Abstract
Purpose
To evaluate the persistence of symptoms after radiotherapy (RT) for localised prostate cancer (PCa) and the association with quality of life (QOL).
Materials and methods
Prospective patient-reported outcome (PRO) from a multi-institutional study on PCa treated with radical RT (2010–2014) was analysed. Data was collected at baseline (BL) and follow-ups (FUPs) up to 5 years. Patients with BL and ≥3 late FUPs (≥6 months) were analysed. PRO was scored by means of the IPSS and ICIQ-SF (urinary), LENT-SOMA (gastrointestinal [GI]), and EORTC-C30 (pain, insomnia, fatigue, and QOL) questionnaires. Symptoms were defined ‘persistent’ if the median score over FUPs was ≥3 (urinary) or ≥2 (GI, pain, insomnia, and fatigue), and worse than BL. Different thresholds were chosen to have enough events for each symptom. QOL was linearly transformed on a continuous scale (0–100). Linear-mixed models were used to identify significant differences between groups with and without persistent symptoms including age, smoking status, previous abdominal surgery, and diabetes as confounders. Mean QOL differences between groups were evaluated longitudinally over FUPs.
Results
The analysis included 293 patients. Persistent urinary symptoms ranged from 2% (straining) to 12% (weak stream, and nocturia). Gastrointestinal symptoms ranged from 7% (rectal pain, and incontinence) to 30% (urgency). Proportions of pain, insomnia, and fatigue were 6, 13, and 18%. Significant QOL differences of small-to-medium clinical relevance were found for urinary incontinence, frequency, urgency, and nocturia. Among GI symptoms, rectal pain and incontinence showed small-to-medium differences. Fatigue was associated with the largest differences.
Conclusions
The analysis showed that symptoms after RT for PCa occur with different persistence and their association with QOL varies in magnitude. A number of persistent urinary and GI symptoms showed differences in a comparable range. Urinary incontinence and frequency, rectal pain, and faecal incontinence more often had significant associations. Fatigue was also prevalent and associated with largely deteriorated QOL.
Introduction
The number of cancer survivors has dramatically increased with improved early detection and treatment efficacy [Citation1]. As a result, the long-term treatment burden on patients’ health and overall well-being has become increasingly important for several cancer sites [Citation2].
Given the excellent prognosis of localised prostate cancer (PCa), the focus is now directed on strategies aimed at reducing treatment-related side effects that can affect quality of life (QOL) [Citation3]. Radiotherapy (RT) is an effective option for localised PCa, especially considering recent technological advances that allow to increase the dose to the target and explore hypofractionated regimes [Citation4]. Although the rare incidence of severe adverse events, mild-to-moderate treatment-related symptoms are still present and might negatively affect daily activities and overall QOL of PCa survivors [Citation5].
Treatment optimisation aims to prevent morbidity based on the relationship between the dose to specific organs and the risk of a given symptom. One of the well-known side effects after RT for PCa and with a defined dose-effect relationship is rectal bleeding, whose risk can be now minimised during treatment planning by keeping the dose to the rectum within defined constraints [Citation6].
While the risk of some symptoms can be effectively reduced with both new technologies and optimal planning, the downstream effect on health-related QOL is less straightforward. Quality of life is in fact a complex concept encompassing functional, psychological, and social aspects of an individual’s health [Citation7]. The impact of a specific side effect on QOL depends not only on its severity, but also on the timing (onset, duration, and pattern of manifestation), location (organ-specific or systemic), and other factors that may be unrelated to treatment [Citation8].
To understand the true burden of radiation-induced symptoms, it is important to know which and to what extent they can impact patients’ QOL. Despite several recent reports on QOL of PCa survivors ranging from intermediate term (4–5 years) up to 15 years of follow-up [Citation9–18], only few have directly evaluated and compared the impact of side effects on QOL [Citation19–23]. However, most of these studies were either cross-sectional, retrospective or with limited follow-up, and in any case not evaluating the long-term temporal evolution of symptoms.
Since it is established that symptoms after RT can have different time patterns, being either irreversible, fluctuating, or also healing with successful interventions, to evaluate the association with long-term QOL it would also be relevant to assess their persistence and not only the severity. Nevertheless, a recent review reported that timing of symptoms is not usually included in prospective RT clinical trials [Citation24].
The aims of this work were: 1) to identify persisting symptoms after radical RT for localised PCa and, 2) to evaluate and rank the association of persistent symptoms with QOL of PCa survivors over 5 years of follow-up. For this purpose, mature patient-reported outcome (PRO) from the prospective DUE01 study was used [Citation4,Citation25,Citation26].
Materials and methods
Patients and treatment
The DUE01 study is an Italian multi-institutional prospective observational study aimed at developing predictive models for radiation-induced urinary and sexual side effects from radical RT for localised PCa. Patients were enrolled from 2010 to 2014. The study was approved by the Ethics Committees of each participating centre. Detailed information on selection criteria, and contouring/planning procedures were previously described [Citation26–28].
Patients were treated with either conventional (1.8–2 Gy/fraction) or moderately hypofractionated RT (2.2–2.7 Gy/fraction) in 5 fractions/week. Set-up included supine position, empty rectum, and full bladder. Image-guided RT was used in 80% of patients. Treatment of pelvic lymph nodes was delivered according to institutional practice.
Symptoms and QOL assessments
PRO was collected at baseline, at the end of RT, at 3 and 6 months after treatment, and thereafter every 6 months up to 5 years. Patients with exclusively biochemical recurrence, e.g., no clinical symptoms and only starting hormone therapy, were not censored during follow-up. For this analysis, patients were included if PRO was available at baseline and at least three late follow-ups (from 6 months onwards).
Urinary (GU) symptoms were scored with the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-SF) and the International Prostate Symptom Score (IPSS) questionnaires. For this analysis, individual items (symptoms) of the two questionnaires were evaluated. ICIQ-SF assesses the frequency and severity of urinary incontinence. IPSS is used to score symptoms of benign prostatic hyperplasia and is commonly used to evaluate urinary outcome after RT for PCa. The overall scores for IPSS and ‘objective’ ICIQ-SF (ICIQ-O, defined as the sum of items 3 and 4 pertaining to frequency and amount of urinary leakage, respectively) were also analysed. Gastrointestinal (GI) symptoms were reported by means of a modified version of the LENT-SOMA scoring system already used in a previous Italian prospective multi-institutional study on rectal toxicity after RT for PCa (AIRO PROS 01-02) [Citation29]. Systemic symptoms (pain, fatigue, and insomnia) were extracted from the European Organisation for Research and Treatment of Cancer (EORTC) C30 questionnaire [Citation30]. EORTC-C30 was also used to evaluate QOL for five functioning (physical, role, emotional, cognitive, and social) scales and a global health/general QOL domain. More information on questionnaires is provided in the Supplementary Material.
Statistics
Definition of symptom persistence
A recently developed methodology to define late, persistent, substantial, treatment-related symptoms (LAPERS) was used [Citation31,Citation32]. Patients were classified with moderate-severe persistent (MSP) symptoms if scores equal or above a pre-defined threshold were reported in at least half of the late follow-ups. As shown in the Supplementary Material, PRO measures included different systems based on either 6 (GU) or 4 (GI and systemic symptoms) points Likert response scales, therefore a score threshold of ≥3 and ≥2 was chosen to capture enough events and have comparable group sizes. In order to identify patients with treatment-related MSP symptoms, they were also divided according to whether or not a worsening beyond baseline condition was present. For overall IPSS and ICIQ-O scores, persistence was defined using thresholds recommended by the literature, requiring patients to report in at least half of follow-ups a score ≥8 or ≥5, respectively [Citation4,Citation33].
Association with QOL
Functioning and global health/QOL scales were linearly transformed into a continuous score (0–100) according to the EORTC manual [Citation34]. Linear mixed-effect models (LMM) were used to identify statistically significant differences in QOL between patients without and with MSP symptoms and with worsening condition compared to baseline. Models were adjusted with relevant patient-related confounders (age, smoking status, diabetes, and previous abdominal surgery). Statistical significance was assigned at a level of p ≤ 0.05 (two-sided). To account for multiple testing, the Benjamini–Hochberg method was used.
Mean differences for functioning scales and global health/QOL domain were calculated across late follow-ups from 6 months up to 5 years. As statistical significance is not always associated with clinical relevance, deteriorations in QOL were also evaluated according to evidence-based guidelines to interpret changes in scores (trivial, small, medium, and large) for the EORTC-C30 questionnaire [Citation35].
Results
Of 554 patients enrolled in the DUE01 study, 293 met the inclusion criteria for this analysis. The complete flowchart for patient selection is available in the Supplementary Material. The median follow-up of the cohort was 36 [range: 18, 60] months. The main patient, disease and treatment characteristics are summarised in . Details on prescribed dose, fractionation, technique, and dose-volume parameters for bladder and rectum are reported in the Supplementary Material.
Table 1. Patient, disease and treatment characteristics in the cohort eligible for analysis.
shows the proportion of patients with MSP symptoms according to the LAPERS methodology with and without worsening compared to baseline condition. Individual symptoms occurred with different persistence: the most frequent GI symptom was faecal urgency, with more patients experiencing worsening as compared to baseline status. On the other hand, fatigue symptoms were also recurrent but with more patients not reporting any worsening compared to BL. The incidences of most GI and GU symptoms were comparable in a ± 5% range. Among GU symptoms, incontinence, frequency, weak stream, and nocturia were more prevalent, while incomplete emptying and straining were rarely reported persistently over time. For GI symptoms, diarrhoea and mucus discharge were slightly more prevalent than incontinence and rectal pain. For most symptoms, most patients who reported persistently also presented a worsening compared to their baseline condition. Most of the patients reported MSP faecal incontinence, blood in stools, and urinary intermittency only after treatment.
Table 2. Proportions of patients with MSP symptoms with and without worsening compared to baseline condition.
Proportions of patients with persistent GI and systemic symptoms defined with a stricter criterion (score ≥3 in at least half of follow-ups) are reported in . The number of patients reporting persistent symptoms dropped dramatically: fatigue symptoms were experienced by ∼2% of the cohort, while GI symptoms were ≤1%. Only faecal urgency was reported by 4.4% of patients. Nevertheless, most of the patients worsened beyond BL condition. No patients had persistent faecal incontinence or rectal pain using this stricter threshold.
Table 3. Proportion of patient with MSP gastrointestinal and systemic (pain, fatigue, and insomnia) symptoms defined using a stricter threshold for persistence (score ≥3).
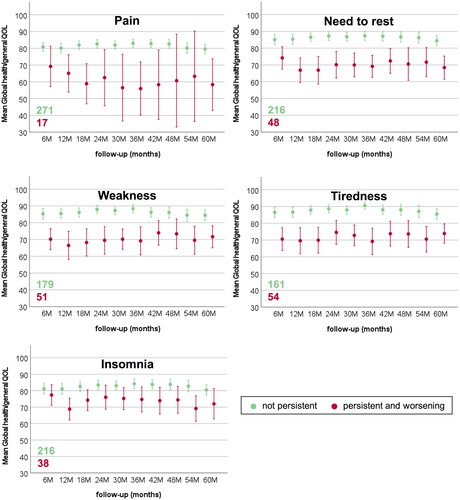
show mean global health/QOL scores for MSP GU, GI, and systemic symptoms over late follow-ups. Only patients with worsening beyond BL condition are reported. As highlighted by the overlap of the confidence intervals, no clear differences emerged for symptoms such as faecal urgency and blood in stools. QOL trends often fluctuated over time (i.e., urinary incontinence and urgency), while for some symptoms were worsening (overall IPSS, tenesmus, and need to rest), and for others improved (diarrhoea and mucus discharge). The QOL of patients without MSP symptoms was stable over time within narrow confidence intervals.
Figure 1. Mean and 95% confidence intervals for EORTC-C30 Global health/QOL during late follow-up for patients without and with MSP urinary symptoms.
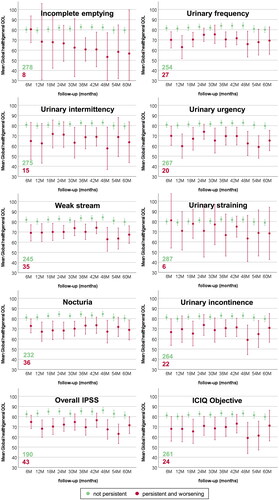
Figure 2. Mean and 95% confidence intervals for EORTC-C30 Global health/QOL during late follow-up for patients without and with MSP gastrointestinal symptoms.
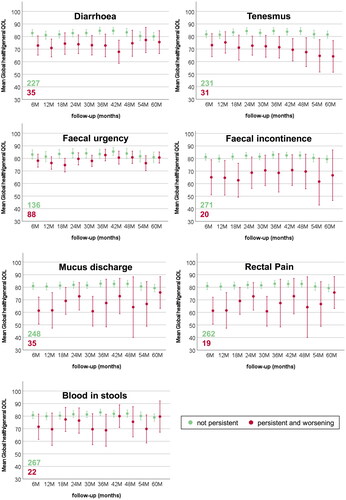
Figure 3. Mean and 95% confidence intervals for EORTC-C30 Global health/QOL during late follow-up for patients without and with MSP pain, fatigue, and insomnia.
After adjusting for confounders and multiple testing, statistically significant differences were found only for some MSP symptoms. The mean differences over time for the five functioning scales and global health/QOL domain are reported in . Differences are listed only for MSP symptoms with significant associations with QOL according to the LMM. The grey scale highlights the clinical relevance of differences according to guidelines for interpreting deteriorations in EORTC-C30 scores [Citation35].
Figure 4. Mean QOL deteriorations over 5 years for patients with MSP symptoms. Values are shown when statistically significance was found with LMM. Clinical relevance was assigned according the guidelines to interpret changes in EORTC-C30 scores [Citation35].
![Figure 4. Mean QOL deteriorations over 5 years for patients with MSP symptoms. Values are shown when statistically significance was found with LMM. Clinical relevance was assigned according the guidelines to interpret changes in EORTC-C30 scores [Citation35].](/cms/asset/9312404d-c180-4abb-9a97-0c42e4378657/ionc_a_2259597_f0004_b.jpg)
Associations with physical functioning were found for multiple MSP symptoms; however, differences were all trivial if considering their clinical relevance. On the other hand, differences for global health/QOL were all medium to large in magnitude. Role functioning was also associated with different symptoms; however, the clinical relevance was small to medium. Social functioning showed mostly differences of only minor clinical relevance. Emotional functioning was the least affected domain, and only fatigue symptoms contributed to small to medium differences. Finally, cognitive functioning was associated only with GU symptoms and with medium clinical relevance.
Some MSP symptoms had a recurrent association with several QOL domains but with varying clinical relevance. This was the case of incontinence, frequency, urgency, and nocturia for GU symptoms, and faecal incontinence and rectal pain for GI symptoms. Objective urinary incontinence (including both frequency and amount of urine) showed differences of medium clinical relevance for both role functioning and global health/QOL domain. On the other hand, the impact of the overall IPSS score, representing the sum of all GU symptoms (excluding incontinence reported in the ICIQ-SF) was not significant for role functioning. Fatigue symptoms were often associated with significant deteriorations, especially for emotional functioning and global health/QOL. Finally, MSP symptoms, such as weak stream, diarrhoea, faecal urgency, tenesmus, and insomnia, even if more prevalent among patients, were rarely associated with significant differences.
Discussion
Based on prospective PROs from PCa survivors over a 5-year follow-up, the current analysis identified symptoms persisting and worsening after RT, also investigating their long-term association with QOL. Urinary incontinence, frequency, and urgency, as well as rectal pain and faecal incontinence impacted several aspects of QOL. Fatigue was also associated with clinically relevant differences in QOL.
The present analysis confirmed previous findings from studies that have directly quantified how the incidence of different symptoms after RT for PCa can affect long-term QOL [Citation19–22], providing new insights on persistence of symptoms and their correlation with QOL.
PROs were used to evaluate a variety of GU, GI, and systemic symptoms. When compared to physician-assessed morbidity, PROs can be more sensitive and capture even milder severity of symptoms and are used to gather a more comprehensive information [Citation2]. Furthermore, physicians often underestimate the severity of symptoms and reporting can be inconsistent, leading to observation and inter-rater biases [Citation2,Citation36].
Persistence and prevalence of symptoms were also considered to evaluate the association with QOL, as mild to moderate but long-lasting symptoms may translate into significant limitations on daily activities and overall well-being [Citation8,Citation21]. The LAPERS methodology developed for EORTC data and validated in a prospective cohort of locally advanced cervical cancer patients [Citation31,Citation32] was modified for this scope. As PROs also refer to symptoms that can occur occasionally in the general healthy population, the concept of persistence was introduced to avoid transient conditions unrelated to treatment. Baseline status was also considered to exclude patients with pre-treatment conditions.
Persistence of symptoms
The LAPERS methodology highlighted that symptoms may last differently. Nocturia and faecal urgency were the most frequent GU and GI MSP symptoms, respectively, with worsening compared to baseline. On the other hand, straining and incomplete emptying rarely persisted over time, indicating that these symptoms are reversible or can potentially be treated with appropriate interventions. A considerable number of patients also experienced worsening MPS fatigue symptoms and insomnia.
Persistence of symptoms is rarely reported in clinical studies, yet it needs to be considered to understand the potentially long lasting or chronic effect on QOL. Karlsdóttir et al. showed that moderate GI symptoms after PCa RT tend to be reversible, while GU symptoms tend to persist [Citation37]. In this analysis, proportions of individual MSP GU and GI symptoms were often in a comparable range. However, scoring systems with different response scales were used to score GU and GI symptoms. As highlighted in , only few patients experienced MSP GI symptoms using a stricter threshold to define persistence.
Association with QOL
The analysis showed that not all symptoms are associated with QOL, regardless of their persistence. Furthermore, not all QOL domains (physical, role, emotional, cognitive, social, and global health/QOL) were affected in the same way and with the same clinical relevance. Statistically significant differences in physical functioning were found for multiple symptoms, but only with trivial relevance according to the guidelines for interpreting change scores for the EORTC-C30 questionnaire [Citation35]. On the other hand, all differences for global health/QOL had a medium-to-large clinical relevance. In addition, multiple symptoms were also associated with role functioning, but with limited clinical relevance, suggesting that MSP symptoms do not heavily impact professional life or leisure activities. Emotional functioning was less affected, and only by fatigue symptoms. The intercorrelation between fatigue, anxiety, and depression in cancer survivors has been already reported in the literature [Citation38,Citation39]. Interestingly, medium differences in cognitive functioning were found only for GU symptoms. A recent study highlighted that cognitive function is strongly associated with dysfunctional urine storage and with overactive bladder syndrome [Citation40]. Finally, a small clinical relevance was found for differences in social functioning. Family life and social activities thus seem to not be strongly affected by most of MSP symptoms.
Several symptoms were recurrently associated to different QOL domains. The impact on QOL of physician-assed urinary incontinence and rectal pain had been already reported by Schaake et al. [Citation21] and it is confirmed here using patient-reported symptoms. In addition, Schaake et al. stated that to the number of patients experiencing faecal incontinence was too small to reliably investigate the association with QOL in their analysis, although it is known to be a disabling condition especially with respect to the social domain [Citation41]. Its detrimental impact is confirmed here, as faecal incontinence was associated with worse role and social functioning, as well as global health/QOL. Other symptoms with similar impact on QOL were also detected, such as urinary frequency, urgency, and nocturia. Of note, despite being one of the most frequently addressed functional endpoints after RT for PCa [Citation42], rectal bleeding did not appear to be the most relevant for QOL.
In contrast to the findings of Bacon et al. indicating that the impact on QOL of GI symptoms after RT for PCa was generally more severe compared with GU and sexual symptoms, this analysis did not highlight clear differences between the two organ systems. This may be due to the questionnaires used to report GI and GU symptoms, including different scoring systems and thresholds used to define their persistence. Doses delivered to the bladder and relevant urinary sub-structures are usually high, and a considerable number of patients experienced MSP GU symptoms [Citation4], while GI symptoms were reported persistently at lower scores.
Fatigue-related symptoms (need to rest, weakness, and tiredness) were associated with almost all QOL domains and with the largest differences. This finding confirms what Lilleby et al. [Citation19] previously reported: fatigue, and physical and emotional functioning emerged as the only predictors of QOL in multivariable analysis. Furthermore, this analysis showed that only fatigue was associated with emotional functioning. Despite being one of the most common symptoms related to cancer and its treatment, fatigue was rarely investigated in clinical studies, probably also owing to its multi-factorial nature [Citation43,Citation44]. Nevertheless, previous works have already evaluated the impact of fatigue on QOL of cancer survivors [Citation45,Citation46]. Other studies have also suggested that fatigue could be related to large irradiated volumes [Citation47,Citation48]. In fact, almost half of the patients in the DUE01 study received prophylactic lymph-nodal irradiation.
Limitations and challenges
This work is not devoid of limitations. First, it relied upon different PRO measures, making the comparison of persistent symptoms less straightforward. A lower severity threshold was chosen for GI and systemic symptoms in order to achieve a sufficient number of events. However, association with QOL was not always dependent on the severity of the score. Indeed, patients reporting faecal incontinence only ‘sometimes’ but persistently had significant differences in QOL. On the other hand, more frequently reported symptoms, such as faecal urgency, diarrhoea, weak stream, or insomnia were usually not associated with worse QOL.
In addition, the analysis was focused on single symptoms, but it is known that they often can co-occur in clusters [Citation8]. Therefore, it would be more relevant to evaluate the impact of clusters on QOL rather than individual symptoms.
Only a limited number of confounders was included in the models, as the primary aim of this work was to evaluate and quantify significant differences associated with symptoms and not the impact of other potential risk factors. However, other aspects may contribute to a worse QOL. Cardiovascular diseases and use of antiaggregants can play a role. Furthermore, the use of antiandrogens (ADT) can increase the risk of GI, GU, and fatigue symptoms after RT for PCa [Citation49–51]. Unfortunately, detailed information on ADT timing before and after RT was not comprehensively recorded in the DUE01 study and therefore the effect of this parameter could not be quantified. Of note, perception of patients’ wellbeing is also modulated by parameters difficult to measure in the context of a clinical study, such as self-esteem, resilience, and spirituality [Citation52,Citation53].
Although PROs offer undeniable advantages, they often lack the quantitative and clinical information found in physician-assessed morbidity. Furthermore, as patients scored both symptoms and QOL, findings might be biased by the extreme response phenomenon, which expresses the tendency to select the extreme endpoints of a response scale [Citation54]. However, since not all symptoms showed clinical relevance in QOL aspects, this issue does not seem to strictly apply here. In general, it would be ideal to use both PRO and physician-assessed morbidity to get a more comprehensive understanding [Citation36].
Moreover, information on morbidity management during follow-up was not recorded in the DUE01 study, while it is known that some symptoms can be successfully treated with interventions and therefore not impacting long-term QOL.
Finally, sexual dysfunction was not addressed in this analysis. Although a comparable burden to GI and GU dysfunctions, its aetiology in relation to treatment, patient-related factors and the impact on QOL is more complex to assess. In fact, sexuality is not limited to the mere erectile dysfunction, but encompasses different aspects such as sexual desire, ability to achieve orgasm, relationships, confidence, self-esteem, etc. Correlations with age, partner status, and use of ADT make interpretations more complicated to disentangle. Sexual dysfunction was reported in the DUE01 study with the International Index of Erectile Function (IIEF) questionnaire that measures domains related to erectile and orgasmic function, sexual desire, intercourse, and overall satisfaction. Future works will be focused on understanding the impact of sexual dysfunction on QOL after RT for PCa [Citation55].
Clinical implications and future perspectives
Identifying persistent symptoms after RT for PCa and evaluating their association with QOL has several clinical implications. It can increase the informational value and comprehensiveness of risk communication with patients, so as to possibly engage them in a shared decision-making and boost their coping strategies with symptoms [Citation32].
Ranking symptoms based on their impact on QOL can also help to define evidence-based priorities in treatment planning to guide dose optimisation [Citation45]. This approach has already been introduced for head and neck cancer, where symptoms with more severe impact on QOL have been also identified [Citation46].
Primary prevention with optimal planning and new technologies is not the only application. Identifying relevant symptoms for specific cancer sites can help to optimise and standardise PRO measures to be used in clinical studies and improve comparability of findings [Citation2]. This can also promote closer follow-up and support measures based on symptoms that can be prevented and/or treated in a timely manner. Finally, knowledge of symptoms that impair QOL can encourage individualised and targeted morbidity management for patients already experiencing them [Citation12]. This may include interventions, such as tailored exercise to reduce symptoms like fatigue. In addition to physical health concerns, anxiety, depression, and overall mental wellbeing should also be addressed before treatment and in the follow-up care [Citation20].
Supplemental Material
Download PDF (88.7 KB)Supplemental Material
Download PDF (156.8 KB)Supplemental Material
Download PDF (110.8 KB)Supplemental Material
Download PDF (221.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, SS, upon reasonable request.
Additional information
Funding
References
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565.
- Remick JS, Kowalski E, Samanta S, et al. Health-related quality of life and patient-reported outcomes in radiation oncology clinical trials. Curr Treat Options Oncol. 2020;21(11):87. doi: 10.1007/s11864-020-00782-4.
- Ferrer M, Guedea F, Suárez JF, et al. Quality of life impact of treatments for localized prostate cancer: cohort study with a 5 year follow-up. Radiother Oncol. 2013;108(2):306–313. doi: 10.1016/j.radonc.2013.05.038.
- Cozzarini C, Rancati T, Palorini F, et al. Patient-reported urinary incontinence after radiotherapy for prostate cancer: quantifying the dose–effect. Radiother Oncol. 2017;125(1):101–106. doi: 10.1016/j.radonc.2017.07.029.
- Ohri N, Dicker AP, Showalter TN. Late toxicity rates following definitive radiotherapy for prostate cancer. Can J Urol. 2012;19(4):6373–6380.
- Rancati T, Fiorino C. Modelling radiotherapy side effects: practical applications for planning optimisation. 1st ed. Boca Raton (FL): CRC Press; 2019.
- Osoba D. Interpreting the meaningfulness of changes in health-related quality of life scores: lessons from studies in adults. Int J Cancer. 1999;83(S12):132–137. doi: 10.1002/(SICI)1097-0215(1999)83:12+<132::AID-IJC23>3.0.CO;2-4.
- Matzka M, Köck-Hódi S, Jahn P, et al. Relationship among symptom clusters, quality of life, and treatment-specific optimism in patients with cancer. Support Care Cancer. 2018;26(8):2685–2693. doi: 10.1007/s00520-018-4102-8.
- Ma TM, Ballas LK, Wilhalme H, et al. Quality-of-Life outcomes and toxicity profile among patients with localized prostate cancer after radical prostatectomy treated with stereotactic body radiation: the SCIMITAR multicenter phase 2 trial. Int J Radiat Oncol Biol Phys. 2023;115(1):142–152. doi: 10.1016/j.ijrobp.2022.08.041.
- Murthy V, Maitre P, Bhatia J, et al. Late toxicity and quality of life with prostate only or whole pelvic radiation therapy in high risk prostate cancer (POP-RT): a randomised trial. Radiother Oncol. 2020;145:71–80. doi: 10.1016/j.radonc.2019.12.006.
- Hoffman KE, Penson DF, Zhao Z, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2020;323(2):149–163. doi: 10.1001/jama.2019.20675.
- Ralph N, Ng SK, Zajdlewicz L, et al. Ten-year quality of life outcomes in men with prostate cancer. Psychooncology. 2020;29(2):444–449. doi: 10.1002/pon.5255.
- Mazariego CG, Egger S, King MT, et al. Fifteen year quality of life outcomes in men with localised prostate cancer: population based Australian prospective study. BMJ. 2020;371:m3503. doi: 10.1136/bmj.m3503.
- Nakamura K, Konishi K, Komatsu T, et al. Quality of life after external beam radiotherapy for localized prostate cancer: comparison with other modalities. Int J Urol. 2019;26(10):950–954. doi: 10.1111/iju.14026.
- Punnen S, Cowan JE, Chan JM, et al. Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE registry. Eur Urol. 2015;68(4):600–608. doi: 10.1016/j.eururo.2014.08.074.
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220.
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221.
- Kerleau C, Guizard AV, Daubisse-Marliac L, et al. Long-term quality of life among localised prostate cancer survivors: QALIPRO population-based study. Eur J Cancer. 2016;63:143–153. doi: 10.1016/j.ejca.2016.05.020.
- Lilleby W, Fosså SD, Waehre HR, et al. Long-term morbidity and quality of life in patients with localized prostate cancer undergoing definitive radiotherapy or radical prostatectomy. Int J Radiat Oncol Biol Phys. 1999;43(4):735–743. doi: 10.1016/s0360-3016(98)00475-1.
- Bacon CG, Giovannucci E, Testa M, et al. The association of treatment-related symptoms with quality-of-life outcomes for localized prostate carcinoma patients. Cancer. 2002;94(3):862–871. doi: 10.1002/cncr.10248.
- Schaake W, Wiegman EM, De Groot M, et al. The impact of gastrointestinal and genitourinary toxicity on health related quality of life among irradiated prostate cancer patients. Radiother Oncol. 2014;110(2):284–290. doi: 10.1016/j.radonc.2013.11.011.
- Sveistrup J, Mortensen OS, Bjørner JB, et al. Prospective assessment of the quality of life before, during and after image guided intensity modulated radiotherapy for prostate cancer. Radiat Oncol. 2016;11(1):117. doi: 10.1186/s13014-016-0689-4.
- Marvaso G, Gugliandolo SG, Bellerba F, et al. Phase II prospective trial “give me five” short-term high precision radiotherapy for early prostate cancer with simultaneous boost to the dominant intraprostatic lesion: the impact of toxicity on quality of life (AIRC IG-13218). Med Oncol. 2020;37(8):37. doi: 10.1007/s12032-020-01397-3.
- Vittrup AS, Kirchheiner K, Fokdal LU, et al. Reporting of late morbidity after radiation therapy in large prospective studies: a descriptive review of the current status. Int J Radiat Oncol Biol Phys. 2019;105(5):957–967. doi: 10.1016/j.ijrobp.2019.08.040.
- Carillo V, Cozzarini C, Rancati T, et al. Relationships between bladder dose-volume/surface histograms and acute urinary toxicity after radiotherapy for prostate cancer. Radiother Oncol. 2014;111(1):100–105. doi: 10.1016/j.radonc.2014.02.006.
- Palorini F, Cozzarini C, Gianolini S, et al. First application of a pixel-wise analysis on bladder dose-surface maps in prostate cancer radiotherapy. Radiother Oncol. 2016;119(1):123–128. doi: 10.1016/j.radonc.2016.02.025.
- Improta I, Palorini F, Cozzarini C, et al. Bladder spatial-dose descriptors correlate with acute urinary toxicity after radiation therapy for prostate cancer. Phys Med. 2016;32(12):1681–1689. doi: 10.1016/j.ejmp.2016.08.013.
- Onjukka E, Fiorino C, Cicchetti A, et al. Patterns in ano-rectal dose maps and the risk of late toxicity after prostate IMRT. Acta Oncol. 2019;58(12):1757–1764. doi: 10.1080/0284186X.2019.1635267.
- Valdagni R, Vavassori V, Rancati T, et al. Increasing the risk of late rectal bleeding after high-dose radiotherapy for prostate cancer: the case of previous abdominal surgery. Results from a prospective trial. Radiother Oncol. 2012;103(2):252–255. doi: 10.1016/j.radonc.2012.03.012.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The european organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365.
- Kirchheiner K, Pötter R, Nout RA, et al. Late, persistent, substantial, Treatment-Related symptoms after radiation therapy (LAPERS): a new method for longitudinal analysis of late morbidity—applied in the EMBRACE study. Int J Radiat Oncol Biol Phys. 2020;106(2):300–309. doi: 10.1016/j.ijrobp.2019.10.027.
- Vittrup AS, Tanderup K, Bentzen SM, et al. Persistence of late substantial patient-reported symptoms (LAPERS) after radiochemotherapy including image-guided adaptive brachytherapy for locally advanced cervical cancer: a report from the EMBRACE study. Int J Radiat Oncol Biol Phys. 2021;109(1):161–173. doi: 10.1016/j.ijrobp.2020.08.044.
- Hopland-Nechita FV, Andersen JR, Beisland C. IPSS “bother question” score predicts health-related quality of life better than total IPSS score. World J Urol. 2022;40(3):765–772. doi: 10.1007/s00345-021-03911-2.
- Fayers P, Bottomley A. Quality of life research within the EORTC-the EORTC QLQ-C30. European organisation for research and treatment of cancer. Eur J Cancer. 2002;38(4):S125–S33. doi: 10.1016/s0959-8049(01)00448-8.
- Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713–1721. doi: 10.1016/j.ejca.2012.02.059.
- Petersen SE, Thorsen LB, Hansen S, et al. A phase I/II study of acute and late physician assessed and patient-reported morbidity following whole pelvic radiation in high-risk prostate cancer patients. Acta Oncol. 2022;61(2):179–184. doi: 10.1080/0284186X.2021.1979246.
- Karlsdóttir Á, Muren LP, Wentzel-Larsen T, et al. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys. 2008;70(5):1478–1486. doi: 10.1016/j.ijrobp.2007.08.076.
- Agasi-Idenburg SC, Thong MSY, Punt CJA, et al. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer. 2017;25(2):625–632. doi: 10.1007/s00520-016-3451-4.
- Schellekens MPJ, Wolvers MDJ, Schroevers MJ, et al. Exploring the interconnectedness of fatigue, depression, anxiety and potential risk and protective factors in cancer patients: a network approach. J Behav Med. 2020;43(4):553–563. doi: 10.1007/s10865-019-00084-7.
- Koitabashi R, Uchida Y. Analysing the relationship between cognition and urine storage function. Int J Urol Nurs. 2019;13(2):51–56. doi: 10.1111/ijun.12184.
- Bartlett L, Nowak M, Ho YH. Impact of fecal incontinence on quality of life. World J Gastroenterol. 2009;15(26):3276–3282. doi: 10.3748/wjg.15.3276.
- Yamazaki H, Nakamura S, Nishimura T, et al. Transitioning from conventional radiotherapy to intensity-modulated radiotherapy for localized prostate cancer: changing focus from rectal bleeding to detailed quality of life analysis. J Radiat Res. 2014;55(6):1033–1047. doi: 10.1093/jrr/rru061.
- Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127.
- Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23(2):127–135. doi: 10.1016/j.soncn.2007.01.004.
- Spampinato S, Tanderup K, Lindegaard JC, et al. Association of persistent morbidity after radiotherapy with quality of life in locally advanced cervical cancer survivors. Radiother Oncol. 2023;181:109501. doi: 10.1016/j.radonc.2023.109501.
- van der Laan HP, Van den Bosch L, Schuit E, et al. Impact of radiation-induced toxicities on quality of life of patients treated for head and neck cancer. Radiother Oncol. 2021;160:47–53. doi: 10.1016/j.radonc.2021.04.011.
- Smet S, Spampinato S, Pötter R, et al. Risk factors for late persistent fatigue after chemoradiotherapy in patients with locally advanced cervical cancer: an analysis from the EMBRACE-I study. Int J Radiat Oncol Biol Phys. 2022;112(5):1177–1189. doi: 10.1016/j.ijrobp.2021.11.022.
- Joseph N, Cicchetti A, McWilliam A, et al. High weekly integral dose and larger fraction size increase risk of fatigue and worsening of functional outcomes following radiotherapy for localized prostate cancer. Front Oncol. 2022;12:937934. doi: 10.3389/fonc.2022.937934.
- Feigenberg SJ, Hanlon AL, Horwitz EM, et al. Long-term androgen deprivation increases grade 2 and higher late morbidity in prostate cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62(2):397–405. doi: 10.1016/j.ijrobp.2004.10.021.
- Taaffe DR, Newton RU, Spry N, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol. 2017;72(2):293–299. doi: 10.1016/j.eururo.2017.02.019.
- Luo YH, Yang YW, Wu CF, et al. Fatigue prevalence in men treated for prostate cancer: a systematic review and meta-analysis. World J Clin Cases. 2021;9(21):5932–5942. doi: 10.12998/wjcc.v9.i21.5932.
- Kobayashi M, Ohno T, Noguchi W, et al. Psychological distress and quality of life in cervical cancer survivors after radiotherapy do treatment modalities, disease stage, and self-esteem influence outcomes? Int J Gynecol Cancer. 2009;19(7):1264–1268. doi: 10.1111/IGC.0b013e3181a3e124.
- Wenzel LB, Donnelly JP, Fowler JM, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: a gynecologic oncology group study. Psychooncology. 2002;11(2):142–153. doi: 10.1002/pon.567.
- Liu M, Harbaugh AG, Harring JR, et al. The effect of extreme response and non-extreme response styles on testing measurement invariance. Front Psychol. 2017;8:726. doi: 10.3389/fpsyg.2017.00726.
- Cozzarini C, Rancati T, Badenchini F, et al. Baseline status and dose to the penile bulb predict impotence 1 year after radiotherapy for prostate cancer. Strahlenther Onkol. 2016;192(5):297–304. doi: 10.1007/s00066-016-0964-1.

