Abstract
Deer bone extract has the potential to relieve the discomfort or the articular cartilaginous damage associated with osteoarthritic (OA) and may be useful as a natural supplement for OA treatment without serious side effects. We analyzed the expression of pro-inflammatory cytokine and cartilage-related genes in monosodium iodoacetate-induced OA rats. Increases in the levels of serum pro-inflammatory cytokines, such as interleukin-1β, interleukin-6, and tumor necrosis factor-α were significantly inhibited by the administration of deer bone extract (p < 0.05). Decreases in the expression of collagen type II (COL2) and tissue inhibitors of metalloproteinases (TIMPs) mRNAs in the cartilage were significantly inhibited by deer bone extract treatment (p < 0.05). The deer bone extract significantly suppressed the expression of matrix metalloproteinases (MMPs) mRNAs in the cartilage. The deer bone extract induced the up-regulation of COL2 and TIMP mRNAs and the down-regulation of MMP mRNAs by suppressing the expression of pro-inflammatory cytokine mRNAs.
Graphical Abstract
Deer bone extract alleviated inflammatory symptoms by altering the expression of pro-inflammatory cytokine in osteoarthritic rats.
Osteoarthritis (OA) is defined as a heterogeneous condition that leads to joint symptoms and signs that are related to defects in the integrity of articular cartilage, in addition to related changes in the underlying bone at the joint margins.Citation1) Fifty-five recent studies indicated that OA is related to an excess of matrix metalloproteinases (MMPs) that can collectively degrade all of the structural proteins of the extracellular matrix (ECM), including interstitial collagen type II (COL2).Citation2–4) MMPs are controlled by a group of inhibitor proteins known as the tissue inhibitors of metalloproteinases (TIMPs).Citation5) Previous studies have demonstrated that various cytokines can affect MMP and TIMP gene expression.Citation6,7) OA chondrocytes secret pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α).Citation8) 62 The key events in the irreversible degradation of the cartilaginous matrix are up-regulated MMP expression and down-regulated TIMP expression by these pro-inflamatory cytokines.Citation9)
It was previously believed that once damaged articular cartilage could not be restored. Treatments were, therefore, targeted toward symptomatic relief with analgesics and anti-inflammatory agents, as well as lubricating and cushioning agents. However, research has provided evidence suggesting that some forms of intervention may support the body’s ability to repair damaged articular cartilage.Citation10) The administration of non-steroidal anti-inflammatory drugs is the conventional treatment of OA management. These medications often relieve pain or OA symptoms but can causes serious side effects, for example, hepatic or renal failure and peptic ulcers.Citation11,12) Many people with OA try complementary and alternative therapies. Therefore, efforts are being made to elucidate the potential of natural products in OA treatment.Citation13,14) In recent years, several investigators have suggested that certain supplements may stimulate the repair of damaged articular cartilage or at least decelerate its progressive degradationCitation14); these supplements include chondroitin sulfate and collagen hydrolysate.Citation15)
Deer bone extract is a proprietary nutraceutical grade powder containing various components associated with cartilage, such as glucosamine, calcium, and ganglioside, as well as chondroitin sulfate and collagen hydrolysate. Deer bone extract has the potential to relieve the discomfort or the articular cartilaginous damage associated with OA, and may be useful as a natural supplement for OA treatment without serious side effects. However, the pharmacological actions of deer bone extract on OA have not been well documented. As a plausible remedy for OA with no side effects, the pharmaceutical effect of deer bone extract should be evaluated.
Therefore, the objective of this study was to evaluate the anti-osteoarthritic effect of deer bone extract using an OA animal model. We investigated the expression of pro-inflammatory cytokine and cartilage-related genes in OA rats treated with deer bone extract.
Materials and methods
Preparation of deer bone extract
Dried antlers (Cervi elaphus) from adult male New Zealand elks were obtained from the Nongshim Co. (Seoul, Korea). The distal end portion of the deer bone was extracted with distilled water under pressure and cooling. The lipid layer of extract was removed by oil separator. The separated solution was filtered and concentrated using a vacuum evaporator and was then dried. Table shows the components of deer bone extract used in this study.
Table 1. Contents of components in deer bone extract.
Experimental animals
Six-week-old male Wistar rats were obtained from Daehan Biolink (Daejeon, Korea). The rats were individually housed in plastic cages. The temperature (24 ± 1 °C), atmospheric humidity (60%), and light–dark cycle (12 h/12 h) in the room were controlled. Before the experiment, the rats had ad libitum access to water and a commercial diet mainly composed of plant (grain) sources those have been confirmed as no direct effects on inflammation (Samyang Co., Seoul, Korea).
OA induction
OA induction was performed as previously described by Duhan et al.Citation16) with some modifications. Briefly, the rats were anesthetized with isoflurane (Abbott Lab., IL, USA) and given a single intra-articular injection of 3 mg of monosodium iodoacetate (MIA; 101 Sigma Co., MO, USA) through the infrapatellar ligament of the knee joint. MIA was dissolved in phosphate-buffed saline (PBS) and injected in a volume of 50 μL using a 27-gauge, 0.5-inch needle.
Experimental protocol
The rats were randomly divided into the following five groups (10 rats/group): sham control (SC: non-treatment without OA induction); negative control (NC: non-treatment after OA induction); positive control (PC: 125 mg/kg glucosamine sulfate (GS) + 125 mg/kg chondroitin sulfate (CS) after OA induction); low dosage of deer bone extract (LDB: 250 mg/kg deer bone extract after OA induction); and high dosage of deer bone extract (HDB: 500 mg/kg deer bone extract after OA induction). Each substance was administered orally in a volume of 10 mL/kg once per day for 50 days from day after MIA was injected. All of the rats were anesthetized by an intra-peritoneal injection of sodium pentobarbital (30 mg/kg) and killed by cervical dislocation at 50th day after MIA injection. All of the experiments were reviewed and approved by the Korea University Animal Care Committee (KUIACUC-2012-165).
IL-1β, IL-6, and TNF-α in serum
At the end of the experimental feeding period, the rats fasted 24 h before being sacrificed. Blood samples were collected into serum-separation tubes. The tubes were maintained for 30 min at 25 °C and centrifuged at 3000×g for 10 min at 4 °C. The IL-1β, IL-6, and TNF-α levels in serum were determined using enzyme-linked immunosorbent assay kits (R&D Systems, MN, USA) according to the manufacturer’s instruction.
Expression of COL2 MMP and TIMP mRNAs in articular cartilage
The expression of COL2, MMP, and TIMP mRNAs was evaluated using real-time quantitative polymerase chain reaction (qPCR) assays. The articular cartilage was extracted, weighed, immediately frozen in liquid nitrogen, and stored in the freezer at −70 °C. RNA was extracted from the articular cartilage and cDNA was synthesized following the method of Marlovits et al.Citation17) The synthesized cDNA (100 ng) was subjected to qPCR amplification using the TaqMan® gene-expression master mix (Applied Biosystems, CA, USA) according to the manufacturer’s instruction. The TaqMan® gene expression assay kits for MMP-2, MMP-3, MMP-9, MMP-13, TIMP-1, and TIMP-2 as the target genes (FAM® 129 MGB probe) and for beta-actin (ACTB) as the endogenous control gene (VIC® 130 MGB probe) were purchased from Applied Biosystems. Respective interrogated sequences for Taqman gene expression assay and tissues from where the mRNA levels were determined are: TIMP1: NM_001044384.1; TIMP2: NM_011594.3; MMP-2: NM_008610.2; MMP-3: NM_010809.1; MMP-9: NM_013599.3; MMP-13: NM_008607.2.
A StepOne real-time PCR system (Applied Biosystems) was used for the qPCR assays. The cycling conditions were 2 min at 50 °C, 10 min at 95 °C, 15 s at 95 °C (40 cycles), and 1 min at 60 °C. The relative expression ratio was calculated using comparative methods.
Statistical analysis
The statistical analysis was performed using the statistical package for social sciences software (SPSS, SPSS Inc., IL, USA). The results are given as mean values ± standard deviation (SD). The differences among groups were analyzed using one-way analysis of variance (ANOVA) with Tukey’s multiple range tests. Values of p < 0.05 were considered to be statistically significant.
Results
Body weight and food intake
The body and organ weights of the OA rats treated with deer bone extract for 50 days are presented in Table . The intra-articular injection of MIA resulted in a significant decrease in body weight (SC: 334.1 g vs. NC: 302.5 g, p < 0.05). When the deer bone extract was administered to the OA rats for 50 days, the body weight was slightly higher compared with that of the rats administered the vehicle. Treatment with the deer bone extract also tended to normalize the food intake that was decreased by OA induction, but there was no significant difference in the food intake of the untreated-OA group and deer bone extract treated-OA group (data not shown). Moreover, the administration of 250–500 mg/kg deer bone extract did not affect the weights of the internal organs in terms of the liver, heart, spleen, and kidneys of the OA rats.
Table 2. Body and organ weights of the MIA-induced OA rats treated with deer bone extract for 50 days.
IL-1β, IL-6, and TNF-α in serum
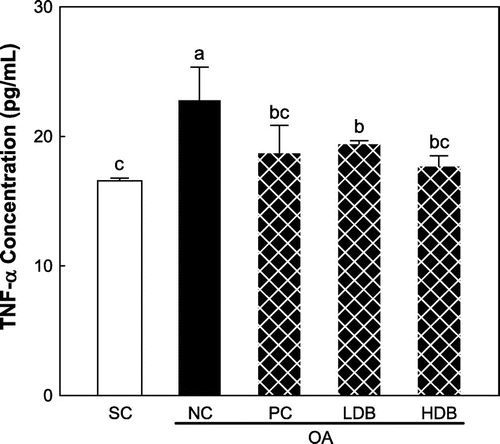
Figs. and show the pro-inflammatory cytokine levels in the serum of OA rats treated with deer bone extract for 50 days. The IL-1β, IL-6, and TNF-α levels were 88.0, 183.6, and 16.6 pg/mL, respectively, in the normal rats. MIA-induced OA significantly increased the IL-1β, IL-6, and TNF-α levels to 102.7 pg/mL, 210.2 pg/mL, and 22.8 pg/mL, respectively (p < 0.05). The increase of the levels of pro-inflammatory cytokines was significantly inhibited by the administration of deer bone extract in a dose-dependent manner (p < 0.05). In particular, the administration of 500 mg/kg deer bone extract resulted in the recovery of the levels of these pro-inflammatory cytokines in OA rats to normal levels.
Fig. 1. IL-1β (A) and IL-6 (B) levels in serum of the MIA-induced OA rats treated with deer bone extract for 50 days.
Note: Values are mean ± SD. Differences between groups (10 rats/group) were analyzed using one-way ANOVA by Tukey’s multiple range tests. Means with different superscript letters are significantly different at p < 0.05. Each group was assigned as follows: SC (PBS injection + non-treatment); NC (MIA injection + non-treatment); PC (MIA injection + 125 mg/kg glucosamine sulfate + 125 mg/kg chondroitin sulfate); LDB (MIA injection + 250 mg/kg deer bone extract); HDB (MIA injection + 500 mg/kg deer bone extract).
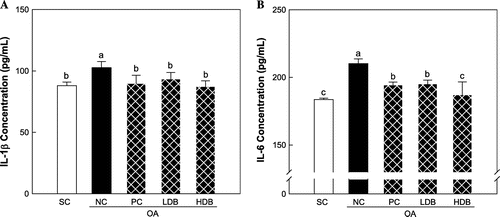
Fig. 2. TNF-α level in serum of the MIA-induced OA rats treated with deer bone extract for 50 days.
Note: Values are mean ± SD. Differences between groups (10 rats/group) were analyzed using one-way ANOVA by Tukey’s multiple range tests. Means with different superscript letters are significantly different at p < 0.05. Each group was assigned as follows: SC (PBS injection + non-treatment); NC (MIA injection + non-treatment); PC (MIA injection + 125 mg/kg glucosamine sulfate + 125 mg/kg chondroitin sulfate); LDB (MIA injection + 250 mg/kg deer bone extract); HDB (MIA injection + 500 mg/kg deer bone extract).
Expression of COL2, MMP, and TIMP mRNAs in articular cartilage
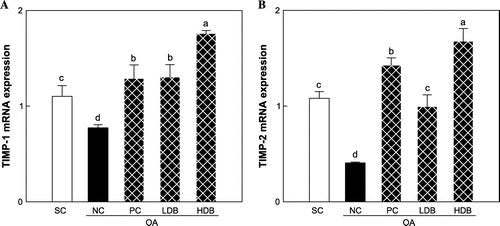
Figs. – present the expression levels of COL2, MMPs, and TIMPs mRNAs in the articular cartilage of OA rats treated with deer bone extract for 50 days. Compared with the normal group, COL2 expression was down-regulated significantly in the MIA-induced OA group (SC: 1.2 vs. NC: 0.1, p < 0.05) (Fig. ). The intra-articular injection of MIA resulted in significant increases in the expression of MMP-2, -3, -9, and -13 mRNAs (p < 0.05) (Fig. ). In addition, the expression of TIMP-1 and -2 declined significantly following OA induction (p < 0.05) (Fig. ). The MIA-induced decrease in COL2 expression was lessened significantly by the administration of deer bone extract (p < 0.05) and the HDB extract (HDB: 0.8) was significantly more effective in preserving COL2 expression than the low dosage (LDB: 0.5) or GS + CS (PC: 0.6) (p < 0.05). Administration of deer bone extract significantly suppressed the increase in MMP-2, -171, 3, -9, and -13 mRNA expression induced by MIA injection in a dose-dependent manner, compared with administration of the vehicle. In particular, the administration of 500 mg/kg deer bone extract maintained MMP-9 and -13 expression in the OA rats at normal levels. TIMP-1 and TIMP-2 expression levels were significantly higher in the cartilage of the deer bone extract treated-OA group than in the deer bone extract untreated-OA group. The effects of the deer bone extract on TIMP-1 and TIMP-2 expression were also dose-dependent: TIMP-1 (LDB: 1.3 vs. HDB: 1.8, p < 0.05) and TIMP-2 (LDB: 1.0 vs. HDB: 1.7, p < 0.05).
Fig. 3. COL2 mRNA expression in articular cartilage of the MIA-induced OA rats treated with deer bone extract for 50 days.
Note: Values are mean ± SD. Differences between groups (10 rats/group) were analyzed using one-way ANOVA by Tukey’s multiple range tests. Means with different superscript letters are significantly different at p < 0.05. Each group was assigned as follows: SC (PBS injection + non-treatment); NC (MIA injection + non-treatment); PC (MIA injection + 125 mg/kg glucosamine sulfate + 125 mg/kg chondroitin sulfate); LDB (MIA injection + 250 mg/kg deer bone extract); HDB (MIA injection + 500 mg/kg deer bone extract).
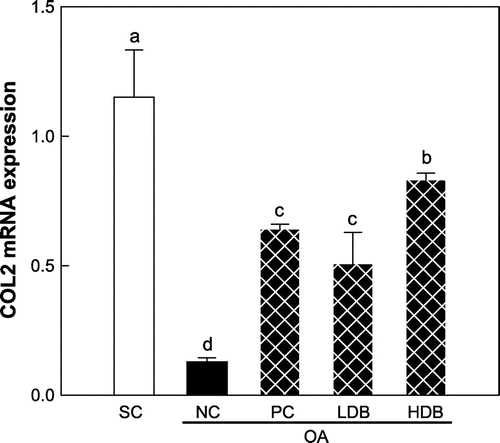
Fig. 4. COL2 mRNA expression in articular cartilage of the MIA-induced OA rats treated with deer bone extract for 50 days.
Note: Values are mean ± SD. Differences between groups (10 rats/group) were analyzed using one-way ANOVA by Tukey’s multiple range tests. Means with different superscript letters are significantly different at p < 0.05. Each group was assigned as follows: SC (PBS injection + non-treatment); NC (MIA injection + non-treatment); PC (MIA injection + 125 mg/kg glucosamine sulfate + 125 mg/kg chondroitin sulfate); LDB (MIA injection + 250 mg/kg deer bone extract); HDB (MIA injection + 500 mg/kg deer bone extract).
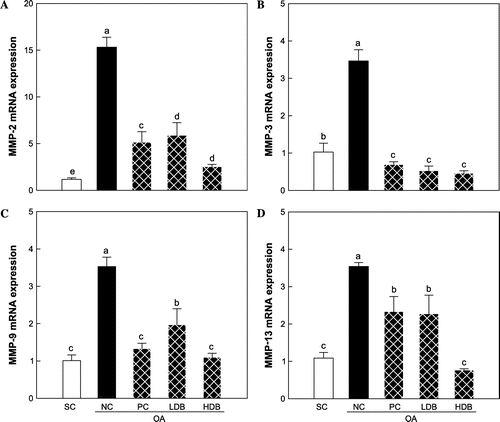
Fig. 5. TIMPs mRNA expression in articular cartilage of the MIA-induced OA rats treated with deer bone extract for 50 days.
Note: Values are mean ± SD. Differences between groups (10 rats/group) were analyzed using one-way ANOVA by Tukey’s multiple range tests. Means with different superscript letters are significantly different at p < 0.05. Each group was assigned as follows: SC (PBS injection + non-treatment); NC (MIA injection + non-treatment); PC (MIA injection + 125 mg/kg glucosamine sulfate + 125 mg/kg chondroitin sulfate); LDB (MIA injection + 250 mg/kg deer bone extract); HDB (MIA injection + 500 mg/kg deer bone extract).
Discussion
OA is regarded as a non-inflammatory disease, but some research has demonstrated that inflammation contributed to the integral progression of OA regarding the development of symptoms.Citation18) Phagocytosis of cartilaginous fragments detached from the synovium could cause inflammation. The inflamed synovium releases pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. These pro-inflammatory cytokines bind to chondrocyte receptors, causing chondrocytes to release MMPs and inhibit COL2 expression. These responses enhance cartilage degradation.Citation19,20) Therefore, inhibiting pro-inflammatory cytokines could be an important approach to managing OA. In this study, the administration of deer bone extract effectively inhibited the progression of OA by suppressing the expression of pro-inflammatory cytokines in MIA-induced OA rats.
To further evaluate the molecular changes in subchondral bone following MIA injection, the expression of genes related to the cartilage metabolism was investigated using qPCR. MIA-induced OA by causing chondrocyte apoptosis and disturbing cartilage and subchondral bone metabolism.Citation21) In addition, the MIA-induced imbalance between anabolism and catabolism leads to cartilage degeneration. OA chondrocytes reduce their expression of COL2 and up-regulate the expression of matrix-degradation proteases, such as gelatinase (MMP-2 and -9), stromelysin (MMP-3), and collagenase (MMP-13).Citation22) The activity of the MMPs is controlled by TIMPs. Therefore, disturbances in the regulation of MMPs and TIMPs cause the loss of cartilage.Citation23) In the progression of OA, the expression of catabolic genes such as those encoding MMPs is elevated, but the expression of anabolic genes such as those encoding COL2 and TIMPs declines.Citation21,24) 200 The ability of the combination of GS and CS to inhibit cartilage erosion may be attributed to the regulation of MMPs and TIMPs.Citation25,26) We found that deer bone extract also induced the up-regulation of COL2 and TIMP genes and the down-regulation of MMP genes when it had been administered to OA rats.
Currently, among all of the dietary supplements indicated for OA-associated symptoms, chondroitin sulfate is most commonly used. Studies have shown that chondroitin sulfate induces the production of proteoglycans and the IL-1β-based reduction of proteoglycan production in OA chondrocytes.Citation27,28) Chondroitin sulfate is reported to provide building blocks for the synthesis of proteoglycans and increase sulfate incorporation by OA proteoglycans.Citation29,30) Chondroitin sulfate stimulated the accumulation of molecules such as COL2 in the ECM, due in part to the down-regulation of MMPs.Citation28) It, therefore, was thought that the chondroitin sulfate in the deer bone extract could account for its beneficial effects in the treatment of OA.
There is also growing interest in collagen hydrolysate as a nutraceutical supplement because collagen-derived peptides possess a variety of interesting biological properties. First, collagen hydrolysate has been shown to stimulate chondrocytes to produce COL2.Citation31) Second, collagen hydrolysate has been shown to be deposited into the articular cartilage.Citation32) In addition, collagen hydrolysate led to the presence of various di- and tripeptides, including proline-hydroxyproline (Pro-Hyp) dipeptide as the major form.Citation33) Pro-Hyp dipeptides inhibit the loss of chondrocytes and thinning of the articular cartilage caused by phosphorus treatment suggesting that collagen hydrolysate-derived Pro-Hyp may promote chondrocyte differentiation, resulting in cartilage protection under stressful conditions.Citation34) These results raise the intriguing possibility that collagen hydrolysate, which is contained in the deer bone extract, may repair or regenerate deteriorating cartilage.
It was reported that the disease-modifying effect—the ability to retard the progression of cartilage degeneration, of the modified components related to OA, was more efficacious than any component alone.Citation25) Also, immunosuppressive activity of deer antler extract in vitro as well as in vivo was well reported.Citation35,36) Therefore, although the chondroitin sulfate and collagen hydrolysate in the deer bone extract are the major active components in OA treatment, it is believed that the various other OA-related components and modified application of deer components might be beneficial in the OA. One possible modified application can be found in the report from elsewhere that deer aqua-acupuncture has protective effect on arthritis.Citation37)
In conclusion, we found that treatment of OA with the deer bone extract induced the up-regulation of COL2 and TIMPs and the down-regulation of MMPs by suppressing pro-inflammatory cytokines. The deer bone extract exhibited recovery effects similar to those of a positive supplement (i.e. the GS and CS combination) for all of the parameters evaluated in the MIA-induced OA rats. Therefore, our results suggest that the deer bone extract could be a potent neutral nutraceutical agent for OA treatment.
Funding
This study was supported by the R&D Program of Nong Shim Co., Ltd.
References
- Kean WF, Kean R, Buchanan WW. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacol. 2004;12:3–31.10.1163/156856004773121347
- Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr. Pharm. Biotechnol. 2008;9:47–54.10.2174/138920108783497659
- Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front. Biosci. 2006;11:529–543.10.2741/1817
- Tardif G, Reboul P, Pelletier JP, Martel-Pelletier J. Ten years in the life of an enzyme: the story of the human MMP-13 (collagenase-3). Mod. Rheumatol. 2004;14:197–204.10.3109/s10165-004-0292-7
- Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract. Res. Clin. Rheumatol. 2001;15:805–829.10.1053/berh.2001.0195
- Vincenti MP. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol. Biol. 2001;151:121–148.
- Tamai K, Ishikawa H, Mauviel A, Uitto J. Interferon-gamma coordinately upregulates matrix metalloprotease (MMP)-1 and MMP-3, but not tissue inhibitor of metalloproteases (TIMP), expression in cultured keratinocytes. J. Invest. Dermatol. 1995;104:384–390.10.1111/jid.1995.104.issue-3
- Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α in human traumatic spinal cord injury. Spine (Phila Pa 1976). 2004;29:966–971.10.1097/00007632-200405010-00004
- Aida Y, Maeno M, Suzuki N, Shiratsuchi H, Motohashi M, Matsumura H. The effect of IL-1β on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci. 2005;77:3210–3221.10.1016/j.lfs.2005.05.052
- Monfort J, Pelletier JP, Garcia-Giralt N, Martel-Pelletier J. Biochemical basis of the effect of chondroitin sulphate on osteoarthritis articular tissues. Ann. Rheum. Dis. 2008;67:735–740.10.1136/ard.2006.068882
- Rashad S, Low F, Revell P, Hemingway A, Rainsford K, Walker F. Effect of non-steroidal anti-inflammatory drugs on course of osteoarthritis. Lancet. 1989;334:1149.10.1016/S0140-6736(89)91503-1
- Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part I. Osteoarthritis of the hip. American College of Rheumatology. Arthritis Rheum. 1995;38:1535–1540.10.1002/(ISSN)1529-0131
- Huang J, Zhu M, Tao Y, Wang S, Chen J, Sun W, Li S. Therapeutic properties of quercetin on monosodium urate crystal-induced inflammation in rat. J. Pharm. Pharmacol. 2012;64:1119–1127.10.1111/jphp.2012.64.issue-8
- Jiang Y, You XY, Fu KL, Yin WL. Effects of extract from Mangifera indica leaf on monosodium urate crystal-induced gouty arthritis in rats. Evid. Based Complement Alternat. Med. 2012;2012:967573.
- Bello AE, Oesser S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: a review of the literature. Clin. Orthop. Relat. Res. 2006;22:2221–2232.
- Dunham J, Hoedt-Schmidt S, Kalbhen DA. Prolonged effect of iodoacetate on articular cartilage and its modification by an anti-rheumatic drug. Int. J. Exp. Pathol. 1993;74:283–289.
- Marlovits S, Hombauer M, Truppe M, Vècsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Joint Surg. 2004;86:286–295.10.1302/0301-620X.86B2.14918
- Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44:7–16.10.1093/rheumatology/keh344
- Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 1997;24:365–371.
- Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, Ding C. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartilage. 2010;18:1441–1447.
- Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036.10.1371/journal.pone.0045036
- Kourí-Flores JB, Abbud-Lozoya KA, Roja-Morales L. Kinetics of the ultrastructural changes in apoptotic chondrocytes from an osteoarthrosis rat model: a window of comparison to the cellular mechanism of apoptosis in human chondrocytes. Ultrastruct. Pathol. 2002;26:33–40.10.1080/01913120252934314
- Fransès RE, McWilliams DF, Mapp PI, Walsh DA. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthr. Cartilage. 2010;18:563–571.10.1016/j.joca.2009.11.015
- Baragi VM, Becher G, Bendele AM, Biesinger R, Bluhm H, Boer J, Deng H, Dodd R, Essers M, Feuerstein T, Gallagher BM Jr, Gege C, Hochgürtel M, Hofmann M, Jaworski A, Jin L, Kiely A, Korniski B, Kroth H, Nix D, Nolte B, Piecha D, Powers TS, Richter F, Schneider M, Steeneck C, Sucholeiki I, Taveras A, Timmermann A, Van Veldhuizen J, Weik J, Wu X, Xia B. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 2009;60:2008–2018.10.1002/art.v60:7
- Lippiello L, Woodward J, Karpman R, Hammad TA. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin. Orthop. Relat. Res. 2000;381:229–240.
- Chan PS, Caron JP, Orth MW. Short-term gene expression changes in cartilage explants stimulated with interleukin beta plus glucosamine and chondroitin sulfate. J. Rheumatol. 2006;33:1329–1340.
- Nerucci F, Fioravanti A, Cicero MR, Collodel G, Marcolongo R. Effects of chondroitin sulfate and interleukin-1β on human chondrocyte cultures exposed to pressurization: a biochemical and morphological study. Osteoarthr. Cartilage. 2000;8:279–287.10.1053/joca.1999.0302
- Wang L, Wang J, Almqvist KF, Veys EM, Verbruggen G. Influence of polysulphated polysaccharides and hydrocortisone on the extracellular matrix metabolism of human articular chondrocytes in vitro. Clin. Exp. Rheumatol. 2002;20:669–676.
- Schwartz NB, Dorfman A. Stimulation of chondroitin sulfate proteoglycan production by chondrocytes in monolayer. Connect. Tissue Res. 1975;3:115–122.10.3109/03008207509152169
- Schwartz NB. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J. Biol. Chem. 1977;252:6316–6321.
- Oesser S, Seifert J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res. 2003;311:393–399.
- Oesser S, Adam M, Babel W, Seifert J. Oral administration of 14C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 1999;129:1891–1895.
- Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, Higashi A, Kido Y, Nakabo Y, Ohtsuki K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005;53:6531–6536.10.1021/jf050206p
- Nakatani S, Mano H, Sampei C, Shimizu J, Wada M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartilage. 2009;17:1620–1627.10.1016/j.joca.2009.07.001
- Kang SK, Kim KS, Kim SI, Chung KH, Lee IS, Kim CH. Immunosuppressive activity of deer antler extracts of Cervus korean TEMMINCK var. mantchuricus Swinhoe, on type II collagen-induced arthritis. In Vitro Cell. Dev. Biol. Anim. 2006;42:100–107.10.1290/0510067.1
- Choi HS, Kim SR, Hong SH, Ku JM, Kim MK, Seo HS, Cho SG, Shin S, Shin YC, Ko SG. Water extract of deer bones activates macrophages and alleviates neutropenia. Evid. Based Complement. Alternat. Med. Forthcoming 2014.
- Kim KS, Choi YH, Kim KH, Lee YC, Kim CH, Moon SH, Kang SG, Park YG. Protective and anti-arthritic effects of deer antler aqua-acupuncture (DAA), inhibiting dihydroorotate dehydrogenase, on phosphate ions-mediated chondrocyte apoptosis and rat collagen-induced arthritis. Int. Immunopharmacol. 2004;4:963–973.10.1016/j.intimp.2004.04.010

