?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
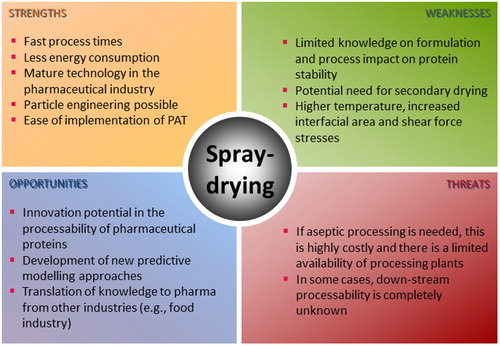
Spray-drying is an inherently continuous and well-established industrial drying process. It can accelerate manufacturing of biopharmaceuticals and vaccine products, resulting in both an economic and health benefit. In this review, we cover a systematic assessment and discuss the spray-drying of diverse protein pharmaceuticals and excipients included therein, solvent systems applicable to these formulations, equipment used and, respective process parameters. Further, key quality aspects of spray-dried protein solids are discussed. Based on the overall trends, we present a concise perspective into the future of protein pharmaceuticals spray-drying.
1. Introduction
Protein pharmaceuticals are, normally, formulated as aqueous dosage forms. However, liquid dosage forms are often unstable, presenting limited shelf life that frequently requires storage and transport under refrigerated conditions. To overcome these limitations, protein pharmaceuticals can be formulated as a dry powder. Ideally, when in the powder form, the biomolecule, will remain stable and retain its activity for the intended periods (3 years or more) under ambient storage conditions. Traditionally, freeze-drying is the process of choice, when drying protein pharmaceuticals. In freeze-drying, the liquid formulation is first frozen and then the ice is removed by sublimation and desorption.[Citation1] As of 2014, there are over 400 approved freeze-dried products by the United States Food and Drug Administration (U.S. FDA). The estimated annual growth of freeze-dried products for 2018 was 13.5%.[Citation2] Many approved freeze-dried products are protein formulations (i.e. vaccines, antibodies, enzymes, peptide hormones, etc.) that emerged rapidly over the last years.[Citation3] Likewise, the expansion of alternative drying processes able to sustainably support the rapid development of biopharmaceutical technologies, are a central need of this industry in years to come.
While freeze-drying presents numerous advantages, there are some inherent challenges. Namely, the difficulty to control the particle/cake properties and microstructures, inter-vial variability, challenge to process large quantities of material, enormous power, time, and resource consumption are some of the major hurdles. These have led many to the quest of finding alternative drying technologies, spray-drying being one of them.[Citation4] Unlike spray-drying, other exploratory drying technologies reported for the processing of protein therapeutics, tend to be costly, time consuming, and not mature enough for industrial implementation. Moreover, one of the main advantages of spray-drying, and a critical driver in generating the interest of the industry in this technique, is the possibility for “continuous processing.”[Citation4] This provides a promising and rapid solution in terms of large volume manufacturing in particular cases like the recent crisis caused by the coronavirus disease 2019 (COVID-19).
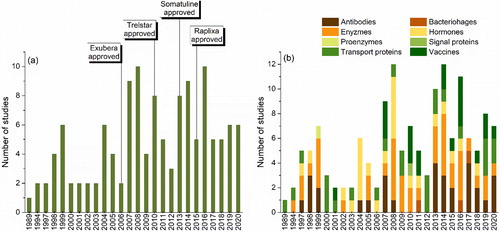
In the last 15 years, spray-drying has, in fact, been successfully applied in the production of a few protein pharmaceuticals (). In 2006, the inhaled insulin powder, Exubera® (Pfizer), became the first commercial spray-dried protein hormone (later withdrawn from the market). Spray-dried alternatives of Poly(lactic-co-glycolic acid) (PLGA) microspheres for depot liquid crystal formulation of triptorelin pamoate and lanreotide acetate were approved in 2010 and 2013, respectively. More recently, in 2015, Raplixa® (ProFibrix BV) became the first approved protein drug manufactured via aseptic spray-drying. Beyond these, other protein pharmaceuticals produced via spray-drying in a wide array of dosage forms are, presently under clinical development.[Citation5]
Table 1. Commercially approved protein pharmaceuticals produced via spray-drying.
In general, drying of protein pharmaceuticals can pose a risk to their chemical and physical stability.[Citation6] Thus, it is of utmost importance to understand how spray-drying’s formulation and process parameters (and their interactions) impact the quality of protein pharmaceuticals. To that end, this review will extensively discuss the learnings achieved over the last 30 years of research conducted in the field of solid protein drug formulations produced by one-step spray-drying process. The following subtopics will be covered: classes of protein pharmaceuticals and excipients used in spray-drying; solvent systems applicable to these types of formulations; spray-dryer equipment used and process parameters applicable to protein formulations; quality aspects of spray-dried protein pharmaceuticals. At the end, we concisely discuss the perspectives on the protein spray-drying based on the identified trends, and identify some opportunities and gaps for the future research in the field.
2. Literature search and statistical analysis
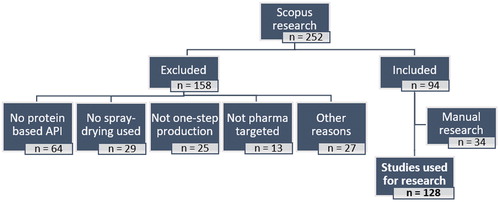
The literature research was carried out between October and November 2020 using Scopus (https://www.scopus.com/). Research papers on the subject area of “Pharmacology, Toxicology, and Pharmaceutics” containing the keywords “spray-drying*” and “protein*” were searched. Considering nanotechnology platforms as a formulation strategy was out of the scope of our review. Any papers with keywords containing the prefix “nano-” were not considered. Patents were also not surveyed, because we considered that, given the enormous number of documents on the subject, there would be great potential in gathering a disproportionate amount of heterogenous information, that would possibly pose a challenge to present a coherent message to our readers. Consequently, the literature search yielded 252 references that were manually screened and an additional 158 works were excluded because:
no protein was used as the active pharmaceutical ingredient (n = 64);
spray-drying was not used to process the formulations (n = 29);
spray-drying was not used in a single step (e.g. pre-complexation of the protein products with inorganic materials, pretreatment of the spray-drying feed solutions with high-speed homogenization, extrusion after drying, particle cross-linking after spray-drying) (n = 25);
no pharmaceutical application (n = 13);
the reference was not a research paper (n = 12);
complex plant extracts were used and a clear distinguishment between active pharmaceutical ingredient and excipients could not be made (n = 5);
DNA/RNA based products were used as the active pharmaceutical ingredient (n = 4);
the protein was used in combination with small organic drugs (n = 3);
no information about the spray-drying process or formulation was available (n = 2);
no version in English was available (n = 1).
Additionally, two relevant reviews on the topic and the internet were manually screened[Citation5,Citation7] and 34 works not found in the Scopus research were deemed adequate to be added. Consequently, a total of 128 experimental research papers were considered for the evaluation of the trends in spray-drying of protein therapeutics over the last 30 years. A resume of the literature research strategy is schematically presented in .
Relevant information (e.g. class of protein pharmaceutical, excipients and equipment used, atomization principle, etc.) taken from the papers, was statistically analyzed using MS-Excel 2016 (Microsoft, USA).
3. Classes of protein pharmaceuticals employed in spray-drying studies
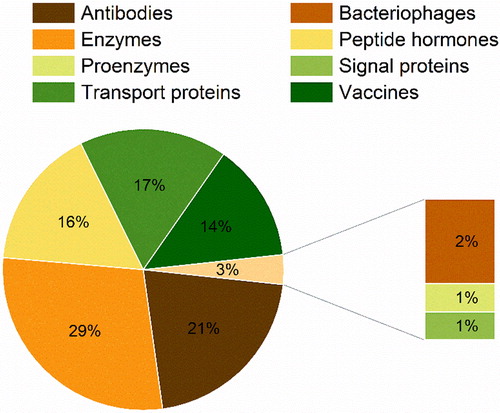
The inclusion of the word “protein*” led to the appearance of proteins, peptide hormones, and vaccines within the search. The vaccines identified had as targets virus, bacteria or cancer cells and used live attenuated organisms, non-virulent recombinant versions of these or their antigen proteins as the active pharmaceutical ingredient (API). Bacteriophages, viruses able to infect and kill bacteria, were also considered. By analyzing 128 works, we categorized the protein pharmaceuticals found in the spray-drying literature in eight main functional classes (): antibodies (21%), bacteriophages (2%), enzymes (29%), peptide hormones (16%), proenzymes (1%), signal proteins (1%), transport proteins (17%), and vaccines (14%).
Antibody proteins have the ability to bind with a high specificity and affinity to a variety of molecules.[Citation8] In the human body, monoclonal antibodies (mAbs) act as a defence against antigens. Since the first large scale production of mAbs,[Citation9] the rapid technological advancement in the field has resulted in mAbs dominating the pharmaceutical market and pipelines, especially in recent years. Commercially available mAbs are used to treat a wide variety of diseases: from cancer to auto-immune diseases and respiratory disorders.[Citation10] While all the commercial formulations of mAbs are produced by freeze-drying, spray-drying could represent a more cost-effective way of processing these products and revolutionize their manufacturing. Thus, there have been a major stride and intensive research and developmental effort in employing spray-drying to mAbs.[Citation11–36]
Bacteriophages can be used alone or in combination with antibiotics in order to treat bacterial infections.[Citation37] Recently published literature explored the potential to spray-dry these viruses for the treatment of respiratory bacterial infections via the pulmonary route.[Citation38–40]
Enzymes are catalyst proteins essential for the biochemical reactions in living organisms.[Citation41] Therapeutic enzymes are mainly applied as replacement therapies in genetic disorders, such as Gaucher disease.[Citation42] Other approved enzymes are applied in blood clotting diseases, cancers, immunodeficiency, etc.[Citation42] The accessibility and the ease of functional analysis[Citation43] of enzymes made them the most extensively studied group of protein therapeutics via spray-drying.[Citation21,Citation24,Citation28,Citation44–75]
A number of endocrine hormones are polypeptides and are vital for regulating biological processes such as metabolism, reproduction, ion balance as well as development and growth.[Citation76] Insulin was the first therapeutically approved peptide to treat Diabetes Mellitus I and was/is available as an injectable liquid formulation.[Citation77] The approval of the spray-dried insulin for inhalation (Exubera® Pfizer) in 2006 made spray-drying of large biological molecules a palpable reality. Since then, spray-drying of many other peptide hormones such as human growth hormone,[Citation24,Citation78–84] octreotide acetate,[Citation85] parathyroid hormone[Citation86,Citation87], salmon calcitonin,[Citation88,Citation89] and ceterolix-acetate[Citation90] have been highly investigated and some have been approved (i.e. triptorelin pamoate and lanreotide acetate).
Proenzymes are the inactive precursors of enzymes and regulate catalytic activity, guaranteeing that this only takes place whenever necessary.[Citation91] Proenzymes also have been the object of research with respect to their activity-stability when spray-dried.[Citation92]
Fibroblast growth factor (bFGF) is a signal protein approved for the treatment of burns[Citation93] and is being investigated as a potential agent for the treatment of pulmonary diseases: i.e. spray-drying of bFGF is reported to generate inhalable particles that could be a promising alternative for the therapy of asthma and chronic obstructive pulmonary disease (COPD).[Citation94]
Transport proteins carry gases, sugars, amino acids, lipids, synthetic drugs, etc., through the vascular system and tissue. Two well-known families of transport proteins are globins and serum albumins.[Citation95] Hemoglobin is the transporter of oxygen.[Citation96] Our literature research found that oxyhemoglobin was one of the first proteins to be successfully spray-dried within a pharmaceutical context.[Citation97] Also spray-drying studies of myoglobin are reported, given its ease of availability and low cost.[Citation47,Citation98] Serum albumins are the most abundant proteins in plasma and they maintain the osmotic pressure in blood, the acid-base balance in plasma, and transport various compounds.[Citation95] Due to its low cost and availability, bovine serum albumin (BSA) has been extensively used as a model protein for spray-drying.[Citation24,Citation98–115] β-lactoglobulin, another protein form bovine source, has also been employed as model.[Citation110]
Vaccines are crucial for the health of populations around the world. Since the advent of the first vaccine against smallpox, many technological advances have made the development of BCG (Bacillus Calmette-Guérin), DPT (diphtheria, pertussis and tetanus), measles, poliomyelitis, hepatitis B (HBV), Haemophilus influenzae serotype b (HiB), human papilloma virus (HPV), COVID-19, and other vaccines a reality.[Citation116] Given the importance of vaccines, it is necessary to guarantee that they are able to reach populations worldwide, alleviating cold-chain burdens. Thus, vaccines should, ideally, be stable during an adequate period of time at elevated temperatures (i.e. 25 °C or more). To this end, powder forms are preferable to liquid formulations. For this, it is not surprising that the spray-drying of diverse types of vaccines has gathered the deep interest of various research groups.[Citation71,Citation117–135]
In , we present the chronological trends of the studies in the spray-drying of protein pharmaceuticals. We can observe an extensive research of peptide hormones during 2002–2011, and their numbers dwindling slightly in recent years. The market introduction of Exubera®, Trelstar® LA and Somatuline® LA between 2006 and 2013 seemed to be a result of the excitement in peptide spray-drying during earlier years. In recent years, from 2013 to 2014, spray-drying of antibodies and vaccines has gathered more attention in terms of the published research. This trend reflects the tremendous rise and clinical success of antibody therapies witnessed over the past decade. Moreover, the quest of facile manufacturing processes to cope with the supply of the antibodies and vaccines for global pandemics like COVID-19 will potentially rise the spray-drying research for these therapeutics. In addition, spray-drying studies on transport proteins (e.g. BSA) and enzymes (e.g. lysozyme, catalase, etc.) have been kept steady throughout the years. Certainly, because these constitute a good choice of model molecules, when trying to limit costs and ease functional analysis.
4. Overview of the excipients used in the formulation of protein pharmaceuticals via spray-drying
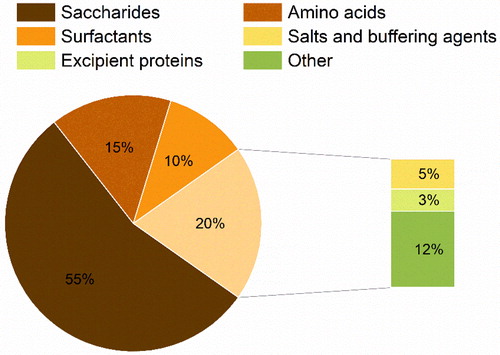
Functional excipients are pharmacologically inactive ingredients that play a crucial role in improving delivery, processability and stability of formulated drugs, ensuring pharmaceutical performance. Typically, diverse excipients are included when formulating the spray-dried proteins (see ). Excipients can aid in interfacial stabilization during dehydration/drying steps, improve yield and processability of the spray-dried powders as well as enhance their dispersity and reconstitution. Our analysis considered the excipients used in the formulation of the feed solutions prior to spray-drying. We categorized our findings of the excipients into six chemical classes (): saccharides (55%), amino acids (15%), surfactants (10%), salts and buffering agents (5%), excipient proteins (3%), and other molecules (12%).
Figure 4. Classes of excipients used in the formulation of protein pharmaceuticals via spray-drying.
4.1. Saccharides
Carbohydrates are the excipients of choice, when formulating protein pharmaceuticals. Various theories outline the mechanisms, by which saccharides stabilize protein molecules and have been the subject of review elsewhere.[Citation136] However, in resume, the vitrification hypothesis postulates that amorphous carbohydrates kinetically stabilize biomolecules above their glass transition temperature (Tg) by arresting their global molecular mobility (α-relaxation).[Citation137] The water replacement theory proposes that carbohydrates could also thermodynamically stabilize proteins by replacing the hydrogen bonds with water present in the hydrated state and thus, allow the biomolecules to maintain their native conformation.[Citation138] More recently, it has also been shown that carbohydrates are able to retard the local molecular motions of proteins (β-relaxation), thereby providing protein stability in the sub-Tg range.[Citation139] Alternatively, certain saccharides are proposed to affect the packing density and interact with proteins, thus stabilizing them.[Citation136]
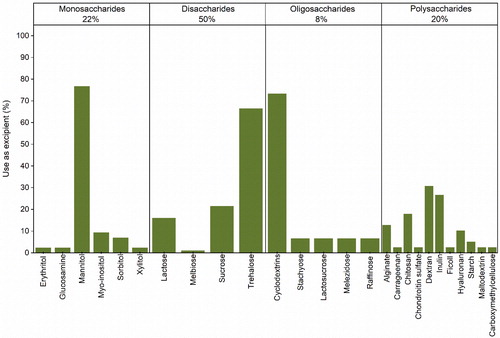
When analyzing the carbohydrates used in spray-drying research, we further sub-categorized them (), according to the number of monomer (glucose) units they have.[Citation140] Likewise, carbohydrates with one and two monomers are monosaccharides and disaccharides, respectively. Saccharides with the number of monomers between 3 and 9 were sub-categorized as oligosaccharides and that with more than 9 were considered polysaccharides.
Figure 5. Sub-categorization of the saccharides used in the formulation of protein pharmaceuticals for spray-drying.
4.1.1. Monosaccharides
Monosaccharides account for 22% of the saccharides used for formulating spray-dried protein pharmaceuticals. These are all sugar alcohols and an amino sugar, i.e. glucosamine. Mannitol is the most used monosaccharide. Sorbitol and myo-inositol seem also to be periodically employed. The other remaining monosaccharides (i.e. erythritol, glucosamine, and xylitol) were found only in one study each.[Citation48,Citation100,Citation128] Even though mannitol is one of the most well-used sugars in commercialized biologic products,[Citation141] when used alone to spray-dry certain classes of proteins (e.g. mAbs), its crystallization during processing has shown the potential to deleteriously affect product stability.[Citation11,Citation13,Citation17,Citation24,Citation28,Citation31,Citation35,Citation44,Citation46,Citation142] Other evidence shows that mannitol alone could be useful in stabilizing peptide hormones[Citation85,Citation88] in such a way that the monosaccharide is actually used in the formulations of Exubera®, Trelstar® LA, and Somatuline® LA. In combination with amorphous sugars, mannitol could also provide some advantage, as the partially crystalline powders expected to be formed could, for example, improve reconstitution of parenteral dosage forms.[Citation143] In turn, sorbitol has shown to be able to stabilize immunoglobulin G (IgG) and glutamate dehydrogenase in spray-dried powders, demonstrating even stronger stabilization when used in combination with trehalose or other disaccharides.[Citation33,Citation53,Citation56] Finally, while the effect of using myo-inositol alone is unknown, its combined use with other excipients was found to be useful, when stabilizing vaccine products.[Citation118,Citation120,Citation132,Citation135]
4.1.2. Disaccharides
Disaccharides are the most used carbohydrates when formulating protein pharmaceuticals for spray-drying (50%). Among them, trehalose is the most preferred, followed by sucrose, lactose and melibiose. However, lactose and melibiose are reducing sugars, so their free aldehyde groups can undergo the Maillard reaction when in contact with the amino groups of proteins.[Citation22,Citation48,Citation136] Although, the reducing disaccharides have been extensively selected to study protein spray-drying[Citation17,Citation22,Citation24,Citation44,Citation48,Citation51,Citation80,Citation81,Citation83,Citation84,Citation86,Citation94,Citation111,Citation128,131,144] and are used in some marketed bio-formulations,[Citation141] it is crucial to guarantee that no detrimental chemical interaction takes place, during processing and storage. Within a spray-drying context, it has been reported that trehalose could be more successful than other sugars, when stabilizing proteins.[Citation32,Citation46] Compared to sucrose, for example, it has been suggested that trehalose presents superior abilities to form H-bond networks with proteins[Citation145] as well as produce formulations with a higher Tg . Likewise, the use of trehalose alone or in combination with other excipients is preferred, when developing protein formulations via spray-drying.
4.1.3. Oligosaccharides
Oligosaccharides are used less frequently than other saccharides, when spray-drying protein pharmaceuticals (8%). However, cyclic oligosaccharides like cyclodextrins have shown great potential in stabilizing proteins. This is potentially due to their cyclic structure bearing a hydrophobic pocket, which can encapsulate the hydrophobic residues of proteins.[Citation146] The use of β-cyclodextrin alone and in combination with other excipients was reported to stabilize IgG,[Citation12,Citation15] trastuzumab,[Citation12] a tuberculosis vaccine,[Citation118] and lysozyme[Citation54] in spray-dried formulations. Stabilization was also achieved by employing (2-Hydroxypropyl)-β-cyclodextrin alone or in combination with other saccharides, when spray-drying IgG,[Citation12,Citation15] trastuzumab,[Citation12] BSA,[Citation101] β-galactosidase,[Citation45] and insulin.[Citation147] In turn, dimethyl-β-cyclodextrin was able to stabilize recombinant human growth hormone, when used alone[Citation81] and α-cyclodextrin helped maintain the activity of porcine trypsin.[Citation44] The potential stabilizing effect of linear oligosaccharides such as stachyose, lactosucrose, melezidose, and raffinose has also been the subject of investigation.[Citation11,Citation72,Citation148,Citation149] However, these are seldom used when spray-drying proteins ().
4.1.4. Polysaccharides
Polysaccharides are also commonly used in the formulation of protein pharmaceuticals via spray-drying and include a wide variety of molecules (20%). High molecular weight amorphous polysaccharides frequently possess higher Tgs than other smaller saccharides. According to the vitrification hypothesis, excipients presenting a higher Tg could offer an advantage when stabilizing proteins.[Citation150] Additionally, some polysaccharides also present mucoadhesive and modifying release properties that could be of interest when formulating certain types of dosage forms (i.e. sustained release, orally administered vaccines). Dextran, inulin, chitosan, alginate and hyaluronan seem to be preferred in comparison to other polysaccharides, i.e. carrageenan,[Citation100] chondroitin sulfate,[Citation100] ficoll,[Citation148] starch,[Citation149] maltodextrin,[Citation48] and carboxymethylcellulose.[Citation60]
Dextran is composed of α-(1,6)-linked glucan with side chains attached to the C-3 position of the backbone.[Citation151] The side chains vary in length, originating dextran molecules with distinct degree of polymerization (DP) and, hence different physicochemical properties (e.g. molecular weight, solubility, flexibility, etc.). Dextran having various molecular weights, from 1 to 70 kDa, have been applied to the formulation of proteins via spray-drying, with weights between 40 and 70 kDa being preferred. Dextran in combination with mannitol and trehalose have successfully been applied in the stabilization of recombinant viral vaccines[Citation130,Citation131] and bacteriophages formulations.[Citation134] However, dextran with 1 kDa and 60–70 kDa were not so successful, when stabilizing IgG formulations[Citation11] and a tuberculosis vaccine[Citation118] via spray-drying. Used alone, dextran of 20, 40, and 70 kDa have been unsuccessful stabilizing formulations of methionyl human hormone growth[Citation148] and transport proteins.[Citation98] One report describes the successful use of 10 kDa dextran to stabilize an investigational peptide hormone.[Citation152] However, the presence of a larger percentage of reducing groups on smaller molecular weight dextran[Citation150] should be carefully considered when selecting these as excipients.
Inulin is a fructan, consisting of β-(2γ1)-linked D-fructosyl residues (n = 2–60), usually with an α-(1 ↔ 2)-d-glucose end group. Inulin is available from a DP range of 3 to 80.[Citation153] Used alone, inulin has shown to be able to stabilize an influenza vaccine,[Citation117] lysozyme,[Citation54] and recombinant human deoxyribonuclease (RhDNase) I,[Citation154] when these were spray-dried. The non-reducing nature of inulin makes it an elegant choice of stabilizing excipient for protein formulations.
Chitosan is a deacetylated derivate of chitin, (a mucopolysaccharide consisting of 2-acetamido-2-deoxy-β-(1γ4)-linked D-glucose.[Citation155] Chitosan has mucoadhesive and controlled release properties that could confer a formulation advantage. It has been shown that chitosan is able to stabilize spray-dried insulin[Citation142] and BSA.[Citation102] Moreover, the use of chitosan has enabled the production of inhalable protein particles with controlled release properties and improved systemic delivery.[Citation104] In combination with mannitol and gastro-resistant polymers, chitosan has demonstrated to be able to successfully produce inhalable particles of salmon calcitonin[Citation89] and microparticles of BSA for oral delivery,[Citation115] respectively.
Alginates are natural polysaccharides extracted from brown algae. Alginates are constituted by linear chains of α-(1γ4)-linked-l-guluronic acid blocks (GG), β-(1γ4)-linked-d-mannuronic acid blocks (MM) and hetero-polymeric sequences of M and G (MG blocks).[Citation156] Alginate is mucoadhesive and due to its anionic nature in the presence of di-cations can form hydrogels with modifying release properties. Likewise, alginate and its sodium salt used alone and in combination with mannitol were able to produce stable microparticles of insulin.[Citation142,Citation157] When cross-linked with zinc, inhalable particles of BSA were produced using alginate.[Citation100] In combination with gastro-resistant polymers, alginate was used as a mucoadhesive agent to successfully produce an oral vaccine of Vibrio cholera.[Citation121]
Hyaluronan is a glycosaminoglycan originating from repeated disaccharides of d-glucuronic acid and N-acetyl-d-glucosamine linked by a glucuronidic β-(1γ3) bond.[Citation158] Like alginate, it also presents mucoadhesive and modifying release properties. Hyaluronan and its sodium salt have been successfully used to produce spray-dried BSA particles with controlled release properties alone[Citation104] and in combination with other excipients (i.e. alginate and PLGA).[Citation100,Citation114] For subcutaneous delivery, spray-drying of sodium hyaluronate in combination with lecithin was shown to be able to originate particles of human growth hormone with sustained release properties.[Citation82]
4.2. Amino acids
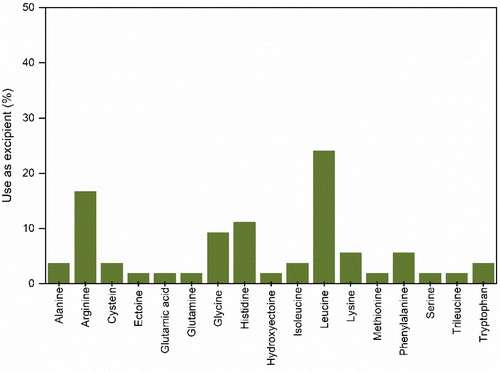
Amino acids are the monomer units of the long polypeptide chains that construct proteins.[Citation159] More than 300 types of amino acids having distinct chemical structures and a variety of physicochemical properties occur in nature. Although amino acids are known to stabilize liquid and solid forms of protein formulations, the exact mechanism(s) by which they do it has not been fully elucidated yet. It has been proposed that amino acids can fill “free volume holes” in amorphous formulations, improving stability[Citation160] or in the case of charged hydrophilic amino acids by the formation of H-bonds.[Citation23] However, amino acids also have shown other advantages, such as the improvement of particle aerosolization[Citation161] and parental dosage reconstitution.[Citation162] In other cases, amino acids can also act as anti-oxidants, avoiding the degradation of formulation components.[Citation30. We found 17 amino acids used during spray-drying of protein pharmaceuticals (). Leucine, arginine, glycine, and histidine seem to be the amino acids most frequently used.
Leucine is a hydrophobic neutral amino acid constituted by an aliphatic side chain[Citation159] and its use is intimately associated with the production of flowable protein powders. Thus, the recurring use of leucine seems to be more associated to its technological advantages in terms of particle dispersibility[Citation16,Citation38–40,Citation94,Citation118,Citation129,Citation131,Citation135] than to its potential stabilization effects.[Citation119,Citation120,Citation134] It has been proposed that during spray-drying leucine migrates to the air-liquid interface accumulating at the particle crust, rigidifying it and originating crumpled particles that present better flow.[Citation161]
Arginine is a hydrophilic amino acid, containing a positively charged side chain[Citation159] and has shown both technological and stabilizing advantages in parental applications, particularly in high concentration formulations of mAbs. In these types of formulations, arginine has shown viscosity lowering abilities and expedite powder reconstitution.[Citation36,Citation162] Its superior stabilizing ability is proposed to be associated to its side chain ability to form H-bonds with proteins.[Citation23] However, in aerosolisable formulations of IgG, arginine was not successful in maintaining long-term stability.[Citation16] Additionally, it has also been shown that used alone and in combination with other excipients, arginine was able to stabilize β-galactosidase,[Citation46] catalase, lysozyme,[Citation71] IgG4,[Citation32] BSA[Citation112,Citation113] as well as an attenuated measles[Citation132] and influenza antigen vaccine.[Citation71]
Histidine is also a hydrophilic amino acid, containing a positively charged side chain (112), hence it is normally applied as a buffering agent (to be discussed later) in liquid formulations of proteins. However, histidine also seems to confer some advantage, when used in solid formulations and has been successfully applied as a stabilizer in combination with sugars[Citation13,Citation18,Citation32] or other amino acids.[Citation71] The use of histidine alone does not seem to offer sufficient stabilization of proteins after spray-drying.[Citation23]
Glycine is a hydrophilic neutral amino acid constituted by an aliphatic side chain.[Citation159] Similarly to histidine, glycine seems to stabilize proteins when used with sugars[Citation11,Citation13,Citation78] or other amino acids,[Citation71] i.e. arginine or histidine, but is less effective when used alone.[Citation23] Exubera® used a combination of glycine and mannitol in its formulation.
4.3. Surfactants
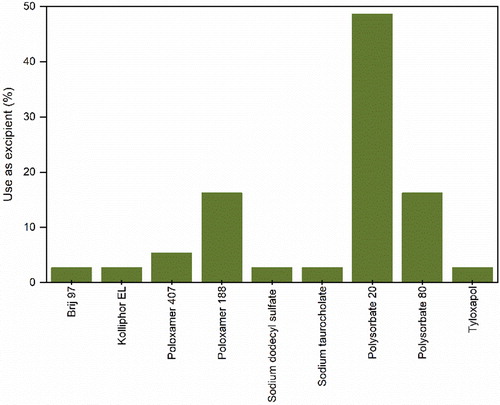
Surfactants are amphiphilic molecules constituted by hydrophobic and hydrophilic groups that confer them the ability to adsorb at interfaces.[Citation163] During drying, large molecules, like proteins, have a tendency to irreversibly accumulate at air-liquid interfaces. This can lead to the physical degradation of proteins through interfacial aggregation and denaturation.[Citation164] Surfactants are known to stabilize protein formulations and are widely applied in both powder and liquid forms.[Citation165] Two main mechanisms or a combination of thereof, seem to be responsible for the stabilization of proteins by surfactants. One of the mechanisms hypothesizes that stabilization takes place through interfacial competition, where the surfactant occupancy of the liquid-air interface is more favorable than that of proteins.[Citation166] The other mechanism proposed, postulates that stabilization of proteins occurs due to the formation of complexes through hydrophobic binding with surfactant molecules.[Citation164,Citation165] Most studies on the subject seem to indicate that interfacial competition is the prevailing mechanism at work during spray-drying.[Citation24,Citation44,Citation73,Citation75,Citation79] Thus, the optimal surfactant concentration depends on the total area of the air-liquid interface. As shown in , most of the surfactants used are nonionic, with the exception of sodium dodecyl sulfate (SDS) and sodium taurocholate. Contrary to ionic surfactants, nonionic ones are unable to denature proteins, even when used at high concentrations[Citation164] and thus, preferred. Pharmaceutical nonionic surfactants are the derivatives of poly(ethylene oxide) (PEO) and are extensively used in formulations of biologics.[Citation165] Polysorbate 20 and 80 as well as poloxamer 188 are the most used surfactants in spray-drying of proteins. However, the potential auto-oxidation of susceptible surfactants like polysorbates at high temperatures has to be considered, as this can jeopardize the long-term storage stability of protein powders.[Citation30,Citation73] The use of anti-oxidant molecules, like the amino acid cysteine, could be useful in preventing oxidation and guaranteeing longer stability of bio-powders containing polysorbates.[Citation30] Lastly, it is important to note that the use of surfactants alone is not enough to stabilize protein formulations, so they are usually used in combination with other excipients (e.g. carbohydrates, amino acids, salts, etc.). From a technological perspective, the use of surfactants during spray-drying has shown to produce particles with a smoother surface.[Citation24]
4.4. Salts and buffering agents
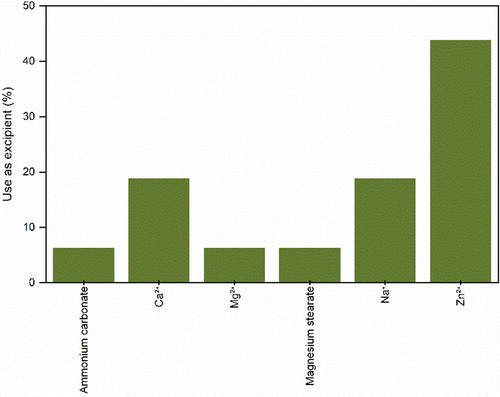
Naturally, salts are used to produce buffered solutions necessary to stabilize biomolecules. However, here in this section, we will explore the advantageous effects that the addition of certain specific salts has on the physical stability and technological performance of bio-powders. As observed in , some salts and their respective cations have been applied as excipients when spray-drying proteins. Zinc is shown to suppress aggregation of growth hormone[Citation79–81] and BSA[Citation105] when these are spray-dried. Moreover, zinc was also used to stabilize spray-dried insulin[Citation149] and in combination with calcium was found to improve the long-term stability of a measles vaccine.[Citation132] Additionally, zinc has also been used to form hydrogel particles for the inhaled delivery of protein therapeutics.[Citation100] With respect to sodium, its phosphate salt has been successfully used to inhibit the detrimental crystallization of mannitol during spray-drying of mAbs.[Citation31] During spray-drying, sodium chloride is reported to crystallize recombinant human DNase I[Citation74] and act as osmotic pressure regulator of a bacterial vaccine.[Citation119] Concerning calcium, this has shown to be able to protect bovine pancreatic DNase I from proteolytic action during spray-drying.[Citation57] In contrast, magnesium was unable to improve the stability of a measles vaccine.[Citation132] Ammonium carbonate, a known pore former agent, has been used to produce aerosolisable particles of octreotide acetate.[Citation85] Finally, magnesium stearate has been applied to the production of microspheres of an oral vaccine of Actinobacillus pleuropneumoniae,[Citation124] probably as agglomeration preventing agent.[Citation167]
4.5. Excipient proteins
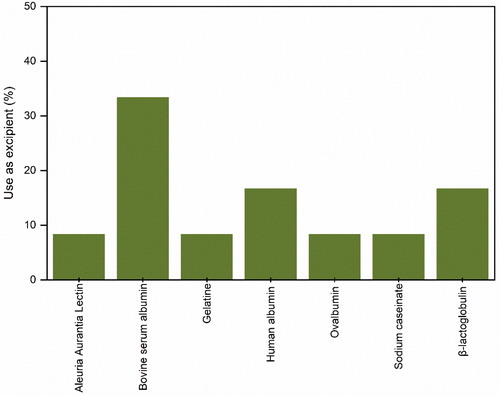
Certain proteins can be used as excipient molecules to stabilize other proteins. Some excipient proteins even present the ability to modify the release or target specific biological marks when included in the formulation of other proteins. shows the proteins identified through our literature research as being employed as excipients in spray-drying. For instance, the concomitant investigation of BSA and β-lactoglobulin has demonstrated that these are able to protect enzymes from loss of activity during spray-drying, due to protein-protein interactions that reduce unfolding and aggregation.[Citation75] When spray-drying the BSA and β-lactoglobulin together, it has also been shown that the biomolecule with the higher surplus amount decreases the apparent surface load of the protein present in lower concentration.[Citation110] These works demonstrated that globins could stabilize other large biomolecules either by competitive surface adsorption or by the formation of hetero-protein complexes. BSA has also shown to successfully stabilize oral vaccines against melanoma.[Citation122,Citation123] Human serum albumin in combination with other excipients has shown to improve the stability of a measles vaccine.[Citation132] However, its use as an excipient in the spray-drying of parathyroid hormone, although successful in protecting the peptide from surface adsorption, has shown to be detrimental to the hormone bioavailability.[Citation86] Another globin, ovalbumin, has demonstrated to successfully sustain the release and change the bioavailability of inhalable protein microspheres.[Citation104] Gelatin, used in the same study was not so successful in achieving a similar end. In contrast, sodium caseinate, a mixture of four phosphoproteins, has been shown to be successful in maintaining the activity of an inhalable formulation of bacteriophages.[Citation40] Lastly, aleuria aurantia lectin has been applied to oral vaccines to target M-cells in the intestine and ensure the uptake of the formulation.[Citation99]
4.6. Other excipients
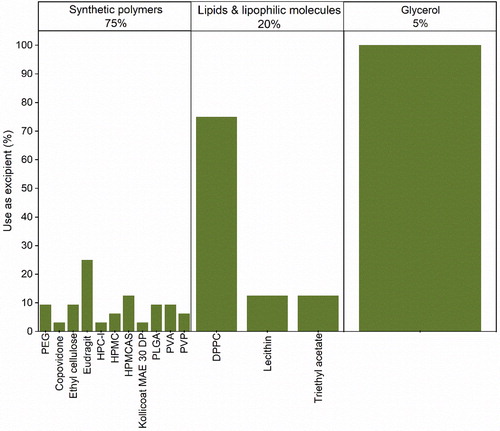
We classified other excipients as a miscellaneous group of molecules with various ends when added to the formulation of large biomolecules via spray-drying. As in , we sub-categorized the molecules into three different groups: synthetic polymers (75%), lipids and lipophilic molecules (20%), and glycerol (5%).
Figure 10. Sub-categorization of other various excipients used in the formulation of protein pharmaceuticals for spray-drying.
4.6.1. Synthetic polymers
Synthetic polymers such as Eudragit®, ethyl cellulose, hydroxypropyl methylcellulose (HPMC) and HPMCAS (hydroxypropyl methylcellulose acetate succinate) are applied to spray-dry formulations of protein therapeutics as enteric coating agents for oral delivery. The release of proteins in the stomach will lead to their degradation due to the low pH in the gastric environment. Consequently, an enteric coating is applied to protect the biomolecules until these can be released in a more amiable environment (i.e. intestine). Thus, Eudragit® L-30 D-55,[Citation121,Citation133] Eudragit® S100 in combination with HPMC[Citation115] and Eudragit® FS 30 with HPMCAS[Citation99] have been used in the formulation of Vibrio cholera vaccines and BSA to produce particles that could potentially be used for oral delivery. Ethyl cellulose and HPMCAS have been used in combination to investigate the possible production of oral vaccines for melanoma and Actinobacillus pleuropneumoniae.[Citation122–124] Particularly HPMC, that has been studied in-depth, showed that when used alone, it can migrate to the surface of spray-dried droplets, coating proteins in-situ and exerting a beneficial protective effect on biomolecules.[Citation106] Moreover, the same study also showed that HPMC produced irregularly surfaced particles that could have an advantage in terms of powder flowability and provide the sustained release of a drug. PLGA is applied to modify the release of injectable bio-microparticles (Trelstar® LA and Somatuline® LA).[Citation52,Citation114] Additionally, some research groups have also tried to apply PLGA as a modifying release agent for the formulation of inhalable protein particles.[Citation104] However, the local lung toxicity of degraded acid products of PLGA still remains a major concern to its successful pulmonary application. The same study showed that low viscosity grade hydroxypropyl cellulose (HPC) was compatible with the lung and produced inhalable particles of BSA via spray-drying with improved delivery efficiency. Polyvinyl alcohol (PVA) alone, also showed, to produce inhalable particles of BSA with modified release profile.[Citation103] In combination with trehalose, PVA was able to stabilize and improve the aerosolization of lysozyme and catalase.[Citation58] Polyvinylpyrrolidone (PVP) has been used in combination with other excipients (i.e. saccharides and amino acids) to successfully formulate tuberculosis (PVP with a molecular weight of 8 kDa) and HPV vaccines.[Citation118,Citation120] In combination with polyethylene glycol (PEG), PVP has shown to produce large porous particles that could be used to deliver proteins to the lung.[Citation168] It has also been demonstrated, that PEG 4000 in combination with chitosan and HPβCD is able to produce sustained release microspheres of BSA.[Citation101] Another study showed that poly(vinylpyrrolidone-co-vinyl acetate) (PVP-VA) was successful in stabilizing lysozyme and trypsin upon spray-drying.[Citation50]
4.6.2. Lipids and lipophilic molecules
With respect to lipid excipients, dipalmitoyl phosphatidylcholine (DPPC), an endogenous phospholipid to the lung, has been frequently applied to protein formulations, particularly, inhaled ones. In combination with sugars (i.e. lactose, mannitol, trehalose), DPPC has been used to produce spray-dried inhalable particles of bFGF, human growth and parathyroid hormones, wherein the lipid showed to induce aggregation of bFGF.[Citation94] For human growth and parathyroid hormones, DPPC was shown to enrich the surface of the spray-dried particles, thus stabilizing them. The lipid acted as barrier to moisture uptake, thereby preventing the crystallization of the sugar present in the formulations of the hormones.[Citation84,Citation86] In combination with Eudragit® E100, DPPC was used to produce microparticles of albumin that exhibited sustained release profiles.[Citation169] Another lipid, egg lecithin (Lipoid E80®), was used in combination with hyaluronic acid to produce sustained release formulations of human growth hormone for subcutaneous injection.[Citation82] Triethyl acetate, a lyophilic molecule, was applied as a plasticizer for the enteric coating of oral vaccines of Vibrio cholera.[Citation121]
4.6.3. Glycerol
The polyol, glycerol, is an interesting molecule and its mechanism of stabilization of large biomolecules, like proteins, largely unknown. Although glycerol is a plasticizer and thus, able to increase the primary molecular mobility of protein formulations, it is surprisingly reported to actually retard the secondary or local molecular motions.[Citation170] This way, it has been proposed that the stabilizing effect of the polyol is due to its anti-plasticization effect on the local molecular mobility (e.g. β-relaxation) of solid formulations containing biomolecules. In the case of spray-drying, glycerol has been applied in combination with other excipients in the formulation of measles and BCG vaccines.[Citation119,Citation132]
5. Feed media used in the spray-drying of protein pharmaceuticals
As spray-drying is a process by which a solution/suspension is dried and a powder obtained, prior to the solution/suspension being processed, the formulations of proteins will have to be dissolved or dispersed in a given medium. The characteristics of the feed medium are very important, and the interplay involving feed components and their properties as well as process conditions will determine the powder quality of the final formulation. In general, spray-drying of proteins is carried out using water-based solutions (). Bacterial and some viral products are an exception, where a suspension of the agent in an aqueous growth medium is normally used.
5.1. Impact of the feed media on the stability of protein pharmaceuticals
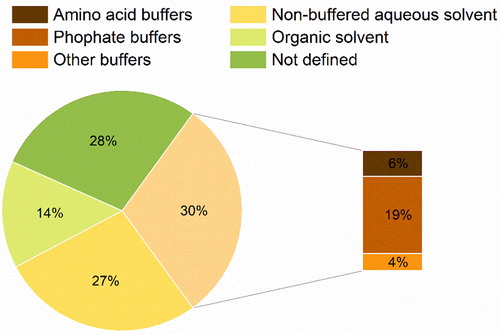
The pH of the media containing biomolecules, e.g. proteins, is critical for their activity. Evidence shows that although the conformational stability of proteins allows for slight fluctuations in pH, their most favorable conformation is tightly correlated to their optimal activity.[Citation171] Likewise, one important function of buffers is to regulate shifts in pH and guarantee that the molecules of interest are maintained in their optimal conformation. Indeed, it has been observed that in lyophilized solids, the ionization state of proteins (microenvironmental pH) is important for its activity and stability.[Citation172] In the case of spray-drying, it has been shown that spray-drying of β-lactoglobulin at acidic pH leads to higher loss in activity than when a neutral one is used.[Citation46] In contrast, lowering the pH of feed solution for a measles vaccine from 7 to 6 on, resulted in an improvement of the formulation potency after spray-drying.[Citation132] Given the importance of guaranteeing an adequate environment for the stability of protein pharmaceuticals, a notable number of reported feed media used in the spray-drying of large biomolecules contain buffering salts (30%).
Phosphate buffers seem to be mostly applied (19%) as they allow for a wide range of pH (pH = 5.8–8.0) and are frequently applied in commercial formulations of biologics.[Citation173] Within spray-drying context, phosphate-buffered saline (PBS) was able to maintain the potency of an inactivated influenza virus vaccine, better than HEPES buffered saline (HBS) at pH = 7.4.[Citation117] The authors attributed this to the ability of PBS, but not HBS, to stabilize pH up to 70 °C. The use of different salt concentrations of a citrate-phosphate buffer did not notably impact the activity of β-lactoglobulin when spray-dried.[Citation46] However, when different concentrations of potassium phosphate were used to spray-dry a measles vaccine, a salt concentration between 25 and 40 mM was found to be better in preventing loss of potency during processing.[Citation132] Moreover, the same authors showed that it made no difference if potassium or sodium phosphate were used. In a mAb formulation, the use of different concentrations of sodium phosphate showed that the presence of sodium had an influence on the solid-phase of the sugar excipient (i.e. mannitol). Sodium tended to amorphize mannitol and, thereby, minimize protein aggregation during spray-drying.[Citation31] The use of amino acid buffers (6%) has also been investigated pertaining to the spray-drying of mAbs. In a recent study, it has been shown that the use of histidine buffer is more beneficial than lactate, when spray-drying an IgG mAb.[Citation23]
Studies on the use of buffering versus non-buffered solutions, have demonstrated that the former have a beneficial impact on the stability and retention of potency of vaccines for hepatitis B and measles.[Citation118,Citation132] Moreover, when spray-drying lysozyme in aqueous mixtures of ethanol, it was found that the use of PBS instead of non-buffered water improved the retention of enzymatic activity after spray-drying.[Citation49] On the contrary, for the spray-drying of a tuberculosis vaccine, the use of non-buffered water or PBS had a similar effect on the survival of the virus during spray-drying.[Citation144]The notable use of non-buffered solutions (27%) also seems to indicate that many biomolecules might be sufficiently stable to withstand spray-drying without the addition of buffering salts. However, a systematic study explicitly examining the buffering effect of the feed solution on the quality of spray-dried protein powders, is yet missing.
Organic solvents (14%), are generally considered to have a deleterious effect on biomolecules, such as rigidification of their conformation, dehydration and damage to the molecular structure.[Citation174] However, some works in literature indicate that in spray-drying the use of organic solvents might not always be critically detrimental. In most reported cases, the organic solvents used are water miscible and volatile (high vapor pressure). In an interesting study, Saß and Lee evaluated the spray-drying of enzyme formulations (trypsin and lysozyme) using mannitol and trehalose from binary mixture of water with sixteen different organic solvents and found that the degree of stabilization and residual solvent content were strongly dependent upon the solvent types used.[Citation175] Using lysozyme, other studies showed that the enzyme could be, in fact, spray-dried from 20:80 wt.% ethanol:water and methanol:n-butyl acetate mixtures without any significant loss in activity.[Citation49,Citation72] In the case of bFGF, when this was spray-dried from a 70:30 wt.% ethanol:water solution, it was observed that aggregation of the biomolecule led to its destabilization.[Citation94] Indeed, there is some evidence that the use of lower organic solvent concentrations in aqueous mixtures or their use alone is more beneficial than the mixture of these with water at concentrations >60 wt.%.[Citation176]
5.2. Impact of feed medium properties on the formation of solid particles
The properties of the medium and its solutes will inevitably impact the drying process and the characteristics of the resulting solid particles, i.e. particle size distribution (PSD), morphology and surface-to-bulk chemistry, phase miscibility, and residual solvent contents. For instance, the geometric diameter of solid dry particles (dg) formed by spray-drying depends on the following mass-balance[Citation177]:
(1)
(1)
where CF is the feed concentration, ρp particle density and dD the droplet diameter. In general, for all atomization devices, the diameter of the generated droplets will correlate to liquid properties such as interfacial tension, viscosity, density, etc. Thus, the latter are crucial to thoroughly characterize and control in the course of the droplet size engineering to obtain the desired particle size distribution (PSD). Another important parameter to consider is the solvent volatility and thus the evaporation rate of the feed solution. During spray-drying, evaporation is controlled by the difference between the temperature of the inlet gas (Tin) and the wet-bulb temperature (Twb) of the droplet[Citation177,Citation178]:
(2)
(2)
Further, the temperature at the droplet is partially dependent on the boiling point (Tb) of the solvent. Thus, the decrease in Tb for a fixed also decreases the Twb, leading to an increase in the
and thereby the evaporation rate.[Citation178] Generally, the evaporation rate (k) relates to the Peclet number (Pe), a dimensionless parameter describing solute (i) diffusion (D) during droplet drying, by following equation[Citation177]:
(3)
(3)
For Peclet numbers < 1, the velocity with which the solute recesses from the interface is similar to the shrinking rate of the droplet surface leading to the formation of dense and round particles with a uniform radial concentration profile. Moreover, if the solute has a large solubility in the solvent, precipitation will take place later and a homogenous droplet, of which the true density is similar to the raw material, is obtained.[Citation178,Citation179] For Peclet numbers > 1, the solute diffusion does not accompany surface recession rate, and a shell or skin is originated leading to the formation of hollow particles.[Citation178,Citation179] The surface enrichment of each solute during drying is expressed by a polynomial equation of its
value.
(4)
(4)
In an effort to regulate evaporation during spray-drying and manufacture large hollow porous particles of disaccharides, Ógáin et al., for example, have tested different ratios of solvent mixtures of methanol and n-butyl acetate. They found that a ratio of methanol to n-butyl acetate of 80:20 vol.% was ideal to produce inhalable particles of sugar that could potentially deliver protein therapeutics to the lung.[Citation72] Other authors reported the use of hydroalcoholic feed solutions to obtain irregular particles of protein formulations. For example, the increase of ethanol in aqueous medium containing insulin led to the formation of crumpled particles with lower bulk density and thus, more aerosolisable.[Citation180] Similarly, increasing the ethanol content also originated a higher fraction of inhalable particles of lysozyme.[Citation61] Considering that protein molecules are surface active, another important phenomenon to consider is the Marangoni effect.[Citation178] This convective surface flow occurs once a gradient in interfacial tension is present. The gradient exerts shear stresses at the interface, inducing the movement of the fluid from lower to higher surface tension areas.[Citation181] Prolonged action of the Marangoni effect during particle formation might produce shear-induced aggregation of sensitive protein molecules, detrimentally impacting their stability. The addition of surfactant (excipient with a higher surface activity than protein) can circumvent this problem by replacing the biomolecule at the droplet surface during drying and inducing the diffusion of the protein from the surface to the bulk of the droplet. From a technological perspective, the addition of surfactant can avoid the potential formation of a shell and promote a more homogenous chemical distribution of protein and excipients within the droplet, originating a smoother surface.[Citation24,Citation73,Citation79,Citation80,Citation105,Citation148]
6. Processability of protein pharmaceuticals via spray-drying
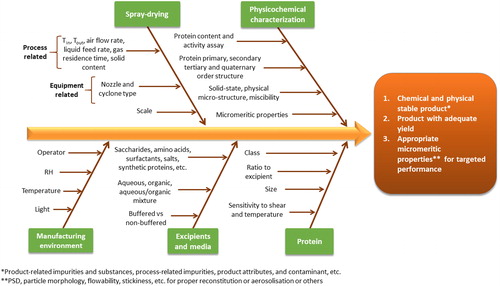
With a longer history of use, freeze-drying presents a plethora of fundamentally important research works on the mechanism behind cryo-stabilization and lyo-stabilization of proteins. In comparison, the literature on pharmaceutical protein spray-drying is only a few decades old. Every step in the spray-drying process of proteins needs precise understanding, optimization and control to prevent the loss of activity in the final product. The extent to which the different steps contribute to the (in)stability of the protein may significantly differ for different process equipment and processing conditions as well as protein formulation types and involved excipients. For spray-drying of a defined formulation of a particular protein, considerations about manufacture can be split into equipment and process-related factors. In terms of equipment related factors, the type of spray-dryer itself, the scale, the nozzle type, and the separator/cyclone design can affect the final product quality.
6.1. Atomization nozzles
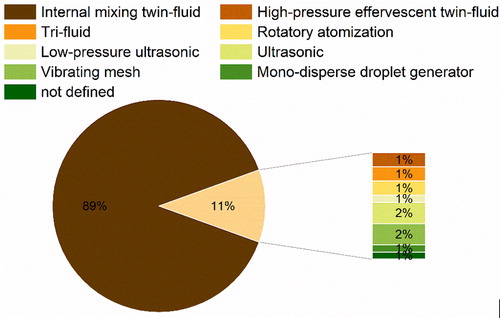
Atomization is a process by which a liquid stream is broken into droplets that are dispersed into a surrounding gas.[Citation182] In general, disintegration of the liquid jet can be achieved by its destabilization, which is intimately dependent on the operating conditions of the spray-dryer and the nozzle selected. As it can be observed in , twin-fluid nozzles are most preferred for the atomization used in the spray-drying of protein pharmaceuticals. Also, other types of nozzles have been applied with varying degrees of success, which are presented in the next section.
6.1.1. Internal mixing twin-fluid nozzles
During gas-liquid atomization (hence, “twin-fluid”) the mixing of the two phases can occur in various ways. In internal mixing twin-fluid nozzles, the liquid and gas phases are mixed in a chamber inside the device before being discharged.[Citation163] By default, most laboratory scale and some larger scale (e.g. Büchi B-290, B-190, B-191, ProCept R&D, Anydro MS-35, Anhydro MS-150, LabPlant SD, etc.) spray-dryers come equipped with these atomizers. Thus, internal mixing twin-fluid atomization is the most commonly used in protein spray-drying. In this type of atomization, the liquid is injected directly into a high velocity gas stream inside the nozzle. Once both phases are discharged through and meet at the nozzle, the constriction leads to the breakage of the jet by the venturi principle.[Citation183] Using water and air, the size of the generated droplets is mainly dependent on the air-to-liquid ratio (ALR) and air pressure,[Citation182,Citation183] i.e. higher ALR and air pressures form smaller droplets. The high surface to volume of smaller droplets might be problematic as larger air-liquid interfaces might lead to higher aggregation of protein-based products.[Citation184] Indeed, this was observed when aqueous solutions of lysozyme were spray-dried, and the collection of finer fractions of the powders revealed higher loss of protein activity.[Citation55] Besides some isolated cases, the damage during atomization from a twin-fluid nozzle is relatively minimal or at maximum comparable to that from other steps, such as pumping, evaporation, and collection.[Citation70] To ameliorate such issues, especially when generating fine particles (like in inhaled formulations of 1–5 µm), surface active excipients could be used. In cases where oxidatively susceptible agents are to be spray-dried, inert drying gases such as nitrogen and argon could be used. However, so far, no direct comparison with air was made in terms of their differing effects at the interfacial level.
6.1.2. High-pressure effervescent twin-fluid nozzle
The high-pressure effervescent twin-fluid nozzle is an adoption from the diesel industry.[Citation185] In effervescent twin-fluid atomization, the gas is also mixed with the liquid before discharge. However, this nozzle design leads to the formation of bubbles within the bulk of the liquid. Likewise, when the feed is discharged the bubbles will squeeze the liquid, breaking it into fine droplets.[Citation183,Citation185] Here also, the droplet size depends on the hydrodynamic properties of the feed liquid, gas to liquid ratio, and gas pressure.[Citation186] This type of nozzle generates much smaller droplets (mean droplet diameter < 30 µm). For protein pharmaceuticals, the high-pressure effervescent nozzle has been successfully applied in combination with a Büchi B-190 to produce formulations of a mAb[Citation25] and a vaccine[Citation126] with sucrose. Likewise, high-pressure effervescent atomization could be of advantage when aiming to produce powders with a smaller particle size, such as for inhalation. Again, the magnitude of local shear and the relative sensitivity of the involved protein to the shear-induced denaturation should be prudently assessed.
6.1.3. Tri-fluid nozzle
The design of tri-fluid nozzles allows the concomitant atomization of two different liquids (instead of one like in twin-fluid atomization). The two liquids are fed into the nozzle, mixed with a gas and then atomized.[Citation187] Likewise, it is possible to spray-dry incompatible materials and/or more easily promote the in-situ coating of active material with an excipient.[Citation187] Evidently, this type of nozzle has been used to produce PLGA coated microparticles of therapeutic proteins such as BSA[Citation114] and lysozyme.[Citation52] As PLGA is water insoluble, the use of a tri-fluid nozzle where the organic phase containing PLGA can be separately spray-dried from the aqueous solution containing the biomolecule, is of great advantage (preventing any potential organic solvent-induced protein denaturation). Also, here, the droplet size is impacted by the gas to liquid ratio. However, for tri-fluid nozzles the feeding ratio between the two liquids also has to be considered in order to guarantee adequate engineering of the particle surface. For example, for the lysozyme microparticles it was found that, when increasing the feeding ratio from the outer to the inner solution in the 4:1 to 10:1 range, the enzymatic surface enrichment diminished.[Citation52]
6.1.4. Rotatory atomization nozzle
Rotatory atomization uses centrifugal energy from the high-speed rotation (10,000 to 50,000 rpm) of a disk or wheel to atomize a feed liquid stream.[Citation188] The feed is supplied to the center of the disk and forced to the brim where it is rapidly disintegrated into droplets.[Citation189] Although, rotatory nozzles present the most efficient mode of atomization in spray-drying, its larger spray cone is best accommodated in large-diameter drying chambers. Thus, these types of atomizers are mostly applied at larger scale (e.g. pilot-to-manufacturing). The use of rotatory atomizers in narrower towers will produce large wall deposits leading to significant product losses and thus, are generally not recommended at smaller scales.[Citation188,Citation189] Rotatory atomization nozzles have been used at larger scales to evaluate the drying behavior of alcohol dehydrogenase encapsulated in trehalose matrices[Citation75] and to successfully produce microspheres of Actinobacillus pleuropneumoniae antigens intended for oral vaccination.[Citation124]
6.1.5. Low-pressure ultrasonic nozzle
These types of nozzle use a hybrid principle between ultrasonic and twin-fluid atomization. The liquid is mixed with a pressurized gas inside a nozzle and its tip vibrates at a high frequency.[Citation190] Likewise, the liquid feed can be atomized into very fine droplets (e.g. mass mean diameter < 7 µm) at low pressures (< 3.5 bar).[Citation132,Citation190] Besides the physicochemical properties of the liquid and feed rate, in these devices, the droplet diameter will be mainly impacted by the amplitude and frequency of vibration. Naturally, higher amplitude and frequencies will generate droplets of a smaller size.[Citation190] By using this form of atomization in combination with a Büchi B-190, Ohtake et al. have successfully produced a heat-stable measles vaccine via spray-drying.[Citation132]
6.1.6. Ultrasonic nozzle
In ultrasonic nozzles, atomization is achieved by the use of high-frequency sound waves generated piezoelectrically.[Citation188] The vibrations are transferred and amplified by a titanium nozzle tip.[Citation189] Likewise, a vibration at the nozzle tip aids the liquid atomization. Compared to twin-fluid nozzles, ultrasonic atomizers generate larger droplets with a narrower size distribution. Beside the physiochemical properties of the liquid, the droplet size will be controlled by the liquid feed rate as well as the amplitude and frequency of vibration.[Citation188] In the presence of disaccharides, spray-drying of catalase with an ultrasonic nozzle, led to higher loss of enzymatic activity than when a twin-fluid device was used. The authors hypothesized that this was probably due to the heating of the ultrasonic nozzle, that contrary to the twin-fluid one, was not cooled with ambient atomizing air. Similar observations were also reported by Ziaee et al., when spray-drying aqueous solutions of lysozyme.[Citation59] Moreover, in both works it was also observed that, when compared to twin-fluid atomization, the ultrasonic one led to powders with a higher water content. This could pose a potential problem regarding long-term stability. Lastly, ultrasonic atomization could also present a challenge in terms of scale-up, considering that a maximum feed rate of 50 ml/min can be employed when using these devices.[Citation188]
6.1.7. Vibrating mesh
The vibrating mesh technology is a special adaptation made by Büchi from nebulizer aerosol delivery in order to generate nano-sized droplets with their B-90 equipment. In this, a liquid is fed through a mesh that vibrates due to the high-frequency of a piezoelectric piece. The fast up and downward movement of the mesh breaks the liquid jet into millions of precisely sized droplets that are then injected into the dryer.[Citation191] The droplet size will depend on the mesh size and the hydrodynamic properties of the liquid. When comparing the spray-drying of l-lactic dehydrogenase and trehalose using twin-fluid and vibrating mesh nozzles, Grasmeijer et al. observed that the latter device led to higher enzymatic losses. The authors then showed that this was mainly due to the heating of the nozzle during spray-drying. Other parameters, i.e. atomization, re-circulation, evaporation and collection also led to enzymatic loss, but to a lower extent.[Citation70] In another study, the generated heat by the vibrating mesh was associated with the gelling of mAb solutions.[Citation34] When spray-drying bacteriophages, the vibrating mesh atomization had distinct effects on the loss of activity of the bioactive material depending of the type of phage and excipients being applied.[Citation40] Lastly, scale-up of this type of atomization could also be a challenge considering the lower feed rates used in the Büchi B-90.[Citation34]
6.1.8. Mono-disperse droplet generator
The mono-disperse droplet generator (MDG) technology has been adopted from ink-jet printing.[Citation192] In MDG nozzles, the jet break-up is a consequence of the hydrodynamic instabilities within the liquid being fed.[Citation193] In MDGs, droplets can be generated using various different forces originating from distinct types of nozzles, i.e. hydrodynamic, electro-hydrodynamic, and mechano-hydrodynamic.[Citation192]
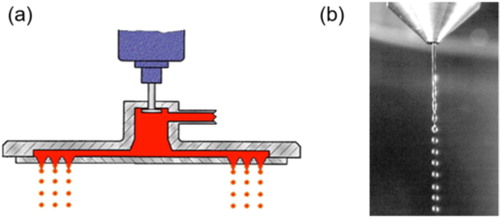
Among said equipment the DROPPO® generator from Maag Germany GmbH allows the generation of uniform spherical particles with a narrow size distribution by the application of a die-head where the liquid is subjected to harmonic vibration (). The liquid flow, escaping from the die holes which are arranged in concentric circles, will break into small droplets. The size of the droplets will be determined by the diameter of the die holes and by the pressure applied to the jet in the distribution chamber. To our knowledge for the spray-drying of protein pharmaceuticals, only one other MDG has been tested, so far. In this device, a piezoelectric piece pre-set at a given frequency, induced vibrations into the liquid stream, breaking it into droplets.[Citation192] As a result of the low-frequency employed, when a MDG nozzle was used in combination with a ProCept R&D spray-dryer, large mono-disperse flowable (mean particle size ≈ 50 µm) particles of lactose and catalase were produced.[Citation51] However, the authors did not report the enzymatic activity after drying. Moreover, the yield was also quite low due to the incomplete drying arising from droplet collision and coalescence, which was observed to begin around 40 cm below the nozzle orifice.[Citation51]
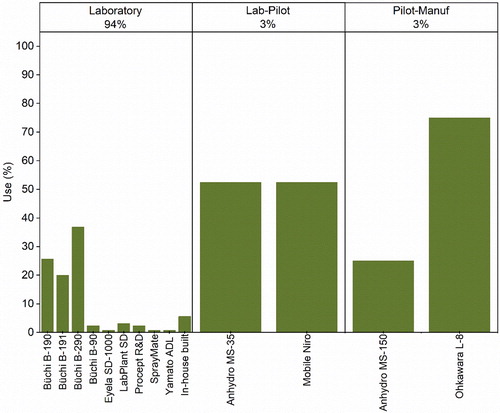
6.2. Spray-drying scales
In literature included here, the major works describing the spray-drying of protein pharmaceuticals () have been carried out using laboratory scale spray-dryers (94%). Even though, spray-drying at smaller scales might not entirely mimic larger ones, as pilot-manufacturing equipment normally presents higher evaporation capacities and throughput, laboratory scale spray-drying can still be highly valuable. Specifically, laboratory scale spray-drying allows to investigate the processability of protype formulations using small quantities of material, this being particularly, useful for high value pharmaceuticals like proteins.[Citation188] Moreover, contrary to larger scale where stainless steel equipment is normally used, at the laboratory level borosilicate glass is usually preferred, allowing the privileged observation of the drying process.[Citation188] Thus, during the early stages of research and development, laboratory scale equipment is normally preferred. Nonetheless, it is important to understand if the processes developed at smaller scales are transferable to larger ones. Obviously, the commercialization of products such as Exubera®, Trelstar® LA, Somatuline® LA, and Raplixa®, as well as other additional evidence in literature show that dry powder inhalers, wound therapies, vaccines[Citation124,Citation144] and injectables[Citation34,Citation36,Citation82] of proteins can be successfully produced at larger scales. A potential difference between spray-dryers across scales, due to differences in equipment and throughput, is the particle size of the obtained powders. At smaller scales, most often powders present a size between 3 and 10 µm, at larger ones particle sizes between 30 and 200 µm are usually achieved.[Citation188] At greater production scales, the larger dimensions of the drying towers allow the droplets to reside more time inside the dryer, leading to larger particle sizes and powders with a lower moisture content.[Citation34,Citation188] This could be the reason why it has been reported that for mAb formulations, larger scale spray-drying was more efficient than using benchtop Büchi equipment.[Citation34] For example, production of formulations with a larger particle size and lower moisture could potentially have led to powders that have less tendency to stick to the spray-dryer and be able to be collected more efficiently. In contrast, laboratory scale spray-dryers like the ProCepT R&D, where a larger drying tower (allowing larger residence times) and less turbulent airflow are used, also enable the generation and drying of larger droplets sizes, better mimicking larger scales.[Citation188] Other approaches looking to better mimic larger scale processing, have also involved the customization of laboratory Büchi scale dryers. Using Büchi B-190, Maa et al., were able to efficiently reduce the drying air flow resistance, by replacing the bag filter unit for a vacuum system that allowed to reduce the inlet air temperature to dry protein products at small scale.[Citation22] Although, evidence seems to indicate that Büchi spray-dryers might not be able to entirely mimic larger scale processes, they have the advantage of a long tradition of use in pharmaceutical development (including the knowledge necessary for their translation to larger scales).[Citation34]
Another important feature to consider across scales is the particle separation from the gas phase. For this and across scales, cyclones are usually preferred. The solid particles are separated using centrifugal forces created by a fast-rotational motion.[Citation189] All the equipment identified, with the exception of the Büchi B-90, use cyclones to separate the solid particles from the gas flow. Given that the Büchi B-90 is designed to generate particles in the nano-sized range and traditional cyclone separators are unable to collect particles below 2 µm, an electrostatic collector is used instead.[Citation191] Although the B-90 is primarily used to produce nano-sized particles, this has also been employed to produce microparticles of protein therapeutics.[Citation34,Citation40,Citation70] However, its potential complex scale-up must be considered when selecting it in the early stages of development. Returning to cyclone based separation, it has been reported that in lab-scale dryers this is less efficient than at a larger scale.[Citation22,Citation34] Likewise, in an effort to improve collection at small scale, a study showed that although the use of different cyclone designs in a Büchi B-190 led to an increase in production without using higher air inlet temperatures, it did not improve the overall product recovery.[Citation22] The same authors have suggested that in order to collect particles below 2 µm and those in a larger range, the combined use of electrostatic and cyclone separation principles could merit a more in-depth investigation. However, in another study the use of a smaller dimensioned cyclone design with Büchi B-290 has shown a higher level of inactivation of lysozyme (in aqueous solution) when the smaller separator was used.[Citation55] Hence, it begs the question if extensive efforts should be employed in improving the collection of the finer particles produced by laboratory scale spray-dryers, when it is expected that at larger scales, greater particle sizes will be obtained. A more in-depth look on the strategies employed to scale-up spray-drying processes is outside the scope of this review and, can be found elsewhere.[Citation188,Citation194]
6.3. Process-related parameters
In spray-drying, protein drugs experience a number of thermal, interfacial, and mechanical stresses at the various stages of the journey between the bulk liquid and dried particles. During drying, the temperature of the atomized feedstock rapidly rises, reaching the point where the surface of the droplets maintains 100% RH in the system (Twb). The drybulb temperature (Tdb) is reached after evaporation, when the particle temperature is close to Tout.[Citation164] Throughout the reviewed studies, it was observed that the inlet temperatures range from 45 to 225 °C. Using EquationEq. (2)(2)
(2) and assuming a water boiling temperature of 100 °C and that the drying gas used is air, a maximum Twb could be in the range of 25–51 °C within the aforementioned range. Likewise, the highest temperature that protein formulations will experience during the process will be the Tout and therefore, it is no surprise, that this has been reported as a critical process parameter.[Citation49,Citation59,Citation195,Citation196] Hence, while residing in the collection chamber, it is important to guarantee that protein powders do not experience an elevated Tout as this might lead to their degradation over time, especially during longer process times.[Citation197] When spray-drying protein formulations, amorphous powders are normally obtained, thus the relation between the Tg and Tout must also be considered. It is usually accepted that the higher the Tg – Tout, the lesser the powder will be sticking, resulting in an improvement of the recovery. Incomplete drying, leading to an excess in moisture could also yield powders with Tg values (e.g. below room temperature) that might have a detrimental effect on the long-term stability of bio-formulations. Maltesen et al., for example, deemed the moisture content a critical quality attribute (CQA) when spray-drying inhalable insulin powders.[Citation198] Considering that the water content of spray-dried protein formulations is affected by the residence time of the gas in the dryer and the Tout,[Citation34] we further emphasize the importance of optimizing the Tout. However, it must be considered that the Tout will be dependent on the liquid feed rate and concentration as well as the gas airflow rate, relative humidity (RH) and atomization principle. Concerning the gas airflow, when this is given, values between 100 and 1600 l/h have been found across the scales that we reviewed. For vaccine materials, for example, besides the gas inlet temperature (and consequent Tout), also the gas flow rate was found to be a critical parameter to conserve viral vector activity.[Citation135] For the spray-drying of mAbs, the airflow rate was not so critical, but it was found that besides the atomization principle, the liquid feed concentration and rate can also impact particle size distribution, impacting the bulk to surface solute distribution, affecting powder performance (i.e. stability, reconstitution).[Citation32] This spray-drying process (parameter) complexity and the sensitive nature of protein drugs certainly warrants the need of further rigorous research, including a more in-depth mechanistic description of the phenomena critically affecting the quality of bio-powders.
7. Quality aspects of spray-drying of protein pharmaceuticals
During spray-drying of protein pharmaceuticals, one has to guarantee their stability, safety, efficacy, and purity/variability. The molecular, particulate, bulk, and surface properties governing the quality, performance and stability of lyophilized protein formulations and the various analytical tools applicable to this end are extensively reviewed elsewhere.[Citation199] Most of the analysis and the intrinsic and derived properties discussed therein are also equally valid for spray-dried protein products. The physical (change in solid-state, powder properties, reconstitution and wetting behavior, reversible aggregation, etc.) and chemical (protein degradation, irreversible aggregation) stability of protein formulations are affected by temperature, moisture content, mechanical perturbation, excipients, and the physical state of the formulation (e.g. amorphous vs. crystalline).[Citation200,Citation201] Eon-Duval et al. investigated the quality attributes of protein formulations and classify them into: (1) product‐related impurities and substances, (2) process‐related impurities, product attributes, and (3) contaminants. Their impact on biological activity, pharmacokinetics and pharmacodynamics, immunogenicity, and the overall safety/toxicity was evaluated as well establishing the link between the quality attributes of a new recombinant protein product and its clinical efficacy and patient safety.[Citation202] Yet another study of Eon-Duval et al. presented a detailed study on the application of quality by design (QbD) principles to the drug substance manufacturing process of a fragment crystallizable (Fc) fusion protein. Here, the CQA were classified in four main categories namely, (1) product-related impurities and substances, (2) process-related impurities, (3) contaminants, and (4) other product attributes. After defining the quality attributes and process parameters, their impacts on CQAs were evaluated using a multivariate design of experiments (DoE) during the process characterization phase. A global multi-step design space was established, defining operational limits for the entire drug substance manufacturing process to ensure that the drug substance quality targets were met, by using predictive statistical models developed during the characterization and then confirmed by performing the entire process, from cell bank thawing to final drug substance, at its limits during the robustness.[Citation203] Important studies like the ones from Eon-Duval et al., are sparsely found and, so far, it appears that our understanding between quality attributes and clinical outcomes, especially for formulations of biologics prepared via advancing technologies like spray-drying, is far from being complete. However, some studies in protein spray-drying literature have taken a much needed first step, in furthering the knowledge on how critical quality attributes can be identified, monitored, and controlled.
Schaefer and Lee have demonstrated that by employing empirical models, the activation energy of critical chemical interactions contributing to enzymatic degradation could be predicted.[Citation51,Citation64] The authors studied the inactivation of catalase during spray-drying over a range of outlet temperatures and represented them in the Arrhenius equation. From this, a minimum activation energy for the damage of catalase was calculated and the onset temperature for the thermally induced degradation of the enzyme determined.[Citation64] In another study, by using the combination of a DoE and forced deactivation studies, Ziaee et al. have demonstrated that some enzymes, like lysozyme, do not follow the Arrhenius relationship of the temperature dependence. Thus, the authors suggested that, the combined use of a DoE with kinetic modeling, would be a more efficient approach, when determining the critical formulation and process factors impacting the quality of spray-dried protein pharmaceuticals.[Citation59]
On another study, focusing on understanding the solid-state properties of a spray-dried peptide hormone, insulin, a complex interaction between the peptide structure, excipient and process parameters was identified using a DoE and multivariate data analysis. It was shown that the insulin concentration was the most important parameter, followed by inlet drying air temperature and the atomization gas flow rate. The insulin concentration mainly affected the particle size, yield and tapped density of the powder, while the inlet drying air temperature mainly affected the moisture content. No change was observed in physical and chemical integrity of insulin, however the moisture content of the powder was found to be the CQA in relation to the storage stability of the formulation.[Citation198]
Employing a DoE, Batens et al. developed several statistical models describing the influence of process and formulation parameters on the product characteristics of a spray-dried mAb formulation. The Tin, concentration of the mAb and nozzle diameter were shown to influence the droplet size and solute diffusion during drying, impacting powder properties.[Citation32] By applying Plackett–Burman factorial DoE, Ramezani et al. also investigated the stability and particle properties of mAb formulations obtained via spray-drying. The authors showed the critical effect that the atomization airflow and the type and ratio of saccharide used on the formulation had on powder stability.[Citation29]
Concerning the spray-drying of vaccines, systematic investigations into formulation and process parameters, showed that the use of excipients able to immobilize viral vectors was critical in preventing their unfolding, aggregation, and inactivation.[Citation131] The same authors subsequently examined the relevance of viral activity in the optimization of spray-drying process parameters for the development of thermally stable vaccine powders. By the use of a DoE with a response surface methodology (RSM), it was found that, in general, good conditions for maintaining viral activity led to reduced yield and fewer particles of the desired size.[Citation130] Moreover, the results also showed that the most significant spray-drying parameters were the Tin and atomization gas flow rate.
In , we present an overview of the factors affecting the spray-drying of protein pharmaceuticals. However, we highlight that the relative importance of each will depend on the CQAs for the intended product application, i.e. a formulation for inhalation will have distinct CQAs than the one for injectables.
8. Perspective and future considerations
With biopharmaceuticals holding prominent shares on the approved, marketed, and current clinical developments, there is a tremendous need to intensify and accelerate manufacturing technologies to expedite the product development process. The isolation, formulation, and product manufacturing steps of these pharmacologically advantageous biologics are complex and challenging, partly due to their inherent sensitivity to a variety of (in-process) stress factors.[Citation204] From a product perspective, sterile liquid formulations and freeze-dried powders to be reconstituted for injection are the common norm for these types of drugs. Therefore, therapeutic modalities like protein, peptide, enzymes, antibodies, and various forms of vaccines require cold storage and supply chain (cold chain) to preserve their integrity. The solid formulations containing dehydrated proteins stabilized by embedding them into glassy matrices of sugar/polymer or encapsulating lipids are more stable than their liquid counterparts and, thus provide an advantage, when considering the complex logistics of global distribution. While protein formulations are often transformed to solid-state by freeze-drying, one of the limitations is that it takes several days to convert the solution into a solid powder cake. In situations, where accelerated manufacturing and supply are needed (e.g. pandemic like COVID-19), the one-step transformation of liquid formulations to particles through atomization and rapid evaporation in spray-drying can offer an enormous advantage. Spray-drying, an inherently continuous process, can adapt production capacity to product demand, allowing to dramatically reduce the lead time, effort and energy consumption of drug powder production, thereby resulting in both an economic and health benefit. Also, the process can be further advanced to be operated and controlled with minimal manual intervention. Freeze-drying, does not offer the same possibility, and being a multi-step batch process with inefficient and uneven heat and mass transfer, often raises concerns in terms of vial-to-vial variability and inhomogeneous quality of the dried products. To this end, the application of innovative process analytical technologies (PAT) in spray-drying, i.e. in-line monitoring of droplet size distribution, moisture content, particle size distribution, etc. can enable real time quality monitoring of the intermediate and final products, potentially avoiding large laboratory testing efforts. Also, continuous recording of process-related data using soft sensors for RH, temperature, pressure, viscosity, etc. can bring a great advantage. Besides analytical and hardware advancements, heat and mass transfer modeling of the spray-drying process using computational fluid dynamics (CFD), various drying kinetics and flow sheet engineering models are state-of-the art and their use holds a great potential for the further enhancement of the protein spray-drying field. Overall, the combination of PAT and mechanistic modeling can add a great incentive to widen the industrial implementation of protein pharmaceuticals via spray-drying.
Although relatively new to the pharmaceutical protein drying industry and limited in number of products approved, contemporary research suggests that the use of spray-drying is rapidly progressing. The main downfall of spray-drying tends to be the high processing temperatures/shear forces used, which certainly pose a risk, when processing thermolabile/shear sensitive biologics. When high drying temperatures can lead to degradation, the use of non-aqueous volatile solvents in the feed solution could provide an opportunity to significantly reduce the drying time and temperature and thus, avoid product degradation. Also, in the case of oxidation sensitive products, the use of an inert gas is possible and can avoid any detrimental effects caused by the use of air. One of the recurring concerns regarding spray-dried protein formulations is that the moisture content of the final product is slightly higher than the one typically achieved through freeze-drying. Thus, further secondary drying via vacuum dehydration might be necessary. The impact of secondary drying to the formulation microstructure, mechanical relaxation of the powders and protein integrity and eventual reconstitution behavior is important to understand and deserves a thorough scientific investigation in future. Given that sufficient scientific bases and a rational workflow for the rapid design, optimization and production of protein drug products are established, spray-drying opens up the possibility to tighter process control (employing PAT) and offers a wider array of opportunities in terms of particle and powder engineering.
One of the immense challenges, when applying spray-drying to the production of sterile protein product manufacturing, is establishing and maintaining an aseptic operation. While limited number of spray-drying companies and contract manufacturers offer nowadays the technology and services for aseptic spray-drying, its wider adoption for diverse parenteral manufacturing is prohibitively expensive. In this context, the development of a partially sterile spray-drying process could be a viable alternative. In this, processing would be executed under conditions that could guarantee low bioburden of the final product. Under conditions, which are not fully aseptic, yet provide dosage forms that meet the desired quality criteria, the aforementioned could provide an overall high benefit-to-cost ratio, when manufacturing sterile protein drug products. Downstream processing of spray-dried powders intended for reconstitution include: handling, transport, and metering into vials, followed by stoppering. Automated powder filling into vials is based on a wide variety of principles dependent upon the filling machine types, i.e. dosator nozzle-based, tamp, pneumatic, vacuum, etc. Design, development and application of an optimized vial filling process are critical steps for mitigating the variability of product quality namely, fill weight variability, agglomeration, moisture content, re-dispersity, and stability. Dry powder filling is a complex process and the filling dose accuracy depends upon the rational selection of filling principle and process parameters with respect to the specific powder properties such as bulk density, flowability, stickiness, electrostatics, etc. Also types of vials, siliconized versus normal ones, and their surface properties can impact of powder emptying or wetting during reconstitution and thus, require a due research. While pharmaceutical protein spray-drying literature is emerging rapidly, surprisingly, there is a scarcity of studies dealing with the automated vial filling of these products. This could be due to the limited availability of small-scale filling devices. Thus, the success of spray-drying in biopharmaceutical industries will need an integrated mindset where a holistic approach to both spray-drying and filling is adopted.
The fundamental knowledge concerning the formulation principles behind the development of spray-dried protein biopharmaceuticals are far behind the ones available for freeze-dried products. While the mechanism of cryo-protection and lyo-protection of protein structure during freeze-drying by some common sugar and amino acid excipients are recently unraveled to some extent, similar mechanistic studies encompassing the spray-drying stress (higher temperature, increase interfacial area of droplets, and shear forces) are largely missing. Very recent studies using (levitated or sessile) single droplets of some food and pharmaceutical proteins, present encouraging results on particle morphology formation and drying/diffusion kinetics.[Citation205–207] Further availability of such data from a wide range of protein and excipient types will build the foundation for developing universal mechanisms that can potentially help deriving spray-drying models that are truly predictive of product quality. The excipients used during spray-drying need, essentially to stabilize the biopharmaceutical conformations and be suitable to be utilized during processing (e.g. Tg of excipient ≫ Tout). At the same time, these excipients should also help fulfill product CQAs, e.g. proper aerosolization performance, rapid reconstitution, etc. Most of the reported studies simply chose and utilize the freeze-drying excipients in the spray-drying process and are limited to a few sets of saccharides. With a wide diversity of physical and chemical properties of protein biopharmaceuticals, one cannot simply assume that these few excipients provide the best stabilization of spray-dried products. Therefore, we stress here the importance of further fundamental research on the formulation’s aspects impacting protein spray-drying, rather than basing excipient selection on assumptions taken from freeze-drying knowledge. Other than excipients, the extent of in-process protein aggregation can also impact the final particle morphology of the spray-dried powders. This is especially important to consider when proteins are used as excipients for other biomolecules. Likewise, there is a greater need for the identification of the factors influencing the choice of a certain type of pH modifiers and buffering agents over others for the spray-drying of a given protein. Not only this will impact the solute diffusion and drying processes, it could also change local pH and micro-viscosity during particle formation. Therefore, temperature dependence of pH, pKa, and rheological behavior of feeds containing varying salts, need a more in-depth research. Also, besides some isolated published cases, the scientific guidance to organic solvent selection for protein spray-drying is still currently missing. Like process models, any predictive modeling, using molecular dynamics simulation, thermodynamic models applicable to formulation design and describing the phase dynamics relevant to the spray-drying process would be highly valuable.[Citation208] Protein therapeutics are in general expensive, thus very less data is published on real world samples (that include in-vivo studies of spray-dried protein products). Therefore, workflows and standardized protocols aiding the prediction of processing and formulation performance using none to less amount of protein would also be immensely advantageous. Finally, and on the top of a sound scientific basis, one has to consider the long-term techno-economic impact of selecting a certain excipient (e.g. the cost of sucrose versus trehalose) and solvent (drying and recovery) for the production of protein pharmaceuticals via spray-drying.
At the end, we present below a generic analysis representing the strengths, weaknesses, opportunities and threats (SWOT) associated with the spray-drying of protein pharmaceuticals (). Overall, the perspective presented above and SWOT analysis can also be extended to other emerging pharmaceutical protein drying technologies associated to spray-drying, such as electro-spray-drying, spray-freeze-drying, and supercritical fluid technology.
Conflict of interest
All authors declare the following interests: MD is a permanent employee of the Baxter AG (part of Takeda); CM and MN are employees of the Maag Germany GmbH; JTP, EF, JD and AP are employees of the Research Center Pharmaceutical Engineering GmbH, which received funding for their work from Baxter AG (part of Takeda) and Maag Germany GmbH.
| Abbreviations | ||
| ALR | = | Air-to-liquid ratio |
| API | = | Active pharmaceutical ingredient |
| BCG | = | Bacillus Calmette-Guérin |
| bFGF | = | Fibroblast growth factor |
| BSA | = | Bovine serum albumin |
| CFD | = | Computational fluid dynamics |
| COPD | = | Chronic obstructive pulmonary disease |
| COVID-19 | = | Coronavirus disease 2019 |
| CQA | = | Critical quality attribute |
| DoE | = | Design of experiments |
| DP | = | Degree of polymerization |
| DPPC | = | Dipalmitoyl phosphatidylcholine |
| DPT | = | Diphtheria, pertussis and tetanus |
| Fc | = | Fragment crystallizable |
| HBS | = | HEPES buffered saline |
| HBV | = | Hepatitis B virus |
| HiB | = | Haemophilus influenzae serotype b |
| HPC | = | Hydroxypropyl cellulose |
| HPMC | = | Hydroxypropyl methylcellulose |
| HPMCAS | = | Hydroxypropyl methylcellulose-acetate succinate |
| HPV | = | Human papilloma virus |
| HPβCD | = | (2-Hydroxypropyl)-β-cyclodextrin |
| IgG | = | Immunoglobulin G |
| IM | = | Intramuscular |
| mAb | = | Monoclonal antibody |
| MDG | = | Mono-dispersed droplet generator |
| PAT | = | Process analytical technologies |
| PBS | = | Phosphate-buffered saline |
| PEG | = | Polyethylene glycol |
| PEO | = | Poly(ethylene oxide) |
| PLGA | = | Poly(lactic-co-glycolic acid) |
| PSD | = | Particle size distribution |
| PVA | = | Polyvinyl alcohol |
| PVP | = | Polyvinylpyrrolidone |
| PVP-VA | = | Poly(vinylpyrrolidone-co-vinyl acetate) |
| QbD | = | Quality by design |
| RH | = | Relative humidity; RhDNase I = Recombinant human DNase I |
| DNase I | = | Deoxyribonuclease I |
| RSM | = | Response surface methodology |
| SDS | = | Sodium dodecyl sulfate |
| SWOT | = | Strengths, weaknesses, opportunities and threats |
| U.S. FDA | = | United States Food and Drug Administration |
| Nomenclature | ||
| ρg | = | particle density |
| CF | = | feed concentration |
| dD | = | droplet diameter |
| dg | = | geometric diameter of solid particles |
| Di | = | diffusion of solute i |
| Ei | = | surface enrichment of solute i |
| k | = | evaporation rate |
| Pei | = | Peclet number of solute i |
| Tb | = | boiling temperature |
| Tdb | = | drybulb temperature |
| Tg | = | glass transition temperature |
| Tin | = | inlet gas temperature |
| Tout | = | outlet gas temperature |
| Twb | = | wet-bulb temperature |
Additional information
Funding
References
- McAndrew, P. T.; Hostetler, D.; DeGrazio, F. Container and Reconstitution Systems for Lyophilized Drug Products. In Lyophilization of Pharmaceuticals and Biologicals: New Technologies and Approaches; Ward, K. R., Matejtschuk, P., Eds.; Humana Press: Totowa, NJ, 2019; pp 193–214.
- DiFranco, N. Lyophilization of Pharmaceuticals: An Overview. https://lubrizolcdmo.com/blog/lyophilization-of-pharmaceuticals-an-overview/ (accessed Sep 16, 2020).
- Comission, E. Study on the Competitiveness of the European Biotechnology Industry – The Financing of Biopharmaceutical Product Development in Europe, 2010. DOI: 10.2769/33524.
- Emami, F.; Vatanara, A.; Park, E. J.; Na, D. H. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics. 2018, 10, 131. DOI: 10.3390/pharmaceutics10030131.
- Vehring, R.; Snyder, H.; Lechuga-Ballesteros, D. Spray Drying. In Drying Technologies for Biotechnology and Pharmaceutical Applications; Ohtake, S., Izutsu, K., Lechuga-Ballesteros, D., Eds.; Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2020; pp 179–166. DOI: 10.1002/9783527802104.ch7.
- Cicerone, M. T.; Pikal, M. J.; Qian, K. K. Stabilization of Proteins in Solid Form. Adv. Drug Deliv. Rev. 2015, 93, 14–24. DOI: 10.1016/j.addr.2015.05.006.
- Ameri, M.; Maa, Y. F. Spray Drying of Biopharmaceuticals: Stability and Process Considerations. Dry. Technol. 2006, 24, 763–768. DOI: 10.1080/03602550600685275.
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic Antibodies: Successes, Limitations and Hopes for the Future. Br. J. Pharmacol. 2009, 157, 220–223. DOI: 10.1111/j.1476-5381.2009.00190.x.
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. DOI: 10.1038/256495a0.
- Lu, R. M.; Hwang, Y. C.; Liu, I. J.; Lee, C. C.; Tsai, H. Z.; Li, H. J.; Wu, H. C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1. DOI: 10.1186/s12929-019-0592-z.
- Schüle, S.; Schulz-Fademrecht, T.; Garidel, P.; Bechtold-Peters, K.; Frieb, W. Stabilization of IgG1 in Spray-Dried Powders for Inhalation. Eur. J. Pharm. Biopharm. 2008, 69, 793–807. DOI: 10.1016/j.ejpb.2008.02.010.
- Ramezani, V.; Vatanara, A.; Najafabadi, A. R.; Shokrgozar, M. A.; Khabiri, A.; Seyedabadi, M. A. A Comparative Study on the Physicochemical and Biological Stability of IgG1 and Monoclonal Antibodies During Spray Drying Process. Daru 2014, 22, 31. DOI: 10.1186/2008-2231-22-31.
- Schüle, S.; Friess, W.; Bechtold-Peters, K.; Garidel, P. Conformational Analysis of Protein Secondary Structure during Spray-Drying of Antibody/Mannitol Formulations. Eur. J. Pharm. Biopharm. 2007, 65, 1–9. DOI: 10.1016/j.ejpb.2006.08.014.
- Nayak, P. K.; Goode, M.; Chang, D. P.; Rajagopal, K. Ectoine and Hydroxyectoine Stabilize Antibodies in Spray-Dried Formulations at Elevated Temperature and during a Freeze/Thaw Process. Mol. Pharm. 2020, 17, 3291–3297. DOI: 10.1021/acs.molpharmaceut.0c00395.
- Ramezani, V.; Vatanara, A.; Seyedabadi, M.; Nabi Meibodi, M.; Fanaei, H. Application of Cyclodextrins in Antibody Microparticles: Potentials for Antibody Protection in Spray Drying. Drug Dev. Ind. Pharm. 2017, 43, 1103–1111. DOI: 10.1080/03639045.2017.1293679.
- Faghihi, H.; Vatanara, A.; Najafabadi, A. R.; Ramezani, V.; Gilani, K. The Use of Amino Acids to Prepare Physically and Conformationally Stable Spray-Dried IgG with Enhanced Aerosol Performance. Int. J. Pharm. 2014, 466, 163–171. DOI: 10.1016/j.ijpharm.2014.03.020.
- Andya, J. D.; Maa, Y. F.; Costantino, H. R.; Nguyen, P. A.; Dasovich, N.; Sweeney, T. D.; Hsu, C. C.; Shire, S. J. The Effect of Formulation Excipients on Protein Stability and Aerosol Performance of Spray-Dried Powders of a Recombinant Humanized anti-IgE Monoclonal Antibody. Pharm. Res. 1999, 16, 350–358. DOI: 10.1023/A:1018805232453.
- Sane, S. U.; Wong, R.; Hsu, C. C. Raman Spectroscopic Characterization of Drying-Induced Structural Changes in a Therapeutic Antibody: Correlating Structural Changes with Long-Term Stability. J. Pharm. Sci. 2004, 93, 1005–1018. DOI: 10.1002/jps.20014.
- Deokar, V.; Sharma, A.; Mody, R.; Volety, S. M. Comparison of Strategies in Development and Manufacturing of Low Viscosity, Ultra-High Concentration Formulation for IgG1 Antibody. J. Pharm. Sci. 2020, 109, 3579–3589. DOI: 10.1016/j.xphs.2020.09.014.
- Dani, B.; Platz, R.; Tzannis, S. T. High Concentration Formulation Feasibility of Human Immunoglubulin G for Subcutaneous Administration. J. Pharm. Sci. 2007, 96, 1504–1517. DOI: 10.1002/jps.20508.
- Maa, Y. F.; Nguyen, P. A.; Andya, J. D.; Dasovich, N.; Sweeney, T. D.; Shire, S. J.; Hsu, C. C. Effect of Spray Drying and Subsequent Processing Conditions on Residual Moisture Content and Physical/Biochemical Stability of Protein Inhalation Powders. Pharm. Res. 1998, 15, 768–775. DOI: 10.1023/A:1011983322594.
- Maa, Y. F.; Nguyen, P. A.; Sit, K.; Hsu, C. C. Spray-Drying Performance of a Bench-Top Spray Dryer for Protein Aerosol Powder Preparation. Biotechnol. Bioeng. 1998, 60, 301–309. DOI: 10.1002/(SICI)1097-0290(19981105)60:3<301::AID-BIT5>3.0.CO;2-L.
- Massant, J.; Fleurime, S.; Batens, M.; Vanhaerents, H.; Van den Mooter, G. Formulating Monoclonal Antibodies as Powders for Reconstitution at High Concentration Using Spray-Drying: Trehalose/Amino Acid Combinations as Reconstitution Time Reducing and Stability Improving Formulations. Eur. J. Pharm. Biopharm. 2020, 156, 131–142. DOI: 10.1016/j.ejpb.2020.08.019.
- Maa, Y. F.; Costantino, H. R.; Nguyen, P. A.; Hsu, C. C. The Effect of Operating and Formulation Variables on the Morphology of Spray-Dried Protein Particles. Pharm. Dev. Technol. 1997, 2, 213–223. DOI: 10.3109/10837459709031441.
- Abdul-Fattah, A. M.; Truong-Le, V.; Yee, L.; Nguyen, L.; Kalonia, D. S.; Cicerone, M. T.; Pikal, M. J. Drying-Induced Variations in Physico-Chemical Properties of Amorphous Pharmaceuticals and Their Impact on Stability (I): Stability of a Monoclonal Antibody. J. Pharm. Sci. 2007, 96, 1983–2008. DOI: 10.1002/jps.20859.
- Koshari, S. H. S.; Ross, J. L.; Nayak, P. K.; Zarraga, I. E.; Rajagopal, K.; Wagner, N. J.; Lenhoff, A. M. Characterization of Protein-Excipient Microheterogeneity in Biopharmaceutical Solid-State Formulations by Confocal Fluorescence Microscopy. Mol. Pharm. 2017, 14, 546–553. DOI: 10.1021/acs.molpharmaceut.6b00940.
- Lassner, P.; Adler, M.; Lee, G. Formation of Insoluble Particulates in a Spray-Dried F(ab')(2) fragment. J. Pharm. Sci. 2014, 103, 1021–1031. DOI: 10.1002/jps.23891.
- Maa, Y. F.; Nguyen, P. A.; Sweeney, T.; Shire, S. J.; Hsu, C. Protein Inhalation Powders: Spray Drying vs Spray Freeze Drying. Pharm. Res. 1999, 16, 249–254. DOI: 10.1023/A:1018828425184.
- Ramezani, V.; Vatanara, A.; Rouholamini Najafabadi, A.; Gilani, K.; Nabi-Meybodi, M. Screening and Evaluation of Variables in the Formation of Antibody Particles by Spray Drying. Powder Technol. 2013, 233, 341–346. DOI: 10.1016/j.powtec.2012.07.038.
- Faghihi, H.; Najafabadi, A. R.; Vatanara, A. Optimization and Characterization of Spray-Dried IgG Formulations: A Design of Experiment Approach. Daru 2017, 25, 22. DOI: 10.1186/s40199-017-0187-8.
- Costantino, H. R.; Andya, J. D.; Nguyen, P. A.; Dasovich, N.; Sweeney, T. D.; Shire, S. J.; Hsu, C. C.; Maa, Y. F. Effect of Mannitol Crystallization on the Stability and Aerosol Performance of a Spray-Dried Pharmaceutical Protein, Recombinant Humanized anti-IgE monoclonal antibody. J. Pharm. Sci. 1998, 87, 1406–1411. DOI: 10.1021/js9800679.
- Batens, M.; Massant, J.; Teodorescu, B.; Van den Mooter, G. Formulating Monoclonal Antibodies as Powders for Reconstitution at High Concentration Using Spray Drying: Models and Pitfalls. Eur. J. Pharm. Biopharm. 2018, 127, 407–422. DOI: 10.1016/j.ejpb.2018.02.002.
- Maury, M.; Murphy, K.; Kumar, S.; Mauerer, A.; Lee, G. Spray-Drying of Proteins: Effects of Sorbitol and Trehalose on Aggregation and FT-IR Amide I Spectrum of an Immunoglobulin G. Eur. J. Pharm. Biopharm. 2005, 59, 251–261. DOI: 10.1016/j.ejpb.2004.07.010.
- Bowen, M.; Turok, R.; Maa, Y. F. Spray Drying of Monoclonal Antibodies: Investigating Powder-Based Biologic Drug Substance Bulk Storage. Dry Technol. 2013, 31, 1441–1450. DOI: 10.1080/07373937.2013.796968.
- Moussa, E. M.; Wilson, N. E.; Zhou, Q. T.; Singh, S. K.; Nema, S.; Topp, E. M. Effects of Drying Process on an IgG1 Monoclonal Antibody Using Solid-State Hydrogen Deuterium Exchange with Mass Spectrometric Analysis (SsHDX-MS). Pharm. Res. 2018, 35, 12. DOI: 10.1007/s11095-017-2318-9.
- Gikanga, B.; Turok, R.; Hui, A.; Bowen, M.; Stauch, O. B.; Maa, Y. F. Manufacturing of High-Concentration Monoclonal Antibody Formulations via Spray Drying-the Road to Manufacturing Scale. PDA J. Pharm. Sci. Technol. 2015, 69, 59–73. DOI: 10.5731/pdajpst.2015.01003.
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. DOI: 10.3389/fphar.2019.00513.
- Leung, S. S. Y.; Parumasivam, T.; Gao, F. G.; Carter, E. A.; Carrigy, N. B.; Vehring, R.; Finlay, W. H.; Morales, S.; Britton, W. J.; Kutter, E.; et al. Effects of Storage Conditions on the Stability of Spray Dried, Inhalable Bacteriophage Powders. Int. J. Pharm. 2017, 521, 141–149. DOI: 10.1016/j.ijpharm.2017.01.060.
- Leung, S. S. Y.; Parumasivam, T.; Gao, F. G.; Carrigy, N. B.; Vehring, R.; Finlay, W. H.; Morales, S.; Britton, W. J.; Kutter, E.; Chan, H.-K. Production of Inhalation Phage Powders Using Spray Freeze Drying and Spray Drying Techniques for Treatment of Respiratory Infections. Pharm. Res. 2016, 33, 1486–1496. DOI: 10.1007/s11095-016-1892-6.
- Matinkhoo, S.; Lynch, K. H.; Dennis, J. J.; Finlay, W. H.; Vehring, R. Spray-Dried Respirable Powders Containing Bacteriophages for the Treatment of Pulmonary Infections. J. Pharm. Sci. 2011, 100, 5197–5205. DOI: 10.1002/jps.22715.
- Kunamneni, A.; Ogaugwu, C.; Goli, D. Enzymes as Therapeutic Agents. In Enzymes in Human and Animal Nutrition; Nunes, C. S., Kumar, V., Eds.; Academic Press: Cambridge, MA, 2018; pp 301–312. DOI: 10.1016/B978-0-12-805419-2.00015-0.
- Baldo, B. A. Enzymes Approved for Human Therapy: Indications, Mechanisms and Adverse Effects. BioDrugs. 2015, 29, 31–55. DOI: 10.1007/s40259-015-0116-7.
- Bisswanger, H. Enzyme Assays. Perspect. Sci. 2014, 1, 41–55. DOI: 10.1016/j.pisc.2014.02.005.
- Millqvist-Fureby, A.; Malmsten, M.; Bergenståhl, B. Spray-Drying of Trypsin – Surface Characterisation and Activity Preservation. Int. J. Pharm. 1999, 188, 243–253. DOI: 10.1016/S0378-5173(99)00226-4.
- Branchu, S.; Forbes, R. T.; York, P.; Petrén, S.; Nyqvist, H.; Camber, O. Hydroxypropyl-Beta-Cyclodextrin Inhibits Spray-Drying-Induced Inactivation of Beta-Galactosidase. J. Pharm. Sci. 1999, 88, 905–911 DOI: 10.1021/js9804819.
- Broadhead, J.; Rouan, S. K. E.; Hau, I.; Rhodes, C. T. The Effect of Process and Formulation Variables on the Properties of Spray-Dried Beta-Galactosidase. J. Pharm. Pharmacol. 1994, 46, 458–467. DOI: 10.1111/j.2042-7158.1994.tb03828.x.
- Wilson, N. E.; Topp, E. M.; Zhou, Q. T. Effects of Drying Method and Excipient on Structure and Stability of Protein Solids Using Solid-State Hydrogen/Deuterium Exchange Mass Spectrometry (SsHDX-MS). Int. J. Pharm. 2019, 567, 118470. DOI: 10.1016/j.ijpharm.2019.118470.
- Lipiäinen, T.; Räikkönen, H.; Kolu, A. M.; Peltoniemi, M.; Juppo, A. Comparison of Melibiose and Trehalose as Stabilising Excipients for Spray-Dried β-Galactosidase Formulations. Int. J. Pharm. 2018, 543, 21–28. DOI: 10.1016/j.ijpharm.2018.03.035.
- Ji, S.; Thulstrup, P. W.; Mu, H.; Hansen, S. H.; van de Weert, M.; Rantanen, J.; Yang, M. Investigation of Factors Affecting the Stability of Lysozyme Spray Dried from Ethanol-Water Solutions. Int. J. Pharm. 2017, 534, 263–271. DOI: 10.1016/j.ijpharm.2017.10.021.
- Haj-Ahmad, R. R.; Mamayusupov, M.; Elkordy, E. A.; Elkordy, A. A. Influences of Copolymers (Copovidone, Eudragit RL PO and Kollicoat MAE 30 DP) on Stability and Bioactivity of Spray-Dried and Freeze-Dried lysozyme. Drug Dev. Ind. Pharm. 2016, 42, 2086–2096. DOI: 10.1080/03639045.2016.1200068.
- Schaefer, J.; Lee, G. Making Large, Flowable Particles of Protein or Disaccharide in a Mini-Scale Spray Dryer. Pharm. Dev. Technol. 2016, 21, 803–811. DOI: 10.3109/10837450.2015.1063649.
- Wan, F.; Maltesen, M. J.; Andersen, S. K.; Bjerregaard, S.; Foged, C.; Rantanen, J.; Yang, M. One-Step Production of Protein-Loaded PLGA Microparticles via Spray Drying Using 3-Fluid Nozzle. Pharm. Res. 2014, 31, 1967–1977. DOI: 10.1007/s11095-014-1299-1.
- Lorenzen, E.; Lee, G. Trehalose and Sorbitol Alter the Kinetic Pattern of Inactivation of Glutamate Dehydrogenase during Drying in Levitated Microdroplets. J. Pharm. Sci. 2013, 102, 4268–4273. DOI: 10.1002/jps.23743.
- Haj-Ahmad, R. R.; Elkordy, A. A.; Chaw, C. S.; Moore, A. Compare and Contrast the Effects of Surfactants (PluronicF-127 and CremophorEL) and Sugars (β-Cyclodextrin and Inulin) on Properties of Spray Dried and Crystallised Lysozyme. Eur. J. Pharm. Sci. 2013, 49, 519–534. DOI: 10.1016/j.ejps.2013.05.004.
- Bögelein, J.; Lee, G. Cyclone Selection Influences Protein Damage during Drying in a Mini Spray-Dryer. Int. J. Pharm. 2010, 401, 68–71. DOI: 10.1016/j.ijpharm.2010.09.023.
- Hulse, W. L.; Forbes, R. T.; Bonner, M. C.; Getrost, M. Do Co-Spray Dried Excipients Offer Better Lysozyme Stabilisation than Single Excipients? Eur. J. Pharm. Sci. 2008, 33, 294–305. DOI: 10.1016/j.ejps.2007.12.007.
- Elkordy, A. A.; Forbes, R. T.; Barry, B. W. Study of Protein Conformational Stability and Integrity Using Calorimetry and FT-Raman Spectroscopy Correlated with Enzymatic Activity. Eur. J. Pharm. Sci. 2008, 33, 177–190. DOI: 10.1016/j.ejps.2007.11.002.
- Liao, Y. H.; Brown, M. B.; Jones, S. A.; Nazir, T.; Martin, G. P. The Effects of Polyvinyl Alcohol on the in Vitro Stability and Delivery of Spray-Dried Protein Particles from Surfactant-Free HFA 134a-Based Pressurised Metered Dose Inhalers. Int. J. Pharm. 2005, 304, 29–39. DOI: 10.1016/j.ijpharm.2005.07.013.
- Ziaee, A.; Albadarin, A. B.; Padrela, L.; Ung, M.-T.; Femmer, T.; Walker, G.; O'Reilly, E. A Rational Approach towards Spray Drying of Biopharmaceuticals: The Case of Lysozyme. Powder Technol. 2020, 366, 206–215. DOI: 10.1016/j.powtec.2020.02.057.
- Li, H. Y.; Song, X.; Seville, P. C. The Use of Sodium Carboxymethylcellulose in the Preparation of Spray-Dried Proteins for Pulmonary Drug Delivery. Eur. J. Pharm. Sci. 2010, 40, 56–61. DOI: 10.1016/j.ejps.2010.02.007.
- Ji, S.; Thulstrup, P. W.; Mu, H.; Hansen, S. H.; van de Weert, M.; Rantanen, J.; Yang, M. Effect of Ethanol as a Co-Solvent on the Aerosol Performance and Stability of Spray-Dried Lysozyme. Int. J. Pharm. 2016, 513, 175–182. DOI: 10.1016/j.ijpharm.2016.09.025.
- Grohganz, H.; Lee, Y. Y.; Rantanen, J.; Yang, M. The Influence of Lysozyme on Mannitol Polymorphism in Freeze-Dried and Spray-Dried Formulations Depends on the Selection of the Drying Process. Int. J. Pharm. 2013, 447, 224–230. DOI: 10.1016/j.ijpharm.2013.03.003.
- Lorenzen, E.; Lee, G. Anomalous Redispersibility Behavior of Glycerophosphate Deyhydrogenase Microparticles Dried in an Acoustic Levitator or Bench-Top Spray Dryer. Int. J. Pharm. 2016, 498, 316–317. DOI: 10.1016/j.ijpharm.2015.12.039.
- Schaefer, J.; Lee, G. Arrhenius Activation Energy of Damage to Catalase during Spray-Drying. Int. J. Pharm. 2015, 489, 124–130. DOI: 10.1016/j.ijpharm.2015.04.078.
- Ehrhardt, C. Inhalation Biopharmaceutics: Progress towards Comprehending the Fate of Inhaled Medicines. Pharm. Res. 2017, 34, 2451–2453. DOI: 10.1007/s11095-017-2304-2.
- Forbes, R. T.; Barry, B. W.; Elkordy, A. A. Preparation and Characterisation of Spray-Dried and Crystallised Trypsin: FT-Raman Study to Detect Protein Denaturation after Thermal Stress. Eur. J. Pharm. Sci. 2007, 30, 315–323. DOI: 10.1016/j.ejps.2006.11.019.
- Elkordy, A. A.; Forbes, R. T.; Barry, B. W. Integrity of Crystalline Lysozyme Exceeds That of a Spray-Dried Form. Int. J. Pharm. 2002, 247, 79–90. DOI: 10.1016/S0378-5173(02)00379-4.
- Suihko, E. J.; Forbes, R. T.; Apperley, D. C. A Solid-State NMR Study of Molecular Mobility and Phase Separation in Co-Spray-Dried Protein-Sugar Particles. Eur. J. Pharm. Sci. 2005, 25, 105–112. DOI: 10.1016/j.ejps.2005.02.002.
- Hulse, W. L.; Forbes, R. T.; Bonner, M. C.; Getrost, M. Influence of Protein on Mannitol Polymorphic Form Produced during Co-Spray Drying. Int. J. Pharm. 2009, 382, 67–72. DOI: 10.1016/j.ijpharm.2009.08.007.
- Grasmeijer, N.; Tiraboschi, V.; Woerdenbag, H. J.; Frijlink, H. W.; Hinrichs, W. L. J. Identifying Critical Process Steps to Protein Stability during Spray Drying Using a Vibrating Mesh or a Two-Fluid Nozzle. Eur. J. Pharm. Sci. 2019, 128, 152–157. DOI: 10.1016/j.ejps.2018.11.027.
- Ajmera, A.; Scherließ, R. Stabilisation of Proteins via Mixtures of Amino Acids during Spray Drying. Int. J. Pharm. 2014, 463, 98–107. DOI: 10.1016/j.ijpharm.2014.01.002.
- Ógáin, O. N.; Li, J.; Tajber, L.; Corrigan, O. I.; Healy, A. M. Particle Engineering of Materials for Oral Inhalation by Dry Powder Inhalers. I-Particles of Sugar Excipients (Trehalose and Raffinose) for Protein Delivery. Int. J. Pharm. 2011, 405, 23–35. DOI: 10.1016/j.ijpharm.2010.11.039.
- Adler, M.; Lee, G. Stability and Surface Activity of Lactate Dehydrogenase in Spray-Dried Trehalose. J. Pharm. Sci. 1999, 88, 199–208. DOI: 10.1021/js980321x.
- Chan, H. K.; Clark, A.; Gonda, I.; Mumenthaler, M.; Hsu, C. Spray Dried Powders and Powder Blends of Recombinant Human Deoxyribonuclease (ase) for Aerosol Delivery. Pharm. Res. 1997, 14, 431–437. DOI: 10.1023/A:1012035113276.
- Yoshii, H.; Buche, F.; Takeuchi, N.; Terrol, C.; Ohgawara, M.; Furuta, T. Effects of Protein on Retention of ADH Enzyme Activity Encapsulated in Trehalose Matrices by Spray Drying. J. Food Eng. 2008, 87, 34–39. DOI: 10.1016/j.jfoodeng.2007.03.014.
- Hyman, P.; Kelner, P. Pharmacotherapeutic Uses of Hormones. Nurs. Clin. North Am. 2007, 42, 1–18. DOI: 10.1016/j.cnur.2006.11.002.
- Edwards, C. M. B.; Cohen, M. A.; Bloom, S. R. Peptides as Drugs. QJM. 1999, 92, 1–4. DOI: 10.1093/qjmed/92.1.1.
- Lechuga-Ballesteros, D.; Charan, C.; Stults, C. L. M.; Stevenson, C. L.; Miller, D. P.; Vehring, R.; Tep, V.; Kuo, M. C. Trileucine Improves Aerosol Performance and Stability of Spray-Dried Powders for Inhalation. J. Pharm. Sci. 2008, 97, 287–302. DOI: 10.1002/jps.21078.
- Maa, Y. F.; Nguyen, P. A. T.; Hsu, S. W. Spray-Drying of Air-Liquid Interface Sensitive Recombinant Human Growth Hormone. J. Pharm. Sci. 1998, 87, 152–159. DOI: 10.1021/js970308x.
- Jalalipour, M.; Gilani, K.; Tajerzadeh, H.; Najafabadi, A. R.; Barghi, M. Characterization and Aerodynamic Evaluation of Spray Dried Recombinant Human Growth Hormone Using Protein Stabilizing Agents. Int. J. Pharm. 2008, 352, 209–216. DOI: 10.1016/j.ijpharm.2007.10.053.
- Jalalipour, M.; Najafabadi, A. R.; Gilani, K.; Esmaily, H.; Tajerzadeh, H. Effect of Dimethyl-Beta-Cyclodextrin Concentrations on the Pulmonary Delivery of Recombinant Human Growth Hormone Dry Powder in Rats. J. Pharm. Sci. 2008, 97, 5176–5185. DOI: 10.1002/jps.21353.
- Hahn, S. K.; Kim, S. J.; Kim, M. J.; Kim, D. H. Characterization and in Vivo Study of Sustained-Release Formulation of Human Growth Hormone Using Sodium Hyaluronate. Pharm. Res. 2004, 21, 1374–1381. DOI: 10.1023/B:PHAM.0000036910.41224.de.
- Bosquillon, C.; Préat, V.; Vanbever, R. Pulmonary Delivery of Growth Hormone Using Dry Powders and Visualization of its Local Fate in Rats. J. Control Release. 2004, 96, 233–244. DOI: 10.1016/j.jconrel.2004.01.027.
- Bosquillon, C.; Rouxhet, P. G.; Ahimou, F.; Simon, D.; Culot, C.; Préat, V.; Vanbever, R. Aerosolization Properties, Surface Composition and Physical State of Spray-Dried Protein Powders. J. Control Release. 2004, 99, 357–367. DOI: 10.1016/j.jconrel.2004.07.022.
- Hou, A.; Li, L.; Huang, Y.; Singh, V.; Zhu, C.; Pan, X.; Quan, G.; Wu, C. Fragmented Particles Containing Octreotide Acetate Prepared by Spray Drying Technique for Dry Powder Inhalation. Drug Deliv. Transl. Res. 2018, 8, 693–701. DOI: 10.1007/s13346-018-0515-7.
- Codrons, V.; Vanderbist, F.; Verbeeck, R. K.; Arras, M.; Lison, D.; Préat, V.; Vanbever, R. Systemic Delivery of Parathyroid Hormone (1–34) Using Inhalation Dry Powders in Rats. J. Pharm. Sci. 2003, 92, 938–950. DOI: 10.1002/jps.10346.
- Shoyele, S. A.; Sivadas, N.; Cryan, S. A. The Effects of Excipients and Particle Engineering on the Biophysical Stability and Aerosol Performance of Parathyroid Hormone (1-34) Prepared as a Dry Powder for Inhalation. AAPS PharmSciTech. 2011, 12, 304–311. DOI: 10.1208/s12249-011-9585-2.
- Chan, H. K.; Clark, A. R.; Feeley, J. C.; Kuo, M. C.; Lehrman, S. R.; Pikal-Cleland, K.; Miller, D. P.; Vehring, R.; Lechuga-Ballesteros, D. Physical Stability of Salmon Calcitonin Spray-Dried Powders for Inhalation. J. Pharm. Sci. 2004, 93, 792–804. DOI: 10.1002/jps.10594.
- Yang, M.; Velaga, S.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y.; Hovgaard, L.; van de Weert, M.; Frokjaer, S. Characterisation of Salmon Calcitonin in Spray-Dried Powder for Inhalation. Effect of Chitosan. Int. J. Pharm. 2007, 331, 176–181. DOI: 10.1016/j.ijpharm.2006.10.030.
- Irngartinger, M.; Camuglia, V.; Damm, M.; Goede, J.; Frijlink, H. W. Pulmonary Delivery of Therapeutic Peptides via Dry Powder Inhalation: Effects of Micronisation and Manufacturing. Eur. J. Pharm. Biopharm. 2004, 58, 7–14. DOI: 10.1016/j.ejpb.2004.03.016.
- Guevara, T.; Yiallouros, I.; Kappelhoff, R.; Bissdorf, S.; Stöcker, W.; Gomis-Rüth, F. X. Proenzyme Structure and Activation of Astacin Metallopeptidase. J. Biol. Chem. 2010, 285, 13958–13965. DOI: 10.1074/jbc.M109.097436.
- Tzannis, S. T.; Prestrelski, S. J. Activity-Stability Considerations of Trypsinogen during Spray Drying: Effects of Sucrose. J. Pharm. Sci. 1999, 88, 351–359. DOI: 10.1021/js980011e.
- Li, X. Protein Structure and Modification of FGFs. In Fibroblast Growth Factors, 1st ed.; Li, X. B. T.-F. G. F., Ed.; Academic Press, 2018; pp 385–476. DOI: 10.1016/B978-0-12-816142-5.00007-2.
- Ibrahim, B. M.; Jun, S. W.; Lee, M. Y.; Kang, S. H.; Yeo, Y. Development of Inhalable Dry Powder Formulation of Basic Fibroblast Growth Factor. Int. J. Pharm. 2010, 385, 66–72. DOI: 10.1016/j.ijpharm.2009.10.029.
- Schaller, J.; Gerber, S.; Kämpfer, U.; Lejon, S.; Trachsel, C. Transport and Storage. In Human Blood Plasma Proteins: Structure and Function; Schaller, J., Gerber, S., Kämpfer, U., Lejon, S., Trachsel, C., Eds.; John Wiley & Sons, Ltd., 2008; pp 451–486.
- Burmester, T. Evolution of Respiratory Proteins across the Pancrustacea. Integr. Comp. Biol. 2015, 55, 792–801. DOI: 10.1093/icb/icv079.
- Labrude, P.; Rasolomanana, M.; Vigneron, C.; Thirion, C.; Chaillot, B. Protective Effect of Sucrose on Spray Drying of Oxyhemoglobin. J. Pharm. Sci. 1989, 78, 223–229. DOI: 10.1002/jps.2600780311.
- Wilson, N. E.; Mutukuri, T. T.; Zemlyanov, D. Y.; Taylor, L. S.; Topp, E. M.; Zhou, Q. T. Surface Composition and Formulation Heterogeneity of Protein Solids Produced by Spray Drying. Pharm. Res. 2019, 37, 14. DOI: 10.1007/s11095-019-2738-9.
- Chablani, L.; Tawde, S. A.; D'Souza, M. J. Spray-Dried Microparticles: A Potential Vehicle for Oral Delivery of Vaccines. J. Microencapsul. 2012, 29, 388–397. DOI: 10.3109/02652048.2011.651503.
- Möbus, K.; Siepmann, J.; Bodmeier, R. Zinc-Alginate Microparticles for Controlled Pulmonary Delivery of Proteins Prepared by Spray-Drying. Eur. J. Pharm. Biopharm. 2012, 81, 121–130. DOI: 10.1016/j.ejpb.2012.01.018.
- Cho, H. J.; Oh, D.; Kim, D. D. Polysaccharides-Based Spray-Dried Microspheres for Maintained Stability and Controlled Release of Protein. J. Pharm. Investig. 2012, 42, 83–88. DOI: 10.1007/s40005-012-0013-8.
- Kusonwiriyawong, C.; Pichayakorn, W.; Lipipun, V.; Ritthidej, G. C. Retained Integrity of Protein Encapsulated in Spray-Dried Chitosan Microparticles. J. Microencapsul. 2009, 26, 111–121. DOI: 10.1080/02652040802190937.
- Salama, R. O.; Traini, D.; Chan, H. K.; Sung, A.; Ammit, A. J.; Young, P. M. Preparation and Evaluation of Controlled Release Microparticles for Respiratory Protein Therapy. J. Pharm. Sci. 2009, 98, 2709–2717. DOI: 10.1002/jps.21653.
- Sivadas, N.; O'Rourke, D.; Tobin, A.; Buckley, V.; Ramtoola, Z.; Kelly, J. G.; Hickey, A. J.; Cryan, S.-A. A Comparative Study of a Range of Polymeric Microspheres as Potential Carriers for the Inhalation of Proteins. Int. J. Pharm. 2008, 358, 159–167. DOI: 10.1016/j.ijpharm.2008.03.024.
- Jalalipour, M.; Rouholamini Najafabadi, A.; Tajerzadeh, H.; Gilani, K.; Barghi, M. The Effect of Protein Stabilizers on the Physical State and Aerosol Performance of Spray-Dried Albumin Microparticles. J. Drug Deliv. Sci. Technol. 2007, 17, 149–153. DOI: 10.1016/S1773-2247(07)50023-7.
- Elversson, J.; Millqvist-Fureby, A. In Situ Coating-An Approach for Particle Modification and Encapsulation of Proteins during Spray-Drying. Int. J. Pharm. 2006, 323, 52–63. DOI: 10.1016/j.ijpharm.2006.05.066.
- Chew, N. Y. K.; Chan, H. K. Use of Solid Corrugated Particles to Enhance Powder Aerosol Performance. Pharm. Res. 2001, 18, 1570–1577. DOI: 10.1023/A:1013082531394.
- Adi, S.; Adi, H.; Chan, H. K.; Tong, Z.; Yang, R.; Yu, A. Effects of Mechanical Impaction on Aerosol Performance of Particles with Different Surface Roughness. Powder Technol. 2013, 236, 164–170. DOI: 10.1016/j.powtec.2012.02.051.
- Adler, M.; Unger, M.; Lee, G. Surface Composition of Spray-Dried Particles of Bovine Serum Albumin/Trehalose/Surfactant. Pharm. Res. 2000, 17, 863–870. DOI: 10.1023/A:1007568511399.
- Landström, K.; Alsins, J.; Bergenståhl, B. Competitive Protein Adsorption between Bovine Serum Albumin and β-Lactoglobulin during Spray-Drying. Food Hydrocoll. 2000, 14, 75–82. DOI: 10.1016/S0268-005X(99)00047-8.
- Fäldt, P.; Bergenståhl, B. The Surface Composition of Spray-Dried Protein-Lactose Powders. Colloids Surf. A Physicochem. Eng. Asp. 1994, 90, 183–190. DOI: 10.1016/0927-7757(94)02914-8.
- Shmool, T. A.; Batens, M.; Massant, J.; Van den Mooter, G.; Zeitler, J. A. Tracking Solid State Dynamics in Spray-Dried Protein Powders at Infrared and Terahertz Frequencies. Eur. J. Pharm. Biopharm. 2019, 144, 244–251. DOI: 10.1016/j.ejpb.2019.09.013.
- Reslan, M.; Demir, Y. K.; Trout, B. L.; Chan, H. K.; Kayser, V. Lack of a Synergistic Effect of Arginine-Glutamic Acid on the Physical Stability of Spray-Dried Bovine Serum Albumin. Pharm. Dev. Technol. 2017, 22, 785–791. DOI: 10.1080/10837450.2016.1185116.
- Wan, F.; Maltesen, M. J.; Andersen, S. K.; Bjerregaard, S.; Baldursdottir, S. G.; Foged, C.; Rantanen, J.; Yang, M. Modulating Protein Release Profiles by Incorporating Hyaluronic Acid into PLGA Microparticles via a Spray Dryer Equipped with a 3-Fluid Nozzle. Pharm. Res. 2014, 31, 2940–2951. DOI: 10.1007/s11095-014-1387-2.
- Shastri, P. N.; Ubale, R. V.; D’Souza, M. J. Implementation of Mixture Design for Formulation of Albumin Containing Enteric-Coated Spray-Dried Microparticles. Drug Dev. Ind. Pharm. 2013, 39, 164–175. DOI: 10.3109/03639045.2012.664148.
- Greenwood, B. The Contribution of Vaccination to Global Health: Past, Present and Future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130433. DOI: 10.1098/rstb.2013.0433.
- Saluja, V.; Amorij, J. P.; Kapteyn, J. C.; de Boer, A. H.; Frijlink, H. W.; Hinrichs, W. L. J. A Comparison between Spray Drying and Spray Freeze Drying to Produce an Influenza Subunit Vaccine Powder for Inhalation. J. Control Release. 2010, 144, 127–133. DOI: 10.1016/j.jconrel.2010.02.025.
- Jin, T. H.; Tsao, E.; Goudsmit, J.; Dheenadhayalan, V.; Sadoff, J. Stabilizing Formulations for Inhalable Powders of an Adenovirus 35-Vectored Tuberculosis (TB) Vaccine (AERAS-402). Vaccine. 2010, 28, 4369–4375. DOI: 10.1016/j.vaccine.2010.04.059.
- Wong, Y. L.; Sampson, S.; Germishuizen, W. A.; Goonesekera, S.; Caponetti, G.; Sadoff, J.; Bloom, B. R.; Edwards, D. Drying a Tuberculosis Vaccine without Freezing. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 2591–2595. DOI: 10.1073/pnas.0611430104.
- Kunda, N. K.; Peabody, J.; Zhai, L.; Price, D. N.; Chackerian, B.; Tumban, E.; Muttil, P. Evaluation of the Thermal Stability and the Protective Efficacy of Spray-Dried HPV Vaccine, Gardasil® 9. Hum. Vaccin. Immunother. 2019, 15, 1995–2002. DOI: 10.1080/21645515.2019.1593727.
- López, Y.; Pastor, M.; Infante, J. F.; Díaz, D.; Oliva, R.; Fernández, S.; Cedré, B.; Hernández, T.; Campos, L.; Esquisabel, A.; et al. Repeated Dose Toxicity Study of Vibrio cholerae-Loaded Gastro-Resistant Microparticles. J. Microencapsul. 2014, 31, 86–92. DOI: 10.3109/02652048.2013.808278.
- Bhowmik, T.; D'Souza, B.; Shashidharamurthy, R.; Oettinger, C.; Selvaraj, P.; D'Souza, M. J. A Novel Microparticulate Vaccine for Melanoma Cancer Using Transdermal Delivery. J. Microencapsul. 2011, 28, 294–300. DOI: 10.3109/02652048.2011.559287.
- Lai, Y. H.; D'Souza, M. J. Formulation and Evaluation of an Oral Melanoma Vaccine. J. Microencapsul. 2007, 24, 235–252. DOI: 10.1080/02652040601162608.
- Liao, C. W.; Cheng, I. C.; Yeh, K. S.; Lin, F. Y.; Weng, C. N. Release Characteristics of Microspheres Prepared by Co-Spray Drying Actinobacillus Pleuropneumoniae Antigens and Aqueous Ethyl-Cellulose Dispersion. J. Microencapsul. 2001, 18, 285–297. DOI: 10.1080/02652040010019442.
- Garcea, R. L.; Meinerz, N. M.; Dong, M.; Funke, H.; Ghazvini, S.; Randolph, T. W. Single-Administration, Thermostable Human Papillomavirus Vaccines Prepared with Atomic Layer Deposition Technology. NPJ Vaccines. 2020, 5, 45. DOI: 10.1038/s41541-020-0195-4.
- Abdul-Fattah, A. M.; Truong-Le, V.; Yee, L.; Pan, E.; Ao, Y.; Kalonia, D. S.; Pikal, M. J. Drying-Induced Variations in Physico-Chemical Properties of Amorphous Pharmaceuticals and Their Impact on Stability II: Stability of a Vaccine. Pharm. Res. 2007, 24, 715–727. DOI: 10.1007/s11095-006-9191-2.
- Kanojia, G.; Raeven, R. H. M.; van der Maas, L.; Bindels, T. H. E.; van Riet, E.; Metz, B.; Soema, P. C.; Ten Have, R.; Frijlink, H. W.; Amorij, J.-P.; et al. A. Development of a Thermostable Spray Dried Outer Membrane Vesicle Pertussis Vaccine for Pulmonary Immunization. J. Control. Release. 2018, 286, 167–178. DOI: 10.1016/j.jconrel.2018.07.035.
- Morgan, B. A.; Xing, Z.; Cranston, E. D.; Thompson, M. R. Acoustic Levitation as a Screening Method for Excipient Selection in the Development of Dry Powder Vaccines. Int. J. Pharm. 2019, 563, 71–78. DOI: 10.1016/j.ijpharm.2019.03.026.
- Sou, T.; Morton, D. A. V.; Williamson, M.; Meeusen, E. N.; Kaminskas, L. M.; McIntosh, M. P. Spray-Dried Influenza Antigen with Trehalose and Leucine Produces an Aerosolizable Powder Vaccine Formulation That Induces Strong Systemic and Mucosal Immunity after Pulmonary Administration. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 361–371. DOI: 10.1089/jamp.2014.1176.
- LeClair, D. A.; Cranston, E. D.; Xing, Z.; Thompson, M. R. Optimization of Spray Drying Conditions for Yield, Particle Size and Biological Activity of Thermally Stable Viral Vectors. Pharm. Res. 2016, 33, 2763–2776. DOI: 10.1007/s11095-016-2003-4.
- Leclair, D. A.; Cranston, E. D.; Xing, Z.; Thompson, M. R. Evaluation of Excipients for Enhanced Thermal Stabilization of a Human Type 5 Adenoviral Vector through Spray Drying. Int. J. Pharm. 2016, 506, 289–301. DOI: 10.1016/j.ijpharm.2016.04.067.
- Ohtake, S.; Martin, R. A.; Yee, L.; Chen, D.; Kristensen, D. D.; Lechuga-Ballesteros, D.; Truong-Le, V. Heat-Stable Measles Vaccine Produced by Spray Drying. Vaccine. 2010, 28, 1275–1284. DOI: 10.1016/j.vaccine.2009.11.024.
- Año, G.; Esquisabel, A.; Pastor, M.; Talavera, A.; Cedré, B.; Fernández, S.; Sifontes, S.; Aranguren, Y.; Falero, G.; García, L.; et al. A New Oral Vaccine Candidate Based on the Microencapsulation by Spray-Drying of Inactivated Vibrio cholerae. Vaccine. 2011, 29, 5758–5764. DOI: 10.1016/j.vaccine.2011.05.098.
- Saboo, S.; Tumban, E.; Peabody, J.; Wafula, D.; Peabody, D. S.; Chackerian, B.; Muttil, P. Optimized Formulation of a Thermostable Spray-Dried Virus-Like Particle Vaccine against Human Papillomavirus. Mol. Pharm. 2016, 13, 1646–1655. DOI: 10.1021/acs.molpharmaceut.6b00072.
- Kunda, N. K.; Wafula, D.; Tram, M.; Wu, T. H.; Muttil, P. A Stable Live Bacterial Vaccine. Eur. J. Pharm. Biopharm. 2016, 103, 109–117. DOI: 10.1016/j.ejpb.2016.03.027.
- Mensink, M. A.; Frijlink, H. W.; van der Voort Maarschalk, K.; Hinrichs, W. L. J. How Sugars Protect Proteins in the Solid State and during Drying (Review): Mechanisms of Stabilization in Relation to Stress Conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. DOI: 10.1016/j.ejpb.2017.01.024.
- Ubbink, J. Structural and Thermodynamic Aspects of Plasticization and Antiplasticization in Glassy Encapsulation and Biostabilization Matrices. Adv. Drug Deliv. Rev. 2016, 100, 10–26. DOI: 10.1016/j.addr.2015.12.019.
- Carpenter, J. F.; Crowe, J. H. An Infrared Spectroscopic Study of the Interactions of Carbohydrates with Dried Proteins. Biochemistry. 1989, 28, 3916–3922. DOI: 10.1021/bi00435a044.
- Batens, M.; Shmool, T. A.; Massant, J.; Zeitler, J. A.; Van Den Mooter, G. Advancing Predictions of Protein Stability in the Solid State. Phys. Chem. Chem. Phys. 2020, 22, 17247–17254. DOI: 10.1039/d0cp00341g.
- Livesey, G. Health Potential of Polyols as Sugar Replacers, with Emphasis on Low Glycaemic Properties. Nutr. Res. Rev. 2003, 16, 163–191. DOI: 10.1079/NRR200371.
- Ionova, Y.; Wilson, L. Biologic Excipients: Importance of Clinical Awareness of Inactive Ingredients. PLoS One. 2020, 15, e0235076. DOI: 10.1371/journal.pone.0235076.
- Razavi Rohani, S. S.; Abnous, K.; Tafaghodi, M. Preparation and Characterization of Spray-dried powders intended for Pulmonary Delivery of Insulin with Regard to the Selection of Excipients. Int. J. Pharm. 2014, 465, 464–478. DOI: 10.1016/j.ijpharm.2014.02.030.
- Kulkarni, S. S.; Patel, S. M.; Bogner, R. H. Reconstitution Time for Highly Concentrated Lyophilized Proteins: Role of Formulation and Protein. J. Pharm. Sci. 2020, 109, 2975–2985. DOI: 10.1016/j.xphs.2020.05.029.
- Chen, D.; Kapre, S.; Goel, A.; Suresh, K.; Beri, S.; Hickling, J.; Jensen, J.; Lal, M.; Preaud, J. M.; Laforce, M.; et al. Thermostable Formulations of a Hepatitis B Vaccine and a Meningitis a Polysaccharide Conjugate Vaccine Produced by a Spray Drying Method. Vaccine. 2010, 28, 5093–5099. DOI: 10.1016/j.vaccine.2010.04.112.
- Malferrari, M.; Savitsky, A.; Lubitz, W.; Möbius, K.; Venturoli, G. Protein Immobilization Capabilities of Sucrose and Trehalose Glasses: The Effect of Protein/Sugar Concentration Unraveled by High-Field EPR. J. Phys. Chem. Lett. 2016, 7, 4871–4877. DOI: 10.1021/acs.jpclett.6b02449.
- Serno, T.; Geidobler, R.; Winter, G. Protein Stabilization by Cyclodextrins in the Liquid and Dried State. Adv. Drug Deliv. Rev. 2011, 63, 1086–1106. DOI: 10.1016/j.addr.2011.08.003.
- De Rosa, G.; Larobina, D.; Immacolata La Rotonda, M.; Musto, P.; Quaglia, F.; Ungaro, F. How Cyclodextrin Incorporation Affects the Properties of Protein-Loaded PLGA-Based Microspheres: The Case of Insulin/Hydroxypropyl-Beta-Cyclodextrin System. J. Control. Release. 2005, 102, 71–83. DOI: 10.1016/j.jconrel.2004.09.030.
- Abdul-Fattah, A. M.; Lechuga-Ballesteros, D.; Kalonia, D. S.; Pikal, M. J. The Impact of Drying Method and Formulation on the Physical Properties and Stability of Methionyl Human Growth Hormone in the Amorphous Solid State. J. Pharm. Sci. 2008, 97, 163–184. DOI: 10.1002/jps.21085.
- Mollmann, S. H.; Bukrinsky, J. T.; Elofsson, U.; Elversson, J.; Frokjaer, S.; Thalberg, K.; Millqvist-Fureby, A. The Stability of Insulin in Solid Formulations Containing Melezitose and Starch. Effects of Processing and Excipients. Drug Dev. Ind. Pharm. 2006, 32, 765–778. DOI: 10.1080/03639040600712458.
- Tonnis, W. F.; Mensink, M. A.; De Jager, A.; Van Der Voort Maarschalk, K.; Frijlink, H. W.; Hinrichs, W. L. J. Size and Molecular Flexibility of Sugars Determine the Storage Stability of Freeze-Dried Proteins. Mol. Pharm. 2015, 12, 684–694. DOI: 10.1021/mp500423z.
- Song, E.-H.; Shang, J.; Ratner, D. M. Polysaccharides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M. B. T.-P. S. A. C. R., Eds.; Elsevier: Amsterdam, 2012; pp 137–155. DOI: 10.1016/B978-0-444-53349-4.00246-6.
- Kuehl, P. J.; Boyden, T.; Dobry, D. E.; Doyle-Eisele, M.; Friesen, D. T.; McDonald, J. D.; Murri, B. G.; Vodak, D. T.; Lyon, D. K. Inhaled PYY(3-36) Dry-Powder Formulation for Appetite Suppression. Drug Dev. Ind. Pharm. 2016, 42, 150–156. DOI: 10.3109/03639045.2015.1036067.
- Mensink, M. A.; Frijlink, H. W.; Van Der Voort Maarschalk, K.; Hinrichs, W. L. J. Inulin, a Flexible Oligosaccharide I: Review of Its Physicochemical Characteristics. Carbohydr. Polym. 2015, 130, 405–419. DOI: 10.1016/j.carbpol.2015.05.026.
- Zijlstra, G. S.; Ponsioen, B. J.; Hummel, S. A.; Sanders, N.; Hinrichs, W. L. J.; De Boer, A. H.; Frijlink, H. W. Formulation and Process Development of (Recombinant Human) Deoxyribonuclease I as a Powder for Inhalation. Pharm. Dev. Technol. 2009, 14, 358–368. DOI: 10.1080/10837450802662820.
- Ravi Kumar, M. N. V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. DOI: 10.1016/S1381-5148(00)00038-9.
- Rinaudo, M. Biomaterials Based on a Natural Polysaccharide: Alginate. TIP. 2014, 17, 92–96. DOI: 10.1016/S1405-888X(14)70322-5.
- Bowey, K.; Swift, B. E.; Flynn, L. E.; Neufeld, R. J. Characterization of Biologically Active Insulin-Loaded Alginate Microparticles Prepared by Spray Drying. Drug Dev. Ind. Pharm. 2013, 39, 457–465. DOI: 10.3109/03639045.2012.662985.
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinology. 2012, 4, 253–258. DOI: 10.4161/derm.21923.
- Kennelly, P. J.; Rodwell, V. W. 3 – Amino Acids & Peptides. In Harper’s Illustrated Biochemistry, 31st ed.; Rodwell, V. W., Bender, D. A., Botham, K. M., Kennelly, P. J., Weil, A. P., Eds.; McGraw-Hill Education: New York, 2018; pp 14–22.
- Forney-Stevens, K. M.; Bogner, R. H.; Pikal, M. J. Addition of Amino Acids to Further Stabilize Lyophilized Sucrose-Based Protein Formulations: I. Screening of 15 Amino Acids in Two Model Proteins. J. Pharm. Sci. 2016, 105, 697–704. DOI: 10.1002/jps.24655.
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A Review. Pharmaceutics. 2020, 12, 55. DOI: 10.3390/pharmaceutics12010055.
- Liu, J.; Shire, S. High Concentration Antibody and Protein Formulations. U.S. Patent 2004/0197324 A1, Oct 7, 2004.
- Dave, N.; Joshi, T. A Concise Review on Surfactants and its Significance. Int. J. Appl. Chem. 2017, 13, 663–672.
- Abdul-Fattah, A. M.; Kalonia, D. S.; Pikal, M. J. The Challenge of Drying Method Selection for Protein Pharmaceuticals: Product Quality Implications. J. Pharm. Sci. 2007, 96, 1886–1916. DOI: 10.1002/jps.20842.
- Khan, T. A.; Mahler, H. C.; Kishore, R. S. K. Key Interactions of Surfactants in Therapeutic Protein Formulations: A Review. Eur. J. Pharm. Biopharm. 2015, 97, 60–67. DOI: 10.1016/j.ejpb.2015.09.016.
- Korang-Yeboah, M.; Rahman, Z.; Shah, D.; Mohammad, A.; Wu, S.; Siddiqui, A.; Khan, M. A. Impact of Formulation and Process Variables on Solid-State Stability of Theophylline in Controlled Release Formulations. Int. J. Pharm. 2016, 499, 20–28. DOI: 10.1016/j.ijpharm.2015.11.046.
- Momoh, M. A.; Kenechukwu, F. C.; Nnamani, P. O.; Umetiti, J. C. Influence of Magnesium Stearate on the Physicochemical and Pharmacodynamic Characteristics of Insulin-Loaded Eudragit Entrapped Mucoadhesive Microspheres. Drug Deliv. 2015, 22, 837–848. DOI: 10.3109/10717544.2014.898108.
- Tewes, F.; Tajber, L.; Corrigan, O. I.; Ehrhardt, C.; Healy, A. M. Development and Characterisation of Soluble Polymeric Particles for Pulmonary Peptide Delivery. Eur. J. Pharm. Sci. 2010, 41, 337–352. DOI: 10.1016/j.ejps.2010.07.001.
- Kohane, D. S.; Anderson, D. G.; Yu, C.; Langer, R. PH-Triggered Release of Macromolecules from Spray-Dried Polymethacrylate Microparticles. Pharm. Res. 2003, 20, 1533–1538. DOI: 10.1023/A:1026162628965.
- Cicerone, M. T.; Soles, C. L. Fast Dynamics and Stabilization of Proteins: Binary Glasses of Trehalose and Glycerol. Biophys. J. 2004, 86, 3836–3845. DOI: 10.1529/biophysj.103.035519.
- Talley, K.; Alexov, E. On the PH-Optimum of Activity and Stability of Proteins. Proteins. 2010, 78, 2699–2706. DOI: 10.1002/prot.22786.
- Zbacnik, T. J.; Holcomb, R. E.; Katayama, D. S.; Murphy, B. M.; Payne, R. W.; Coccaro, R. C.; Evans, G. J.; Matsuura, J. E.; Henry, C. S.; Manning, M. C. Role of Buffers in Protein Formulations. J. Pharm. Sci. 2017, 106, 713–733. DOI: 10.1016/j.xphs.2016.11.014.
- Sek, D. Breaking Old Habits: Moving Away From Commonly Used Buffers in Pharmaceuticals. https://www.europeanpharmaceuticalreview.com/article/13699/breaking-old-habits-moving-away-from-commonly-used-buffers-in-pharmaceuticals/ (accessed Nov 27, 2020).
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catal. 2013, 3, 2823–2836. DOI: 10.1021/cs400684x.
- Sass, A.; Lee, G. Evaluation of Some Water-Miscible Organic Solvents for Spray-Drying Enzymes and Carbohydrates. Drug Dev. Ind. Pharm. 2014, 40, 749–757. DOI: 10.3109/03639045.2013.782554.
- Griebenow, K.; Klibanov, A. M. On Protein Denaturation in Aqueous-Organic Mixtures but Not in Pure Organic Solvents. J. Am. Chem. Soc. 1996, 118, 11695–11700. DOI: 10.1021/ja961869d.
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. DOI: 10.1007/s11095-007-9475-1.
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M. R.; Clasen, C.; Van den Mooter, G. Unraveling Particle Formation: From Single Droplet Drying to Spray Drying and Electrospraying. Pharmaceutics. 2020, 12, 625. DOI: 10.3390/pharmaceutics12070625.
- Vehring, R.; Foss, W. R.; Lechuga-Ballesteros, D. Particle Formation in Spray Drying. J. Aerosol Sci. 2007, 38, 728–746. DOI: 10.1016/j.jaerosci.2007.04.005.
- Ung, K. T.; Rao, N.; Weers, J. G.; Huang, D.; Chan, H. K. Design of Spray Dried Insulin Microparticles to Bypass Deposition in the Extrathoracic Region and Maximize Total Lung Dose. Int. J. Pharm. 2016, 511, 1070–1079. DOI: 10.1016/j.ijpharm.2016.07.073.
- Stetten, A. Z.; Iasella, S. V.; Corcoran, T. E.; Garoff, S.; Przybycien, T. M.; Tilton, R. D. Surfactant-Induced Marangoni Transport of Lipids and Therapeutics within the Lung. Curr. Opin. Colloid Interf. Sci. 2018, 36, 58–69. DOI: 10.1016/j.cocis.2018.01.001.
- Nguyen, D. a.; Rhodes, M. J. Producing Fine Drops of Water by Twin-Fluid Atomisation. Powder Technol. 1998, 99, 285–292. DOI: 10.1016/S0032-5910(98)00125-9.
- Lefebvre, A. H. Some Recent Developments in Twin-Fluid Atomization. Part. Part. Syst. Charact. 1996, 13, 205–216. DOI: 10.1002/ppsc.19960130307.
- Duerkop, M.; Berger, E.; Dürauer, A.; Jungbauer, A. Impact of Cavitation, High Shear Stress and Air/Liquid Interfaces on Protein Aggregation. Biotechnol. J. 2018, 13, DOI: 10.1002/biot.201800062.
- Sovani, S. D.; Chou, E.; Sojka, P. E.; Gore, J. P.; Eckerle, W. A.; Crofts, J. D. High Pressure Effervescent Atomization: Effect of Ambient Pressure on Spray Cone Angle. Fuel. 2001, 80, 427–435. DOI: 10.1016/S0016-2361(00)00105-8.
- Truong-Le, V.; Scherer, T. High Pressure Spray-Dry of Bioactive Materials. U.S. Patent US7378110B2, May 27, 2008.
- Sunderland, T.; Kelly, J. G.; Ramtoola, Z. Application of a Novel 3-Fluid Nozzle Spray Drying Process for the Microencapsulation of Therapeutic Agents Using Incompatible Drug-Polymer Solutions. Arch. Pharm. Res. 2015, 38, 566–573. DOI: 10.1007/s12272-013-0261-9.
- Gaspar, F.; Vicente, J.; Neves, F.; Authelin, J.-R. Spray-Drying: Scale-up and Manufacturing. In Amorphous Solid Dispersions.Advances in Delivery Science and Technology;Shah, N., Sandhu, H., Choi, D. S., Chokshi, H., Malick, A. W., Eds.; Springer: New York, NY, 2014; pp 261–302.DOI: 10.1007/978-1-4939-1598-9_8
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. DOI: 10.1002/jps.21886.
- Truong-Le, V.; Ohtake, S.; Martin, R. A.; Pham, B. V.; Yee, L. Sonic Low Pressure Spray Drying. U.S. Patent US8673357B2, March 18, 2014.
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S. M. Nano Spray Drying for Encapsulation of Pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. DOI: 10.1016/j.ijpharm.2018.05.037.
- Wu, W. D.; Patel, K. C.; Rogers, S.; Chen, X. D. Monodisperse Droplet Generators as Potential Atomizers for Spray Drying Technology. Dry Technol. 2007, 25, 1907–1916. DOI: 10.1080/07373930701727176.
- Lin, S. P.; Reitz, R. D. Drop and Spray Formation from a Liquid Jet. Annu. Rev. Fluid Mech. 1998, 30, 85–105. DOI: 10.1146/annurev.fluid.30.1.85.
- Poozesh, S.; Bilgili, E. Scale-up of Pharmaceutical Spray Drying Using Scale-up Rules: A Review. Int. J. Pharm. 2019, 562, 271–292. DOI: 10.1016/j.ijpharm.2019.03.047.
- Ziaee, A.; Albadarin, A. B.; Padrela, L.; Femmer, T.; O'Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization Approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. DOI: 10.1016/j.ejps.2018.10.026.
- Ståhl, K.; Claesson, M.; Lilliehorn, P.; Lindén, H.; Bäckström, K. The Effect of Process Variables on the Degradation and Physical Properties of Spray Dried Insulin Intended for Inhalation. Int. J. Pharm. 2002, 233, 227–237. DOI: 10.1016/S0378-5173(01)00945-0.
- Rajagopal, K.; Chang, D.; Nayak, P.; Izadi, S.; Patapoff, T.; Zhang, J.; Kelley, R.; Sreedhara, A. Trehalose Limits Fragment Antibody Aggregation and Influences Charge Variant Formation in Spray-Dried Formulations at Elevated Temperatures. Mol. Pharm. 2019, 16, 349–358. DOI: 10.1021/acs.molpharmaceut.8b01002.
- Maltesen, M. J.; Bjerregaard, S.; Hovgaard, L.; Havelund, S.; van de Weert, M. Quality by Design – Spray Drying of Insulin Intended for Inhalation. Eur. J. Pharm. Biopharm. 2008, 70, 828–838. DOI: 10.1016/j.ejpb.2008.07.015.
- Wahl, V.; Khinast, J.; Paudel, A. Lyophilized Protein Powders: A Review of Analytical Tools for Root Cause Analysis of Lot-to-Lot Variability. TrAC – Trends Anal. Chem. 2016, 82, 468–491. DOI: 10.1016/j.trac.2016.05.012.
- Lai, M. C.; Topp, E. M. Solid-State Chemical Stability of Proteins and Peptides. J. Pharm. Sci. 1999, 88, 489–500. DOI: 10.1021/js980374e.
- Chang, L. L.; Pikal, M. J. Mechanisms of Protein Stabilization in the Solid State. J. Pharm. Sci. 2009, 98, 2886–2908. DOI: 10.1002/jps.21825.
- Eon-Duval, A.; Broly, H.; Gleixner, R. Quality Attributes of Recombinant Therapeutic Proteins: An Assessment of Impact on Safety and Efficacy as Part of a Quality by Design Development Approach. Biotechnol. Prog. 2012, 28, 608–622. DOI: 10.1002/btpr.1548.
- Eon-Duval, A.; Valax, P.; Solacroup, T.; Broly, H.; Gleixner, R.; L. E.; Strat, C.; Sutter, J. Application of the Quality by Design Approach to the Drug Substance Manufacturing Process of an Fc Fusion Protein: Towards a Global Multi-Step Design Space. J. Pharm. Sci. 2012, 101, 3604–3618. DOI: 10.1002/jps.23273.
- Thorat, B. N.; Sett, A.; Mujumdar, A. S. Drying of Vaccines and Biomolecules. Dry. Technol. 2020, DOI: 10.1080/07373937.2020.1825293.
- Malafronte, L.; Ruoff, D.; Gunes, D. Z.; Lequeux, F.; Schmitt, C.; Windhab, E. J. Morphology Development in Single Drop Drying for Native and Aggregated Whey Protein Dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 1235549. DOI: 10.1016/j.colsurfa.2019.06.015.
- Both, E. M.; Tersteeg, S. M. B.; Boom, R. M.; Schutyser, M. A. I. Drying Kinetics and Viscoelastic Properties of Concentrated Thin Films as a Model System for Spray Drying. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124075. DOI: 10.1016/j.colsurfa.2019.124075.
- Doerr, F. J. S.; Burns, L. J.; Lee, B.; Hinds, J.; Davis-Harrison, R. L.; Frank, S. A.; Florence, A. J. Peptide Isolation via Spray Drying: Particle Formation, Process Design and Implementation for the Production of Spray Dried Glucagon. Pharm. Res. 2020, 37, 255. DOI: 10.1007/s11095-020-02942-5.
- Dohrn, S.; Reimer, P.; Luebbert, C.; Lehmkemper, K.; Kyeremateng, S. O.; Degenhardt, M.; Sadowski, G. Thermodynamic Modeling of Solvent-Impact on Phase Separation in Amorphous Solid Dispersions during Drying. Mol. Pharm. 2020, 17, 2721–2733. DOI: 10.1021/acs.molpharmaceut.0c00418.