Abstract
Calciphylaxis is a rare cutaneous vascular disease that manifests with intolerable pains, non-healing skin wounds, histologically characterized by calcification, fibrointimal hyperplasia, and microvessel thrombosis. Currently, there are no standardized guidelines for this disease. Recent studies have recognized a high prevalence of thrombophilias and hypercoagulable conditions in calciphylaxis patients. Here, we report a case of uremic calciphylaxis patient whom was refractory to conventional treatments and then received a salvage strategy with intravenous and local hAMSC application. In order to investigate the therapeutic mechanism of hAMSCs from the novel perspective of hypercoagulability, coagulation-related indicators, wound status, quality of life and skin biopsy were followed up. Polymerase chain reaction (PCR) was performed to determine the distribution of hAMSCs in multiple tissues including lung, kidney and muscle after infusion of hAMSCs for 24 h, 1 week and 1 month in mice aiming to investigate whether hAMSCs retain locally active roles after intravenous administration. Improvement of hypercoagulable condition involving correction of platelet, D-dimer and plasminogen levels, skin regeneration and pain alleviation were revealed after hAMSC administration over one-year period. Skin biopsy pathology suggested regenerative tissues after 1 month hAMSC application and full epidermal regeneration after 20 months hAMSC treatment. PCR analysis indicated that hAMSCs were homing in lung, kidney and muscle tissues of mice even until tail vein injection of hAMSCs for 1 month. We propose that hypercoagulability is a promising therapeutic target of calciphylaxis patients, which can be effectively improved by hAMSC treatment.
Introduction
Uremic calciphylaxis, also known as calcific uremic arteriolopathy (CUA), is a rare but life-threating cutaneous vascular disease. It primarily affects patients with end-stage kidney disease (ESKD) and characterized by rapidly progressing, intensely painful, ischemic skin lesions [Citation1]. Pathologically, the disease is typified by calcification, microthrombosis, and fibrointimal hyperplasia of small dermal and subcutaneous arteries and arterioles [Citation2]. An estimated incidence of calciphylaxis in patients undergoing dialysis is reported to range from 0.04% to 4% [Citation3], with 1-year mortality up to 45%-80% [Citation4,Citation5]. The sepsis secondary to infected ulcerations is considered the most common cause of death [Citation1,Citation2]. Skin biopsy remains the gold standard for the diagnosis of clinically suspected calciphylaxis.
The pathogenesis of this devastating disease remains uncertain despite the gradually increasing reports. A multidisciplinary approach including wound care, analgesia, anti-inflammatory treatment, elimination of risk factors might be effective, however, nonstandard treatment has been established [Citation3,Citation6]. Due to the stimulation of cell proliferation, inhibition of vascular calcification, neovascularization, anti-inflammation and antioxidative effects, MSCs treatment is considered promising for skin regeneration and kidney repair [Citation7–9]. Notably, hAMSCs were particularly attractive due to their no-tumorigenicity, low immunogenicity and no ethical concerns [Citation10], thus might be a promising therapeutic option for CUA. According to our previous study, we speculated that hAMSCs played therapeutic roles through the inhibition of vascular calcification, stimulation of neovascularization and myogenesis, as well as enhanced anti-inflammatory activity, immunoregulatory actions, and re-epithelialization [Citation11].
Although microvascular calcification is regarded as the basic histopathological characteristic, it is noteworthy that hypercoagulable states seem to be crucial in the development of CUA [Citation6,Citation12]. Studies have shown that anticoagulants including tissue plasminogen activator or low molecular weight heparin have been reported effective in treating CUA [Citation6,Citation12–14]. We have reported an innovative therapeutic strategy for a CUA patient via intravenous combined with local treatment of hAMSCs, with speculated mechanisms including the inhibition of vascular calcification, the stimulation of neovascularization and myogenesis, as well as anti-inflammation, immunoregulatory actions, and re-epithelialization [Citation11]. This study aims to investigate the impact of hAMSCs on the improvement of hypercoagulation in CUA patient and analyze the possible mechanisms.
Case report
Case summary
We reported a 34-year-old female calciphylaxis patient who underwent peritoneal dialysis (PD) for 5 years. She was admitted to our hospital in July 2018 with multiple skin lesions accompanied by intolerable pain for more than 1 month. The skin of her back, thighs, lower limbs, and buttocks were presented with induration, plaques, purpura, livedo reticularis, and ecchymosis, gradually progressing into malodorous necrotic ulcerations surrounded by leather-like skin. Based on the clinical manifestation, medical history and skin histopathology [Citation11], the patient was diagnosed as CUA. The comorbidities included secondary hyperparathyroidism (SHPT), skin and soft tissue infections, PD-related tunnel infection, malnutrition and hypertension.
Salvage treatment strategy with hAMSCs for CUA patient
Due to the ineffectiveness to multiple conventional therapies, she was treated with hAMSCs, a rescue therapeutic strategy approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital (2018-QT-001). hAMSCs were provided by the State Key Laboratory of Reproductive Medicine in our hospital [Citation15]. A series of hAMSC preclinical tests including validity and safety assessments were performed before clinical administration [Citation11]. The CUA patient was administered hAMSCs intravenously at a dosage of 1.0 × 106 cells/kg, along with a local intramuscular injection at the edge of the wound (2.0 × 104 cells/cm2) and external application of cell culture supernatants on the wound surface [Citation11]. A detailed flow chart of hAMSC treatment was demonstrated in . Written informed consent was signed prior to hAMSC therapy.
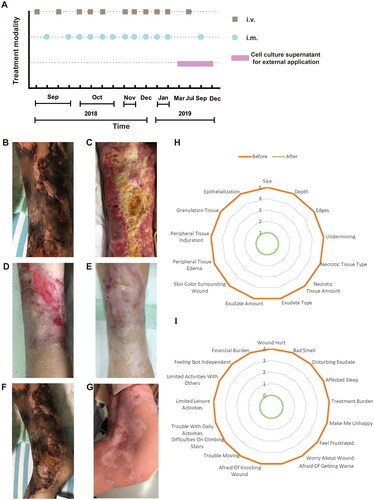
Figure 1. Flow chart of hAMSC treatment and skin regeneration, quality of life in the CUA patient. (A) Flow chart of hAMSC treatment. (B-E) Healing process of the skin lesions on the right thigh during the course of hAMSC treatment. (B) The anterior of right thigh presenting with large areas of irregular black scab, superficial ulceration and slight exudation before hAMSC treatment. (C) Patchy shallow ulcer covered with granulation tissues, pale yellow necrotic tissues and crusting in the center after hAMSC treatment for 2 months and debridement. (D) Striated scar with reddish dry ulcer in the center, pigment reduction in the peripheral, dilated blood vessels and a small amount of local crusting after hAMSC treatment for 9 months. (E) Fully regenerated skin accompanied with irregularly shaped scar, localized hypopigmentation and few scabs in the right thigh after hAMSC treatment for 20 months. (F) The left knee presenting with large black eschar and scattered deep ulcers before treatment. (G) Scar healing on the left knee with hypopigmentation after hAMSCs for 15 months. (H-I) Wound assessment based on BWAT(H) and quality of life evaluated by Wound-QoL(I) after hAMSC treatment for 15 months. hAMSC: human amnion-derived mesenchymal stem cell; CUA: calcific uremic arteriolopathy; BWAT: Bates-Jensen Wound Assessment Tool; Wound-QoL: Wound-Quality of Life.
Observed efficacy indicators
During the course of hAMSC treatment, the dynamic changes of blood parameters including coagulation indicators (platelet, D-dimer, fibrinogen), C reactive protein (CRP) and albumin, clinical symptoms, pain level, quality of life and pathological characteristics of skin biopsy were followed up. Pain Visual Analog Scale (VAS) was applied to rate pain level on a scale of zero to 10 [Citation16]. Bates-Jensen Wound Assessment Tool (BWAT), containing 13 evaluation items, was used to assess wound status during interventions. The score for each item ranges from 1 to 5, and a total score of 13 represents the healthiest, while a score of 65 indicates the unhealthiest status [Citation17]. Wound-Quality of Life (Wound-QoL) was also implemented to measure quality of life with 17 assessment items divided into three subscales on everyday life, body and psyche. Each item is scored from 0 to 4, representing low to high level of quality of life. Overall score ranges from 0, which represents the best imaginable health status, to 68, which represents the worst imaginable health status [Citation18]. Detailed scoring items were shown in . Hematoxylin and eosin (H&E) staining of skin biopsy obtained at different time points (before, 1 month after, and 20 months after hAMSC treatment) were investigated by light microscopy and the skin tissue was taken by deep incisional wedge biopsy [Citation19].
Animal study
hAMSC DNA in multiple tissues at different times after the mice were injected by hAMSCs via the tail vein
C57BL/6 mice (9–10 weeks old, 8 mice/group including equal number of male and female mice) were acclimatized in a specific pathogen free (SPF) animal house for 7 days before the experiments (Ethical number: IACUC 6131808004). hAMSCs (cell line: AMSC10; karyotype: 46, XY) were injected via the tail vein into mice weighing 18.3–21.7g (mean weight 19.6 g) at a dose of 8.50 × 106 cells/kg in females and 22.5–25.7g (mean weight 23.9 g) at a dose of 8.33 × 106 cells/kg in males, which is approximately one times the clinically equivalent dose (1 × 106 cells/kg) based on the body surface area of a patient weighing 60 kg.
We took lung, kidney and muscle tissues from the mice at 24 h, 1 week and 1 month after injection of hAMSCs, and extracted DNA for polymerase chain reaction (PCR) analysis of hAMSC colonization. Receptor-associated protein of the synapse (Rapsn, primer sequences: Rapsn-F: accccacccatcctgcaaat; Rapsn-R: acctgtccgtgctgcagaa) was used to label the receptor mouse tissue DNA. The human Y chromosome sex determining gene (SRY: sex determining region Y; primer sequences: SRY-609F: cccgaattcgacaatgcaatcatatgcttctgc; SRY-609R. ctgtagcggtcccgttgctgcggtg) was used to mark the hAMSC DNA. Amplification of Rapsn was performed as a normal PCR with one round of amplification followed by gel electrophoresis and amplification of SRY was performed as a nested PCR with a second round of amplification followed by gel electrophoresis.
Results
We performed skin biopsy on this CUA patient before hAMSC treatment, which is consistent with the characteristic pathological manifestations of calciphylaxis. Multidisciplinary therapies for this CUA patient involving analgesics, antibiotic administration, wound care, nutrition support, continuous kidney replacement therapy (CKRT), sodium thiosulfate [intravenous infusion of 7.68 g of sodium thiosulfate (each vial contained 0.64 g sodium thiosulfate and 12 vials were used each time), 30 min before the completion of hemodialysis] and anticoagulation [Citation1,Citation2] were applied, but without improvement in skin lesions and pain relief. As there were no alternative therapeutic options available, we rescue this CUA patient with hAMSCs as a candidate [Citation11]. The dosage of sodium thiosulfate remained unchanged during the initial 4 months of hAMSC treatment, but it was withdrawn afterwards due to its high cost and improvements in her skin lesions. During the entire follow-up process, the dosage of anticoagulants continued at the original dose.
The skin manifestations, wound status and quality of life were shown in . We followed up the healing process of skin lesions on the right thigh before treatment () and after 2 months (), 9 months (), 20 months () of hAMSC application. It was noteworthy that the right thigh obtained full skin regeneration when administered hAMSCs for 20 months. Similarly, the left knee presented with large areas of black eschar and scattered deep ulcers before hAMSC treatment (), which were healed after 15 months hAMSC application ().
In addition, the patient’s skin lesions on the lower limbs and buttock also shown significant improvement after treatment. Before hAMSC administration, the patient had widespread irregular ulcers on the buttocks and lower extremities, with scabs and purulent odor secretions on some wounds. Additionally, brown pigmentation was visible at the edge of the ulcers. After 4 months of treatment, the buttock ulcer had notably reduced in size and become shallow, with some regenerative tissues visible. Pain had considerably reduced, with the infection under control, and no odor present. After 12 months, most of the buttocks’ wounds had healed, except for three small ulcers and a few erosive surfaces. Pain was almost non-existent. After 15 months, the skin scar on the buttock had healed and became painless [Citation11,Citation20]. Hence, the patient got great relief of wound pain and the scores of pain VAS decreased from 10 to 0 after 15 months hAMSC treatment. Likewise, the score of BWAT decreased from 65 to 13 points, accompanying with substantial improvement in quality of life after hAMSC application for 15 months (). Either inflammation status or immune disorder was ameliorated after hAMSC administration for one year, with tumor markers in normal ranges [Citation11].
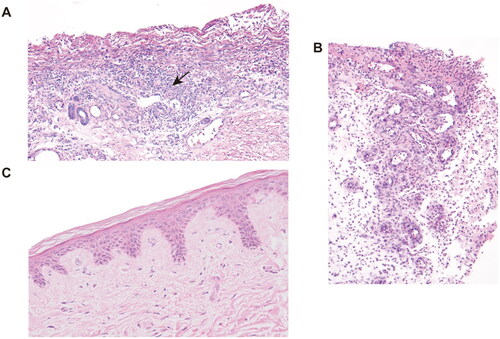
As hypercoagulability has been identified as a potential risk factor for CUA, we monitored coagulation-related indicators including platelet, D-dimer and fibrinogen (), which were all above normal levels before hAMSC treatment, indicating a potentially hypercoagulable and inflammatory condition. After hAMSC treatment for 3 months, platelet and fibrinogen levels tended to normalize. After 6 months of hAMSC therapy, D-dimer showed a fluctuating downward trend to physiological level. The inflammation related marker, CRP, decreased substantially after hAMSC therapy for 3 months. The pathological characteristics of skin biopsy before hAMSC treatment contained exfoliation of epidermis with extensive inflammatory cells infiltration (); whereas, the administration of hAMSCs for 1 month displayed regenerative tissues and reduced inflammation (). After 20 months of treatment, the skin biopsy showed intact epidermis, well-aligned collagen, without visible inflammatory reaction ().
Figure 2. The levels of blood coagulation-related markers of CUA patient before and during the course of hAMSC treatment. (A) Platelets, (B) Fibrinogen, (C) D-dimer and (D) CRP were followed up. The rectangular shaded area represented the normal reference range for each index and the recommended ranges were based on the normal reference values of laboratory tests. CUA: calcific uremic arteriolopathy; hAMSC: human amnion-derived mesenchymal stem cell; CRP: C reactive protein, m: month, d: day, w: week, y: year.
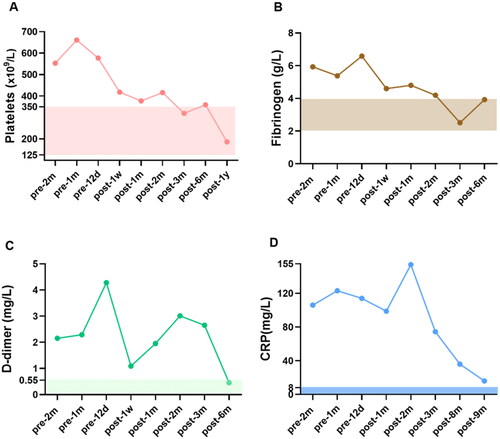
Figure 3. H&E staining of skin biopsy from the calciphylaxis patient before and during hAMSC treatment. (A) Specimen of biopsy obtained from the margin of an ulcer on the thigh, presented exfoliation of epidermis, necrosis, and extensive inflammatory cells infiltration around microvessels (black arrow) before hAMSC treatment (200×). (B) After hAMSC treatment for 1month, skin biopsy showed regenerative tissue with reduced inflammation and absence of epidermis (200×). (C) Intact epidermis, well-aligned collagen without visible inflammatory reaction after hAMSC treatment for 20 months (400×). H&E: Hematoxylin and eosin; hAMSC: human amnion-derived mesenchymal stem cell.
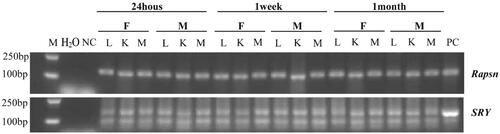
In order to investigate whether hAMSCs play roles in local tissues, we performed PCR analysis for the distribution of hAMSCs after infusion in mice. The results showed that hAMSC DNA were detected in the lung, kidney and muscle tissues of the mice after accepting hAMSC injection for 24 h, 1 week and 1 month (), indicating hAMSCs were homing in multiple tissues after intravenous infusion.
Figure 4. Tissue distribution of hAMSC DNA in C57BL/6 mice after 24 h, 1 week and 1 month of hAMSC infusion. M: DNA gradient labeling; H2O: sterile water; NC: negative control (AMSC10 cells cultured in vitro in the Rapsn primer amplification system and mice muscle tissues without injection of AMSC10 cells in the SRY primer amplification system); PC: positive control (mice muscle tissues without injection of AMSC10 cells in the Rapsn primer amplification system and AMSC10 cells cultured in vitro in the SRY primer amplification system). F: female mice; M: male mice; L: lung; K: kidney; M: muscle, Rapsn: receptor-associated protein of the synapse; SRY: sex determining region Y; hAMSC: human amnion-derived mesenchymal stem cell.
Discussion
Calciphylaxis is a rare and severely morbid disorder with high mortality that predominantly affects ESKD patients. Various comorbidities, such as obesity, female, disordered calcium-phosphate metabolism, hypoalbuminemia, warfarin, thrombophilia associated with protein C and protein S deficiencies, are considered as risk factors [Citation21]. Although clinical and histological descriptions of CUA have been characterized, its pathogenic mechanisms remain poorly understood and the important role of hypercoagulability has been noticed [Citation12,Citation22].
Case reports of calciphylaxis linked to hereditary and acquired thrombophilic conditions such as protein S deficiency, protein C deficiency, lupus anticoagulant and antithrombin deficiency, have been reported in the literature [Citation22,Citation23]. It has been reported that 60% calciphylaxis patients have severe thrombophilia [Citation23]. Harris revealed that 38% and 43% of calciphylaxis patients had decreased protein C and protein S levels, respectively [Citation22]. Additionally, elevated fibrinogen (47%) and D-dimer (41%) levels in the plasma were observed in calciphylaxis cohort [Citation23]. Calciphylaxis patients treated with tissue plasminogen activator had approximately 30% greater survival than controls, but significant difference was not observed [Citation13]. Therapeutic success in a case of calciphylaxis is thought to be associated with low molecular weight heparin for hypercoagulable condition [Citation14]. Hence, as a potential risk factor, hypercoagulability plays an important role in the pathogenic mechanism and is proposed to be the treatment target for calciphylaxis.
D-dimer is a plasmin-derived soluble degradation product of cross-linked fibrin, served as a valuable marker of coagulation and fibrinolysis activation. Of note, D-dimer is a sensitive indicator for detection of thrombosis, but its specificity is not ideal [Citation24]. Fibrinogen, the soluble precursor of fibrin, is a major determinate of blood viscosity and red blood cells aggregation. Elevated fibrinogen levels may provide a hypercoagulable state through increasing the aggregation and reactivity of platelet [Citation25]. Prior to hAMSC treatment, the patient in this case had elevated levels of plasma D-dimer and fibrinogen, indicative of an underlying hypercoagulable condition. The patient also displayed significantly elevated levels of CRP and leukocytes before hAMSC administration, indicating a high inflammatory status. Increasing evidence indicates that systemic inflammation can induce the activation of coagulation system, which may result from tissue factor (TF)-mediated thrombin generation or the dysfunction of anticoagulant pathway, such as the impaired protein C system [Citation26]. Compared with physiological serum level of albumin, platelet aggregation was significantly higher in the presence of low serum albumin level [Citation27]. This case had hypoalbuminaemia at the onset of hAMSC administration. Thus, either acquired thrombophilic conditions like protein C and protein S deficiency or hypercoagulability induced by inflammation and hypoalbuminaemia may be correlated with the development of CUA.
After hAMSC treatment, plasma D-dimer and fibrinogen levels decreased immediately after one week administration. However, the level of D-dimer presented a re-elevation after hAMSC treatment for 1 month and gradually showed a tendency to normal range accompanied by controlled inflammatory state, significant improvement in skin lesions and skin pathological biopsy. Increasing evidence indicates that inflammation can lead to the activation of coagulation and thrombosis [Citation26,Citation28]. During the course of hAMSC treatment for 2 months, the patient had a transient infection aggravation and the level of CRP rose from 98.8 mg/L (after hAMSC treatment for 1 month) to 154 mg/L (after hAMSC treatment for 2 months). Thus, we speculated that the exacerbated inflammatory state was correlated with elevated D-dimer. Conclusively, based on the significant improvement of coagulation and inflammation-related indicators accompanied with skin regeneration after hAMSC administration, we presumed that hAMSCs may exert therapeutic roles in CUA by correcting hypercoagulable conditions. Netsch et al. [Citation29] demonstrated that MSCs originating from bone marrow, adipose and cord blood inhibited the agonist-induced activation and aggregation of platelets in platelet-rich plasma (PRP) and whole blood, expressing anticoagulant features. The underlying mechanism could be attributed to the CD73-mediated adenosine generation, and MSCs exerted hemostatic functions through adenosinergic pathway [Citation29]. Additionally, placenta MSCs were reported to act a protective role in agonist-induced platelet activation, inhibiting thrombosis and atherosclerosis, partially through decreasing CD36-mediated platelet activation [Citation30]. In this case, we presume that hAMSCs may alleviate hypercoagulability of CUA either directly or indirectly through improving the inflammatory state and hypoalbuminemia. Of note, the thrombogenic risk induced by MSCs associated with expression of procoagulant molecules such as TF has drawn attention in recent studies [Citation31] and the activation of coagulation after MSCs infusion were reported to be in a cell-dose-dependent manner [Citation32,Citation33]. In our preclinical acute toxicity animal tests, high-dosage hAMSCs (over 6 times and 20 times of the clinical equivalent dose in mice and rats, respectively) can cause pulmonary embolism and death in mice. During hAMSC treatment for this CUA patient, the intravenous dosage(1.0 × 106 cells/kg) was lower than the maximum tolerated dosage in C57BL/6 mice (equivalent to 6.0 × 106 cells/kg in humans) and in Sprague–Dawley rats (equivalent to 20.0 × 106 cells/kg in humans) [Citation11]. Hence, the intravenous dose we currently administered is considered safe and will not directly result in the complication of embolism.
Furthermore, numerous studies demonstrated that hAMSCs had great advantages over other stem cells, one of which was no-tumorigenicity [Citation10,Citation34]. Although hAMSCs were capable of self-renewing and differentiating into multi-linage, no aberrant carcinogenetic signals were identified in the carcinogenicity analysis test [Citation35] and thus hAMSCs could be a biosafe source of stem cells for clinical therapy [Citation11].
The process of wound repairment may involve the colonization, incorporation, proliferation and differentiation of MSCs. In order to trace the transplanted human umbilical cord mesenchymal stem cells (hUC-MSCs) in rat model of diabetic foot ulcers, Shi et al. performed fluorescent immunostaining to detect the ZsGreen labeled hUC-MSCs in frozen sections of ulcerated tissues after injecting hUC-MSCs for 24h, 3 days, 8 days and 16 days, which demonstrated that the transplanted hUC-MSCs reached and survived in the ulcer tissues at all the time points [Citation36]. Similarly, in our study, we detected the colonization of hAMSCs in lung, kidney and muscle tissues of C57BL/6 mice after intravenous infusion for 1 month.
Limitations
Here we report that hAMSC treatment may play crucial role in improving hypercoagulability in CUA patient via a clinical case study, thus the causal relationships between hypercoagulability and calciphylaxis can not be concluded. A complete thrombophilia tests involving protein C, protein S, antithrombin III or other related hypercoagulability indicators were not performed in this case. In addition, before hAMSC treatment, the patient had progressive and severe skin lesions refractory to conventional multidisciplinary therapies, it was uncertain whether the rescue hAMSC therapy would be effective for calciphylaxis. Hence, we took little skin tissue with biopsy before hAMSC treatment, and the histopathological features presented with necrotic components and inflammatory response around microvessels. Due to the limited amount of biopsy tissue, we can not find more figures to display typical pathological manifestations of calciphylaxis including intimal hyperplasia and microvessels lesions.
Conclusion
Our findings implied that skin lesions and inflammatory status were improved via salvage hAMSC therapy based on the conventional multidisciplinary therapies, which may partly due to the improvement of hypercoagulability in the calciphylaxis patient. There is strong support that identification of coagulation profile and a thorough search for both congenital and acquired thrombophilias should be assessed for the new onset calciphylaxis patients. In order to improve our understanding of this complex disease, future research should focus on early causative factors for hypercoagulability in CUA and the identification of driving forces that cause the progression of vasculopathy as dichotomic event. Prospective multi-institutional collaborative efforts to clarify the safety and efficacy of novel anticoagulants and hAMSC therapy in calciphylaxis patients are suggested when there was risk of thrombosis predisposing to ulceration.
Ethical approval and consent to participate
The study was performed after an approval by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital (2018-QT-001). Healthy pregnant women in their 20s or 30s who provided written informed consent donated human amniotic membranes, which was approved by the Ethics Committee of Jiangsu Province Hospital (2012-SR-128).
Acknowledgements
The authors thank the patient, her family and all medical staff who participated in the treatment and data collection. The study was supported by the International Society of Nephrology (ISN) Mentorship Program and the authors thank Professor Marcello Tonelli (University of Calgary, Canada) for his helpful comments on the draft of the manuscript. The patient and her family gave the consent for publication of the data obtained in the present study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Garcia-Lozano JA, Ocampo-Candiani J, Martinez-Cabriales SA, et al. An update on calciphylaxis. Am J Clin Dermatol. 2018;19(4):1–8.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378(18):1704–1714.
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37(12):4797–4807.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61(6):2210–2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421–3429.
- Marin BG, Aghagoli G, Hu SL, et al. Calciphylaxis and kidney disease: a review. Am J Kidney Dis. 2022;81(2):232–239.
- Jo H, Brito S, Kwak BM, et al. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci. 2021;22:2410.
- Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8(1):273.
- Liu Y, Guo Y, Bao S, et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5. Cell Death Dis. 2022;13(3):278.
- Liu QW, Huang QM, Wu HY, et al. Characteristics and therapeutic potential of human Amnion-Derived stem cells. Int J Mol Sci. 2021;22:970.
- Qin L, Zhang J, Xiao Y, et al. A novel long-term intravenous combined with local treatment with human amnion-derived mesenchymal stem cells for a multidisciplinary rescued uremic calciphylaxis patient and the underlying mechanism. J Mol Cell Biol. 2022;14:mjac010.
- Dobry AS, Ko LN, St John J, et al. Association between hypercoagulable conditions and calciphylaxis in patients with renal disease: a Case-Control study. JAMA Dermatol. 2018;154(2):182–187.
- el-Azhary RA, Arthur AK, Davis MDP, et al. Retrospective analysis of tissue plasminogen activator as an adjuvant treatment for calciphylaxis. JAMA Dermatol. 2013;149(1):63–67.
- Carter A, Ortega-Loayza AG, Barrett J, et al. Calciphylaxis with evidence of hypercoagulability successfully treated with unfractionated heparin: a multidisciplinary approach. Clin Exp Dermatol. 2016;41(3):275–278.
- Liu H, Jiang C, La B, et al. Human amnion-derived mesenchymal stem cells improved the reproductive function of age-related diminished ovarian reserve in mice through ampk/FoxO3a signaling pathway. Stem Cell Res Ther. 2021;12(1):317.
- Reed MD, Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol. 2014;54(3):241–244.
- Harris C, Bates-Jensen B, Parslow N, et al. Bates-Jensen wound assessment tool: pictorial guide validation project. J Wound Ostomy Continence Nurs. 2010;37(3):253–259.
- Blome C, Baade K, Debus ES, et al. The "Wound-QoL": a short questionnaire measuring quality of life in patients with chronic wounds based on three established disease-specific instruments. Wound Repair Regen. 2014;22(4):504–514.
- Neitzel CD. Biopsy techniques for skin disease and skin cancer. Oral Maxillofac Surg Clin North Am. 2005;17(2):143–146.
- Wang NN, Qin LJ, Liu K, et al. Multidisciplinary regenerative treatment and mechanisms for rescuing a severe calciphylaxis patient with human amnion-derived mesenchymal stem cells. Natl Med J China. 2022;102:2217–2221.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66(1):133–146.
- Harris RJ, Cropley TG. Possible role of hypercoagulability in calciphylaxis: review of the literature. J Am Acad Dermatol. 2011;64(2):405–412.
- El-Azhary RA, Patzelt MT, McBane RD, et al. Calciphylaxis: a disease of pannicular thrombosis. Mayo Clin Proc. 2016;91(10):1395–1402.
- Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: d -Dimer. J Am Coll Cardiol. 2017;70(19):2411–2420.
- de Moerloose P, Boehlen F, Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Semin Thromb Hemost. 2010;36(1):7–17.
- Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–S34.
- Paar M, Rossmann C, Nusshold C, et al. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS One. 2017;12(8):e0182997.
- Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–918.
- Netsch P, Elvers-Hornung S, Uhlig S, et al. Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73-converted adenosine. Stem Cell Res Ther. 2018;9(1):184.
- Al Subayyil A, Basmaeil YS, Alenzi R, et al. Human placental mesenchymal stem/stromal cells (pMSCs) inhibit agonist-induced platelet functions reducing atherosclerosis and thrombosis phenotypes. J Cell Mol Med. 2021;25(19):9268–9280.
- Coppin L, Sokal E, Stephenne X. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: current status and future perspectives. Cells. 2019;8(10):1160.
- Liao L, Shi B, Chang H, et al. Heparin improves BMSC cell therapy: anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7(1):106–116.
- Coppin L, Najimi M, Bodart J, et al. Clinical protocol to prevent thrombogenic effect of Liver-Derived mesenchymal cells for Cell-Based therapies. Cells. 2019;8(8):846.
- Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, et al. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front Immunol. 2019;10:238.
- Phermthai T, Thongbopit S, Pokathikorn P, et al. Carcinogenicity, efficiency and biosafety analysis in xeno-free human amniotic stem cells for regenerative medical therapies. Cytotherapy. 2017;19(8):990–1001.
- Shi R, Lian W, Jin Y, et al. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim Biophys Sin (Shanghai). 2020;52(6):620–630.
