Abstract
Objective
Rheumatoid cachexia (RC) is prevalent among patients with established rheumatoid arthritis (RA). Although changes in muscle mass and fat mass have been reported in early RA, these findings have not been classified according to existing RC definitions. This study aimed to describe the prevalence of RC and associated variables in patients with early RA.
Method
This cross-sectional study included 87 patients. Body composition was evaluated with dual-energy X-ray absorptiometry after a median disease duration of 15 months. RC was defined as a fat-free mass index < 10th percentile and fat mass index > 25th percentile. We also assessed the erythrocyte sedimentation rate (ESR), C-reactive protein, Disease Activity Score in 28 joints, aerobic capacity, physical activity, traditional cardiovascular disease risk factors, functional disability, and sociodemographic data. Associations between RC and the independent variables were determined with logistic regression analyses.
Results
The prevalence of RC was 24%. RC was significantly associated [odds ratio (95% confidence interval)] with aerobic capacity [0.28 (0.09–0.89), p = 0.030], low-intensity physical activity [0.77 (0.60–0.99), p = 0.048], body mass index [0.78 (0.70–0.92), p = 0.002], waist circumference [0.96 (0.92–0.99), p = 0.023], body weight [0.94 (0.90–0.98), p = 0.004], and ESR at the time of diagnosis [1.02 (1.00–1.05), p = 0.033]. All of these associations remained significant after adjusting for age and gender.
Conclusion
RC was highly prevalent in early RA. Patient outcome may be improved by detecting this condition early and applying treatments for improving inflammation, aerobic capacity, physical activity, and body composition.
Rheumatoid arthritis (RA) has negative consequences on functional ability, quality of life, and work disability (Citation1). RA also confers a higher risk of several comorbidities, such as cardiovascular disease (CVD) and infections (Citation2). Patients can be affected by rheumatoid cachexia (RC), which is characterized by reduced muscle mass and stable or increased fat mass (Citation3, Citation4). Cachexia comes from the Greek word for ‘bad condition’ (Citation5). Originally, the tumour necrosis factor-α molecule, which has an important role in the inflammatory process in RA, was named cachectin, indicating its relation to the development of cachexia (Citation6).
This condition has been described by several authors. Engvall et al (Citation7) defined RC as a fat-free mass index (FFMI; kg/height2) below the 10th percentile and a fat mass index (FMI; kg/height2) above the 25th percentile. Elkan et al (Citation8) defined RC as an FFMI below the 25th percentile and an FMI above the 50th percentile. The mechanism underlying RC is not fully understood, but it is probably multifactorial. However, systemic inflammation, long-term medication with corticosteroids, physical inactivity, and poor diet may comprise an underlying network.
Studies on the prevalence of RC among patients with established RA have reported highly varied results; a 2018 meta-analysis showed that the prevalence of RC varied between 1% and 54% (Citation9). This variation may be due to the different criteria used to diagnose RC, the different assessment methods, and the heterogeneity of the studied populations. In early RA, two studies reported that patients with less than 1 year of disease duration had lower lean mass and higher fat mass than controls (Citation4, Citation10). Those results suggested that preclinical and/or early changes in body composition occurred during the course of the disease. To the best of our knowledge, RC in early RA has not been described previously, according to existing criteria.
The clinical importance of determining the prevalence of RC is that this information may assist in detecting patients with a poor prognosis, i.e. patients with an increased risk of metabolic diseases (Citation11), CVD (Citation12), infections (Citation2), reduced physical function (Citation13), and, potentially, an increased risk of premature death (Citation12, Citation14). Factors associated with RC may form the basis for hypotheses about treatments that aim to improve patient outcome.
The present cross-sectional study aimed to describe the prevalence of RC, and associated variables, in a cohort of patients with early RA.
Method
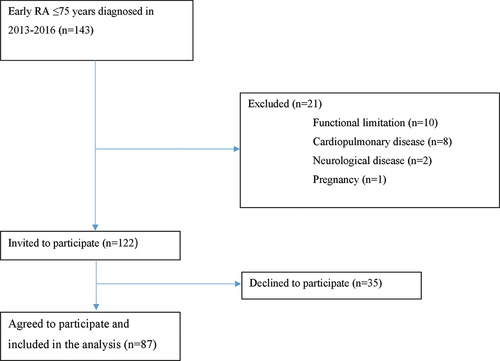
This study was a part of a continuing structured programme on early RA conducted at the Department of Rheumatology, University Hospital of Umeå, Umeå, Sweden. Eligible patients (n = 143) had RA symptoms for no longer than 12 months before RA diagnosis in 2013–2016 (). Inclusion criteria were: age 18 to ≤ 75 years and an RA diagnosis, based on criteria established by the 2010 American College of Rheumatology/European League against Rheumatism (ACR/EULAR) (Citation15). Exclusion criteria were: severe functional limitations, severe cardiopulmonary disease, and/or severe neurological disease.
The following were assessed in the 87 included patients at inclusion in the study, i.e. after a median disease duration of 15 months (range 11–30 months): total fat, lean mass, and fat mass distribution were examined with dual-energy X-ray absorptiometry (DXA; Lunar Prodigy X-ray Tube Housing Assembly, Brand BX-11, Model 8743; GE Medical Systems, Madison, WI, USA) (Citation16). The percentage of fat-free mass was calculated by dividing the fat-free mass (g) by the total mass (g). The FMI was defined as the fat mass (kg)/[height (m)]2; the FFMI was defined as the fat-free mass (kg)/[height (m)]2. RC was defined as an FFMI below the 10th percentile and an FMI above the 25th percentile, based on data from a healthy reference population (Citation17), as proposed by Engvall et al (Citation7). For comparison, we also categorized patients according to the Elkan et al (Citation8) definition for RC, which was an FFMI below the 25th percentile and an FMI above the 50th percentile.
Disease activity was calculated with the Disease Activity Score in 28 joints (DAS28) (Citation18), which included the physician’s examination of tenderness and swelling, the erythrocyte sedimentation rate (ESR), and the patient’s global health, rated on a visual analogue scale. Analyses of C-reactive protein (CRP), ESR, total cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were performed with routine clinical laboratory methods. Functional ability was assessed with the Stanford Health Assessment Questionnaire (HAQ) (Citation19). Ten-year cardiovascular mortality risk was assessed with the Systematic Coronary Risk Evaluation (SCORE) (Citation20). Waist circumference (WC) was measured with a measuring tape. The subjects stood erect, and the waist was measured to the nearest 0.5 cm, midway between the iliac crest and the lower rib margin (Citation21).
Aerobic capacity was assessed by the Åstrand submaximal cycle test (Citation22, Citation23). The calculation was adjusted with the HUNT formula, which has been shown to predict the maximal heart rate better than the original Åstrand predictor (Citation24). Physical activity data were collected with the Actiheart monitor (CamNtech, Cambridge, UK), which is a combined heart rate monitor and movement sensor. Details on collecting aerobic capacity and physical activity data were described previously (Citation25, Citation26).
All patients were interviewed by medical staff to collect data on risk factors for CVD, previous CVD events, chronic diseases, current medication, educational level, and employment situation. All cardiovascular events were validated by reviewing the patients’ medical files. We also obtained ESR, CRP, and DAS28 from the time of diagnosis and after 6 months to assess longitudinal associations between inflammation and RC. Inflammatory load was calculated with the area under the curve (AUC) (Citation27) for ESR, CRP, and DAS28, based on values collected at the time of diagnosis, at 6 months, and the time of this study. Patients were also classified as responders or no-responders to treatment according to the EULAR criteria for treatment response (Citation28).
All included patients provided informed consent before the assessments. Patients received both verbal and written information about the study, in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committee at Umeå University (Dnr 2014/356-31). All patients were treated in accordance with standard clinical practices.
Statistics
Descriptive statistics are presented as the number and percentage, the mean value and standard deviation (sd), or the median value and interquartile range (Q1–Q3), depending on the variable and the data distribution. Differences between groups were evaluated with the Mann–Whitney U-test and chi-squared test, as appropriate. Univariate logistic regression analyses were performed to analyse associations between the dependent variable (RC) and the independent variables (age, gender, disease-related variables, aerobic capacity, physical activity, CVD risk factors, and drug prescriptions). The results are expressed as the odds ratio (OR) and 95% confidence interval (CI). In the next phase, each independent variable was analysed in a separate model adjusted for age and gender. Finally, the variables that showed significance in the univariate analysis were entered into multiple logistic regression modelling. The Hosmer and Lemeshow test was used to assess model estimations, and higher p-values indicated a better fit. Nagelkerke’s pseudo-R2 value is reported with each model. The level of significance was set at p < 0.05. The sample size calculation was based on aerobic capacity data, originally performed for the first study on this study cohort (Citation25). We used R2 = 0.2 for the calculations. A test to assess the deviation of R2 from zero in a linear regression model was used to calculate the required sample size, i.e. the sample size needed to reject R2 = 0. The calculations suggested that a sample size of at least 34 was needed to achieve a power of 80% with a significance level of 0.05. The calculation was determined using G*Power (version 3.1.9.2; University of Dusseldorf, Germany). All other statistical calculations were performed with the SPSS (version 26; IBM, Armonk, NY, USA).
Results
The prevalence of RC in this study was 24% (n = 21), according to the Engvall et al definition, and 32% (n = 28), according to the Elkan et al definition (). Patients with RC had significantly lower aerobic capacity, FFMI, BMI, WC, and body weight, and were also responders to treatment to a higher degree compared to those without RC. There were no significant differences between the groups in terms of age, gender, disease activity, physical activity level, serology, FMI, prescription of drugs, blood pressure, 10 year CVD mortality risk, functional ability, smoking habits, current working situation, or educational level. The characteristics of the total group of 87 patients are shown in .
Table 1. Characteristics of 87 patients with early rheumatoid arthritis (RA) and subgroups with and without rheumatoid cachexia (RC).†
Rheumatoid cachexia and associated variables
Univariate logistic regression analyses were performed to explore the associations between the dependent variable (RC) and independent variables of disease activity (CVD risk factors, body composition, physical activity, and aerobic capacity). Cross-sectional analyses showed that with every unit of decrease in aerobic capacity, physical activity, BMI, WC, or body weight, the probability of RC increased. Longitudinal analyses indicated that, with every unit of increase in ESR measured at the time of diagnosis, the probability of RC 15 months later increased. All remained significant after adjusting for age and gender (). Logistic regression modelling showed the contribution of each input variable to the probability of RC and the significance of the association ().
Table 2. Univariate logistic regression results for 87 patients with early rheumatoid arthritis, with rheumatoid cachexia (RC)† as the dependent variable.
Table 3. Logistic regression model results for patients with early rheumatoid arthritis, with rheumatoid cachexia.†
Discussion
Prevalence
In this study, nearly one-quarter of the patients with early RA had RC (24%). This percentage is slightly lower than the 32% reported in a previous meta-analysis (Citation9) on patients with established RA. All the studies included in that meta-analysis used the same assessment technique (DXA) and the same clinical definition of RC (Citation7) that were used in the present study. However, another study on patients with established RA found an RC prevalence of only 13%, based on the same definition (Citation29). The fact that RC was prevalent in these patients, and to a similar extent in patients with established RA (Citation7, Citation9), implied that the changes in body composition occurred preclinically, or very early in the disease. An increased awareness regarding this early consequence of RA may guide healthcare providers in choosing appropriate interventions to improve patient outcome.
Rheumatoid cachexia and associated variables
Chronic inflammation is considered one of the most important underlying causes of RC. In this study, RC after 15 months of disease duration was positively associated with the ESR measured at the time of diagnosis, even after adjusting for age and gender. Disease activity was previously reported to be associated with increased fat mass in early RA (Citation4, Citation10), and it was reported to be associated with low lean body mass in established RA (Citation7, Citation30). Those findings implied that measurements of ESR early in the disease may be important indicators of RC. Inflammatory load over time was, however, not associated with RC, probably because of the low disease activity achieved at the time of measurement of RC. Nor was response to treatment associated with RC, which implies that successful treatment per se does not reduce the probability of RC. Similar phenomena have been observed by others (Citation13), and may call for rehabilitative interventions.
One subcriterion for an RC diagnosis is a reduced FFMI. This criterion may be particularly important, because even a 5% loss of body cell mass, which comprises mostly muscle mass, has been associated with increases in the risk of infection and impaired cell metabolism (Citation31). The other criterion for an RC diagnosis is maintained or increased fat mass; thus, we expected RC to be positively associated with variables related to body mass. However, we found that the BMI, body weight, and WC were all inversely associated with RC, i.e. the probability of RC increased as these variables decreased. This finding may be explained by fact that our patients with RC had significantly lower FFMIs, but similar FMIs, compared to those without RC. Previous research suggests that FFMI can be improved with targeted strength exercise programmes (Citation32).
RC was inversely associated with aerobic capacity, when it was expressed in L O2/min, but not when it was adjusted for body weight. This result may reflect another aspect of the lower FFMI, BMI, WC, and body weight found in patients with RC compared to those without RC in this study. Earlier studies linked RC with low subjectively reported physical activity (Citation29) and poor physical function (Citation13, Citation33). In this study, low-intensity physical activity was inversely associated with RC. This study may be the first to measure physical activity objectively in patients with RC. In the clinical setting, assessments of aerobic capacity and physical activity may contribute towards identifying patients with increased risk of RC. Interventions that aim to improve aerobic capacity and physical activity may improve RC.
Systemic and local effects of corticosteroids may contribute to the loss of muscle mass (Citation34, Citation35). In this study, 26% of patients were prescribed corticosteroids; however, no association was found between RC and current corticosteroid prescriptions, or any other medication. A potential explanation for this finding could be the low disease activity, which required low corticosteroid doses. Our data were categorized as non-prescription or prescription; thus, we did not analyse specific doses.
A uniform definition of rheumatoid cachexia
To date, no uniform definition of RC has been established. In a meta-analysis conducted in 2018 (Citation9), six out of eight studies used the Engvall et al (Citation7) definition of RC. However, an additional definition was proposed by Elkan et al (Citation8). In the present study, the prevalence of RC based on the Engvall et al definition was lower than the prevalence based on the Elkan et al definition. Similar differences were reported in previous studies. One study found a more obvious difference between the Engvall et al and the Elkan et al definitions (Citation29), but others reported more subtle differences (Citation8). The definition from Engvall et al provided a lower prevalence, probably due to the subcriterion of FFMI below the 10th percentile. Until standard criteria have been established, we suggest using caution when comparing results from different studies.
Strengths and limitations
The main strength of this study was the well-defined population of patients with early RA. Other strengths are that all patients were recruited from the only unit for rheumatology in the region, which cares for all patients with early RA, and that a large proportion of patients who were invited agreed to participate. The study results may therefore be applicable to other populations with early RA in Sweden and northern Europe. This study also had some limitations. First, the cross-sectional design prevented us from drawing conclusions regarding causality between the variables. Secondly, the patients who received beta-blocking agents, which reduce the maximal heart rate, did not perform the aerobic capacity test. Consequently, the group of patients who may have had the lowest aerobic capacity did not participate in the analysis. Thirdly, we lack data on potential confounders, such as longitudinal dietary data and long-term exercise data, which may have influenced the results.
Conclusion
RC was highly prevalent in this cohort of patients with early RA. Thus, RC may be considered an early consequence of RA. Our findings suggested that RC was more likely to develop in patients with a high initial ESR, low levels of aerobic capacity and physical activity, and low BMI, body weight, and WC. Treatment methods to improve these factors and an increased awareness of this condition may have a positive effect on patient outcome.
References
Acknowledgements
We thank Jeremy Pomeroy, Clinical Research Center, Marshfield Clinic Research Institute, Marshfield Clinic Health System, Marshfield, WI, USA, for his expertise in the analysis of physical activity data.
This report was supported by grants from the Swedish Research Council, Knut and Alice Wallenberg Foundation, the Swedish Rheumatism Association, the Swedish Rheumatism Association in the Västerbotten County, King Gustaf V’s 80-year Fund, and Region Västerbotten.
Disclosure statement
No potential conflict of interest was reported by the authors.
- Scott DL, Smith C, Kingsley G. What are the consequences of early rheumatoid arthritis for the individual? Best Pract Res Clin Rheumatol 2005;19:117–36.
- Michaud KWF. Comorbidities in RA. Best Pract Res Clin Rheumatol 2007;21:885–906.
- Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 1994;93:2379–86.
- Turk SA, van Schaardenburg D, Boers M, De Boer S, Fokker C, Lems WF, et al. An unfavorable body composition is common in early arthritis patients: a case control study. PLoS One 2018;13:e0193377.
- Rajbhandary R, Khezri A, Panush RS. Rheumatoid cachexia: what is it and why is it important? J Rheumatol 2011;38:406–8.
- Tracey KJ, Wei H, Manogue KR, Fong Y, Hesse DG, Nguyen HT, et al. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med 1988;167:1211–27.
- Engvall IL, Elkan AC, Tengstrand B, Cederholm T, Brismar K, Hafstrom I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand J Rheumatol 2008;37:321–8.
- Elkan AC, Håkansson N, Frostegård J, Cederholm T, Hafström I. Rheumatoid cachexia is associated with dyslipidemia and low levels of atheroprotective natural antibodies against phosphorylcholine but not with dietary fat in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 2009;11:R37.
- Santo RCE, Fernandes KZ, Lora PS, Filippin LI, Xavier RM. Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2018;9:816–25.
- Book C, Karlsson MK, Åkesson K, Jacobsson LTH. Early rheumatoid arthritis and body composition. Rheumatology 2009;48:1128–32.
- da Cunha VR, Brenol CV, Brenol JCT, Fuchs SC, Arlindo EM, Melo IMF, et al. Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scand J Rheumatol 2012;41:186–91.
- Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum 2004;50:3450–7.
- Lemmey AB, Wilkinson TJ, Clayton RJ, Sheikh F, Whale J, Jones HSJ, et al. Tight control of disease activity fails to improve body composition or physical function in rheumatoid arthritis patients. Rheumatology 2016;55:1736–45.
- Tyrovolas S, Panagiotakos D, Georgousopoulou E, Chrysohoou C, Tousoulis D, Haro JM, et al. Skeletal muscle mass in relation to 10 year cardiovascular disease incidence among middle aged and older adults: the ATTICA study. J Epidemiol Community Health 2020;74:26–31.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81.
- Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring) 2012;20:30–9.
- Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord 2002;26:953–60.
- Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, Van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8.
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45.
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003.
- Ciudin A, Salvador R, Budoy A, Spinu C, Diaconu MG, Constantin V, et al. Measurement of waist circumference for retrospective studies - prospective validation of use of CT images to assess abdominal circumference. Endocrinol Nutr 2014;61:147–52.
- Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol 1954;7:218–21.
- Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl 1960;49:1–92.
- Nes BM, Janszky I, Wisløff U, Støylen A, Karlsen T. Age-predicted maximal heart rate in healthy subjects: the HUNT fitness study. Scand J Med Sci Sports 2013;23:697–704.
- Angstrom L, Hornberg K, Sundstrom B, Jonsson SW, Sodergren A. Aerobic capacity is associated with disease activity and cardiovascular risk factors in early rheumatoid arthritis. Physiother Res Int 2020;25:e1833.
- Hörnberg K, Pomeroy J, Sandberg C, Södergren A, Ångström L, Sundström B, et al. Physical activity in rheumatoid arthritis: relationship to cardiovascular risk factors, subclinical atherosclerosis, and disease activity. Scand J Rheumatol 2020;49:112–21.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230–5.
- Fransen J, Van Riel PL. The disease activity score and the EULAR response criteria. Rheum Dis Clin North Am 2009;35:745–57, vii–viii.
- Santo RC, Silva JM, Lora PS, Moro ALD, Freitas EC, Bartikoski BJ, et al. Cachexia in patients with rheumatoid arthritis: a cohort study. Clin Rheumatol 2020;39:3603–13.
- Baker JF, Von Feldt J, Mostoufi-Moab S, Noaiseh G, Taratuta E, Kim W, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:1612–18.
- Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev 1991;49:163–75.
- Marcora SM, Lemmey AB, Maddison PJ. Can progressive resistance training reverse cachexia in patients with rheumatoid arthritis? Results of a pilot study. J Rheumatol 2005;32:1031–9.
- Santillán-Díaz C, Ramírez-Sánchez N, Espinosa-Morales R, Orea-Tejeda A, Llorente L, Rodríguez-Guevara G, et al. Prevalence of rheumatoid cachexia assessed by bioelectrical impedance vector analysis and its relation with physical function. Clin Rheumatol 2018;37:607–14.
- Klein GL. The effect of glucocorticoids on bone and muscle. Osteoporos Sarcopenia 2015;1:39–45.
- Lemmey AB, Wilkinson TJ, Perkins CM, Nixon LA, Sheikh F, Jones JG, et al. Muscle loss following a single high-dose intramuscular injection of corticosteroids to treat disease flare in patients with rheumatoid arthritis. Eur J Rheumatol 2018;5:160–4.