Abstract
Vibrio species pose significant threats worldwide, causing mortalities in aquaculture and infections in humans. Global warming and the emergence of worldwide strains of Vibrio diseases are increasing day by day. Control of Vibrio species requires effective monitoring, diagnosis, and treatment strategies at the global scale. Despite current efforts based on chemical, biological, and mechanical means, Vibrio control management faces limitations due to complicated implementation processes. This review explores the intricacies and challenges of Vibrio-related diseases, including accurate and cost-effective diagnosis and effective control. The global burden due to emerging Vibrio species further complicates management strategies. We propose an innovative integrated technology model that harnesses cutting-edge technologies to address these obstacles. The proposed model incorporates advanced tools, such as biosensing technologies, the Internet of Things (IoT), remote sensing devices, cloud computing, and machine learning. This model offers invaluable insights and supports better decision-making by integrating real-time ecological data and biological phenotype signatures. A major advantage of our approach lies in leveraging cloud-based analytics programs, efficiently extracting meaningful information from vast and complex datasets. Collaborating with data and clinical professionals ensures logical and customized solutions tailored to each unique situation. Aquaculture biotechnology that prioritizes sustainability may have a large impact on human health and the seafood industry. Our review underscores the importance of adopting this model, revolutionizing the prognosis and management of Vibrio-related infections, even under complex circumstances. Furthermore, this model has promising implications for aquaculture and public health, addressing the United Nations Sustainable Development Goals and their development agenda.
Introduction
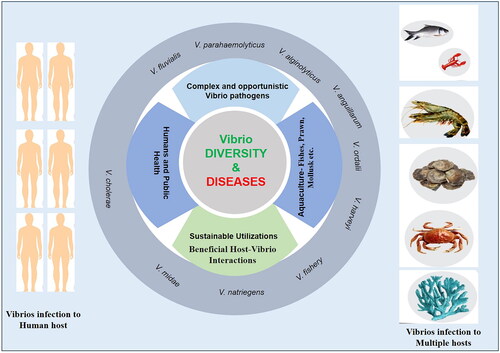
Vibrio species are a group of bacteria that have been associated with both aquaculture and human infections on a global scale. These bacteria can be found in various aquatic and marine habitats, either in a free-living state, attached to biotic or abiotic surfaces, or in a symbiotic or host-pathogen relationship with other organisms due to their metabolic versatility [Citation1]. Vibrio bacteria belong to the Vibrionaceae family and this family currently holds more than 190 species classified in over nine genera [Citation1–3]. Of these, ∼12 Vibrio species are known to cause human infections [Citation3]. The most common Vibrio species responsible for human infections include V. parahemolyticus, V. vulnificus, and V. cholera [Citation1–3]. Some important Vibrio species that cause serious infections in humans include V. cholerae, V. parahaemolyticus and V. vulnificus, while other Vibrio species, such as Grimontia hollisae, Photobacterium damselae, V. alginolyticus, V. cincinnatiensis, V. fluvialis, V. furnisii, V. harveyi, V. metschnikovii, and V. mimicus cause less severe infections [Citation4]. Several species of Vibrio, including V. anguillarum, V. ordalii, and V. harveyi, are responsible for classic vibriosis in marine life and are involved in the mass mortality of marine animals [Citation5,Citation6]. Aquaculture hosts, including shrimp, shellfish, prawn, and finfish, are particularly vulnerable to Vibrio infections [Citation4–6]. As Vibrio pathogens are ubiquitous in halophilic marine environments, the risk of infection is increasing worldwide, and many aquaculture organisms can act as vectors for human pathogenic bacteria. V. parahaemolyticus, V. vulnificus, and non-O1/non-O139 V. cholerae are the most commonly transmitted Vibrio species from seafood and different reservoirs to humans [Citation7]. The emergence of new species and strains, as well as rising global temperatures, all contribute to an increase in the burden of Vibrio-related diseases [Citation2–4,Citation7]. Despite advances in Vibrio spp. research, a knowledge gap must be filled to reduce the pathogens’ foodborne and waterborne hazard of pathogens [Citation5]. It is imperative to manage these Vibrio infections properly to avoid declines in aquaculture production and public health consequences. Continued food control and surveillance are necessary due to the high prevalence of Vibrios in environments. Therefore, biotechnology that places a premium on sustainability may have a substantial impact not only on human health, but also on the fish sector during the avoidance of hazardous Vibrio species. In this review, we focus on the most recent studies that have altered our perspective on these pathogens, identify the gaps in our knowledge, and speculate on the likelihood of technological adoption in the near future.
Vibrios worldwide distribution and epidemiological parameters
Vibrio cholerae can cause serious infections in humans. Cholera is caused by V. cholerae by eating or drinking contaminated food or water [Citation1,Citation3]. Vibrio species, such as V. parahaemolyticus and V. vulnificus cause vibriosis [Citation1,Citation3]. Vibrio species infections arise from various factors, including host variety (fish, shrimp, human, and others), immune status, pathogen types (species and strains), and environmental conditions (such as temperature, salinity of the water, and quality) [Citation1–3,Citation5]. Vibriosis symptoms can include mild gastroenteritis or primary septicemia, while contaminated water can cause wound infection and secondary septicemia [Citation3]. The distribution of Vibrio species is widespread across different continents (including the Americas, Asia, Europe, Africa, and Australia) ( and ), indicating a diverse range of host (). , emphasizes its cosmopolitan role and informs about the presence of diverse species. These Vibrio species were later converted into an opportunistic pathogen and continue to participate in the evolutionary pathway to contribute to the mechanism of disease through new adaptations and pathways. Geographic locations and diverse Vibrio pathogens referenced in the list represent a better understanding of the Vibrio diversity and global impact.
Figure 1. A geographical representation of the distribution of Vibrio species and Vibrio genera across all continents is presented here.
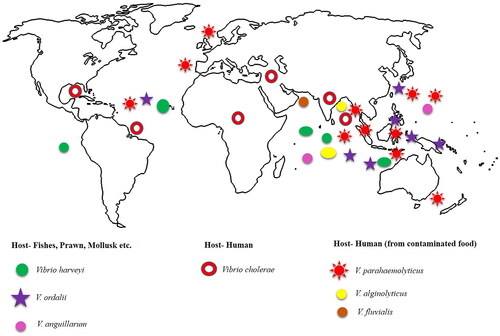
Table 1. Geographic locations and various Vibrio pathogens mentioned in the list represent a better understanding of Vibrio diversity and global impact.
The virulence of V. alginolyticus is demonstrated by mass mortality outbreaks in cultured tiger prawns (Penaeus monodon) in Taiwan [Citation18]. Additionally, the whiteleg shrimp P. vannamei is commonly cultivated and distributed from Asia to America (including Thailand, China, Vietnam, Brazil, Mexico, Texas, Hawaii, among others) and can be affected by the V. cholerae pathogen, which can infect both fish and humans [Citation19]. Overall, vibriosis has been reported to affect diverse hosts across different regions, such as the Indo-Pacific region (P. monodon), from Japan (P. japonicas), Europe, and South and Central America (P. vannamei), among others [Citation7,Citation8,Citation18–20] ( and ). illustrates the host variations and reports Vibrio species across different geographical regions. The information presented here can provide insights into the epidemiology of vibriosis worldwide, including its impact on different continents and countries and the role of climate and progression.
Table 2. Observed variation in the hosts as well as the species of Vibrio that have been reported across the various geographical regions with reference.
Human health risk and Vibrios infections
The incidence of Vibrio sp. infections, including V. cholerae, V. parahaemolyticus, and V. vulnificus, is increasing due to climate change, posing a significant threat to human health [Citation2]. V. cholerae, which can survive in both aquatic reservoirs and human hosts, is responsible for causing cholera disease in humans, with an estimated 3–5 million cases and 100,000–120,000 deaths occurring annually, and over 1.3 billion people at risk of the disease [Citation12]. Vibrio parahaemolyticus is the primary cause of human acute gastroenteritis following the consumption of raw or undercooked marine products that are mishandled, with approximately half of the cases of food poisoning outbreaks in Asia attributed to this pathogen [Citation21]. Vibrio vulnificus, found in oysters and other molluscan shellfish, is the most lethal foodborne pathogen, with a 50% fatality rate [Citation22]. In addition, V. fluvialis and V. mimicus are other foodborne pathogens that pose significant public health hazards worldwide [Citation23] demonstrates the diverse hosts of Vibrio that are involved in posing a risk to humans and aquaculture. A few Vibrio species (V. vulnificus, V. parahaemolyticus, etc.) are fatal foodborne pathogens with high case fatality rates and adopt two lifestyles in coastal water/shellfish and human disease. Meanwhile, a few Vibrio species (V. cholerae) directly affect humans. Therefore, understanding the ways of infection is important for creating a control plan.
Aquaculture risk and economic burden
Fishes, shrimp, molluscs, and prawns are important sources of food that provide essential nutrients, such as proteins, vitamins, minerals, and essential fatty acids, which are necessary for maintaining good health. Aquaculture is the fastest-growing food industry, and it plays an important role in ensuring food security, creating employment opportunities, and promoting social development in middle-income countries [Citation24,Citation25]. With the global population expected to reach 9 billion by 2050, a 70% increase in food production will be required to meet the demand. Aquaculture has the potential to address malnutrition and food insecurity issues in low-income countries [Citation25,Citation26]. However, aquaculture also poses risks to human health and the environment, as foodborne illnesses can have a significant economic impact and challenge public health [Citation27].
Clinical phenotype and pathogenesis
Clinical variations in phenotype are often the first indications of pathogen infections. Although certain phenotypes, such as reduced motility and specific lesions, are common, complexity is associated with strain variation, geographic regions, and host-specific factors [Citation6,Citation13]. In fish, symptoms of severe infection include skin ulcers, hemorrhage on fins and body surfaces, fin and tail rot, and abdominal distension. Other important clinical features of infected fish include darkened body coloration, loss of appetite, head floating, and abnormal swimming behavior. In humans, V. cholerae infection can cause symptoms, such as diarrhea, dehydration, and vomiting, while V. parahaemolyticus infection can lead to diarrhea, abdominal cramps, nausea, vomiting, headaches, fever, and chills [Citation21]. Understanding the virulence systems of fish pathogens is essential to ensure fish health. Pathogenicity involves various cellular, genetic, and immunological factors that increase the severity of the infection, leading to clinical complications and apoptosis [Citation28]. The study also highlights the importance of cellular processes, such as capsule formation, iron acquisition, and flagellar motility, in host infection. Genetic virulence depends on several factors, including virulence genes [Citation29]. Immune virulence refers to the inability of the host to protect against pathogens and the virulent biomarkers of the pathogens [Citation30–32]. Understanding how pathogens such as phagocyte activity and oxidative killing, can break through the immune barrier is essential to develop effective vaccines [Citation31,Citation32].
Diagnosis and identification
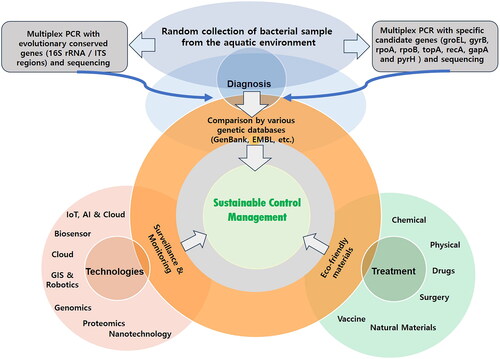
Diagnosing Vibrio disease requires clinical evaluation and histological confirmation of the Vibrio pathogen. Identification involves several methods, including Gram staining, measurement, oxidase test, glucose utilization mode, nitrate reduction, and luminescence [Citation20]. Molecular diagnosis based on multiplex PCR (Polymerase Chain Reaction, Real-time PCR, etc.) using a set of defined primers is widely used for rapid and effective detection of Vibrio species and strains [Citation1,Citation33,Citation34]. Microarrays are also important methods for the identification of Vibrio species isolates [Citation33,Citation34]. Multilocus sequence-based phylogeny accurately defines Vibrio clades and has led to the identification of new species such as V. tritonius [Citation35]. Advanced genomics has also helped in differentiating Vibrios [Citation36]. The GroEL gene, which encodes the chaperonin GroEL and controls cellular stress, is useful for detecting Vibrio species [Citation37–39]. The groEL gene is preferred over other evolutionary genes, such as 16S rRNA and 23S rRNA, and is applied in a groEL-based LAMP method (Loop-mediated isothermal amplification, LAMP is a single-tube technique for the amplification of DNA) due to its higher sensitivity and accuracy [Citation37–39].
Prevention
Disease control using chemicals, antimicrobials, vaccination, and environmental strategies, is frequently used depending on requirements.
Chemical and antimicrobial control
Vibrio-related infections can spread through water and initially affect external body parts such as fins and gills. Therefore, liquid-type disinfectants like copper sulfate and formalin may be the best treatment option, as they can disperse in the water medium and promptly act on pathogens. The study shows that compounds used in aquaculture water treatment and disease control have been found to reduce crayfish innate immunity and disease resistance [Citation40]. The analysis focused on the most commonly used fish drugs and water treatment chemicals, including norfloxacin, calcium hypochlorite, quick lime, povidone iodine, and copper sulfate. The results suggest that calcium hypochlorite led to a significant decrease in the survival rates of crayfish infected with V. alginolyticus [Citation40]. In aquaculture, various antibiotics are employed depending on the fish species and region. For instance, florfenicol and oxolinic acid are used for cod fry in Norway, while quinolones and flumequine are used for classical and cold-water vibriosis [Citation41]. However, it is worth noting that according to the Centers for Disease Control and Prevention (CDC), antibiotics are occasionally used to treat severe or long-term human illnesses like cholera, even though there is no evidence that they are effective (source: www.cdc.gov/vibrio/diagnosis.html). The residues from widespread antibiotic use can lead to antibiotic resistance and contaminate food, water, and sediments, posing risks to human and animal health [Citation41]. As a result, antimicrobial control in aquaculture is approached cautiously.
Immune protection
Proper studies are necessary to determine the ability of Vibrio immunostimulatory effects to improve innate immune activity and generate protection against various bacterial agents in several fish species. Parameters, such as hemotaxis, respiratory burst activity, phagocytic activity and lysozyme activity, must be studied in order to establish the most effective stimulants. Vaccines and immunostimulants generate innate immune protection for aquaculture by stimulating pathogen-associated molecular patterns (PAMPs) [Citation42,Citation43]. Recently, dietary intake of chemical agents, bacterial components, polysaccharides, animal-derived nutrients, plant extracts, nutritional factors, and cytokines have been reported to be effective as immunostimulants in fish for adequate innate immune protection [Citation42,Citation44]. The use of immunostimulants in fish larval aquaculture is a good option [Citation45]. It has been reported that probiotic chemicals (UltraZyme-P-FS and BioRemid-Aqua) and probiotic bacteria (Lactobacillus sp.) are effective immune protection for giant tiger shrimp (P. monodon) [Citation46]. Some vaccines have been optimized and are now regularly available for fish, which can raise measurable immune protection, primarily against V. anguillarum pathogens [Citation47]. Various methods, such as parenteral injection, incorporation into the diet, hyperosmotic infiltration, direct immersion of fish into vaccine suspensions, and spraying or showering fish with vaccine preparation have been regulated for the establishment of vaccines for aquaculture [Citation48].
Environmental protection
A high-quality sanitary system is crucial in aquaculture to prevent infection, disease, and mortalities. Previous research on fish has reported that the environment also impacts the innate immune system. Therefore, any burden related to culture systems may increase disease susceptibility and become a source of infection [Citation49]. Vibrio infections become deadly when many factors contribute together, such as poor water quality, crowding, high water temperature and salinity which can be controlled by water sanitation [Citation50,Citation51]. Several studies have suggested appropriate strategies to control vibriosis by designing and selecting ponds, water exchanges, draining, administering lime/dolomite, or regular partial harvesting. Environmental factors, such as temperature and salinity variables, offer improved capability for V. parahaemolyticus [Citation52]. In a climate change scenario, interdisciplinary studies are needed to properly understand the connection between ocean warming and vibriosis through epidemiology [Citation53]. Research on the disease is essential to advance the knowledge of pathogenesis, vaccination, and treatment [Citation54].
Aquatic animals can also be controlled by directly applying several natural compounds, such as herbal extracts, prebiotics, probiotics, immunostimulants, and non-antibiotics, in water, which can enhance protection against Vibrio infections without harmful side effects. Establishing ideal and standard conditions is essential to maintain high-quality water management. Filtration techniques based on various mechanical and biological methods also provide an effective alternative to control aquaculture infections in their natural habitats without any harmful effects. It is important to adopt several methods studied earlier for better results, including changing water, monitoring fish infection, using cage systems, etc. [Citation55].
This review focuses on updated progress on vibriosis, including epidemiology and technological aspects, such as diagnosis, treatment, pathogenesis, and available control measures of vibriosis. We emphasize the utilization of recent advanced technology for vibriosis management.
Technology-oriented Vibrio research
Biotechniques used in a preventive manner
Several biotechniques are employed for preventive studies in aquaculture. The use of probiotics, bacteriophages, natural products, and potential therapeutic compounds against vibriosis may be considered useful biotechnologies [Citation56–58]. To control multidrug-resistant Vibrio, antimicrobials can be developed using phage endolysins, based culture techniques, and studies on fish microbial contamination [Citation59,Citation60]. Although several techniques have been developed for aquaculture, such as the control studies mentioned in , further research is needed specifically for Vibrio control to achieve high control potential.
Table 3. The use of biotechnology-based mechanisms in the performance of studies on the avoidance of infections caused by Vibrio.
Biosensors-based research
Biosensors are analytical devices that convert biochemical/biological reactions into measurable physicochemical signals proportional to the analyte concentration [Citation67]. presents important Vibrio sensor, signaling, and functionalization research in aquaculture [Citation68–75]. Sensor-based Vibrio research, based on electrochemical, resonance, light-scattering, lateral flow, and colorimetric aptasensor systems, provides enough information about the sensor mechanism and is useful for further steps toward diagnosis and differentiation [Citation68–73]. Sensors can produce a wealth of data visually or on an indicator basis, which can be correlated with pathogens or indicate the possibility of environmental growth. The mechanism of biosensors, which identifies and captures the target molecule onto the electrode surface, is useful for detecting foodborne pathogens [Citation68]. Sensor-based research of Vibrio pathogens has identified several candidate biomolecules involved in important biological mechanisms related to pathogenicity. One of the essential aspects achieved through sensor-based studies is the study of immuno-functionalisation through the capture and detection of Vibrio species in the marine environment [Citation73]. The cell-to-cell communication process is called quorum sensing and this collective behavior covers bioluminescence, virulence factor production, secondary metabolite production, competence for DNA uptake and biofilm formation orchestrate as important functionalities [Citation5,Citation74–76]. It is important to understand the vibrios functionality as a nitrifier, where they compete with other heterotrophic bacteria in a nitrogen-rich environment and increase the population size, which causes the production of quorum sensing signals and secretion of toxins and biofilms [Citation76]. Quorum sensing and α-Hydroxyketone (AHKs) synthesis and sensing in Vibrio represent significant signaling events [Citation75]. Although limited information is available on aquaculture hosts, research on human host V. cholera can be correlated with other aquaculture species to create a defined database to understand the disease mechanism and Vibrio differentiation through sensing technologies.
Table 4. Combining sensor-based diagnosis with a number of different applied biotechnological methods involving Vibrio species in research.
Geographical information
The Geographic Information System (GIS) is a valuable tool for effectively gathering temperature data, airflow speed, and water conditions or currents in both land and water areas through the use of satellites [Citation77]. GIS utilizes computer-assisted technologies based on hardware and software to collect and update data. GIS-based models can identify the most suitable and sustainable locations for Aquaculture Management Areas (AMAs) [Citation78]. Waterborne diseases related to Vibrio can be efficiently monitored through advanced designed systems, which can cover and analyze large geographical regions simultaneously to obtain useful and comparative information. In the field of fisheries, some studies have reported the application of remote sensing [Citation79]. Although the use of GIS in aquaculture is limited, it can be more extensively utilized for Vibrio control strategies to enhance their effectiveness ().
Table 5. The application of cutting-edge technologies to the study of Vibrio in order to guarantee effective aquaculture and species management.
Important organizations associated with the Vibrio research
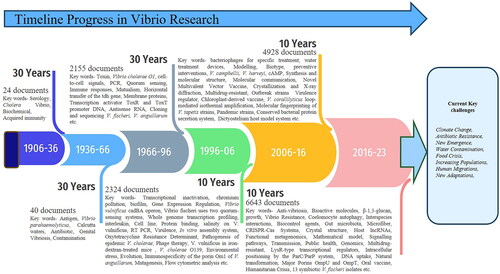
Scopus data with the keyword ‘Vibrio’ reveals 15,031 documents related to research (Figure S1a). This data demonstrates the top ten organizations and funding institutions associated with Vibrio research, which have made substantial contributions to progress in this field [Citation86]. The data (Figure S1b) indicates that, on a country-wise basis, the USA is the leader in research, followed by China and France. The organizations associated with the number of documents they have produced are as follows: Harvard Medical School, USA, with 462 documents; International Center for Diarrheal Disease Research, Bangladesh, with 380 documents; CNRS Center National de la Recherche Scientifique, France, with 360 documents; Chinese Academy of Sciences, China, with 360 documents; Howard Hughes Medical Institute, USA, with 291 documents; Pilot National Laboratory for Marine Science and Technology, China, with 268 documents; Ministry of Agriculture of the People’s Republic of China, China, with 254 documents; IFREMER Institut Français de Recherche pour l‘Exploitation de la Mer, France, with 228 documents; Ministry of Education of the People’s Republic of China, China, with 222 documents; and Massachusetts General Hospital, USA, with 211 documents. These organizations have made significant contributions to Vibrio scientific research and technology, impacting the field [Citation86]. Additionally, here are some important organizations associated with funding Vibrio control research, based on the number of documents published in Scopus data [Citation86]. The top 10 funding organizations (Figure S1c) involved in funding were the National Institute of Allergy and Infectious Diseases (1779 documents), the National Natural Science Foundation of China (1113 documents), the National Institutes of Health (1006 documents), the National Institute of General Medical Sciences (899 documents), China’s National Science Foundation (550 documents), National Key Research and Development Programme (381 documents), Japan Society for the Promotion of Science (283 documents), the National Institute of Diabetes and Digestive and Kidney Diseases (218 documents), the National Research Foundation of Korea (167 documents), and the National Center for Research Resources with 162 documents.
Key challenges under current practices of Vibrio research
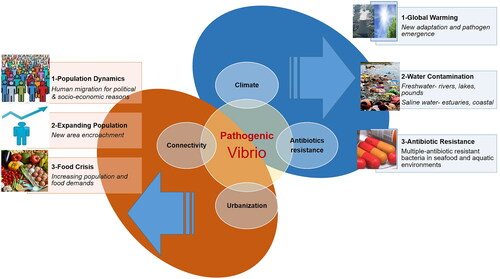
Vibrio-related infections are increasing worldwide both in humans and aquatic animals. Increasing global population and food demand are important issues that intersect with human health [Citation25–27]. Climate is another challenge that gives rise to emerging Vibrio’s challenges [Citation7,Citation13,Citation50,Citation52,Citation53,Citation76]. An effective control management system for Vibrio in aquaculture is missing various fundamental elements, which can compromise both the quality and quantity of the yield. Disease control using antimicrobials, vaccination, and environmental strategies is associated with various downstream challenges, which will be discussed in detail in the sections below. Schematic representation of the key challenges associated with vibriosis is shown in , which demonstrate the multiple factors connecting urbanization, climate changes and multiple-antibiotic-resistant bacteria interrelations in humans and aquaculture system.
Figure 3. Schematic representation of the key challenges associated with vibriosis. Vibriosis caused by Vibrio increases due to human urbanization and increasing connectivity. Climate changes and multiple-antibiotic-resistant bacteria in seafood and aquatic environments burden humans and aquaculture production.
Climate change and new emergence
In vibriosis, the clinical presentation depends on several factors, making it difficult to determine the precise cause of the infection. The clinical phenotypes of Vibrio are influenced by several epidemiological factors, such as strain diversity, host specificity, environment, etc., complicating their identification. Climate change has a significant impact on marine organisms, resulting in varied environmental conditions that lead to genetic divergence and probable convergence for Vibrio species [Citation2,Citation76]. The increase in disease outbreaks caused by V. harveyi under elevated sea surface temperature and starvation potentially reinforces the pathogenicity of the organism, as evidenced by the higher expression of many virulence genes [Citation5].
Generalized additive models revealed that long-term increase in Vibrio abundance is promoted by increasing sea surface temperatures (up to ∼1.5 °C over the past 54 y) [Citation7]. At Gulf Coast sites, the numbers of V. vulnificus increased with water temperatures up to 26 °C and were constant at higher temperatures [Citation50]. The rise in global sea surface temperature (SST), which is approximately 1 °C higher now than 140 years ago and is one of the primary physical impacts of global warming, has been associated with the rate of bacterial growth, culturability, expression of pathogenic traits, and interactions with aquatic organisms and abiotic surfaces [Citation53]. Global warming effects on Vibrio interactions with zooplankters; therefore, there is a need for interdisciplinary studies to understand the connection of epidemiology of the vibriosis. PCR fingerprinting study highlights the complex genotypic variations that occur in Vibrio strains (V. alginolyticus and V. harveyi) could affect genomic variations, depending on the environmental conditions of the culture [Citation53]. Due to varied environmental conditions, genetic divergence and/or probable convergence occur, which subsequently lead to the organismal adaptive variation, which results in their ability to cause a productive infection in aquatic organisms or generation of new strains. Recent studies regularly report diversity and evolutionary mechanisms in emerging strains of the family Vibrionaceae [Citation2].
Research on species diversity and virulence
Vibrio spp. are key pathogens in many species of cultured and wild fishes and vibriosis is a major fish disease among aquaculture leading to significant economic losses. Presently, eight genera have been identified within the family Vibrionaceae with 172 validated species, and amongst the many such species: V. harveyi, V. vulnificus, V. alginolyticus, and V. parahaemolyticus are the frequently encountered fish pathogens that are associated with significant economic losses in the aquaculture industry [Citation87]. The prevalence of zoonotic agents in fishes varies seasonally in both wild and cultured fish and mostly these zoonotic diseases are transmitted to humans mainly via the improperly cooked or raw fish or fish products [Citation88]. Furthermore, asymptomatic fishes act as pathogen reservoirs, and their existence always generates the possibilities of infections and diseases [Citation88]. Diagnosing subclinical infections is considered the major challenge in controlling further infections. Global warming, increasing human connectivity, urbanization, antibiotic resistance, and food crisis, present challenges to both human and aquatic life, where Vibrio’s pathogenic mechanism plays a crucial role [Citation89]. All major risks affecting the socioeconomic aspects of human life are represented in . Virulence factors move the infection mechanism in the host. Pathogenic Vibrio spp. possess virulence factors, such as the membrane and secretory proteins, polysaccharide capsule, outer membrane components, siderophores and biofilm forming proteins etc., and lead to disease development in the host tissues [Citation87]. Regarding the virulence and lethality of Vibrio species, extracellular products produced by Vibrio have been identified and examined, and they play an essential role in pathogenicity depending on the organism and host. The extracellular products (ECPs, including proteases) produced by Vibrio-diseased shellfish and marine fish are considered important determinants of the pathogenesis of Vibrio in fish. The major exotoxin of the pathogen is a cysteine protease, and ECPs possess hemolytic and cytotoxic activities that contribute to pathogenicity. It has been reported that phospholipase and hemolysin also play a significant role in the pathogenicity of vibriosis. Certain Vibrio species impose a greater burden on the aquaculture industry due to their species-specific pathogenic mechanisms. Numerous studies have examined the diversity associated with pathogenicity in aquaculture.
Vibrios adapt quickly to environmental stresses. Different environmental conditions allow Vibrio species to adopt and modify, increasing the chance of gene exchange that improves fitness for nutrients and survival and leads to virulent species. The study found that 70 of 130 Vibrio isolates identified by sequencing a recA fragment had virulence-associated genes, such as ctx, ace, tcpA, tdh, trh, vvhA, vllY, and toxRS, confirming the spread of virulence determinants across environmental isolates [Citation90]. Using virulence genes as identification markers may be significant because their presence may be linked to pathogenesis. However, there is a potential risk of misidentification when applying this approach to environmental samples, as these genes may be transferred among bacteria. Pathogenicity and genomic variations have been found among V. harveyi and V. alginolyticus isolates, and several genetic mechanisms (such as horizontal gene transfer, prophage and plasmid integration, phages, and conjugative transposons) are involved in generating pathogenic Vibrios from environmental isolates [Citation6,Citation76]. Vibrio species are mesophilic and chemoorganotrophic, possessing facultative fermentative metabolism, ubiquitous inhabitants of aquatic environments, except for V. cholerae and V. mimicus (halophilic organism, salinity adaptations commonly occurring at 30–35 ppt) work as commensal, mutualistic, and pathogenic species [Citation91]. The role of Vibrio spp. in marine organic carbon cycling is diverse and support their pathogenic behavior in varied warming environmental conditions, which suppress fish immunity and increase their susceptibility to vibriosis [Citation91]. Virulence-related factors are often associated with mobile genetic elements (such as prophages and genomic islands). A well-known example of prophage-associated virulence is in V. cholera, where key toxins (CTX and Zonula occludens toxin (zot)) are encoded by prophages [Citation92]. Prophage-associated toxins have also been identified in diverse marine Vibrios, such as V. coralliilyticus, V. anguillarum, and V. parahaemolyticus, indicating that prophage-encoded virulence genes are disseminated among environmental Vibrio populations [Citation92].
Immune protection and vaccine
The pathogenesis of vibriosis is influenced by several factors, including host and bacterial isolates as well as ecological conditions. Immune protection is limited by cost-effectiveness and insufficient knowledge of virulence, and intramuscular vaccination is laborious and stressful for fish [Citation93,Citation94]. Furthermore, various downstream complications have been associated with vaccine preparation and optimization. Vaccine efficacy is often challenged by factors such as morphology, biochemical assays (serotype), pathogenicity (virulence), immunogenicity, etc. [Citation93,Citation94].
Providing long-term protection is an essential characteristic of vaccines; however, booster doses can also be considered. Nevertheless, extensive use of vaccines in culture systems is limited by cost factors. In the case of human infections, oral cholera vaccines (OCVs) have recently been widely accepted as a tool for cholera control. However, vaccines have limitations in terms of long-term protection, requiring multiple doses [Citation95,Citation96].
Multi-host pathogens and cost-effective diagnosis
Due to the proximity of humans to the aquaculture industry, they are constantly facing infection problems caused by zoonotic bacterial agents [Citation97,Citation98]. Humans also face the risk of contracting vibriosis disease by consuming fish carrying zoonotic agents. Several Vibrio species are multi-host pathogens, and some such as V. cholerae, V. parahaemolyticus, and V. vulnificus are pathogenic to humans, posing a major threat to public health [Citation1,Citation5,Citation97,Citation98]. illustrates a diagrammatic representation of Vibrio infection and disease hosts, emphasizing the diverse host range. Vibrio species are also considered opportunistic pathogens, and humans sometimes act as reservoirs. In humans, approximately 75% of V. cholerae infections are asymptomatic. Among symptomatic infections, about 5% of cases are mild, 35% are considered moderate, and approximately 60% of infections are categorized as severe, according to Baker-Austin et al. [Citation1]. Asymptomatic individuals act as carriers and sources of the infection. V. vulnificus is another opportunistic pathogen, commonly associated with liver diseases, such as cirrhosis or hepatitis, diabetes mellitus, and malignancies. Infection conditions lead to elevated serum iron levels, which the bacterium requires for successful tissue growth and invasion [Citation1].
Regarding fish and marine animals as hosts, several Vibrio species, particularly V. harveyi, function as opportunistic pathogens [Citation99]. Since asymptomatic infections can also lead to disease, research involving multiple hosts, including aquaculture animals and humans, is essential for a better understanding of control mechanisms [Citation99]. The diagnosis of vibriosis primarily relies on clinical symptoms, encompassing external and internal lesions. However, the immediate and cost-effective diagnosis of aquatic organisms remains challenging [Citation100]. Diagnosing subclinical infections is considered the primary challenge in controlling further infections because, phenotypically, they exhibit no clinical signature, yet they carry the pathogen asymptomatically, contributing to the spread of infections [Citation1]. Furthermore, PCR-based diagnosis can incur costs and potential misidentifications due to the close genetic diversity within Vibrios, necessitating further examinations through sequencing with additional markers [Citation101–103].
Traditional bacterial identification methods based on culture, morphology, or biochemical reactions are time-consuming and resource-intensive. Moreover, identification methods are dependent on diverse biochemical and molecular methodologies, which require a comparative analysis of pathogen and host clinical phenotypes. The complexity of phenotypes, diversity of Vibrio strains, and highly variable virulence factors pose significant challenges in achieving accurate identification. Given the vast number of aquaculture species, it is challenging to diagnose all possible pathogens in a large water system. Thus, several issues must be addressed to improve the diagnostic process.
Evolutionary mechanism and environment
Vibrio genomes undergo frequent recombination in the aquatic environment, leading to zoonotic infections [Citation98,Citation101]. High gene similarity poses difficulties in differentiating between related species of Vibrionaceae (e.g. V. campbellii, V. harveyi, V. parahaemolyticus). Even important evolutionary genes, such as 16S rRNA, 23S rRNA, 16S-23S intergenic spacer region, or gyrB may not differentiate Vibrio species [Citation98,Citation101]. Thompson et al. sequenced and compared seven housekeeping genes (topA, pyrH, ftsZ, mreB, gyrB, recA, gapA) to distinguish V. campbellii from V. harveyi. To overcome misidentification of Vibrio species and strains, PCR-based analysis uses other housekeeping genes in differentiation analysis [Citation6,Citation102]. PCR identifies Vibrios at the species or sub-species level and is useful in epidemiological investigations [Citation6,Citation102,Citation103].
The environmental conditions may cause false-positive or false-negative results when analyzing virulence genes. Vibrio species can exchange genetic elements, including virulence genes. Identifying virulence markers to differentiate environmental and clinical isolates is crucial in controlling this pathogen [Citation104,Citation105]. Due to the ability of Vibrio species to cause co-infection in multiple hosts (e.g. shrimp farms and animals), multimarker-based PCRs for all hosts are necessary, which increases the overall time and cost of the process.
Comparative genomic analyses identified the V. harveyi clade, which contains known pathogens from fish, corals, mollusks, and humans [Citation106]. V. cholerae is known for its genomic plasticity and its ability to exchange genes through natural transformation, conjugation, and transduction. Virulence is generated by continuous evolution in this bacterium due to acquisition or loss. Chun et al. [Citation107] proposed a hypothetical evolutionary pathway for the emergence of the seventh strain of V. cholerae pandemic driven by environmental factors. This pathway was proposed for the purpose of evolutionary speculation. Furthermore, virulence has evolved through diversification of Vibrio strains, and studies on evolutionary links are still missing [Citation108]. In this sense, having a better understanding of the evolutionary processes that led to the appearance of Vibrio pandemic strains could result in the development of innovative strategies to combat this pathogen.
Antibiotic resistance
The use of antibiotics and antibacterial cleaners, such as benzalkonium chloride or Virkon, has several drawbacks in downstream systems, such as pathogen drug resistance, host clinical complications, and environmental pollution. However, the interaction between host-pathogen and the environment is critical for disease resistance. Antibiotic-resistant Vibrio species have been reported in many studies [Citation109]. Antimicrobial susceptibility of Vibrio species related to food safety has also been found [Citation109]. Many known antibiotics such as penicillin and streptomycin are limited in use due to cost-effectiveness and resistance issues generated by extensive use. The biofilm that forms Vibrio spp. poses increasing problems with the development of antibiotic resistance that causes severe threats in aquaculture, reported by Arunkumar et al. [Citation110]. Specific structural genes and regulatory systems of the quorum sensing system mediate biofilm formation in Vibrios [Citation110]. Antimicrobials used worldwide to treat Vibrio infections may cause resistant bacterial strains, horizontal gene transfer, and harm to humans, the environment, and aquatic ecosystems [Citation41].
The effective policy for control strategies
Although the aquaculture industry faces many challenges, current technology may provide solutions. However, there is a lack of knowledge and practice regarding confirmed identification methods that can be applied to larger fish cultures regardless of biological material or mixed clinical phenotypes. Currently, phenotype observation along with rapid and random molecular studies is the best strategy to detect any type of infection or carrier at an early stage. According to the United Nations Department of Economic and Social Affairs (UN-DESA 2009), the current world population (6.8 billion) is expected to grow by approximately 32% (to 9 billion) by 2050 with an increasing demand for food [Citation26]. Thus, effective measures are needed for fisheries industries to control vibriosis disease, so that food quality and quantity can be maintained. Based on various studies, it is clear that due to the non-availability of effective and universal vaccines or antibiotics for fish diseases, environmental protective measurements with advanced technology integration may be the best strategies for vibriosis disease control. However, effective control systems can be achieved through advanced technologies and upgraded management of the aquaculture industry. Subject-wise research should focus on fishery science and technology, biological science, environmental conditions, pathogen research, advanced technologies, and management practices for the best results.
Disease control in aquaculture should ideally adopt high hygienic standards and environmental strategies based on upgraded management steps and interbreeding of innately strong fishes. Based on our phylogeographic information in and , we suggest that strategies based explicitly on bacterial species or strains and aquaculture hosts (fish and prawn, etc.) are the most effective control mechanisms. Some bacterial strains are present more predominantly in one region than in others, and likewise, fish species also show dominance in specific geographical areas (; and ). In this article, we suggested specific regional strategies for vibrios and affected hosts. Human diseases due to infections of V. cholerae, V. parahaemolyticus, V. Vulnificus and V. fluvialis, needs public health-based control model while other Vibrio species require more emphasis on an aquaculture model due to fisheries hosts [Citation1,Citation3,Citation5,Citation7,Citation9]. Further, V. cholera is associated with Asian, African and American regions, and consequently requires more public health strategies for human infections control based on sanitization, food, socio-economic and educational strategies [Citation3,Citation5,Citation7,Citation29,Citation34]. Well-known fish pathogens V. parahaemolyticus, and V. Vulnificus etc. need strategies for food-based processing and heating systems to control the infections [Citation3,Citation5,Citation7,Citation111,Citation112]. In the case of control of V. alginolyticus (highly salt tolerant more in south Asian regions) and V. harveyi (found free-swimming in tropical marine waters with up to 35 degrees centigrade), V. anguillarum in marine organisms, need more aquaculture-based control strategies to stop the economic loss in the fishery industry [Citation1,Citation3,Citation5,Citation7,Citation10,Citation18,Citation113].
Effective control management should consider both host variables in the analysis. Among human-affected Vibrio species, it is crucial to observe the emerging strains through advanced technologies and monitor environmental conditions through GIS as an early precaution to stop any possibility of endemicity.
Phenotype and pathogenic research
Advanced research of virulence and public health
To enhance immunity against Vibrio infections, more research should focus on innate and adaptive immunity, cellular-based immunity responses (such as macrophage, T cell, and B cell markers), and humoral elements (including complement factors, cytokines, and various immunoglobulin classes) and their mechanisms. Additionally, continuous research on different pathology-associated assays, such as immunohistochemistry, immunocytochemistry, advanced technology-based diagnosis, flow cytometry, and gene expression will reveal unknown mechanisms about pathways. Furthermore, all this information can be established in vivo to make vaccines or therapeutic mechanisms more effective against Vibrio diseases. A study of V. cholera infection indicates long-lasting immunity or protection against reinfection for the longest interval [Citation114,Citation115]. Studies of toxin function through circulating CtxB-specific immunoglobulin G (IgG) antibodies and CtxB-specific IgG memory B cells are important for immune protection against human cholera [Citation115]. However, there is a need to develop cholera conjugate, multivalent, and live attenuated vaccines for a better immunization schedule [Citation115]. An advanced understanding of human defenses against Vibrio infections also opens the door for control through commensal bacteria [Citation116]. Clinical phenotypes of Vibrios must be correlated with drug resistance, genetic profile, virulence, and mortality to understand their pathogenicity and make better protection plans for aquaculture and public health.
Basic research of virulence and breeding innately improved fish
The mechanisms underlying the innate resistance of specific fish species toward vibriosis infections have not been fully explored. Basic and advanced research on the immune systems of aquaculture organisms and the effectiveness of pathogenic molecules for pathogenicity will provide more knowledge about protection against vibriosis diseases. Further research on virulence factors will provide more knowledge about pathogenesis mechanisms. Effective disease prevention strategies have shown that the host immune system controls Vibrios, whereas virulent strains rely on Vibrio species-specific molecular determinants [Citation117,Citation118]. Earlier studies established that treating fish with vaccines and immunostimulants that can stimulate innate immune responses against β-glucans was a good strategy. A study shows that differential responses of cytokines in the intestine of fish upon exposure to V. anguillarum suggest that both mannan oligosaccharides and β-glucans impact the ability of Atlantic cod to respond to the pathogen [Citation119,Citation120]. The study also shows that modern molecular genetic technology provides a genetically defined mutant construct that can be differentiated from the wild-type strains, confirming that it is the only variable in the evaluated strain. However, this technological approach is limited for pathogenesis in fish due to the lack of adequate systems to achieve the demand for mutants in fish pathogens. Therefore, the development of such approaches, which can manipulate the genomes of aquaculture pathogens, will provide a detailed understanding of susceptibility and resistance mechanisms. Various studies indicate that several factors are possibly involved. Host genomic information can substantially improve selective breeding for disease-resistant traits [Citation121]. Therefore, it is important to propagate and nurture interbreeding programs to develop innately improved fishes, which can show high resistance to bacterial infections. Innately improved varieties can be achieved either by including disease-free fishes in aquaculture sites for breeding programs or through environmentally friendly experiments, which enhance the capability of fish to fight pathogens more effectively. Removing susceptible fishes from aquaculture systems and regularly increasing the innate resistant fishes is considered an optimal strategy for disease control.
Dietary oil sources and symbiotic method to develop innate immunity
Dietary methods offer a better alternative to curing pathogen-related diseases than antibiotic treatments [Citation109,Citation122]. A study has shown that the diet formulated with oleic acid provides the highest fish survival rate and growth rate [Citation122]. The sources of dietary lipids and the fatty acid composition of dietary oils can modulate immune responses. Nutritional food has a long-lasting effect on increasing fish immunity against pathogens [Citation123]. The administration of dietary supplements, such as symbiotics, prebiotics, and probiotics, can be used to improve the health status of aquaculture. Their effects can be evaluated through growth and immunological assays to assess disease resistance capabilities in fisheries [Citation123].
Bacteriophages, probiotics and other methods of protection
Bacteriophages, commonly referred to as phages, are important in controlling infections in an aquatic environment, including fish pathogens [Citation124,Citation125]. Given the detrimental and costly effects of antimicrobial agents, chemical and vaccine control strategies, the use of phages against specific pathogens is becoming increasingly important. Studies have shown that isolated phages with lytic activity against V. harveyi were applied as phage therapy against luminous vibriosis to improve the survival of P. monodon larvae, while bacteriophages were helpful in the biological control of V. alginolyticus and V. anguillarum in aquaculture [Citation58,Citation63]. Several potent phages have been tested against causative agents, such as V. harveyi, V. parahaemolyticus, V. alginolyticus, V. splendidus, V. anguillarum, and V. coralliilyticus, resulting in higher survival rates of cultured animals. The potential effects of phages against vibriosis are mentioned in of Kalatzis et al. [Citation57].
In addition to phages, other methods contribute to the protection of fish against vibriosis, such as probiotics and natural products [Citation56,Citation123]. An earlier study shows that the bacterial storage compound poly-b-hydroxybutyrate (PHB), a polymer of the short-chain fatty acid b-hydroxybutyrate, can protect Artemia nauplii from the virulent V. campbellii strain [Citation60]. Probiotics have been shown to produce inhibitory compounds for pathogens and compete with harmful microorganisms for nutrients, adhesion sites, and energy. Moreover, they should enhance the host’s immune system, improve water quality, and interact with phytoplankton [Citation126]. Best-suited probiotics can colonize intestinal mucus, exhibit resistance to low pH and bile salts, and target quorum sensing systems of pathogenic bacteria [Citation56]. In aquaculture, probiotic bacteria have been used as harmless microbial control strategies in many studies (). Various probiotics have been found to be effective against several Vibrio species, such as V. anguillarum and V. harveyi, which are controlled by quorum sensing. In contrast, V. parahaemolyticus and V. harveyi are controlled by other bacterial strains or Vibrio strains as probiotics, and the V. alginolyticus strain lacks pathogenicity [Citation126,Citation127]. Under the increasing Vibrio risk, we believe that probiotics and selective bacteriophages are the best options for controlling harmful Vibrio species instead of using antibiotics in aquaculture.
Sensor-based research for pathogenicity
Ideally, biomarkers related to disease should prioritize sensitivity, specificity, reliability, rapidity, and low cost for detecting pathogens. In addition, the development of biochips based on Vibrio identification markers for large sample sizes is currently required for food safety and public health. Sensor techniques for Vibrio should be based on pathogenic biomolecules, multidetectors, nanotechnology, and smaller sizes to provide real-time information on the infection burden for a smaller sample with good sensitivity. Several studies have reported accurate sensors based on biological materials of pathogenic bacteria and biomolecule-related interactions, such as microwave-microfluidic, CRISPR-Cas12a-derived biosensing, antibody-antigen, and DNA hybridization mechanisms [Citation67,Citation128–130].
Utilizing the latest material science advancements for sensor technology may be a relevant step toward Vibrio control. Studies based on nanoparticles, graphene, and ratiometric fluorescent sensors have found them effective for pathogen identification and analysis [Citation129,Citation130]. Multiplexed detection of foodborne pathogens through electroanalytical sensor devices may be the leading approach for vibriosis [Citation131]. The above sensor-based mechanism should be studied for Vibrio-related species for more accurate sensor technologies. However, there is growing interest in candidate molecule research and new sensor mechanisms to include in sensor technology, primarily focused on V. cholera.
Techniques for effective diagnosis
Molecular identification methods and genetics research
Identifying Vibrios in aquaculture is essential to take preventive measures early. Understanding aquaculture genetics and available genomics information is necessary for identifying the pathogenesis mechanism based on genetic materials [Citation132]. Molecular strategies have an edge over other standard microbiological culture-based methods as they are fast and cost-effective [Citation100]. Among molecular methods, candidate genes-based multiplex PCR is considered an important approach due to the time, cost, and differentiation efficiency of related species and isolates. Candidate genes that have been well characterized for diversity include 16S rRNA, heat-shock proteins (HSPs), groESL, and tRNA gene intergenic spacer region (ISR) [Citation133]. Genetic variations in any vibriosis strain arise from mutations, insertions, deletions, etc. These variations represent the bacteria’s evolutionary and virulence interrelation [Citation100]. Therefore, studies based on any pathogenic candidate gene can provide information on various strains due to evolution [Citation134]. Among several genes, evolutionarily conserved genes and candidate conserved genes are widely investigated in many Vibrio species through combined genetic analysis. Research on the genetics of diseases, stress, nutrition, metabolomics, and population is helpful for identifying and establishing molecular techniques that are preferable under changing global scenarios. Genetic evidence identifies the divergence of Vibrio species within the Harveyi clade [Citation135]. Furthermore, the latest trends in aquaculture genomics research and technology related to data collection and genome editing using CRISPR/Cas9 are important objectives for future research [Citation132]. Vibrio has a diversified host, so adopting a hologenomic approach is the best strategy to understand human disease resistance and sustainable aquaculture growth [Citation44,Citation136,Citation137].
Recent advancements in genomics
The decreasing cost of genome, transcriptome, and exome sequencing has enabled many new applications in the study of infectious diseases [Citation100]. Whole-genome sequencing can be performed quickly and inexpensively for trend studies, diagnostics, and real-time surveillance to detect disease outbreaks. Functional genomics, selective breeding, quantitative genetics, sex control, genomic prediction, and epigenetics are all exciting areas of research that can aid in understanding disease and environmental risks [Citation132]. The aquaculture industry requires a sophisticated NGS-based system capable of producing immediate results for microbial genomes, transcriptomes, and metabolomes in order that researchers can draw valuable conclusions for implementing effective precaution strategies.
Evolutionarily conserved and genetic virulence gene
Conserved genes, such as 16S rRNA, are preferred over other candidate genes in molecular methods because of their capability to facilitate comparative bacterial identification and further our understanding of virulence and variations within them [Citation134]. Evolutionary gene databases that are well-defined correlate with taxonomic identification and disease epidemiology [Citation102,Citation103,Citation138].
The expression of virulence factors is influenced by ecological conditions and the host environment. Understanding the genetic virulence mechanisms in vibriosis is crucial for better control, but validating a single or a few virulence genes for specific species of bacteria is challenging due to the variations of ecological risk factors and the biological system of Vibrio at both the micro and macro levels. Genetic virulence factors can be potential drug and vaccine candidates or serve as a diagnostic marker. Depending on the disease caused by specific strains, molecular techniques based on virulence genes can be useful for diagnostic purposes. For instance, Kurdi Al-Assafi et al. identified extracellular capsule polysaccharide (CPS), protease, cytotoxins, many hydrolytic enzymes, hemolysin/cytolysin, and other extracellular toxins as virulence determinants for V. vulnificus infections in humans, all encoded by their respective virulence genes.
Currently, a diagnostic system integrating genetic data (virulence and evolutionary) would be a convenient feature where inexpensive and highly sensitive molecular reactions can be used to detect and identify biological material, even in mixed samples, with great efficiency. Once this genetic information is integrated with the management platform, it can deliver efficient outputs useful for preventive measures ().
Sensor-based diagnosis
Phenotypic characteristics associated with Vibrio diseases can be leveraged for sensor-based technologies, as biosensing mechanisms can enable instant disease monitoring. Biosensors that utilize nanoparticles are particularly important for waterborne pathogens due to their specific and sensitive detection capabilities for biotic and abiotic analytes. Biosensors can quickly and continuously monitor and measure metabolic and biological activity, and they can also function within living tissue [Citation139]. Biosensing platforms for Vibrio detection based on whole-cell and nucleic acid analyses are widely used [Citation70,Citation140,Citation141].
Real-time identification of V. parahaemolyticus, V. vulnificus, and V. cholerae colonies using a light-scattering sensor on a solid agar plate has been reported [Citation72]. By combining a biosensor with multiple cross-displacement amplification, V. cholerae was rapidly, visually, and sensitively detected [Citation73]. The above example illustrates the potential of nanotechnology for Vibrio diagnosis.
Key measures for prevention
Eco-friendly materials and breeding control
The best strategy to maintain ecological balance is to avoid the use of chemicals. However, chemicals can provide quick relief from infections in aquaculture, although they have hazardous side effects on the environment. The use of oxidizers, such as hydrogen peroxide, sodium percarbonate, and peracetic acid is a superior control strategy. Most important is to use eco-friendly materials to avoid the negative impact on environment. Nevertheless, the selection and breeding of pathogen-resistant hosts may be crucial, as the use of chemicals can lead to the origin of drug tolerance and resistance and disturb ecological conditions. The use of antibiotics should be avoided, as it can modulate the genetic inheritance pattern of pathogens. It is important to note that pathogens can be present in a dormant stage with adaptation in a host, which can lead to asymptomatic infections in other hosts in the future. Thus, it is crucial to take precautions during the antibiotic treatment strategy in aquaculture. Dilution of the adverse antibiotic effect through inbreeding with naturally strong groups can produce better generations.
Environmental control
Environmental control is considered an effective strategy among all control methods as it can be easily implemented without causing harm to the environment. It can be achieved in various ways through appropriate management decisions (). We suggest regularly monitoring randomly selected aquaculture organisms for their health status to detect any irregularities at an early stage, based on which proper control strategies can be implemented. Intelligent decision-making at the regional level has improved control mechanisms. The optimal control system relies on maintaining fish stocks at numbers as low as possible to ensure all required resources are in sufficient supply and to reduce the chances of spreading infection. A crowded aquaculture community can cause stress and lead to low health status [Citation100]. Surveillance of Vibrio populations in aquaculture water can be used as an indicator of microbial contamination of fish fillets [Citation59]. Using physical barriers through netting and removing moribund fish or aquaculture communities can control the chances of infection. Studies have demonstrated the biotechnology revolution in fish health management [Citation142,Citation143]. An appropriate system with high-quality water reduces the possibility of infection. Research has shown that deteriorating water quality promotes bacterial growth and aquaculture mortality, so this information could be used as a risk [Citation100]. Ecological conditions are important for pathogen infection and survival. Vibrio disease outbreaks also depend on environmental conditions [Citation144]. Factors, such as water salinity, dissolved oxygen, and pH have significant effects on the resistance rates of seawater-derived Vibrio isolates [Citation14]. The current need is to identify effective entities that can play a crucial role in better control.
Photo inactivation in aquaculture
Photo inactivation or light therapy has been studied for its potential use in aquaculture and has shown promising effects in various studies [Citation145,Citation146]. The technique involves the use of various light therapies, such as ultraviolet C irradiation, photodynamic therapy (PDT), and blue light to inhibit bacterial pathogens in aquaculture [Citation73,Citation146]. However, limited knowledge is available about their application in the aquaculture industry. Ultraviolet (UV) irradiation has been found to adversely affect fish, causing skin lesions, reduced mucus production, and downregulation of innate immunity [Citation147]. On the other hand, blue light (400–500 nm) has been found to have an antimicrobial effect and is less harmful than UV irradiation [Citation71]. In addition to light therapy, bacteriophage therapy is another approach to controlling bacterial infections in aquaculture [Citation148].
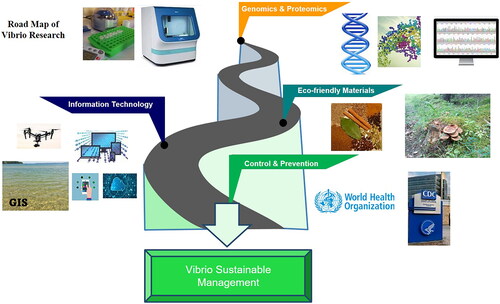
Proposed advanced technologies
Considering recent technological advances, we propose the use of advanced models integrated into a single platform for better results. Various technologies mentioned in have significantly transformed information technology, computer science, and health science and should be incorporated into aquaculture control mechanisms for better outcomes.
Adaption of advanced technologies
Biosensors and disease analysis
Biosensing technology is considered to be the best for mass-level identification due to its sensitivity and ease of use, which increases its reliability. If biological methods or phenomena are combined with electronic technology, those electronic devices can start functioning through a simple operation that involves molecular and cellular modulations and electrical communication. The bio-recognition component is a crucial part of biosensors. This component, fixed on the surface of the electrode by physical or chemical methods, generates communication through the electrical signal and can be carried forward to the next level to achieve qualitative or quantitative analysis. The biosensor can selectively work and produce a wide variety of data depending on the inputs. Studies related to the immunosensing of bacteria or biomolecules and the electrochemical biosensing of molecules using advanced material science have already established biosensor research at the next level [Citation149–151]. The concept of bioelectronics allows capturing any modulation at a cellular or molecular level and, through bio-electrical communication, can enrich information in real-time. Bioelectronic research on the switchable mechanism through auto, light, pH, etc., leads to advanced Vibrio research, which can more efficiently strengthen control strategies [Citation151,Citation152].
Artificial intelligence and machine learning
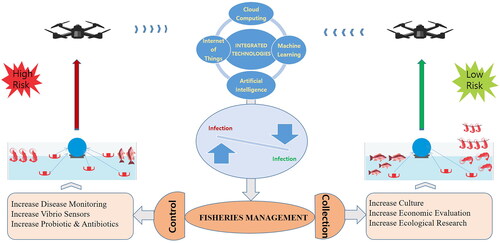
Artificial intelligence (AI) is a field of computer science that enables fast and high-quality decision-making based on multiple analyses. AI involves computer programming for specific traits using mathematical analysis and algorithms for optimal performance. Machine learning (ML) and deep learning are important analysis categories used in AI. AI generates highly intelligent output that can be useful in drones with robotics and camera technology. The use of an AI camera is helpful in aquaculture navigation, localization, and mapping through image analysis services. The information collected from various drone sources can be analyzed through an AI support system, which can provide dynamic maps of ecology and disease alerts. This can help the end-user make timely decisions before any potential losses due to either ecology or pathogens occur.
Internet of things (IoT) devices
IoT devices have become an essential part of the technology industry, as they can collect data and transfer it to research professionals via linked devices. IoT can assist even in remote areas and is helpful in emergencies. IoT technology is useful to speed up communication mechanisms between aquaculture fields to control management systems. Important oceanographic data and their impacts on aquaculture can be tracked to make the industry less prone to errors. Next-generation IoT-related technology is a must in the aquaculture industry, as it is helpful to predict harmful ecological variations, monitor behavior, measure aquaculture information, and transmit it in real-time for further assessment.
Cloud computing and data analytics
Cloud computing and data analytics play an essential role in handling data and inferring its real meaning. Cloud computing involves gathering information, data exchange, connectivity, and storage management. In the ocean, cloud computing is useful for research [Citation153]. However, cloud computing requires analytics programs and data experts to extract real meaning. Data analytics is used to solve all issues related to data based on a specific model or algorithm. Cloud computing can take advanced predictive measures through artificial intelligence. By using a specific AI algorithm and the geographic/GIS (geographic information systems) dimension, cloud computing can justify big databases of health and ecological parameters. It can monitor big industries efficiently through a management platform. A high-performance computing infrastructure is essential for big data storage and analysis [Citation154].
Proposed integrated technology system
The advent of information technology, IoT, ML, and AI has opened new doors for fisheries science to adopt and implement better diagnostics and control mechanisms. Early information can be obtained through diagnostics by monitoring with advanced sensors. Pathogenicity-based sensors in various localities of the same region can help to understand the real impacts of infections. Nanotechnology-oriented sensor-based mechanisms could be adapted as molecular electronics, which can be used to solve aquaculture diseases more frequently. There is an urgent need to create many biosensor-based bioelectronics that can diagnose various pathogenic molecules of Vibrio species and convert them into effective signals.
Machine learning is one of the computational modeling methods that can analyze such varied signals at maximum variable parameters like Vibrio species, pathogenic protein, ecological conditions, etc. and generate the best-fitting algorithm for a big geographical area. Through a generated and defined algorithm, we can obtain instant information for alerts that help us understand the risk factor of diseases in the fishery community to the maximum extent.
The current upgrade in information technology and futuristic 5 G technology has extensively changed scientific technology in all sectors. With the IoT, one can spread messages and get instant alerts. Integrated platforms that use these technologies can control and manage challenges (). The technologies of specific objectives can be integrated into single platforms to make them suitable for diversified activities ( and ). In this study, we propose a model for the Integrated Technology System (ITS) () to control and manage Vibrio-related diseases effectively. Since it is based on several logical and regional issues in an integrated platform, it could be one of the possible solutions enabling better control management for public health and the aquaculture industry.
Available aquaculture tools and technologies
The aquaculture system offers many products related to the technologies listed in . Although these technologies and systems are commercially available, there are limitations and a lack of integrated platforms. Commercial products for aquaculture monitoring, production, and management exist, but they are not specific to Vibrio or have complete applications (). While these products monitor water and the environment, integration with all related requirements is missing (). Thus, there is a need to develop a Vibrio-specific, cost-effective, and technology-based integrated system that can have a broader impact on Vibrio infection control in aquaculture and human healthcare.
Table 6. Products of technology and systems that can be used for the effective management of aquaculture and species of Vibrio.
Advanced fishery management
The aquaculture industry’s expansion highlights the need for policies and management that address both diagnostic and environmentally oriented effective control. However, the lack of information in the fish industry can dilute these efforts. To reach the next step in the control mechanism (), integrated approaches that join multidisciplinary expertise on a single platform are needed. This sequential success or efficient result can then be integrated with an integrated technology system for diversified applications and output.
Adaptive fishery management
Adaptive research is crucial for effective implementation of fishery management. Developing countries often have unorganized fish industries lacking clear regulations, as reported in various studies [Citation155,Citation156]. Therefore, efforts to understand regional aquaculture management are crucial. We suggest a practical aquaculture management module that considers the role of disease and health management for a sustainable system [Citation157].
Cooperation with international partners from countries where Vibrio infections are significant, such as Bangladesh, Chile, India, Thailand, and Vietnam [Citation158], can strengthen the research network. The first step is identifying regional issues and developing solutions to achieve the objective’s structure. All processes must focus on regional issues and address each parameter through sub-sections. Health monitoring and market evaluation are also crucial parameters of the aquaculture industry.
Aquacultural biotechnology with molecular sustainability
Molecular biotechnology serves as the foundation for many biological solutions. A significant focus in biotechnology is the development of molecular technologies that are specific for diagnosis and treatment. Advanced technical aspects and a general approach combine to select the desired biomolecule for the desired solution. Molecular evolution helps to understand DNA sequence-based features through evolutionary processes, such as mutation, recombination, natural selection, genetic drift, and dynamic population effects. The accumulation of transposable elements (TEs) in the human genome increases genetic diversity. TEs act as promoters, enhancers, and insulators, causing this increase. The TEs can produce microRNAs and are suspected of playing a role in infectious disease and cancer progression. Long terminal repeats containing Z-DNA can provide alternative promoters for human functional genes, while analyses of non-coding RNAs have implications for human diseases [Citation159–161].
Genetic ontology (GO) analysis can predict multiple pathogenesis-related genes, such as binding, catalytic, cellular, and metabolic processes. MicroRNA (miR-10a-3p) can be used as a biomarker to detect VHSV in olive flounder and iridovirus in rock bream [Citation162]. MicroRNA is involved in the regulation of expression during pathogen infections. Down-regulation of MiR-15b-5p increases SOCS6 expression during VHSV infection in olive flounder (Paralichthys olivaceus), while the down-regulated pol-miR-140-3p induces kinesin 5 A in olive flounder infected with Streptococcus parauberis [Citation161]. Biotechnology advancements have opened up new opportunities for aquaculture that are more efficient, sustainable, and environmentally friendly. Recent research data suggest that aquaculture will depend more on land than on the sea [Citation163]. Due to a lack of knowledge about virulence, strains, treatments, and control methods, infectious diseases are difficult to control. Early molecular diagnosis, improved cell research, and environmental control through appropriate managerial decisions are the best options [Citation164]. Molecular biotechnology focuses on developing diagnostic and treatment technologies for several pathogens. Urgent action is required for sustainable solutions to address the greatest risks to human health posed by antibiotic use and climate change [Citation165]. Developments in global aquaculture have made the greatest contribution to global production volumes, as well as global food security [Citation166]. Furthermore, sustainable planning for human health is required to achieve sustainable aquaculture in the face of the challenges posed by climate change [Citation165,Citation167].
Sustainable production and interaction
The fastest-growing bacterium, Vibrio natriegens, is a halophilic bacterium considered to be the next-generation workhorse of the biotechnology industry. It excels in rapid molecular biology, protein expression, and metabolic engineering. This study demonstrates its high potential for biotechnological production processes, particularly in cultures with high sodium chloride concentrations. The study revealed that sodium chloride can be entirely replaced with sodium salts, such as disodium hydrogen phosphate, disodium sulfate, and sodium citrate, while maintaining a constant total concentration of sodium ions [Citation168]. In another study, the biosynthesis of pyruvate and its derivatives was explored. The strain PYR32 was capable of producing 54.22 g/L of pyruvate from glucose within 16 h. Moreover, it demonstrated the ability to transform sucrose or gluconate into pyruvate at high titers [Citation169]. Abalones, slow-growing molluscs of the Haliotis genus, benefit from probiotics that improve their growth, health, and vigor. This is achieved through the bacterial isolate Vibrio midae which is naturally present in the intestinal tract of South African abalones, contributing to a probiotic system [Citation170]. The commercial production of V. midae isolates, achieved by regulating process parameters, can lead to an important probiotic product. Understanding the beneficial symbiosis between the Hawaiian bobtail squid (Euprymna scolopes) and the bioluminescent Vibrio bacterium (Vibrio fischeri) is crucial for mapping bacterial genes responsible for signal exchange and rhythmic activities. This symbiosis results in a persistent and beneficial association, evolving into symbiotic competence [Citation171].
The strategies described in the above research can be valuable for engineering Vibrio species as potential microorganisms for the production of various foods, chemicals, and biofuels, as well as for enhancing our understanding of host-environment interactions.
Effective fishery management
The current requirement is the full-fledged development of advanced fishery management systems based on real-world challenges. To a great extent, large-scale aquaculture in the developed world demands a scientific approach toward in-house-based regional challenges. Understanding the nature of small-scale fisheries is also required to deal with control mechanisms properly. Highly developed monitoring programs are urgently needed for small-scale fisheries via integrated multi-disciplinary and participative assessment. Continuous monitoring systems need to be developed that can rapidly monitor minor changes and, coupled with effective management processes, can react effectively according to the situation. Natural climate conditions have always affected fish distribution; thus, the integration of ecological study in management will neutralize the concerns of any potential negative impacts and provide effective strategies in advance. Understanding local fish populations, consumer relations, and fish health status, along with quantitative and qualitative fluctuations, will provide advance notice of specific fish species under high demand and allow the design of control strategies accordingly. Complete knowledge of risk factors will help establish an effective strategy for managing and controlling the disease.
A self-integrated system with biosensors, various statistical models (for specific cases), and a technology-based electronics and cloud system is considered the best-advanced monitoring system. Effective control of fish infection relies on understanding evolutionary mechanisms, the virulence of isolates, and host adaptation; thus, a gene-environment study is warranted, specifically targeting isolates from specific hosts in particular geographical environments. Database management for mass healthcare and data analytics to understand real-time issues can be adopted through integrated models. Discussed technologies have practical implications for Vibrio-related challenges as they can provide effective solutions from diagnosis to control ( and ).
Digital technologies play a crucial role in Vibrio control. A team at the European Center for Disease Prevention and Control (ECDC) has developed a tool, the ECDC Vibrio Map Viewer, which uses remotely sensed data on SST and sea surface salinity to predict the occurrence of environmental conditions that favor Vibrio proliferation [Citation172]. A recent study applied a new generation of models that combine climate, population, and socioeconomic projections to map future distribution scenarios and suitability of the season suitability for pathogenic Vibrio [Citation89]. They used the Coupled Model Intercomparison Project 6 framework. Three datasets were used Geophysical Fluid Dynamics Laboratory’s CM4.0 sea surface temperature and sea surface salinity; the coastline length dataset from the World Resources Institute; and Inter-Sectoral Impact Model Intercomparison Project 2b annual global population data. It should be noted that the incidence of Vibrio disease has been recorded in the United States since 1997 (www.cdc.gov/nationalsurveillance/cholera-vibrio-surveillance.html), while in Europe, with the exception of toxigenic V. cholerae, vibriosis is not a notifiable disease.
Implications of effective policy and proposed technology model
This study highlights the implications of effective policies against vibriosis. The emerging computational modeling in healthcare practice and clinical databases for issue-solving mechanisms lie in upgraded cloud computing and AI integration in transdisciplinary platforms. The first step is to collect risk data for statistical analysis of small and large aquaculture fields and industries. Then, establish an aquaculture management system to gather information about fish health, ecological conditions, infectious agents, hazardous substances, and fish species. Governments or industries could promote such a control management system through the public-private partnership model (PPP model), which generates a control platform for aquaculture industry research centers to discuss issues related to vibriosis. Overall, the ultimate goal should be to maintain the ecological balance for aquaculture organisms with limited pathogens and environmental waste. Establishing such a management system could contribute to disease control through knowledge sharing by technology experts and consensus to develop suitable control policies.
Most importantly, the results of the culture of various bacterial strains and regional disease incidence information must be made available to management to develop further control strategies. Overall, exchanging innovative data, results, modeling, simulation methods, data accessibility, and computational tools, for establishing clinical phenotype databases is an urgent requirement to contribute to improved products and understanding processes of Vibrio disease at the mass level. Finally, the proposed ITS system will facilitate regional idea adaptation and technology-based implementation for appropriate control.
Scope of integrated technology system
The proposed ITS concept is based on the integration of IoT, AI, and ML, and thus is designed to address the challenges posed by Vibrio in water. In this article, we have defined the ITS concept and discussed its framework in relation to research and industry. Furthermore, we have highlighted the potential industrial impacts that are important for the continued development of aquaculture. Finally, we believe that the applications of ITS will transfer to Vibrio-infected aquatic species. In general, having access to more information regarding virulence, strains, diagnosis, treatments, and molecular biotechnology, enables better and more sustainable control management [Citation164,Citation165].
Sustainable integrated technology system and novelty
An integrated technology system for Vibrio control encompasses a diverse array of technologies and approaches to prevent, detect, and manage infections. Its novelty stems from the innovative and emerging technologies seamlessly combined to create a comprehensive Vibrio control system. This includes advanced testing and diagnostics, utilizing instruments, such as digital PCR, fast antigen tests, and CRISPR-based diagnostics. Automation for high-throughput testing allows for quick identification and tracking of Vibrio, while mobile apps with Bluetooth and GPS enable efficient contact tracking. Real-time data analysis identifies potential outbreak clusters, leveraging cloud computing and GPS-enabled mobile applications for contact tracing. Machine learning algorithms predict Vibrio spread in aquaculture and humans, aiding early detection. Behavioral insights and educational apps inform the public about cholera prevention. Sensor networks monitor the Vibrio load in water systems near contaminated areas. Additionally, telemedicine technologies for remote medical consultations during cholera outbreaks are a promising start, wearable technology continuously monitors vital signs and symptoms, and drones are utilized for monitoring and mapping larger areas. Sustainable disposal methods for infected aquaculture waste are adopted as needed, along with sanitation technologies like disinfecting water using ultraviolet-visible (UV-C) light and safe chemicals such as chlorine. IoT devices monitor people with the guidance of medical experts, even from a distance. Vaccine management involves the distribution and administration of vaccines using blockchain technology and digital vaccine passports. Technologies also automate vaccination appointments in outbreak areas. This integrated system’s novelty lies in the seamless fusion of cutting-edge techniques and technologies to form a vast ecosystem for Vibrio control, capable of responding effectively to existing threats and adapting to new challenges. The continuous integration of data and feedback from various sources enables a proactive, data-driven approach to Vibrio control and prevention. Our proposed Integrated Technology System (ITS) model () will integrate all of the above technologies to provide effective control and management of Vibrio-related diseases in aquaculture.
Conclusion and prospects
Vibrio infections have a global impact on aquaculture and human health and are considered a limiting factor for further sustainable development. Emerging Vibrio species due to climate change and ineffective control mechanisms have recently posted significant challenges, resulting in negative impacts on aquaculture production and human health. This review extensively discusses the climate change, virulence, diagnosis, and effective control strategies of Vibrio infections, highlighting important parameters. The development of Vibrio research is illustrated using a timeline in , providing a comprehensive overview of the evolution of scientific information, mechanisms, methods, and technologies related to various Vibrios. This historical context aids in understanding the progress in the field (). The future development relies on identified prioritized fields, as outlined in the roadmap in . This roadmap discusses the scientific and technological aspects of Vibrio research and defines the path for sustainable Vibrio management in the future. We recommend early diagnosis using molecular methods and effective control through ITS that is environment- and technology-oriented. ITS employs nanotechnology, biosensors, bioelectronics systems, IoT, cloud computing, and machine learning approaches to enable fast and effective management decisions for effective control. Finally, the sustainability of aquaculture biotechnology focusing on molecular methods and the implementation of innovative technologies based on regional requirements has an impact on several sustainable development goals.
Supplemental Material
Download PDF (451.9 KB)Supplemental Material
Download PNG Image (180.9 KB)Acknowledgements
Dr Anshuman Mishra acknowledges the PDF fellowship of Genetic Engineering Institute, Pusan National University, South Korea and Prof. Herbert Gliter Fellowship of International Association of Advanced Materials, IAAM, Sweden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Montánchez I, Ogayar E, Plágaro AH, et al. Analysis of Vibrio harveyi adaptation in sea water microcosms at elevated temperature provides insights into the putative mechanisms of its persistence and spread in the time of global warming. Sci Rep. 2019;9:289. doi: 10.1038/s41598-018-36483-0.
- Jiang C, Tanaka M, Nishikawa S, et al. Vibrio Clade 3.0: new Vibrionaceae evolutionary units using genome-based approach. Curr Microbiol. 2022;79:10. doi: 10.1007/s00284-021-02725-0.
- Baker-Austin C, Oliver JD, Alam M, et al. Vibrio spp. infections. Nat Rev Dis Primers. 2018;4:8–19. doi: 10.1038/s41572-018-0005-8.
- Thompson FL, Iida T, Swings J. Biodiversity of vibrios. Microbiol Mol Biol Rev. 2004;68:403–431, table of contents. doi: 10.1128/MMBR.68.3.403-431.2004.
- Onohuean H, Agwu E, Nwodo UU. A global perspective of Vibrio species and associated diseases: three-decade meta-synthesis of research advancement. Environ Health Insights. 2022;16. doi: 10.1177/11786302221099406.
- Hickey ME, Lee JL. A comprehensive review of Vibrio (Listonella) anguillarum: ecology, pathology and prevention. Rev Aquacult. 2018;10:585–610. doi: 10.1111/raq.12188.
- Vezzulli L, Chiara G, Philip C, et al. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. PNAS. 2016;113:E5062–E5071. doi: 10.1073/pnas.1609157113.
- Cervino JM, Thompson FL, Gomez-Gil B, et al. The Vibrio core group induces yellow band disease in Caribbean and Indo‐Pacific reef‐building corals. J Appl Microbiol. 2008;105:1658–1671. doi: 10.1111/j.1365-2672.2008.03871.x.
- Ramamurthy T, Chowdhury G, Pazhani GP, et al. Vibrio fluvialis: an emerging human pathogen. Front Microbiol. 2014;5:91. doi: 10.3389/fmicb.2014.00091.
- Mustapha S, Mustapha EM, Nozha C. Vibrio alginolyticus: an emerging pathogen of food borne diseases. Inte J Sci Technol. 2013;2:302–309.
- Ghenem L, Elhadi N, Alzahrani F, et al. Vibrio parahaemolyticus: a review on distribution, pathogenesis, virulence determinants and epidemiology. Saudi J Med Med Sci. 2017;5:93–103. doi: 10.4103/sjmms.sjmms_30_17.
- Ali M, Nelson AR, Lopez AL, et al. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9:e0003832. doi: 10.1371/journal.pntd.0003832.
- Kokashvili T, Whitehouse CA, Tskhvediani A, et al. Occurrence and diversity of clinically important Vibrio species in the aquatic environment of Georgia. Front Public Health. 2015;3:232. doi: 10.3389/fpubh.2015.00232.
- Wang R, Wang J, Sun Y, Yang B, Wang A. Antibiotic resistance monitoring in Vibrio spp. isolated from rearing environment and intestines of abalone Haliotis diversicolor. Mar Pollut Bull 2015;101(2):701–6. doi: 10.1016/j.marpolbul.2015.10.027.
- Poblete-Morales M, Irgang R, Henríquez-Núñez H, et al. Vibrio ordalii antimicrobial susceptibility testing—modified culture conditions required and laboratory-specific epidemiological cut-off values. Vet Microbiol. 2013;165:434–442. doi: 10.1016/j.vetmic.2013.04.024.
- Davidson N, Edwards F, Harris PNA, et al. Vibrio species bloodstream infections in Queensland, Australia. Intern Med J. 2023;54:157–163. doi: 10.1111/imj.16187.
- Williams NLR, Siboni N, King WL, et al. Latitudinal dynamics of Vibrio along the Eastern Coastline of Australia. Water. 2022;14:2510. doi: 10.3390/w14162510.
- Lee K-K, Yu S-R, Chen F-R, et al. News & notes: virulence of Vibrio alginolyticus isolated from diseased tiger prawn, Penaeus monodon. Curr Microbiol. 1996;32:229–231. doi: 10.1007/s002849900041.
- Cao H, An J, Zheng W, et al. Vibrio cholerae pathogen from the freshwater-cultured whiteleg shrimp Penaeus vannamei and control with Bdellovibrio bacteriovorus. J Invertebr Pathol. 2015;130:13–20. doi: 10.1016/j.jip.2015.06.002.
- Lightner DV. A handbook of shrimp pathology and diagnostic procedures for diseases of cultured penaeid shrimp. Sorrento (LA): World Aquaculture Society; 1996.
- Wong HC, Liu SH, Wang TK, et al. Characteristics of Vibrio parahaemolyticus O3: k 6 from Asia. Appl Environ Microbiol. 2000;66:3981–3986. doi: 10.1128/AEM.66.9.3981-3986.2000.
- Oliver J. The biology of Vibrio vulnificus. Microbiol Spect. 2015;3. doi: 10.1128/microbiolspec.VE-0001-2014.
- Chitov T, Kirikaew P, Yungyune P, et al. An incidence of large foodborne outbreak associated with Vibrio mimicus. Eur J Clin Microbiol Infect Dis. 2009;28:421–424. doi: 10.1007/s10096-008-0639-7.
- Subasinghe R, Soto D, Jia J. Global aquaculture and its role in sustainable development. Rev Aquacult. 2009;1:2–9. doi: 10.1111/j.1753-5131.2008.01002.x.
- Godfray HCJ, Beddington JR, Crute IR, et al. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383.
- King T, Cole M, Farber JM, et al. Food safety for food security: relationship between global megatrends and developments in food safety. Trends Food Sci Technol. 2017;68:160–175. doi: 10.1016/j.tifs.2017.08.014.
- Gormaz JG, Fry JP, Erazo M, et al. Public health perspectives on aquaculture. Curr Environ Health Rep. 2014;1:227–238. doi: 10.1007/s40572-014-0018-8.
- Broberg CA, Calder TJ, Orth K. Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 2011;13:992–1001. doi: 10.1016/j.micinf.2011.06.013.
- Mohammadi-Barzelighi H, Bakhshi B, Lari AR, et al. Characterization of pathogenicity island prophage in clinical and environmental strains of Vibrio cholerae. J Med Microbiol. 2011;60:1742–1749. doi: 10.1099/jmm.0.031732-0.
- Aguirre-Guzmán G, Ascencio F, Saulnier D. Pathogenicity of Vibrio penaeicida for white shrimp Litopenaeus vannamei: a cysteine protease-like exotoxin as a virulence factor. Dis Aquat Organ. 2005;67:201–207. doi: 10.3354/dao067201.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723–1733. doi: 10.1128/IAI.01046-08.
- Weil AA, Becker RL, Harris JB. Vibrio cholerae at the intersection of immunity and the microbiome. MSphere. 2019;4:e00597-19. doi: 10.1128/mSphere.00597-19.
- Luan XY, Chen JX, Zhang XH, et al. Comparison of different primers for rapid detection of Vibrio parahaemolyticus using the polymerase chain reaction. Lett Appl Microbiol. 2007;44:242–247. doi: 10.1111/j.1472-765X.2006.02074.x.
- Park JY, Jeon S, Kim JY, et al. Multiplex real-time polymerase chain reaction assays for simultaneous detection of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus. Osong Public Health Res Perspect. 2013;4:133–139. doi: 10.1016/j.phrp.2013.04.004.
- Sawabe T, Ogura Y, Matsumura Y, et al. Corrigendum: updating the Vibrio clades defined by multilocus sequence phylogeny: proposal of eight new clades, and the description of Vibrio tritonius sp. nov. Front Microbiol. 2014;5:583. doi: 10.3389/fmicb.2014.00583.
- Thompson CC, Vicente ACP, Souza RC, et al. Genomic taxonomy of vibrios. BMC Evol Biol. 2009;9:258. doi: 10.1186/1471-2148-9-258.
- Xie ZY, Hu CQ, Chen C, et al. Investigation of seven Vibrio virulence genes among Vibrio alginolyticus and Vibrio parahaemolyticus strains from the coastal mariculture systems in Guangdong, China. Lett Appl Microbiol. 2005;41:202–207. doi: 10.1111/j.1472-765X.2005.01688.x.
- Silvester R, Alexander D, Antony AC, et al. GroEL PCR-RFLP–an efficient tool to discriminate closely related pathogenic Vibrio species. Microb Pathog. 2017;105:196–200. doi: 10.1016/j.micpath.2017.02.029.
- Yu J, Zhu B, Zhou T, et al. Species‐specific identification of Vibrio sp. based on 16S‐23S rRNA gene internal transcribed spacer. J Appl Microbiol. 2020;129:738–752. doi: 10.1111/jam.14637.
- Ma X, Zhu F, Jin Q. Antibiotics and chemical disease control agents reduce innate disease resistance in crayfish. Fish Shellfish Immunol. 2019;86:169–178. doi: 10.1016/j.fsi.2018.11.015.
- Wong KC, Brown AM, Luscombe GM, et al. Antibiotic use for Vibrio infections: important insights from surveillance data. BMC Infect Dis. 2015;15:226. doi: 10.1186/s12879-015-0959-z.
- Vallejos-Vidal E, Reyes-López F, Teles M, et al. The response of fish to immunostimulant diets. Fish Shellfish Immunol. 2016;56:34–69. doi: 10.1016/j.fsi.2016.06.028.
- Alvarez-Pellitero P. Fish immunity and parasite infections: from innate immunity to immunoprophylactic prospects. Vet Immunol Immunopathol. 2008;126:171–198. doi: 10.1016/j.vetimm.2008.07.013.
- Galindo-Villegas J, García-Moreno D, De Oliveira S, et al. Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proc Natl Acad Sci U S A. 2012;109: e2605–E2614. doi: 10.1073/pnas.1209920109.
- Bricknell I, Dalmo RA. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol. 2005;19:457–472. doi: 10.1016/j.fsi.2005.03.008.
- Ghosh AK, Sarkar S, Bir J, et al. Probiotic tiger shrimp (Penaeus monodon) farming at different stocking densities and its impact on production and economics. Int J Res Fish Aquac. 2013;3:25–29.
- Miccoli A, Saraceni P, Scapigliati G. Vaccines and immune protection of principal Mediterranean marine fish species. Fish Shellfish Immunol. 2019;94:800–809. doi: 10.1016/j.fsi.2019.09.065.
- Du Y, Hu X, Miao L, et al. Current status and development prospects of aquatic vaccines. Front Immunol. 2022;13:1040336. doi: 10.3389/fimmu.2022.1040336.
- Magnadóttir B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006;20:137–151. doi: 10.1016/j.fsi.2004.09.006.
- Motes ML, DePaola A, Cook DW, et al. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl Environ Microbiol. 1998;64:1459–1465. doi: 10.1128/AEM.64.4.1459-1465.1998.
- Nigro OD, James-Davis LTI, De Carlo EH, et al. Variable freshwater influences on the abundance of Vibrio vulnificus in a tropical urban estuary. Appl Environ Microbiol. 2022;88:e01884-21. doi: 10.1128/AEM.01884-21.
- Urquhart EA, Jones SH, Yu JW, et al. Environmental conditions associated with elevated Vibrio parahaemolyticus concentrations in Great Bay Estuary, New Hampshire. PLoS One. 2016;11:e0155018. doi: 10.1371/journal.pone.0155018.
- Vezzulli L, Pezzati E, Brettar I, et al. Effects of global warming on Vibrio ecology. Microbiol Spectr. 2015;3:18. doi: 10.1128/microbiolspec.VE-0004-2014.
- Kashulin A, Seredkina N, Sørum H. Cold‐water vibriosis. The current status of knowledge. J Fish Dis. 2017;40:119–126. doi: 10.1111/jfd.12465.
- Faria EC, Brown BJT, Snook RD. Water toxicity monitoring using Vibrio fischeri: a method free of interferences from colour and turbidity. J Environ Monit. 2004;6:97–102. doi: 10.1039/b311137g.
- Bentzon‐Tilia M, Sonnenschein EC, Gram L. Monitoring and managing microbes in aquaculture–towards a sustainable industry. Microb Biotechnol. 2016;9:576–584. doi: 10.1111/1751-7915.12392.
- Kalatzis PG, Castillo D, Katharios P, et al. Bacteriophage interactions with marine pathogenic vibrios: implications for phage therapy. Antibiotics. 2018;7:15. doi: 10.3390/antibiotics7010015.
- Vinod MG, Shivu MM, Umesha KR, et al. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture. 2006;255:117–124. doi: 10.1016/j.aquaculture.2005.12.003.
- Kim JY, Lee J-L. Correlation of total bacterial and Vibrio spp. populations between fish and water in the aquaculture system. Front Mar Sci. 2017;4:147. doi: 10.3389/fmars.2017.00147.
- Halet D, Defoirdt T, Van Damme P, et al. Poly-β-hydroxybutyrate-accumulating bacteria protect gnotobiotic Artemia franciscana from pathogenic Vibrio campbellii. FEMS Microbiol Ecol. 2007;60:363–369. doi: 10.1111/j.1574-6941.2007.00305.x.
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165.
- Rutherford S, Bassler B. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspect Med. 2012;2:a012427.
- Kalatzis PG, Bastías R, Kokkari C, et al. Isolation and characterization of two lytic bacteriophages, φSt2 and φGrn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS One. 2016;11:e0151101. doi: 10.1371/journal.pone.0151101.
- Matamp N, Bhat SG. Phage endolysins as potential antimicrobials against multidrug resistant Vibrio alginolyticus and Vibrio parahaemolyticus: current status of research and challenges ahead. Microorganisms. 2019;7:84. doi: 10.3390/microorganisms7030084.
- Sotomayor MA, Reyes JK, Restrepo L, et al. Efficacy assessment of commercially available natural products and antibiotics, commonly used for mitigation of pathogenic Vibrio outbreaks in Ecuadorian Penaeus (Litopenaeus) vannamei hatcheries. PLoS One. 2019;14:e0210478. doi: 10.1371/journal.pone.0210478.
- Manilal A, Selvin J, George S. In vivo therapeutic potentiality of red seaweed, Asparagopsis (Bonnemaisoniales, Rhodophyta) in the treatment of Vibriosis in Penaeus monodon Fabricius. Saudi J Biol Sci. 2012;19:165–175. doi: 10.1016/j.sjbs.2011.12.003.
- Szunerits S, Boukherroub R. Graphene-based biosensors. Interface Focus. 2018;8:20160132. doi: 10.1098/rsfs.2016.0132.
- Zhang Z, Zhou J, Du X. Electrochemical biosensors for detection of foodborne pathogens. Micromachines. 2019;10:222. doi: 10.3390/mi10040222.
- Rahman M, Heng LY, Futra D, et al. Ultrasensitive biosensor for the detection of Vibrio cholerae DNA with polystyrene-co-acrylic acid composite nanospheres. Nanoscale Res Lett. 2017;12:474. doi: 10.1186/s11671-017-2236-0.
- Hash S, Martinez-Viedma MP, Fung F, et al. Nuclear magnetic resonance biosensor for rapid detection of Vibrio parahaemolyticus. Biomed J. 2019;42:187–192. doi: 10.1016/j.bj.2019.01.009.
- Huff K, Aroonnual A, Littlejohn AEF, et al. Light‐scattering sensor for real‐time identification of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae colonies on solid agar plate. Microb Biotechnol. 2012;5:607–620. doi: 10.1111/j.1751-7915.2012.00349.x.
- Wang Y, Li H, Wang Y, et al. Nanoparticle-based lateral flow biosensor combined with multiple cross displacement amplification for rapid, visual and sensitive detection of Vibrio cholerae. FEMS Microbiol Lett. 2017;364:fnx234. doi: 10.1093/femsle/fnx234.
- Laczka OF, Labbate M, Seymour JR, et al. Surface immuno-functionalisation for the capture and detection of Vibrio species in the marine environment: a new management tool for industrial facilities. PLoS One. 2014;9:e108387. doi: 10.1371/journal.pone.0108387.
- Milton DL. Quorum sensing in vibrios: complexity for diversification. Int J Med Microbiol. 2006;296:61–71. doi: 10.1016/j.ijmm.2006.01.044.
- Tiaden A, Hilbi H. α-Hydroxyketone synthesis and sensing by Legionella and Vibrio. Sensors. 2012;12:2899–2919. doi: 10.3390/s120302899.
- Santhyia AV, Mulloorpeedikayil RG, Kollanoor RJ, et al. Molecular variations in Vibrio alginolyticus and V. harveyi in shrimp-farming systems upon stress. Braz J Microbiol. 2015;46:1001–1008. doi: 10.1590/S1517-838246420140410.
- Girgis K, Clouse N, Mushe O, et al. The applications of GIS in water resources. 2022 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA; 2022; p. 1515–1520. doi: 10.1109/CSCI58124.2022.00268.
- Longdill P, Healy T, Black K. GIS-based models for sustainable open-coast shellfish aquaculture management area site selection. Ocean Coast Manage. 2008;51:612–624. doi: 10.1016/j.ocecoaman.2008.06.010.
- Klemas VV. editor. Advances in fisheries applications of remote sensing. 2014 IEEE/OES Baltic International Symposium (BALTIC): IEEE; 2014. doi: 10.1109/BALTIC.2014.6887836.
- Erisman B, Aburto-Oropeza O, Gonzalez-Abraham C, et al. Spatio-temporal dynamics of a fish spawning aggregation and its fishery in the Gulf of California. Sci Rep. 2012;2:284. doi: 10.1038/srep00284.
- Assefa WW, Abebe WB. GIS modeling of potentially suitable sites for aquaculture development in the Lake Tana basin, Northwest Ethiopia. Agric Food Sec. 2018;7:1–15.
- Vance TC, Merati N, Yang C, et al. Cloud computing for ocean and atmospheric science. New York: IEEE; 2016.
- Ali A, Syed KS. An outlook of high performance computing infrastructures for scientific computing. Adv Comp. 2013;91:87–118.
- Nižetić S, Šolić P, López-de-Ipiña González-de-Artaza D, et al. Internet of Things (IoT): opportunities, issues and challenges towards a smart and sustainable future. J Clean Prod. 2020;274:122877. doi: 10.1016/j.jclepro.2020.122877.
- Lee PG. Process control and artificial intelligence software for aquaculture. Aquacult Eng. 2000;23:13–36. doi: 10.1016/S0144-8609(00)00044-3.
- Scopus data. Key word vibrio. [accessed 2023 Oct 28]. https://www.scopus.com.
- Manchanayake T, Salleh A, Amal MNA, et al. Pathology and pathogenesis of Vibrio infection in fish: a review. Aquacult Rep. 2023;28:101459. doi: 10.1016/j.aqrep.2022.101459.
- Ziarati M, Zorriehzahra MJ, Hassantabar F, et al. Zoonotic diseases of fish and their prevention and control. Vet Q. 2022;42:95–118. doi: 10.1080/01652176.2022.2080298.
- Trinanes J, Martinez-Urtaza J. Future scenarios of risk of viruses infections on a warming planet: a global mapping study. Lancet Planet Health. 2021;5:e426–E435. doi: 10.1016/S2542-5196(21)00169-8.
- Matteucci G, Schippa S, Di Lallo G, et al. Species diversity, spatial distribution, and virulence associated genes of culturable vibrios in a brackish coastal Mediterranean environment. Ann Microbiol. 2015;65:2311–2321. doi: 10.1007/s13213-015-1073-6.
- Sanches-Fernandes GMM, Sá-Correia I, Costa R. Vibriosis outbreaks in aquaculture: addressing environmental and public health concerns and preventive therapies using gilthead seabream farming as a model system. Front Microbiol. 2022;13:904815. doi: 10.3389/fmicb.2022.904815.
- Castillo D, Kauffman K, Hussain F, et al. Widespread distribution of prophage-encoded virulence factors in marine Vibrio communities. Sci Rep. 2018;8:9973. doi: 10.1038/s41598-018-28326-9.
- Mohamad A, Zamri-Saad M, Amal MNA, et al. Vaccine efficacy of a newly developed feed-based whole-cell polyvalent vaccine against vibriosis, streptococcosis and motile aeromonad septicemia in Asian Seabass, Lates calcarifer. Vaccines. 2021;9:368. doi: 10.3390/vaccines9040368.
- Ma J, Bruce JT, Jones M, et al. Review of fish vaccine development strategies: conventional methods and modern biotechnological approaches. Microorganisms. 2019;7:569. doi: 10.3390/microorganisms7110569.
- Mondiale de la Santé O, Organization WH. Cholera vaccines: WHO position paper–August 2017–vaccins anticholériques: note de synthèse de l’OMS–août 2017. Week Epidemiol Rec = Relevé Épidémiologique Hebdomadaire. 2017;92:477–498.
- Sit B, Zhang T, Fakoya B, et al. Oral immunization with a probiotic cholera vaccine induces broad protective immunity against Vibrio cholerae colonization and disease in mice. PLoS Negl Trop Dis. 2019;13:e0007417. doi: 10.1371/journal.pntd.0007417.
- Rahman MT, Sobur MA, Islam MS, et al. Zoonotic diseases: etiology, impact, and control. Microorganisms. 2020;8:1405. doi: 10.3390/microorganisms8091405.
- Christou L. The global burden of bacterial and viral zoonotic infections. Clin Microbiol Infect. 2011;17:326–330. doi: 10.1111/j.1469-0691.2010.03441.x.
- Triga A, Smyrli M, Katharios P. Pathogenic and opportunistic Vibrio spp. associated with Vibriosis incidences in the Greek aquaculture: the role of Vibrio harveyi as the principal cause of Vibriosis. Microorganisms. 2023;11:1197. doi: 10.3390/microorganisms11051197.
- Loo K-Y, Law JW-F, Teng-Hern Tan L, et al. Diagnostic techniques for the rapid detection of Vibrio species. Aquaculture. 2022;561:738628. doi: 10.1016/j.aquaculture.2022.738628.
- Li H, Zhang X, Long H, et al. Vibrio alginolyticus 16S-23S intergenic spacer region analysis, and PCR assay for identification of coral pathogenic strain XSBZ03. Dis Aquat Organ. 2018;129:71–83. doi: 10.3354/dao03233.
- Hoffmann M, Brown EW, Feng PC, et al. PCR-based method for targeting 16S-23S rRNA intergenic spacer regions among Vibrio species. BMC Microbiol. 2010;10:90. doi: 10.1186/1471-2180-10-90.
- Coyle NM, Bartie KL, Bayliss SC, et al. A hopeful sea-monster: a very large homologous recombination event impacting the core genome of the marine pathogen Vibrio anguillarum. Front Microbiol. 2020;11:1430. doi: 10.3389/fmicb.2020.01430.
- Meparambu Prabhakaran D, Ramamurthy T, Thomas S. Genetic and virulence characterisation of Vibrio parahaemolyticus isolated from Indian coast. BMC Microbiol. 2020;20:62. doi: 10.1186/s12866-020-01746-2.
- Hossain MMM, Uddin MI, Islam H, et al. Diagnosis, genetic variations, virulence, and toxicity of AHPND-positive Vibrio parahaemolyticus in Penaeus monodon. Aquac Int. 2020;28(6):2531–2546. doi: 10.1007/s10499-020-00607-z.
- Lin B, Wang Z, Malanoski AP, et al. Comparative genomic analyses identify the Vibrio harveyi genome sequenced strains BAA‐1116 and HY01 as Vibrio campbellii. Environ Microbiol Rep. 2010;2:81–89. doi: 10.1111/j.1758-2229.2009.00100.x.
- Chun J, Grim CJ, Hasan NA, et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A. 2009;106:15442–15447. doi: 10.1073/pnas.0907787106.
- Kennedy DA, Kurath G, Brito IL, et al. Potential drivers of virulence evolution in aquaculture. Evol Appl. 2016;9:344–354. doi: 10.1111/eva.12342.
- Yano Y, Hamano K, Satomi M, et al. Prevalence and antimicrobial susceptibility of Vibrio species related to food safety isolated from shrimp cultured at inland ponds in Thailand. Food Control. 2014;38:30–36. doi: 10.1016/j.foodcont.2013.09.019.
- Arunkumar M, LewisOscar F, Thajuddin N, et al. In vitro and in vivo biofilm forming Vibrio spp: a significant threat in aquaculture. Process Biochem. 2020;94:213–223. doi: 10.1016/j.procbio.2020.04.029.
- Letchumanan V, Chan K-G, Lee L-H. Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol. 2014;5:705. doi: 10.3389/fmicb.2014.00705.
- Oliver JD, Jones JL. Vibrio parahaemolyticus and Vibrio vulnificus. In: Molecular medical microbiology. Amsterdam: Elsevier; 2015. p. 1169–1186.
- Tan D, Gram L, Middelboe M. Vibriophages and their interactions with the fish pathogen Vibrio anguillarum. Appl Environ Microbiol. 2014;80:3128–3140. doi: 10.1128/AEM.03544-13.
- Ali M, Emch M, Park JK, et al. Natural cholera infection–derived immunity in an endemic setting. J Infect Dis. 2011;204:912–918. doi: 10.1093/infdis/jir416.
- Harris JB. Cholera: immunity and prospects in vaccine development. J Infect Dis. 2018;218: s141–S146. doi: 10.1093/infdis/jiy414.
- Duan F, March JC. Engineered bacterial communication prevents Vibrio cholerae virulence in an infant mouse model. Proc Natl Acad Sci U S A. 2010;107:11260–11264. doi: 10.1073/pnas.1001294107.
- Frans I, Michiels CW, Bossier P, et al. Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J Fish Dis. 2011;34:643–661. doi: 10.1111/j.1365-2761.2011.01279.x.
- Rubio T, Oyanedel D, Labreuche Y, et al. Species-specific mechanisms of cytotoxicity toward immune cells determine the successful outcome of Vibrio infections. Proc Natl Acad Sci U S A. 2019;116:14238–14247. doi: 10.1073/pnas.1905747116.
- Kaper JB. Vibrio cholerae vaccines. Rev Infect Dis. 1989;11(Suppl 3):S568–S573. doi: 10.1093/clinids/11.supplement_3.s568.
- Lokesh J, Fernandes JM, Korsnes K, et al. Transcriptional regulation of cytokines in the intestine of Atlantic cod fed yeast derived mannan oligosaccharide or β-glucan and challenged with Vibrio anguillarum. Fish Shellfish Immunol. 2012;33:626–631. doi: 10.1016/j.fsi.2012.06.017.
- Ødegård J, Baranski M, Gjerde B, et al. Methodology for genetic evaluation of disease resistance in aquaculture species: challenges and future prospects. Aquac Res. 2011;42:103–114. doi: 10.1111/j.1365-2109.2010.02669.x.
- Natnan ME, Low CF, Chong CM, et al. Comparison of different dietary fatty acids supplement on the immune response of hybrid grouper (Epinephelus fuscoguttatus× Epinephelus lanceolatus) Challenged with Vibrio vulnificus. Biology. 2022;11:1288. doi: 10.3390/biology11091288.
- Mendivil CO. Dietary fish, fish nutrients, and immune function: a review. Front Nutr. 2020;7:617652. doi: 10.3389/fnut.2020.617652.
- Nakai T, Park SC. Bacteriophage therapy of infectious diseases in aquaculture. Res Microbiol. 2002;153:13–18. doi: 10.1016/s0923-2508(01)01280-3.
- Pereira C, Silva YJ, Santos AL, et al. Bacteriophages with potential for inactivation of fish pathogenic bacteria: survival, host specificity and effect on bacterial community structure. Mar Drugs. 2011;9:2236–2255. doi: 10.3390/md9112236.
- Verschuere L, Rombaut G, Sorgeloos P, et al. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 2000;64:655–671. doi: 10.1128/MMBR.64.4.655-671.2000.
- Riquelme C, Hayashida G, Toranzo A, et al. Pathogenicity studies on a Vibrio anguillarum-related (VAR) strain causing an epizootic in Argopecten purpuratus larvae cultured in Chile. Dis Aquat Org. 1995;22:135–141. doi: 10.3354/dao022135.
- Liang M, Li Z, Wang W, et al. A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat Commun. 2019;10:3672. doi: 10.1038/s41467-019-11648-1.
- Sanvicens N, Pastells C, Pascual N, et al. Nanoparticle-based biosensors for detection of pathogenic bacteria. TrAC, Trends Anal Chem. 2009;28:1243–1252. doi: 10.1016/j.trac.2009.08.002.
- Svechkarev D, Sadykov MR, Bayles KW, et al. Ratiometric fluorescent sensor array as a versatile tool for bacterial pathogen identification and analysis. ACS Sens. 2018;3:700–708. doi: 10.1021/acssensors.8b00025.
- Pedrero M, Campuzano S, Pingarrón JM. Electroanalytical sensors and devices for multiplexed detection of foodborne pathogen microorganisms. Sensors. 2009;9:5503–5520. doi: 10.3390/s90705503.
- Shen Y, Yue G. Current status of research on aquaculture genetics and genomics-information from ISGA 2018. Aquac Fish. 2019;4:43–47. doi: 10.1016/j.aaf.2018.11.001.
- Clarridge IJE, Attorri SM, Zhang Q, et al. 16S ribosomal DNA sequence analysis distinguishes biotypes of Streptococcus bovis: Streptococcus bovis biotype II/2 is a separate genospecies and the predominant clinical isolate in adult males. J Clin Microbiol. 2001;39:1549–1552. doi: 10.1128/JCM.39.4.1549-1552.2001.
- Nho S-W, Hikima J-i, Park SB, et al. Comparative genomic characterization of three Streptococcus parauberis strains in fish pathogen, as assessed by wide-genome analyses. PLoS One. 2013;8:e80395. doi: 10.1371/journal.pone.0080395.
- Ke H-M, Liu D, Ogura Y, et al. Tracing genomic divergence of Vibrio bacteria in the Harveyi clade. J Bacteriol. 2018;200:e00001-18. doi: 10.1128/JB.00001-18.
- Bonder MJ, Kurilshikov A, Tigchelaar EF, et al. The effect of host genetics on the gut microbiome. Nat Genet. 2016;48:1407–1412. doi: 10.1038/ng.3663.
- Limborg MT, Alberdi A, Kodama M, et al. Applied hologenomics: feasibility and potential in aquaculture. Trends Biotechnol. 2018;36:252–264. doi: 10.1016/j.tibtech.2017.12.006.
- Sakala R, Hayashidani H, Kato Y, et al. Isolation and characterization of Lactococcus piscium strains from vacuum‐packaged refrigerated beef. J Appl Microbiol. 2002;92:173–179. doi: 10.1046/j.1365-2672.2002.01513.x.
- Naresh V, Lee N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors. 2021;21:1109. doi: 10.3390/s21041109.
- Da-Silva E, Baudart J, Barthelmebs L. Biosensing platforms for Vibrio bacteria detection based on whole cell and nucleic acid analysis: a review. Talanta. 2018;190:410–422. doi: 10.1016/j.talanta.2018.07.092.
- Stella RG, Baumann P, Lorke S, et al. Biosensor-based isolation of amino acid-producing Vibrio natriegens strains. Metab Eng Commun. 2021;13:e00187. doi: 10.1016/j.mec.2021.e00187.
- Adams A, Thompson KD. Biotechnology offers revolution to fish health management. Trends Biotechnol. 2006;24:201–205. doi: 10.1016/j.tibtech.2006.03.004.
- Jia B, St-Hilaire S, Singh K, et al. Biosecurity knowledge, attitudes and practices of farmers culturing yellow catfish (Pelteobagrus fulvidraco) in Guangdong and Zhejiang provinces, China. Aquaculture. 2017;471:146–156. doi: 10.1016/j.aquaculture.2017.01.016.
- Ceccarelli D, Hasan NA, Huq A, et al. Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front Cell Infect Microbiol. 2013;3:97. doi: 10.3389/fcimb.2013.00097.
- Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007;11:497–507. doi: 10.1016/j.smrv.2007.06.003.
- Alves E, Faustino MAF, Tomé JPC, et al. Photodynamic antimicrobial chemotherapy in aquaculture: photoinactivation studies of Vibrio fischeri. PLoS One. 2011;6:e20970. doi: 10.1371/journal.pone.0020970.
- Alves RN, Agustí S. Effect of ultraviolet radiation (UVR) on the life stages of fish. Rev Fish Biol Fisheries. 2020;30:335–372. doi: 10.1007/s11160-020-09603-1.
- Liu R, Han G, Li Z, et al. Bacteriophage therapy in aquaculture: current status and future challenges. Folia Microbiol. 2022;67:573–590. doi: 10.1007/s12223-022-00965-6.
- Gupta S, Tiwari A, Jain U, et al. Synergistic effect of 2D material coated Pt nanoparticles with PEDOT polymer on electrode surface interface for a sensitive label free Helicobacter pylori CagA (Ag-Ab) immunosensing. Mater Sci Eng C Mater Biol Appl. 2019;103:109733. doi: 10.1016/j.msec.2019.05.018.
- Ashaduzzaman M, Deshpande SR, Murugan NA, et al. On/off-switchable LSPR nano-immunoassay for troponin-T. Sci Rep. 2017;7:44027. doi: 10.1038/srep44027.
- Parlak O, İncel A, Uzun L, et al. Structuring Au nanoparticles on two-dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens Bioelectron. 2017;89:545–550. doi: 10.1016/j.bios.2016.03.024.
- Mishra S, Ashaduzzaman M, Mishra P, et al. Stimuli-enabled zipper-like graphene interface for auto-switchable bioelectronics. Biosens Bioelectron. 2017;89:305–311. doi: 10.1016/j.bios.2016.03.052.
- Ubina NA, Cheng S-C, Chen H-Y, et al. A visual aquaculture system using a cloud-based autonomous drones. Drones. 2021;5:109. doi: 10.3390/drones5040109.
- Teixeira RR, Puccinelli JB, Poersch L, et al. Towards precision aquaculture: a high performance, cost-effective IoT approach. arXiv preprint arXiv:210511493. 2021.
- Arvanitoyannis IS, Kassaveti A. Fish industry waste: treatments, environmental impacts, current and potential uses. Int J of Food Sci Tech. 2008;43:726–745. doi: 10.1111/j.1365-2621.2006.01513.x.
- Aguilera SE, Cole J, Finkbeiner EM, et al. Managing small-scale commercial fisheries for adaptive capacity: insights from dynamic social-ecological drivers of change in Monterey Bay. PLoS One. 2015;10:e0118992. doi: 10.1371/journal.pone.0118992.
- Bondad-Reantaso MG, Subasinghe RP, Arthur JR, et al. Disease and health management in Asian aquaculture. Vet Parasitol. 2005;132:249–272. doi: 10.1016/j.vetpar.2005.07.005.
- Alter T, Appel B, Bartelt E, et al. Vibrio infections from food and sea water. Introducing the" VibrioNet. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2011;54:1235–1240. doi: 10.1007/s00103-011-1359-1.
- Hon C-C, Ramilowski JA, Harshbarger J, et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374.
- Kim WR, Park EG, Lee YJ, et al. Integration of TE induces cancer specific alternative splicing events. Int J Mol Sci. 2022;23:10918. doi: 10.3390/ijms231810918.
- Park EG, Kim WR, Lee YJ, et al. Downregulated pol-miR-140-3p induces the expression of the kinesin family member 5A against Streptococcus parauberis infection in olive flounder. Fish Shellfish Immunol. 2022;126:178–186. doi: 10.1016/j.fsi.2022.05.043.
- Jo A, Im J, Lee H-E, et al. Evolutionary conservation and expression of miR-10a-3p in olive flounder and rock bream. Gene. 2017;628:16–23. doi: 10.1016/j.gene.2017.07.020.
- Zhang W, Belton B, Edwards P, et al. Aquaculture will continue to depend more on land than sea. Nature. 2022;603: e2–E4. doi: 10.1038/s41586-021-04331-3.
- Mishra A, Nam G-H, Gim J-A, et al. Current challenges of Streptococcus infection and effective molecular, cellular, and environmental control methods in aquaculture. Mol Cells. 2018;41:495.
- Reverter M, Sarter S, Caruso D, et al. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat Commun. 2020;11:1870. doi: 10.1038/s41467-020-15735-6.
- Naylor RL, Hardy RW, Buschmann AH, et al. A 20-year retrospective review of global aquaculture. Nature. 2021;591:551–563. doi: 10.1038/s41586-021-03308-6.
- Stentiford GD, Bateman IJ, Hinchliffe SJ, et al. Sustainable aquaculture through the One Health lens. Nat Food. 2020;1:468–474. doi: 10.1038/s43016-020-0127-5.
- Biener R, Horn T, Komitakis A, et al. High-cell-density cultivation of Vibrio natriegens in a low-chloride chemically defined medium. Appl Microbiol Biotechnol. 2023;107:7043–7054. Sep 23. doi: 10.1007/s00253-023-12799-4.
- Wu F, Wang S, Peng Y, et al. Metabolic engineering of fast-growing Vibrio natriegens for efficient pyruvate production. Microb Cell Fact. 2023;22:172. doi: 10.1186/s12934-023-02185-0.
- Moonsamy G, Zulu NN, Lalloo R, et al. Large-scale production of an abalone probiotic, Vibrio midae, isolated from a South African abalone, Halitotis midae for use in aquaculture. Biocatal Agric Biotechnol. 2020;29:101794. doi: 10.1016/j.bcab.2020.101794.
- Visick KL, Stabb EV, Ruby EG. A lasting symbiosis: how Vibrio fischeri finds a squid partner and persists within its natural host. Nat Rev Microbiol. 2021;19:654–665. doi: 10.1038/s41579-021-00557-0.
- Levy S. ECDC Vibrio Map Viewer: tracking the whereabouts of pathogenic species. Environ Health Perspect. 2018;126:034003. doi: 10.1289/EHP2904.