Abstract
Background
In peripheral lung tumours, stereotactic body radiotherapy (SBRT) is superior to conventional RT. SBRT has also shown high loco-regional control (LC) in centrally located tumours, but there is a high risk of severe toxicity. The STRICTSTARLung trial (NCT05354596) examines if risk-adapted SBRT for central tumours is feasible. In this study, we examined overall survival (OS), Disease-free survival (DSF), LC, and toxicity in patients with central tumours that could have been candidates for SBRT but received conventional RT.
Material and methods
Retrospectively, we evaluated 49 lung cancer patients that between 2008 and 2021 received RT (60–70Gy in 2 Gy fractions) for a solitary tumour or lymph node with a diameter <5cm located <2cm from the bronchial tree, oesophagus, aorta or heart. All tumours were pathologically verified; 30 were primary lung tumours (T1b-T4) and 19 were solitary lymph nodes (T0N1-N2). Chemotherapy was administered as concomitant (29) or sequential (4). OS and LC were analysed using Kaplan Meier. Cox proportional hazards model for OS and disease-free survival (DFS) was performed including tumour volume, histology, sex, T- vs N-site and chemotherapy. Toxicity was scored.
Results
In 42 patients, the tumour was located <1 cm to mediastinum. Median follow-up time was 44 months (range: 7–123). The median OS was 51 months. OS at 1-, 3- and 5-year was 88% (SE:5), 59% (SE:7) and 50% (SE:8). Loco-regional recurrences occurred in 16 patients resulting in 1-, and 3-year LC rates of 77% (SE:6) and 64% (SE:8). The majority occurred within 3 years after RT. Only stage showed significant impact on OS and DFS. No patients experienced grade 4–5 toxicity. Seven patients developed grade 3 toxicity (5 oesophageal stenosis, 2 pneumonitis).
Conclusion
Conventional RT for patients with small central lung tumours or solitary lymph nodes is feasible. Median OS was 51 months, and toxicity was low with no grade 4–5 events.
Keywords:
Background
Non-small cell lung cancer (NSCLC) is one of the leading causes of cancer-related deaths worldwide. The standard treatment for inoperable locally advanced NSCLC patients is concomitant chemo- and radiotherapy at 60–66 Gy/30–33 fractions. Despite an intensive treatment strategy with a risk of severe toxicity [Citation1–3], the five-year progression-free survival (PFS) is as low as 20–30%, with ∼30% of the patients experiencing loco-regional failure [Citation4–9]. Small peripheral tumours with a diameter <5cm may be treated with stereotactic body radiotherapy (SBRT) delivering high doses in a few fractions. Excellent local control rates above 80% after SBRT have been shown in former studies [Citation10–13]. However, a similar strategy for small centrally located tumours is challenging due to the vicinity of critical organs at risk (OAR). Here, delivery of high biologically effective doses may result in unacceptable rates of lethal toxicity [Citation14–20]. An ongoing Danish trial investigates the feasibility of risk-adapted SBRT for central and ultra-central tumours (STRICTSTARLung NCT05354596) [Citation21]. The trial is based on strict dose limits for the OARs being prioritized over target coverage.
In this retrospective study, we analyse LC, DFS, OS and toxicity in small central lung tumours treated with conventionally radiotherapy (RT). Tumours like these may prospectively be candidates for SBRT in the STRICTSTARLung trial and thus, this retrospective study may serve as an investigation of the outcome of the standard treatment.
Materials and methods
Patient data
Between 2008 to 2021, 1100 patients with lung cancer were treated with RT to 60–70Gy in 2–2.083 Gy/fraction at Aarhus University Hospital. Of these, 49 either had a solitary primary lung tumour or relapse after surgery in a solitary lymph node target <5cm in diameter located centrally, i.e., within 2 cm of the bronchial tree, oesophagus, aorta or heart, see . All the patients in this study were considered not suitable for SBRT because of tumour location. No tumour or malignant lymph nodes were left untreated.
Figure 1. Planning CT scans of solitary lung tumour (upper panel) and lymph node target (lower panel). the GTVs are delineated in red.
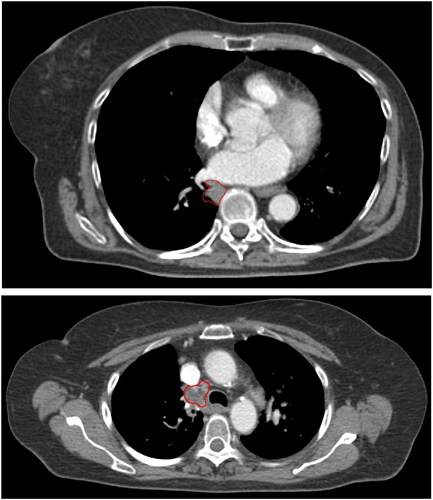
For all patients, staging was based on a diagnostic 18fluoro-deoxy-glucose positron emission tomography (18FDG-PET) scan acquired simultaneously with a CT scan, and endo bronchial ultrasonography (EBUS) including transbronchial biopsy. Tumours were pathologically verified (21 squamous cell carcinoma, 26 adenocarcinomas, 2 other), 30 were primary lung tumours (T1b-T4) and 19 were solitary lymph nodes (T0N1-N2). Four patients had a solitary brain metastasis and were treated radically with surgery or SBRT of the metastasis up front. The patients were treated with RT only (16), concomitant (29), or sequential chemotherapy (4). The chemotherapy consisted of a platinum derivate (carboplatin AUC5 or cisplatin 75 mg/m2) combined with vinorelbine (60 mg/m2 day 1 and day 8 each cycle). When concomitant, RT start coincided with the second cycle of chemotherapy. Sequential chemotherapy was completed 2–4 weeks before RT start. None of the patients received adjuvant durvalumab.
Scanning, contouring and treatment planning
All patients underwent a free-breathing 18FDG PET-CT planning scan with 3 mm slice spacing and intravenous contrast. The patients were positioned with both arms above the head in a standard or individualized immobilisation device. The CT-scan was performed as a time-resolved 4D-scan and the mid-ventilation phase was selected for delineation and treatment planning. For patients treated before April 2013 (8 patients), the gross tumour volume of the tumour (GTV-T) or the lymph node (GTV-N) was uniformly expanded by 5 mm and corrected for bones and large vessels to create the clinical target volume (CTV). CTV was further expanded by 5 mm (left–right, LR), 5 mm (anterior-posterior, AP), 10 mm (cranio-caudal, CC) to create the internal target volume (ITV). ITV was expanded by 5 mm (LR), 5 mm (AP), 8 mm (CC) into a planning target volume (PTV). For patients treated after 2013 (42 patients), the GTV was delineated using the maximum intensity projection thus accounting for respiratory tumour motion [Citation22,Citation23]. The GTV was expanded to the CTV by an isotropic 5 mm margin and corrected for bones and large vessels. PTV was formed by adding 4 mm (LR, AP) and5mm (CC) [Citation24]. Treatment planning was performed in Eclipse version 8–16 (Varian Medical Systems) using AAA (37 patients) or Acuros (13 patients) for dose calculation and either conventional 3D-conformal RT (3D-CRT) or intensity-modulated RT (IMRT). The lung dose constraints were V20Gy < 35%, mean lung dose (MLD) < 19 Gy, and V5Gy < 60%. For the oesophagus, D1cm3 < 66 Gy was required.
Treatment delivery
A 3D CBCT-scan acquired with a kV On-Board Imager system (Varian Medical Systems) was used for daily image-guided patient setup. For patients treated before April 2013, the CBCT-scan was registered automatically to the planning-CT scan based on a match of the thoracic vertebrae. The radiation therapists (RTT) evaluated the registration and the resulting 3D-translational shifts and couch rotation were performed. For all other patients, a GTV-T or GTV-N soft-tissue match was performed. For patients with systematic deviations in the position of the tumour or lymph node above a pre-set limit, adaptive re-planning was performed [Citation25].
Follow up
After RT, patients were followed with CT scans every third month for the first two years. The follow-up interval was then increased to every six months for the next three years. Patients were followed until relapse or death. Loco-regional recurrence was defined as T-site relapse in the same lung lobe as the primary tumour and/or N-site relapse in the mediastinum, ipsi- or contralateral hilus or ipsilateral supraclavicular region (relative to the primary tumour). Recurrence was identified based on biopsy or from progression seen on consecutive CT scans. All medical records were reviewed retrospectively by an experienced oncologist to score the maximum observed radiation pneumonitis and esophagitis using (CTCAE v3.0) within 6 months from RT start. Grade 3–5 treatment-related late toxicity (after 6 months) was scored until relapse or death.
Retrospective data collection
Retrospective data collection was approved by the Danish Data Protection Agency and the National Board of Health. Basic patient characteristics were collected: stage (American Joint Committee on Cancer 7th Edition), histology, age, gender and performance status (PS, Eastern Cooperative Oncology Group ECOG). The distance from GTV volume to the bronchial tree, oesophagus, aorta or heart was measured and categorized into three groups (0–0.9 cm, 1–1.4 cm and 1.5–2cm). The treatment was described in terms of previous surgery, chemotherapy, prescribed RT dose, Mean Lung Dose (MLD), Mean Heart Dose (MHD), V40Gy to oesophagus, and GTV volume. The first failure was recorded as loco-regional or distant. In case of simultaneous loco-regional and distant, they were included in both categories. The recurrence was dated as the first scan (MR, CT or PET-CT) where failure was suspected.
Statistics
The loco-regional control (LC), disease-free survival (DFS) and overall survival (OS) were calculated using the Kaplan and Meier method as a function of time from the start of RT. LC was measured until loco-regional relapse, patients were censored at the time of death or last available follow-up. DSF was measured until relapse (loco-regional and/or distant) or death of all cause. Patients were censored from last follow-up. OS were measured until death of all cause and censored from last follow-up. Association of stage, gender, age (separated at the median), GTV volume, histology, chemotherapy (concomitant, sequential or no chemotherapy), performance status (0, 1 or 2) and T-site versus N-site was investigated in univariate Cox proportional hazard model for all endpoints and hazard ratios extracted. A p-value of less than 0.05 was considered as statistically significant. SPSS software (version 20) was used for data analyses. No Multivariate Cox proportional hazard analysis was made.
Results
Patients and tumour characteristics are given in . The median follow-up time was 44 months (range, 7–123). At the end of the study 30 patients had died, 9 of these died with no evidence of disease. Prior to RT, 31 patients underwent surgery (23 lobectomies, 5 sleeve resections, 2 pneumonectomies’, and 1 bi-lobectomy). The median time from surgery to RT was 10.4 months (range, 0–154 months). Forty-two, one and six lesions were located 0–0.9 cm, 1–1.4 cm, and 1.5–2cm from the bronchial tree, oesophagus, aorta or heart, respectively. Thirty-four lesions were within 2 cm of the bronchial tree, 24 were within 2 cm of the oesophagus, 29 were within 2 cm of the aorta, and 15 were within 2 cm of the heart. Forty-two of the lesions was located within at least two OAR.
Table 1. Patient and tumour characteristics.
The solitary lymph nodes were located in stations 1 L (n = 1), 2–4 L or R (n = 6), 5–6 (n = 3), 7 (n = 4), and hilum 10–11 R or L (n = 4). For the entire cohort, the median OS was 51 months (CI:14.0–88.0). 1-, 2-, 3- and 5-year OS was 88% (SE:5), 70% (SE:7), 59% (SE:7) and 50% (SE:8) (). Of the investigated variables, only stage (stage IV versus stage I-III) showed statistical influence on OS in univariate analysis, HR: 1.4 (SE:0.4) (p = 0.001) (). T-site versus N-site was borderline significant, HR: 0.73 (SE:0.4) (p = 0.069) (). Median OS for patients with solitary lymph node targets was 81 months (CI: 31.8–130.1) and for patients with primary lung tumour, it was 36 months (CI: 13.8–58.2). All patients with stage IV disease suffered from distant failure. Loco-regional recurrences occurred in 16 out of 49 patients. Nine patients experienced loco-regional relapse only, (4 patients at N-site, 2 at T-site and 3 T + N-site). Seven patients experienced both loco-regional and distant failure. Of all loco-regional recurrences, nine occurred within the PTV, six outside, and one was not evaluable.
Figure 2. Left: Loco-regional recurrence occurred in 16 out of 49 patients resulting in a 1-, 2- and 3-year loco-regional control rate of 77% (SE: 6), 66% (SE: 7), and 64% (SE: 8). in the Middle: the median DFS was 27 months. 1-, 2-, 3-, and 5-year DSF was 65% (SE:7), 51% (SE:7), 49% (SE:7), and 44% (SE:7). right: the median OS was 51 months. 1-, 2-, 3-, and 5-year overall survival was 88% (SE:5), 70% (SE:7), 59% (SE:7), and 50% (SE:8).
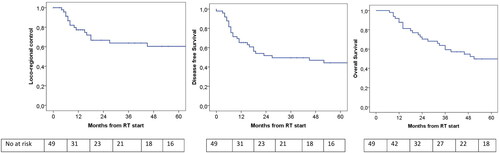
Figure 3. At top the DFS related to stage. The median DFS for stage I-II was 79.0 months, for stage III 19.0 months and for stage IV 3.0 months (p = 0.003). At bottom OS for patients with stage I-II, stage III and stage IV. The median OS for stage I-II was 69.0 months, for stage III 96.0 months and stage IV 9.0 months (p = 0.001).
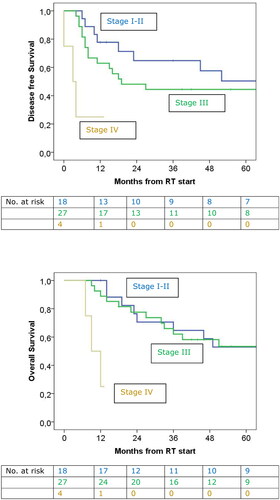
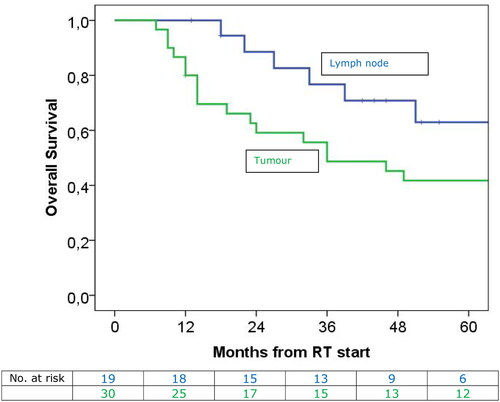
Figure 4. The OS for patients with lymph node targets (green) or primary tumour (blue). the median OS for patients with solitary lymph nodes was 81.0 months and for patients with primary tumours it was 36.0 months (p = 0.069).
The 1-, 2-, and 3-year loco-regional control rates were 78% (SE: 6), 67% (SE: 7), and 64% (SE: 8) (). The majority of recurrences occurred within 3 years after RT. None of the investigated variables showed a statistically significant influence on the loco-regional control rate in univariate analysis (Supplementary Table 1). For the entire cohort, the median disease-free survival (DFS) was 27 months (CI:0–67.7). 1-, 2-, 3- and 5-year DSF was 65% (SE:7), 51% (SE:7), 49% (SE:7) and 44% (SE:7) (). Only stage showed statistical influence on DFS (). The median DFS for stage I-II was 79 months (CI 26.4–131.1), for stage III 19 months, HR 0.71 (CI: 0.3–37.7) and for stage IV 3 months (CI 0–6.9) HR 0.51 (p = 0.003). Nine patients suffered from grade 2–3 pneumonitis (). The median MLD for those was 7.6 Gy (1.9–9.6). For the patients without pneumonitis, the median MLD was 5.8 Gy (1.7–20.5). For all patients with pneumonitis, it resolved. Oesophagitis grade 1–2 occurred in 17 patients. No grade 3–5 were diagnosed. Six patients developed stenosis of the oesophagus, four of these also suffered from early oesophagitis grade 2. The median V40Gy to the oesophagus in the group with stenosis was 6.0% (0–12.7) versus 1.1% (0–12.4) in the group not experiencing stenosis. The patients who died with no evidence of disease died of lung infection (n = 3), cardiac arrest and severe comorbidity (n = 2), head and neck cancer, dissecting aorta aneurism, fall from stairs and one unknown reason. There was no grade 4 or 5 toxicity registered.
Table 2. Early (within 6 months from start of RT) and late toxicity (after 6 months from start of RT).
Discussion
In this retrospective study on conventionally fractionated small solitary tumours or lymph nodes, a median OS of 51 months was found. This is very high compared to the findings of studies including all stages of LA-NSCLC. The RTOG-0617 showed an OS of 28.7 months and a phase III trial with 473 stage III NSCLC patients treated with chemo-RT showed a median time to death or distant metastasis of 28.3 months [Citation2, Citation7]. Similarly, a retrospective study including 255 patients from our institution showed a median OS of 28 months in patients treated with adaptive chemo-RT [Citation8]. The current cohort included stage I-IV patients, with the majority in stage III. However, all tumours were smaller than 5 cm in diameter. Tumour volume has previously been shown to be a prognostic factor for loco-regional recurrence with larger tumours showing higher rates of recurrence [Citation26–28]. The effect of tumour volume was not significant in the current study. However, all tumours included in the study were small with a median GTV volume of 8.7 cm3.
Patients with solitary lymph node targets had much longer (81 months) OS than patients with primary lung tumours (36 months). This agrees with the findings of other studies, including a competing risk model of the first failure site showing that primary tumours were more likely to fail than lymph nodes [Citation26, Citation28,Citation29].
In the univariate analysis of OS, only stage was significant, with patients diagnosed with stage IV disease showing OS of 9 months. Only four patients had stage IV, they all developed new distant metastases and died as a result of that. Prospectively, these patients may not be candidates for long-term RT and may be better suited for SBRT or palliative treatment. However, with only four stage IV patients it is difficult to draw a final conclusion.
Modelling studies [Citation10, Citation30,Citation31] have shown that tumour control may mainly depend on mean tumour dose, which is clearly seen in SBRT, where very high average dose levels ensure good tumour control and retrospective cohort studies have shown better OS for SBRT compared to conventional RT [Citation32,Citation33]. However, delivery of high doses to centrally located tumours nearby radiosensitive OAR may result in high toxicity levels as seen in previous studies [Citation14–20]. The Nordic HILUS-trial was a phase II trial of SBRT for patients with central or ultra-central tumours. Grade 4–5 toxicity occurred in 19% and 3% of the ultra-central and centrally located tumours, respectively [Citation15]. Additionally, a recent meta-analysis including results from controlled trials of SBRT of ultra-central and central tumours showed grade 3–5 toxicity rates of 9.0% and 4.4%, while the grade 5 complication rates were 5.7% and 2.3% [Citation16]. Therefore, the delivery of SBRT needs to focus on the dose to the OAR in order to avoid high toxicity levels. In Denmark, a national trial STRICTSTARLung recruits’ patients with central and ultra-central tumours. The trial is based on inhomogeneous dose distributions and the escalation dose is restricted by strict constraints on the OAR [Citation20]. The tumours in the current study were located centrally and thus, the patients potentially were candidates for the STRICTSTARLung trial.
In the current retrospective study, low levels of toxicity were observed with no grade 4–5 toxicity. Possibly due to the small tumour size and the conventionally dose regimen. However, the loco-regional control rate of 67% at 2-years is far below the 90.4% and 93.7% for ultra-central and central tumours reported in the meta-analysis [Citation16]. Similarly, a retrospective study performed at our hospital showed a local control rate of 89.4% for central lung tumours [Citation34]. A randomized study of SBRT vs conventional RT [Citation35] showed no difference in PFS and OS but they observed a tendency of an improved disease control rate in the SBRT group. Though, conventional RT secures low-toxicity dose delivery it could be at the expense of lower loco-regional control rates.
Dose escalation may be an alternative to SBRT. While the RTOG 0617 trial showed worse OS for the escalated arm, small phase I/II trials have shown increased loco-regional control [Citation2, Citation36,Citation37]. However, increased toxicity has been seen for dose escalation, too [Citation6, Citation38]. Thus, strict constraints for OAR are of high importance for dose escalation like in SBRT trials [Citation39].
The limitations of this study include its retrospective nature, the small size of the cohort and the extent of the treatment period. In the ten-year span of the study, improvements have been made in treatment techniques e.g., by the implementation of adaptive RT, which has led to significantly increased OS [Citation8]. However, the vast majority of the patients in this cohort were treated with adaptive RT. Additionally, improvements have been made in systemic treatment, especially the use of immunotherapy that demonstrated favourable responses in NSCLC patients. [Citation9, Citation40]. While this would impact the OS, the loco-regional control rate would not be affected to the same extent. Furthermore, the current cohort included stage I-IV patients, which makes comparison of outcome to other studies with exclusively stage III difficult, however the majority of patients had stage III and IV. The strengths of this study include a long median follow-up time of 44 months which secures that late toxicity has been registered. Moreover, all patients have been staged using PET securing correct staging.
In conclusion, conventional RT for NSCLC patients with small centrally located lung tumours or solitary lymph nodes is feasible. The toxicity was low with no grade 4–5 events. The median OS was 81 months for patients with solitary lymph node targets and 36 months for patients with primary lung tumours. The 3-year loco-regional control rate was 64%. This rate may be improved by treating the patients with SBRT and prospectively, these patients may be referred to the STRICTSTARLung trial.
Supplemental Material
Download MS Word (17.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study is available upon reasonable request from the corresponding author (MMK). The data are not publicly available as they contain information that could compromise the privacy of research participations.
Additional information
Funding
References
- Khalil AA, Hoffmann L, Moeller DS, et al. New dose constraint reduces radiation-induced fatal pneumonitis in locally advanced non-small cell lung cancer patients treated with intensity-modulated radiotherapy. Acta Oncol. 2015;54(9):1343–1349. doi:10.3109/0284186X.2015.1061216.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi:10.1016/S1470-2045(14)71207-0.
- van Diessen J, De Ruysscher D, Sonke J-J, et al. The acute and late toxicity results of a randomized phase II dose-escalation trial in non-small cell lung cancer (PET-boost trial). Radiother Oncol. 2019;131:166–173. doi:10.1016/j.radonc.2018.09.019.
- Hansen O, Knap MM, Khalil A, et al. A randomized phase II trial of concurrent chemoradiation with two doses of radiotherapy, 60 gy and 66 gy, concomitant with a fixed dose of oral vinorelbine in locally advanced NSCLC. Radiother Oncol. 2017;123(2):276–281. doi:10.1016/j.radonc.2017.03.017.
- De Ruysscher D, van Baardwijk A, Wanders R, et al. Individualized accelerated isotoxic concurrent chemoradiotherapy for stage III non-small cell lung cancer: 5-Year results of a prospective study. Radiother Oncol. 2019;135:141–146. doi:10.1016/j.radonc.2019.03.009.
- Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38(7):706–714. doi:10.1200/JCO.19.01162.
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi:10.1056/NEJMoa1809697.
- Møller DS, Lutz CM, Khalil AA, et al. Survival benefits for non-small cell lung cancer patients treated with adaptive radiotherapy. Radiother Oncol. 2022;168:234–240. doi:10.1016/j.radonc.2022.01.039.
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year survival outcomes From the PACIFIC trial: durvalumab After chemoradiotherapy in stage III Non-Small-Cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi:10.1200/JCO.21.01308.
- Klement RJ, Sonke JJ, Allgäuer M, et al. Correlating dose variables with local tumor control in stereotactic body radiation therapy for Early-Stage Non-Small cell lung cancer: a modeling study on 1500 individual treatments. Int J Radiat Oncol Biol Phys. 2020;107(3):579–586. doi:10.1016/j.ijrobp.2020.03.005.
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic body radiation therapy for operable Early-Stage lung cancer: findings From the NRG oncology RTOG 0618 trial. JAMA Oncol. 2018;4(9):1263–1266. doi:10.1001/jamaoncol.2018.1251.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage nonsmall-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–682. doi:10.1016/j.ijrobp.2008.11.042.
- Hoyer M, Roed H, Hansen AT, et al. Prospective study on stereotactic radiotherapy of limited-stage non small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66(4):S128–S135. doi:10.1016/j.ijrobp.2006.01.012.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating Central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839. 20doi:10.1200/JCO.2006.07.5937.
- Lindberg K, Grozman V, Karlsson K, et al. The HILUS-trial - a prospective nordic multi-center phase II study of ultra-Central lung tumors treated with stereotactic body radiotherapy. J Thorac Oncol. 2021;16(7):1200–1210.
- Rim CH, Shin IS, Yoon WS, et al. Dose-response relationship of stereotactic body radiotherapy for ultracentral tumor and comparison of efficacy with Central tumor: a meta-analysis. Transl Lung Cancer Res. 2020;9(4):1268–1284. doi:10.21037/tlcr-20-503.
- Tekatli H, Duijm M, Oomen-de HE, et al. Normal tissue complication probability modeling of pulmonary toxicity After stereotactic and hypofractionated radiation therapy for Central lung tumors. Int J Radiat Oncol Biol Phys. 2018;100(3):738–747. doi:10.1016/j.ijrobp.2017.11.022.
- Roach MC, Robinson CG, DeWees TA, et al. Stereotactic body radiation therapy for Central Early-Stage NSCLC: results of a prospective phase I/II trial. J Thorac Oncol. 2018;13(11):1727–1732. doi:10.1016/j.jtho.2018.07.017.
- Baker R, Han G, Sarangkasiri S, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys. 2013;85(1):190–195. doi:10.1016/j.ijrobp.2012.03.041.
- Nishimura S, Takeda A, Sanuki N, et al. Toxicities of organs at risk in the mediastinal and hilar regions following stereotactic body radiotherapy for centrally located lung tumors. J Thorac Oncol. 2014;9(9):1370–1376. doi:10.1097/JTO.0000000000000260.
- Hoffmann L, Persson GF, Nygård L, et al. Thorough design and pre-trial quality assurance (QA) decrease dosimetric impact of delineation and dose planning variability in the STRICTLUNG and STARLUNG trials for stereotactic body radiotherapy (SBRT) of Central and ultra-Central lung tumours. Radiother Oncol. 2022;171:53–61. doi:10.1016/j.radonc.2022.04.005.
- Sloth Møller D, Knap MM, Nyeng TB, et al. Difference in target definition using three different methods to include respiratory motion in radiotherapy of lung cancer. Acta Oncol. 2017;56(11):1604–1609. doi:10.1080/0284186X.2017.1373848.
- Wolthaus JWH, Sonke J-J, van Herk M, et al. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys. 2008;70(4):1229–1238. doi:10.1016/j.ijrobp.2007.11.042.
- Hoffmann L, Holt MI, Knap MM, et al. Anatomical landmarks accurately determine interfractional lymph node shifts during radiotherapy of lung cancer patients. Radiother Oncol. 2015;116(1):64–69. doi:10.1016/j.radonc.2015.06.009.
- Møller DS, Holt MI, Alber M, et al. Adaptive radiotherapy for advanced lung cancer ensures target coverage and decreases lung dose. Radiother Oncol. 2016;121(1):32–38. doi:10.1016/j.radonc.2016.08.019.
- Schytte T, Nielsen TB, Brink C, et al. Pattern of loco-regional failure after definitive radiotherapy for non-small cell lung cancer. Acta Oncol. 2014;53(3):336–341. doi:10.3109/0284186X.2013.868035.
- Bradley JD, Ieumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2002;52(1):49–57. doi:10.1016/s0360-3016(01)01772-2.
- van Diessen JN, Chen C, van den Heuvel MM, et al. Differential analysis of local and regional failure in locally advanced non-small cell lung cancer patients treated with concurrent chemoradiotherapy. Radiother Oncol. 2016;118(3):447–452. doi:10.1016/j.radonc.2016.02.008.
- Nygård L, Vogelius IR, Fischer BM, et al. A competing risk model of first failure site after definitive chemoradiation therapy for locally advanced Non-Small cell lung cancer. J Thorac Oncol. 2018;13(4):559–567. doi:10.1016/j.jtho.2017.12.011.
- Brahme A. Dosimetric precision requirements in radiation therapy. Acta Radiol Oncol. 1984;23(5):379–391. doi:10.3109/02841868409136037.
- Webb S, Nahum AE. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol. 1993;38(6):653–666. doi:10.1088/0031-9155/38/6/001.
- von Reibnitz D, Shaikh F, Wu AJ, et al. Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC). Acta Oncol. 2018;57(11):1567–1573. doi:10.1080/0284186X.2018.1481292.
- Haque W, Verma V, Polamraju P, et al. Stereotactic body radiation therapy versus conventionally fractionated radiation therapy for early stage non-small cell lung cancer. Radiother Oncol. 2018;129(2):264–269. doi:10.1016/j.radonc.2018.07.008.
- Khalil AA, Knap MM, Møller DS, et al. Local control after stereotactic body radiotherapy of centrally located lung tumours. Acta Oncol. 2021;60(8):1069–1073. doi:10.1080/0284186X.2021.1914345.
- Nyman J, Hallqvist A, Lund J, et al. SPACE- A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8. doi:10.1016/j.radonc.2016.08.015.
- Belderbos JS, Heemsbergen WD, De JK, et al. Final results of a phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(1):126–134. doi:10.1016/j.ijrobp.2006.04.034.
- Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63(2):324–333. doi:10.1016/j.ijrobp.2005.02.010.
- Cooke SA, de Ruysscher D, Reymen B, et al. 18F-FDG-PET guided vs whole tumour radiotherapy dose escalation in patients with locally advanced non-small cell lung cancer (PET-Boost): results from a randomised clinical trial. Radiother Oncol. 2023;181:109492. doi:10.1016/j.radonc.2023.109492.
- Møller DS, Nielsen TB, Brink C, et al. Heterogeneous FDG-guided dose-escalation for locally advanced NSCLC (the NARLAL2 trial): design and early dosimetric results of a randomized, multi-Centre phase-III study. Radiother Oncol. 2017;124(2):311–317. doi:10.1016/j.radonc.2017.06.022.
- Punekar SR, Shum E, Grello CM, et al. Immunotherapy in non-small cell lung cancer: past, present, and future directions. Front Oncol. 2022;12:877594. doi:10.3389/fonc.2022.877594.

