 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Introduction
Diffuse midline glioma (DMG) is the most common brainstem cancer among children [Citation1]. It is a fatal disease with less than 5% of patients alive 2 years from diagnosis [Citation2]. Despite numerous trials, tumour response has only been demonstrated following radiotherapy (RT). Re-irradiation has been suggested to enhance tumour control and symptom relief, however, the literature regarding re-irradiation is limited as DMG is a rare disease [Citation3–6]. Janssens et al. reported an increased survival benefit for re-irradiated patients with DMG compared to non-re-irradiated patients in a retrospectively matched cohort (3). Likewise, a meta-analysis showed that 87% of the re-irradiated patients had clinical improvement and radiologic response (7) and concluded that re-irradiation appeared safe for patients with DMG, with a caveat on the risk of radionecrosis following re-irradiation.
The European Society for Radiation Oncology and European Organisation for Research and Treatment of Cancer recently published a consensus statement defining re-irradiation as ‘(1) a new course of radiotherapy, (2) overlap of irradiated volumes, or (3) having a concern for toxicity from cumulative doses’ [Citation7]. The paper proposes an improved methodology for evaluating re-irradiation assessing clinical effect, anatomical localisation of the irradiated tumour, and the cumulative doses delivered even for palliative care.
For patients with DMG, with their limited overall survival, any treatment should ensure palliation, have limited toxicity, as well as reduce the overall burden of disease for patients and their families. In this, retrospective case series, we aim to evaluate the clinical efficacy and early toxicity in relation to a dosimetric analysis of re-irradiated patients with DMG.
Method and materials
This study is a retrospective review of DMG patients receiving re-irradiation at our institution. It was conducted in accordance with ethical regulations and permission from the regional ethical board and data approvals were obtained prior to the study (ID: R-21025615).
Patients
Paediatric patients with DMG disease, <18 years at diagnosis, were identified from our databases at Copenhagen University Hospital. Patients had been diagnosed and treated between January 2011 and May 2021. Patients were included for analysis if they had received two radiation treatments. Health records were systematically reviewed for baseline characteristics (age, gender, treatments), time to progression, symptoms at progression, toxicity from treatments, and clinical improvement/deterioration of symptoms at progression at 6 weeks following re-irradiation. All radiotherapy plans were collected.
Radiotherapy
The radiotherapy was delivered as volumetric-modulated arc therapy using a linear accelerator. Dose and fractionation schedules for first treatment (course 1) and re-irradiation (course 2) were retrieved. Treatment plans were evaluated in Varian EclipseTM (ver. 16.00.00, ©1996-2017 Varian Medical Systems Inc.) and Velocity (ver. 4.0 Varian Medical Systems Inc.). Tumour volume (clinical target volume (CTV)) and surrounding critical organs (OARs) were defined (i.e., brainstem, chiasm, pituitary, cochleae, hippocampi, optic nerves, eyes, lenses, and lacrimal glands) and contoured on relevant imaging scans in accordance with national guidelines [Citation8,Citation9]. The computed tomography (CT) scans used for radiotherapy planning in course 1 and 2, respectively, were fused for each re-irradiated patient using rigid registration. The anatomical overlap, if any, of each treatment’s CTV was visually assessed.
Dose-volume histogram
Biologically, equieffective (EQD2) dose-volume histograms (DHV) were plotted for all OARs and CTVs for course 1 and 2, respectively, using the package ‘DVHmetrics’ in Rstudio (ver. 4.1.2) [Citation10]. Withers formula was used to calculate the biologically, equieffective DVHs in 2 Gy fractions for each voxel:
D is the total dose, d is the dose per fraction, and the α/β ratio is a constant defining fractionation sensitivity. The α/β ratio was chosen to 2 Gy based on available literature [Citation11,Citation12].
For the patients with an overlapping CTV, dose summation was done in Velocity with a Dmax and Dmin for the brainstem, chiasm, pituitary, cochlear and optic nerve, respectively. EQD2 from the two courses were then summed using manual rigid registration. Dmax refers to the dose to 0,03cc (3x3x3mm3) of the structure and Dmin to the minimum dose of a voxel.
Results
Patients
We identified 19 patients with DMG and, among these, seven patients had been re-irradiated. All patients received 54 Gy/30F in course 1, and patients were evaluated for re-irradiation if they had effect of their primary treatment, were 6 months from primary treatment, and had a life expectancy which justified re-irradiation. The median age of the re-irradiated patients was six years (range: 2–13 years), and the median overall survival was 20 months (range: 9–30 months). The median time from course 1 to course 2 was 10 months (range: 8–17 months) and median time from course 2 to death was four months (range: 3–10 months).
After re-irradiation, four patients had clinical improvement of symptoms, one patient had stable disease, and one patient had progression of symptoms. No imaging was performed for this patient, and progression of symptoms occurred during re-irradiation. For one patient no follow-up data were available. Toxicities were minor and reported at a maximum of 6 weeks following start of re-irradiation. Three patients had to be anaesthetised during radiation. For more details, cf. .
Table 1. Re-irradiation of DMG.
Complete overlap of the CTVs in the first and the second course of radiotherapy was observed for all but one patient (cf Figure A1 for example of overlapping CTVs). This patient had metastatic progression in the medulla and was treated with focal radiotherapy (25 Gy/5F) to the involved spinal cord.
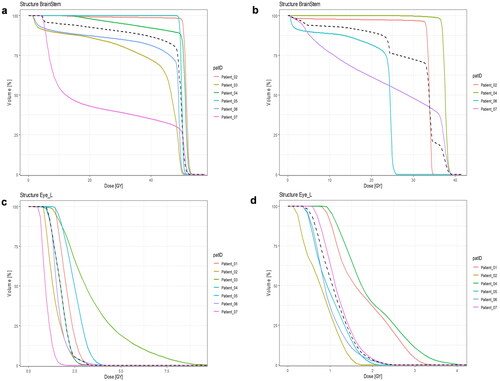
Figure 1. The individual DVHs for two organs at risk (OAR), one OAR close to (brainstem) and one OAR (left eye) far from the CTV. The colours represent the individual patients. The y-axis is percentage of irradiated volume, and the x-axis is the absolute dose in gray (Gy). The dashed line represents the median DVH of all patients. (a) Dose to the brainstem from course 1, (b) dose to the brainstem from course 2. (c) Dose to the left eye from course 1. (d) Dose to the left eye from course 2. This figure shows how OAR close to the CTV received a high dose to a large volume compared to OAR further away from the CTV.
Cumulative dose analysis
The DVH plots of the OARs demonstrate the similar dose distribution between course 1 and course 2 (cf. ). Also, the plots show how anatomical structures close to the CTV, i.e., the chiasm, pituitary, hippocampi, and cochleae, receive a high percentage of dose to a high percentage of volume. Conversely, for OAR further away from the CTV, i.e., the lenses, eyes, and lacrimal glands, a smaller volume is irradiated to relatively low doses (cf. Appendix Figure A2 for all DVHs).
Figure 2. EQD2 dose (Gy), each dot represents a patient and each colours represents a structure in the brain defined as an OAR. The colour code is shown in the legend. The dashed red line is equal to 60 Gy and represents the DNOG recommended Dmax to the brainstem, chiasm, and optic nerves [Citation9].
![Figure 2. EQD2 dose (Gy), each dot represents a patient and each colours represents a structure in the brain defined as an OAR. The colour code is shown in the legend. The dashed red line is equal to 60 Gy and represents the DNOG recommended Dmax to the brainstem, chiasm, and optic nerves [Citation9].](/cms/asset/6a67c4b1-8067-43db-a31a-d5aafd7316e1/ionc_a_2258271_f0002_c.jpg)
shows the cumulative Dmax and demonstrates high cumulative doses to the pituitary gland, the chiasm, cochleae, and brainstem (for more details cf. Appendix Table A1). The red line represents the maximal recommended Dmax according to the DNOG guidelines [Citation9]. As illustrated, the existing dose constraints used in cerebral radiotherapy are, for some patients, clearly violated during re-irradiation.
Discussion
In this retrospective study with seven patients, we found re-irradiation of DMG to be a potentially relevant and acceptable palliative treatment. Four patients experienced clinical improvement and one patient had stable disease at 6 weeks following re-irradiation. Toxicity was minor and no radionecrosis was reported. There was a complete anatomical overlap of the CTVs between the first and second course for all but one patient. Consequently, the cumulated Dmax to the brainstem, chiasm, cochleae, and optic nerves exceeded recommended dose constraints [Citation13–17].
Other studies of re-irradiation for DMG have been published, yet, none have included an analysis of the cumulated doses to the OAR in a paediatric setting [Citation3–5,Citation18–20]. In a study by Stiefel et al. cumulative doses after re-irradiation of brain tumours were assessed, though, the patients were adults and had a longer median OS compared to our patients [Citation21]; acute toxicity and late effects were limited, however, one patient died from radionecrosis (no report of cumulative dose). Re-irradiation appeared to be safe with cumulated doses below 100 Gy (EQD2) to the brainstem and below 75 Gy (EQD2) to the chiasm and optic nerves. In the paediatric setting, Tsang et al. have showed a similar tolerance to high doses in re-irradiation for children with ependymoma, hence an extrapolation of the cumulated Dmax levels suggested by Steifel et al. might be applicable to children [Citation22]. Likewise, Lu et al. concluded that re-irradiation is an effective treatment with minimal toxicities in a well-selected population of patients with DMG [Citation23].
Using re-irradiation in a palliative setting should be weighed against the risk of late effects, symptom relief, weaning of steroids, and acute toxicity [Citation7]. Patients with DMG have limited survival, hence palliative efficacy is of importance. As a third course of radiotherapy to patients with DMG have been suggested [Citation24,Citation25], this will introduce new clinical dilemmas concerning dose and efficacy. Our study demonstrated that dose constraints to avoid late effects were violated, but maybe this should play a smaller role for patients with DMG disease?
In our study, four patients were treated with a hypofractionated schedule (3 Gy/F) and two patients with normo-fractionation (2 Gy/F). For all but one patient, symptom relief or stabilization was reported. One patient progressed clinically during re-irradiation and died shortly thereafter. Unfortunately, it was not possible to conclude from the health records if the progression was due to progressive disease or if RT toxicity may have played a role, as no imaging was performed. In the literature, the recommended re-irradiation schedule is often 2 Gy/F, but in clinical practice there is a great variability in re-irradiation regimens for this patient group [Citation19,Citation23].
A few limitations to the study should be acknowledged. First, only seven patients out of 19 were re-irradiated, and only six were included for our dosimetric analysis. The low number of patients included in the dosimetric analysis limits the ability to generalize our results, however, our patients are comparable to patients in other DMG re-irradiation studies with regards to age, toxicities and OS [Citation3,Citation4,Citation26–28]. Secondly, we were unable to compare our patients to those not selected for re-irradiated. This obvious risk of selection bias has also been reported by others, but has not been systematically addressed in any studies [Citation23]. Likewise, due to the retrospective design of the study there is a risk of interpretation bias from the retrospective evaluation of health records.
In conclusion, most patients had symptom relief and toxicity was reported to be minor. Importantly, the cumulated doses to OAR close to the re-irradiated target were high and above the recommended dose constraints. Hence, re-irradiation is a relevant palliative treatment in DMG. The heterogenous and retrospective data in this small group – like previous studies – highlight the need for a protocolled treatment to ensure comparable evidence for palliative efficacy from re-irradiation. We plan to address this in the REMIT protocol.
Supplemental Material
Download MS Word (11.8 KB)Supplemental Material
Download PDF (250.2 KB)Supplemental Material
Download Zip (459.5 KB)Supplemental Material
Download JPEG Image (160 KB)Supplemental Material
Download MS Word (12.8 KB)Acknowledgements
We would like acknowledge colleagues Thomas Carlslund, Bob Smulders, Nora Forbes, and Deborah Anne Schut at the Department of Clinical Oncology, Section of Radiotherapy, Copenhagen University Hospital, for their help to extract the data. Also, an acknowledgement to Heidi Rønde and Camilla Kronborg from the Danish Centre for Particle therapy, for helping with dose summation.
Data availability statement
Data not available due to ethical/legal restrictions.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. doi: 10.1016/S1470-2045(06)70615-5.
- Jalali R, Raut N, Arora B, et al. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2010;77(1):113–118. doi: 10.1016/j.ijrobp.2009.04.031.
- Lassaletta A, Strother D, Laperriere N, et al. Reirradiation in patients with diffuse intrinsic pontine gliomas: the Canadian experience. Pediatr Blood Cancer. 2018;65(6):e26988. doi: 10.1002/pbc.26988.
- Massimino M, Biassoni V, Miceli R, et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol. 2014;118(2):305–312. doi: 10.1007/s11060-014-1428-z.
- Janssens GO, Gandola L, Bolle S, et al. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer. 2017;73:38–47. doi: 10.1016/j.ejca.2016.12.007.
- Krishnatry R, Manjali JJ, Chinnaswamy G, et al. Clinical approach to re-irradiation for recurrent diffuse intrinsic pontine glioma. Jpn J Clin Oncol. 2021;51(5):762–768. doi: 10.1093/jjco/hyab006.
- Andratschke N, Willmann J, Appelt AL, et al. European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus on re-irradiation: definition, reporting, and clinical decision making. Lancet Oncol. 2022;23(10):e469–e478. doi: 10.1016/S1470-2045(22)00447-8.
- DNOG. Nationale retningslinjer for proton behandling. 2022.
- DNOG. DNOG Retningslinjer for strålebehandling. 2016.
- Daniel Wollschlaeger and Heiko Karle "DVH metrics. RStudio". Germany: University Medical Center Mainz; 2022.
- Joiner MC, van der Kogel A. Basic clinical radiobiology. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2009.
- Jiang P, Zhang X, Jiang W, et al. Analysis of long-term outcome of image-guided volumetric modulated arc therapy (VMAT) for primary malignant tumor of the cervical spine. Cancer Biol Ther. 2020;21(7):623–628. doi: 10.1080/15384047.2020.1743149.
- Mayo C, Martel MK, Marks LB, et al. Radiation dose–volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S28–S35. doi: 10.1016/j.ijrobp.2009.07.1753.
- Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S36–S41. doi: 10.1016/j.ijrobp.2009.08.078.
- Darzy KH, Shalet SM. Hypopituitarism following radiotherapy. Pituitary. 2009;12(1):40–50. doi: 10.1007/s11102-008-0088-4.
- Chan SH, Ng WT, Kam KL, et al. Sensorineural hearing loss after treatment of nasopharyngeal carcinoma: a longitudinal analysis. Int J Radiat Oncol Biol Phys. 2009;73(5):1335–1342. doi: 10.1016/j.ijrobp.2008.07.034.
- Jeganathan VSE, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. 2011;79(3):650–659. doi: 10.1016/j.ijrobp.2010.09.056.
- Fontanilla HP, Pinnix CC, Ketonen LM, et al. Palliative reirradiation for progressive diffuse intrinsic pontine glioma. Am J Clin Oncol. 2012;35(1):51–57. doi: 10.1097/COC.0b013e318201a2b7.
- Amsbaugh MJ, Mahajan A, Thall PF, et al. A phase 1/2 trial of reirradiation for diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2019;104(1):144–148. doi: 10.1016/j.ijrobp.2018.12.043.
- Freese C, Takiar V, Fouladi M, et al. Radiation and subsequent reirradiation outcomes in the treatment of diffuse intrinsic pontine glioma and a systematic review of the reirradiation literature. Pract Radiat Oncol. 2017;7(2):86–92. doi: 10.1016/j.prro.2016.11.005.
- Stiefel I, Schröder C, Tanadini-Lang S, et al. High-dose re-irradiation of intracranial lesions – efficacy and safety including dosimetric analysis based on accumulated EQD2Gy dose calculation. Clin Transl Radiat Oncol. 2021;27:132–138. doi: 10.1016/j.ctro.2021.01.011.
- Tsang DS, Burghen E, Klimo P, et al. Outcomes after reirradiation for recurrent pediatric intracranial ependymoma. Int J Radiat Oncol Biol Phys. 2018;100(2):507–515. doi: 10.1016/j.ijrobp.2017.10.002.
- Lu VM, Welby JP, Mahajan A, et al. Reirradiation for diffuse intrinsic pontine glioma: a systematic review and meta-analysis. Childs Nerv Syst. 2019;35(5):739–746. doi: 10.1007/s00381-019-04118-y.
- Madrid AL, Santa-Maria V, Martinez OC, et al. Hg-37second re-irradiation for DIPG progression, re-considering “old strategies” with new approaches. Neuro Oncol. 2016;18(suppl 3):iii55.4–iii56. doi: 10.1093/neuonc/now073.34.
- Bergengruen PM, Hernaíz Driever P, Budach V, et al. Second course of re-irradiation in pediatric diffuse intrinsic pontine glioma. Strahlenther Onkol. 2023;199(8):773–777. doi: 10.1007/s00066-023-02057-x.
- Vanan MI, Eisenstat DD. DIPG in children – what can we learn from the past? Front Oncol. 2015;5:237. doi: 10.3389/fonc.2015.00237.
- Hayashi A, Ito E, Omura M, et al. Hypofractionated radiotherapy in children with diffuse intrinsic pontine glioma. Pediatr Int. 2020;62(1):47–51. doi: 10.1111/ped.14070.
- Zamora PL, Miller SR, Kovoor JJ. Single institution experience in re-irradiation of biopsy-proven diffuse intrinsic pontine gliomas. Childs Nerv Syst. 2021;37(8):2539–2543. doi: 10.1007/s00381-021-05195-8.

