Abstract
Introduction
Head and neck cancer (HNC) patients’ anatomy may undergo significant changes during radiotherapy (RT). This potentially affects dose distribution and compromises conformity between planned and delivered dose. Adaptive radiotherapy (ART) is a promising technique to overcome this problem but requires a significant workload. This systematic review aims to estimate the clinical and dosimetric benefits of ART using prospective data.
Material and methods
A search on PubMed and Web of Science according to the PRISMA guidelines was made on Feb 6, 2023. Search string used was: ‘adaptive radiotherapy head neck cancer’. English language filter was applied. All studies were screened for inclusion on title and abstract, and the full text was read and discussed in the research group in case of uncertainty. Inclusion criteria were a prospective ART strategy for HNC investigating clinical or dosimetric outcomes.
Results
A total of 1251 articles were identified of which 15 met inclusion criteria. All included studies were published between 2010 and 2023 with a substantial diversity in design, endpoints, and nomenclature. The number of patients treated with ART was small with a median of 20 patients per study (range 4 to 86), undergoing 1-2 replannings. Mean dose to the parotid glands was reduced by 0.4-7.1 Gy. Maximum dose to the spinal cord was reduced by 0.5-4.6 Gy. Only five studies reported clinical outcome and disease control was excellent. Data on toxicity were ambiguous with some studies indicating reduced acute toxicity and xerostomia, while others found reduced quality of life in patients treated with ART.
Conclusion
The literature on clinical ART in HNC is limited. ART is associated with small reductions in doses to organs at risk, but the influence on toxicity and disease control is uncertain. There is a clear need for larger, prospective trials with a well-defined control group.
Background
Head and neck cancer (HNC) is characterized by a tendency to regional rather than distant spread. Disease control is therefore possible with aggressive loco-regional radiotherapy (RT). In most patients this means fractionated RT to a total dose of 66-70 Gy over 5-6 weeks, often combined with systemic chemotherapy. The combined treatment is associated with significant acute and long-term toxicity [Citation1], and recurrence is seen in 20-40% of patients [Citation2–4]. Improvements in RT of HNC is therefore pivotal to reduce side effects and increase tumor control.
While modern day RT planning is detailed and precise, anatomical changes during treatment due to tumor shrinkage, edema, weight loss, etc. may constitute a significant challenge. These volumetric changes may reshape the anatomy of the neck, and modify the primary tumor, nodes and normal tissue to a degree where the anatomy at time of planning becomes inconsistent with the anatomy during treatment [Citation5–7]. Significant anatomical changes have been observed as early as two weeks into treatment [Citation8,Citation9]. This is problematic as some areas may receive less or more dose than planned with a corresponding increased dose to normal tissue. Studies have shown that the parotid glands migrate up to 12 mm toward the high dose areas resulting in overdosage and an increased risk of xerostomia as a result [Citation10–14]. Others have described increases in doses to the brainstem or spinal cord in patients with nasopharyngeal cancer (NPC) [Citation15,Citation16]. Some studies have shown improved target coverage with adaptive radiotherapy (ART) compared to non-ART in presence of large anatomical changes [Citation6,Citation8]. However, the impact on tumor control is more difficult to assess and it should be noted that a number of studies point to centralized locations in the target – as opposed to marginal failures – as the main location of local failures in HNC [Citation17,Citation18].
One way to mitigate these anatomical changes is by frequent replanning, thereby adapting the treatment to the anatomical changes. ART holds the potential of increased treatment accuracy, reduced dose to normal tissue and the possibility of monitoring the actual delivered dose [Citation19]. While the concept is promising, the manual replanning has been resource-heavy and time-consuming, preventing routine clinical use [Citation20].
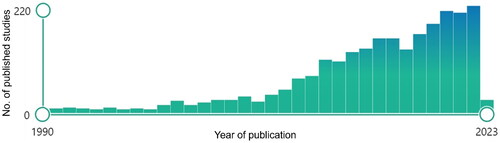
Over the last 15 years there has been an excessive increase in numbers of published studies investigating ART (). Most of these studies are retrospective and investigates the presumed benefit of ART at various points in treatment [Citation21,Citation22]. Retrospective studies offer limited guidance on the potential advantages and disadvantages of ART in routine clinical practice due to a notable risk of selection bias where adapted patients showed unusual anatomical changes. Prospective studies may provide a more representative sample of patients with HNC [Citation6,Citation23].
Figure 1. PubMed Search results: ‘Adaptive radiotherapy head neck cancer’. English language filter was applied. Each column represents the number of published articles per year. Picture is captured from: https://pubmed.ncbi.nlm.nih.gov/. Modified using the windows office package.
The aim of this study was to generate an overview of clinical studies testing ART procedures in a prospective setting and extract data from such studies which can inform about the potential clinical value of ART in HNC.
Materials and methods
Search strategy
A search on PubMed and Web of Science was performed according to the PRISMA 2020 guidelines [Citation24] with the following search string: ‘adaptive radiotherapy head neck cancer’. English language filter was applied. For exact search MeSH please see Supplementary A. The final search was made on 6 February 2023. All search results were screened on title and abstract. When in doubt, full text was read and decision on inclusion was discussed within the group (by AL, KH, IV and JF).
To be included the study should report dosimetric or clinical endpoints of adult human patients treated with curative intended external photon ART for HNC (thyroid carcinomas excluded) with at least one planned rescan/adaption during treatment. Reviews were excluded. However, the reference lists of the papers were manually reviewed for additional relevant studies meeting inclusion criteria. Studies only including patients with unknown primary, recurrent disease or reports of postoperative RT were excluded. Studies concerning planned sequential boost on a new scan were excluded if this was the only reason for replan. An example of an excluded study using sequential boost ART is Nishi et al. [Citation25] despite that the boost was adapted to the changing anatomy.
Dosimetric endpoints selected were mean dose to parotid glands and spinal cord and maximum dose to spinal cord. Clinical endpoints encompassed toxicity grade and reports on quality of life (QoL). Clinical endpoints also encompassed survival, progression or disease control, specifically: Overall survival (OS), local control (LC), regional control (RC), locoregional control (LRC), complete response (CR) and partial response (PR).
There was no attempt to obtain unpublished data.
For complete set of data variables extracted please see Supplementary B.
Results
Screening
shows a PRISMA diagram of the study selection process. One thousand four hundred and seventy-two studies were found on PubMed and Web of Science. Three additional studies were added from manual cross-reference of articles and browsing. Most studies were published within the last decade (see ). We excluded a total of 1202 papers on title or abstract review only. A total of 49 full text articles were screened and 34 of these were excluded. Fifteen studies (31% off full text screened studies) were included in this review. Study characteristics are presented in .
Figure 2. PRISMA diagram of study selection. Diagram made in https://lucidchart.com.
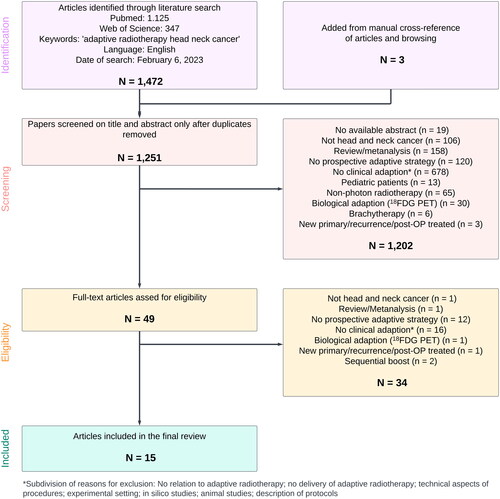
Table 1. Characteristics of studies included in the review.
Patients
All included studies were published between year 2010 and 2023. Eight out of fifteen studies included HNC in various sites (53%), five studies included NPC only (33%) and two studies (13%) oropharyngeal carcinomas (OPSCC) only. The 15 studies included a total of 643 patients of which 392 (61%) received ART. All patients were treated with curative intended RT except for one study that included one patient treated with palliative intend. Generally, a low number of patients treated with ART were included in the studies with a mean of 26 and a median of 20 patients per study (interquartile range: 15-30. See ). Four hundred and forty eight patients (70%) were treated with concomitant chemoradiotherapy while 167 (26%) received RT alone. In one study of 28 patients (4%) no information on use of chemotherapy was provided [Citation26]. Four studies included patients treated with postoperative radio/chemoradiotherapy, two studies included treatment of recurrent disease and one of the studies also included 22 non-HNC patients. The prescribed doses ranged from 60-76 Gy in 30-40 fractions with 70 Gy planned for most patients.
Figure 3. The 392 patients treated with adaptive radiotherapy by year of publication. Each column represents the number of patients treated with ART in each study. Sections marked pink, blue and green represent the share of patients not analyzed, treated off protocol or treated with palliative intend respectively. Mean number of patients treated per study was 26, and the median was 20 (interquartile range: 15-30).
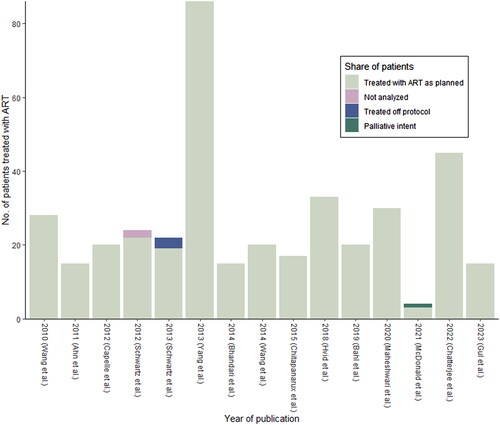
All ART patients (n = 392) had at least one replan during treatment, 63 (9.8%) patients had two replans. No patients had three or more replans. Two-hundred-and forty-nine (39%) patients either belonged in a control group or did not meet the criteria for replanning. Two (0.3%) patients in one study were not analyzed.
Two studies used a zero mm PTV margins for ART patients. Most others used 3-5 mm PTV margins. Three studies did not report size of PTV margin.
Dosimetric outcomes
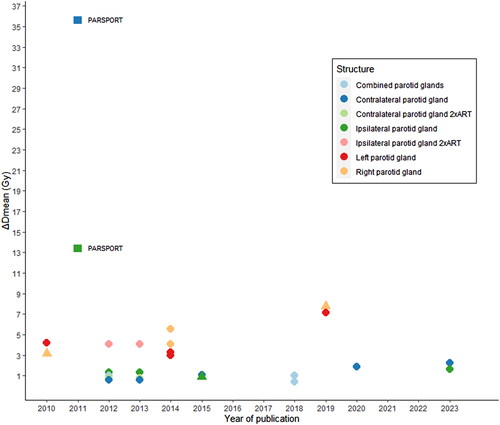
Most studies (13 out of 15) reported dosimetric outcomes [Citation26–38]. The maximum dose to the spinal cord was reduced by 0.5-5 Gy with at least one replanning. One study reported a reduction of the maximum dose to spinal cord by 7-8% with adaption. Three studies reported a reduction in mean dose to the spinal cord by 1.3-6.7 Gy compared to non-ART. Likewise, mean doses to the parotid glands were reduced with one or more replannings by an overall of 0.4-7.1 Gy. Looking on lateralization, a higher reduction was seen in the ipsilateral gland, with 1.3-4.1 Gy compared to 0.6-2.25 Gy in the contralateral gland with one or more replannings. One study found ART to reduce the mean dose to ipsilateral parotid gland by 1.5-3% and to the contralateral parotid gland by 2-5% [Citation29]. Two studies found superior sparing of the right parotid gland by 4.1-5.6 Gy compared to the left parotid gland 3-3.3 Gy [Citation34,Citation35].
Only Schwartz et al. [Citation33] compared benefit of two versus one replan. A second replan was found to reduce the mean dose to the ipsilateral and contralateral parotid glands further from 1.3 to 4.1 Gy and 0.6 to 0.8 Gy respectively.
The magnitude of reduction in mean doses to the parotid glands with ART did not change over the past decade and remained within 0.4-7.1 Gy (see ).
Figure 4. Reduction in mean dose to parotid glands by publication year. Circles represent significant results and triangles represent non-significant results of the reduction in mean dose with ART vs. non-ART. The squares represent the reduction in mean dose found in the PARSPORT trial with parotid sparring IMRT vs. 3D-RT.
Clinical outcomes
Five studies evaluated clinical outcomes of ART [Citation7,Citation28,Citation32,Citation38,Citation39]. Two reported toxicity and QoL data only while three also included disease- and survival specific data. Schwartz et al. [Citation32] found a high disease control rate in patients treated with ART with a LC of 100% and a RC of 95% two years post treatment. Yang et al. [Citation7] found a LRC of 97.2% two years post treatment in patients receiving ART which was significant better compared to patients treated with non-ART who had a two year LRC of 92.4%. However, the two-year OS was not significantly different in patients receiving ART compared to non-ART. Maheshwari et al. [Citation28] found a LRC of 96.7% six months post treatment in patients receiving ART and in 90% of patients treated with non-ART (significance not reported) but did not report survival.
Two studies reported the rate of xerostomia, both studies using the NCI Common Terminology Criteria for Adverse Events (CTCAE). Maheshwari et al. [Citation28] found a distribution of 6.6% grade I, 63.7% grade II and 30% grade III in patients treated with ART assessed 6 months after end of treatment. In patients receiving non-ART the corresponding results were 3.3% grade I, 46.7% grade II and 50% grade III 6 months after end of treatment. Schwartz et al. [Citation32] found 41% grade I xerostomia, 55% grade II and 5% grade III up to 90 days after end of treatment in patients treated with ART. In the same group of patients the rate of mucositis and dermatitis up to 90 days after end of treatment was 100% for grade III mucositis and 45% and 55% for grade I and II dermatitis respectively. Bahl et al. [Citation38] found an acute toxicity risk of 60% for grade II-IV mucositis, 25% grade III dermatitis and 40% grade II dysphagia. In two studies with a comparative group QoL measures were used. Chatterjee et al. [Citation39] observed a reduced QoL in patients treated with ART including problems with xerostomia, oral health and speech three months after end of treatment compared to patients receiving non-ART. The difference between the two groups was not persistent nine months after end of treatment. In a cohort of 129 patients with NPC Yang et al. [Citation7] found ART to improve global QoL significantly compared to patients receiving non-ART.
Median follow-up time was only reported in three studies and were 18, 30 and 31 months.
Discussion
Existing prospective clinical data of ART for HNC are limited and characterized by small patient series without adequate comparative groups. Despite this, there seem to be a dosimetric benefit to normal tissues which should be expected as the dose plan is reoptimized to a new target and OAR delineation. However, if the magnitude of benefit is sufficient to outweigh the uncertainties and render a clinically relevant benefit on toxicity and disease control remains unproven.
Of the 15 studies included in this review only five studies included some form of toxicity or QoL assessment, and in the three studies including a comparative non-ART group, results were ambiguous. Studies without comparative groups are even more difficult to evaluate, but do not point toward a clear benefit. For example, in a study of 20 patients with NPC undergoing ART Bahl et al. [Citation38] reported acute grade III dermatitis in 25%, grade III-IV mucositis in 60% and grade II dysphagia in 40% of patients. This is higher than reported in earlier non-ART studies, for example [Citation40].
One of the reasons convincing toxicity data are not yet available may be because the dose reductions associated with ART are modest. Most studies report reductions in parotid mean dose below 5 Gy, even with several adaptions. Moreover, there seems to be no sign of increased benefit over the past decade (see ). Yet delivered dose to the parotid glands may be significantly higher than the planned dose [Citation41], which means that the actual benefit may be larger than depicted in . Under all circumstances, the clinical outcome is highly dependent on where on the dose-complication curve the reduction takes place, as a 3 Gy reduction in mean dose to the parotids from 26->23 Gy or from 12->9 Gy, will result in different risk reductions. However, it is sobering to compare with the documented benefit of IMRT in reducing xerostomia established in the PARSPORT study [Citation42] as this positive trial was mediated by a mean dose reduction of 13.4 Gy and 35.6 Gy to the ipsilateral and contralateral parotid glands compared to 3D-RT. It is therefore not surprising that the French ARTIX study, which was presented at the 2022 ESTRO meeting, randomizing 132 HNC patients to standard chemo-RT or chemo-RT with weekly ART, failed to show a significant benefit on salivary flow or patient-reported outcomes [Citation43]. A further reduction in toxicity could be obtained by margin reduction, a concept only explored by two of the included studies [Citation32,Citation33]. Experimental data suggest a general benefit on organ-at-risk exposure by an average 1 Gy/mm, but at the expense of target underdosage > 2 Gy in 32% of patients [Citation21]. However, this target underdosage could be mitigated in most patients by adaptive intervention. More studies investigating ART with margin reductions would therefore be of high relevance.
While the main focus of ART in HNC have been on reducing toxicity, ART may also impact disease control. Due to changes in anatomy over the course of treatment the target area may develop dose inhomogeneities with unintended ‘cold spots’, which could be associated with a decrease in local control. Underdosage to the target area is especially important in head-neck cancer as most recurrences after RT occur in the target area [Citation17,Citation18,Citation44]. If ART could mitigate these inhomogeneities by eliminating ‘cold spots’ with frequent adaptions it holds the potential for improved disease control. It is therefore an interesting outcome in studies of ART. In this review, only three of the included studies report disease control, all achieving a 95% or higher loco-regional control at two years post-treatment. In the few prospective studies planned with a comparative group, results have pointed toward improved disease control. Maheshwari et al. [Citation28] reported 96.7% complete response rate six months post-treatment with ART compared to 90% with non-ART in a small randomized study of 60 HNC patients. In patients with NPC, Yang et al. [Citation7] compared disease control in ART and non-ART using a cohort of 129 patients. They found a significant increase in two-year LRC from 92.4% to 97.2% with 1-2 replans during treatment, but with no influence on OS. Using ART in OPSCC exclusively have also generated an impressive disease control [Citation32], but results are not far from what is obtained by conventional non-ART in p16-positive OPSCC, exemplified by a cohort of 150 OPSCC patients treated in Denmark with a two-year LRC rate of ∼95% in p16-positive [Citation45]. A challenge in ART is the definition of GTV during treatment as the tumor is not likely to shrink equally in all directions. In the three studies reporting disease control one did not change the GTV in the replanning. The two others recontoured target volumes but did not specify how the GTV was recontoured.
Retrospective data on ART are at risk of being biased. In a case-control study in NPC patients, Zhao et al. [Citation46] found ART to improve three-year local relapse-free survival in patients with large tumors (T3-4). However, the main reason for replanning in the ART group was tumor and/or nodal shrinkage, and it is therefore likely that receiving ART was also a proxy measure of response. This underlines the importance of prospective trials with a clear ART strategy and a relevant comparison.
Giving the modest benefit so far associated with ART, the resources used needs to be weighed carefully and a scientific approach to implementation is warranted. Offline adaption requires a new planning scan or generation of a synthetic CT from the Cone beam computed tomography (CBCT), followed by recontouring and generation of a new treatment plan. In case of online ART, the prolonged time on the couch puts high demands on the patient, which may be increasingly difficult as the acute toxicities develop during the course of treatment. In both online and offline ART reports on time spent on adaption is therefore central. However, only five out of fourteen studies included any time measures, and with no consistency on reported timeframes. Three studies reported time from rescan to delivery of first adapted plan. In two studies [Citation32,Citation37] it was reported as a ‘median of two days’ or ‘a few workdays’, and in another based on fixed intervals with ‘rescan at 17’th fraction and ART delivery from 21’st fraction’ [Citation36]. The only study providing precise time estimates was Chatterjee et al. [Citation39], where the replanning process was divided into ‘time spent on contouring’ and ‘time spent on replanning’. On average more than four hours were spend each on contouring and replanning, underlining the significant resources used for ART. While all the above time measures are relevant, the lack of reporting consensus makes it challenging to assess the resources used for ART. But an interesting development is the possibility of daily, online ART, aided by artificial intelligence. Results from bladder cancer have proved the feasibility [Citation47], and an automated process may also save resources and justify the potentially modest benefit in head and neck cancer.
A general challenge in interpreting studies on ART, is the inconsistent terminology and lack of nomenclature. E.g., Anatomy-adapted, response-adapted, biology-adapted are all concepts that are used indiscriminately. Also, there is no consensus on the replanning strategy as decision of adaption can be based on e.g. one or multiple complete rescans, a hybridplan or CBCT deformation. A possible way forward would be the endorsement of a common terminology, for example as suggested by Heukelom et al. [Citation48].
In conclusion, ART holds a clear potential to reduce dose to normal tissue and maybe increase disease control. However, the benefits are modest and require well-designed studies with a relevant comparison.
Supplemental Material
Download MS Word (14.3 KB)Supplemental Material
Download MS Word (22.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data derived from public domain resources.
References
- Corry J, Peters LJ, Rischin D. Optimising the therapeutic ratio in head and neck cancer. Lancet Oncol. 2010;11(3):287–291. doi:10.1016/S1470-2045(09)70384-5.
- Bollen H, van der Veen J, Laenen A, et al. Recurrence patterns after IMRT/VMAT in head and neck cancer. Front Oncol. 2021;11:720052. doi:10.3389/fonc.2021.720052.
- Kjems J, Gothelf AB, Håkansson K, et al. Elective nodal irradiation and patterns of failure in head and neck cancer after primary radiation therapy. Int J Radiat Oncol Biol Phys. 2016;94(4):775–782. doi:10.1016/j.ijrobp.2015.12.380.
- Pagh A, Grau C, Overgaard J. Failure pattern and salvage treatment after radical treatment of head and neck cancer. Acta Oncol. 2016;55(5):625–632. doi:10.3109/0284186X.2015.1117136.
- Barker JL, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59(4):960–970. doi:10.1016/j.ijrobp.2003.12.024.
- Hansen EK, Bucci MK, Quivey JM, et al. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64(2):355–362. doi:10.1016/j.ijrobp.2005.07.957.
- Yang H, Hu W, Wang W, et al. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85(1):e47–e54. doi:10.1016/j.ijrobp.2012.09.033.
- Bhide SA, Davies M, Burke K, et al. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: a prospective observational study. Int J Radiat Oncol Biol Phys. 2010;76(5):1360–1368. doi:10.1016/j.ijrobp.2009.04.005.
- Liu Q, Liang J, Zhou D, et al. Dosimetric evaluation of incorporating patient geometric variations into adaptive plan optimization through probabilistic treatment planning in head and neck cancers. Int J Radiat Oncol Biol Phys. 2018;101(4):985–997. doi:10.1016/j.ijrobp.2018.03.062.
- Brouwer CL, Steenbakkers RJHM, Langendijk JA, et al. Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115(3):285–294. doi:10.1016/j.radonc.2015.05.018.
- Qi XS, Santhanam A, Neylon J, et al. Near real-time assessment of anatomic and dosimetric variations for head and neck radiation therapy via graphics processing unit-based dose deformation framework. Int J Radiat Oncol Biol Phys. 2015;92(2):415–422. doi:10.1016/j.ijrobp.2015.01.033.
- Lee C, Langen KM, Lu W, et al. Assessment of parotid gland dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int J Radiat Oncol Biol Phys. 2008;71(5):1563–1571. doi:10.1016/j.ijrobp.2008.04.013.
- van Timmeren JE, Chamberlain M, Bogowicz M, et al. MR-guided adaptive radiotherapy for head and neck cancer: prospective evaluation of migration and anatomical changes of the major salivary glands. Cancers. 2021;13:5404. doi:10.3390/cancers13215404.
- Castelli J, Simon A, Louvel G, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol. 2015;10:6. doi:10.1186/s13014-014-0318-z.
- Wang J, Bai S, Chen N, et al. The clinical feasibility and effect of online cone beam computer tomography-guided intensity-modulated radiotherapy for nasopharyngeal cancer. Radiother Oncol. 2009;90(2):221–227. doi:10.1016/j.radonc.2008.08.017.
- Han C, Chen Y-J, Liu A, et al. Actual dose variation of parotid glands and spinal cord for nasopharyngeal cancer patients during radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(4):1256–1262. doi:10.1016/j.ijrobp.2007.10.067.
- Due AK, Vogelius IR, Aznar MC, et al. Recurrences after intensity modulated radiotherapy for head and neck squamous cell carcinoma more likely to originate from regions with high baseline [18F]-FDG uptake. Radiother Oncol. 2014;111(3):360–365. doi:10.1016/j.radonc.2014.06.001.
- Zukauskaite R, Hansen CR, Brink C, et al. Analysis of CT-verified loco-regional recurrences after definitive IMRT for HNSCC using site of origin estimation methods. Acta Oncol. 2017;56(11):1554–1561. doi:10.1080/0284186X.2017.1346384.
- Dawson LA, Sharpe MB. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol. 2006;7(10):848–858. doi:10.1016/S1470-2045(06)70904-4.
- Schwartz DL. Current progress in adaptive radiation therapy for head and neck cancer. Curr Oncol Rep. 2012;14(2):139–147. doi:10.1007/s11912-012-0221-4.
- van Kranen S, Hamming-Vrieze O, Wolf A, et al. Head and neck margin reduction with adaptive radiation therapy: robustness of treatment plans against anatomy changes. Int J Radiat Oncol Biol Phys. 2016;96(3):653–660. doi:10.1016/j.ijrobp.2016.07.011.
- Wu Q, Chi Y, Chen PY, et al. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys. 2009;75(3):924–932. doi:10.1016/j.ijrobp.2009.04.047.
- Chen AM, Daly ME, Cui J, et al. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck. 2014;36(11):1541–1546. doi:10.1002/hed.23477.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Nishi T, Nishimura Y, Shibata T, et al. Volume and dosimetric changes and initial clinical experience of a two-step adaptive intensity modulated radiation therapy (IMRT) scheme for head and neck cancer. Radiother Oncol. 2013;106(1):85–89. doi:10.1016/j.radonc.2012.11.005.
- Wang W, Yang H, Hu W, et al. Clinical study of the necessity of replanning before the 25th fraction during the course of intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2010;77(2):617–621. doi:10.1016/j.ijrobp.2009.08.036.
- Ahn PH, Chen C-C, Ahn AI, et al. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys. 2011;80(3):677–685. doi:10.1016/j.ijrobp.2010.03.014.
- Maheshwari G, Dhanawat A, Kumar HS, et al. Clinical and dosimetric impact of adaptive intensity-modulated radiotherapy in locally advanced head-and-neck cancer. J Cancer Res Ther. 2020;16(3):600–604. doi:10.4103/jcrt.JCRT_928_19.
- McDonald BA, Vedam S, Yang J, et al. Initial feasibility and clinical implementation of daily MR-guided adaptive head and neck cancer radiation therapy on a 1.5T MR-Linac system: prospective R-IDEAL 2a/2b systematic clinical evaluation of technical innovation. Int J Radiat Oncol Biol Phys. 2021;109(5):1606–1618. doi:10.1016/j.ijrobp.2020.12.015.
- Gul OV, Buyukcizmeci N, Basaran H. Dosimetric evaluation of three-phase adaptive radiation therapy in head and neck cancer. Radiat Phys Chem. 2023;202:110588. doi:10.1016/j.radphyschem.2022.110588.
- Capelle L, Mackenzie M, Field C, et al. Adaptive radiotherapy using helical tomotherapy for head and neck cancer in definitive and postoperative settings: initial results. Clin Oncol (R Coll Radiol). 2012;24(3):208–215. doi:10.1016/j.clon.2011.11.005.
- Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-Neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986–993. doi:10.1016/j.ijrobp.2011.08.017.
- Schwartz DL, Garden AS, Shah SJ, et al. Adaptive radiotherapy for head and neck cancer—dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106(1):80–84. doi:10.1016/j.radonc.2012.10.010.
- Wang R, Zhang S, Zhou L, et al. Volume and dosimetric variations during two-phase adaptive intensity-modulated radiotherapy for locally advanced nasopharyngeal carcinoma. Biomed Mater Eng. 2014;24(1):1217–1225. doi:10.3233/BME-130923.
- Bhandari V, Patel P, Gurjar O, et al. Impact of repeat computerized tomography replans in the radiation therapy of head and neck cancers. J Med Phys. 2014;39(3):164. doi:10.4103/0971-6203.139005.
- Chitapanarux I, Chomprasert K, Nobnaop W, et al. A dosimetric comparison of two-phase adaptive intensity-modulated radiotherapy for locally advanced nasopharyngeal cancer. J Radiat Res. 2015;56(3):529–538. doi:10.1093/jrr/rru119.
- Hvid CA, Elstrøm UV, Jensen K, et al. Cone-beam computed tomography (CBCT) for adaptive image guided head and neck radiation therapy. Acta Oncol. 2018;57(4):552–556. doi:10.1080/0284186X.2017.1398414.
- Bahl A, Elangovan A, Dracham CB, et al. Analysis of volumetric and dosimetric changes in mid treatment CT scan in carcinoma nasopharynx: implications for adaptive radiotherapy. J Exp Ther Oncol. 2019;13:33–39.
- Chatterjee S, Maulik S, Prasath S, et al. Xerostomia quality of life and resource requirements following parotid sparing adaptive radiotherapy in head and neck cancers: results of a prospective cohort study (study ID CTRI/2017/11/010683). Radiother Oncol. 2022;168:250–255. doi:10.1016/j.radonc.2022.01.020.
- Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi:10.1016/j.radonc.2012.08.013.
- O'Daniel JC, Garden AS, Schwartz DL, et al. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys. 2007;69(4):1290–1296. doi:10.1016/j.ijrobp.2007.07.2345.
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136. doi:10.1016/S1470-2045(10)70290-4.
- Castelli J, Benezery K, Hasbini A, et al. OC-0831 results of ARTIX phase III study: adaptive radiotherapy versus standard IMRT in head and neck cancer. Radiother Oncol. 2022;170: s 749–S750. doi:10.1016/S0167-8140(22)02695-0.
- Raktoe SAS, Dehnad H, Raaijmakers CPJ, et al. Origin of tumor recurrence after intensity modulated radiation therapy for oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;85(1):136–141. doi:10.1016/j.ijrobp.2012.02.042.
- Bentzen J, Toustrup K, Eriksen JG, et al. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol. 2015;54(7):1001–1007. doi:10.3109/0284186X.2014.992547.
- Zhao L, Wan Q, Zhou Y, et al. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98(1):23–27. doi:10.1016/j.radonc.2010.10.009.
- Åström LM, Behrens CP, Calmels L, et al. Online adaptive radiotherapy of urinary bladder cancer with full re-optimization to the anatomy of the day: initial experience and dosimetric benefits. Radiother Oncol. 2022;171:37–42. doi:10.1016/j.radonc.2022.03.014.
- Heukelom J, Fuller CD. Head and neck cancer adaptive radiation therapy (ART): conceptual considerations for the informed clinician. Semin Radiat Oncol. 2019;29(3):258–273. doi:10.1016/j.semradonc.2019.02.008.

