Abstract
Background
The socioeconomic differences in survival are pronounced for patients diagnosed with head and neck cancer; disease stage at diagnosis is suggested to be a main driver of this association. This nationwide, population-based study investigates socioeconomic differences in the pre-diagnostic interval and disease stage at diagnosis.
Material and Methods
Information on patient-reported symptoms, symptom onset and disease-specific factors was obtained from the nationwide population-based Danish Head and Neck Cancer Group (DAHANCA) database for patients diagnosed with head and neck squamous cell carcinoma between 2008 and 2019 in Denmark. Socioeconomic position (SEP) was measured by individual-level education, income and cohabitation status obtained from administrative registers. Socioeconomic differences in the interval from symptom onset to diagnosis were investigated in general linear models with 95% confidence intervals (CIs); overall and by subsite, symptom and comorbidity score. Consultation patterns prior to diagnosis were examined using methods for change-point detection. Associations with advanced-stage disease were estimated in logistic regression models.
Results
Patients with low, medium and high SEP had a similar interval from patient-reported symptom onset to diagnosis of 10 weeks. Although this interval varied according to primary symptom and anatomical subsite, no apparent socioeconomic differences were observed within these subgroups. Aligned with the patient-reported symptom onset, a distinct increase in consultation rates was observed at 9 weeks (95% CI [7.3; 10.7]) for patients with low SEP and 7 weeks (95% CI [4.8; 9.2]) for patients with high SEP, with overlapping CIs. Patients with low compared to high SEP had increased odds for advanced-stage glottic and oral cavity squamous cell carcinoma. For the remaining subsites the association varied according to SEP-indicator and TNM-edition.
Conclusion
The interval from symptom onset to diagnosis and consultation patterns were similar across SEP groups. Still, socioeconomic differences in stage at diagnosis were observed for some – but not all – subsites.
Background
Across countries and health care systems, a higher socioeconomic position (SEP) is associated with improved survival after cancer and the socioeconomic gap in survival is pronounced for patients diagnosed with head and neck cancer [Citation1–4]. Differences in disease stage at the time of diagnosis have been identified as a potential main driver of the association between SEP and cancer survival, suggesting timely diagnosis as a potential target for interventions [Citation4–6]. In Denmark, a cancer patient pathway (CPP) for head and neck cancer was implemented in October 2007. This entailed a standardized and expedited strategy for cancer diagnosing, with specified time limits from suspicion of cancer in primary or secondary care to final diagnosis and onset of treatment [Citation7]. Considering the patient interval (from first noticed symptom to first contact with medical care) or the primary care interval (from symptom presentation in primary care to referral to a CPP), this may to a larger degree rely on patient characteristics such as health literacy, comorbidity, use of health care system and communication with health-care professionals [Citation8–12]. These parameters have been observed to differ by socioeconomic position and thus may cause differences in the pre-diagnostic interval [Citation8–11,Citation13,Citation14]. A previous study found that patients diagnosed with laryngeal or oropharyngeal cancer had the longest patient interval compared to other investigated cancer sites [Citation15] and extended time to treatment has been associated with poorer prognosis [Citation16]. Information on symptoms and symptom onset are, however, rarely systematically collected and the impact of socioeconomic differences taking place in the pre-diagnostic interval has primarily been studied for other cancer sites with large variations in methodology (eFigure 1) [Citation8,Citation17–28].
Rather uniquely, the nationwide, population-based clinical database Danish Head and Neck Cancer Group (DAHANCA) routinely registers information on patient-reported symptom onset and primary symptoms at diagnosis of head and neck squamous cell carcinoma (HNSCC). By linking information from nationwide, population-based administrative registers this study investigates the association between SEP and the interval from patient-reported symptom onset to diagnosis. Further, this interval is compared to objectively measured consultation patterns observed in primary health care prior to the HNSCC diagnosis. Finally, the association between SEP and disease stage at diagnosis is examined.
Material and methods
Study design and setting
This nationwide, population-based cohort study is based on the Danish population (5.8 M citizens). The Danish social welfare system provides free tuition from primary to higher education [Citation29]. Health care is tax-funded with no co-payments for diagnostic procedures in e.g., general practice (GP) and ear-nose-throat specialist (ENT), while co-payment exists for all dental procedures in citizens older than 18 years [Citation29]. General practitioners act as gatekeepers to specialized primary and secondary health care services, except for ENT, ophthalmologists and dental care, that can be accessed without a referral [Citation29].
Materials
Denmark has a longstanding tradition of maintaining nationwide, population-based clinical databases and administrative registers [Citation29]. Information between these data sources can be linked using the unique personal identification number, which is assigned to all residents and contains information on e.g., the date of birth and legal gender [Citation29]. In the Danish Head and Neck Cancer Group (DAHANCA) database, we identified all patients diagnosed and registered with HNSCC in Denmark between 2008–2019 (eFigure 2) [Citation30]. From this database, we obtained prospectively collected information on the date of diagnosis (date of first appointment at the oncological center), anatomical subsite, clinical stage (UICC TNM-7 and TNM-8), human papillomavirus (HPV) status (classified as positive in case of a strong and diffuse nuclear or cytoplasmatic p16 immunohistochemistry staining in more than 70% of tumor cells, else negative), presenting main symptoms (according to a pre-specified list (eTable 1)) and date of first noticed symptom onset, which patients report at the first appointment at the oncological center [Citation30].
In Statistics Denmark, we obtained information on three different individual-level indicators for SEP: educational level [Citation31], disposable income [Citation32] and cohabitation status [Citation33]. These indicators have previously been observed to be individually related to cancer outcomes [Citation34–36]. Despite being highly correlated they reflect different aspects of SEP [Citation37] and were analyzed separately. Educational level reflects e.g., a person’s cognitive function and attained knowledge and is highly associated with understanding of health information and health care utilization and is considered as a proxy for health literacy [Citation37]. Income reflects material resources, while cohabitation status reflects social, emotional and practical support [Citation37]. Education was defined as short (mandatory education), medium (secondary or vocational education) and long (higher education) as registered the year before diagnosis [Citation31]. Disposable income for the calendar year before diagnosis was categorized as low (1st quintile), medium (2nd and 3rd quintile) or high (4th and 5th quintile), according to that of the entire Danish population with the same legal gender and birth year [Citation32]. Cohabitation status by 1 January of the year of diagnosis was defined as living alone or cohabiting (married, registered partner or residing at the same address with a person: of the opposite legal gender, above age 16 years and with an age difference of ≤15 years, with no kinship relations, and with no other adults, except for own children, in residence) [Citation33]. For easy reading, when similar results are observed, low SEP refers to: short education, low income or living alone; medium SEP refers to: medium education or income; and high SEP refers to: long education, high income or living with a partner.
From the Danish National Health Service Register [Citation38], we obtained information on all primary care daytime and out-of-hours face-to-face contacts in GP, ENT and dental care. From the Danish National Patient Register [Citation39] we obtained information on hospitalizations and outpatient visits in the period between 10 years and 30 days prior to HNSCC diagnosis. Comorbidity was defined according to the revised Charlson Comorbidity Index for Head and Neck Cancer (HN-CCI) [Citation40] and categorized as no versus prior hospitalization or outpatient visit with: congestive heart failure, cerebrovascular disease, chronic pulmonary disease, gastric ulcer disease, liver disease or diabetes [Citation40].
Study population
In the DAHANCA database, we identified 11 922 patients diagnosed with: laryngeal (glottic and non-glottic), oropharyngeal (HPV+ and HPV−), hypopharyngeal or oral cavity squamous cell carcinoma (SCC) between 2008 and 2019. A total of 11 609 patients fulfilled the inclusion criteria of being: 40 years or older at the date of diagnosis, born after 1920 and residing in Denmark within the year prior to diagnosis (eFigure 2). These criteria were chosen to ensure that included patients had established their socioeconomic position, to avoid fluctuations in consultation patterns for women during pregnancy, and because information on educational attainment is not available for patients born before 1921. The main analyses were based on complete cases, excluding 585 (5%) with either missing information on educational level or HPV status for oropharyngeal SCC (eFigure 2). A further 1,910 (17%) with missing information on the date of symptom onset were excluded in analyses of this parameter (eFigure 2).
Statistical analyses
Initially, directed acyclic graphs were used to identify confounding variables (age, gender and calendar year) of the association between SEP and the pre-diagnostic interval (eFigure 3). Several mediators were identified (e.g., health literacy, health behavior, comorbidity, symptom perception and health care utilization) as being part of the causal pathway between SEP and the pre-diagnostic interval and were therefore not adjusted for (eFigure 3). Socioeconomic differences in the interval from the reported date of first noticed symptom onset to diagnosis were analyzed in general linear models with 95% confidence intervals (CIs), adjusting for age, gender and calendar year of diagnosis. The calculated interval was log-transformed to follow a normal distribution and the slope parameter β was reported as eβ – 1. Due to presumed registration errors, intervals smaller than 0 were coded as 1 day and intervals longer than 548 days as 548 days. In sub-analyses different criteria for the maximum number of days were applied. The change in consultation patterns was estimated as the week for which the weekly mean number of consultations for the analyzed group changed, using the R package changepoint. The 95% CIs were estimated based on 300 bootstraps. In the logistic regression models adjusted for age, gender and calendar year of diagnosis, we estimated odds ratios (ORs) with 95% CIs for presenting with advanced disease (TNM-7: III-IV versus I-II). In sub-analyses, we defined stage according to the TNM-8 edition, which has been applied in recent years to better reflect prognostic outcomes for patients diagnosed with HPV+ oropharyngeal SCC. All analyses were stratified by anatomical subsite as well as HPV status for oropharyngeal SCC, to account for differences in symptom signature and epidemiology. In sub-analyses, the analyses were further stratified by gender, age, year of diagnosis, HN-CCI and type of symptom to examine potential effect modification. Following Danish legislation, strata containing micro-data (less than 5 observations) were replaced with NA. For stage, specifically, missing values (n = 126) were imputed according to the distribution for each subsite in , but omitted in analyses including this parameter.
Table 1. Descriptive characteristics of patients in the analyzed cohort.
Results
Descriptive characteristics of the analyzed cohort
The main analyzed cohort included 11 024 patients (eFigure 1). Patient characteristics varied considerably across the investigated subsites (). The median age ranged from 60 years (IQR, [54;67]) for patients with HPV+ oropharyngeal SCC to 68 years (IQR, [61;74]) for patients with glottic laryngeal SCC. The proportion of females ranged from 13% for glottic laryngeal SCC to 39% for oral cavity SCC. A higher proportion of HPV+ oropharyngeal SCC was diagnosed in more recent years, reflecting the well-known epidemiology of the disease (). Particularly in comparison with HPV- oropharyngeal SCC, a higher proportion of patients with HPV+ oropharyngeal SCC had long education (25% vs. 14%), high income (41% vs. 19%), a cohabiting partner (68% vs. 45%) and no comorbidity (HNSCC =0) (83% vs 63%) (). Finally, the proportion diagnosed with advanced-stage disease (TNM-7) ranged from 19% for glottic laryngeal SCC to 90% for HPV+ oropharyngeal and hypopharyngeal SCC ().
Symptoms
In all 10 000(91%) of the included patients had information on primary symptoms (), with missing information ranging from 5% for patients diagnosed with HPV+ oropharyngeal SCC to 15% for patients diagnosed with oral cavity SCC. The symptom signature varied substantially across subsites. For instance, the proportion of patients reporting neck lymph node as a primary symptom ranged from 1% among patients diagnosed with glottic laryngeal SCC to 55% among patients diagnosed with HPV+ oropharyngeal SCC. In contrast, nearly all patients diagnosed with glottic laryngeal SCC (95%) reported hoarseness as a primary symptom compared to 1% among patients diagnosed with HPV+ oropharyngeal SCC ().
Interval from reported symptom onset to diagnosis
The median interval from patient-reported symptom onset to diagnosis differed according to primary symptom and subsite (eTable 2). Patients with neck lymph node as a primary symptom had the shortest interval (7 weeks (IQR, [4;13])), whereas patients reporting hoarseness as a primary symptom had the longest interval (14 weeks (IQR, [8;27])) (eTable 2). Aligning the distribution of these symptoms across subsites, the interval was shortest for HPV+ oropharyngeal SCC (8 weeks (IQR, [4;16]) and longest for glottic SCC (15 weeks (IQR, [8;29]) (eTable 2).
The presence of comorbidity was associated with a longer interval for patients with glottic laryngeal SCC (1.16 [1.01;1.32]), but a shorter interval for patients with non-glottic laryngeal SCC (0.78 [0.68;0.91]) (). Regarding socioeconomic position, the interval from symptom onset to diagnosis was similar for patients with short, medium and long education; low, medium and high income; and among patients living alone compared to cohabiting (). Further, the interval was similar for patients with short compared to long education across strata of age, gender, calendar year, comorbidity, anatomical subsite and presenting symptom (eFigure 4). However, for glottic laryngeal SCC, patients with short compared to long education had a shorter interval (0.80 [0.67;0.96]) (, eFigure 4). This was primarily observed for older patients, males and for patients with no comorbidity (eFigure 5), and no differences were observed when using income or cohabitation status as SEP indicator.
Table 2. Adjusted estimates with 95% confidence intervals for the interval from reported symptom onset to diagnosis.
Consultation patterns prior to diagnosis
A distinct increase in the average weekly number of consultations was observed two to three months prior to diagnosis (). The interval from this increase to diagnosis was slightly longer for patients with short education (9 weeks, CI [7.3;10.7]) than long education (7 weeks, CI [4.8;9.2]), but the confidence intervals overlapped (). For non-glottic laryngeal SCC, the reverse association was observed, with a shorter interval for patients with short education (11 weeks, CI [4.9;17.1]) than long education (14 weeks, CI [-0.6;28.6]) (), but the confidence intervals were wide and overlapping.
Figure 1. Average weekly number of contacts prior to diagnosis and change-point for which the average weekly number of contacts changed, with 95% confidence intervals, for patients with short (A) and long (B) education.
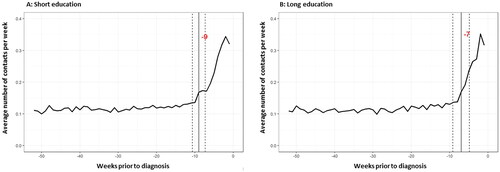
Figure 2. Median interval and IQR between patient-reported symptom onset and diagnosis and change-point for which the average weekly number of consultations changed, by subsite and educational level. IQR: Interquartile range. CI: confidence intervals.
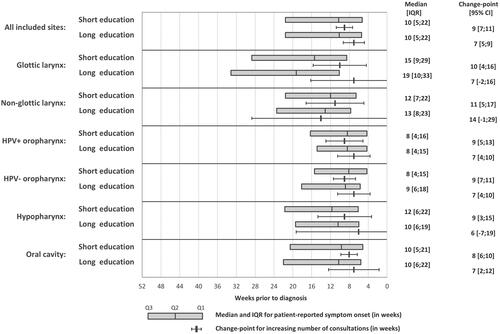
The observed increase in consultation rates prior to diagnosis was aligned with the patient-reported median time of symptom onset (), also across strata of age, gender and presenting symptom (eFigure 6). Furthermore, patients reporting a long interval from symptom onset to diagnosis tended to have a more extended period with an increased number of consultations (eFigure 7). This trend differed, however, for patients with a reported interval from symptom to diagnosis above the 80% percentile (> 5.7 months), for which the interval with increased consultation rates was considerable shorter (< 3 months) (eFigure 7).
Stage at time of diagnosis
Across subsites, patients living alone had increased odds for advanced disease stage at diagnosis than patients living with a partner (). In case of glottic laryngeal and oral cavity SCC, the odds of advanced-stage disease were also increased for patients with short compared to long education (OR: 1.51 [1.01;2.25]) and low (OR: 1.67 [1.18;2.38]) and medium (OR: 1.66 [1.21;2.27]) compared to high income (). For HPV+ oropharyngeal specifically, the proportion diagnosed with advanced stage was 88% using the TNM-7 edition and 10% using the TNM-8 edition. For this subsite, patients with low compared to high income had decreased odds for advanced stage when applying TNM-7 (OR: 0.64 [0.46;0.90]) (), but increased odds when applying TNM-8 (OR: 1.78 [1.31;2.42]) (eTable 3). No consistent differences for education and income were observed for the remaining subsites.
Table 3. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for advanced disease stage (TNM-7: III-IV vs. I-II), by subsite.
Regarding the interval from symptom onset to diagnosis, a long (4th quartile) compared to short (1st quartile) interval was associated with increased odds for advanced stage for glottic laryngeal SCC () as well as HPV+ oropharyngeal SCC for the TNM-8 edition (eTable 3), while no clear patterns were observed for the remaining subsites.
Discussion
Despite large variations in the interval from patient-reported symptom onset to HNSCC diagnosis according to primary symptom and across subsites, no consistent socioeconomic differences were observed. This finding aligned with objectively observed trends in consultation patterns. Still, patients living alone, and patients diagnosed with glottic laryngeal or oral cavity SCC with short education or low income, had increased odds for being diagnosed with advanced-stage disease compared to patients with high SEP.
Previous studies of socioeconomic differences in the interval between symptom onset and cancer diagnosis have primarily included other cancer sites (eFigure 1) [Citation8,Citation17–28]. In line with our results, the observed socioeconomic differences were small and ambiguous with overlapping confidence intervals; however, with a tendency towards a longer interval for patients with a low compared to a high socioeconomic position (eFigure 1) [Citation8,Citation17–28]. Based on the observed large differences in the interval from symptom onset to diagnosis according to symptom and subsites, direct comparison of results on the pre-diagnostic interval across cancer sites appear challenging [Citation41]. Even within subsites we observed large variability in this interval. This may reflect a complex relationship between tumor-specific factors (i.e., symptom signature and progression of the disease), individual characteristics (i.e., symptom appraisal, health literacy, comorbidity, health care seeking behavior and communication with health care professionals) and contextual factors (i.e., the selection of patients to referral and the following diagnostic process) [Citation42]. For instance, the shortest interval was observed for patients diagnosed with HPV+ oropharyngeal SCC, for which the majority (55%) reported swollen lymph node as a primary symptom; a well-established and apparent cancer alarm symptom. In contrast, the longest interval was observed for patients diagnosed with glottic laryngeal SCC, for which hoarseness is the primary symptom and likely requires laryngoscopy by ENT specialist to detect. Although a referral to ENT specialist is not required, there may be differential knowledge about this by SEP, which the socioeconomic differences in advanced stage glottic laryngeal SCC may reflect. Particularly the patient-related interval appears extended for glottic laryngeal SCC, compared to the other subsites, suggesting that hoarseness may be considered a less severe symptom or ascribed other illnesses. In this regard, it is interesting that for glottic laryngeal SCC, patients with comorbidity have a longer interval, whereas having comorbidity for the remaining subsites is associated with a shorter interval. For HPV+ oropharyngeal SCC, the interval decreased over time, which may reflect increased awareness of cancer alarm symptoms in this increasing and distinct patient group in Denmark.
Owing the detailed information from the DAHANCA database and administrative registries, we were able to investigate individual-level socioeconomic differences in both self-reported and objectively measured proxy indicators for the pre-diagnostic interval. The date of symptom onset was patient-reported and reported retrospectively at the date of diagnosis. This may have introduced bias due to difficulties in recalling the specific time of symptom onset and the reported data do not take into account potential differences in symptom appraisal [Citation42]. Across strata, the observed trends in patient-reported interval from symptom onset to diagnosis aligned, however, with the observed objectively measured trends in consultation patterns, which may be taken as a validation of the patient-reported symptom onset. This alignment was, however, less good for patients with a long interval (> 5.7 months) from symptom onset to diagnosis. Patients with a long interval may be a distinct patient group with diverging consultation patterns. It may, however, also indicate misclassification or reporting errors. If the latter is the case, this calls for careful interpretation of results based on the frequently used method in other studies comparing patients with an interval above or below the 75% percentile [Citation8,Citation17,Citation18,Citation24,Citation28]. Nevertheless, in this study, we observed similar results on the association with education in sub-analyses when applying different maximum limits to the self-reported interval.
The nationwide design is another major strength, particularly when investigating socioeconomic differences, as information obtained from smaller, selected cohorts may underestimate socioeconomic differences. Further, the proportion of patients with missing values was substantially lower compared to previous studies (eTable 1). Still, missing values were not conclusively non-differential, with a lower proportion of missing values for HPV+ oropharyngeal SCC, for patients cohabiting or those diagnosed in later calendar year (eTable 4). Despite the nationwide design, sub-analyses included a relatively low number of patients with less robust estimates. To validate the observed null-finding, we conducted a large number of sub-analyses which may have introduced chance findings. Finally, as no other studies of this association exist for the HNSCC population, the lack of an association between socioeconomic position and the pre-diagnostic interval may be exclusive for Denmark or other countries with universal access to health care and standardized cancer patient pathways. Still, for some anatomical subsites patients with low compared to high SEP had increased odds for being diagnosed with advanced-stage disease. The poor alignment between the interval from symptom to diagnosis and stage at diagnosis, may be due to the waiting-time paradox, for which some patients with more progressive or advanced cancer may have more apparent symptoms initiating a short time from symptom to diagnosis. For glottic laryngeal SCC, patients with short compared to long education had e.g., a shorter interval from symptom onset to diagnosis, but a higher risk of advanced-stage diseases.
Summarizing previous findings (eFigure 1) and the findings from this large nationwide, population-based study, with unique information on symptom onset, primary symptoms and consultations patterns, there is only weak evidence for an association between SEP and the pre-diagnostic interval. This may be because other factors drive the association between SEP and stage at diagnosis or that the associations cannot be identified with the data available or the chosen methodologies. A recent Danish study of multiple cancer sites observed delayed referral for patients with low compared to high health literacy specifically [Citation8]. Future studies could further study such specific mediating factors or investigate more distinct socioeconomic vulnerable groups or geographical differences in the pre-diagnostic interval. However, to diminish the pronounced socioeconomic differences in outcomes among patients diagnosed with head and neck SCC other mediating factors [Citation4] e.g., smoking behavior and comorbidity [Citation34] appear as more relevant targets [Citation1–4,Citation34].
Conclusion
Despite large differences in the interval from symptom onset to HNSCC diagnosis across subsites and symptoms, this interval was similar across SEP groups and aligned with objectively observed trends in consultation patterns prior to diagnosis. Still, for some – but not all – subsites, patients with low compared to high SEP had increased odds for being diagnosed with advanced-stage disease. Acknowledging the complex mechanisms involved in the pre-diagnostic interval and the methodological challenges in measuring this interval, other identified drivers behind the socioeconomic differences in HNSCC outcomes i.e., lifestyle factors, comorbidity, and treatment may be more relevant targets for intervention. Increased awareness of persistent hoarseness as a potential cancer alarm symptom may, however, reduce the longer interval observed for glottic SCC.
Supplemental Material
Download MS Word (936 KB)Data availability statement
The data used in this study were de-identified, linked and accessed through the Danish Cancer Institute’s secure server at Statistics Denmark. The authors do not have permission to share the data. In agreement with the General Data Protection Regulation the study is registered in the Danish Cancer Society’s internal project register database (journal number 2018-DCRC-0034).
Disclosure statement
The authors report no conflicts of interest.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Dalton SO, Olsen MH, Johansen C, et al. Socioeconomic inequality in cancer survival - changes over time. A population-based study, Denmark, 1987-2013. Acta Oncol. 2019;58(5):737–744. doi:10.1080/0284186X.2019.1566772.
- Jansen L, Kanbach J, Finke I, et al. Estimation of the potentially avoidable excess deaths associated with socioeconomic inequalities in cancer survival in Germany. Cancers (Basel). 2021;13(2):357. doi:10.3390/cancers13020357.
- Afshar N, English DR, Blakely T, et al. Differences in cancer survival by area-level socio-economic disadvantage: a population-based study using cancer registry data. PLoS One. 2020;15(1):e0228551. doi:10.1371/journal.pone.0228551.
- Afshar N, English DR, Milne RL. Factors explaining Socio-Economic inequalities in cancer survival: a systematic review. Cancer Control. 2021;28:10732748211011956. doi:10.1177/10732748211011956.
- Olsen MH, Bøje CR, Kjær TK, et al. Socioeconomic position and stage at diagnosis of head and neck cancer - a nationwide study from DAHANCA. Acta Oncol. 2015;54(5):759–766. doi:10.3109/0284186X.2014.998279.
- Vaccarella S, Lortet-Tieulent J, Saracci R, et al. editors 2019). Reducing social inequalities in cancer: evidence and priorities for research (IARC scientific publication no. 168). Lyon, France: international Agency for Research on Cancer.
- Lyhne NM, Christensen A, Alanin MC, et al. Waiting times for diagnosis and treatment of head and neck cancer in Denmark in 2010 compared to 1992 and 2002. Eur J Cancer. 2013;49(7):1627–1633. doi:10.1016/j.ejca.2012.11.034.
- Petersen GS, Laursen SGW, Jensen H, et al. Patients’ health literacy is associated with timely diagnosis of cancer-A cross-sectional study in Denmark. Eur J Cancer Care (Engl). 2022;31(1):e13532. doi:10.1111/ecc.13532.
- Humphrys E, Burt J, Rubin G, et al. The influence of health literacy on the timely diagnosis of symptomatic cancer: a systematic review. Eur J Cancer Care (Engl). 2019;28(1):e12920. doi:10.1111/ecc.12920.
- Jensen H, Moller H, Vedsted P. Characteristics of customary non-attenders in general practice who are diagnosed with cancer: a cross-sectional study in Denmark. Eur J Cancer Care (Engl). 2019;28(6):e13143. doi:10.1111/ecc.13143.
- Balasubramaniam K, Rasmussen S, Haastrup PF, et al. Women’s barriers for contacting general practice when experiencing gynecological cancer symptoms: a population-based study. BMC Fam Pract. 2021;22(1):167. doi:10.1186/s12875-021-01518-5.
- Morris M, Woods LM, Bhaskaran K, et al. Do pre-diagnosis primary care consultation patterns explain deprivation-specific differences in net survival among women with breast cancer? An examination of individually-linked data from the UK west Midlands cancer registry, national screening programme and clinical practice research datalink. BMC Cancer. 2017;17(1):155. doi:10.1186/s12885-017-3129-4.
- Jorgensen JT, Andersen JS, Tjonneland A, et al. Determinants of frequent attendance in danish general practice: a cohort-based cross-sectional study. BMC Fam Pract. 2016;17(1):9. doi:10.1186/s12875-016-0412-4.
- Weller D, Vedsted P, Rubin G, et al. The aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–1267. doi:10.1038/bjc.2012.68.
- Lyratzopoulos G, Saunders CL, Abel GA, et al. The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br J Cancer. 2015;112(Suppl 1):S35–S40. doi:10.1038/bjc.2015.40.
- Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84(1):5–10. doi:10.1016/j.radonc.2007.04.001.
- Virgilsen LF, Pedersen AF, Vedsted P, et al. Alignment between the patient’s cancer worry and the GP's cancer suspicion and the association with the interval between first symptom presentation and referral: a cross-sectional study in Denmark. BMC Fam Pract. 2021;22(1):129. doi:10.1186/s12875-021-01480-2.
- van Erp NF, Helsper CW, Slottje P, et al. Time to diagnosis of symptomatic gastric and oesophageal cancer in The Netherlands: where is the room for improvement? United European Gastroenterol J. 2020;8(5):607–620. doi:10.1177/2050640620917804.
- Foroozani E, Ghiasvand R, Mohammadianpanah M, et al. Determinants of delay in diagnosis and end stage at presentation among breast cancer patients in Iran: a multi-center study. Sci Rep. 2020;10(1):21477. doi:10.1038/s41598-020-78517-6.
- Barros ÂF, Murta-Nascimento C, Abdon CH, et al. Factors associated with time interval between the onset of symptoms and first medical visit in women with breast cancer. Cad Saude Publica. 2020;36(2):e00011919.
- Walter FM, Mills K, Mendonça SC, et al. Symptoms and patient factors associated with diagnostic intervals for pancreatic cancer (SYMPTOM pancreatic study): a prospective cohort study. Lancet Gastroenterol Hepatol. 2016;1(4):298–306. doi:10.1016/S2468-1253(16)30079-6.
- Walter FM, Emery JD, Mendonca S, et al. Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: results from a prospective cohort study. Br J Cancer. 2016;115(5):533–541. doi:10.1038/bjc.2016.221.
- Walter FM, Rubin G, Bankhead C, et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br J Cancer. 2015;112(Suppl 1):S6–S13. doi:10.1038/bjc.2015.30.
- Guldbrandt LM, Fenger-Gron M, Rasmussen TR, et al. The role of general practice in routes to diagnosis of lung cancer in Denmark: a population-based study of general practice involvement, diagnostic activity and diagnostic intervals. BMC Health Serv Res. 2015;15(1):21. doi:10.1186/s12913-014-0656-4.
- Robinson KM, Christensen KB, Ottesen B, et al. Socio-demographic factors, comorbidity and diagnostic delay among women diagnosed with cervical, endometrial or ovarian cancer. Eur J Cancer Care (Engl). 2011;20(5):653–661. doi:10.1111/j.1365-2354.2011.01259.x.
- Dalton SO, Frederiksen BL, Jacobsen E, et al. Socioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001-2008. Br J Cancer. 2011;105(7):1042–1048. doi:10.1038/bjc.2011.342.
- Smith SM, Campbell NC, MacLeod U, et al. Factors contributing to the time taken to consult with symptoms of lung cancer: a cross-sectional study. Thorax. 2009;64(6):523–531. doi:10.1136/thx.2008.096560.
- Hansen RP, Olesen F, Sørensen HT, et al. Socioeconomic patient characteristics predict delay in cancer diagnosis: a danish cohort study. BMC Health Serv Res. 2008;8(1):49. doi:10.1186/1472-6963-8-49.
- Schmidt M, Schmidt SAJ, Adelborg K, et al. The danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi:10.2147/CLEP.S179083.
- Overgaard J, Jovanovic A, Godballe C, et al. The danish head and neck cancer database. Clin Epidemiol. 2016;8:491–496. doi:10.2147/CLEP.S103591.
- Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7 Suppl):91–94. doi:10.1177/1403494810394715.
- Statistics Denmark. DISPON_13 2022 [cited 2023 17 May]. Available from: https://www.dst.dk/da/Statistik/dokumentation/Times/moduldata-for-sociale-forhold–sundhedsvaesen–retsvaesen/livskvalitet/dispon-13.
- Statistics Denmark. Households and families 2022 [cited 2023 17 May]. Available from: https://www.dst.dk/en/Statistik/emner/borgere/husstande-familier-og-boern/husstande-og-familier.
- Olsen MH, Frederiksen K, Lassen P, et al. Association of smoking, comorbidity, clinical stage, and treatment intent With socioeconomic differences in survival After oropharyngeal squamous cell carcinoma in Denmark. JAMA Netw Open. 2022;5(12):e2245510. doi:10.1001/jamanetworkopen.2022.45510.
- Ammitzbøll G, Levinsen AKG, Kjær TK, et al. Socioeconomic inequality in cancer in the nordic countries. Acta Oncol. 2022;61(11):1317–1331.
- Dalton SO, Steding-Jessen M, Gislum M, et al. Social inequality and incidence of and survival from cancer in a population-based study in Denmark, 1994-2003: background, aims, material and methods. Eur J Cancer. 2008;44(14):1938–1949. doi:10.1016/j.ejca.2008.06.010.
- Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. 2006;60(1):7–12. doi:10.1136/jech.2004.023531.
- Andersen JS, Olivarius Nde F, Krasnik A. The danish national health service register. Scand J Public Health. 2011;39(7 Suppl):34–37. doi:10.1177/1403494810394718.
- Lynge E, Sandegaard JL, Rebolj M. The danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi:10.1177/1403494811401482.
- Bøje CR, Dalton SO, Primdahl H, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiother Oncol. 2014;110(1):91–97. doi:10.1016/j.radonc.2013.11.009.
- Koo MM, Hamilton W, Walter FM, et al. Symptom signatures and diagnostic timeliness in cancer patients: a review of current evidence. Neoplasia. 2018;20(2):165–174. doi:10.1016/j.neo.2017.11.005.
- Andersen RS, Vedsted P, Olesen F, et al. Patient delay in cancer studies: a discussion of methods and measures. BMC Health Serv Res. 2009;9(1):189. doi:10.1186/1472-6963-9-189.

