Abstract
Background
This study investigated changes in body weight, lean body mass (LBM), fat mass (FM), muscle strength and functional performance during radiation treatment in head and neck cancer (HNSCC) patients. Secondly, it investigated the impact of cisplatin-based chemoradiation (CCRT) on LBM loss compared with radiation alone.
Methods
48 patients (all tumor sites) received either 6 weeks of radiation alone (n = 16) with 66–68 Gy in 33–34 Fx, 5-6 Fx/week or CCRT, adding weekly cisplatin or carboplatin (n = 32). LBM and FM was evaluated using Dual-energy X-ray Absorptiometry bi-weekly from pre- to two weeks post-treatment. Maximal muscle strength (knee extension, leg - and chest press) and functional performance (stair climb, chair rise, and arm curl) were assessed pre- and post-treatment.
Results
Body weight and LBM had declined significantly already week 2 into treatment and declined significantly further through week 4 and 6 before leveling off after week 6. Bi-weekly, from treatment start to week 2, 2–4, and 4–6, LBM declined 1.2 ± 0.4 kg (p = .002; 95% CI: 0.4;2.0), 2.0 ± 0.4 kg (p < .0001; 1.2;2.8) and 1.4 ± 0.4 kg (p = .001; 0.6;2.2). With a two-week delay, FM declined significantly from week 2–8. All measures of muscle strength declined significantly from pre- to post-treatment. Functional performance was unchanged. LBM loss from pre- to post-treatment was significantly associated with impaired muscle strength (R2 = 0.3–0.5). CCRT patients lost 3.1 ± 0.8 kg of LBM (p = .0001; 1.5;4.7) more from pre- to post-treatment compared with patients receiving radiation alone. Analyses adjusting for nimorazole, tumor stage, baseline BMI, mean radiation dose to constrictor muscles and oral cavity confirmed this.
Conclusion
Accelerated and substantial LBM loss was already initiated within the first two weeks of treatment - before the onset of radiation-induced mucositis. LBM loss was associated with muscle strength impairment. Patients receiving CCRT experienced significantly larger LBM loss than patients receiving radiation alone. Registered on clinincaltrials.gov (Identifier: NCT05890859).
Background
Weight loss is common following treatment in patients with head and neck squamous cell carcinoma (HNSCC) [Citation1,Citation2] and is associated with a reduction in quality of life, patient-reported functional performance, as well as disease-specific survival [Citation3,Citation4]. Overall, weight loss may comprise as much as 10% of pre-treatment body weight and is caused by the cancer disease per se (i.e., cancer cachexia) and treatment-related side effects such as mucositis and dysphagia leading to a decrease in energy intake [Citation1,Citation2].
A recent review of the literature by Ferrão et al. [Citation5] suggests that loss of body weight during treatment in HNSCC patients constitutes both loss of fat and lean body mass (LBM). It has been suggested that up to three-quarters of body mass loss is constituted by LBM [Citation6]; however, the current evidence is characterized by rather heterogeneous studies performed in mixed HNSCC populations in terms of tumor sites, disease extent, and treatment modalities with either concurrent chemoradiation therapy (CCRT) or radiation alone. Investigations taking such potential effect modifiers into account are needed.
Most studies have used less validated or indirect evaluation methods of LBM, such as bioelectrical impedance analysis (BIA), skinfold thickness measurements, or calculated whole-body muscle mass estimates from computed tomography (CT) scans at the lumbar level. Only four [Citation6–9] of the twelve studies reviewed by Ferrão et al. [Citation5] assessed body composition using Dual-Energy Absorptiometry (DXA), which is considered the gold standard of body composition evaluation in HNSCC patients [Citation10]. Furthermore, no studies have evaluated LBM multiple times during treatment, but have been limited to evaluations of changes from pre-treatment to one or two time points, months after treatment [Citation5]. Thus, studies using accurate and validated measures of LBM in HNSCC patients with detailed temporal changes in body composition from treatment onset to the time of post-radiation tumor evaluation are needed.
Previously, we have shown that LBM loss is associated with loss of muscle strength and functional performance in HNSCC patients post-treatment [Citation11]. Only one [Citation9] of the DXA studies mentioned above assessed maximal muscle strength (by handgrip strength), while other publications on objective and patient-reported measures of functional performance have reported diverging findings [Citation6–9]. Thus, the relationship between LBM changes during radiation treatment and changes in muscle strength and functional performance in HNSCC patients remains under-investigated. In addition to the negative consequences on muscle function, LBM loss has been shown to predict chemotherapy dose-limiting toxicity [Citation12] and has been associated with overall and disease-specific survival in HNSCC patients [Citation13]. Even though a causal relationship has not been demonstrated prospectively, investigations of LBM loss remains important.
The majority of HNSCC patients in Denmark receive CCRT with weekly cisplatin. As recently reviewed by Fairman et al. [Citation14], there is robust pre-clinical evidence that muscle mass loss is influenced by chemotherapy administration – including cisplatin [Citation15–18]. A low number of studies in various clinical cancer cohorts have hypothesized that LBM may be negatively affected during chemotherapy in different drug combinations [Citation19,Citation20]. However, to our knowledge, no studies have investigated the impact of cisplatin on LBM in a clinical setting.
The primary aim of the present study was to investigate the change in body composition (LBM, body weight, and fat mass), maximal muscle strength and functional performance during radiation treatment in HNSCC patients and to investigate the association between the LBM loss and impairments in maximal muscle strength and functional performance. Secondly, the study investigated whether adding cisplatin during chemoradiation was associated with exacerbated LBM loss compared to radiation alone.
Methods
This study was a single-arm, prospective, cohort study. Eligible HNSCC patients were included at the Department of Oncology, Odense University Hospital, Denmark over 26 months. Eligibility criteria were: (1) histologically proven epithelial cell carcinoma of the larynx, pharynx, oral cavity, or unknown primary tumor, (2) prescribed to radical or post-operative radiotherapy of at least 60 Gy with or without concomitant chemotherapy. Exclusion criteria were palliative radiation, participation in competing research protocols or age under 18 years.
Patient demographics and additional data were retrieved from the national Danish Head and Neck Cancer Group (DAHANCA) database and patients’ electronic medical records. This included age, gender, body weight, height, WHO performance status, HPV or p16 status, tumor site (pharynx, oral cavity, supraglottic and glottic larynx, and unknown primary tumor), tumor stage, treatment dose (Gy), fractionation (5 versus 6 Fx/week), number of treatment days, weekly albumin, and hemoglobin levels, and whether patients received cisplatin and the hypoxic radiosensitizer nimorazole. Patients prescribed concomitant chemotherapy received 40 mg/m2 of cisplatin weekly, or in case of hearing impairment, carboplatin AUC 1.5 according to DAHANCA guidelines [Citation21].
Baseline evaluations on all patients included physical examination, anthropometric assessment, and blood sampling (hemoglobin and albumin). Patients were followed weekly with dietary counseling and pain management and offered educational sessions with relatives, and referral to a dietician when clinically indicated. Standardized nutritional support provided complete meal replacement drinks. Specific dietary intake was not recorded.
Feeding tube insertion followed a ‘reactive’ institutional policy, with decisions based on concerns regarding weight loss, insufficient food or liquid intake, and difficulty taking prescribed medication, including analgesics and nimorazole.
The study received approval from the regional committee on Health Research for Southern Denmark (journal number S-10140144) and was registered on clinicaltrials.gov (Identifier: NCT05890859).
Body composition
The primary endpoint was whole-body LBM. Secondary body composition measures included appendicular (ALBM), lower and upper extremity LBM (LELBM and UELBM, respectively), and whole-body fat mass (FM). All body composition endpoints were measured at baseline just before treatment start and continuously two, four, six, and eight weeks after the onset of treatment using total body Dual-energy X-ray Absorptiometry (DXA) scans (Hologic QGR Discovery, Hologic Inc., Bedford, MA, US.). All patients were scanned in the standardized supine position in underwear only. Due to ethical considerations when studying a potential malnourished cohort, patients did not undergo DXA scans in the fasting state but were encouraged to standardize pre-scanning eating and drinking between each visit. This was to ensure as consistent hydration levels as possible since this has been reported to affect the validity of LBM determined by DXA [Citation22]. Previously, on the exact same DXA scanner and under the same pre-scanning circumstances, we reported a 0.4% mean coefficient of variance of whole-body LBM in a comparable HNSCC cohort [Citation23]. On the same occasion as the DXA scan, total body weight was measured using a standard clinical scale after patients removed shoes and heavy clothing.
Maximal muscle strength and functional performance
Patients’ maximal muscle strength and functional performance were assessed twice: pre-treatment and immediately post-treatment. The assessment involved a standardized 5-minute ergometer cycling warm-up protocol. Subsequently, patients performed maximal functional performance tests in the same order during both visits. Upper body functional performance was evaluated using the 30-s arm curl test, where seated patients attempted to lift a 3 kg (for women) or 4 kg (for men) dumbbell as many times as possible in 30 s using the dominant arm. Lower body functional performance was assessed with the 30 s chair rise test, where patients aimed to stand up from a chair as many times as possible in 30 s. From the same test, lower extremity muscle power (Watt and Watt/kg BW) was calculated as described and validated elsewhere [Citation24] using the patients’ body mass and height, chair height (43 cm), and the number of repetitions completed. The power estimate was included to provide a measure of a more explosive-type muscle function, which cannot be derived from the 1RM tests.
Body weight-carrying functional performance was determined through the stair climbing test, where patients tried to ascend two flights of stairs as quickly as possible. These standardized tests have been extensively described and utilized in studies involving HNSCC patients [Citation11,Citation25,Citation26]. During the functional performance tests, patients were provided with two attempts, separated by a three-minute rest period, and the best attempt was recorded for analysis.
Immediately following the functional performance tests, patients completed three tests to assess maximal muscle strength. The tests included seated knee extension, chest press, and leg press, using standardized training equipment (Technogym, Cesena, Italy). Unilateral knee extension and leg press were performed using the patients’ dominant leg, while bilateral chest press was conducted. Patients underwent 4–6 submaximal warm-up attempts, which progressively increased in intensity. Subsequently, patients attempted one repetition at a time with an individually set weight. If the weight was lifted successfully with a full range of motion, a three-minute rest period was provided, and the weight increased. One-repetition maximum (1RM) was to be obtained within 6–8 attempts. The highest weight achieved represented the 1RM.
Statistics
The estimated sample size of the study was based upon the assumption that a minimum of ten events (i.e., patients) are needed per variable included in an adjusted linear regression analysis to ensure appropriate statistical power [Citation27]. Thus, 50 patients were to be included in the study when accounting for a 10% drop-out rate. All data were tested for normal distribution using Q-plots and histograms. Endpoint changes between several time points during treatment were analyzed using linear mixed models and post hoc analyses. Crude and adjusted linear mixed model analyses were used to investigate whether changes in body composition from pre- to post-treatment differed between patients receiving CCRT (cisplatin or carboplatin) compared to radiation alone.
The following potential effect modifiers were all included in the adjusted analyses: nimorazole (dichotomized to yes or no), tumor stage (dichotomized to I + II vs III + IV), baseline BMI (dichotomized to ≤ or > the median of 26), oral cavity mean radiation dose (Gy) and pharynx constrictor muscles mean radiation dose (Gy). These variables were chosen since they were anticipated to be related to changes in total BW during treatment in HNSCC patients [Citation2,Citation28]. Instead of tumor site as a surrogate for radiation treatment volume and dysphagia or mucositis, the mean radiation dose (Gy) to the pharyngeal constrictor muscles and oral cavity was introduced. The organs at risk were delineated, as described elsewhere [Citation29], in the treatment planning system (Pinnacle, Philips) from which individual mean doses were retrieved. Pre-treatment to post-treatment changes in muscle strength and functional performance were analyzed using Students Paired T-tests. All endpoints were tested statistically using a 5% level of significance. Data are presented as mean values ± SEM. All statistical analyses were performed using STATA software version 17 (Stata Corp LLC, Texas, US).
Results
Fifty-five patients were recruited from April 2015 to July 2017. Seven patients were excluded, leaving 48 patients for primary outcome analyses. Reasons for exclusion were: 1) withdrawal of consent (n = 2), absence from three or more DXA appointments (n = 1), hospitalization because of comorbidity (n = 3), and prophylactic tracheostomy (n = 1). shows that 48 HNC patients in performance status 0–1 were included. Sixteen patients received radiation alone and 32 patients received concurrent chemoradiation (27 cisplatin and 7 patients carboplatin).
Table 1. Patient characteristics.
Body composition
From pre-treatment to 8 weeks after treatment start, total BW declined by 7.2 ± 0.5 kg (8.8%) from 86.6 ± 2.6 to 79.7 ± 2.5 kg (p < .0001; 95% CI: 6.2;8.3 kg), total LBM declined by 4.7 ± 0.4 kg (9.3%) from 59.7 ± 1.8 to 55.4 ± 1.7 kg (p < .0001; 95% CI: 3.9;6 kg), total FM declined by 2.5 ± 0.2 kg (9.0%) from 26.9 ± 1.4 to 24.3 ± 1.5 kg (p < .0001; 95% CI: 2.1;2.9 kg) ( and ).
Figure 1. Changes in total body weight, LBM (lean body mass) and fat mass (FM) during treatment. Specific p-values denote significant difference from previous time-point according to the linear mixed models and post hoc analyses. Data are presented as mean values ± SEM.
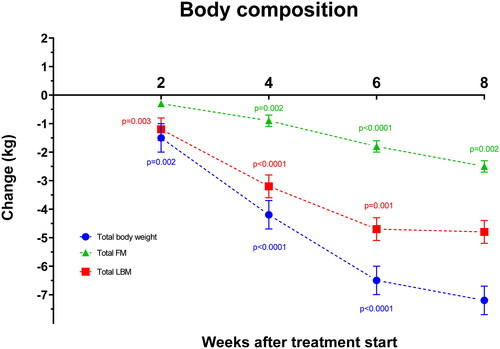
Table 2. Body composition and lower extremity power during the course of treatment.
ALBM declined by 2.1 ± 0.2 kg (8.1%) from 25.8 ± 0.9 to 23.9 ± 0.8 kg (p < .0001; 95% CI: 1.7;2.4 kg), UELBM declined by 0.7 ± 0.08 kg (10.0%) from 7.0 ± 0.3 to 6.4 ± 0.3 kg (p < .0001; 95% CI: 0.5;0.8) and LELBM declined by 1.5±.0.1 kg (8.0%) from 18.7 ± 0.6 to 17.4 ± 0.6 kg (p < .0001; 95% CI: 1.2;1.8 kg) ( and ).
Figure 2. Changes in appendicular lean body mass (ALBM), Upper extremity lean body mass (UELBM) and lower extremity lean body mass (LELBM) during treatment. Specific p-values denote significant difference from previous time-point according to the linear mixed models and post hoc analyses. Data are presented as mean values ± SEM.
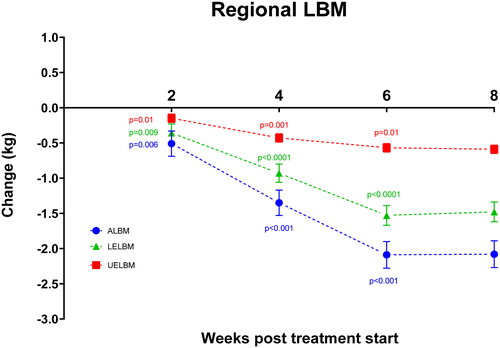
Total BW and all measures of LBM declined significantly already at week 2 and continued to decline significantly through week 4 and 6 before leveling off from week 6 to 8. Thus, every two weeks from treatment start to week 2, week 2–4, and 4–6, total LBM declined by 1.2 ± 0.4 kg (p = .002; 95% CI: 0.4;2.0 kg), 2.0 ± 0.4 kg (p < .0001; 95% CI: 1.2;2.8 kg) and 1.4 ± 0.4 kg (p = 0.001; 95% CI: 0.6;2.2 kg). Similarly, ALBM declined by 0.5 ± 0.2 kg (p = .006; 95% CI: 0.2;0.9 kg), 0.8 ± 0.2 kg (p < .0001; 95% CI: 0.5;1.2 kg) and 0.7 ± 0.2 kg (p < .0001; 95% CI: 0.4;1.1 kg).
The decline in total FM was delayed compared to LBM and was unchanged from pre-treatment to week 2 (p = .2), after which it declined from week 2–4, 4–6, and 6-8 by 0.7 ± 0.2 kg (p = .002; 95% CI: 0.3;0.7 kg), 0.9 ± 0.2 kg (p < .0001; 95% CI: 0.5;1.3 kg) and 0.7 ± 0.2 kg (p = .002; 95% CI: 0.2;1.1 kg).
Maximal muscle strength and functional performance
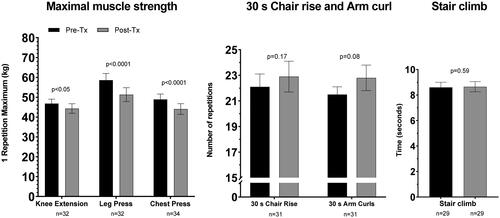
One RM knee extension declined significantly by 2.5 ± 1.2 kg (5.3%) from 47.0 ± 2.3 to 44.5 ± 2.5 kg (n = 32; p = .045; 95% CI: 0.04; 5.0 kg), 1RM leg press declined significantly by 7.2 ± 1.4 kg (12.7%) from 59.2 ± 3.5 to 52.0 ± 3.6 kg (n = 32; p < .0001; 95% CI: 4.4; 10.0 kg) and 1RM chest press declined significantly by 5.0 ± 1.1 kg (10%) from 50.0 ± 2.8 to 44.6 ± 2.7 kg (n = 34; p = .0001; 95% CI: 2.8; 7.2 kg). As shown in none of the functional performance evaluations changed from pre- to post-treatment (n = 29–31). Total and BW-adjusted lower extremity muscle power also did not change during treatment ().
Figure 3. Levels of maximal muscle strength (1 repetition maximum test) and functional performance pre- and post-treatment (30 s chair rise, 30 s arm curl, stair climb). Values expressed as means ± SEM. Specific p-value denotes significant or non-significant difference between time points according to the student’s Paired T-test.
The pre- to post-treatment change in total LBM was significantly associated with a change in 1RM knee extension (R2 = 0.5; p < .0001), 1 RM leg press (R2 = 0.3; p = .002), and 1RM chest press (R2 = 0.4; p = .0001). Similarly, the pre- to post-treatment change in ALBM was associated with the same measures of muscle strength with R2 values equaling 0.5 (p < .0001), 0.3 (p = .002), and 0.4 (p = .0001), respectively.
Changes in body composition after CCRT compared with radiation alone
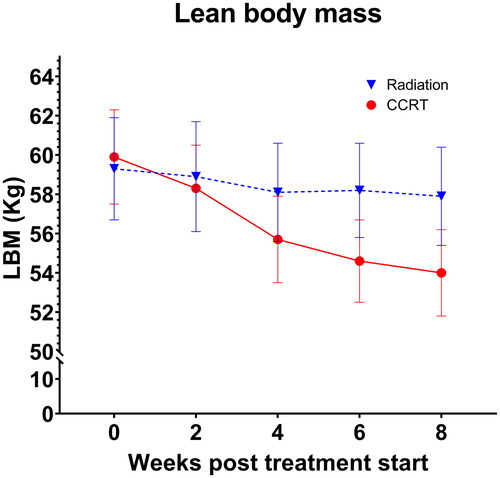
shows the reported levels of total LBM for patients receiving radiation alone and CCRT at various time points. According to the crude linear mixed model analyses, patients receiving CCRT experienced significantly larger body composition changes compared to patients receiving radiation alone. Thus, according to the analysis estimates, total BW declined from pre-treatment to two weeks post-treatment by 4.6 ± 1.0 kg more in CCRT patients compared with patients receiving radiation alone (p < .0001; 95% CI: 2.7;6.6 kg), total LBM declined by 3.1 ± 0.8 kg more (p < .0001; 95% CI: 1.5; 4.7 kg), FM declined by 1.5 ± 0.4 kg (p = .001; 95% CI: 0.7; 2.4 kg) and ALBM declined by 1.2 ± 0.4 kg more (p = .001; 95% CI: 0.5; 2.0 kg).
Figure 4. Observed levels of whole body lean body mass (LBM) during the course of radiation alone or concurrent chemoradiation (CCRT). Data are presented as mean values ± SEM.
The results from the linear mixed model analyses adjusting for tumor stage, mean radiation dose to the oral cavity, mean radiation dose to the constrictor muscles, nimorazole, and baseline BMI were aligned with the crude analyses’ findings. Thus, the estimations from the adjusted mixed models provided larger declines from pre-treatment to two weeks post-treatment in CCRT compared with radiation-alone patients: Total BW decline was 4.1 ± 1.1 kg (p < .0001; 95% CI: 1.9; 6.3 kg) larger for CCRT patients, total LBM was 2.8 ± 0.9 kg (p = .004; 95% CI: 0.9;4.6 kg) larger, total FM was 1.3 ± 0.5 kg (p = .007; 95% CI: 0.4; 2.3 kg) larger and ALBM was 1.1 ± 0.4 kg (p = .009; 95% CI: 0.3;2.0 kg) larger.
Discussion
Our findings are that the 4.7 kg loss of LBM constitutes the greatest part of the total 7.7 kg body mass loss from pre-treatment to two weeks post-treatment. LBM was reduced significantly already 2 weeks into treatment and continued with a substantial 1.2–2 kg bi-weekly decline until the end of treatment, from where it leveled off. Similar results were observed for ALBM. In contrast, the decline pattern in FM was shifted right, not showing a significant decline until four weeks into treatment but was ongoing until two weeks after the end of treatment.
Interestingly, the decline in LBM, ALBM, UELBM, and LELBM were significant and substantial already before the onset of radiation-induced mucositis that have been reported to occur approximately two-three weeks into treatment [Citation30,Citation31]. This could be attributable to nausea or vomiting among patients from cisplatin or nimorazole administration [Citation32]. However, a review of patients records revealed no registrations of nausea during the first four weeks, and previously, in a comparable cohort of patients, only 12–13% of patients reported grade 2 or worse nausea and vomiting over the whole treatment course of cisplatin-based CCRT [Citation33]. In addition, our adjusted linear mixed model analyses did not discern nimorazole, with nausea as a known side effect, as an independent factor of weight loss or loss of LBM, while a significantly larger loss of LBM following the course of CCRT per se was demonstrated. It cannot be excluded that early loss of LBM is affected by tumor/disease burden inducing cancer cachexia and causing a catabolic state and increased systemic inflammation already pre-treatment as reported in comparable HNSCC cohorts previously [Citation34–36].
We acknowledge that the patients’ energy intake and physical activity levels are relevant confounders to the observed early LBM loss. We have no records of individual energy intake and are unable to adjust the analyses for this. Data on physical activity levels was obtained from Physical Activity Scale questionnaires (PAS) [Citation37] collected pre- and two weeks post-treatment. However, the data was regarded insufficient in terms of estimating changes in levels of physical activity with a satisfactory frequency and thus, unreliable as an explanatory confounder for early LBM loss and excluded.
The early and substantial LBM loss occurs despite nutritional support, educational sessions on diet and prophylactic tube insertion at our institution and emphasizes both the severity and complexity of the problem as well as a potential need for even earlier and more effective prophylactic intervention. This may include multimodal approaches such as enhanced nutritional intervention and support [Citation38,Citation39] combined with resistance exercise as shown in the DAHANCA 25 studies [Citation23,Citation26] and other trials [Citation40,Citation41]. Specifically, the randomized controlled DAHANCA 25B trial reported a mean 2.4 kg LBM increase following 12 weeks of resistance training initiated two months post-treatment. Thus, it is likely that resistance training after completion of treatment can reverse the LBM loss to a large extent, however despite ongoing initiatives [Citation40], it remains to be investigated whether resistance training from the onset of treatment is feasible and can prevent or reduce LBM loss. In HNSCC patients who likely struggle to meet the nutritional demands, aerobic exercise should be avoided since this does not increase LBM, but may increase energy expenditure and exacerbate the loss of LBM and FM [Citation42].
All measures of maximal muscle strength were significantly impaired post-treatment, and the changes were significantly associated with the reduction in both whole body LBM and ALBM. This aligns with cross-sectional data from the DAHANCA 25 studies based on similar evaluation methods [Citation11] and a previous study assessing muscle strength objectively [Citation9,Citation43]. The associations were low to moderate (R2 between 0.3 and 0.5) leaving 30–50% of the variation in muscle strength decline explained by variation in LBM decline. Other factors that affect maximal muscle strength include changes in neural muscle activation and interaction between the working muscles, as well as motor skills [Citation44]. Impairment in these parameters may contribute to declines in muscle strength, however, the present study has no data to investigate this.
In contrast, functional performance measures remained unchanged during treatment, contradicting our hypothesis and previous findings indicating a significant but weaker relationship between levels of LBM and functional performance [Citation11]. Typically, functional performance relies not only on muscle mass and strength but also on neural factors and motor skills [Citation23]. Consequently, if patients engage daily in regular activities such as climbing stairs and getting up from chairs, they may maintain their performance levels during treatment even with LBM reductions. Furthermore, a sustained higher level of performance induces the risk of a ceiling effect in the performance tests, and this could affect the sensitivity of the tests to identify any changes. We also acknowledge the potential for a learning effect among patients from pre- to post-treatment; however, the two tests were conducted approximately eight weeks apart, and any learning effect was mitigated through familiarization before each test.
Remarkably, our data shows that cisplatin-based CCRT was associated with significantly larger decrements in whole-body LBM (3.1 kg) and ALBM (1.2 kg) compared with radiation alone. Thus, our observations lend support to the hypothesis that platinum may exert a direct toxic effect on muscle cells. This has been proposed from data derived from animal-models [Citation18,Citation45].
Given the reported association between LBM loss and loss of muscle strength in the present cohort as well as other cohorts of HNSCC patients [Citation11], the accelerated LBM loss following CCRT may potentially worsen the physical impairments in this sub-group. A subsequent, explorative analysis showed that change in 1RM chest press was significantly larger (p = .03; Students unpaired T-test) in patients receiving CCRT (−7.0 ± 1.4 kg) compared with radiation alone (−2.1 ± 1.4 kg). Showing similar trends, but statistically insignificant, 1RM leg press and 1RM knee extension change were larger in patients receiving CCRT (−8.6 ± 2.2 kg and −4.0 ± 1.6 kg, respectively) compared with radiation alone (−5.2 ± 1.0 kg and −0.3 ± 1.6 kg, respectively). The explorative nature and low sample size should be considered when interpreting these findings and further studies are warranted. Nonetheless, together with the exacerbated LBM loss, these findings provide interesting characteristics of the short-term treatment impact in the CCRT subgroup which could be important arguments in terms of initiating more intense dietary and exercise interventions as early as possible.
Strengths and limitations
In contrast to other studies [Citation6,Citation9], this study meticulously investigated changes in body composition bi-weekly during treatment in HNSCC patients from the gold standard, Dual-energy X-ray Absorptiometry. This enabled reliable investigations within a shorter time frame of ongoing radiation treatment, providing a more comprehensive understanding of the impact of treatment in HNSCC patients. To our knowledge, the present study is the first clinical study to investigate the impact of cisplatin-based chemotherapy on LBM – not only in HNSCC patients but in cancer patients in general.
Despite a low coefficient of variation of LBM using DXA scans in the present cohort, we acknowledge that the hydration status of the patients may not be fully standardized between DXA scans. Thus, there is a risk that the variations in the different measures of body composition are increased which should be considered when interpreting the specific changes in all DXA endpoints.
Given the rather small sample size, the number of confounding factors that can be included in the analyses are limited. Specifically, energy intake and physical activity levels would have been relevant to include. As mentioned earlier insufficient data on physical activity, disqualified the inclusion of this confounder in the analyses of early LBM loss. Preliminary analyses of the data revealed no significant difference between the fraction of patients not participating in regular exercise (68–77% of the cohort) [Citation37] and thus, this parameter was excluded from further analyses of overall changes in body composition endpoints.
We acknowledge the risk of selection-bias in generalizing the findings of the study since the single-center population is both relatively small and represents a group of well-functioning patients (WHO performance score 0–1). Specifically, a number of patients were unable to complete the tests for muscle strength and functional performance post-treatment due to dizziness, fatigue, and low energy-levels which reduced statistical power. Consequently, the present findings should be translated only cautiously to other populations, and should not act as general predictions for body composition changes.
Conclusion
This study demonstrated accelerated, and substantial, loss of whole-body LBM already within the first two weeks of treatment before the onset of radiation-induced mucositis. In comparison, FM loss was delayed by two weeks. Maximal muscle strength, but not functional performance, declined significantly during treatment; the former was associated with loss of LBM. Additionally, this is the first clinical study to show that cisplatin-based CCRT is associated with exacerbated loss of LBM compared with radiation alone. Altogether, the findings add novel information and details that further emphasize the clinical importance of developing more effective strategies including targeted resistance training and nutritional therapy to combat the substantial reductions in LBM and muscle strength in HNSCC patients – particularly following cisplatin-based CCRT.
Acknowledgements
We acknowledge the A.P. Møller Foundation for the Advancement of Medical Science (ID 13-259) for funding the present study; Dorthe Grunske Schmidt, Registered Nurse, Dept. of Oncology, Odense University Hospital, Denmark, for handling logistics and patient flow excellently; and Bo Martin Bibby, associate professor, and Nina Sjørup Simonsen, M.Sc., from the Dept. of Public Health, Aarhus University, Denmark for their competent statistical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author (SL). The data are not publicly available due to the General Data Protection Regulation (GDPR) to ensure privacy and security of personal data.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Ottosson S, Zackrisson B, Kjellen E, et al. Weight loss in patients with head and neck cancer during and after conventional and accelerated radiotherapy. Acta Oncol. 2013;52(4):711–718. doi: 10.3109/0284186X.2012.731524.
- Lønbro S, Petersen GB, Andersen JR, et al. Prediction of critical weight loss during radiation treatment in head and neck cancer patients is dependent on BMI. Support Care Cancer. 2016;24(5):2101–2109. doi: 10.1007/s00520-015-2999-8.
- Langius JA, van Dijk AM, Doornaert P, et al. More than 10% weight loss in head and neck cancer patients during radiotherapy is independently associated with deterioration in quality of life. Nutr Cancer. 2013;65(1):76–83. doi: 10.1080/01635581.2013.741749.
- Langius JA, Bakker S, Rietveld DH, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer. 2013;109(5):1093–1099. doi: 10.1038/bjc.2013.458.
- Ferrão B, Neves PM, Santos T, et al. Body composition changes in patients with head and neck cancer under active treatment: a scoping review. Support Care Cancer. 2020;28(10):4613–4625. doi: 10.1007/s00520-020-05487-w.
- Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck. 2007;29(10):893–900. doi: 10.1002/hed.20607.
- Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34(7):631–636. doi: 10.1055/s-0032-1323746.
- Ng K, Leung SF, Johnson PJ, et al. Nutritional consequences of radiotherapy in nasopharynx cancer patients. Nutr Cancer. 2004;49(2):156–161. doi: 10.1207/s15327914nc4902_6.
- Jager-Wittenaar H, Dijkstra PU, Vissink A, et al. Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck. 2011;33(6):863–870. doi: 10.1002/hed.21546.
- Almada-Correia I, Neves PM, Mäkitie A, et al. Body composition evaluation in head and neck cancer patients: a review. Front Oncol. 2019;9:1112. doi: 10.3389/fonc.2019.01112.
- Lønbro S, Dalgas U, Primdahl H, et al. Lean body mass and muscle function in head and neck cancer patients and healthy individuals–results from the DAHANCA 25 study. Acta Oncol. 2013;52(7):1543–1551. doi: 10.3109/0284186X.2013.822553.
- Wendrich AW, Swartz JE, Bril SI, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017;71:26–33. doi: 10.1016/j.oraloncology.2017.05.012.
- Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of Radiotherapy-Treated head and neck squamous cell carcinoma. JAMA Oncol. 2016;2(6):782–789. doi: 10.1001/jamaoncol.2015.6339.
- Fairman CM, Lønbro S, Cardaci TD, et al. Muscle wasting in cancer: opportunities and challenges for exercise in clinical cancer trials. JCSM Rapid Commun. 2022;5(1):52–67. doi: 10.1002/rco2.56.
- Hojman P, Fjelbye J, Zerahn B, et al. Voluntary exercise prevents cisplatin-induced muscle wasting during chemotherapy in mice. PLOS One. 2014;9(9):e109030. doi: 10.1371/journal.pone.0109030.
- Hiensch AE, Bolam KA, Mijwel S, et al. Doxorubicin-induced skeletal muscle atrophy: elucidating the underlying molecular pathways. Acta Physiol. 2020;229(2):1–18. doi: 10.1111/apha.13400.
- Schneider CM, Dennehy CA, Roozeboom M, et al. A model program: exercise intervention for cancer rehabilitation. Integr Cancer Ther. 2002;1(1):76–82; discussion 82. doi: 10.1177/153473540200100117.
- Coletti D. Chemotherapy-induced muscle wasting: an update. Eur J Transl Myol. 2018;28(2):153–157. doi: 10.4081/ejtm.2018.7587.
- Møller AB, Lønbro S, Farup J, et al. Molecular and cellular adaptations to exercise training in skeletal muscle from cancer patients treated with chemotherapy. J Cancer Res Clin Oncol. 2019;145(6):1449–1460. doi: 10.1007/s00432-019-02911-5.
- Mijwel S, Cardinale DA, Norrbom J, et al. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. Faseb J. 2018;32(10):5495–5505. doi: 10.1096/fj.201700968R.
- Jensen K, Friborg J, Hansen CR, et al. The danish head and neck cancer group (DAHANCA) 2020 radiotherapy guidelines. Radiother Oncol. 2020;151:149–151. doi: 10.1016/j.radonc.2020.07.037.
- Toomey CM, McCormack WG, Jakeman P. The effect of hydration status on the measurement of lean tissue mass by dual-energy X-ray absorptiometry. Eur J Appl Physiol. 2017;117(3):567–574. doi: 10.1007/s00421-017-3552-x.
- Lønbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy – Results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108(2):314–319. doi: 10.1016/j.radonc.2013.07.002.
- Alcazar J, Kamper RS, Aagaard P, et al. Relation between leg extension power and 30-s sit-to-stand muscle power in older adults: validation and translation to functional performance. Sci Rep. 2020;10:16337. doi: 10.1038/s41598-020-73395-4.
- Lønbro S. The effect of progressive resistance training on lean body mass in post-treatment cancer patients – A systematic review. Radiother Oncol. 2014;110(1):71–80. doi: 10.1016/j.radonc.2013.07.008.
- Lønbro S, Dalgas U, Primdahl H, et al. Feasibility and efficacy of progressive resistance training and dietary supplements in radiotherapy treated head and neck cancer patients-the DAHANCA 25A study. Acta Oncol. 2013;52(2):310–318. doi: 10.3109/0284186X.2012.741325.
- Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3.
- Zhao JZ, Zheng H, Li LY, et al. Predictors for weight loss in head and neck cancer patients undergoing radiotherapy: a systematic review. Cancer Nurs. 2015;38(6):E37–E45. doi: 10.1097/NCC.0000000000000231.
- Brouwer CL, Steenbakkers RJHM, Bourhis J, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(1):83–90. doi: 10.1016/j.radonc.2015.07.041.
- Dische S, Saunders M, Barrett A, et al. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol. 1997;44(2):123–136. doi: 10.1016/s0167-8140(97)00094-7.
- Mortensen HR, Overgaard J, Specht L, et al. Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6&7 randomised trial with accelerated radiotherapy for head and neck cancer. Radiother Oncol. 2012;103(1):69–75. doi: 10.1016/j.radonc.2012.01.002.
- Metwally MAH, Frederiksen KD, Overgaard J. Compliance and toxicity of the hypoxic radiosensitizer nimorazole in the treatment of patients with head and neck squamous cell carcinoma (HNSCC). Acta Oncol. 2014;53(5):654–661. doi: 10.3109/0284186X.2013.864050.
- Driessen CML, Janssens GO, Van Der Graaf WTA, et al. Toxicity and efficacy of accelerated radiotherapy with concurrent weekly cisplatin for locally advanced head and neck carcinoma. Head Neck. 2016;38(Suppl 1):E559–E565. doi: 10.1002/hed.24039.
- Jager-Wittenaar H, Dijkstra PU, Dijkstra G, et al. High prevalence of cachexia in newly diagnosed head and neck cancer patients: an exploratory study. Nutrition. 2017;35:114–118. doi: 10.1016/j.nut.2016.11.008.
- Solís-Martínez O, Álvarez-Altamirano K, Cardenas D, et al. Cancer cachexia affects patients with head and neck cancer in all stages of disease: a prospective cross-sectional study. Nutr Cancer. 2022;74(1):82–89. doi: 10.1080/01635581.2020.1869792.
- Ohmae N, Yasui-Yamada S, Furumoto T, et al. Muscle mass, quality, and strength; physical function and activity; and metabolic status in cachectic patients with head and neck cancer. Clin Nutr ESPEN. 2023;53:113–119. doi: 10.1016/j.clnesp.2022.12.006.
- Møller PK, Schmidt DG, Kaalund I, et al. PO-1092: weight loss and patient-reported daily activity after curative radiotherapy for head and neck cancer. Radiother Oncol. 2018;127:S614–S615.
- Ackerman D, Laszlo M, Provisor A, et al. Nutrition management for the head and neck cancer patient. Cancer Treat Res. 2018;174:187–208.
- Talwar B, Donnelly R, Skelly R, et al. Nutritional management in head and neck cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S32–S40. doi: 10.1017/S0022215116000402.
- Lonkvist CK, Lønbro S, Vinther A, et al. Progressive resistance training in head and neck cancer patients during concomitant chemoradiotherapy – design of the DAHANCA 31 randomized trial. BMC Cancer. 2017;17(1):400. doi: 10.1186/s12885-017-3388-0.
- Rogers LQ, Anton PM, Fogleman A, et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck. 2013;35(8):1178–1188. doi: 10.1002/hed.23118.
- Mavropalias G, Sim M, Taaffe DR, et al. Exercise medicine for cancer cachexia: targeted exercise to counteract mechanisms and treatment side effects. J Cancer Res Clin Oncol. 2022;148(6):1389–1406.
- Jager-Wittenaar H, Dijkstra PU, Vissink A, et al. Malnutrition and quality of life in patients treated for oral or oropharyngeal cancer. Head Neck. 2011;33(4):490–496. doi: 10.1002/hed.21473.
- Schenkman M, Hughes MA, Samsa G, et al. The relative importance of strength and balance in chair rise by functionally impaired older individuals. J Am Geriatr Soc. 1996;44(12):1441–1446. doi: 10.1111/j.1532-5415.1996.tb04068.x.
- Conte E, Bresciani E, Rizzi L, et al. Cisplatin-Induced skeletal muscle dysfunction: mechanisms and counteracting therapeutic strategies. Int J Mol Sci. 2020;21:1242. doi: 10.3390/ijms21041242.

