Abstract
Background
The aim of this retrospective registry-based Danish patterns of care study was (1) to evaluate the real-world utilisation of short-course hypofractionated radiotherapy (HFRT) in glioblastoma (GBM) patients over time, and (2) to evaluate the impact of short-course HFRT by assessing trends in multimodality treatment utilisation, compliance, and outcome.
Material and methods
Data of all adults with newly diagnosed pathology-confirmed GBM between 2011 and 2019 were extracted from the nationwide Danish Neuro-Oncology Registry. Short-course HFRT was defined as a fraction size of > 2 Gy to a planned dose of > 30 Gy. Patterns of care were assessed. To analyse trends in the assignment to short-course HFRT, and in radiotherapy (RT) compliance, multivariable logistic regression was applied. To analyse trends in survival, multivariable Cox regression was used.
Results
In this cohort of 2416 GBM patients, the utilisation of short-course HFRT significantly increased from ca. 10% in 2011 to 33% in recent years. This coincided with the discontinued use of palliative regimens and a decreased use of conventional fractionation. The proportion of patients proceeding to RT remained stable at ca. 85%. The proportion of patients assigned to chemoradiotherapy (CRT) remained stable at ca. 60%; the use of short-course hypofractionated CRT increased with ca. 10%, while the use of conventionally fractionated CRT decreased with ca. 10%. Compliance with conventionally fractionated and short-course HFRT was respective 92% and 93%, and significantly increasing in recent years. In the complete cohort, the median overall survival remained stable at ca. 11 months. Assignment to short-course HFRT was independently associated with shorter survival.
Conclusion
In Denmark, the use of short-course HFRT significantly increased in recent years. Nonetheless, the overall utilisation of RT and chemotherapy did not increase on a population level. Nor did survival change. In contrast, compliance with both conventionally fractionated RT and short-course HFRT increased.
Background
Glioblastoma (GBM) is the most common and most aggressive malignant brain tumour in adults [Citation1]. Standard-of-care consists of maximal safe resection and postoperative chemoradiotherapy, followed by adjuvant chemotherapy [Citation2]. Traditionally, radiotherapy dose is prescribed in 1.8–2 Gy per fraction, one fraction per day, and five fractions per week, which is called conventionally fractionated radiotherapy (RT). Using conventional fractionation, a total dose of 60 Gy is administered over 6–7 weeks, also known as long-course RT for GBM [Citation2–4]. Long-course RT is combined with concomitant and adjuvant chemotherapy (i.e. temozolomide), since the publication of the landmark paper by Stupp et al. in 2005 [Citation5]. This combination resulted in a median overall survival (OS) of approximately 15 months in clinical trials [Citation5,Citation6]. Yet, in patients with poor prognostic factors, in particular an elderly age [Citation7], who are usually not selected for clinical trials, the median OS drops to only a few months [Citation8–11]. This is the reason why long-course RT may not be considered justifiable. Hence, shorter RT courses have been developed for poor prognosis GBM patients.
Short-course RT in GBM employs hypofractionation, i.e. the use of doses per fraction higher than 2 Gy. Also, a lower total radiation dose is used, both contributing to the reduced treatment time of 2–3 weeks instead of 6 weeks. Comparable survival outcomes for GBM patients treated with short-course hypofractionated radiotherapy (HFRT) or long-course RT were found in two randomised trials including patients aged 60 years or older [Citation8,Citation12]. The first of those trials was published in 2004 by Roa et al. [Citation12]. The second trial, named the Nordic trial, was published in 2012 and accrued patients in among other Danish centres between 2000 and 2009. In their subgroup analysis including only patients older than 70 years (n = 81), the median OS was significantly longer with short-course HFRT (7.0 months) vs long-course RT (5.2 months) [Citation8]. Of note, in both trials RT was not combined with chemotherapy, and patients eligible for combined modality treatment were excluded. Later in 2017, Perry et al. published a phase 3 trial including patients aged 65 years or older and randomising between short-course HFRT with or without chemotherapy. In this trial, a survival benefit was shown with the combination (9.3 vs 7.6 months with radiotherapy-only) [Citation13]. Randomised trials comparing short- and long-course chemoradiotherapy (CRT) are being conducted but have not yet been published to the best of our knowledge.
The abovementioned trials thus generated new high-level evidence for the use of short-course HFRT in poor prognosis GBM patients, defined by elderly age in these trials. Short-course HFRT for poor prognosis patients has thus been incorporated in the Danish (dnog.dk) and EANO guidelines [Citation2,Citation14,Citation15], first established in 2008 and 2014, respectively. Consequently, the real-world utilisation of short-course HFRT is expected to increase. In addition, short-course HFRT is considered a more feasible and patient-friendly regimen, potentially resulting in more patients proceeding to and complying with RT. Moreover, the outcome may hypothetically be positively affected. Thus far, the real-world utilisation of short-course HFRT has been sparsely studied in small non-nationwide populations [Citation11,Citation16], and trends in the utilisation over time have been exclusively studied in selected elderly GBM patients [Citation17,Citation18]. To fully assess any potential positive effect of emerging evidence supporting the use of short-course HFRT, it is essential to study an unselected GBM population over time.
The first aim of the present Danish patterns of care study was thus to evaluate the real-world utilisation of short-course HFRT in an unselected nationwide GBM population over time. The second aim was to evaluate the impact of short-course HFRT by assessing trends in multimodality treatment utilisation, compliance, and outcome.
Material and methods
Nationwide patterns of care study
This is a retrospective registry-based study of pathology-confirmed GBM patients, operated in Denmark between 2011 and 2019. Follow-up was completed on 8 April 2021.
Neuro-oncology in Denmark
In Denmark, neuro-oncological treatment (surgery, RT, and chemotherapy) has been traditionally centralised to four university hospitals. Free access has been provided by the Danish government. National guidelines regarding neuro-oncological care have been established by the Danish Neuro-Oncology Group since 2008. These have been following the EANO guidelines to a large degree.
Danish Neuro-Oncology Registry
The Danish Neuro-Oncology Registry (DNOR) was used to identify newly diagnosed GBM patients and to extract all relevant data. The DNOR is a nationwide cancer database, established in 2009 and monitored by the Danish Clinical Quality Programme. For each GBM patient, data on diagnosis and treatment have been collected at the neurosurgery and oncology departments. The assessment of MGMT promotor methylation status at initial diagnosis has been incorporated nationwide since 2015. IDH mutation status has not been systematically obtained throughout the cohort period. Mortality data in the DNOR have been automatically obtained from the Danish Civil Registration System. In an evaluation study, very high completeness of patient registration in the DNOR and good validity of the registered data have been shown [Citation19].
Selection of patients
All adults (≥18 years at the time of first surgery) with newly diagnosed, pathology-confirmed GBM in accordance with the World Health Organisation (WHO) criteria at the time (SNOMED codes M94403, M94404, M94406, M94407, M94409, M94413, M94423), were selected. There were no exclusion criteria applied.
Extent of surgery
In the DNOR, codes for the surgical procedure and the result of the direct postoperative MRI have been registered since 2013. Extent of surgery (EOS) was deducted from these variables and categorised as follows [Citation20]: complete resection if the MRI showed no residual contrast-enhancing tumour; near total resection if the MRI showed non-measurable residual contrast-enhancing tumour (diameter < 10 mm); partial resection if a code for resection was registered in combination with measurable contrast-enhancing residual tumour (both diameters ≥ 10 mm) on MRI if available; biopsy if a code for biopsy was registered in combination with a measurable contrast-enhancing residual tumour on MRI if available; unknown extent of surgery if both surgical code and MRI result were missing. In the case of direct complementary surgery, surgery was categorised to the largest extent reported.
Radiotherapy regimens
RT was considered planned if registered as such and if any RT variable was completed. RT was classified into four types (prescribed dose): conventionally fractionated based on a fraction size of 1.8–2 Gy and/or > 15 fractions; short-course hypofractionated based on a fraction size of > 2 Gy to a planned total dose of >30 Gy [Citation8,Citation12]; palliative based on a fraction size of > 2 Gy to a planned dose of ≤ 30 Gy; unknown type based on ≤ 15 fractions without registered details on fraction size and total dose.
Chemotherapy, concomitant and adjuvant
Chemotherapy was considered planned if any chemotherapy variable was completed. Chemotherapy was considered as concomitant treatment if registered as such and/or if it overlapped with RT. It was considered an adjuvant treatment if registered as such, and/or if it not overlapped with RT.
Statistical analysis
The utilisation of RT and chemotherapy was measured by planned treatment, and compliance with RT and chemotherapy was measured by administered treatment. Utilisation and compliance were calculated as the proportion of respective eligible patients.
To analyse trends, patients were grouped per year of primary surgery. Multivariable logistic regression was used to assess the association of the year of surgery to RT-type assignment and to RT compliance while correcting for potential confounders. In secondary analyses, the variables MGMT promotor methylation status and IDH mutation status were added as potential confounders. Only patients from the years 2013 to 2019 were included due to missing data regarding the direct postoperative MRI in 2011–2012 determining the EOS.
OS, measured from (first) surgery to death or the last follow-up, was estimated using the Kaplan–Meier method. An event was defined as death from any cause, with patients censored at the last follow-up. Multivariable cox regression analysis was used to analyse the association between the year of surgery and survival while correcting for potential confounders. In a secondary analysis, the variable IDH mutation status was added. Only patients from the years 2015 to 2019 were included due to a lack of systematically obtained MGMT promotor methylation status from 2011–2014. All statistical analyses were performed using SPSS. Significance was set at p < 0.05.
Results
Study population
The Danish cohort consisted of 2416 newly diagnosed adult GBM patients. No patient was excluded. The number of patients per year fluctuated between 230 and 299 without a trend (p= 0.5, ).
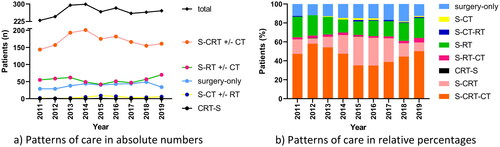
Figure 1. Patterns of care over time in (a) absolute numbers and (b) relative percentages in the Danish glioblastoma cohort 2011–2019 (n = 2416). In Figure 1(a), no subdivision between S-CRT followed or not followed by adjuvant CT, S-RT followed or not followed by adjuvant CT, and S-CT followed or not followed by RT, is made. Note in 2015 and 2016 known registration gap in adjuvant chemotherapy at one hospital, explaining the lower percentage of S-CRT-CT in those years to an unknown extent. S: surgery; CT: chemotherapy; RT: radiotherapy; CRT: chemoradiotherapy.
Patient, tumour, and treatment characteristics
The median age was 66.3 (range 18.1–93.4) years at diagnosis. The majority of patients (69%) had a WHO performance status (PS) of 0–1 at diagnosis (). MGMT promotor methylation status was methylated in 32% of patients, unmethylated in 36%, and unknown in 32%. From 2015 to 2019, MGMT promotor methylation status was methylated in 45% of patients and unmethylated in 51%.
Table 1. Patient, tumour, and treatment characteristics of 2416 glioblastoma patients in the Danish cohort 2011–2019, and specified for patients assigned to conventionally and short-course hypofractionated radiotherapy.
Patterns of care
Between 2011 and 2019, 15% of patients underwent surgery only (). The proportion of patients undergoing surgery only increased from 13% in 2011 to 19% in 2018, after which it dropped to 13% again in 2019 (). This was consistent with the proportion of patients receiving postoperative RT, fluctuating between 81% and 88%. The most frequently used treatment was surgery in combination with postoperative concomitant CRT ± adjuvant chemotherapy (64% of patients), peaking at 67% in 2014, and returning to 59% in 2019.
Utilisation of conventionally fractionated RT and short-course HFRT
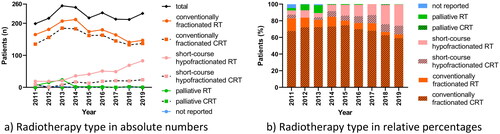
In patients treated with direct postoperative RT, the utilisation of conventionally fractionated and short-course HFRT was 78% and 19%, respectively (3% palliative regimen, 1% regimen not reported). Over time, the utilisation of conventional fractionation decreased from 83% in 2011 to 64% in 2019 (). This coincided with increased utilisation of hypofractionation from 10% in 2011 to 36% in 2019.
Figure 2. Utilisation of radiotherapy regimens for patients treated with postoperative radiotherapy over time in (a) absolute numbers and (b) relative percentages in the Danish glioblastoma cohort 2011–2019 (n = 2026). CT: chemotherapy; RT: radiotherapy; CRT: chemoradiotherapy.
In multivariable analysis, treatment assignment to short-course HFRT was significantly more likely in each year between 2014 and 2019 compared with 2013 (ORs of 4.1–19.3, Supplementary Table 1). In addition, treatment assignment to short-course HFRT was significantly more likely with higher age, lower PS before the start of RT, lacking MGMT promotor methylation, multifocal tumour, tumour crossing midline, and biopsy as EOS.
Utilisation of concomitant and adjuvant chemotherapy
Overall, the utilisation of concomitant chemotherapy was 64% in patients treated with postoperative RT, with the lowest percentages in 2018 (59%) and 2019 (59%) (). In patients assigned to postoperative RT, the use of conventionally fractionated CRT peaked at 75% in 2015, decreasing to 59% in 2019 (). The use of hypofractionated CRT steadily increased over time to a maximum of 10% in 2019.
In patients who started conventionally fractionated RT, concomitant chemotherapy was utilised in 89% (). This increased from 82% in 2011 to 94% in 2018 and 93% in 2019 (). Of the patients who started conventionally fractionated CRT, 72% proceeded to adjuvant chemotherapy overall, and 86% in 2019.
Figure 3. Treatment utilisation of and compliance with multimodality treatment, in patients treated with (a) conventionally fractionated and (b) short-course hypofractionated postoperative radiotherapy, in the Danish glioblastoma cohort 2011–2019.
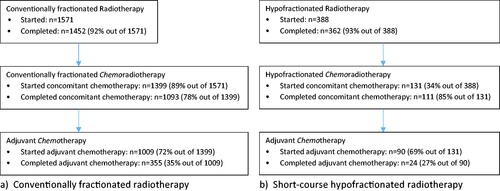
Figure 4. Utilisation of concomitant chemotherapy over time in patients treated with (a) conventionally fractionated (n = 1571) and (b) short-course hypofractionated postoperative radiotherapy (n = 388) in the Danish glioblastoma cohort 2011–2019.
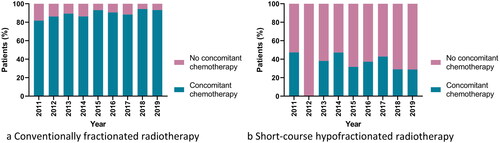
In patients who started short-course HFRT, concomitant chemotherapy was utilised in 34% (), with no clear trend over time (). In the patients who started hypofractionated CRT, 69% proceeded to adjuvant chemotherapy overall, with no clear trend.
Compliance with conventionally fractionated RT and short-course HFRT
Overall, RT compliance was 92% and 93% in patients planned for conventional and hypofractionated RT, respectively. With conventional fractionation, RT compliance fluctuated over the years between 87% and 99% with an especially high compliance in 2017–2019 (). With short-course HFRT, RT compliance fluctuated over the years between 86% and 100% with no clear trend ().
Figure 5. Compliance with postoperative radiotherapy over time in patients treated with (a) conventionally fractionated (n = 1571) and (b) short-course hypofractionated regimens (n = 388) in the Danish glioblastoma cohort 2011–2019. Numbers in (a) and (b) include both patients treated with radiotherapy-only and those treated with chemoradiotherapy.
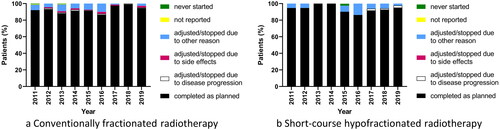
In multivariable analysis, RT compliance was significantly more likely in each year between 2017 and 2019 compared with 2013 (ORs of 0.2–0.3, Supplementary Table 2). RT regimen was not significantly associated with compliance.
Compliance with concomitant and adjuvant chemotherapy
In patients assigned to conventionally fractionated CRT, 78% completed concomitant chemotherapy as planned (), with no clear trend. Concomitant chemotherapy was adjusted/stopped due to disease progression in 0.5%, side-effects in 11%, other reasons in 7%, never started in 0.1%, and not reported in 3% of patients. Adjuvant chemotherapy was completed as planned in 35% of patients (no clear trend), and was stopped/adjusted due to disease progression in 44%, side effects in 8%, other reasons in 5%, and not reported in 8% of patients.
Of patients assigned to hypofractionated CRT, 85% completed concomitant chemotherapy as planned (); 100% in 2011–2015, thereafter dipping to 60% in 2018 and increasing to 88% in 2019. Concomitant chemotherapy was adjusted/stopped due to disease progression in 1%, side effects in 1%, other reasons in 13%, and never started in 1% of patients. Adjuvant chemotherapy was completed as planned in 27% of patients (no clear trend), and was stopped/adjusted due to disease progression in 37%, side effects in 13%, other reasons in 18%, and not reported in 6% of patients.
Survival
In the complete cohort, at a median follow-up time of 11 (range 0–122) months, 2258 (94%) patients had died, 155 were alive, and 3 were lost to follow-up. Median OS was 11.0 (95% CI 10.4–11.6) months, with 1- and 2-year survival rates of respective 48% and 19%. In patients assigned to conventionally fractionated RT, the median OS was 15.0 (95% CI 14.4–15.6) months. In patients assigned to short-course HFRT, the median OS was 7.0 (95% CI 6.5–7.5) months (Supplementary Figure 1(a–c)).
In multivariable analysis, survival did not significantly change over time (Supplementary Table 3). Assignment to short-course HFRT was independently associated with a shorter survival compared with assignment to conventionally fractionated RT (HR 1.8, 95% CI 1.4–2.2, p <0.001). Known prognostic factors for survival were confirmed.
Discussion
In this nationwide Danish patterns of care study from 2011 to 2019, the utilisation of short-course HFRT in GBM patients was 19%. This seems similar to the 21% reported at University College London Hospitals between 2011 and 2015 [Citation11], and lower than the 30% in a Canadian province-wide registry covering an earlier time period of 2006–2012 [Citation16]. Here, it should be mentioned that Canada has been driving the development of short-course HFRT in clinical trials [Citation12,Citation13,Citation21]. Our results furthermore showed a significantly increased use of short-course HFRT, from ca. 10% in 2011 to one-third in recent years. The increased utilisation was especially pronounced since 2014. Thus, approximately 1 year after the publication of the Nordic trial [Citation8], in which Denmark participated, and coinciding with the publication of the first EANO guideline [Citation15]. Therefore, the adoption of short-course HFRT in Denmark seems to be a clear response to those publications, but not to the earlier published Canadian Roa trial [Citation12]. This may be further illustrated by the predominant use of the ‘Nordic regimen’ of 34 Gy in 10 fractions, and minimal use of the ‘Canadian regimen’ of 40 Gy in 15 fractions, in this cohort. Altogether, these findings illustrate the importance of clinical trial participation for the real-world adoption of evidence-based treatments.
Next, we assessed whether the utilisation of the short-course regimen led to increased use of postoperative RT and concomitant chemotherapy in general, which we did not observe. The proportion of patients undergoing postoperative RT remained namely stable at ca. 85%, which is comparable to the series from Norway, Spain, France, Italy, Germany, and Hong Kong [Citation22–27]. The increase in hypofractionation in our cohort, indeed coincided with the discontinued use of more palliative regimens and a decreased use of conventional fractionation (). This indicates that a part of the GBM patients treated with palliative or conventionally fractionated radiotherapy in earlier years were being treated with HFRT in more recent years. Similarly, our results suggest a shift in the fractionation schedule within the CRT subgroup instead of expanding the group of patients being treated with the combination (). The utilisation of CRT remained stable over time at ca. 60%, comparable to what has been reported in the literature [Citation11,Citation22–26]. When assessing the subgroup of patients assigned to short-course HFRT, the use of concomitant chemotherapy did not increase despite the publication of the Perry trial in 2017 [Citation13]. In more detail, the use of concomitant chemotherapy was 34%, which is significantly lower than the 50% reported in the US National Cancer Database and France [Citation28,Citation29]. With our study, we cannot conclude on the optimal use of short-course HFRT, nor on the optimal use of concomitant chemotherapy, as data on treatment decisions were not registered. On that note, patients eligible for long-course CRT have been excluded from the Nordic and Perry trial, and randomised trials comparing short-course CRT (34 and 40 Gy) to long-course CRT are still ongoing. In accordance, the guidelines have reserved short-course HFRT without chemotherapy (based on MGMT promotor methylation status) for only those elderly patients who are not considered candidates for combination CRT [Citation2,Citation14,Citation15].
Subsequently, we assessed whether the adoption of short-course HFRT led to improved treatment compliance. RT compliance was 92% and 93% in patients planned for conventionally and HFRT, respectively. These high compliance rates are comparable to those reported in clinical trials [Citation5,Citation8,Citation12]. Moreover, for the patients older than 70 years in our cohort, the compliance rate to conventional fractionation was 92% (data not shown), which is clearly higher than the 72–74% reported in the Nordic trial and Roa-trial [Citation8,Citation12]. Importantly, we observed a significant increase in RT compliance in recent years, irrespective of the type of regimen. We acknowledge that this can be explained by several factors, including the adoption of short-course HFRT and changed patient selection. On the other hand, compliance with concomitant chemotherapy showed no clear trends. The observed concomitant chemotherapy compliance is close to that reported in clinical trials [Citation5,Citation13]. Altogether, these compliance rates show the high feasibility of multimodality treatments in a real-world setting with centralised neuro-oncology care.
Lastly, we assessed whether the adoption of short-course HFRT would improve survival. In this Danish cohort, the median OS was 11 months without signs of improvement over time, also not after increased use of short-course HFRT in recent years. This seems logical considering that the proportion of patients proceeding to adjuvant treatment remained stable, and only small improvements in treatment compliance were found. In other nationwide cohorts, the median OS is comparable [Citation22–24,Citation26,Citation27]. The median OS of 16 and 7 months in patients assigned to conventional and hypofractionation in our cohort, respectively, is comparable to what is reported in clinical trials [Citation5,Citation8,Citation12,Citation13,Citation21]. Furthermore, assignment to short-course HFRT was independently associated with shorter survival in our cohort. As we cannot completely correct for treatment assignment bias, we need to refer to the randomised trials to assess the efficacy of altered fractionation schemes [Citation8,Citation12]. Interestingly, the evaluation of hypofractionated regimens in clinical trials is currently being expanded to younger GBM patients with a good PS. The Canadian phase 3 non-inferiority trial (NCT02206230), randomising GBM patients aged 18–70 years with a WHO PS 0–2 between conventionally fractionated (60 Gy in 30 fractions of 2 Gy + temozolomide) and hypofractionated RT (60 Gy in 20 fractions of 3 Gy + temozolomide), presented non-inferior survival at ASTRO 2022. Of importance is that their hypofractionated 4-week regimen employs a significantly higher radiation dose than what we currently use for poor prognosis elderly patients. Other randomised trials are ongoing (NCT05439278, NCT05781321), all combining HFRT with temozolomide.
This study has several strengths and limitations inherent to the registry-based design. One particular strength is that we used a nationwide registry with detailed high-quality high-completeness data, including MGMT promotor methylation status. Limitations are that patients were diagnosed according to the WHO criteria at the time and that the registry lacks patients diagnosed with GBM without pathology confirmation. Addressing these limitations would make our results even more representative of current clinical practice. On that note, we would like to acknowledge that there is an interplay between offering surgery and proceeding to adjuvant treatment. However, we do not expect that our results are influenced by a change in surgical strategy over time as we observed a stable yearly number of operated patients. In addition, we are missing data on comorbidity and surgical morbidity and mortality, shown to influence treatment decisions [Citation17,Citation22,Citation23,Citation25,Citation30]. Lastly, we are missing data on complementary end-points to fully evaluate the impact of short-course HFRT, such as quality of life, progression-free survival, and secondary treatment upon disease progression.
To summarise, in GBM patients operated on in Denmark between 2011 and 2019, the use of short-course HFRT increased especially since 2014, in line with emerging evidence. This increase coincided with the discontinued use of more palliative regimens and a decreased use of conventional fractionation. The availability of short-course HFRT, considered a more feasible and patient-friendly regimen, did not lead to increased use of RT or chemotherapy, nor to improved survival on a population level. In contrast, it did lead to an improved compliance with RT, in both conventional and hypofractionated regimens. Lastly, the observed compliance with RT and chemotherapy is very close to what has been reported in clinical trials, showing the high feasibility of these treatments outside of clinical trials. To conclude, while the real-world adoption of short-course HFRT was extensive in Denmark, enabling patients to benefit from a reduced treatment time, the impact on multimodality treatment utilisation, compliance and survival on a population level was minimal.
Ethical approval
This study was deemed exempt by the Central Denmark Region Committees on Health Research Ethics. This study was approved by the Danish Patient Safety Authority (Reg.no. 31-1521-174) and the Danish Clinical Quality Program - National Clinical Registries (Reg.no. DNOR-2020-03-02). The study was registered on the internal registry of research projects of Central Denmark Region (Reg.no. 1-16-02-74-20).
Supplemental Material
Download MS Word (190.6 KB)Supplemental Material
Download MS Word (17.6 KB)Supplemental Material
Download MS Word (16.8 KB)Supplemental Material
Download MS Word (18 KB)Acknowledgements
The authors would like to acknowledge the Danish Clinical Quality Program – National Clinical Registries, especially Henriette Engberg, for the contributions regarding the data, and Erik Thorlund Parner, Department of Public Health – Department of Biostatistics, Aarhus University, for the support regarding the statistical analyses.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The used data are freely available by request to the Danish Clinical Quality Program – National Clinical Registries, Danish Neuro-Oncology Registry.
Additional information
Funding
References
- Berger TR, Wen PY, Lang-Orsini M, et al. World health organization 2021 classification of Central nervous system tumors and implications for therapy for adult-type gliomas: a review. JAMA Oncol. 2022;8(10):1493–1501. doi: 10.1001/jamaoncol.2022.2844.
- Michael W, Martin van den B, Matthias P. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–186.
- Gzell C, Back M, Wheeler H, et al. Radiotherapy in glioblastoma: the past, the present and the future. Clin Oncol (R Coll Radiol). 2017;29(1):15–25. doi: 10.1016/j.clon.2016.09.015.
- Niyazi M, Andratschke N, Bendszus M, et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiother Oncol. 2023;184:109663.
- Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330.
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma a randomized clinical trial. JAMA 2015;314(23):2535–2543. doi: 10.1001/jama.2015.16669.
- Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9(1):29–38. doi: 10.1016/S1470-2045(07)70384-4.
- Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6.
- Hulshof MCCM, Schimmel EC, Andries Bosch D, et al. Hypofractionation in glioblastoma multiforme. Radiother Oncol. 2000;54(2):143–148.
- Brodbelt A, Greenberg D, Winters T, et al. Glioblastoma in England: 2007-2011. Eur J Cancer. 2015;51(4):533–542.
- Brown NF, Ottaviani D, Tazare J, et al. Survival outcomes and prognostic factors in glioblastoma. Cancers (Basel). 2022;:14(13):3161.
- Roa W, Brasher PMA, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082.
- Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. doi: 10.1056/NEJMoa1611977.
- Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. doi: 10.1016/S1470-2045(17)30194-8.
- Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403.
- Morgan ER, Norman A, Laing K, et al. Treatment and outcomes for glioblastoma in elderly compared with non-elderly patients: a population-based study. Curr Oncol. 2017;24(2):e92–e98. doi: 10.3747/co.24.3424.
- Nead KT, Swisher-McClure S. Utilization of hypofractionated radiation therapy in older glioblastoma patients. J Geriatr Oncol. 2019;10(1):155–158. doi: 10.1016/j.jgo.2018.06.006.
- Haque W, Verma V, Butler EB, et al. Patterns of care and outcomes of hypofractionated chemoradiation Versus conventionally fractionated chemoradiation for glioblastoma in the elderly population. Am J Clin Oncol. 2018;41(2):167–172. doi: 10.1097/COC.0000000000000417.
- Hansen S. The Danish neuro-oncology registry. Clin Epidemiol. 2016;8:629–632. doi: 10.2147/CLEP.S99459.
- Karschnia P, Vogelbaum MA, van den Bent M, et al. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur J Cancer. 2021;149:23–33. doi: 10.1016/j.ejca.2021.03.002.
- Roa W, Kepka L, Kumar N, et al. International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. doi: 10.1200/JCO.2015.62.6606.
- Gulati S, Jakola AS, Johannesen TB, et al. Survival and treatment patterns of glioblastoma in the elderly: a population-based study. World Neurosurg. 2012;78(5):518–526. doi: 10.1016/j.wneu.2011.12.008.
- Graus F, Bruna J, Pardo J, et al. Patterns of care and outcome for patients with glioblastoma diagnosed during 2008-2010 in Spain. Neuro Oncol. 2013;15(6):797–805. doi: 10.1093/neuonc/not013.
- Fabbro-Peray P, Zouaoui S, Darlix A, et al. Association of patterns of care, prognostic factors, and use of radiotherapy-temozolomide therapy with survival in patients with newly diagnosed glioblastoma: a French national population-based study. J Neurooncol. 2019;;142(1):91–101. doi: 10.1007/s11060-018-03065-z.
- Brandes AA, Franceschi E, Ermani M, et al. Pattern of care and effectiveness of treatment for glioblastoma patients in the real world: results from a prospective population-based registry. Could survival differ in a high-volume center? Neurooncol Pract. 2014;;1(4):166–171. doi: 10.1093/nop/npu021.
- Woo PYM, Yau S, Lam T-C, et al. Patterns of care and survival of Chinese glioblastoma patients in the temozolomide era: a Hong Kong population-level analysis over a 14-year period. Neurooncol Pract. 2023;10(1):50–61. doi: 10.1093/nop/npac069.
- Efremov L, Abera SF, Bedir A, et al. Patterns of glioblastoma treatment and survival over a 16-years period: pooled data from the German Cancer Registries. J Cancer Res Clin Oncol. 2021;147(11):3381–3390. doi: 10.1007/s00432-021-03596-5.
- Haque W, Verma V, Butler EB, et al. Addition of chemotherapy to hypofractionated radiotherapy for glioblastoma: practice patterns, outcomes, and predictors of survival. J Neurooncol. 2018;136(2):307–315. doi: 10.1007/s11060-017-2654-y.
- Biau J, Chautard E, De Schlichting E, et al. Radiotherapy plus temozolomide in elderly patients with glioblastoma: a “real-life” report. Radiat Oncol. 2017;12(1):197. doi: 10.1186/s13014-017-0929-2.
- Amsbaugh MJ, Yusuf MB, Gaskins J, et al. Patterns of care and predictors of adjuvant therapies in elderly patients with glioblastoma: an analysis of the National Cancer Data Base. Cancer 2017;123(17):3277–3284. doi: 10.1002/cncr.30730.

