Abstract
Background. Intake of n‐3 polyunsaturated fatty acids (n‐3 PUFA) either from natural sources or dietary supplementation is inversely associated with atherothrombosis.
Aim. A double‐blind pilot study was designed to address the impact of n‐3 PUFA on atherosclerosis, haemostasis and vascular status in patients with combined hyperlipoproteinemia.
Methods. Carotid intima‐media thickness (C‐IMT), texture of intima‐media complex (T‐IMC), lipids and platelet function were evaluated in 64 patients with combined hyperlipoproteinemia who received placebo or n‐3 PUFA (6 g/day) for 2 years. C‐IMT and T‐IMC were assessed by B‐mode ultrasound. Lipids and platelet function were determined by validated methods.
Results. C‐IMT increased in placebo, but not in n‐3 PUFA group with respect to baseline. In contrast T‐IMC decreased in n‐3 PUFA, but not in placebo; in both cases, however, treatment effect did not reach statistical significance. A fall of triglycerides, concomitant to a rise of high‐ and low‐density lipoprotein cholesterol (HDL and LDL), was observed in the active treated group. Platelet function was significantly reduced by n‐3 PUFA.
Conclusions. Results show a favourable effectiveness of n‐3 PUFA on IMT progression and T‐IMC that deserves to be confirmed in larger studies. Despite the small sample size, the beneficial effect of n‐3 PUFA on platelet function, triglycerides and HDL‐C is clearly highlighted.
| Abbreviations | ||
| n‐3 PUFA | = | n‐3 polyunsaturated fatty acids |
| C‐IMT | = | carotid intima‐media thickness |
| T‐IMC | = | texture of intima‐media complex |
| CAD | = | coronary artery disease |
| EPA | = | eicosapentaenoic acid |
| DHA | = | docosahexaenoic acid |
| CC | = | common carotid |
| Bif | = | bifurcation |
| ICA | = | internal carotid artery |
| Mean‐IMT | = | mean intima‐media thickness of the whole carotid tree |
| ROI | = | region of interest |
| AC50 | = | collagen concentration inducing 50% decrease of optical density in platelet‐rich plasma |
Introduction
Elevated plasma triglyceride levels affect millions of people in the world. Although associated with an increased risk of coronary artery disease (CAD) Citation1,2, the contribution of hypertriglyceridemia to the progression of atherosclerosis remains to be defined Citation3.
Carotid intima‐media thickness (C‐IMT) is a widely used surrogate index of the atherosclerotic burden. Besides the correlation with the presence of carotid plaque Citation4, C‐IMT is directly associated with the presence and extent of CAD Citation5 and with the incidence of new vascular events Citation6,7. C‐IMT is also largely accepted to assess the impact of pharmacologic/dietary interventions on atherosclerosis Citation8.
Although the relationship between C‐IMT and conventional risk factors (e.g. hypercholesterolemia, hypertension etc) is well defined Citation9,10, the one with hypertriglyceridemia is not so clear; elevated triglyceride concentrations are, in fact, often associated with alterations of other lipid variables, mainly high density lipoprotein (HDL) Citation11,12. Even less information is available on the effects of triglyceride‐lowering on IMT progression, particularly in asymptomatic patients.
It is well known that n‐3 polyunsaturated fatty acids (n‐3 PUFA), from either natural sources or dietary supplementation, have a consistent triglyceride‐lowering effect Citation13; however, no long‐term prospective studies on the efficacy of these agents on IMT progression in primary prevention exist Citation14. Indeed, although two epidemiological cross‐sectional studies documented an inverse relationship between the intake of n‐3 PUFA from fish and C‐IMT Citation15,16, only one secondary prevention study addressed their effect when supplemented by capsules Citation17. IMT also correlates with some haemostatic variables, such as platelet aggregation Citation18, that have been reported to be influenced by n‐3 PUFA Citation19. Finally, n‐3 PUFA can reduce the incidence of vascular events by enhancing atherosclerotic plaque stability Citation20.
A double‐blind 2‐year single centre pilot study was therefore carried out, in a group of asymptomatic patients with combined hyperlipoproteinemia, to explore the potential effects of n‐3 PUFA administration on (i) IMT progression, (ii) texture of intima‐media complex (T‐IMC), (iii) lipids and haemostasis.
Key messages
In patients with combined hyperlipoproteinemia, n‐3 polyunsaturated fatty acids (PUFA) exert a beneficial effect on carotid intima‐media thickness progression and texture of intima‐media complex that, however, deserves to be confirmed in larger scale studies.
The beneficial effect of n‐3 PUFA on platelet function, triglycerides and high‐density lipoprotein cholesterol (HDL‐C) is clearly highlighted in patients with combined hyperlipoproteinemia.
Patients and methods
Patients
Males and post‐menopausal females without history of cardiovascular events were selected. Patients aged 45–75 years, with at least one carotid lesion ⩾1.3 mm, low density lipoprotein (LDL) cholesterol levels <4.93 mmol/L, triglyceride levels between 1.93 and 4.55 mmol/L and a 10‐year cardiovascular risk <20% (according to Framingham algorithm) Citation21, were eligible. The triglyceride cutpoint of 1.7 mmol/L, suggested by ATPIII guidelines Citation3 to classify hypertriglyceridemia, was raised to 1.93 mmol/L to increase the probability that the condition of hypertriglyceridemia could be satisfied even after 2 months of prudent diet.
Patients with ‘very high’ LDL cholesterol levels (>4.93 mmol/L) according to ATPIII guidelines, were excluded for ethical reasons because n‐3 PUFA were not expected to exert a positive effect on LDL cholesterol. Patients with ‘high’ LDL cholesterol (4.14–4.89 mmol/L) were considered as acceptable on condition that their 10‐year cardiovascular risk was lower than 20%.
Exclusion criteria were: blood pressure >135/90 mmHg, diabetes, renal insufficiency or other endocrine syndromes, excessive alcohol consumption, heavy cigarette smoking (>10 cigarettes/day), and treatment with lipid‐lowering drugs, anti‐hypertensives, or hormone replacement therapy. Each patient signed an informed consent prior to randomization. The study was approved by the Ethical Committee of Niguarda Hospital (Milan, Italy).
Intervention
At baseline, patients who satisfied the inclusion criteria received a low fat content prudent diet (26% fats, 22% proteins, 52% carbohydrates; polyunsaturated/saturated fat ratio: 0.43), as recommended by the European Atherosclerosis Society. The presence of hypertriglyceridemia even after dietary intervention was confirmed 2 months later. Patients were thereafter randomized to placebo or n‐3 PUFA. An appropriate software was used to obtain two groups balanced for sex, age and smoking. Each n‐3 PUFA capsule contained 1 g of fatty acid mixture (19% eicosapentaenoic acid (EPA), 13% docosahexaenoic acid (DHA), 19% palmitic acid, 18% oleic acid, 2% linoleic acid and 29% other minor components). Six capsules, corresponding to a daily intake of 1.08 g EPA, 0.72 g DHA, 0.01 g tocopherol acetate, were prescribed. The placebo group received 6 g/day olive oil prepared in opaque, identical‐looking soft gelatine capsules. Patients were instructed to divide capsules among breakfast, lunch and dinner.
Patients were examined as outpatients at month 0, 3, 6, 12, 18, and 24. Compliance was assessed by counting returned capsules at each visit and by measuring EPA and DHA levels at month 24.
Carotid ultrasound
Carotid ultrasound was carried out at month 0, 6, 12, and 24 by a single operator (author M.A.) using an Esaote AU4 system, equipped with a 10–13 MHz linear array probe and recorded on sVHS videotapes. The far wall of the whole common carotid (CC), of the bifurcation (Bif) and of the first proximal centimetre of the internal carotid artery (ICA) was visualized in anterior, lateral and posterior projections.
Ultrasonic measurements were performed by a single operator (author B.F.) with appropriate software (Eurequa System) Citation22, which allows the automatic edge detection of echogenic lines limiting the intima‐media complex. Progressions of C‐IMTs were estimated by assuming a linear trend with time.
The ultrasonic scans performed at months 0 and 24 were repeated within 2 weeks; the mean of the two independent C‐IMT determinations was adopted for statistical analyses and to assess reproducibility. The absolute differences (mean±SD) between replicate scans at baseline were 0.024±0.019 mm, 0.072±0.060 mm, 0.052±0.045 mm and 0.039±0.034 mm for CC‐IMT, Bif‐IMT, ICA‐IMT and Mean‐IMT, respectively. The same absolute differences at month 24 were 0.024±0.017 mm, 0.048±0.033 mm, 0.039±0.033 mm, 0.026±0.019 mm, respectively.
Texture of intima‐media complex (T‐IMC) by video‐densitometric analysis
CC‐IMT video‐densitometric analysis Citation23 was performed at months 0 and 24. Briefly, two bi‐dimensional optimal images of the far wall of both CC arteries were digitized in longitudinal projection, with a resolution of 576×768 pixels, and 256‐degree grey‐scale per pixel.
A region of interest (ROI) of 10 mm, including the intima‐media complex, was selected 10 mm below the bifurcation. To adjust for different ultrasound attenuation and different gain settings in different subjects, two calibration steps were introduced into the analysis: the grey‐scale value of each ROI was adjusted linearly, so that the mean value of blood was 0 and that of adventitia was 256. All recordings were evaluated by a single reader (author M.K.). A computer‐driven image analysis system (Medical Imaging Processing) was used to analyse the ROIs by first‐ and second‐order parameters. First‐order parameters, reflecting IMT mean grey level, are arithmetic mean and standard deviation. Mean grey levels are positively related to the content of smooth muscle cells (SMCs) and negatively to that of macrophages Citation23.
Second‐order parameters, reflecting IMT heterogeneity, are entropy, contrast and second angular moment. Entropy and contrast correlate positively, whereas second angular moment correlates negatively, with the prevalence of SMCs over macrophages Citation23.
Plasma lipids
Lipids were measured two months and one week before the start of the study and thereafter at months 3, 6, 12, 18, and 24. Total and HDL cholesterol and triglycerides were determined by enzyme methods in serum, after an overnight fast Citation24,25. Serum LDL was calculated by Friedewald's formula Citation26 in samples with triglyceride levels <4.55 mmol/L (400 mg/dL); otherwise measurement was performed after separation of LDL fraction by ultracentrifugation.
Platelet function
Platelet aggregation was carried out in platelet‐rich plasma at months 0, 6, 12, and 24 using collagen (Mascia Brunelli, Milano, Italy) as stimulus. For each patient, a collagen concentration that induces 50% decrease of optical density (AC50) was calculated on the basis of a linear regression between collagen concentrations and corresponding percentages of aggregation Citation27, measured 5 minutes after collagen addition. Platelet thromboxane B2 was determined by enzyme‐linked immuno‐assay (Cayman Chemical, Spi Bio, Massy Cedex France) in platelet‐rich plasma samples (3×1011 platelets/L) incubated for 5 min (1,000 rpm stirring) with collagen (5 µg/mL).
Plasma fatty acid analysis
Plasma fatty acid analysis was performed as described Citation28. Briefly, fatty acid methyl esters were prepared by acid trans‐methylation from total lipid extracts and separated by high‐pressure liquid chromatography. Peaks were identified using pure reference compounds and quantified by an internal standard (C19:0), added to the lipid extract before methylation. Data are reported as percentages of total fatty acids.
Statistical methods
The ultrasonic variables considered for statistical analyses were the mean IMT of common carotids (CC‐IMT), of bifurcations (Bif‐IMT), of ICAs (ICA‐IMT) and of the whole carotid tree (Mean‐IMT). C‐IMT progression was estimated by weighted linear regression of up to eight serial measurements versus time; weights were proportional to the number of visualised arterial walls used to compute C‐IMTs.
Treatment efficacy was evaluated by comparing the slope of the progression lines IMT/time in placebo and active treated group. Mean C‐IMT progressions of the two groups were compared by analysis of covariance (ANCOVA). Progression rates were also weighted proportionally to the inverse of the estimated variance of progression slope. Baseline C‐IMTs were used as covariates.
The individual extent of lipid‐lowering was calculated as differences between baseline and the mean of all on‐trial measurements obtained between months 6 and 24. All statistical tests were two‐tailed with a significance level of P<0.05. Comparison versus baseline (within‐group analysis) was performed by ANOVA completed by Dunnett's test. ANOVA for repeated measures was carried out to compare the two treatment groups over time (between‐group analysis). Version 6.12 Windows of SAS software was used to perform statistical analyses.
Results
Treatment
Six hundred and forty‐two patients were screened, and 82 matched the inclusion/exclusion criteria. After 2 months of prudent diet, hypertriglyceridemia was confirmed in 64 patients who were therefore randomized for the treatments (32/group). One patient left the study after 3 months because he moved to another city and was therefore excluded from statistical analyses. Two patients were excluded because of major deviation from the protocol during the follow‐up (anti‐hypertensive assumption) and four because of non‐compliance on the basis of returning capsules (compliance <70%). The final analysed group included 57 patients (30 on active treatment).
shows the clinical characteristics of the patients. The two groups were fully comparable; no significant differences were, indeed, observed for any variable considered.
Table I. Characteristics of the patients at baseline.
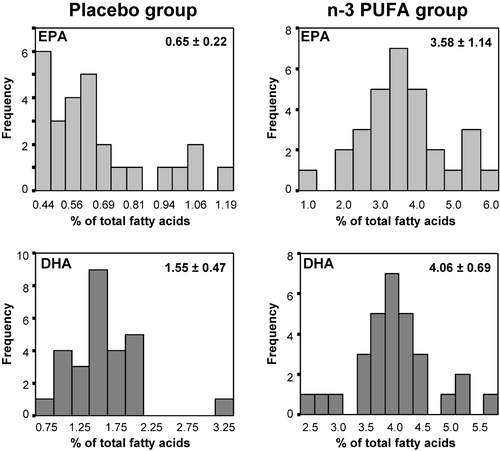
The adherence to treatment was documented by the different frequency distribution of plasma EPA and DHA in the two groups at the end of the study (). In the active treatment group one case of myocardial infarction occurred. Four cases of newly diagnosed hypertension were recorded; two in the n‐3 PUFA and two in the placebo treated group. None of the adverse events were considered by physician as attributable to treatment.
A rise in body weight (+3%, P<0.01) was observed at the end of the study in both groups. Blood pressure and heart rate were unchanged throughout the study.
Effects on plasma lipids/lipoproteins
A significant reduction of triglycerides, ranging from −20% at the 3rd month to −30% at the 24th month, was observed in the n‐3 PUFA‐treated group. Triglyceridemia was essentially unchanged until month 18 in the placebo group with a significant increase observed at month 24 (+13.1%; P<0.05). n‐3 PUFA significantly reduced plasma triglycerides at each time point considered (P values between‐group ranging from 0.005 to 0.0001).
The levels of LDL significantly increased in the n‐3 PUFA group; the rise approximated 10% (P = 0.002) and 16.4% (P = 0.005) after month 3 and 24, respectively. The treatment effect was significant at month 18 and 24 (P = 0.01 and P = 0.003, respectively). HDL increased significantly in the active treated group, reaching a 7% rise at month 6 (P = 0.003) that persisted throughout the study. No change versus baseline was observed in the placebo group. The between‐group analysis did not reach statistical significance at any time point considered.
Effect on platelets
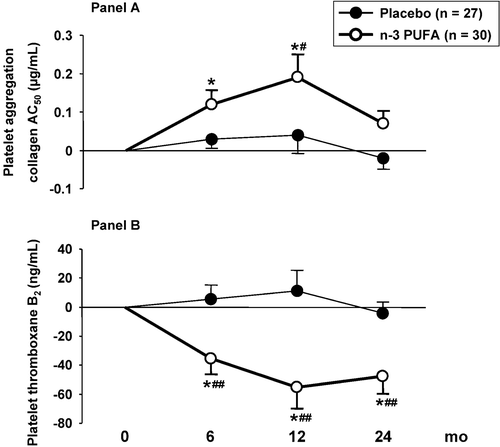
Changes of platelet aggregation compared to baseline are shown in (Panel A). Collagen AC50 increased significantly at month 6 and 12 in the n‐3 PUFA group. The effect of treatment (between‐group analysis) reached statistical significance (P = 0.03) at month 12.
The effect of n‐3 PUFA on platelet aggregation was concomitant with the inhibition of thromboxane B2 synthesis in platelet‐rich plasma (, Panel B). As for platelet aggregation, the highest treatment effect was observed at month 12 (P = 0.002).
Effect on intima‐media thickness
All ultrasonic variables increased significantly versus baseline in the placebo group (). In contrast, no IMT progression was observed in n‐3 PUFA group, except for CC‐IMT. Despite these differences, the effect of n‐3 PUFA did not reach statistical significance when the between‐group analysis was performed.
Table II. Intima‐media thickness (IMT) progression in n‐3 polyunsaturated fatty acids (n‐3 PUFA) and placebo groups.
Effect on IMT composition
shows the effect of n‐3 PUFA on IMT video‐densitometric variables. No change versus baseline was observed in the placebo group when mean grey levels (first‐order parameters) were considered. Among second‐order parameters that reflect IMT heterogeneity, only entropy was significantly reduced. In contrast, patients included in the n‐3 PUFA group showed a significant reduction of almost all the video‐densitometric variables, with the exception of the second angular moment that increased significantly. The effect of n‐3 PUFA treatment did not reach statistical significance when the between‐group analysis was performed. Similar results were obtained when an analogue segment was analysed on the left CC (data not shown).
Table III. Effect of n‐3 polyunsaturated fatty acids (n‐3 PUFA) on ultrasound video‐densitometry of right common carotid intima‐media thickness (CC‐IMT) segments.
Discussion
Firstly, results from this study show that carotid IMT progression in patients with combined hyperlipoproteinemia can be appreciated as significant even after a relatively short period of follow‐up (2 years), notwithstanding IMT changes are smaller than those observed in patients with other vascular risk factors Citation10. In addition, despite the lack of significance of the between‐group analysis, likely due to the small sample size, the within‐group analysis clearly shows that IMT progression is blunted in the n‐3 PUFA group but not in placebo. These results encourage the design of a larger trial to confirm the potential favourable effectiveness of these agents on IMT progression observed in this pilot study.
Only few studies have so far addressed the effects of n‐3 PUFA on carotid arteries. Results from a longitudinal (24 months' duration) placebo‐controlled study in CAD patients, supplemented with a dose of n‐3 PUFA similar to that adopted in our study, did not show any change in IMT progression Citation17. Of interest, an inverse relationship between fish consumption and carotid atherosclerosis has been reported by two Japanese epidemiological cross‐sectional studies Citation15,16. Besides the different nature of the studies (prospective versus cross‐sectional), it can be hypothesized that this discrepancy can be explained by a more efficient incorporation of n‐3 PUFA in plasma lipids when ingested as fish rather than as capsules Citation29 or by a different efficacy of n‐3 PUFA on early or advanced carotid atherosclerosis.
Our data from IMT video‐densitometry may provide an additional explanation for this controversy. It has been reported that mean grey levels are positively related to the content of smooth muscle cells (SMCs) and negatively to that of macrophages, and that the prevalence of SMCs over macrophages correlates positively with entropy and contrast and negatively with the second angular moment Citation23. On this basis, our results suggest that, if an effect of n‐3 PUFA on IMT exists, this is directed toward a reduction of SMC content rather than a reduction of macrophages. Although these results have to be interpreted with caution in consideration of the relatively small scale of the study, they may provide a further explanation for the positive effect of n‐3 PUFA observed in population‐based studies but not in CAD patients. Indeed, since ultrasound techniques do not distinguish between media and intima, an effect of n‐3 PUFA on IMT in terms of reduction of SMCs can be more easily appreciated when evaluated in patients with early atherosclerosis in which the media contributes to IMT measurements much more than the intima Citation30. In contrast, in CAD patients, in whom the contribution of intima to IMT measurement is higher, this effect could be less appreciable. Of interest, a beneficial effect of EPA on the composition of atherosclerotic lesions has been reported in an animal model of the twice‐injured carotid artery Citation31.
A clear‐cut effect of n‐3 PUFA emerges when coronary atherosclerosis progression and/or incidence of cardiovascular events are considered Citation32–35. The effect has been attributed to a favourable activity on lipid profile Citation36,37, to an anti‐arrhythmic effect Citation38 and also to an anti‐thrombotic potential Citation19. As already reported Citation39, results from our study confirm that n‐3 PUFA exert an antiplatelet effect both in terms of inhibition of platelet aggregation and thromboxane synthesis.
Of interest, our data show that at least one year of treatment is required to reach the maximal effect on platelet function. A trend toward baseline values for platelet aggregation was observed at month 24, concomitantly with the maximal increase of LDL, a finding explainable by the pro‐aggregatory effect of LDL observed in vitroCitation40 and ex vivoCitation27,Citation41.
The role of hypertriglyceridemia as a predictor of carotid atherosclerosis is also controversial. Several cross‐sectional studies have identified a correlation between postprandial triglyceridemia and C‐IMT Citation42. Less information is, however, available on the role of fasting triglyceridemia on C‐IMT and even less on C‐IMT progression. As expected Citation36, a reduction of triglycerides, accompanied by a significant increase of HDL and LDL in the active treated group, was observed also in our study. The increase of LDL was, however, even higher than that reported by others Citation34,35,Citation43. In spite of this, IMT progression was clearly reduced in the n‐3 PUFA group but not in placebo, suggesting that the rise of LDL, a well known determinant of IMT Citation44, does not completely obscure the effects of n‐3 PUFA. A possible explanation is that the increase of LDL induced by n‐3 PUFA is mainly due to a rise of LDL bigger in size, known to be less atherogenic Citation45. In addition, also the concomitant rise of HDL may have contributed to the inhibition of IMT progression observed in the n‐3 PUFA group Citation46.
Taken together, our results may give grounds for the reported protection of n‐3 PUFA against vascular events, and indicate that, in patients with combined hyperlipoproteinemia, the antiplatelet effect emerges more clearly than the anti‐atherosclerotic one.
Results on the effect of n‐3 PUFA on IMT progression herein described strongly encourage an adequately dimensioned primary prevention trial in hypertriglyceridemic patients, or in patients with combined hyperlipoproteinemia, to be performed in order to strengthen not only the potential benefit of n‐3 PUFA on IMT progression but also their effect on SMC content as one of the mechanisms responsible for the reduction of IMT progression.
Acknowledgements
The study was supported by Institut De Recherche Pierre Fabre, Departement Recherche Clinique. The sponsor had no role in the collection, interpretation of data, in the writing of the report or in the decision to submit the report for publication.
We appreciated the expertise of Professor C. Galli for fatty acid analyses.
References
- Assmann G., Schulte H., Funke H., von Eckardstein A. The emergence of triglycerides as a significant independent risk factor in coronary artery disease. Eur Heart J 1998; 19: M8–M14
- Hopkins P. N., Wu L. L., Hunt S. C., Brinton E. A. Plasma triglycerides and type III hyperlipidemia are independently associated with premature familial coronary artery disease. J Am Coll Cardiol 2005; 45: 1003–12
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report., Circulation. 106. 3143–421
- Rosfors S., Hallerstam S., Jensen‐Urstad K., Zetterling M., Carlstrom C. Relationship between intima‐media thickness in the common carotid artery and atherosclerosis in the carotid bifurcation. Stroke 1998; 29: 1378–82
- Bisoendial R. J., Hovingh G. K., de Groot E., Kastelein J. J., Lansberg P. J., Stroes E. S. Measurement of subclinical atherosclerosis: beyond risk factor assessment. Curr Opin Lipidol 2002; 13: 595–603
- O'Leary D. H., Polak J. F., Kronmal R. A., Manolio T. A., Burke G. L., Wolfson S. K., for the Cardivascular Health Study Collaborative Research Group. Carotid‐artery intima‐media thickness as a risk factor for myocardial infarction and stroke in older adults. New Engl J Med 1999; 340: 14–22
- Van Der Meer I. M., Bots M. L., Hofman A., Iglesias Del Sol A., Van Der Kuip D. A., Witteman J. C. Predictive Value of Noninvasive Measures of Atherosclerosis for Incident Myocardial Infarction. The Rotterdam Study. Circulation 2004; 109: 1089–94
- Grobbee D. E., Bots M. L. Atherosclerotic disease regression with statins: studies using vascular markers. Int J Cardiol 2004; 96: 447–59
- Safar M. E., Girerd X., Laurent S. Structural changes of large conduit arteries in hypertension. J Hypertens 1996; 14: 545–55
- Simon A., Gariepy J., Chironi G., Megnien J. L., Levenson J. Intima‐media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens 2002; 20: 159–69
- Krauss R. M. Triglycerides and atherogenic lipoproteins: rationale for lipid management. Am J Med 1998; 105: 58S–62S
- Hodis H. N., Mack W. J. Triglyceride‐rich lipoproteins and progression of atherosclerosis. Eur Heart J 1998; 19: A40–4
- Roche H. M., Gibney M. J. Effect of long‐chain n‐3 polyunsaturated fatty acids on fasting and postprandial triglyceride metabolism. Am J Clin Nutr 2000; 71: 232S–7S
- Balk E. M., Lichtenstein A. H., Chung M., Kupelnick B., Chew P., Lau J. Effects of omega‐3 fatty acids on coronary restenosis, intima‐media thickness, and exercise tolerance: a systematic review. Atherosclerosis 2006; 184: 237–46
- Yamada T., Strong J. P., Ishii T., Ueno T., Koyama M., Wagayama H., et al. Atherosclerosis and n‐3 fatty acids in the populations of a fishing village and a farming in Japan. Atherosclerosis 2000; 153: 469–81
- Hino A., Adachi H., Toyomasu K., Yoshida N., Enomoto M., Hiratsuka A., et al. Very long chain N‐3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis 2004; 176: 145–9
- Angerer P., Kothny W., Störk S., von Schacky C. Effect of dietary supplementation with n‐3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovasc Res 2002; 54: 183–90
- Salonen R., Salonen J. T. Progression of carotid atherosclerosis and its determinants: a population‐based ultrasonography study. Atherosclerosis 1990; 81: 33–40
- Knapp H. R. Dietary fatty acids in human thrombosis and hemostasis. Am J Clin Nutr 1997; 65: 1687S–98S
- Thies F., Garry J. M., Yaqoob P., Rerkasem K., Williams J., Shearman C. P., et al. Association of n‐3 polyunsaturated fatty acids with stability of atherosclerosis plaques: a randomised controlled trial. Lancet 2003; 361: 477–85
- Wilson P. W., D'Agostino R. B., Levy D., Belanger A. M., Silbershatz H., Kannel W. B. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97: 1837–47
- Touboul P. J., Prati P., Scarabin P. Y., Adrai V., Thibout E., Ducimetiere P. Use of monitoring software to improve the measurement of carotid wall thickness by B‐mode imaging. J Hypertens 1992, Suppl 10: S37–41
- Puato M., Faggin E., Rattazzi M., Paterni M., Kozakova M., Palombo C., et al. Study Group on Arterial Wall Structure. In vivo noninvasive identification of cell composition of intimal lesions: a combined approach with ultrasonography and immunocytochemistry. J Vasc Surg 2003; 38: 1390–5
- Bucolo G., David M. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 1973; 19: 476–82
- Röschlau P., Bernt E., Gruber W. Enzymatische Bestimmung des Gesamt Cholesterins in Serum. Z Klin Chem Klin Biochem 1974; 12: 403–7
- Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502
- Tremoli E., Maderna P., Colli S., Morazzoni G., Sirtori M., Sirtori C. R. Increased platelet sensitivity and thromboxane B2 formation in type‐II hyperlipoproteinaemic patients. Eur J Clin Invest 1984; 14: 329–33
- Tremoli E., Maderna P., Marangoni F., Colli S., Eligini S., Catalano I., et al. Prolonged inhibition of platelet aggregation after ethyl ester ingestion by healthy volunteers. Am J Clin Nutr 1995; 61: 607
- Visioli F., Rise P., Barassi M. C., Marangoni F., Galli C. Dietary intake of fish vs. formulations leads to higher plasma concentrations of n‐3 fatty acids. Lipids 2003; 38: 415–18
- Salonen J. T., Salonen R. Ultrasound B‐mode imaging in observational studies of atherosclerotic progression. Circulation 1993; 87((3 Suppl))II 56–65
- Kawano H., Yano T., Mizuguchi K., Mochizuki H., Saito Y. Changes in aspects such as the collagenous fiber density and foam cell size of atherosclerotic lesions composed of foam cells, smooth muscle cells and fibrous components in rabbits caused by all‐cis‐5, 8, 11, 14, 17‐icosapentaenoic acid. J Atheroscler Thromb 2002; 9: 170–7
- Burr M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989; 2: 757–61
- Singh R. B., Niaz M. A., Sharma J. P., Kumar R., Rastogi V., Moshiri M. Randomized, double‐blind, placebo‐controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival. Cardiovasc Drugs Ther 1997; 11: 485–91
- von Schacky C., Angerer P., Kothny W., Theisen K., Mudra H. The effect of dietary n‐3 fatty acids on coronary atherosclerosis. A randomized, double‐blind, placebo‐controlled trial. Ann Intern Med 1999; 130: 554–62
- GISSI‐Prevenzione Investigators. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial., Lancet. 354. 447
- Nestel P. J. Fish oil and cardiovascular disease: lipids and arterial function. Am J Clin Nutr 2000; 71: 228S–31S
- Harris W. S., Park Y., Isley W. L. Cardiovascular disease and long‐chain omega‐3 fatty acids. Curr Opin Lipidol 2003; 14: 9–14
- Leaf A., Kang J. X., Xiao Y. F., Billman G. E. Dietary n‐3 fatty acids in the prevention of cardiac arrhythmias. Curr Opin Clin Nutr Metab Care 1998; 1: 225–8
- Kristensen S. D., Schmidt E. B., Dyerberg J. Dietary supplementation with n‐3 polyunsaturated fatty acids and human platelet function: a review with particular emphasis on implications for cardiovascular disease. J Intern Med 1989, Suppl 731: 141–50
- Colli S., Maderna P., Tremoli E., Baraldi A., Rovati G. E., Gianfranceschi G., et al. Prostacyclin‐lipoprotein interactions. Studies on human platelet aggregation and adenylate cyclase. Biochem Pharmacol 1985; 34: 2451–7
- Tremoli E., Folco G., Agradi E., Galli C. Platelet thromboxanes and serum cholesterol. Lancet 1979; 1: 107–8
- Karpe F., Boquist S., Tang R., Bond G. M., de Faire U., Hamsten A. Remnant lipoproteins are related to intima‐media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res 2001; 42: 17–21
- Harris W. S. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res 1989; 30: 785–807
- Poli A., Tremoli E., Colombo A., Sirtori M., Pignoli P., Paoletti R. Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients. A new model for the quantitation and follow‐up of preclinical atherosclerosis in living human subjects. Atherosclerosis 1988; 70: 253–61
- Suzukawa M., Abbey M., Howe P. R., Nestel P. J. Effects of fish oil fatty acids on low density lipoprotein size, oxidizability, and uptake by macrophages. J Lipid Res 1995; 36: 473–84
- Baldassarre D., Amato M., Pustina L., Tremoli E., Sirtori C. R., Calabresi L., et al. Increased carotid artery intima‐media thickness in subjects with primary hypoalphalipoproteinemia. Arterioscler Thromb Vasc Biol 2002; 22: 317–22