Abstract
Background
The randomized clinical trial ESO-SPARE investigates if oesophagus-sparing radiotherapy (RT) can reduce dysphagia in patients with metastatic spinal cord compression (MSCC). Patient-reported outcome (PRO) is the only follow-up measure. Due to the fragile patient population, low respondent compliance was anticipated. We performed a planned interim analysis of dosimetry and respondent compliance, to ensure that the protocol requirements were met.
Methods
Patients >18 years referred for cervical/thoracic MSCC radiotherapy in 1-10 fractions were included from two centres. Patients were randomized (1:1) to standard RT or oesophagus-sparing RT, where predefined oesophageal dose constraints were prioritized over target coverage. Patients completed a trial diary with daily reports of dysphagia for 5 weeks (PRO-CTC-AE) and weekly quality of life reports for 9 weeks (QLQ-C30, EQ-5D-5L). According to power calculation, 124 patients are needed for primary endpoint analysis. The sample size was inflated to 200 patients to account for the fragile patient population. The co-primary endpoints, peak patient-reported dysphagia, and preserved ability to walk (EQ-5D-5L), are analysed at 5 and 9 weeks, respectively. The interim analysis was conducted 90 days after the inclusion of patient no 100. Respondent compliance was assessed at 5 and 9 weeks. In all RT plans, oesophagus and target doses were evaluated regarding adherence to protocol constraints.
Results
From May 2021 to November 2022, 100 patients were included. Fifty-two were randomized to oesophagus-sparing RT. In 23% of these plans, oesophagus constraints were violated. Overall, the dose to both target and oesophagus was significantly lower in the oesophagus-sparing plans. Only 51% and 41% of the patients were evaluable for co-primary endpoint analysis at five and nine weeks, respectively. Mortality and hospitalization rates were significantly larger in patients who completed <4 days PRO questionnaires.
Conclusion
Compliance was lower than anticipated and interventions to maintain study power are needed.
Introduction
Metastatic spinal cord compression (MSCC) is a feared complication of metastatic cancer, affecting 5–10% of all cancer patients [Citation1]. In addition to high-dose corticosteroids most patients are treated with palliative radiotherapy (RT), either alone or in combination with decompressive surgery [Citation1,Citation2].
Patients diagnosed with MSCC compose a heterogeneous and fragile cohort, with a median survival of a few months and only a minority of long-term survivors [Citation3–7]. The primary aim of palliative RT is to improve quality of life (QoL) by reducing pain and preventing loss of motor and sensory function distal to the lesion. Paradoxically some patients experience acute toxicity after treatment, potentially affecting their quality of life [Citation8]. Since life expectancy for most MSCC patients is short, acute toxicity of any grade is important to consider.
There is little focus on preventing acute toxicity in MSCC treatment planning, where organs at risk (except the spinal cord) are not routinely delineated [Citation9].
Post-RT esophagitis is a well-known acute toxicity in palliative lung cancer patients [Citation10]. The PROACTIVE [Citation11] trial randomized patients with advanced central lung tumours to either standard or oesophagus-sparing intensity-modulated radiotherapy (IMRT). Even though there was no significant improvement in oesophageal QoL in the oesophagus-sparing arm, the treatment did significantly reduce symptomatic oesophagitis [Citation11]. Similarly, sparing of the contralateral oesophagus wall resulted in reduced risk of oesophagitis in a phase 1 study including patients with locally advanced lung cancer treated with high-dose chemoradiation [Citation12].
In six [Citation3–7,Citation13] randomized trials comparing different fractionations schedules for MSCC, five trials [Citation4–7,Citation13] reported grade 1–2 dysphagia in 3–14% of patients, and three trials reported grade 3 dysphagia in 1% of patients [Citation4–6]. One trial did not report toxicity below grade 2 [Citation3].
Only one prospective study systematically investigated upper gastrointestinal toxicity after irradiation for MSCC. Gram et al. [Citation8] found that 79% of patients treated with 30 Gy/10 fractions (fx) at the level of the oesophagus experienced oesophageal toxicity lasting 11 days on average, suggesting that acute oesophageal toxicity is underreported [Citation8].
The ESO-SPARE trial investigates if oesophagus-sparing RT can decrease patient-reported dysphagia in patients with MSCC in the cervical and thoracic spine. Follow-up consists solely of patient-reported outcomes (PRO).
The study design faces several challenges.
First, previous randomized trials of patients with MSCC reported a high-drop-out rate due to high early mortality [Citation3,Citation6,Citation7,Citation13].
Second, the two trials employing PRO measures (PROMs) in their follow-up procedure report compliance rates of only 55% and 70–75% at four and five weeks, respectively [Citation14,Citation15].
Third, in a feasibility study, sparing of the oesophagus led to dose plans with underdosing of the anterior vertebrae, potentially compromising clinical target volume (CTV) and planning target volume (PTV) coverage [Citation9]. The feasibility of oesophagus-sparing treatment planning has not been clinically tested prior to study initiation.
To ensure that these challenges were adequately addressed, we performed a planned interim analysis to evaluate the dosimetry parameters, compliance, and early mortality rate in the first 100 included patients.
Methods
We used the CONSORT 2010 reporting guidelines when writing our report [Citation16], for CONSORT 2010 checklist see Supporting Information 1.
The ongoing ESO-SPARE trial is a multicentre, single-blind, parallel-group phase 3 clinical trial, with 1:1 randomization. Patients are enrolled directly in the radiotherapy departments from two academic cancer centres (Copenhagen University Hospital – Herlev and Gentofte, and Copenhagen University Hospital – Rigshospitalet). The trial is conducted after approval by the local ethic committee (Reginal Scientific Ethical Committee, Capital Region, Denmark) and is performed in accordance with the Helsinki Declaration. It is registered at clinicaltrials.gov (registration date 25 October 2021, identifier: NCT05109819).
Inclusion criteria are verified cancer of any type, >18 years of age, and referred for cervical or thoracic MSCC RT of maximum 10 fractions. Patients with soft tissue involvement, prior RT, and patients referred for postoperative treatment are not excluded.
After ensuring informed consent, patients are randomized 1:1 to either standard or oesophagus-sparing radiotherapy. Randomization is stratified by centre and fractionation. Patients are blinded to randomization outcomes. Investigators remain blinded to primary outcomes throughout the study including this interim analysis.
Patients are treated with either volumetric modulated arc therapy (VMAT) or IMRT. The CTV includes the affected vertebral body and any adjacent soft tumour tissue. Cranial-caudal delineation is limited by the upper and lower edge of the affected vertebrae. A PTV margin of 0.5 cm is added. The oesophagus is delineated in all patients. For patients treated in the cervical spine, the posterior pharyngeal wall, from the top of C1 to the beginning of the oesophagus, is delineated as well.
In the experimental arm, dose constraints to the oesophagus and posterior pharyngeal are prioritized by compromising the dose to target. The constraints differ between fractionation schemes, but all correspond to the same biological equivalent dose, Supporting Information Table A. In the standard arm, we aim to cover the PTV with at least 90% of the prescribed dose. In special cases, e.g., soft tissue involvement where the CTV compromise is deemed too large, deviation from protocol is at the discretion of the treating physician.
The nine-week follow-up consists exclusively of patient-reported outcomes. Dysphagia, dyspepsia, nausea, and treatment site pain on a Numeric Pain Rating Scale (NPRS) are reported daily for 5 weeks and subsequently weekly for four weeks. Medicine consumption, weight, EQ-5D-5L, and EORTC QLQ-30 are reported weekly.
The trial investigates if oesophagus-sparing radiotherapy can reduce dysphagia in patients with MSCC in the cervical and thoracic spine. Co-primary endpoints are (a) early patient-reported dysphagia, measured as NCI PRO-CTCAE v.1.0 peak score within the first five weeks after treatment start and (b) preserved ability to walk nine weeks after treatment start, measured by the EQ-5D-5L mobility dimension According to power calculations 62 patients in each arm are needed for primary endpoint analysis.
To account for the fragile study population sample size was inflated to include 200 patients.
Data were analysed 90 days after the inclusion of patient no. 100.
Tokuhashi score was computed retrospectively [Citation17]. The compliance rate was assessed at five and nine weeks, corresponding to time points of co-primary outcome analysis. Patients who complete less than four days of questionnaires were defined as nonrespondents. Patients who completed more than four days of questionnaires were defined as respondents. Survival analysis comparing respondents and nonrespondents was done using the Kaplan–Meier method and Log-rank test. Dose plans were analysed with respect to the volume of the PTV that is covered by the 90% isodose (PTV V90%), the volume of the CTV covered by the 95% isodose (CTV V95%), and the minimum dose to the hottest 0.027 cm3 and 1 cm3 of the combined volume of the oesophagus and the pharyngeal wall (EsoD0.027 cm3, and EsoD1cm3, respectively). For patients with several concurrent plans, the plan with the highest oesophageal/pharyngeal dose was included in the analysis. Data analysis was performed using R version 4.2.2 and IBM SPSS Statistics 28.
Results
From May 2021 to December 2022, 100 patients were included. Patient and treatment characteristics are listed in . The majority were males (64%), and the most common diagnoses were lung, prostate, and breast cancer (27%, 23%, and 12%, respectively). Most patients (60%) were in PS 0-1. Only 8% of patients received single fraction treatment. Most patients had multiple spinal targets (77%), and most targets were in the thoracic spine (n = 204, 65%).
Table 1. Baseline characteristics of the first 100 patients of the ESO-SPARE trial.
Thirty-four (34%) patients were nonrespondents and only 51% and 41% of patients were evaluable for co-primary endpoint analysis at five and nine weeks, respectively. Patient and treatment characteristics for respondents and nonrespondents are listed in .
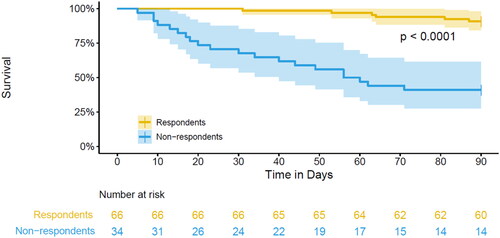
Nonrespondents had a significantly higher performance status, a lower Tokuhashi score and were more hospitalized during the nine-week follow-up, . Survival was significantly worse for nonrespondents, with a 90-day mortality of 67% compared to 9% of respondents, . Despite the high-mortality rate, only 12% of nonrespondents received single-fraction treatment.
Figure 1. Overall survival in respondents and nonrespondents of PROMs in the first 100 patients of the ESO-SPARE trial.
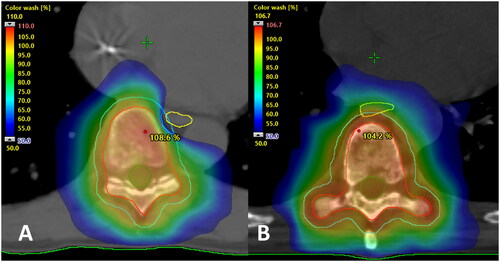
Dosimetry parameters are summarized in . Eight patients received single fraction treatment, five in the oesophagus-sparing arm and three in the standard arm. There was a seemingly less dosimetric difference between the arms for the single fraction group. This is caused by there being few patients in this group, violation of oesophageal constraints in two of five oesophagus-sparing plans (the posterior pharyngeal wall was not delineated), and one plan in the standard arm had a low oesophageal dose (5.4 Gy) due to anatomical location far away from the target.
Table 2. Dosimetry parameters of the first 100 patients in the ESO-SPARE trial.
In the 25 Gy/5 fx and 30 Gy/10 fx groups, the oesophageal dose was lower in the oesophagus-sparing arm, at the cost of PTV and CTV coverage. An example of an oesophagus-sparing plan is shown in .
Overall, oesophageal constraints were compromised in 12 (23%) of the oesophagus-sparing plans. The median constraint deviation was 5.6 Gy, range 0.2–21.1 Gy. This was due to protocol violations (organ at risk not delineated, n = 6), and physicians’ choice due to concerns about CTV/PTV coverage (n = 6).
Discussion
For patients with MSCC in the cervical and thoracic spine, treated with five or ten fractions, sparing of the oesophagus was accomplished at the cost of PTV and CTV coverage, as prescribed in the protocol. Trial diary compliance was lower than anticipated, with only 51% and 41% of the patients being evaluable for co-primary endpoint analysis at five and nine weeks, respectively. As expected, nonrespondents were more fragile, had significantly lower performance status, higher hospitalization rate, and higher mortality compared to respondents.
To our knowledge, ESO-SPARE is the first randomized trial introducing organs at risk delineation to prevent acute toxicity in patients with MSCC. Furthermore, it is the first randomized trial in this patient cohort to employ PRO questionnaires as the only follow-up.
Previous randomized trials of patients with MSCC reported a high-drop-out rate due to high early mortality with a median survival of 3.2–6 months [Citation3–7]. One noninferiority trial, comparing 10 Gy/1 fx to 20 Gy/5 fx emphasized the difference in median survival and performance status between eligible and evaluable patients [Citation7]. Median survival was three months in eligible patients compared to 6.4 months in evaluable patients. Correspondingly eligible patients had a median Karnofsky performance status of 50% compared to 70% in evaluable patients, indicating that the most fragile patients do not complete follow-up [Citation7]. These findings are in accordance with our study, where we found a significant difference in performance status, hospitalization rate, and survival between nonrespondents and respondents.
PRO assessment of acute toxicity was deemed the most relevant outcome in this patient cohort where the primary treatment aim is to improve or maintain QoL. To avoid burdening the patients, additional hospital visits and examinations were excluded from follow-up. Focus was exclusively on a short daily PRO questionnaire (five questions from the PRO-CTC-AE library) in addition to weekly reports of quality of life, weight, and medicine consumption. Daily PRO questionnaires were chosen to document the full scope of acute oesophageal toxicity.
Two other randomized trials comparing different radiation schedules for MSCC, included PROs as part of their follow-up procedure [Citation14,Citation15]. The SCORE-2 trial [Citation15] investigated patient-reported distress and pain at baseline, directly after, and at 1, 3, and 6 months after RT. Overall, 149 (73%) and 138 (70%) patients were evaluable for distress and pain assessment one month after RT [Citation15].
In the ICOG 05-03 trial [Citation14], patients-reported QoL by EORTC QLQ-C30 prior to treatment, at five weeks and every three months thereafter until death. At five weeks 57 (49%) had completed QoL questionnaires and only 35 (30%) of patients were evaluable at three months [Citation14].
The use of PRO questionnaires in the ESO-SPARE trial is more extensive compared to the previously mentioned trials. Completion of daily questionnaires relies highly on patient involvement. Despite our intentions to simplify the follow-up procedure, it has proven difficult for the most fragile MSCC patients to complete follow-up. This shows that, although highly relevant, unaided PRO questionnaires are not the optimal follow-up tool in this patient population.
To mirror the real-world patient population, the ESO-SPARE trial was pragmatically designed with no objective exclusion criteria regarding performance status or predicted life expectancy. Patients who were able to complete follow-up were subjectively selected by the recruiting physicians and RTT’s from the two centres. Furthermore, objective scoring systems, predicting outcome and survival after RT are not routinely used to guide RT prescription in our clinics [Citation17–20]. These factors may have contributed to the low compliance rate, high-mortality rate, and low share of single-fraction treatments in this study population.
Evidence suggests that single-fraction treatment is equivalent to longer fractionation schemes in terms of preserved motor function and pain control [Citation5–7,Citation21]. This is emphasized in the updated ESTRO-ACROP guidelines where a single dose of 8–10 Gy is recommended for all MSCC patients not fit for surgery [Citation2]. The results of this interim analysis demonstrate that multifraction regimes are still widely used, even in patients with low life expectancy. In nonrespondents, a group of patients with a median survival of less than two months, only four (12%) received single fraction treatment.
Due to missing delineations of oesophagus and posterior pharyngeal wall, oesophageal constraints were violated in 6 (11.5%) of oesophagus-sparing plans. Patients with MSCC, especially fragile patients treated with a single fraction, often start treatment the same day they are planned. This leaves little time for plan quality assurance. Automation of the planning process including AI-driven autocontouring of organs at risk may help to solve this problem.
Conclusion
In this interim analysis of the randomized ESO-SPARE trial PRO compliance was lower than expected. Nonrespondents were more fragile with a significantly lower performance status, higher hospitalization rate, and higher mortality compared to respondents. Sparing of the oesophagus was accomplished in most patients in the intervention arm.
Supplemental Material
Download MS Word (25.1 KB)Supplemental Material
Download MS Word (15 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author, AMN, upon reasonable request, after the termination of the ESO-SPARE trial.
Additional information
Funding
References
- Cole JS, Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7(5):459–466. doi: 10.1016/S1474-4422(08)70089-9.
- Oldenburger E, Brown S, Willmann J, et al. ESTRO ACROP guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother Oncol. 2022;173:240–253. doi: 10.1016/j.radonc.2022.06.002.
- Rades D, Šegedin B, Conde-Moreno AJ, et al. Radiotherapy with 4 Gy × 5 versus 3 Gy × 10 for metastatic epidural spinal cord compression: final results of the SCORE-2 trial (ARO 2009/01). J Clin Oncol. 2016;34(6):597–602. doi: 10.1200/JCO.2015.64.0862.
- Maranzano E, Bellavita R, Rossi R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23(15):3358–3365. doi: 10.1200/JCO.2005.08.193.
- Maranzano E, Trippa F, Casale M, et al. 8 Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol. 2009;93(2):174–179. doi: 10.1016/j.radonc.2009.05.012.
- Hoskin PJ, Hopkins K, Misra V, et al. Effect of single-fraction vs multifraction radiotherapy on ambulatory status among patients with spinal canal compression from metastatic cancer: the SCORAD randomized clinical trial. JAMA. 2019;322(21):2084–2094. doi: 10.1001/jama.2019.17913.
- Thirion PG, Dunne MT, Kelly PJ, et al. Non-inferiority randomised phase 3 trial comparing two radiation schedules (single vs. five fractions) in malignant spinal cord compression. Br J Cancer. 2020;122(9):1315–1323. doi: 10.1038/s41416-020-0768-z.
- Gram V, Fog LS, Hemer M, et al. Patient reported upper gastro-intestinal symptoms associated with fractionated image-guided conformal radiotherapy for metastatic spinal cord compression. Tech Innov Patient Support Radiat Oncol. 2020;13:1–5. doi: 10.1016/j.tipsro.2019.11.008.
- Gram VR, Gram D, Persson GF, et al. Reduction of oesophageal toxicity with VMAT dose-sparing radiotherapy in thoracic metastatic spinal cord compression: a feasibility study. Tech Innov Patient Support Radiat Oncol. 2022;23:8–14. doi: 10.1016/j.tipsro.2022.07.001.
- Stevens R, Macbeth F, Toy E, et al. Palliative radiotherapy regimens for patients with thoracic symptoms from non‐small cell lung cancer. Cochrane Database Syst Rev. 2015;1:CD002143.
- Louie AV, Granton PV, Fairchild A, et al. Palliative radiation for advanced Central lung tumors with intentional avoidance of the esophagus (PROACTIVE): a phase 3 randomized clinical trial. JAMA Oncol. 2022;8(4):1–7. doi: 10.1001/jamaoncol.2021.7664.
- Kamran SC, Yeap BY, Ulysse CA, et al. Assessment of a contralateral esophagus-sparing technique in locally advanced lung cancer treated with high-dose chemoradiation. A phase 1 nonrandomized trial. JAMA Oncol. 2021;7(6):910–914. doi: 10.1001/jamaoncol.2021.0281.
- Abu-Hegazy M, Wahba HA. Single-versus multi-fraction radiation treatment for metastatic spinal cord compression: functional outcome study. Chin Ger J Clin Oncol. 2011;10(9):535–540. doi: 10.1007/s10330-011-0832-5.
- Lee KA, Dunne M, Small C, et al. ICORG 05-03: prospective randomized non-inferiority phase III trial comparing two radiation schedules in malignant spinal cord compression (not proceeding with surgical decompression); the quality-of-life analysis. Acta Oncol. 2018;57(7):965–972. doi: 10.1080/0284186X.2018.1433320.
- Rades D, Šegedin B, Conde-Moreno AJ, et al. Patient-reported outcomes-secondary analysis of the SCORE-2 trial comparing 4 Gy × 5 to 3 Gy × 10 for metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2019;105(4):760–764. doi: 10.1016/j.ijrobp.2019.08.002.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232.
- Tokuhashi Y, Uei H, Oshima M, et al. Scoring system for prediction of metastatic spine tumor prognosis. World J Orthop. 2014;5(3):262–271. doi: 10.5312/wjo.v5.i3.262.
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119(4):897–903. doi: 10.1002/cncr.27849.
- Rades D, Rudat V, Veninga T, et al. A score predicting posttreatment ambulatory status in patients irradiated for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2008;72(3):905–908. doi: 10.1016/j.ijrobp.2008.02.018.
- Bartels RHMA, Feuth T, Van Der Maazen R, et al. Development of a model with which to predict the life expectancy of patients with spinal epidural metastasis. Cancer. 2007;110(9):2042–2049. doi: 10.1002/cncr.23002.
- Donovan EK, Sienna J, Mitera G, et al. Systematic review single versus multifraction radiotherapy for spinal cord compression: a systematic review and meta-analysis. Radiother Oncol. 2019;134:55–66. doi: 10.1016/j.radonc.2019.01.019.