Abstract
Introduction
We examined the role of receptor profiles and other prognostic factors in survival outcomes after stereotactic radiosurgery (SRS) for brain metastases in breast cancer patients, to help improve selection of candidates for SRS.
Material and methods
We included 149 consecutive patients who received SRS between 2012 and 2019 at the University Hospital of Copenhagen, Rigshospitalet, Denmark. Overall survival (OS) following SRS was determined through the Kaplan–Meier method, while CNS progression-free survival (CNS-PFS) was determined through competing risk analysis. Prognostic factors for both OS and CNS-PFS were evaluated through uni- and multivariate Cox regression and Fine-Gray models, respectively. The proportional hazards assumptions were tested through Schoenfeld residuals, and non-proportionality was accounted for by the inclusion of time-dependent variables.
Results
Median OS was 14.8 months for the entire cohort and was as follows for the four receptor profiles: 33.3 months for ER+/HER2+ (ER: estrogen receptor, HER2: human epidermal growth factor receptor 2), 11.0 months for ER+/HER2−, 17.7 months for ER−/HER2+, and 5.3 months for ER−/HER2−. In the multivariate model, the ER−/HER2− receptor profile (hazard ratio (HR): 2.00, 95% confidence interval (CI): 1.09–3.67) and the presence of extracranial visceral metastases (HR: 2.90, 95% CI: 1.53–5.50) were associated with worse OS. The ER+/HER2+ receptor profile (HR: 0.43, 95% CI: 0.19–0.96) and 5+ lines of treatment (HR: 0.40, 95% CI: 0.20–0.82) were both associated with improved OS. For CNS-PFS, 5+ lines of treatment (sub-distributional hazard ratio (SHR): 2.88, 95% CI: 1.06–7.81) was associated with worse CNS-PFS, while extracranial visceral metastases (SHR: 0.54, 95% CI: 0.30–0.97) was associated with reduced risk of CNS progression – which is primarily due to patients with extracranial metastases dying before developing new CNS progression.
Conclusion
Extracranial visceral disease and the ER−/HER2− receptor profile were associated with poor survival outcomes following SRS.
Background
Treatment options for patients with metastatic breast cancer (mBC) have been rapidly evolving over the last decades, resulting in prolonged survival following diagnosis [Citation1]. With this prolonged survival as well as improved imaging techniques, breast cancer brain metastases (BCBM) are becoming an increasingly common diagnosis [Citation2,Citation3]. Approximately 15%–30% of patients with metastatic breast cancer will develop brain metastases, making it the second-most common cause of brain metastases after lung cancer [Citation4–7].
The prognosis after the development of brain metastases remains poor. Median overall survival (OS) ranges from 4.5 to 13.8 months [Citation8]. The incidence of BCBM varies greatly based on ER (estrogen receptor) and HER2 (human epidermal growth factor receptor 2) status, with a significantly higher incidence among HER2+ and ER−/HER2− cancers (22-36% for HER2+, 15%–37% for ER−/HER2−) than ER+/HER2− cancers (15%) [Citation9,Citation10]. Survival following a diagnosis of brain metastases also varies based on subtype, with ER−/HER2− having the poorest prognosis with a median OS of 4.4 months [Citation6].
Systemic treatment options for BCBM are limited, and treatment therefore still relies on local treatment therapies [Citation11,Citation12]. The three major treatment options are surgical resection, stereotactic radiosurgery (SRS), and whole brain irradiation (WBI). SRS is administered as one or a few high-dose treatments, causing minimal disruptions to systemic treatment schedules. It also ensures high levels of intracranial control with fewer risks and side effects than WBI, making it the preferred choice of treatment in patients with 1–3 brain metastases [Citation13–15].
While the efficacy of SRS is well-documented, less is known about how factors such as receptor subtype affect survival outcomes in patients following SRS. This information could be used to provide a better understanding of how to select patients for SRS more efficiently. The aim of the study was to establish which clinical factors had the greatest impact on survival outcomes and how this information can be used to improve patient selection for SRS, with a special focus on the role of receptor subtypes. We retrospectively analyzed a cohort of 149 Danish breast cancer patients who had received SRS in order to examine which clinical factors were associated with overall survival (OS) and CNS progression-free survival (CNS-PFS) following SRS.
Material and methods
Study population
We included patients with metastatic breast cancer who had brain metastases that were treated with SRS at University Hospital Copenhagen, Rigshospitalet, Denmark, from January 1st, 2012, through to December 2nd, 2019. The final follow-up date was May 31st, 2022 and patients were followed according to guidelines at the time of treatment. The population included patients who received SRS as their only radiotherapy treatment, as well as patients who had had whole brain irradiation or post-surgical radiation before or after SRS. All patients were selected for SRS at a multidisciplinary conference. Patients with more than one primary cancer where the histology of the brain metastases could not be confirmed were excluded. Patients from Greenland and patients who received systemic treatment prior to 2000 were also excluded due to the lack of access to medical records. All patient information was gathered from the Danish Breast Cancer Group’s (DBCG) metastatic database and a local SRS database at the Department of Clinical Oncology, Rigshospitalet. ER and HER2 status were determined using results from biopsies taken at the time of patients’ primary breast cancer diagnosis. The study was approved by the DBCG and the Capital Region’s research overview as per Danish legislation, with approval number P-2020-70.
Stereotactic radiosurgery technique
Before treatment initiation, MRI and CT were used to scan patients, with the images being fused in three dimensions. The CT scan was used to ensure proper positioning of the patient and for dosage calculations, while the MRI was used to identify and delineate the targets and organs at risk. Treatment was delivered via VMAT (volumetric modulated arc therapy) on a linear accelerator. Varian Novalis TrueBeam® was used to ensure optimal conformity to the target. Patient fixation was achieved through the thermoplastic mask, and the patient was placed on a couch moveable in six dimensions to allow optimal positioning. Geometric setup uncertainty was kept below 1 mm by a 2D X-ray system (ExacTrac®, Varian Medical Systems). The gross tumor volume (GTV) was defined as a macroscopic tumor (or postoperative cavity) on MRI, and the planning target volume (PTV) was the GTV +1mm isocentric margin for optimisation. Treatment was delivered in 1–3 fractions, with most patients receiving single-fraction SRS with a prescribed dose of 18 Gy. The dose was prescribed to the PTV with 95% of the PTV receiving 98% of the dose, and the maximum dose was 105–125% of the prescribed dose (<110% of dose delivered outside of the GTV).
Statistical analysis
Our cohort was described using descriptive statistics, with the cohort split into four subgroups based on receptor profiles (ER+/HER2+, ER+/HER2−, ER−/HER2+, and ER−/HER2−). Our primary endpoint was overall survival (OS), and our secondary endpoint was CNS progression-free survival (CNS-PFS). The prognostic factors examined for both endpoints were receptor profiles (four types), ER±, HER2±, age (dichotomized at median), the total volume of treated tumors (dichotomized at median), number of treated brain metastases (dichotomized at median), lines of treatment at the time of SRS (dichotomized at median), WBI before SRS yes/no, presence of extracranial visceral metastases yes/no, and time from breast cancer diagnosis to a diagnosis of BCBM (dichotomized at median). ER and HER2 status were examined in a univariate setting, but they were not included in our multivariate models due to risk of covariance. All statistical analyses were performed using R version 4.2.1.
OS following SRS was defined as the time from the patient’s first SRS until death from any cause. OS was estimated using the Kaplan-Meier product limit method. Prognostic factors and their impact on OS were then evaluated using uni- and multivariate Cox proportional hazards regression. Factors significant (p < .1) in a univariate Cox model were combined in a multivariate Cox model, providing multivariate hazard ratios (HR) and confidence intervals (CI).
CNS-PFS was defined as the time from SRS until confirmed CNS progression (the date when CNS progression following SRS was first confirmed via scans). CNS progression was defined as local recurrence, new remote lesions or both and was analyzed in a competing risk setting, with death before CNS progression as the competing event. Cumulative incidence functions were evaluated using Gray’s test. The Fine-Gray model was used to calculate subdistributional hazard ratios and confidence intervals for prognostic factors. Factors significant (p < .1) in a univariate Fine-Gray model were then included in the final multivariate model.
The proportional hazards assumptions were tested using the Schoenfeld residuals, and non-proportionality was accounted for by including time-dependent variables in the models. Non-proportional variables were analyzed utilizing a time-split at 12 months, meaning the variables were split into the first 12 months following SRS and after 12 months following SRS.
Results
Patient characteristics
We included 149 consecutive patients with a median age of 59 years at the time of SRS. 88 patients (59%) had ER + disease, and 76 patients (51%) had HER2+ disease. When looking at all four possible receptor subtypes across the cohort, 26% were ER+/HER2+, 33% ER+/HER2−, 25% ER−/HER2+, and 15% ER−/HER2−, respectively, with one patient having an unknown receptor profile due to unknown HER2 status. Invasive ductal carcinoma was the most prevalent histological type of breast cancer, making up 88% of the cancers. 28% of the patients presented with no extracranial disease, while 50% had bone metastases, 40% lung metastases, and 38% liver metastases, respectively. A detailed description of patient and treatment characteristics is shown in .
Table 1. Baseline characteristics at the time of SRS.
A total number of 267 lesions were treated across the cohort. The majority of patients had one lesion treated, with two outliers receiving treatment for four and five lesions, respectively. 25 patients (17%) had received WBI prior to SRS. Single-fraction SRS was the most common treatment across all the receptor subtypes (65% of total number of patients) except for the ER+/HER2+ group, where only 41% received single-fraction SRS, and 59% received fractionated SRS. An overview of radiotherapy characteristics of the cohort can be seen in .
Table 2. Radiotherapy characteristics at time of SRS.
Overall survival following stereotactic radiosurgery
Median OS for the entire cohort was 14.8 months (95% CI: 11.40–19.00) and there were 129 events (deaths) in the cohort. ER+/HER2+ had the longest median OS (33.31 months, 95% CI: 21.91–52.21), and ER−/HER2− had the shortest (5.29 months, 95% CI: 2.20–14.75), with ER+/HER2− and ER−/HER2+ having a median OS of 11.04 (95% CI: 7.39–18.04) and 17.74 (95% CI: 11.89–27.83) months, respectively (). 75% of the cohort was still alive at six months, and 25% was alive at 32 months.
Figure 1. Kaplan–Meier Curves for OS by (a) receptor profiles, (b) HER2 status, (c) lines of treatment dichotomized at the median (four lines), and (d) presence of extracranial visceral metastases yes/no.
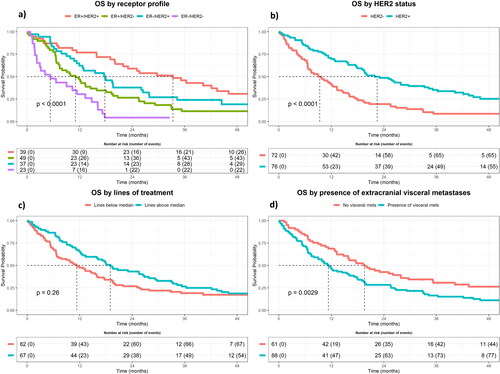
In univariate analysis (UVA), the ER−/HER2− receptor profile (HR: 2.19, 95% CI: 1.30–3.71, p = .003), age above the median (HR: 1.92, 95% CI: 1.34–2.73, p = .0003), and presence of extracranial visceral metastases (HR: 1.72, 95% CI: 1.20–2.47, p = .003) were all shown to be significant prognostic factors of shorter OS. Isolated HER2+ status was associated with superior OS (HR: 0.50, 95% CI: 0.35–0.71, p = .0001), as were the receptor profile ER+/HER2+ (HR: 0.36, 95% CI: 0.17–0.78, p = .009) and lines of treatment above four (HR: 0.55, 95% CI: 0.33–0.91, p = .02) after the first 12 months after SRS.
In multivariate analysis (MVA), the ER−/HER2− receptor profile (HR: 2.00, 95% CI: 1.09–3.67, p = .02) and presence of extracranial visceral metastases (HR: 2.90, 95% CI: 1.53–5.50, p = .001) remained significantly associated with poor survival. Age above 59 years was not significant in MVA. After the first 12 months after SRS, both the ER+/HER2+ receptor profile (HR: 0.43, 95% CI: 0.19–0.96, p = .04) and lines of treatment above four (HR: 0.40, 95% CI: 0.20–0.82, p = .01) remained associated with improved OS. An overview of our Cox regression analysis can be seen in .
Table 3. Univariate and multivariate Cox regression analysis for OS.
CNS-PFS
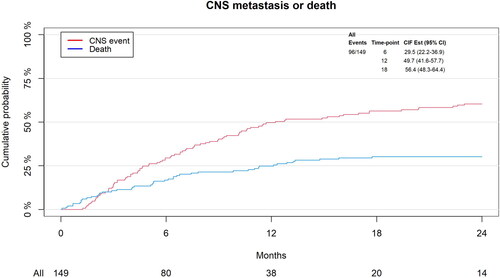
At 6 months, 29.5% of patients had developed CNS progression, and 16.8% had died without confirmed CNS progression; at 12 months, these numbers rose to 49.7% and 24.8%, respectively (). At the time of final follow-up, 96 patients had developed CNS progression, 46 had died without confirmed CNS progression, and 7 patients had experienced neither event.
In UVA, the only proportional prognostic factor significantly associated with decreased risk of CNS progression was the presence of extracranial visceral metastases (sub-distributional hazard ratio (SHR): 0.71, p = .95, ). Time from BC diagnosis to development of brain metastases was also associated with decreased risk of CNS progression (SHR: 0.64, p = .06), but only when looking at ≥12 months after SRS. When looking at the first 12 months following SRS, the receptor profile ER+/HER2+ (SHR: 2.44, p = .06), isolated positive HER2 status (SHR: 2.43, p = .05), and lines of treatment above four (SHR: 4.54, p = .002) were all significantly associated with increased risk of CNS progression, while age above median (59 years) was associated with decreased risk of CNS progression (SHR: 0.38, p = .03).
Figure 3. Cumulative incidence for CNS-PFS illustrated by presence of extracranial visceral metastases Yes/no.
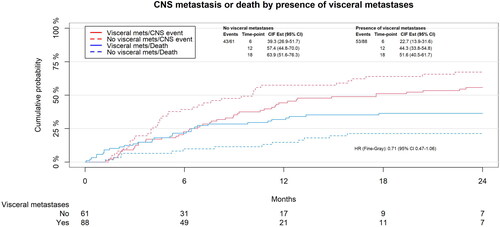
In MVA, presence of extracranial visceral metastases was significantly associated with decreased risk of CNS progression (SHR: 0.54, p = .04). Lines of treatment above four (SHR: 2.88, p = .04) was the only prognostic factor found to be significantly associated with worse CNS-PFS in the first 12 months following SRS. An overview of our Fine-Gray analysis can be seen in .
Table 4. Univariate and multivariate Fine-Gray regression analysis for CNS-PF.
Discussion
Although SRS has been shown to have improved outcomes when compared to WBI, our findings confirm that the prognosis following SRS treatment of BCBM still remains poor [Citation16,Citation17]. In our cohort, we found a median overall survival of 14.8 months (95% CI: 11.37–18.99). While receptor profiles were found to be highly significant in relation to OS, there was no statistically significant connection between receptor profiles and CNS-PFS in our multivariate model.
No statistically significant connection was found between tumor volume and OS, nor CNS-PFS. Due to the retrospective nature of our study, our cohort was already preselected as candidates for SRS, meaning they had smaller total tumor volumes and 1–4 BM at the time of SRS (with two outliers having 5 and 6, respectively). This most likely explains why we could not detect tumor volume as being significant around our median of 3.37 cm3, with other studies reporting an increased risk in patients at >10 cm3 total tumor volume [Citation18]. It is also possible that increased risk could be detected when dividing the cohort into quartiles rather than at the median, with risk having been shown to increase at a tumor volume of >2.6 cm3 [Citation19].
Overall survival following stereotactic radiosurgery
When looking at OS, the receptor profile ER−/HER2− (triple-negative breast cancer, TNBC) was the most significant predictor of shorter OS, consistent with findings in other retrospective studies [Citation18,Citation20]. The decreased OS seen in the ER−/HER2− group is most likely due to the lack of effective systemic treatments available for TNBC in combination with the more aggressive nature of this subtype of breast cancer [Citation21,Citation22]. There are several treatment agents available for ER + and HER2+ cancers where studies have shown improved control of brain metastases and OS following BCBM, but the lack of targets in TNBC means that systemic treatment options remain limited [Citation23–26]. To improve the prognosis for TNBC patients, some studies suggest that WBI in addition to SRS improves intracranial tumor control, but more data is needed to validate the possible benefit for TNBC patients [Citation27–29].
The HER2+ receptor profiles had the longest OS and the ER+/HER2+ receptor profile was associated with improved OS. Like TNBC, HER2+ cancers are known to have a higher risk of developing brain metastases [Citation30]. In our study, we also found that next to the TNBC subgroup, the ER−/HER2+ subgroup had the shortest time from BC diagnosis to development of BCBM (34 months for ER−/HER2+ and 30 months for TNBC) [Citation30]. However, compared to TNBC, systemic treatment options for HER2+ cancers have greatly improved, leading to improved longterm survival in spite of the increased risk of BCBM [Citation31].
Patients who receive WBI alone or in combination with SRS have been found to experience long-term neurocognitive impairment more frequently than patients who received only SRS [Citation32–34]. SRS is, therefore, a preferable choice of treatment in patients with a longer OS. our institution, SRS is only offered routinely to patients with 1–4 brain metastases. We found that the ER+/HER2+ and ER−/HER2+ patients had a long median OS following SRS (33.31 months, 95% CI: 21.91–52.21 and 17.74 months, 95% CI: 11.89–27.83 respectively). These findings suggest that there may be a benefit of SRS for patients with HER2+ receptor profiles with more than four brain metastases in order to provide these patients an improved quality of life following radiotherapy.
Conversely, patients with HER2− cancers had a shorter OS following SRS (11.0 months for ER+/HER2− (95% CI: 7.39–18.04) and 5.29 months for ER−/HER2− (95% CI: 2.20–14.75)), and the impact of long-term adverse effects from WBI may be less of a concern in this group of patients. Studies suggest that combining SRS with WBI decreases the risk of new CNS metastases but SRS + WBI has not been associated with improved OS [Citation28,Citation35]. As the addition of WBI only increases intracranial control and not OS, other factors, such as SRS being delivered in fewer fractions than WBI, still make SRS a strong option, even in patients with a poor prognosis.
The presence of extracranial visceral metastases (liver and/or lung metastases) at the time of SRS was found to be strongly associated with poor OS. In this study, we only looked at the presence of extracranial visceral metastases yes vs. no and not as active vs. stable extracranial disease. In a retrospective study from 2014, poor OS was only associated with extracranial disease when looking at active vs. stable disease, and no association was observed between the presence of extracranial metastases and decreased OS [Citation20]. This connection between progressive extracranial disease and decreased OS has also been detected in other retrospective studies [Citation18,Citation36]. This may suggest that the strongest predictor for inferior OS is not the presence of systemic disease in itself but rather how controlled it is.
We found that after the first year after SRS, patients who had started SRS on their 5th or above line of treatment had improved OS. This can possibly be explained by the fact that patients who did not receive SRS until their 5th or later line developed brain metastases late in their disease, compared to patients who had SRS on their 1st–4th line of treatment. This could indicate that these patients have less aggressive disease, meaning that if they do survive the first year following SRS, they have better OS compared to patients who developed brain metastases on earlier lines of treatment.
CNS-PFS
When looking at the role of receptor profiles in relation to CNS-PFS, the ER+/HER2+ receptor profile and HER2+ status alone were found in UVA to increase the risk of CNS progression in the first 12 months after SRS, which can potentially be explained by HER2+ cancers being known to have a higher risk of developing brain metastases [Citation30]. In our overall survival analysis, the ER+/HER2+ receptor profile was associated with increased overall survival, largely due to the availability of systemic treatment options. This seems paradoxical in relation to findings showing an increased risk of CNS progression, However, in a competing risk setting the two possible events (death and CNS progression) are compared to each other. For the ER+/HER2+ patients with long OS, this means they have a higher risk of developing CNS progression than of dying, consistent with our findings of increased overall survival in this group. It is important to note that the receptor profile ER+/HER2+ was not found to be significant in MVA which means that based on this analysis, we can only draw limited conclusions on the significance of the ER+/HER2+ receptor profile. Overall, we could not detect a significant association between any of the receptor profiles and CNS-PFS.
The ER−/HER2− receptor profile has previously been found to be associated with an increased risk of CNS progression [Citation37–39]. The cohorts in these studies were of a similar size to ours, but none of them treated their CNS-PFS data in a competing risk model. Our data suggest that not treating the data in a competing risk model may overestimate the influence of the ER−/HER2− receptor profile as a risk factor for poorer CNS-PFS. As such, it remains difficult for us to compare our results to previous studies, and more data is needed to establish the role of receptor profiles as prognostic factors for CNS-PFS in a competing risk setting.
In UVA, both age and the presence of extracranial visceral metastases were found to decrease the risk of CNS progression (with the presence of extracranial visceral metastases also found to be significant in MVA). However, these results are not indicative of advanced age and systemic disease lowering the risk of CNS progression but rather that these factors increase the risk of dying before developing CNS progression.
To the best of our knowledge, only one retrospective study has been able to detect extracranial disease as a risk factor for CNS progression [Citation20]. They detected an increased risk when looking at progressive extracranial disease, which we did not examine. However, five or more lines of treatment at the time of SRS was found to be significant in both UVA and MVA as a risk factor for CNS progression. A number of treatment lines can potentially be viewed as an approximation of progressive disease, as patients on their 5th line of treatment have already progressed at least four times during their treatment and can thus be viewed as patients with a high burden of disease and a higher likelihood of progressive disease. Our results therefore also suggest a potential link between progressive extracranial disease and increased risk of CNS progression, but more research is needed to test this assumption.
Strengths and limitations
The primary strength of our study lies in its unselected cohort, which covers a large geographical area and includes all breast cancer patients in the relevant time frame who received SRS. Limitations include the retrospective nature of the study as well as the inherent preselection of patients in the cohort due to them already having been evaluated as candidates for SRS. Variables for model selection were chosen based on clinical significance and based on results of previous studies with the use of forward selection in the model reduction, leaving us with a risk of bias which may limit the possibilites for external validation.
Conclusions
In summary, we found a median overall survival following SRS of 14.8 months, and the presence of extracranial visceral metastases was found to be the strongest prognostic factor both in terms of OS and CNS-PFS. Advanced systemic disease, age >59 years, and the ER−/HER2− receptor profile were the strongest predictors of poor survival following SRS. In order to truly evaluate the benefit of SRS for these patients, we need more research focused on the quality of life of the patients by looking at toxicity levels and side effects as well as patient-reported outcomes, which were not included in this study. We also found that patients with ER+/HER2+ had a median overall survival of 33.3 months and these patients may be good candidates for SRS even in cases with more than four brain metastases, as they have a lot to gain in terms of living without neurological impairment or side effects from more traditional radiotherapy techniques.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are not publicly available due to institutional restrictions. The data can be made available to qualified researchers through application to the Danish Breast Cancer Group.
Additional information
Funding
References
- Sundquist M, Brudin L, Tejler G. Improved survival in metastatic breast cancer 1985–2016. Breast. 2017;31:46–50. doi: 10.1016/j.breast.2016.10.005.
- Rostami R, Mittal S, Rostami P, et al. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol. 2016;127(3):407–414. doi: 10.1007/s11060-016-2075-3.
- Sacks P, Rahman M. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020;31(4):481–488. doi: 10.1016/j.nec.2020.06.001.
- Tabouret E, Chinot O, Metellus P, et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32:4655–4662.
- Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and Central nervous system metastases. Breast. 2014;23(5):623–628. doi: 10.1016/j.breast.2014.06.009.
- Darlix A, Louvel G, Fraisse J, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000. doi: 10.1038/s41416-019-0619-y.
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149.
- Sim H-W, Morris PG, Patil S, et al. Brain metastases in breast cancer. Expert Rev Anticancer Ther. 2014;14(2):173–183. doi: 10.1586/14737140.2014.863468.
- Komorowski AS, Warner E, MacKay HJ, et al. Incidence of brain metastases in nonmetastatic and metastatic breast cancer: is there a role for screening? Clin Breast Cancer. 2020;20(1):e54–e64. doi: 10.1016/j.clbc.2019.06.007.
- Kuksis M, Gao Y, Tran W, et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021;23(6):894–904. doi: 10.1093/neuonc/noaa285.
- Lukas RV, Gabikian P, Garza M, et al. Treatment of brain metastases. Oncology. 2014;87(6):321–329. doi: 10.1159/000362389.
- Bailleux C, Eberst L, Bachelot T. Treatment strategies for breast cancer brain metastases. Br J Cancer. 2021;124(1):142–155. doi: 10.1038/s41416-020-01175-y.
- Specht HM, Combs SE. Stereotactic radiosurgery of brain metastases. J Neurosurg Sci. 2016;60:357–366.
- van Grinsven EE, Nagtegaal SHJ, Verhoeff JJC, et al. The impact of stereotactic or whole brain radiotherapy on neurocognitive functioning in adult patients with brain metastases: a systematic review and meta-analysis. Oncol Res Treat. 2021;44(11):622–636. doi: 10.1159/000518848.
- Lippitz B, Lindquist C, Paddick I, et al. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40(1):48–59. doi: 10.1016/j.ctrv.2013.05.002.
- Gullhaug A, Hjermstad MJ, Yri O, et al. Use of radiotherapy in breast cancer patients with brain metastases: a retrospective 11-year single center study. J Med Imaging Radiat Sci. 2021;52(2):214–222. doi: 10.1016/j.jmir.2021.01.002.
- Halasz LM, Uno H, Punglia RS. Comparative effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for patients with brain metastases from breast or non-small cell lung cancer: WBRT vs SRS for brain metastases. Cancer. 2016;122(20):3244–3245. doi: 10.1002/cncr.30009.
- Wilson TG, Robinson T, MacFarlane C, et al. Treating brain metastases from breast cancer: outcomes after stereotactic radiosurgery. Clin Oncol. 2020;32(6):390–396. doi: 10.1016/j.clon.2020.02.007.
- Caballero JA, Sneed PK, Lamborn KR, et al. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):303–309. doi: 10.1016/j.ijrobp.2011.06.1987.
- Yang TJ, Oh JH, Folkert MR, et al. Outcomes and prognostic factors in women with 1 to 3 breast cancer brain metastases treated with definitive stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2014;90(3):518–525. doi: 10.1016/j.ijrobp.2014.06.063.
- Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161(2):279–287. doi: 10.1007/s10549-016-4059-6.
- Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–269. doi: 10.1007/s00404-015-3859-y.
- Figura NB, Potluri TK, Mohammadi H, et al. CDK 4/6 inhibitors and stereotactic radiation in the management of hormone receptor positive breast cancer brain metastases. J Neurooncol. 2019;144(3):583–589. doi: 10.1007/s11060-019-03260-6.
- Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2 − positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775.
- Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2 − positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. doi: 10.1158/1078-0432.CCR-08-1080.
- Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. doi: 10.1200/JCO.18.01511.
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8.
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483.
- Medikonda R, Srivastava S, Kim T, et al. Development of new brain metastases in triple negative breast cancer. J Neurooncol. 2021;152(2):333–338. doi: 10.1007/s11060-021-03702-0.
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820.
- Deluche E, Antoine A, Bachelot T, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016.
- Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68(5):1388–1395. doi: 10.1016/j.ijrobp.2007.03.048.
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. doi: 10.1016/S1470-2045(17)30441-2.
- Monaco EA, Faraji AH, Berkowitz O, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer: leukoencephalopathy after WBRT, not SRS. Cancer. 2013;119(1):226–232. doi: 10.1002/cncr.27504.
- Duan L, Zeng R, Yang K-H, et al. Whole brain radiotherapy combined with stereotactic radiotherapy versus stereotactic radiotherapy alone for brain metastases: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(2):911–915. doi: 10.7314/apjcp.2014.15.2.911.
- Kondziolka D, Kano H, Harrison GL, et al. Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer: clinical article. J Neurosurg. 2011;114(3):792–800. doi: 10.3171/2010.8.JNS10461.
- Mills MN, Thawani C, Figura NB, et al. Breast cancer subtype predicts clinical outcomes after stereotactic radiation for brain metastases. J Neurooncol. 2021;152(3):591–601. doi: 10.1007/s11060-021-03735-5.
- Vern-Gross TZ, Lawrence JA, Case LD, et al. Breast cancer subtype affects patterns of failure of brain metastases after treatment with stereotactic radiosurgery. J Neurooncol. 2012;110(3):381–388. doi: 10.1007/s11060-012-0976-3.
- Cho E, Rubinstein L, Stevenson P, et al. The use of stereotactic radiosurgery for brain metastases from breast cancer: Who benefits most? Breast Cancer Res Treat. 2015;149(3):743–749. doi: 10.1007/s10549-014-3242-x.