Abstract
Alzheimer’s disease and type 2 diabetes are very serious diseases with the latter having been suggested to cause the former. We prepared super-hard rice bread blended with black rice bran (SRBBB), which contained a high amount of resistant starch that showed strong inhibitory activities against β-secretase and acetylcholinesterase even after heating. Black rice bran showed greater β-secretase inhibitory activity (3.6-fold) than Koshihikari rice. The bran contained more oleic acid and anthocyanin, meaning that it is potentially a biofunctional food with a high antioxidant capacity. Furthermore, aged mice, which were fed a SRBBB diet for four weeks, showed lower amyloid β 40 peptide in the blood than mice fed a commercial diet (p < 0.01). Additionally, their initial blood glucose levels (BGLs) after 12 weeks of being fed SRBBB were significantly lower than those in the control group. Taken together, our results indicate SRBBB shows promise for inhibiting not only amyloid β production, but also abrupt increases in postprandial BGLs.
Graphical abstract
Characterization of amyloid β-40 protein species with ELISA method.
Alzheimer’s disease affects more than 25 million people worldwide and is the most common form of dementia.Citation1) β-amyloid precursor protein (APP) is processed to generate β-amyloid (Aβ) by β- and γ-secretase, in a highly regulated process. Many drugs have been approved for the treatment of Alzheimer’s disease, at different stages of the disease, although they all have limited efficacy.
Recent epidemiological studies have suggested a link between Alzheimer’s disease and type 2 diabetes mellitus associated with insulin resistance.Citation2–5) Diabetes is a lifestyle disease, and its prevention and treatment are extremely important. Low glycemic index (GI) foods inhibit rapid increases in blood glucose and insulin secretion after meals. The glycemic effect of food depends on numerous factors such as the structure of amylose and amylopectin.Citation6) The amylopectin found in amylose extender (ae) mutants of rice contains more long-chain glucans, thereby making the texture of the rice grains very hard and non-sticky after boiling, which renders it less palatable as boiled rice.Citation7) Thus, the gelatinization temperatures of ae mutant rice starches are very high; however, they are promising in the production of low-GI foods such as breads or noodles, and may help prevent diabetes because they contain a substantial amount of resistant starch even after boiling.Citation8,9) The ae mutant rice cultivar Konayukinomai (KOY) has been developed by the National Agricultural and Food Research Organization (NARO). The amylopectin starch of KOY has fewer short-branched glucans and more long chains, is resistant to gelatinization, and the boiled rice grains are non-sticky and brittle. In a previous study, we developed a novel method for inhibiting postprandial blood glucose levels (BGLs) in Sprague–Dawley rats by preparing boiled rice grains of KOY soaked with functional food ingredients.Citation10,11) Moreover, this has recently been tested in human subjects (article currently under submission). Several studies have reported the development of highly resistant starch riceCitation12,13) as well as high-amylose and high-dietary fiber riceCitation14) via physical or chemical mutation. The hydrolysis of starch is a key factor in controlling the GI of foods. Functional foods that have α-glucosidase inhibitory activities have proven effective in controlling blood sugar levels in people at risk of developing diabetes.Citation15)
Furthermore, epidemiological studies suggest that the low incidences of certain chronic diseases in rice-consuming regions of the world may be attributable to the antioxidant compounds found in rice. The molecules with antioxidant activity contained in rice include phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Rice bran also contains various functional substances, such as γ-oryzanol, ferulic acid, sterol, wax, ceramide, phytin, inositol, and protein.Citation16) Rice bran oil, the only domestic edible vegetable oil made from the rice bran produced in Japan, is known to have high oxidative stability and serum cholesterol-lowering activity.Citation17,18) You et al. showed that in mice fed a ferulic acid-enriched diet, exercise endurance capacity was enhanced and fatigue was reduced by elevating antioxidative potentials.Citation19) Matsuzaki et al. showed that bran rice and γ-oryzanol reduced hypothalamic endoplasmic reticulum stress and attenuated the preference for dietary fat in mice.Citation20) Parthasarathy et al. showed that in rabbits fed a oleic acid-enriched diet, the progression of atherosclerosis may have been slowed by resistance to oxidative modification.Citation21) Matsuoka et al. showed that plant sterols/stanols decreased blood cholesterol levels through the inhibition of cholesterol absorption in the intestines.Citation22) Intake of fermented brown rice could minimize insulin secretion, thus attenuating any subsequent rise in the levels of blood sugar.Citation23) Abe et al. showed that rice components including inositol hexaphosphate significantly inhibited Aβ production in neuroblastoma cells without causing cytotoxicity, suggesting such foods may prevent Alzheimer’s disease.Citation24)
Pigmented rice contains naturally occurring colored substances that belong to the flavonoid group called anthocyanins. Positive health effects of these pigments present in the bran layer of rice have been reported.Citation25) Ling et al. showed that red and black rice decreases atherosclerotic plaque formation and increase antioxidant status in rabbits due to their enhanced serum high-density lipoprotein (HDL) cholesterol and apolipoprotein A1 concentrations.Citation26)
The consumption of a western diet, which is characterized by a high intake of red meats and high-fat dairy products, may contribute to obesity and metabolic syndrome, as well as increase the risk of developing type 2 diabetes and cardiovascular disease. In contrast, the traditional Asian diet, which is rich in soy and fish but low in animal protein and fat, may help reduce the frequency of severe chronic diseases.Citation27) There is also a significant association between a Mediterranean diet and reduced risk of major chronic degenerative diseases, including Alzheimer’s disease. The optimal diet for the prevention of cardiovascular and other major chronic diseases has rapidly evolved.Citation28)
In this study, we sought to develop a novel product that inhibited both Aβ protein production and the abrupt increase in postprandial BGLs using super-hard brown rice bread blended with black rice bran (SRBBB).
Materials and methods
Materials
The ae mutant rice cultivar KOY and the high-quality premium rice Koshihikari were cultivated in an experimental field at Hokuriku Research Center in the Central Agricultural Research Center, NARO, Japan in 2014. Okunomurasaki (OKM) rice was purchased from a local market. All of the rice samples were stored at 4 °C before the experiments. Commercial feed (MF) was produced by Oriental Yeast Co., Ltd. Rice bran oil (Tsuno Food Co.), dry yeast (Pioneer Planning Co.), sugar (Nisshin Seito Co.), and sodium chloride (Ajinomoto Co.) were commercially available products.
Preparation of rice bran and brown rice flour samples
Rice bran was made from polished brown rice using an experimental friction-type rice milling machine (Yamamotoseisakusyo Co. Ltd., Tendoh, Japan) to obtain a milling yield (yield after polishing) of 90–91%. Brown rice flour was prepared using a cyclone mill (SFC-S1; Udy, Corp., Fort Collins, Co., Ltd.) with a screen containing 1-mm diameter pores. OKM rice bran was treated at 400 MPa at room temperature for 15 min in an HP machine (Ishikawajima-Harima Heavy industries Co., Ltd., Tokyo, Japan).
Measurement of the moisture content of rice flour
The moisture content of the brown rice and brown rice bread flour were measured using an oven-drying method. Samples of 2 g were dried for 1 h at 135 °C. All samples were stored in a freezer at −80 °C, followed by lyophilizing with a freeze-dryer (FD-1, Tokyo Rikakikai Co., Ltd.). Rice flour, bran, and bread flour were prepared using a cyclone mill (SFC-SI, Udy Corp., Fort Collins Co., Ltd.) with a screen with 1 mm diameter pores.
Measurement of the fatty acid composition of rice bran
Measurement of the fatty acid composition of rice bran was carried out by the Food Analysis Technology Center (using a gas chromatography method).
Measurement of the ferulic acid and inositol composition of rice bran
Measurement of the ferulic acid and inositol composition of rice bran was carried out by the Japan Food Research Laboratories (using microbiological assays and high performance liquid chromatography–mass spectrometry).
α-glucosidase inhibitory activity
Measurement of the inhibitory activity of α-glucosidase of freeze-dried rice bran samples was carried out as described by Yamaki et al.Citation29) α-Glucosidase inhibitory activity was measured using an enzyme extracted from the small intestine of rats (Sigma Chemical Co.) The enzyme (25 mg/mL) was used as an ultrasonic treatment in cold water for 0.5 h. A kit for measuring α-glucosidase activity (Kikkoman Biochemicals Corp.) was also used. One milliliter of 0.01 M acetate buffer solution (pH 5.0, including 0.5% NaCl) was added to the rice bran sample and bread sample (SRBBB and 100% wheat flour bread) (0.2 g), and the mixture was extracted at 5 °C for 16 h, and then centrifuged for 5 min at 3000 × g. For removing the colored component from the extraction solution, an adsorption filter (MILLEX-GN33 mm: Millipore Ireland Ltd.) was used. The extraction solution was diluted serially with extraction buffer. The extraction solution (0.5 mL) was mixed with the enzyme solution (0.5 mL), and then pre-warmed at 37 °C for 5 min and mixed with 0.5 mL PNPG substrate solution (the blank was set without enzyme). This was then heated at 37 °C for 30 min, followed by the addition of 0.5 mL of stopping solution (0.2 M Na2CO3) and stirring. The absorbance of the sample solution was measured at 400 nm. The linear least-squares problem was used in statistical regression analysis.
β-secretase inhibitory activity
The β-secretase (BACE1) inhibitory activity of freeze-dried rice bran, brown rice breads, and MF were determined using a BACE1 activity detection kit (Fluorescent; Sigma-Aldrich Co. LLC.). To obtain the measurements, test sample (0.1 g) was extracted with 0.5 mL of 10 mM acetate buffer solution (pH 5.0, including 0.1% Triton and 0.05% CHAPS) at room temperature for 1 h, and then centrifuged for 15 min at 1000 × g. For removing the colored component from the extraction solution, an adsorption filter (MILLEX-GN33 mm: Millipore I reland Ltd.) was used. For fluorometry, a 96-well plate (tissue culture plate: AS One Corporation) was used. The fluorescent buffer (78 μL), extraction solution (30 μL) (the blank was set without fluorescent buffer), and 0.03 U/μL enzyme (10 μL) were added in order, and then pre-incubated at 37 °C for 10 min. Next, 50 μM substrate (20 μL) was added, covered with Parafilm, and incubated at 37 °C for 2 h. The absorbance values were measured at 320 nm (excitation) and 405 nm (emission) using a fluorescent microplate reader (Grating Based Multimode Reader SH-9000: Corona Electric Co, Ltd.). The enzyme concentration was 0.03 U/μL for the rice bran, and 0.015 U/μL for the brown rice bread and MF samples.
Acetylcholinesterase inhibitory activity
The acetylcholinesterase (AChE) inhibitory activities of freeze-dried rice bran, brown rice breads, and MF samples were measured as described by Ellman et al.Citation30), Augustinsson et al.Citation31), and Dawson et al.Citation32) To obtain the measurements, test sample (0.03 g) was extracted with 0.3 ml of 80% ethanol for 15 min in ice, and then centrifuged for 15 min at 3000 × g. The extraction solution was diluted eightfold in water. To remove the colored component from the extraction solution, an adsorption filter (MILLEX-GN33 mm: Millipore Ireland Ltd.) was used. For fluorometry, a 96-well microtiter plate (AS ONE Corporation) was used. Phosphate buffer at pH 8.0 (50 mM, 50 μL), 0.226 U/ml (1.65 ng/mL) of AChE (from the electric eel: Sigma-Aldrich Co. LLC) (25 μL), and coenzyme (10 mM, 125 μL: 39.6 mg of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) were dissolved in 10 mL of 0.1 M phosphate buffer at pH 7.0 and 15 mg of sodium bicarbonate). And 25 μL of extraction solution (the blank was set with 0.1 M phosphate buffer at pH 7.0) were added, and then pre-incubated at 37 °C for 5 min. Next, 25 μL of 3 mM (0.99 mg/mL) of substrate (MATP+: Dojindo Laboratories Co., Ltd.) was added, and the solution was immediately measured at 412 nm using a fluorescent microplate reader (Grating Based Multimode Reader SH-9000: Corona Electric Co, Ltd.).
Measurement of hydrophilic and lipophilic oxygen radical absorbance capacity
The hydrophilic and lipophilic oxygen radical absorbance capacities of rice bran, brown rice bread, and MF samples were measured as described by Prior et al.,Citation33) Ito et al.,Citation34) and Watanabe et al.Citation35,36) For the hydrophilic antioxidant assay, freeze-dried rice bran, brown rice bread, and MF samples (0.1 g) were extracted with 10 mL of hexane at room temperature for 2 min, and then centrifuged for 15 min at 3000 × g. The hexane layer was removed, and then the process was repeated. Residual hexane was evaporated using a water bath at 70 °C for 10 min, and then the residue was extracted with 10 mL of acetone/water/acetic acid (70:29.5:0.5, v/v/v). After this, the solution was sonicated (Ultrasonic cleaner 3510J-MTH: Branson Ultrasonics Co, Ltd.) at 37 °C for 15 min. The tube remained at room temperature for 15 min, and was then centrifuged for 15 min at 3000 × g. The supernatant was removed and transferred to a volumetric flask and diluted to 25 mL total volume.
For the lipophilic antioxidant assay, freeze-dried rice bran, brown rice bread, and MF samples (0.1 g) were extracted with 1 mL of hexane at room temperature for 2 min, and then centrifuged for 15 min at 3000 × g. The hexane layer was collected and the process was repeated. The hexane was evaporated using a water bath at 70 °C for 10 min, and then the dried hexane extract was dissolved in 250 μL of acetone and then diluted with 750 μL of 7% randomly methylated β-cyclodextrin (RMCD; 0.7 g of methyl-β-cyclodextrin (Sigma Chemical Co.) was dissolved in 10 mL of 50% acetone). Trolox calibration solutions (50, 25, 12.5, and 6.25 μmol/L in assay buffer solution) were made to obtain a standard curve. A fluorometer 96-well plate (AS One Corporation) was used. Absorbance values were measured at 485 nm (excitation) and 530 nm (emission) using a fluorescent microplate reader (Grating Based Multimode Reader SH-9000: Corona Electric Co, Ltd.). The trolox calibration solutions, blank (dilute buffer), and diluted test samples (35 μL), fluorescein solution (115 μL, 110.7 nmol/L), and AAPH (2, 2′-azobis (2-amidinopropane) dihydrochloride) solution (50 μL, 31.7 mmol/L: H-ORAC, 63.4 mmol/L: L-ORAC) were added in order, and measurements were taken immediately at 37 °C for 90 min.
Measurement of resistant starch
The RS of the starch in rice flour was measured according to the Association of Analytical Communities method using an RS assay kit (Megazyme, Ltd, Wicklow, Ireland). Each sample (100 mg) was digested with pancreatin and amyloglucosidase at 37 °C for 6 h, and the glucose content was measured using a spectrophotometer at 510 nm.
Measurement of polyphenol content
The polyphenol content of freeze-dried brown rice bread and MF samples was determined using the Folin–Ciocalteu method.Citation37) The polyphenol content of freeze-dried brown rice bread and MF samples were measured by extracting 0.1 g of the rice flour sample by shaking with 4 mL of 80% ethanol at room temperature for 30 min, and then centrifuging for 10 min at 3000 × g. The supernatant (1 mL) was mixed with the same volume of Folin–Ciocalteu solution (1 mL) and incubated at room temperature for 3 min, followed by the addition of 5 mL of sodium carbonate and incubation at 50 °C for 5 min. Finally, the sample solution was cooled and allowed to stand for 1 h at 10 °C. Absorbance was measured at 765 nm. Gallic acid (0.1 mg/mL) was used for calibration.
Measurement of L-glutamic acid
The L-glutamic acid content was measured using an F-kit (Roche Diagnostics, Mannheim, Germany). Each sample (1 g) was subjected to extraction by shaking with DW (1 mL) for 30 min at room temperature. The L-glutamic acid content of the sample was measured based on the generation of formazan, according to the manufacturer’s instructions. Absorbance was measured at 510 nm.
Bread-making for feed
Based on the recipe for wheat flour blended with rice flour,Citation38) 100% KOY (ae mutant rice) brown rice bread was made as follows: 280 g of brown rice flour (dry matter: 244.3 g), 5.0 g of sucrose, 1.0 g of NaCl, 20 g of rice bran oil, 2.7 g of commercial dried yeast, 180 mL of water. The moisture content was 39.6%. The SRBBB was prepared as follows: 94.6% KOY brown rice flour and 5.4% OKM (black rice) bran were blended into bread dough, of which the recipe was as follows: 266 g of KOY brown rice flour (dry matter: 231.1 g), 14g of OKM rice bran (dry matter: 12.6 g), 5.0 g of sucrose, 1.0 g of NaCl, 20 g of rice bran oil, 2.7 g of commercial dried yeast, 180 mL of water. The moisture content was 38.4%. Each dough sample was baked in an SD-BT103 automatic home bakery machine (Panasonic Co. Ltd., Kadoma, Japan) using the normal dough procedure. The bread-making procedure, involving dough processing, proofing, and baking, took 4 h.
Commercial rodent diet (MF, Oriental Yeast, Tokyo: moisture content: 7.1%) and 0.02% ferulic acid (MP Biomedicals, LLC) were mixed with 0.06 g of ferulic acid dissolved in 244 mL of hot (55 °C) water for 3 h, and then mixed with 300 g of commercial diet (MF) that had been dried at 55 °C for 8 h with a convection oven (MOV-212 F (U): SANYO Electric Co., Ltd.). The moisture content was 6.9%.
Animal feeding test and diets
Twenty-four-week-old C57BL/6 mice were obtained from Charles River Co. Ltd. The mice were housed individually in an air-conditioned room at 23–24°C under a 12-h light cycle. After acclimatization with commercial rodent diet (MF, Oriental Yeast, Tokyo) for seven days, the mice were divided into four groups of eight mice each (A: Rice bread of 100% KOY brown rice flour; B: Rice bread of 94.6% KOY brown rice flour mixed with 5.4% OKM rice brown (SRBBB); C: MF diet; D: MF diet mixed with 0.02% ferulic acid.) The carbohydrate content of the feed was adjusted to 2.7 g/mouse/d of dry matter). In this way, group A were fed 8.3 g/mouse (wet base), group B were fed 8.6 g/mouse (wet base), group C were fed 5.4 g/mouse (wet base), and group D were fed 5.4 g/mouse (wet base). After cutting into 5-mm cubes, the breads were given to the mice once every day and the mice were allowed to eat freely.
We took blood samples at 0, 4, 8, and 12 weeks during the experiment. The fasting BGL was measured, after 12 weeks, using an MS-FR501W (Terumo blood sugar measurement: Terumo Co., Ltd. Japan). Moreover, the BGL was measured at 0, 30, and 90 min after feeding, which was rice bread of 94.6% KOY brown rice flour mixed with 5.4% OKM rice brown (SRBBB) and 100% wheat flour bread. The animal feeding test was conducted with the formal approval of the Ethics Committee on Animal Care according to the “Guide for the Care and Use of Laboratory Animals” of the Faculty of Agriculture, Niigata University.
Enzyme-linked immunosorbent assay (ELISA) method
We used the enzyme-linked immunosorbent assay (ELISA) method with site-specific monoclonal antibodies to detect Aβ40 using a human/rat β Amyloid (40) Sandwich ELISA kit II (Wako Pure Chemical Industries, Ltd.). Blood plasma diluted with dilution buffer (4-fold dilution) was mixed with standard solution, and then set overnight in the refrigerator, before being washed five times. Horseradish peroxidase antibody standard product (100 μL) was then added, and the mixture was set in the refrigerator for 1 h, followed by washing five times. Finally, 100 μL of TMB (3,3′,5,5-tetramethylbenzidine) was added, and the mixture was incubated at room temperature for 0.5 h, covered with aluminum foil. Stop solution (100 μL) was added and absorbance was measured immediately at 450 nm using a fluorescent microplate reader (Grating Based Multimode Reader SH-9000: Corona Electric Co, Ltd.). Aβ40 content was calculated according to the calibration using a standard sample and test samples.
Statistical analyses
All of the results, including the significance of regression coefficients, were subjected to t-testing and one-way ANOVA using Excel Statistics (ver. 2006, Microsoft Corporation, Tokyo, Japan).
Results and discussion
Measurement of the fatty acid composition of rice bran
Rice bran is a potential fiber and mineral source for the nutritional enhancement of cereals. The germ and bran layer (about 10% of a rice kernel) removed during the milling procedure are rich in proteins, lipids (γ-oryzanol, ferulic acid, sterol, wax, ceramide, phytin, and inositol), fiber, minerals, tocotrienols, tocopherols, and B-complex vitamins (B1 and B6).Citation16,39) Extracted from rice bran, rice bran oil is a high-quality cooking oil with an excellent balance of fatty acids. It has higher oxidative stability than soybean or cottonseed oil.Citation40) Both animal and human studies have shown that rice bran oil lowers low-density lipoprotein (LDL) cholesterol levels as well as corn oil, and better than olive oil.Citation41,42) Fujiwara et al. showed that a high concentration of oleic acid in the diet had a lipid-lowering effect similar to that of a polyunsaturated fatty acid-rich diet.Citation43) Mattson et al. showed that oleic acid is as effective as linoleic acid in lowering LDL cholesterol levels, and oleic acid seemingly reduces HDL cholesterol levels less frequently than does linoleic acid.Citation44) The long-term intake of a high-oleic acid diet may, therefore, help to prevent atherosclerosis in its initial stages.
As shown in Table , the oleic acid (monounsaturated fatty acid) content of OKM (pigmented rice; 46.0%) rice bran was significantly higher than that of Koshihikari (41.2%) and KOY (44.2%) (p < 0.05). The stearic acid (saturated fatty acid) content of OKM (2.0%) rice bran was significantly higher than that of Koshihikari (1.5%) and KOY (1.6%) (p < 0.05). Nevertheless, the linoleic acid (polyunsaturated fatty acid) content of OKM (32.0%) rice bran was significantly lower than that of Koshihikari (36.7%) and KOY (34.1%) (p < 0.05). Moreover, the ratio of oleic acid to polyunsaturated fatty acid (i.e. linoleic acid + α-linolenic acid) is 1.09, 1.26, and 1.40 in Koshihikari, KOY, and OKM, respectively. As a result, OKM bran was significantly higher than Koshihikari at p < 0.05.Therefore, the rice bran of OKM may potentially be a biofunctional food with high antioxidant capacity.
Table 1. Fatty acid composition of rice bran.
Ferulic acid is used as an antioxidant and an antimicrobial agent. It is also recognized that ferulic acid exhibits a preventive effect on discoloration in various food products, and a variety of physiological functions such as suppression of Alzheimer’s disease, prevention of muscular fatigue, improvement of hypertension, and antitumor activity in the breast, liver, and colon.Citation16) As shown in Table , the ferulic acid content of KOY rice bran (1.6 mg) was significantly higher than that of Koshihikari rice bran (0.9 mg) and OKM rice bran (1.1 mg) at p < 0.05. Inositol (also called myoinositol) is a nutrient in the vitamin B complex group that the body needs in small amounts to function and stay healthy. Myoinositol helps cells to make membranes and respond to messages in their environment. It is water-soluble and must be consumed every day.Citation16) As shown in Table , the inositol content of Koshihikari rice bran (1.7 g) was significantly higher than that of KOY rice bran (1.1 g) and OKM rice bran (1.4 g) at p < 0.05.
Table 2. Components, inhibition of enzyme reaction, and anti-oxidative activities of rice bran.
β-secretase and acetylcholinesterase inhibitory activities
Functional foods which have α-glucosidase inhibitory activity are effective in controlling blood sugar levels in people at risk of developing diabetes.Citation29) Watanabe et al. showed that tochu-cha inhibits α-glucosidase activity,Citation45) and Matsui et al.Citation46) and Kim et al. showed that the α-glucosidase inhibitory activity of luteolin (a flavonoid) was very strong.Citation47) Moreover, Watanabe et al. showed that an α-glucosidase inhibitor such as acarbose has a beneficial effect on postprandial hyperglycemia and postprandial hyperinsulinemia in non-insulin dependent diabetes mellitus patients treated with conventional insulin therapy.Citation48) As shown in Table , the α-glucosidase inhibitory activity of OKM bran, given as an IC50 value, was 17.2 μg/μL, which was 0.8 times that of Koshihikari bran (IC50 of 22.0 μg/μL) and 0.9 times that in KOY bran (IC50 of 20.0 μg/μL). Therefore, the α-glucosidase inhibitory activity of the OKM rice bran was significantly higher than that of the Koshihikari rice bran and KOY rice bran (p < 0.05).
Pigmented rice contains naturally occurring colored substances that belong to the flavonoid group called anthocyanins. Tsuda et al. showed that anthocyanins extracted from purple corn prevent obesity and ameliorate hyperglycemia in mice.Citation49) Moreover, Suzuki et al. showed that the calcium content of pigmented brown rice was higher than that of the Koshihikari cultivar.Citation50) These experiments suggest that anthocyanins, as a functional food component, can aid in the prevention of obesity and diabetes.
BACE1 is the first protease in the process of converting APP into Aβ in the brain. Therefore, BACE1 is a major therapeutic target for the development of inhibitory drugs.Citation51) Abe et al. identified myoinositol hexaphosphate as a BACE1 inhibitory molecule in rice grain extract and digests.Citation26) As shown in Table , the BACE1 inhibition rate of OKM rice bran (61.4%) was significantly higher than that of Koshihikari rice bran (17.0%) and KOY rice bran (12.1%) (p < 0.01). Supplemental Figure 1 shows that phytic acid (reference material) inhibited 0.03 U/μL enzyme solution (10 μL) of BACE1 activity using fluorometry. The IC50 of phytic acid was 0.27 μg/μL (6.75 μg/well). The equation has a determination coefficient (R2) of 0.97. The BACE1 inhibition rate was converted to a phytic acid equivalent: Koshihikari rice bran (0.05 μg–PA eq./μL), KOY rice bran (0.02 μg–PA eq./μL), and OKM rice bran (0.39 μg–PA eq./μL). This shows that the BACE1 inhibitory activity of OKM (pigmented rice) rice bran was much stronger than that of Koshihikari (ordinary brown rice) bran and KOY (ae mutant rice) bran.
The leading Alzheimer’s disease therapeutics involve AChE inhibitors, resulting in increased acetylcholine concentrations in the synaptic cleft and enhanced cholinergic transmission.Citation52) Donepezil, galantamine, and tacrine are therapeutic AChE inhibitors used for the treatment of Alzheimer’s disease.Citation53) But, there are many possible side effects for the medicines, mild and moderate inhibitors are necessary to be developed. Pervin et al. showed that grape skin anthocyanin could be an excellent source of antioxidants and its inhibition of cholinesterase is of interest with regard to neurodegenerative disorders such as Alzheimer’s disease.Citation54) Vladimir-Knežević et al. showed that the Lamiaceae species are a rich source of various natural AChE inhibitors and antioxidants that could be useful in the prevention and treatment of Alzheimer’s disease and other related diseases.Citation55) As shown in Table , the AChE inhibition rate of KOY rice bran (2.4%)was significantly higher than that of Koshihikari rice bran (0.8%) (p < 0.05).
Supplemental Figure 2 shows that tacrine (the reference material) inhibited 0.007 U/μL of AChE activity. The IC50 of tacrine was 0.0916 ng/μL (2.29 ng/well). The equation has a determination coefficient (R2) of 0.99.
The AChE inhibition rate was converted to a tacrine equivalent: Koshihikari rice bran (0.018 ng–TCeq./μL), KOY rice bran (0.021 ng–TCeq./μL), and OKM rice bran (0.020 ng–TCeq./μL). This shows that the AChE inhibitory activity of KOY (ae mutant rice) rice bran and OKM rice bran were higher than Koshihikari (ordinary brown rice) rice bran.
High pressure treatment (HPT) is very useful for the food industry. In this study, we tried to develop functional materials, such as black rice bran of which AChE inhibitory activity was accelerated by the HPT, for example, its AChE inhibitory rate was 2.1 times that of the bran before HPT (data not shown).
Reactive oxygen species damage biological molecules, including proteins, lipids, and nucleic acids. Chronic and degenerative diseases such as cancer, heart disease, and Alzheimer’s disease are thought to be caused, in part, by reactive oxygen species produced by the body.Citation35,36) Many dietary compounds have been suggested to be important antioxidants; the evidence for a key role of vitamins E and C is strong, but that for carotenoids and related plant pigments is weaker.Citation56) In a study by Cazzola et al., aqueous and methanol extracts of dry sage, rosemary, basil, parsley, chili, garlic, and onion were analyzed to investigate their antioxidant and anti-glycant activities and their in vitro inhibitory potentials against enzymes involved in glycemic regulation.Citation57) As shown in Table , the lipophilic oxygen radical absorbance capacity (L-ORAC) of Koshihikari rice bran was 132.7 μmolTE/100 gFW), which was 1.3 times that of the OKM rice bran (102.2 μmolTE/100 gFW), and that of KOY rice bran (126.4 μmolTE/100 gFW) showed a similar tendency. The hydrophilic oxygen radical absorbance capacity (H-ORAC) of OKM rice bran was 1349.3 μmolTE/100 gFW, which was 4.2 times that of the Koshihikari rice bran (318.6 μmolTE/100 gFW) and 4.8 times that of the KOY rice bran (279.6 μmolTE/100 gFW). The H-ORAC of the OKM rice bran was significantly higher than that of the Koshihikari rice bran and the KOY rice bran (p < 0.01).
Component analysis and enzyme inhibition of feed
We prepared four kinds of feed for the mice. A: Rice bread of 100% KOY brown rice flour; B: Rice bread of 94.6% KOY brown rice flour mixed with 5.4% OKM rice brown (SRBBB); C: MF diet; D: MF diet mixed with 0.02% ferulic acid. As shown in Table , the L-ORAC value of the C feed was 115.4 μmolTE/100 gFW, which was 3.3 times that of the A feed (35.5 μmolTE/100 gFW), 2.9 times that of the B feed (40.0 μmolTE/100 gFW), and 1.1 times that of the D feed. Moreover, the H-ORAC value of the D feed (195.5.4 μmolTE/100 gFW) was 1.1 times that of the C feed (35.5 μmolTE/100 gFW), 2.0 times that of the B feed (40.0 μmolTE/100 gFW), and 3.6 times that of the A feed. The L-ORAC and H-ORAC values of the C and D feeds were significantly higher than those of the A and B feeds (p < 0.05). The L-ORAC value was positively correlated with the H-ORAC value (r = 0.95; p < 0.05).
Table 3. Component analysis, enzyme inhibition, and anti-oxidative activity of mouse feeds.
Resistant starch is starch that escapes digestion in the small intestine and that may be fermented in the large intestine. Four main subtypes of resistant starch have been identified based on their source or structure.Citation58,59) The cooked grains of ae mutant rice cultivars are hard and non-sticky because they lack starch branching enzyme IIb and SLC. They are promising in terms of their bio-functionality, such as diabetes prevention.Citation7–9) As shown in Table , the resistant starch content of the A feed was 4.9%, which was 9.8 times that of the D feed (0.5%), 6.1 times that of the C feed (0.8%), and 1.5 times that of the B feed: the resistant starch contents of the A and B feeds were significantly higher than those of the C and D feeds (p < 0.01). The H-ORAC value was negatively correlated with resistant starch content (r = 0.99; p < 0.01).
As shown in Table , the glutamic acid content of the C and D feeds was 32.0 mg/100 g and 33.0 mg/100 g, respectively. This was 4.8–4.9 times that in the A feed (6.7 mg/100 g), and 3.4–3.5 times that in the B feed (9.4 mg/100 g): the glutamic acid contents of the C and D feeds were significantly higher than those in the A and B feeds (p < 0.01).
As shown in Table , the polyphenol contents of the C and D feeds were 27.6 mg/100 g and 29.2 mg/100 g, which were 3.1–3.2 times that in the A feed (9.0 mg/100 g) and 1.7–1.8 times that in the B feed (16.7 mg/100 g): the polyphenol contents of the C and D feeds were significantly higher than those of the A and B feeds (p < 0.01 and p < 0.05, respectively). Resistant starch content was negatively correlated with glutamic acid content (r = 0.96; p < 0.05) and polyphenol content (r = −1.00; p < 0.01), and glutamic acid content was positively correlated with polyphenol content (r = 0.97; p < 0.05).
As shown in Table , the BACE1 inhibition rate of B was 8.1%, and those of A, C, and D were all 0%. Even after heating, the B feed showed very little BACE1 inhibitory activity.
As shown in Table , the AChE inhibition rates of A and B were 20.4 and 29.0%, respectively; 1.6–2.1 times that of the C feed (8.9%) and 1.5–1.9 times that of the D feed (9.3%). The AChE inhibition rates of the A and B feeds were significantly higher than those of the C and D feeds (p < 0.05 and p < 0.01, respectively). The AChE inhibition rates were converted to tacrine equivalents: A feed (0.048 ng–TCeq./μL), B feed (0.062 ng–TCeq./μL), C feed (0.031 ng–TCeq./μL), and D feed (0.031 ng–TCeq./μL). Even after heating, the AChE inhibitory activities of A and B were high.
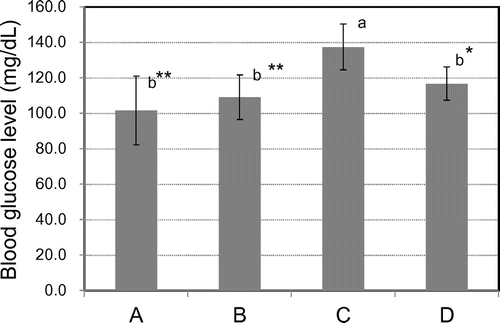
Determination of the initial BGL of aged mice kept for 12 weeks
Jung et al. showed that ferulic acid may be beneficial for the treatment of type 2 diabetes because it regulates BGLs by elevating glucokinase activity and production of glycogen in the liver.Citation60) The initial BGL after the fasting period of 24 h of aged mice after 12 weeks is shown in Fig. . A: Rice bread of 100% KOY brown rice flour; B: Rice bread of 94.6% KOY brown rice flour mixed with 5.4% OKM rice brown (SRBBB); C: MF diet; D: MF diet mixed with 0.02% ferulic acid. As shown in Fig. , the initial BGLs of mice fed the A, B, and D feeds were significantly lower than that of mice fed the C feed (p < 0.01 or p < 0.05), respectively. Initial blood glucose values of aged mice after 12 weeks were significantly correlated with resistant starch feed content (p < 0.01). The initial BGLs of mice kept for 12 weeks on SRBBB were correlated with resistant starch contents. In a previous study, we investigated the inhibition of abrupt increases in postprandial blood glucose after consuming boiled ae mutant rice (KOY) using Sprague–Dawley rats.Citation11,61) An article is also under submission describing a human study on single-dose administration of cooked KOY rice.
Fig. 1. The initial blood glucose level after the fasting period of 24 h of aged mice after 12 weeks.
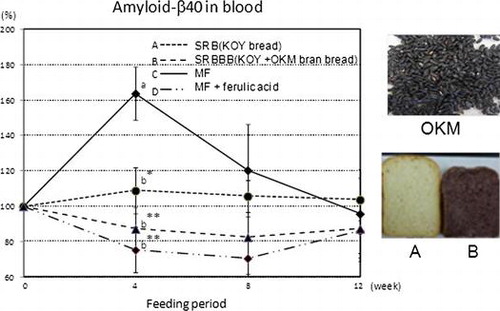
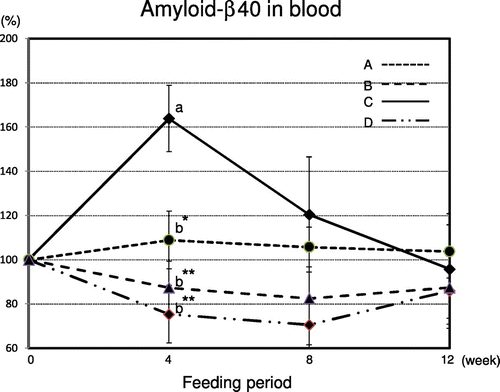
Characterization of amyloid β-40 protein species with the ELISA method
We took blood samples from aged mice after 0, 4, 8, and 12 weeks. We performed an ELISA assay with site-specific monoclonal antibodies to detect Aβ40. Figure A: (_ _ _) Rice bread of 100% KOY brown rice flour; B: (_ _) Rice bread of 94.6% KOY brown rice flour mixed with 5.4% OKM rice brown (SRBBB); C: (_) MF diet; D: (_ . .) MF diet mixed with 0.02% ferulic acid. As shown in Fig. , the Aβ40 species levels of aged mice after four weeks when fed the B and D feeds were significantly lower than those of mice fed the C feed (p < 0.01) and the A feed (p < 0.05). Moreover, the Aβ40 species levels of aged mice after eight weeks of receiving the A, B, and D feeds were lower than those in mice fed the C feed. The Aβ40 species levels of aged mice after four weeks were significantly correlated with resistant starch, glutamic acid, and polyphenol contents, and with AChE and BACE1 inhibitory activities (p < 0.01). The Aβ40 species levels of aged mice after eight weeks were significantly correlated with resistant starch content and BACE1 inhibitory activity (p < 0.01), and with glutamic acid and polyphenol contents, and AChE inhibitory activity (p < 0.05). The Aβ40 species levels of aged mice after 12 weeks were similar.
Fig. 2. Characterization of amyloid β-40 protein species with ELISA method.
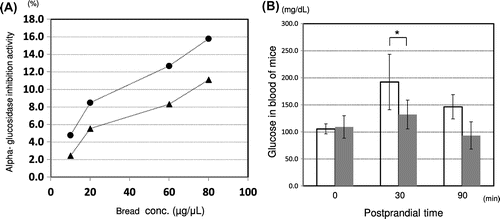
It can be seen that the Aβ40 species levels after 4–8 weeks were correlated with resistant starch content and BACE1 inhibitory activity. Moreover, the α-glucosidase inhibitory activity of rice bread of 94.6% KOY brown rice flour mixed with 5.4% OKM rice brown (SRBBB) was 1.5 times higher than that of 100% wheat flour bread (Fig. ), and that of postprandial BGL at 30 min was significantly lower than that for 100% wheat flour bread (p < 0.05) in aged mice (Fig. ). Tokutake et al. showed that naturally secreted Aβ induced hyperphosphorylation of Tau and impaired insulin signal transduction.Citation5)
Fig. 3. Comparison of SRBBB and control bread in alpha-glucosidase inhibitory activity and blood glucose.
Conclusions
As it has been reported that type2 diabetes increase the risk of Alzheimer disease, we investigated about the prevention of amyloidβ generation by foodstuff which is resistant to digestion. To find a neuroactive and slow-digestive compounds with a potent inhibitory effect on BACE1 and AChE from natural resources, we evaluated black rice bran and ae mutant brown rice.
| (1) | Rice bran from OKM has high ratio of oleic acid in fatty acid composition and it has higher BACE1 inhibitory activity and H-ORAC than the ordinary rice. | ||||
| (2) | KOY brown rice bread (bread mixed 94.6% KOY brown rice flour and 5.4% OKM rice bran) had high levels of resistant starch and inhibitory activities against BACE1 and AChE. | ||||
| (3) | After feeding aged mice, KOY brown rice bread showed significant lower Aβ40 in blood than ordinary rodent diet (p < 0.01). | ||||
Therefore, we propose KOY brown rice bread produced with black rice bran, which would be promising because of inhibitory activity against Aβ production and prevention of abrupt increase in postprandial blood glucose.
Author contributions
Nakamura S, Yamazaki A, Ikeuchi T, and Ohtsubo K designed the experiments; Nakamura S, Hara T, Joh T, Kobayashi A, Kasuga K performed experiments; Nakamura S wrote the paper, and Ohtsubo K commented on the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Cabinet Office, Government of Japan (SIP), The Ministry of Education, Culture, Sports, Science and Technology (Grant-in-aid for scientific research (B)).
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.otg/10.1080/09168451.2016.1240605.
TBBB_1240605_Supplemental_Material.ppt
Download MS Power Point (173.5 KB)Acknowledgment
A part of this research was supported by a Grant in Aid for Scientific Research B (15H02891), MEXT Japan, and Grant for Cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for creating next-generation agriculture, forestry and fisheries”, The Council for Science, Technology and Innovation (CSTI). We express our gratitude to Dr Hideo Maeda for kind gift of super hard rice, Konayukinomai as a sample and Dr Masatoyo Nishizawa for his precious comments on the research
Notes
Abbreviations: ae mutants, amylose extender mutants of rice; Aβ, amyloid β protein; AChE, acetylcholinesterase; BACE1, β-secretase; KOY rice, Konayukinomai rice cultivar; OKM rice, Okunomurasaki rice cultivar; PA, Phytic acid; TC, Tacrine.
References
- Rissman RA, Trojanowski JQ, Shaw LM, et al. Longitudinal plasma amyloid beta as a biomarker of Alzheimer’s disease. J Neural Transm. 2012;119:843–850.10.1007/s00702-012-0772-4
- Ohara T, Doi Y, Ninomiya T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama Study. Neurology. 2011;77:1126–1134.10.1212/WNL.0b013e31822f0435
- Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Brain Stimulation. 2006;5:64–74.
- Kasuga K, Kaneko H, Nishizawa M, et al. Generation of intracellular domain of insulin receptor tyrosine kinase by gamma-secretase. Biochem Biophys Res Commun. 2007; 17;360:90–96.10.1016/j.bbrc.2007.06.022
- Tokutake T, Kasuga K, Yajima R, et al. Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent AKt-GSK3β signaling pathway. J Biol Chem. 2012;287:35222–35233.10.1074/jbc.M112.348300
- Nishi A, Nakamura Y, Tanaka N, et al. Biochemical and genetic analysis of the effects of amylase-extender mutation in rice endosperm. Plant Physiol. 2001;127:459–472.10.1104/pp.010127
- Nakamura S, Satoh H, Ohtsubo K. Palatable and bio-functional wheat/rice products developed from pre-germinated brown rice of super-hard cultivar EM10. Biosci Biotechnol Biochem. 2010;74:1164–1172.10.1271/bbb.90850
- Nilsson AC, Ostman EM, Granfeldt Y, et al. Effect of cereal test breakfast differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects 1, 2, 3. Am J Clin Nutr. 2008;87:645–654.
- Henry CJ, Lightowler HJ, Tydeman EA, et al. Use of low-glycaemic index bred to reduce 24-h blood glucose: implications for dietary advice to non-diabetic and diabetic subjects. J Food Nutr. 2006;57:273–278.
- Nakamura S, Nakano Y, Satoh H, et al. Improved palatability and bio-functionality of super-hard rice by soaking in a barley- koji miso suspension. Biosci Biotechnol Biochem. 2013;77:2419–2429.10.1271/bbb.130528
- Nakamura S, Ohtsubo K. Improvement of palatability and inhibition of abrupt increase in postprandial blood glucose level by the boiled rice after soaking with functional food ingredients. J Appl Glycosci. 2015;62:53–63.10.5458/jag.jag.JAG-2014_014
- Yang CZ, Shu XL, Zhang LL, et al. Starch properties of mutant rice high in resistant starch. J Agric Food Chem. 2006;54:523–528.10.1021/jf0524123
- Goddard M, Yong G, Marcus R. The effect of amylose content on insulin and glucose responses to ingested rice. Am J Clin Nutr. 1984;39:388–392.
- Kang HJ, Wang IKH, Kim KS, et al. Comparative strecture and physicochemical properties of Ilpumbyeo, a high-quality Japonica rice, and its mutant, Suwon 464. J Agric Food Chem. 2003;51:6598–6603.10.1021/jf0344946
- Kimura T. Development of mulberry leaf product with α- glucosedase inhibitor for the prevention of type 2 diabetes mellitus. Nippon Shokuhin Kagaku Kogaku Kaishi. 2010;57:57–62.10.3136/nskkk.57.57
- Taniguchi H, Hashimoto H, Hosoda A, et al. Functionality of compounds contained in rice bran and their improvement. Nippon Shokuhin Kagaku Kogaku Kaishi. 2012;59:301–318.10.3136/nskkk.59.301
- Plat J, Mensink RP. Plant stanol and sterol esters in the control of blood cholesterol levels: mechanism and safety aspects. Am J Cardiol. 2005;96:15–22.10.1016/j.amjcard.2005.03.015
- Ha TY, Han S, Kim SR, et al. Bioactive components in rice bran oil improve lipid profiles in rats fed a high—cholesterol diet. Nutr Res. 2005;25:597–606.10.1016/j.nutres.2005.05.003
- You Y, Park J, Yoon HG, et al. Stimulatory effects of ferulic acid on endurance exercise capacity in mice. BiosciBiotechnol Biochem. 2009;73:1392–1397.10.1271/bbb.90062
- Kozuka C, Yabiku K, Sunagawa S, et al. Brown rice and its component, γ Oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes. 2012;61:3084–3093.10.2337/db11-1767
- Parthasarathy S, Khoo JC, Miller E, et al. Low density lipoprotein rich in oleic acid is protected against oxidative modification: implications for dietary prevention of atherosclerosis. Proc. Natl. Acad. Sci. USA.1990;87:3894–3898.10.1073/pnas.87.10.3894
- Matsuoka K. Study on mechanism of inhibitive cholesterol absorption by plant sterol/stanols. Jpn Oil Chem Soc. 2011;11:119–125.
- Yamauchi A. Dietary fermented brown rice attenuates the level of blood sugar and minimizes insulin secretion in rats. J Jpn Soc Nutr Food Sci. 2012;65:271–276.10.4327/jsnfs.65.271
- Abe T, Taniguchi M. Identification of myo-inositol hexakisphosphate (IP6) as a β-secretase 1(BACE1) inhibitory molecule in rice grain extract and digest. FEBS Open Bio. 2014;4:162–167.10.1016/j.fob.2014.01.008
- Sutharut J, Sudarat J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int Food Res J. 2012;19:215–221.
- Ling WH, Cheng QX, Ma J, et al. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J Nutr. 2001;131:1421–6.
- Iso H. Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb. 2010;18:83–88.
- Sofi F, Macchi C, Abbate R, et al. Effectiveness of the mediterranean diet: can it help delay or prevent Alzheimer’s disease? J Alzheimers Dis. 2010;20:795–801.
- Yamaki K, Mori Y. Evaluation of α-glucosidase inhibitory activity in colored foods: a trial using slope factors of regression curves. Nippon Shokuhin Kagaku Kogaku Kaishi. 2006;53:229–231.10.3136/nskkk.53.229
- Ellman GL, Courtney KD, Andres VJ, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95.10.1016/0006-2952(61)90145-9
- Augustinsson KB, Eriksson H. Effects of two disulphides on cholinesterase activity in the spectrophotometric assay. Biochem J. 1974;139:123–127.10.1042/bj1390123
- Dawson RM. The effect of some amine oxides and disulphide compounds on the activity of acetylcholinesterase. Biochem J. 1975;149:293–295.10.1042/bj1490293
- Prior RL, Hoang H, Gu L, et al. Assay for hydrophilic and lipophilic antioxidant capacity(oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–3279.10.1021/jf0262256
- Ito M, Ohara E, Kobayashi A, et al. Antioxidant capacities and polyphenol content of colored rice cultivars. Nippon Shokuhin Kagaku Kogaku Kaishi. 2011;58:576–582.10.3136/nskkk.58.576
- Watanabe J, Oki T, Takebayashi J, et al. Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for the determination of antioxidant capacities of antioxidant solutions and food extracts. Anal Sci. 2012 Feb;28:159–165.10.2116/analsci.28.159
- Watanabe J, Oki T, Takebayashi J, et al. Improvement of the lipophilic-oxygen radical absorbance capacity (L-ORAC) method and single-laboratory validation. Biosci Biotechnol Biochem. 2013;77:857–859.10.1271/bbb.120786
- Folin O, Denis W. A colorimetric method for the determination of phenols (and phenol derivatives) in purine. J Biol Chem. 1915;22:305–308.
- Nakamura S, Suzuki K, Ohtsubo K. Characteristics of bread prepared from wheat flour blended with various kinds of newly developed rice flours. J Food Sci. 2009;74:E121–E130.10.1111/jfds.2009.74.issue-3
- BRAN of Rice. Rice, chemistry and technology. St. Paul (MO): American Association of Cereal Chemists; 2004; p. 480.
- Rice Bran Oil of Rice. Rice, chemistry and technology. St. Paul (MO): American Association of Cereal Chemists; 2004; p. 481.
- Kahlon TS, Saunders RM, Sayre RN, et al. Cholesterol-lowering effects of rice bran and rice bran oil fractions in hypercholesterolemic hamsters. Cereal Chem. 1992;69:485–489.
- Spritz N, Mishkel MA. Effects of dietary fats on plasma lipids and lipoproteins: an hypothesis for the lipid–lowering effect of unsaturated fatty acids. J Clin Invest. 1969;48:78–86.10.1172/JCI105976
- Fujiwara Y, Satsuka A, Matsumoto A, et al. Effect of a diet in high oleic acid on plasma lipids in guinea pigs and humans. Jpn Soc Home Econ. 2005;56:41–49.
- Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26:194–202.
- Watanabe J, Kawabata J, Kurihara H, et al. Isolation and identification of α-glucosidase inhibitors from tochu-cha (Eucommia ulmoides). Biosci Biotechnol Biochem. 1997;61:177–178.
- Matsui T, Kobayashi M, Hayashida S, et al. Luteolin, a flavones, does not suppress postprandial glucose absorption through an inhibition ofα-glucosidase action. Biosci Biotechnol Biochem. 2002;66:689–692.10.1271/bbb.66.689
- Kim JS, Kwon CS, Son KH. Inhibition of α-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64:2458–2461.10.1271/bbb.64.2458
- Watanabe N, Fukumoto Y, Namba M, et al. J Beneficial effect of an α-glucosidase inhibitor combined with conventional insulin therapy in non-insulin-dependent diabetics. Jpn Diab Soc. 1996;39:679–686.
- Tsuda T, Horio F, Uchida K, et al. Dietary cyaniding 3-O-beta-D-glucoside—rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–2130.
- Suzuki M, Kataoka T, Ohtsubo K. Variation in the mineral content of 8 cultivars of pigmented brown rice. Nippon Shokuhin Kagaku Kogaku Kaishi. 2014;61:427–432.10.3136/nskkk.61.427
- Arun K, Margherita B, Jordan T. Developing β-secretase inhibitors for treatment of Aizheimer’s disease. J Neurochem. 2012;120:71–83.
- Martorana A, Esposito Z, Koch G. Beyond the cholinergic hypothesis: do current drugs work in Alzheimer’s disease? CNS Neurosci Ther. 2010;16:235–245.
- Takatori Y. Mechanisms of neuroprotective effect of therapeutic accetylcholinesterase inhibitors used in treatment of Alzheimer’s disease. Yakugaku Zasshi. 2006;126:607–616.10.1248/yakushi.126.607
- Pervin M, Hasnat MA, Lee YM, et al. Antioxidant activity and acetylcholinesterase inhibition of grape skin anthocyanin (GSA). Molecules. 2014;19:9403–9418.10.3390/molecules19079403
- Vladimir-Knežević S, Blažeković B, Kindl M, et al. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the lamiaceae family. Molecules. 2014;19:767–782.10.3390/molecules19010767
- Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–50.10.1146/annurev.nu.16.070196.000341
- Cazzola R, Camerotto C, Cestaro B. Anti-oxidant, anti-glycant, and inhibitory activity against α-amylase and α-glucosidase of selected spices and culinary herbs. J Food Sci Nutr. 2011;62:175–184.
- Englyst HN, Kingmen SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50.
- Englyst HN, Cummings JH. Digestion of the polysaccharides of some cereal foods in the human small intestine. J Clin Nutr. 1985;42:778–787.
- Jung EH, Kim SR, Hwang IK, et al. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/mice. J Agric Food Chem. 2007;55:9800–9804.10.1021/jf0714463
- Maeda S, Kazama Y, Kobayashi A, et al. Improvement of palatability and prevention of abrupt increases in postprandial blood glucose level by Hokurikukona243 after high pressure treatment. J Appl Glycosci. 2015;62:127–134.10.5458/jag.jag.JAG-2015_013

