall and 35 Gy through an anterior supraclavicular–axillary field with a posterior axillary boost. Finally, the internal mammary field received a dose of 35 Gy. All the fields were treated with cobalt-60. In addition patients with estrogen receptor positive tumor were sub-randomized to receive ovarian ablation induced by radiation and prednisolone. Twenty years clinical follow-up was obtained for all patients.
The DBCG 82b trial enrolled 1708 high-risk premenopausal patients from 1982 to 1989 [Citation6]. The inclusion criterion was lymph-node-positive disease and/or tumor size larger than 5 cm and/or invasion of tumor to surrounding skin or pectoral fascia. Like the BC trial, all patients had mastectomy, axillary dissection, received adjuvant CMF and were randomized to postmastectomy RT or no RT. The intended dose of RT was 55 Gy (given in 25 fractions) or 53 Gy (given in 22 fractions) delivered through an anterior electron field to the chest wall and internal mammary nodes and an anterior photon field against the supraclavicular, infraclavicular and axillary regions. The use of posterior axillary fields was advised in patients in whom the ratio of the anterior to posterior diameter was too large to limit the maximal absorbed dose. The closing date for the assessment of recurrence and vital status was 1 January, 2012. The potential median observation time was 25.1 years.
Gene expression profiling
A flowchart for the patients included is shown in Supplementary Figure 1. From the 318 enrolled patients in BC-trial, 159 (50%) had formalin-fixed paraffin-embedded (FFPE) tissues available for RNA extraction. The gene expression profiles of the PAM50 genes essential for intrinsic subtype classification were collected using Nanostring nCounter® system [Citation13,Citation17]. Expression of each gene was normalized relative to the expression of the five housekeeper genes including ACTB, MRPL19, PSMC4, RPLP0 and SF3A. In 145/159 cases, intrinsic subtyping by PAM50 was technically successful.
To enhance the comparability between the studies only material from DBCG 82b-patients with lymph-node positive disease were included. Fresh frozen tumor (FFT) samples were available from 83 patients. Extraction of mRNA from FFT and microarray analysis was performed as described previously [Citation18]. Whole gene expression profiles were obtained using the Applied Biosystem Human Genome Survey Microarray v2.0 (Applied Biosystem, Foster City CA). Microarray data was log2-transformed and quantile normalized. The 83 patients are part of a previously published data set (GEO: GSE24117).
In our 148 samples, we have 49 ER-positive patients, 74 ER-negative patients, and 25 patients without ER status. To match the clinicopathological heterogeneity of the training cohort, we use all the 49 ER positive patients, and randomly select 49 ER-negative from the 74 (subsetting), calculate the average expression of these 49 pairs of samples, and use this average expression as the normalization vector. Instead of row (gene) median centering, we subtract this normalization vector from the whole data matrix (148 samples) and use the residue as our normalized data matrix to perform PAM50 analysis [Citation3,Citation19].
Statistical analysis
Primary endpoint was local–regional relapse (LRR) for both trials, defined as relapse in the ipsilateral chest wall or an axillary, internal mammary or supraclavicular lymph-node. Cumulative incidence curves for LRR were plotted using a competing risk model, considering distant metastasis and death as competing events. Crude hazard ratios (HR) were computed for all end-points using Cox proportional hazards regression. Patient and clinicopathological parameters were compared by chi-squared test. All tests were two-tailed and p value <.05 were considered significant. All statistical tests were performed using STATA version 12.1 (Stata Corp, College Station, TX) and R 3.0.1.
Results
The patient and clinicopathological parameters had a similar distribution within the randomization arms in both studies (). Similar distributions were also found between patients from the study cohorts and the original BC and DBCG 82b trials, except for lymph node status and tumor size (DBCG 82b) and malignancy grades (BC) (Supplementary Table 2).
Table 1. Distribution of patient and clinicopathological parameters among patients from the BC and DBCG 82b study cohorts.
In the BC cohort, 39% patients were assigned as Luminal A (56/145), 16% Luminal B (23/145), 17% HER2-E (25/145), 19% Basal-like (27/145) and 10% as Normal-like (14/145). In the DBCG 82b validation-cohort of 83 patients, the distribution of intrinsic subtypes was similar to the BC-study (p = .94); 36% patients were assigned to Luminal A (30/83), 18% to Luminal B (15/83), 14% to HER2-E (12/83), 19% to Basal-like (16/83) and 12% to Normal-like (10/83) ().
Association of locoregional recurrence with radiation therapy, stratified by intrinsic subtypes
Overall, adjuvant RT decreased the locoregional recurrence significantly in both BC study and DBCG 82b study (). After 20 years of follow-up, the cumulative incidence proportion of LRR was 15% in the BC trial among women assigned to RT compared to 36% in the control group, giving a 22% (95CI: 8-36%) absolute risk reduction of LRR associated with RT (HR = 0.35 (0.17–0.72). A similar effect was demonstrated in the DBCG 82b trial, wherein the 20-years risk of LRR was 11% in the RT arm versus 37% in the control, giving a 26% (8-44%) absolute LRR risk reduction, HR = 0.30 (0.11–0.83). In the BC study, patients with Luminal A tumors had a significant reduced risk of LRR (4% in the RT arm vs. 31% in the control arm) when treated with RT, giving a 20-years absolute LRR risk difference of 27% (9–46%), HR = 0.17 (0.01–0.92) (). A reduction of LRR was also found among the Basal-like cases (). No statistically significant difference of LRR was observed between the radiation- and control arm at 20 years for patients with Luminal B and the few HER2-E tumors, respectively ().
Figure 1. Loco-regional recurrence as a function of randomization assignment to adjuvant postmastectomy radiotherapy (RT) within the study cohorts of the BC-trial (left) and the DBCG 82b-trial (right).
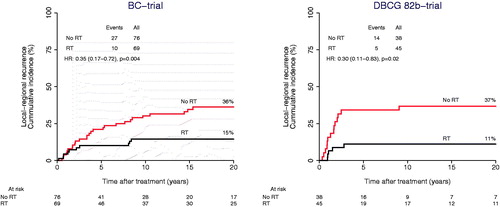
Figure 2. Loco-regional recurrence among patients with a Luminal A tumor as a function of randomization to adjuvant postmastectomy radiotherapy (RT) within the BC-trial (left) and the DBCG 82b-trial (right).
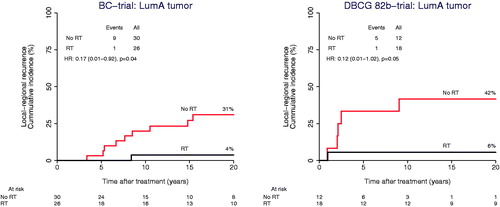
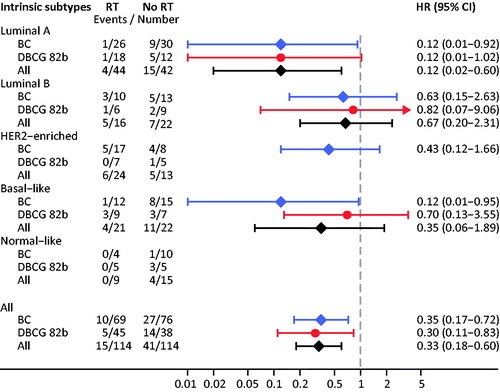
Figure 3. Forest plot showing the association between radiotherapy (RT) and the incidence of local-regional recurrence within different intrinsic subtype subgroups. BC-trial (Blue bar), DBCG 82b-trial (Red bar) and Merged data (Black bar). In subgroups with no events, HR cannot be estimated, nor can the overall HR.
In the DBCG 82b validation-cohort, among patients with Luminal A tumors, those who received RT had a significantly reduced risk of LRR (6% in the RT arm vs. 42% in the control arm). The 20-years absolute LRR risk difference was 36% (6-66%), HR = 0.12 (0.01–1.02) (). LRR risk differences did not reach statistical significance, with hazard ratio confidence intervals crossing 1.0 observed between the radiation and control arm at 20 years for patients with Luminal B, Basal-like and HER2-E tumors respectively ()
An overall estimate was calculated by merging the individual data from the two trials. Adjuvant RT reduced the incidence of LRR significant in the merged cohort (HR = 0.33 (0.18–0.60)) (). The overall estimate within each intrinsic subtype generally favorable outcome was observed in the RT arm. This benefit was greatest for Luminal A (HR = 0.12 (0.02–0.60)) and to a lesser extent for the Basal-like tumors. In the smaller Luminal B and HER2-E tumor subsets, no significant differences were observed in the risk of LRR between the RT – and control arm.
Discussion
We studied intrinsic subtyping of patients from the original post-mastectomy randomized radiation studies, BC- and DBCG 82b-trial, and confirmed that our translational study had demonstrated a reduced risk of LRR associated with RT among young high-risk patients treated with adjuvant systemic therapy as the original trials.
Our data supports that premenopausal lymph-node-positive patients with Luminal A tumors do benefit from postmastectomy RT. The other intrinsic subtypes generally have favorable outcomes in the RT arm, but because of the low numbers within each of these subgroups, it was not possible to prove the benefit of RT.
On a larger material from the DBCG 82b and 82-c trials, molecular subtypes were approximated by an immuno-histochemical panel of estrogen, progesterone and HER2 [Citation20]. Luminal A-like tumors had beneficial effect of RT (3% in RT arm vs. 32% in the control arm), and the 15-year overall survival was improved from 33% vs. 44%, HR = 0.78 (0.64–0.93). They did also find an equivalent (3% vs. 48%) association between RT and LRR among Luminal B-like tumors (defined as estrogen and/or progesterone receptor positive and HER2 positive).
However, Liu et al. found among post-menopausal lymph-node-negative patients receiving tamoxifen and randomized to ± RT, which intrinsic subtype classification had prognostic impact on the risk of developing local failure, but was not predictive of benefit from RT [Citation14]. Interestingly, the author observed no effect of RT among low-risk patients older than 60 patients with Luminal A tumors. These opposite findings of RT effects on Luminal tumors in our study may reflect the a priori prognosis of the different study populations. In the current study and the Kyndi paper, the cohort consisted of high-risk lymph-node-positive patients, whereas in the Liu study, all the patients had lymph-node negative disease. One could speculate that in the first case the less aggressive tumor type, Luminal A, had beneficial effect of RT, whereas it is more doubtful with the more aggressive tumor subtypes, because those patients suffer from distant metastases. Luminal A tumors are local slow growing and have such a good prognosis after adjuvant systemic treatment, which the patients do not develop LRR and as a consequence do not obtain any beneficial effect from RT. It is also likely that the Luminal A tumors harbor further heterogeneity in regard to cellular radiosensitivity. In a previous study, a differential effect of postmastectomy RT in Luminal A tumors has been observed, when examining a 7-gene profile predictive of response to postmastectomy RT (DBCG-RT profile) [Citation16].
Recently, Sjøgren et al also reported no predictive value of intrinsic subtype related to RT among lymph-node negative patients randomized to ± RT after breast conservative surgery, but low-risk patients with Luminal A tumors had beneficial effect of RT [Citation15]. The inconsistent results of the subanalysis restricted to older low-risk Luminal A patients could be due to all patients receiving tamoxifen in the paper from Lui et al, whereas only 8% received adjuvant systemic treatment in the Sjøgren study.
A limitation of our present study was the low number of patients with available material in comparison with the total number of patients accrued in the original trial. Another limitation is that at the time of enrollment in the trials the standard treatment for all premenopausal high-risk patients was adjuvant CMF; if the patients were treated today they would have received anthracycline and/or taxane-based systemic treatment. We also acknowledge that the expression profiles were obtained from two different technology platforms: the expression profiles from the BC-study were based on FFPE-derived RNA analyzed on the Nanostring nCounter whereas that from the DBCG 82b trial was based on frozen-tissue derived RNA applied to whole genome microarrays. However, despite different gene technologies applied, the PAM50 intrinsic calls and their association of outcome to radiation therapy were similar in the two trials. Hence our results suggest that PAM50 assignments are robust across technology platforms and patient populations, as Luminal A tumors did benefit from RT in both studies.
In summary, we demonstrate using material from two independent randomized trials that postmastectomy RT significantly decreases local-regional recurrences among pre-menopausal high-risk patients treated with chemotherapy. Because of limited material, when breaking into the major intrinsic molecular subtypes, it was only possible to evaluate the effect of RT among patients with Luminal A tumors. In both trials, RT lowered the risk of LRR, and thus there is no molecular subtype indication to omit adjuvant chest wall radiation in pre-menopausal high-risk patients with Luminal A tumors treated by mastectomy.
Tinne_Laurberg_et_al._Supplementary_material.pdf
Download PDF (161.5 KB)Acknowledgments
We thank Sherman Lau at the Genetic Pathology Evaluation Centre (Vancouver) for his assistance in RNA extraction of samples from the BC trial.
Disclosure statement
C.M.P. is equity stock holders of, and T.O. consults for BioClassifier LLC. C.M.P., T.O. and M.C.U.C. have filed a patent on the PAM50 assay. T.T, J.A., S.M, T.S. and J.O holds a patent for a gene signature associated with efficacy of radiotherapy in breast cancer (international patent publication no. WO 2013/132354A2). The patent is not related to the present work.
Additional information
Funding
References
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752.
- Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874.
- Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167.
- Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345.
- Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962.
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish breast cancer cooperative group 82b Trial. N Engl J Med. 1997;337:949–955.
- Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–5232.
- Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–1511.
- Prat A, Bianchini G, Thomas M, et al. Research-based PAM50 subtype predictor identifies higher responses and improved survival outcomes in HER2-positive breast cancer in the NOAH study. Clin Cancer Res. 2014;20:511–521.
- Cheang MC, Voduc KD, Tu D, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res. 2012;18:2402–2412.
- Chia SK, Bramwell VH, Tu D, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res. 2012;18:4465–4472.
- Nielsen TO, Jensen M-B, Burugu S, et al. High-Risk Premenopausal luminal A breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: results from the DBCG77B clinical trial. Clin Cancer Res. 2017; 23:946–953.
- Jørgensen CLT, Nielsen TO, Bjerre KD, et al. PAM50 breast cancer intrinsic subtypes and effect of gemcitabine in advanced breast cancer patients. Acta Oncol. 2014;53:776–787.
- Liu F-F, Shi W, Done SJ, et al. Identification of a low-risk luminal A breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol. 2015;33:2035–2040.
- Sjöström M, Lundstedt D, Hartman L, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol. 2017;35:3222–3229.
- Tramm T, Kyndi M, Myhre S, et al. Relationship between the prognostic and predictive value of the intrinsic subtypes and a validated gene profile predictive of loco-regional control and benefit from post-mastectomy radiotherapy in patients with high-risk breast cancer. Acta Oncol. 2014;53:1337–1346.
- Fortina P, Surrey S. Digital mRNA profiling. Nat Biotechnol. 2008;26:293–294.
- Myhre S, Mohammed H, Tramm T, et al. In silico ascription of gene expression differences to tumor and stromal cells in a model to study impact on breast cancer outcome. PLoS One. 2010;5:e14002.
- Zhao X, Rødland EA, Tibshirani R, et al. Molecular subtyping for clinically defined breast cancer subgroups. Breast Cancer Res. 2015;17:29
- Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish breast cancer cooperative group. J Clin Oncol. 2008;26:1419–1426.
