Abstract
Tumor-secreted exosomes are critical for the functional regulation of tumor-associated macrophages (TAMs). This study aimed to explore how exosomes secreted by ovarian carcinoma cells regulate the phenotype and function of macrophages. Hypoxic treatment of A2780 cells was postulated to mimic the tumor microenvironment, and exosomes were co-cultured with TAMs. miR-1225-5p was enriched in hypoxic exosomes and contributed to M2 macrophage polarizationby modulating Toll-like receptor 2 expression (TLR2). Furthermore, hypoxia-treated macrophages promote ovarian cancer cell viability, migration, and invasion via the wnt/β-catenin pathway. This study clarified that exosomal miR-1225-5p promotes macrophage M2-like polarization by targeting TLR2 to promote ovarian cancer, which may via the wnt/β-catenin pathway.
1. Introduction
Ovarian cancer is the third most common gynecologic malignancy globally; however, the mortality is the highest among all gynecologic tumors [Citation1]. Practically, high-grade serous ovarian cancer (HGSOC) accounts for about 80% of death among ovarian cancer [Citation2]. HGSOC is usually diagnosed at an advanced stage, with a median overall survival of just four years [Citation3]. However, effective treatments for HGSOC are still lacking clinically. Hypoxia is a crucial driver of ovarian cancer tumorigenesis and progression. Importantly, hypoxia affects tumor microenvironment to induce angiogenesis, immune escape, and damage-associated pattern molecule release [Citation4,Citation5]. Therefore, it is of great significance to elucidate the regulatory mechanism of ovarian cancer in hypoxic environment for exploring new therapeutic strategies.
The solid tumor cells are generally encompassed by an extracellular matrix, new blood vessels that support tumour growth, as well as multiple cell types [Citation6]. Extracellular vesicles are recognized as key players in this process because of their ability to transmit a variety of complex messages to recipient cells [Citation7]. Exosomes, the most widely studied type of extracellular vesicles, are considered as crucial regulators of tumor immune escape [Citation8,Citation9].
Recent evidence suggests that exosomal miRNAs can modulate molecular signalings in recipient cells, thereby affecting tumor incidence and development, such as vascularization[Citation9], immune evasion [Citation10], epithelial-mesenchymal transformation [Citation11], metastasis [Citation12], and resistance [Citation13]. Exosomal miRNAs have been reported to regulate the progress of ovarian cancer [Citation14–16]. The miR-1225 gene is located on chromosome 16 and functions as an oncogene or anti-oncogene in diverse cancers. Laryngeal carcinoma tissues express low levels of miR-1225-5p, which upregulation suppresses cellular proliferation and promotes apoptosis [Citation17]. In contrast, miR-1225-5p expression is increased in pancreatic cancer, whose decrease can impede pancreatic cancer cell apoptosis and promote cell proliferation and survival [Citation18]. Furthermore, the secretion of exosomal miR-1225-5p in the peritoneal fluid has the potential to predict the peritoneal dissemination of gastric cancer [Citation19]. However, whether exosomal miR-1225-5p affects the progression of ovarian cancer has not yet been reported.
Previous studies have suggested that tumor-associated macrophages (TAMs) are mainly M2-phenotype macrophages (M2) involved in angiogenesis and promoting tumor progression [Citation20]. Studies have shown that tumor-derived exosomes can deliver non-coding RNA to stromal cells in the tumor microenvironment, altering their phenotypes and functions. Exosomal miRNA regulation of the tumor microenvironment has being increasingly recognized [Citation21,Citation22]. TAM is an essential component of ovarian cancer [Citation14,Citation23]. Therefore, understanding the mechanism of tumor exosomal miRNAs in macrophage polarization may provide a new direction for clinical tumor treatment. Herein, we sought to investigate the effects of ovarian cancer cell released exosomal miR-1225-5p on macrophage polarization. The underlying mechanisms were also clarified.
2. Experimental details
2.1. Cell culture and treatment
The A2780 and OV90 cell lines, THP-1 monocytes, and HEK293T cells were purchased from the Cell Culture Center of the Chinese Academy of Sciences. The cell experiments were carried out strictly in the sterile environment of an ultra-clean station, and cell culture conditions were as follows: 37 °C, 5% CO2, saturated humidity incubator. A2780 and OV90 cells were incubated in RPMI-1640 (HyClone, SH30809.01B) containing 10% fetal bovine serum (FBS; Gibco, 10099141). THP-1 cells were maintained in high-glucose Dulbecco’s Modified Eagle Medium (DMEM; HyClone, SH30243.01B) with 10% FBS.
A2780 cells were maintained in 1% O2 conditions to simulate hypoxic cells, while a normal oxygen content of 20% O2 was used for normoxic A2780 cells [Citation24].
After 1 × 106 THP-1 cells were planted in culture flasks, phorbol 12-myristate 13-acetate (100 ng/mL; Sigma, P8139) was added, and THP-1 cells fully differentiated into adherent macrophages after treatment for 48 h [Citation25].
HEK293T cells used for the dual-luciferase reporter assay were cultured as described previously [Citation26].
2.2. Bioinformatics analysis
We used a miRNA array (GSE52743) to study the differential expression of miRNAs under hypoxic conditions in A2780 cells [Citation27]. Cells cultured under normoxia were used as controls. The screening conditions of miRNA were as follows: p < 0.05, |logFC| >1.0. TargetScan was used to predict the binding domain between the miRNA and target gene.
2.3. Exosome isolation
Exosomes were separated and extracted as previously described [Citation28,Citation29]. Briefly, after 24 h of A2780 cell culture, the medium was collected, and then centrifuged to obtain the supernatants. Subsequently, Exosome Isolation Reagent (Life Technologies, 4478359) was added for one night, and centrifugation was performed at 4 °C to collect exosomes. Finally, after discarding the supernatant, PBS was added to prepare the exosome suspension.
2.4. Exosome identification
Transmission electron microscopy (TEM; JEOL) analyze exosome morphological features. Nanoparticle tracking analysis (NTA) was carried out using Zetaview (Particle Metrix) to determine the granulometric distribution of the exosomes [Citation30]. Western blotting was used to detect surface markers CD63 (ab216130, Abcam) and CD81 (ab109201, Abcam).
2.5. Cell transfection
The plasmid pLenti-III-miR-Off (1225-5p-KO) and its control (scramble) were acquired from Applied Biological Materials (Canada). HEK293T cells were co-transfected with miR-1225-5p-KO/scramble plasmids together with lentivirus packaging vectors by Lipofectamine 3000 (Invitrogen, USA). Post-transfection of 72 h, the culture medium was collected, filtered, and used to transduce A2780 cells. Stably expressed cells were filtrated using puromycin (0.3 μg/mL) for a week. The knockout efficiency was determined using quantitative reverse-transcription PCR (RT-qPCR).
The FAM-labeled miR-1225-5p mimic, nc mimic, and GFP-labeled TLR2 overexpressed vectors were acquired from GenePharma (Shanghai, China). A2780 cells were placed at the 2 × 105 cells/well density in 6-well plates. Corresponding plasmids were transiently transfected into A2780 cells by Lipofectamine 3000, following the manufacturer’s protocol.
2.6. Conditioned medium (CM) preparation
Macrophages treated with hypoxia A2780-derived exosomes (hypo-exo) and normoxic A2780-derived exosomes (nor-exo) were cultured in complete DMEM. The supernatants were discarded and the culture continued for another 24 h in serum-free medium, after which new supernatants of these cells were obtained as CM.
2.7. Tracing of miR-1225-5p and TLR2
After transfection of FAM-labeled miR-1225-5p mimic and GFP-labeled TLR2 overexpressed vector into hypoxia A2780 cells, exosomes were isolated. Macrophages were treated with these exosomes. The macrophages were visualised under a light microscope. The fluorescence was observed using a confocal laser-scanning microscope.
2.8. Luciferase reporter assay
HEK293T cells were co-transfected mimic or NC and the wild-type or mutant reporter vectors using the cell transient transfection technique, and the experiment was performed in line with the protocol of the Dual Luciferase Reporter Gene Detection Kit (Promega).
2.9. Western blotting
After cell lysis, the protein concentration was examined through the BCA method (Beyotime, P0012). Each protein sample was 40 μg. The lysis products were run on polyacrylamide gel electrophoresis (10%) and transferred to poly(vinylidene fluoride) membrane (Millipore) with gel imprinting. The membranes were sealed in 5% defatted milk and crossbred with primary antibodies including anti-CD63 (ab134045), anti-CD81 (ab278545), anti-inducible type of nitric oxide synthase (anti-iNOS, ab178945), anti-IL-1β (ab216995), anti-IL-6 (ab233706), anti-Arginase-1 (Arg-1, ab133543), anti-IL-10 (ab133575), anti-β-catenin (ab32572), anti-GSK-3β (ab93926), anti-p-GSK-3β (ab75814), anti-PI3K (ab32089), anti-p-PI3K (ab278545), anti-AKT (ab235958), anti-p-AKT (ab38449), anti-c-Myc (ab32072), anti-Notch1 (ab52627), anti-p65 (ab32536), anti-p-p65 (ab76302), anti-STAT3 (ab68153), and anti-p-STAT3 (ab267373) and incubated overnight at 4 °C. The strips were incubated with the secondary antibody for 2 h at room temperature, and Electrochemical luminescence was developed. Densitometric analyses was performed to quantify the intensity of each band using the QuantityOne software (BioRad).
2.10. qRT-PCR
qRT-PCR was conducted to measure mRNA expression. Total RNA from each exosome (or culture medium) was extracted using TRIzol reagent (Beyotime, R0016), and synthesis of cDNA was determined using a reverse transcription kit (Toyobo). The cDNA was used as a template for qRT-PCR amplification (Bio-Rad, CFX-96). Relative mRNA expression was quantified using the 2−ΔΔCt method. Primer sequences are listed below (5′→3′): iNOS, F: TTCAGTATCACAACCTCAGCAAG and R: TGGACCTGCAAGTTAAAATCCC; IL-1β, F: ATGATGGCTTATTACAGTGGCAA and R: GTCGGAGATTCGTAGCTGGA; IL-6, F: ACTCACCTCTTCAGAACGAATTG and R: CCATCTTTGGAAGGTTCAGGTTG; CD206, F: TCCGGGTGCTGTTCTCCTA and R: CCAGTCTGTTTTTGATGGCACT; Arg-1, F: GTGGAAACTTGCATGGACAAC and R: GTGGAAACTTGCATGGACAAC; IL-10, F: GACTTTAAGGGTTACCTGGGTTG and R: TCACATGCGCCTTGATGTCTG; miR-1225-5p, F: GCCGAGGTGGGTACGGCCCA and R: CTCAACTGGTGTCGTGGA; TLR2, F: AAGGAGGTGCGGACTGTTTC and R: GAGCCAAAGAGCTCGTAGC; GAPDH, F: TGTGGGCATCAATGGATTTGG and R: ACACCATGTATTCCGGGTCAAT; U6, F: CTCAACTGGTGTCGTGGA and R: AACGCTTCACGAATTTGCGT.
2.11. Cellular function evaluation
Ovarian cancer cell viability was assessed by a CCK-8 kit (Beyotime). In short, 2 × 103 A2780/OV90 cells seeded in 96-well plates were maintained in 50 μL macrophage culture medium for 48 h. After 5 days of routine cultivation, the CCK8 liquor was added, and the absorbancy was recorded at 450 nm to assess cell viability.
To assess cell aggressiveness, A2780/OV90 cells (5 × 104) were inoculated in the upper layer of a 24-well Transwell chamber (Corning). The macrophage cell culture medium was then added to the substratum. After 12 h of incubation, migrated cells were immobilized in methanol and stained with crystal violet (1%; C0121, Beyotime). Cell ability to invade was observed after the addition of Matrigel (BD Biosciences) based on migration experiments.
2.12. Statistical analysis
The data were analyzed by the GraphPad Prism software version 8.3 (GraphPad, USA), and the data operation between the two groups was represented by mean ± SD and compared with t test. A one-way ANOVA compared the means of multiple groups. Tukey’s post-hoc test was used after ANOVA. p < 0.05 means a statistically significant difference.
3. Results
3.1. A2780 cell-released exosomes under hypoxia promote macrophages into M2-like macrophages
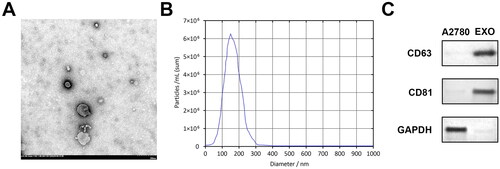
Firstly, A2780 cell derived exosomes were identified; shows exosomes with membrane structures observed under TEM. The diameter was less than 200 nm, and the shape was similar to that of teacup saucer-like nanoparticles. NTA analysis showed that exosomes were distributed at 33 and 199 nm but were mainly distributed at 148 nm (). CD63 and CD81 were highly expressed in exosomes, but low or no expression was observed in cells (). The data confirmed that exosomes derived from A2780 can be phagocytosed by macrophages.
Figure 1. Identification of A2780 cell-released exosomes. (A) Morphological characteristics of exosomes under TEM. (B) NTA of isolated exosomes shows the distribution of particle size. (C) Western blotting of CD63 and CD81 levels on cell lysates and extracted exosomes. TEM: transmission electron microscope; NTA: nanoparticle tracking analysis.
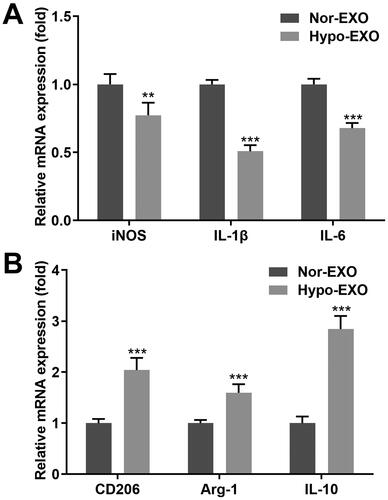
Next, we tested the expression of macrophage surface markers after co-incubation with exosomes from the different groups. The iNOS, interleukin (IL)-1β, and IL-6 expression, which represent the markers of the M1-type polarization of macrophages, was prominently lower in macrophages co-incubated with hypo-exo, compared with those in macrophages co-incubated with nor-exo (). Meanwhile, hypo-exo prominently elevated CD206, Arg-1, and IL-10 expression, representing the M2-type macrophages polarization, compared with the nor-exo group (). In conclusion, exosomes secreted from ovarian cancer cells under hypoxic conditions may induce the differentiation of macrophages into M2 macrophages.
Figure 2. Hypoxic A2780 cell derived exosomes promote macrophage M2 polarisation. (A, B) RT-qPCR measured the iNOS, IL-1β, IL-6, CD206, Arg-1, and IL-10 expression ratio of macrophages co-incubated with hypo-exos and nor-exos. Hypo-exo: hypoxia A2780-derived exosome; nor-exo: normoxic A2780-derived exosome. **p < 0.01; ***p < 0.001.
3.2. Exosomal miR-1225-5p contributed to the differentiation of macrophages
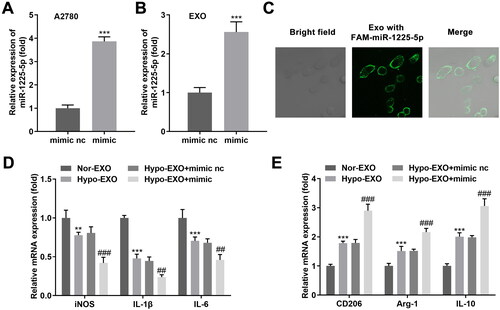
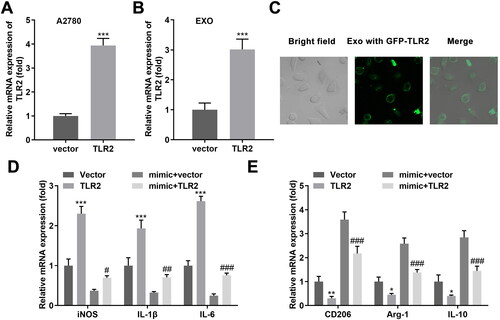
Exosomes can contain a variety of active components, including miRNAs, through which they can transmit specific information to target cells. Therefore, we hypothesized that differences in miRNA expression in the exosomes of ovarian cancer origin lead to the polarization of M2-type macrophages. Our bioinformatics results demonstrated that five upregulated and fifteen downregulated miRNAs were identified by hypoxia, compared to normoxia (). Next, the two miRNAs (miR-1225-5p and miR-1207-5p), with the most significant upregulation, were selected for further analysis. Our data revealed that miR-1225-5p was markedly elevated in hypoxia-treated A2780 cells (). The miR-1225-5p was knocked out in A2780 cells (), and exosomes were derived from A2780 cells. Exosomes deficient in miR-1225-5p facilitated the differentiation of macrophages into M1 phenotype macrophages and inhibited the differentiation to M2 type ()). Elevation of miR-1225-5p was found in A2780 cells after transfected with mimic (), and the exosomes were isolated. As expected, miR-1225-5p levels were also elevated in exosomes (). Moreover, macrophages showed green fluorescence, suggesting macrophages internalized exosomes secreted by FAM-labeled miR-1225-5p transfected A2780 cells (). Overexpression of miR-1225-5p ultimately decreased iNOS, IL-1β, and IL-6 in macrophages delaying the M1-type polarization of macrophages. CD206, Arg-1, and IL-10, however, were dramatically elevated in macrophages (). Thus, exosomal miR-1225-5p may promote macrophage differentiation into the M2 phenotype.
Figure 3. Exosomal miR-1225-5p contributed to the differentiation of macrophages. (A) Volcano plot of aberrant expressed miRNAs in hypoxia and normoxia groups. (B) miR-1225-5p and miR-1207-5p expression in A2780 cells treated with hypoxia and normoxia. (C) miR-1225-5p levels in A2780 cells after its knockout. (D, E) RT-qPCR detected the iNOS, IL-1β, IL-6, CD206, Arg-1, and IL-10 expression of macrophages co-incubated with hypo-exos and nor-exos. *p < 0.05; **p < 0.01; ***p < 0.001 (vs. Nor, scramble, and nor-EXO). #p < 0.05; ##p < 0.01; ###p < 0.001 (vs. Hypo-EXO + scramble).
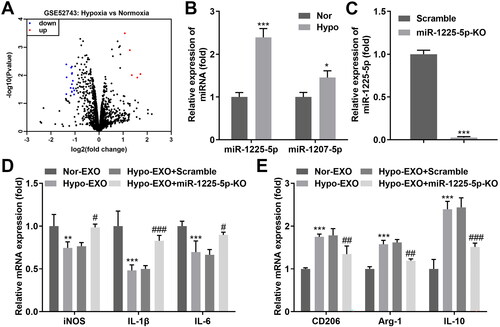
Figure 4. Exosomal miR-1225-5p facilitates polarization of macrophages. mRNA levels of exosomal miR-1225-5p isolated from (A) A2780 cells and (B) exosomoes derived from A2780 cells after transfection. (C) FAM-labeled miR-122-5p were transfected into A2780 cells, the released exosomes were incubated with macrophages. The trail of miR-1225-5p was observed under a confocal microscope. (D, E) RT-qPCR was used to detect the iNOS, IL-1β, IL-6, CD206, Arg-1, and IL-10 expression of macrophages co-incubated with hypo-exos and nor-exos. **p < 0.01; ***p < 0.001 (vs. mimic nc, and nor-EXO). ##p < 0.01; ###p < 0.001 (vs. Hypo-EXO + mimic nc).
3.3. Exosomal TLR2 reversed the effects of exosomal miR-1225-5p on macrophage differentiation
TLR2 was considered as a miR-1225-5p downstream target gene (), and was affirmed using the dual-luciferase reporter assay (). Additionally, elevated miR-1225-5p expression markedly decreased TLR2 expression in A2780 cells (). Next, we investigated whether exosomal miR-1225-5p facilitates macrophage polarization by regulating TLR2. TLR2 expression was increased after TLR2 overexpression in A2780 and exosomes (). Green fluorescence existed in macrophages after incubation with GFP-TLR2 exosomes (). Elevated TLR2 dramatically upregulated iNOS, IL-1β, IL-6, and downregulated CD206, Arg-1, and IL-10 expression. Exosomal miR-1225-5p reversed the levels of the six markers induced by TLR2 ()), suggesting TLR2 mediated by exosomal miR-1225-5p restraining the differentiation of macrophages into the M2 phenotype.
Figure 5. TLR2 was a miR-1225-5p downstream gene. (A) The binding domain between miR-1225-5p and TLR2 was predicted by bioinformatics. (B) Dual-luciferase reporter analysis confirmed the association between TLR2 and miR-1225-5p. (C) qRT-PCR as well as (D) western blot analyses for the expression of TLR2. ***p < 0.001 (vs. mimic nc).
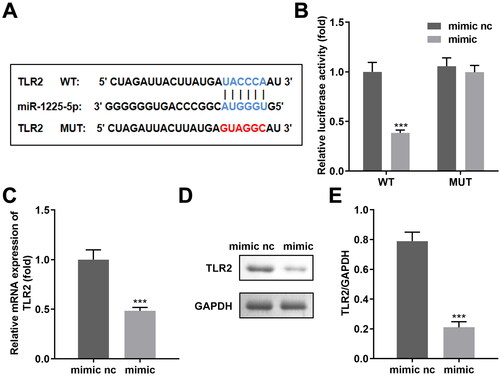
Figure 6. Exosomal miR-1225-5p promotes polarisation of macrophages into M2-like macrophages by modulating TLR2. mRNA levels of exosomal TLR2 isolated from (A) A2780 cells and (B) exosomes derived from A2780 cells after transfection. (C) Exosomes released from GFP-labeled TLR2 transfected A2780 cells were incubated with macrophages. The trail of TLR2 was observed under a confocal microscope. (D, E) RT-qPCR was used to detect the iNOS, IL-1β, IL-6, CD206, Arg-1 and IL-10 expression of macrophages co-incubated with hypo-exos overexpressing miR-122-5-5p and TLR2. **p < 0.01; ***p < 0.001 (vs. vector, mimic + vector).
3.4. Hypoxia activated the wnt/β-catenin pathway to promote the migration-promoting function of M2-like macrophages on ovarian cancer cells
To investigate the effects of hypoxia on the interactions of tumor cells and macrophages, the cellular behaviors were evaluated. CM from hypoxia-treated macrophages increased A2780 and OV90 cell viability ()), migration ()), as well as invasion ()). Several pathways have been studied in ovarian cancer. Results of western blot showed that hypo-exo-treated CM significantly activated the wnt/β-catenin pathway ()) in A2780 and OV90 cells, whereas the PI3K ()), Notch ()), p65 ()), and STAT3 ()) pathways were not significantly affected.
Figure 7. Hypoxia promoted the migration-promoting function of M2-like macrophages on ovarian cancer cells by the activation of the wnt/β-catenin pathway. (A, B) Cell viability, (C, D) migration, (E, F) and invasion of ovarian cancer cells cultured in CM hypo-exo treated macrophages. (G–S) Proteins of the wnt/β-catenin, PI3K, Notch, p65, and STAT3 pathways were examined using Western blotting. ***p < 0.001 [vs. CM(nor-exo)].
![Figure 7. Hypoxia promoted the migration-promoting function of M2-like macrophages on ovarian cancer cells by the activation of the wnt/β-catenin pathway. (A, B) Cell viability, (C, D) migration, (E, F) and invasion of ovarian cancer cells cultured in CM hypo-exo treated macrophages. (G–S) Proteins of the wnt/β-catenin, PI3K, Notch, p65, and STAT3 pathways were examined using Western blotting. ***p < 0.001 [vs. CM(nor-exo)].](/cms/asset/9ecf8328-9b8f-45e0-8171-59c7ba252ac6/iaut_a_2281226_f0007_c.jpg)
4. Discussion
TAMs are an important component of tumor microenvironment closely related to immunosuppression and poor prognosis of ovarian cancer. They can promote tumor progression by releasing exosomes [Citation31]. Exosomes mediate the interactions between cancer and immune cells, including macrophages, cancer-associated fibroblasts, and stem cells, and affect tumor progression [Citation32–35]. Therefore, the interactions between tumor-derived exosomes and macrophages need to be studied. In our study, macrophages were treated with exosomes which isolated from hypoxic-induced ovarian carcinoma cells. After co-incubation, the exosomes induced the differentiation of macrophages into M2 macrophages. We also identified that miR-1225-5p in hypoxic cancer cells was prominently upregulated. In addition, exosomal miR-1225-5p promoted macrophage polarization by regulating its downstream gene, TRL2. Furthermore, hypoxia promoted the migration-promoting function of M2-like macrophages on ovarian carcinoma cells via activating the wnt/β-catenin pathway.
As one of the main immune cells in the microenvironment, macrophages are mainly divided into the M1 and M2 types with antitumor and pro-tumor effects, respectively. Accumulating evidence suggests that a variety of cancer cells promote the M2 macrophage polarization by secreting exosomes to deliver miRNAs. For example, tumor-released exosomal miR-934 promote metastasis of colorectal cancer to liver by inducing macrophage M2 polarization [Citation36]. Exosomal miR-21-5p derived from esophageal cancer contributes to the progression of esophageal cancer through destruction of M1 polarizations [Citation37]. In addition, an anoxic microenvironment is a characteristic of solid tumors. Studies have shown that hypoxia promotes tumor growth. Hence, we speculated that hypoxia may promote tumor progression by modulating macrophage polarization, which may be associated with the secretion of tumor-derived exosomal miRNAs induced by hypoxia. The current data revealed that miR-1225-5p was elevated in hypoxic exosomes derived from ovarian cancer cells and that hypoxic exosomes promoted the transformation of macrophages into M2-like macrophages.
Subsequently, we identified the function of the miR-1225-5p downstream candidates. The toll-like receptors (TLRs), which initiate inflammation, are considered as a bridge between acquired and innate immunity [Citation38]. TLR2 is commonly present on the immune cell surface, such as monocytes, macrophages, dendritic cells, and suppressor cells derived from the bone marrow [Citation39] and is also widely involved in tumorigenesis and cancer development [Citation40]. The relationship between TLR2 and tumorigenesis has been reported by several studies. In a study on liver cancer, autophagy and apoptosis of cells in the liver of mice with a TLR2 gene knockout were reduced, and the number of infiltrating macrophages was markedly decreased, leading to rapid tumor progression in mice [Citation41]. However, in a study on human hepatoma cell lines, Shi et al. reported that downregulation of TLR2 resulted in a decrease in the activity of HCC cell lines [Citation42]. In a study on colorectal cancer, TLR2 was found to have the opposite regulatory outcome [Citation43,Citation44]. We speculated that this may be related to the different polarized forms of macrophages. TLR2 was found to bind to miR-1225-5p, and exosomal TLR2 dramatically reversed the effects of exosomal miR-1225-5p on modulating macrophage polarization.
Subsequently, we investigated whether hypoxic treatment could promote M2 macrophage differentiation through exosomes to promote tumor formation. As speculated, the CM from hypo-exo-treated macrophages prominently facilitated tumor cell viability and metastasis. Subsequently, the pathways related to macrophage differentiation and tumor growth was studied. For example, activated wnt/β-catenin pathway stimulates M2 polarization in macrophages, and activation of this pathway was also observed in ovarian cancer [Citation45,Citation46]. PI3K, Notch, p65, and STAT3 pathways also participate in regulating macrophage polarization [Citation47–50]. This study indicated that hypoxia facilitated the wnt/β-catenin pathway activation to promote the migration-promoting function of M2-like macrophages on ovarian cancer cells.
5. Conclusion
Hypoxia increased miR-1225-5p levels in ovarian cancer cell-derived exosomes endocytosed by macrophages to promote M2-like polarization by targeting TLR2. Induced macrophages promote cell aggressive behaviors of ovarian cancer by activating the wnt/β-catenin pathway.
Author contributions
HY performed the experiments, ZZ and GL conceived the study, YL analysed the data; ST wrote the manuscript and designed the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:1. doi:10.1136/bmj.m3773
- Xu J, Fang Y, Chen K, et al. Single-Cell RNA sequencing reveals the tissue architecture in human high-Grade serous ovarian cancer. Clin Cancer Res. 2022;28(16):3590–10. doi:10.1158/1078-0432.CCR-22-0296
- Millstein J, Budden T, Goode EL, et al. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann Oncol. 2020;31(9):1240–1250. doi:10.1016/j.annonc.2020.05.019
- Chen X, Lan H, He D, et al. Multi-omics profiling identifies risk hypoxia-related signatures for ovarian cancer prognosis. Front Immunol. 2021;12:645839. doi:10.3389/fimmu.2021.645839
- Pereira M, Matuszewska K, Jamieson C, et al. Characterizing endocrine status, tumor hypoxia and immunogenicity for therapy success in epithelial ovarian cancer. Front Endocrinol. 2021;12:772349. doi:10.3389/fendo.2021.772349
- Poltavets V, Kochetkova M, Pitson SM, et al. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front Oncol. 2018;8:431. doi:10.3389/fonc.2018.00431
- Maia J, Caja S, Strano MM, et al. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18. doi:10.3389/fcell.2018.00018
- Feng W, Dean DC, Hornicek FJ, et al. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol Cancer. 2019;18(1):124. doi:10.1186/s12943-019-1049-4
- He L, Zhu W, Chen Q, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–8220. doi:10.7150/thno.37455
- Neviani P, Wise PM, Murtadha M, et al. Natural Killer-Derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019;79(6):1151–1164. doi:10.1158/0008-5472.CAN-18-0779
- Wang L, Yang G, Zhao D, et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol Cancer. 2019;18(1):86. doi:10.1186/s12943-019-0997-z
- Au YC, Co NN, Tsuruga T, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150.
- Kanlikilicer P, Bayraktar R, Denizli M, et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. Ebiomedicine. 2018;38:100–112. doi:10.1016/j.ebiom.2018.11.004
- Nowak M, Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells. 2020;9(5):1299. doi:10.3390/cells9051299
- Yoshida K, Yokoi A, Kato T, et al. The clinical impact of intra- and extracellular miRNAs in ovarian cancer. Cancer Sci. 2020;111(10):3435–3444. doi:10.1111/cas.14599
- Pan C, Stevic I, Muller V, et al. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol Oncol. 2018;12(11):1935–1948. doi:10.1002/1878-0261.12371
- Sun P, Zhang D, Huang H, et al. MicroRNA-1225-5p acts as a tumor-suppressor in laryngeal cancer via targeting CDC14B. Biol Chem. 2019;400(2):237–246. doi:10.1515/hsz-2018-0265
- Zhong R, Li S, Fang K, et al. microRNA-1225 inhibit apoptosis of pancreatic cancer cells via targeting JAK1. Cell Cycle. 2019;18(9):990–1000. doi:10.1080/15384101.2019.1608127
- Tokuhisa M, Ichikawa Y, Kosaka N, et al. Exosomal miRNAs from peritoneum lavage fluid as potential prognostic biomarkers of peritoneal metastasis in gastric cancer. PLOS One. 2015;10(7):e130472.
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi:10.1038/nature12034
- Yang F, Ning Z, Ma L, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16(1):148–110. doi:10.1186/s12943-017-0718-4
- Mori MA, Ludwig RG, Garcia-Martin R, et al. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30(4):656–673. doi:10.1016/j.cmet.2019.07.011
- Song M, Yeku OO, Rafiq S, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages. Nat Commun. 2020;11(1):6298. doi:10.1038/s41467-020-20140-0
- Chen X, Ying X, Wang X, et al. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. 2017;38(1):522–528. doi:10.3892/or.2017.5697
- Genin M, Clement F, Fattaccioli A, et al. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15(1):577. doi:10.1186/s12885-015-1546-9
- Klemm P, Behnke M, Solomun JI, et al. Self-assembled PEGylated amphiphilic polypeptides for gene transfection. J Mater Chem B. 2021;9(39):8224–8236. doi:10.1039/D1TB01495A
- Rupaimoole R, Wu SY, Pradeep S, et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat Commun. 2014;5(1):5202. doi:10.1038/ncomms6202
- El-Andaloussi S, Lee Y, Lakhal-Littleton S, et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7(12):2112–2126. doi:10.1038/nprot.2012.131
- Rehman FU, Liu Y, Yang Q, et al. Heme oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. J Control Release. 2022;345:696–708. doi:10.1016/j.jconrel.2022.03.036
- Helwa I, Cai J, Drewry MD, et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLOS One. 2017;12(1):e170628.
- El-Arabey AA, Alkhalil SS, Al-Shouli ST, et al. Revisiting macrophages in ovarian cancer microenvironment: development, function and interaction. Med Oncol. 2023;40(5):142. doi:10.1007/s12032-023-01987-x
- Han Q, Zhao H, Jiang Y, et al. HCC-derived exosomes: critical player and target for cancer immune escape. Cells. 2019;8(6):558. doi:10.3390/cells8060558
- El-Arabey AA, Abdalla M. GATA3 as a regulator for naughty cancer-associated fibroblasts in the microenvironment of high-grade serous ovarian cancer. Hum Cell. 2021;34(6):1934–1936. doi:10.1007/s13577-021-00598-w
- El-Arabey AA, Denizli M, Kanlikilicer P, et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell Signal. 2020;68:109539. doi:10.1016/j.cellsig.2020.109539
- El-Arabey AA, Abdalla M, Abd-Allah AR. GATA3 and stemness of high-grade serous ovarian carcinoma: novel hope for the deadliest type of ovarian cancer. Hum Cell. 2020;33(3):904–906. doi:10.1007/s13577-020-00368-0
- Zhao S, Mi Y, Guan B, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13(1):156. doi:10.1186/s13045-020-00991-2
- Song J, Yang P, Li X, et al. Esophageal cancer-derived extracellular vesicle miR-21-5p contributes to EMT of ESCC cells by disorganizing macrophage polarization. Cancers. 2021;13(16):4122. doi:10.3390/cancers13164122
- Ospelt C, Gay S. TLRs and chronic inflammation. Int J Biochem Cell Biol. 2010;42(4):495–505. doi:10.1016/j.biocel.2009.10.010
- Girkin J, Loo SL, Esneau C, et al. TLR2-mediated innate immune priming boosts lung anti-viral immunity. Eur Respir J. 2021;58(1):2001584. doi:10.1183/13993003.01584-2020
- McCoy MG, Nascimento DW, Veleeparambil M, et al. Endothelial TLR2 promotes proangiogenic immune cell recruitment and tumor angiogenesis. Sci Signal. 2021;14(666):eabc5371. doi:10.1126/scisignal.abc5371
- Lin H, Yan J, Wang Z, et al. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in toll-like receptor 2-deficient mice. Hepatology. 2013;57(1):171–182. doi:10.1002/hep.25991
- Shi W, Su L, Li Q, et al. Suppression of toll-like receptor 2 expression inhibits the bioactivity of human hepatocellular carcinoma. Tumour Biol. 2014;35(10):9627–9637. doi:10.1007/s13277-014-2268-3
- Lowe EL, Crother TR, Rabizadeh S, et al. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLOS One. 2010;5(9):e13027. doi:10.1371/journal.pone.0013027
- Scheeren FA, Kuo AH, van Weele LJ, et al. A cell-intrinsic role for TLR2-MYD88 in intestinal and breast epithelia and oncogenesis. Nat Cell Biol. 2014;16(12):1238–1248. doi:10.1038/ncb3058
- Feng Y, Ren J, Gui Y, et al. Wnt/β-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol. 2018;29(1):182–193. doi:10.1681/ASN.2017040391
- Arend RC, Londoño-Joshi AI, Straughn JM, et al. The wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013;131(3):772–779. doi:10.1016/j.ygyno.2013.09.034
- Zhao SJ, Kong FQ, Jie J, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3beta/beta-catenin pathway. Theranostics. 2020;10(1):17–35. doi:10.7150/thno.36930
- Chen W, Liu Y, Chen J, et al. The notch-signaling pathway regulates macrophage polarization in liver diseases. Int Immunopharmacol. 2021;99:107938. doi:10.1016/j.intimp.2021.107938
- Liu C, Hu F, Jiao G, et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFkappaB P65 signaling pathway after spinal cord injury. J Nanobiotechnology. 2022;20(1):65. doi:10.1186/s12951-022-01273-4
- Yin Z, Ma T, Lin Y, et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119(11):9419–9432. doi:10.1002/jcb.27259


