Abstract
Background
Adenocarcinoma (AC) and squamous cell carcinoma (SCC) are the most frequent histological subtypes of non-small cell lung cancer (NSCLC). The aim of this study was to investigate how patients with AC and SCC benefit from image-guided adaptive radiotherapy (ART) with tumour match.
Material and methods
Consecutive patients diagnosed with AC or SCC of the lung treated with definitive chemo-radiotherapy before and after the implementation of ART and tumour match were retrospectively included for analyses. Data collection included baseline patient and treatment characteristics in addition to clinical data on radiation pneumonitis (RP), failure, and survival. Patients were divided into four categories based on their histology and treatment before (n = 173 [89 AC and 84 SCC]) and after implementation of ART (n = 240 [141 AC and 99 SCC]).
Results
Median follow-up was 5.7 years for AC and 6.3 years for SCC. Mean lung dose decreased for both histologies with ART, whereas mean heart dose only decreased for patients with AC. Incidences of grade 3 and 5 RP decreased for both histologies with ART. Loco-regional failure (LRF) rates decreased significantly for patients with SCC after ART (p = .04), no significant difference was observed for AC. Overall survival (OS) increased significantly for SCC after ART (p < .01): the 2-year OS increased from 31.0% (95% confidence interval [CI] [22.5–42.6]) to 54.5% (95% CI [45.6–65.3]). No significant effect on OS was observed for patients with AC.
Conclusion
ART and tumour match in the radiotherapeutic treatment of patients with locally advanced NSCLC primarily led to decreased LRF and improved OS for patients with SCC.
Background
Lung cancer is the leading cause of cancer-related deaths worldwide [Citation1]. Pathologically, lung cancer is subdivided into small cell lung cancer and non-small cell lung cancer (NSCLC) [Citation2]. NSCLC accounts for approximately 85% of all lung cancer cases, with adenocarcinoma (AC) and squamous cell carcinoma (SCC) as the most frequent subtypes [Citation3,Citation4]. Regardless of subtype, the standard of care for all patients with non-resectable locally advanced NSCLC is combined chemo-radiotherapy (cCRT) [Citation5,Citation6]. However, AC and SCC differ both anatomically and aetiologically. While both subtypes are highly related to tobacco exposure, SCC is typically placed in the proximal part of the lungs, while AC is typically placed in the distal part of the lungs [Citation7] and more common in female and younger patients [Citation8]. Furthermore, the microscopic extension, and expression of different oncogenetic drivers, such as EGFR mutations and ALK rearrangements, vary between the two histologies [Citation9,Citation10]. It may therefore not be surprising that recent studies indicate a difference in patterns of failure after cCRT for locally advanced disease [Citation11,Citation12]: patients with AC seem to be more prone to distant failure while patients with SCC appear more prone to loco-regional failure (LRF). Thus, the effect of changes in a localized treatment, such as radiotherapy, might differ between SCC and AC.
During the last decades, radiotherapy techniques in the treatment of locally advanced NSCLC have evolved drastically. Modern-day state-of-the-art precision radiotherapy techniques include image-guided and intensity-modulated radiotherapy with adaptive strategies. In 2013, daily online tumour match in combination with an adaptive radiotherapy (ART) strategy was implemented at Aarhus University Hospital, simultaneously increasing treatment precision and decreasing treatment volumes as well as unnecessary doses to lungs and heart [Citation13]. We have previously shown improvement in both progression-free survival (PFS) and overall survival (OS) in a cohort of 439 patients with locally advanced NSCLC, including both SCC and AC [Citation14].
The aim of this current study is to investigate how patients with AC and SCC benefit from increased precision in local treatment, here exemplified by image-guided treatment with tumour match and an adaptive treatment strategy.
Material and methods
Patients
Consecutive patients diagnosed with locally advanced NSCLC treated with definitive cCRT at Aarhus University Hospital between 2010 and 2018 were retrospectively included in analyses (n = 439). Inclusion criteria were treatment with curative intended cCRT with homogenous doses ranging from 50 to 66 Gy in 25–33 fractions. Patients with stage I–II disease not eligible for surgery or stereotactic body radiation therapy (SBRT) were included as well as patients with stage IV oligometastatic disease who were treated prior to or simultaneously with cCRT with either surgery or SBRT. This cohort has been described extensively in a previous publication [Citation14], including details on patient and treatment characteristics and the retrospective collection of these. Patients who did not have a pathologically proven diagnosis of either SCC or AC were excluded from this study (n = 26), leaving 413 patients for final analysis: 173 patients treated before (pre-ART), and 240 patients treated after (ART) the implementation of the tumour match and ART strategy in April 2013.
Staging and treatment
All patients were staged based on a diagnostic fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET)-CT scan and endobronchial ultrasonography (EBUS). Patients had pathologically verified diagnoses by biopsy of tumour and/or potential malignant lymph nodes and all patients were staged and treated independently of their histopathology. Chemotherapy consisted of either cisplatin or carboplatin in combination with vinorelbine on days 1 and 8 in each cycle. Chemotherapy was given either sequentially or, for the majority of patients, concomitantly with radiotherapy. However, a minor proportion of patients did not receive chemotherapy (). If concomitant chemotherapy was administered, radiotherapy was usually initiated on the first day of the second cycle of chemotherapy.
Table 1. Patient and treatment characteristics.
Radiotherapy planning was performed using Eclipse (Varian Medical Systems). Before ART, clinical target volume (CTV) was created by an isotropic expansion of the gross tumour volume (GTV) by 5 mm, corrected for bones and large blood vessels. The internal target volume (ITV) was performed by an expansion of the CTV by 5 mm left–right (LR), 5 mm anterior–posterior (AP), and 10 mm craniocaudal (CC) to account for respiratory motion. The ITV was further expanded 5 mm LR, 5 mm AP, and 8 mm CC to create the planning target volume (PTV). In the ART group, respiration was accounted for by transferring the mid-ventilation-defined GTV to every phase of the respiratory cycle on the 4D-CT to generate an internal GTV (iGTV) as the sum of all phases. Subsequently, the CTV was created by an isotropic expansion of 5 mm, corrected for bones and large vessels. The PTV was created by an expansion of the CTV by 4 mm LR, 4 mm AP, and 5 mm CC for primary tumour, while margins of 7 mm LR, 7 mm AP, and 8 mm CC were applied for the lymph nodes. Same margins were applied for SCC and AC before and after ART.
Dose was delivered normofractionated with 2 Gy/fraction/5 weekly fractions. All patients had daily cone beam computed tomography (CBCT) scans performed prior to treatment. In the pre-ART group, patients were positioned by a match on the thoracic vertebrae while patients in the ART group were positioned based on a match on tumour volumes. As part of the adaptive strategy in the ART group, radiotherapists (RTTs) performed systematic daily evaluation of the CBCT for anatomical changes. Treatment was adapted by performing a new CT and treatment plan in case anatomical changes had clinically relevant impact on the dose distribution (loss of target coverage or increased dose to organs at risk). A schematic and detailed overview of how the adaptive treatment strategy was performed is published in previous work [Citation13,Citation14]. A stepwise learning programme qualified all RTTs to manage ART in the clinic [Citation15].
Retrospective data collection
Data collection was approved by the Danish Data Protection Agency and the Danish National Board of Health (3-3013-2756/1). In addition to the previously reported data collection [Citation14], data on the administration of immunotherapy at relapse were considered for the current report. The role of immunotherapy in the treatment of NSCLC has drastically evolved during the study period [Citation16,Citation17], and hence a retrospective review of pharmacological registries and electronic patient files was performed to collect additional relevant data. shows patient- and treatment characteristics. If GTV was not available, CTV was used (n = 8).
Index date was the first day of radiotherapy. Date of failure was defined as the date of the first scan where failure was detected and clinically confirmed. Failures were classified as either loco-regional, distant, or simultaneous loco-regional and distant failure. If patients did not experience failure, they were either classified as alive without failure or dead with no evidence of disease (DNED). Patients were censored for further failure analysis after first failure. Radiation pneumonitis (RP) during treatment and follow-up was scored using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 grading system. The maximum score of RP in the follow-up period was noted.
Analysis
The cohort was subdivided into four groups prior to analyses based on their treatment period (pre-ART or ART) and histology (AC or SCC). All data were analysed using R version 4.2.2 for statistical analyses. Results are reported with a 95% confidence interval (95% CI) and p-values of .05 or less were considered statistically significant. Patient- and treatment characteristics were compared between groups using Pearson’s chi-square test (two-sided) for categorical variables and Mann–Whitney U tests for continuous variables. OS, LRF, and fatal RP were analysed using Kaplan–Meier (KM) estimates (1 minus the KM estimates for LRF and fatal RP) and log-rank tests for differences between groups. Cox proportional hazards regression for OS was performed for each histology, including pre-specified factors assumed to impact outcome: performance status (PS), stage, age, volume of GTV, chemotherapy (concomitant vs. sequential or no chemotherapy), mean lung dose (MLD), mean heart dose (MHD), and ART vs. no-ART as factors. Assumption of proportional hazards was checked by visual inspection of the Schoenfeld residuals.
Cox proportional hazards models for LRF were performed for each histology, including stage, chemotherapy, volume of GTV, and ART vs. no-ART. In the LRF analysis, both LRF alone and simultaneous loco-regional and distant failure counted as an event of LRF.
Results
Median follow-up was 5.7 years for AC (95% CI [5.1–7.0]) and 6.3 years for SCC (95% CI [5.7–7.7]), estimated by reverse KM. Baseline patient and treatment characteristics are presented in . In total, 230 patients with AC and 183 patients with SCC were included. Baseline patient characteristics were equally distributed for each histology before and after the implementation of ART. Treatment evolved significantly during the period of the study. Thus, for both histology subtypes, the use of concomitant chemotherapy increased significantly after the introduction of ART. As a result of reduced PTV margins in the ART group, MLD was significantly reduced in the ART group for both histologies (with no significant difference in GTV volumes). For patients with AC, MHD was significantly reduced after implementing ART. Significantly more SCC patients in the ART group received radiotherapy with 66 Gy rather than 60 Gy compared to SCC in the pre-ART group. The proportion of patients receiving immunotherapy as relapse treatment in the ART group was higher for SCC than AC (14.1% vs. 9.2%), however, the difference was non-significant (Pearson’s chi-square p-value = .235).
Although the OS time increased for patients with AC after ART, the difference was non-significant (p = .31) (). There was, however, a significant increase of OS after ART for patients with SCC (p < .01): the 2-year OS increased from 31.0% (95% CI [22.5–42.6]) to 54.5% (95% CI [45.6–65.3]) (). In the Cox proportional hazards model, factors influencing OS differed between AC and SCC. For AC, only GTV was a significant factor influencing OS (). For SCC, ART, GTV, MLD, and PS all showed a significant impact on OS (). For both AC and SCC, the effect of MHD on OS was insignificant.
Figure 1. Overall survival (OS) for patients divided by histopathology before (pre-ART) and after (ART) the implementation of adaptive radiotherapy. No significant improvement of 2-year OS for adenocarcinomas (AC) from 53.9% (95% CI [44.5%:65.4%]) to 58.9% (95% CI [51.3%:67.6%]) was observed (A). Meanwhile, 2-year OS for squamous cell carcinomas (SCC) increased significantly from 31% (95% CI [22.5%:42.6%]) to 54.5% (95% CI [45.6%:65.3%]) (B).
![Figure 1. Overall survival (OS) for patients divided by histopathology before (pre-ART) and after (ART) the implementation of adaptive radiotherapy. No significant improvement of 2-year OS for adenocarcinomas (AC) from 53.9% (95% CI [44.5%:65.4%]) to 58.9% (95% CI [51.3%:67.6%]) was observed (A). Meanwhile, 2-year OS for squamous cell carcinomas (SCC) increased significantly from 31% (95% CI [22.5%:42.6%]) to 54.5% (95% CI [45.6%:65.3%]) (B).](/cms/asset/0e793934-eac5-4e18-a2b1-517fc5941144/ionc_a_2260944_f0001_c.jpg)
Table 2. Multivariate Cox proportional hazards model for overall survival for each histology.
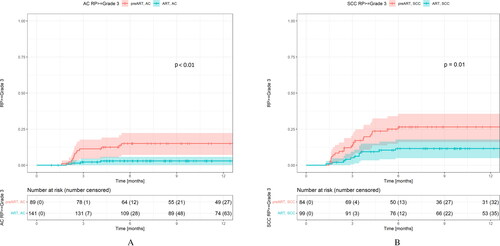
Regarding fatal RP, incidence of grade 5 pneumonitis was significantly reduced from the pre-ART group to the ART group for both AC (p = .01) and SCC (p = .02) (KM estimates, log-rank test). In crude numbers, four patients with AC (4.5%) and six patients with SCC (7.1%) experienced grade 5 pneumonitis pre-ART. After implementing ART, no patients with AC and one patient with SCC (1.0%) experienced grade 5 pneumonitis. Additionally, incidences of grade 3 pneumonitis were significantly reduced from the pre-ART group to the ART group for both AC (p < .01) and SCC (p = .01) (KM estimates, log-rank test) ().
Figure 2. Kaplan–Meier curves showing incidences of radiation pneumonitis (RP) grade 3 or higher. Incidences declined significantly after implementing an adaptive strategy (ART) for both patients with adenocarcinoma (AC) (A) and squamous cell carcinoma (SCC) (B).
The log-rank estimated differences in LRF pre-ART and after ART were non-significant for AC (p = .62) but improved significantly for patients with SCC after ART (p = .044): 2-year freedom from LRF increased from 32.8% (95% CI: [22.6–47.7]) pre-ART to 42.3% (95% CI: [33.1–54.2]) after ART for SCC. The KM estimates for LRF before and after ART stratified by histology are presented in . In the univariate Cox regression model of LRF for SCC, ART showed a significant protective effect (HR: 0.668, 95% CI [0.45–0.992], p = .045). The effect size was maintained in the multivariable Cox regression model, although with only borderline significance (HR: 0.679, 95% CI [0.445–1.036], p = .073). GTV showed a significant correlation for LRF for both patients with AC and SCC in the multivariate Cox regression model (see ).
Figure 3. Loco-regional failure (LRF) for patients divided by histopathology (AC: adenocarcinoma; SCC: squamous cell carcinoma) before (pre-ART) and after (ART) the implementation of ART. Two-year freedom from LRF was 62.4% (95% CI: [51.0–76.3] for AC pre-ART and 52.9% (95% CI [43.6–64.2]) for AC after ART (A). For SCC, 2-year freedom for LRF was 32.8% (95% CI [22.6–47.7]) pre-ART and 42.3% (95% CI [33.1–54.2]) (B).
![Figure 3. Loco-regional failure (LRF) for patients divided by histopathology (AC: adenocarcinoma; SCC: squamous cell carcinoma) before (pre-ART) and after (ART) the implementation of ART. Two-year freedom from LRF was 62.4% (95% CI: [51.0–76.3] for AC pre-ART and 52.9% (95% CI [43.6–64.2]) for AC after ART (A). For SCC, 2-year freedom for LRF was 32.8% (95% CI [22.6–47.7]) pre-ART and 42.3% (95% CI [33.1–54.2]) (B).](/cms/asset/0455badf-1e31-4b71-b7ff-c177d58fd008/ionc_a_2260944_f0003_c.jpg)
Table 3. Multivariate Cox proportional hazards model for loco-regional failure for each histology.
Discussion
As shown in previous work [Citation14], PFS and OS improved significantly with the introduction of ART and tumour match in the radiotherapeutic treatment of patients with locally advanced NSCLC. In this study, we show that patients with SCC mainly drive the effect, while OS for patients with AC remains unchanged. This difference between SCC and AC is observed both for survival and LRF, and it seems therefore probable that the increased OS for SCC after ART is a direct consequence of improved loco-regional control for this patient group.
There are many possible explanations for this. The ART and tumour match strategy has been shown to improve treatment precision and ensure target coverage [Citation13]. The differences in pattern of failure between SCC and AC, described for patients with NSCLC treated with both cCRT [Citation11,Citation12], surgery, or SBRT [Citation18–20], were observed in our data as well (). Since patients with SCC are more likely to experience loco-regional recurrence, and we observe a large reduction in LRF as well as increase in OS in the same patient group, it is possible that patients with SCC simply benefit more from the increased local treatment precision provided by ART than patients with AC.
Apart from increased treatment precision, other obvious benefits resulting from introducing ART that might influence OS, such as reduced dose to normal tissue and a resulting reduction in toxicity, should also be considered. Although we observe a significant reduction in MLD and RP with ART and tumour match [Citation13,Citation14], the slight difference in lethal RP before and after ART cannot explain the advantage of ART for patients with SCC in our study. MHD was not significantly reduced for patients with SCC after ART, likely because tumours with SCC histology tend to be placed in the central part of the lungs and hence closer to the mediastinum and heart. Since the SCC group showed no reduction of MHD but an increase in OS, while the AC group presented a significant reduction of MHD and no significant increase in OS, the results suggest that the correlation between high cardiac dose and high mortality risk found in some studies [Citation21,Citation22] might not be straightforward – or that the cardiac doses in this study for all groups were too low to have an effect on OS ().
A significantly higher proportion of SCC patients in the ART group received 66 Gy rather than 60 Gy. Nevertheless, it seems unlikely that the total extra dose of 6 Gy to a higher proportion of patients could solely explain the significantly improved OS for patients with SCC after the introduction of ART. This is supported by the Danish randomized phase II NARLAL trial, where Hansen et al. showed that final doses of either 60 or 66 Gy resulted in similar rates of local control and OS [Citation23]. However, baseline clinical information on tumour location and proportion of patients with T4 and N3 disease could have further strengthened this study.
Treatment with immunotherapy for recurrences was administered significantly more in the ART group for both histologies. This treatment difference was inevitable, as treatment with immunotherapy was introduced late in the observation period of this study (2015) and thus, more patients in the ART group were alive to receive immunotherapy when they experienced recurrent disease. We chose not to include immunotherapy in the Cox proportional hazards model for OS as the assumption of proportional hazards over time was not fulfilled. Including immunotherapy as a time-dependent variable was not straightforward, because the treatment was administered exclusively to patients who experienced recurrent disease and thus introduced a strong selection bias. It is likely that the administration of immunotherapy as palliative treatment has an impact on the OS since it has been shown to increase median OS 3–8.7 months depending on the PD-L1 expression of the tumour [Citation24,Citation25]. However, since only a few patients in total received immunotherapy in this study (), it cannot explain the improvement of OS entirely. This is supported by the fact that relapse treatment with immunotherapy was administered to a comparable higher proportion of patients in the ART group for both SCC and AC, without resulting in a significantly improved OS for AC ().
As the adaptive strategy and tumour match result in increased treatment precision with clear benefits by decreasing symptomatic and lethal RP as well as other factors previously demonstrated to be associated with OS like MLD [Citation26] and MHD [Citation22,Citation27], we do not recommend to omit ART and tumour match for patients with AC. Instead, results from this study should merely be interpreted as additional evidence that patients with NSCLC exhibit great variability in response to treatment. Sub-distributed histology may provide important informative data that could be missed with the general term NSCLC. Our results clearly demonstrate different responses to increased precision of radiotherapy, depending on histology. Although more studies are needed to support these findings, it is arguable whether the general term of NSCLC remains sufficient for this highly heterogeneous disease in the future.
In conclusion, this study shows that although an adaptive strategy and tumour match in the radiotherapeutic treatment of patients with locally advanced NSCLC reduces RP for both histologies, decreased LRF and improved OS were primarily observed for patients with SCC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Additional information
Funding
References
- WHO.int [Internet]. Geneva (Switzerland): WHO; 2023 [cited 2023 Feb 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
- Travis WD, Brambilla E, Nicholson AG, WHO Panel, et al. The 2015 World Health Organization classification of lung tumors. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630.
- Molina JR, Yang P, Adjei AA, et al. Non–small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584.
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183.
- Duma N, Santana-Davila R, Molina JR. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013.
- Evison M, on behalf of AstraZeneca UK Limited. The current treatment landscape in the UK for stage III NSCLC. Br J Cancer. 2020;123(Suppl 1):3–9. doi: 10.1038/s41416-020-01069-z.
- Marcussen N, Sørensen FB, Steiniche T, et al. Patologi. 1st ed. Roskilde (Denmark). FADLs Forlag; 2010.
- Wang B-Y, Huang J-Y, Cheng Y-F, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. 2020;146(1):43–52. doi: 10.1007/s00432-019-03079-8.
- Relli V, Trerotola M, Alberti S, et al. Abandoning the notion of Non-Small cell lung cancer. Trends Mol Med. 2019;25(7):585–594. doi: 10.1016/j.molmed.2019.04.012.
- Giraud P, Antoine M, Touboul E, et al. Evaluation of microscopic tumor extension in non–small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48(4):1015–1024. doi: 10.1016/S0360-3016(00)00750-1.
- Nygård L, Vogelius IR, Bentzen SM, et al. A competing risk model of first failure site after definitive chemoradiation therapy for locally advanced Non-Small cell lung cancer. J Thorac Oncol. 2018;13(4):559–567. doi: 10.1016/J.JTHO.2017.12.011.
- Katagiri Y, Jingu K, Kadoya N, et al. Differences in patterns of recurrence of squamous cell carcinoma and adenocarcinoma after radiotherapy for stage III non-small cell lung cancer. Jpn J Radiol. 2021;39(6):611–617. doi: 10.1007/s11604-021-01091-y.
- Møller DS, Holt MI, Hoffmann L, et al. Adaptive radiotherapy for advanced lung cancer ensures target coverage and decreases lung dose. Radiother Oncol. 2016;121(1):32–38. doi: 10.1016/j.radonc.2016.08.019.
- Møller DS, Lutz CM, Hoffmann L, et al. Survival benefits for non-small cell lung cancer patients treated with adaptive radiotherapy. Radiother Oncol. 2022;168:234–240. doi: 10.1016/j.radonc.2022.01.039.
- Boejen A, Vestergaard A, Grau C, et al. A learning programme qualifying radiation therapists to manage daily online adaptive radiotherapy. Acta Oncol. 2015;54(9):1697–1701. doi: 10.3109/0284186X.2015.1062914.
- Brahmer J, Reckamp KL, Spigel DR, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627.
- Spigel DR, Faivre-Finn C, Antonia SJ, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi: 10.1200/JCO.21.01308.
- Kelsey CR, Marks LB, Boyd JA, et al. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer. 2009;115(22):5218–5227. doi: 10.1002/cncr.24625.
- Woody NM, Stephans KL, Abazeed ME, et al. A histologic basis for the efficacy of SBRT to the lung. J Thorac Oncol. 2017;12(3):510–519. doi: 10.1016/j.jtho.2016.11.002.
- Hörner-Rieber J, Bernhardt D, Rieken S, et al. Histology of non-small cell lung cancer predicts the response to stereotactic body radiotherapy. Radiother Oncol. 2017;125(2):317–324. doi: 10.1016/j.radonc.2017.08.029.
- Defraene G, Dankers FJWM, Ruysscher DD, et al. Multifactorial risk factors for mortality after chemotherapy and radiotherapy for non-small cell lung cancer. Radiother Oncol. 2020;152:117–125. doi: 10.1016/j.radonc.2019.09.005.
- Pan L, Lei D, Wang D, et al. Heart dose linked with cardiac events and overall survival in lung cancer radiotherapy: a meta-analysis. Medicine. 2020;99(38):e21964. doi: 10.1097/MD.0000000000021964.
- Hansen O, Knap MM, Schytte T, et al. A randomized phase II trial of concurrent chemoradiation with two doses of radiotherapy, 60 Gy and 66 Gy, concomitant with a fixed dose of oral vinorelbine in locally advanced NSCLC. Radiother Oncol. 2017;123(2):276–281. doi: 10.1016/j.radonc.2017.03.017.
- Herbst RS, Garon EB, Baas P, et al. Five year survival update From KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1–positive advanced NSCLC. J Thorac Oncol. 2021;16(10):1718–1732. doi: 10.1016/j.jtho.2021.05.001.
- Borghaei H, Gettinger S, Brahmer J, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non–small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733. doi: 10.1200/JCO.20.01605.
- Dupic G, Biau J, Bellière-Calandry A, et al. Significant correlation between overall survival and mean lung dose in lung stereotactic body radiation therapy (SBRT). Front Oncol. 2020;10:1577. doi: 10.3389/fonc.2020.01577.
- Bradley JD, Paulus R, Choy H, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0.

