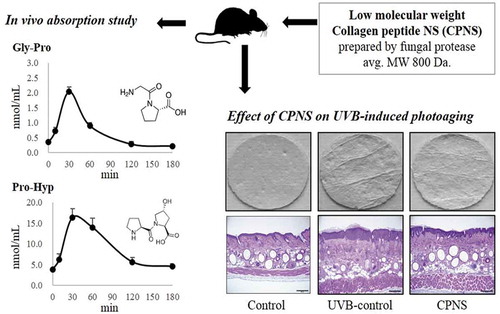
ABSTRACT
Collagen hydrolysate is a well-known nutritional supplement for the improvement of healthy skin. Here, collagen peptide NS (CPNS) from fish scale was prepared, and its physicochemical properties were investigated. Gly-Pro was revealed as a representative low molecular weight peptide of CPNS, by performing prep-HPLC and LC-MS/MS. CPNS treatment attenuated matrix metalloproteinase-1 production and increased the synthesis of type 1 procollagen in HDF cells. After orally administering CPNS to rats, the plasma concentrations of Gly-Pro and Pro-Hyp increased dramatically. To examine the protective effects of CPNS against ultraviolet B (UVB)-induced photoaging in vivo, the dorsal skins of hairless mice were exposed to UVB and supplemented with CPNS for 12 weeks. The CPNS consumption significantly attenuated UVB-induced wrinkle formation, transepidermal water loss, and epidermis thickness, and increased skin hydration. Collectively, these results suggest that bioactive peptides of CPNS, Gly-Pro and Pro-Hyp, exert beneficial effects on skin health.
Graphical abstract_V1
Low molecular weight collagen peptides, Gly-Pro and Pro-Hyp, effectively prevent skin aging.
Several factors, including nutrition deficiency, photoaging, hormonal disorders, and environmental factors, affect skin appearance and integrity [Citation1]. Besides topical treatments, dietary supplements, such as collagen, have emerged as a new strategy to delay skin aging and improve skin health [Citation2]. Matrix metalloproteinases (MMPs) are zinc-containing endopeptidases that are well known to degrade virtually every component of the extracellular matrix [Citation3,Citation4]. Although MMPs are expressed in mammalian skin, MMP-1 is considered as a principal matrix enzyme which cleaves fibrillar type 1 and 3 collagen [Citation3,Citation5,Citation6]. Since collagen is one of the major constituents of connective tissues, collagen deficiency is considered the distinct feature of skin aging. Type 1 collagen is the main structural protein in the dermis and forms large, eosinophilic fibers known as collagen fibers [Citation7].
Gelatin- and collagen-based products have been extensively researched and are used in many sectors of the cosmetics, foods, medicines, and bioengineering industries [Citation8]. Gelatin is a water-soluble, high molecular weight polymer derived from partially hydrolyzed collagen that is often used as a delivery vehicle for the controlled release of bioactives. When gelatin is degraded, for example, by using specific enzymes, the resultant mixture of various small peptides is termed collagen hydrolysate. There is increasing scientific evidence that demonstrates the efficacy of collagen hydrolysate to benefit skin parameters related to cutaneous physiology and aging, such as transepidermal water loss (TEWL), elasticity, and wrinkles [Citation9], as well as to improve glucose tolerance [Citation10], wound healing [Citation11,Citation12], and joint pains [Citation13]. It has been shown that collagen peptides exert stimulatory effects on type 1 collagen and other extracellular matrix molecules in human fibroblasts [Citation14]. Moreover, in vivo studies have demonstrated that the administration of collagen peptides increases the density and diameter of fibroblasts and collagen fibrils [Citation7]. Although the efficacy of collagen hydrolysates to improve skin conditions has been demonstrated, little is known about the functions and physiological effects of bioactive peptides from collagen hydrolysates [Citation15,Citation16].
Bioactive peptides of collagen hydrolysates are defined as specific short chains of amino acid monomers combined by peptide bonds that have a positive impact on body functions and may regulate the human system [Citation17]. For instance, Gly-Pro-Hyp interacts with platelets [Citation18,Citation19] and the central nervous system [Citation20]. Pro-Hyp dipeptide stimulates cellular proliferation, hyaluronic acid synthesis [Citation21], and joint cartilage protection [Citation22]. Furthermore, pharmacological bioavailability trials have revealed that two types of collagen dipeptides, Pro-Hyp and Hyp-Gly, are available at high concentrations in the human blood after oral administration of collagen hydrolysate, suggesting that they play an important role in skin health [Citation23–Citation25]. In addition, Yamamoto et al. [Citation26] reported that small but significant amounts of Ala-Hyp, Ala-Hyp-Gly, Pro-Hyp-Gly, Leu-Hyp, Ile-Hyp, and Phe-Hyp are also present in the blood after oral ingestion of collagen hydrolysate. Among the bioactive peptides of collagen hydrolysates, Gly-Pro is often a repeated sequence of collagen and is produced by enzymatic degradation of Gly-Pro containing peptides (Gly-Pro-X). Gly-Pro is exceptionally resistant to intestinal proteolytic enzymes and degradation by enterocytes, leading to its partial absorption into the bloodstream [Citation27,Citation28]. The dipeptide has also shown antihypertensive activity following gastrointestinal digestion [Citation29]. However, specific receptors for Gly-Pro have not been characterized, and the effectiveness of Gly-Pro- or Gly-Pro-containing collagen hydrolysates on skin health have not been well investigated [Citation30].
The molecular weight and peptide composition of hydrolyzed collagen are two of the most important factors influencing the functional properties of collagen hydrolysate. Enzymatic hydrolysis is preferred over chemical hydrolysis to produce collagen hydrolysate, and various commercial proteases have been used for this purpose. The type of proteases used in the process has a substantial impact on the physicochemical properties of the collagen hydrolysate. In this study, we investigated the physicochemical properties of collagen hydrolysate, prepared by hydrolysis with specific multiple fungal proteases and studied its absorption and beneficial effects on skin health in vitro and in vivo.
Materials and methods
Preparation of collagen hydrolysate
Collagen peptide NS (CPNS) used in this study was prepared by Nong Shim Co., Ltd. (Seoul, Korea). Briefly, CPNS was prepared derived from fish scale collagens, which were hydrolyzed with multiple fungal proteases (Amano Enzyme Inc., Nagoya, Japan). The average molecular weight of CPNS was determined by gel permeation chromatography on an Ultra-hydrogel 120 column (Waters, Milford, MA, USA), using high-performance liquid chromatography (HPLC; Agilent 1200 series, Waldbronn, Germany). CPNS was dissolved in distilled water at 10 mg/mL, serially diluted, and then filtered to remove debris. The chromatographic separation was achieved at a flow rate of 0.5 mL/min for 60 min, with isocratic 45% acetonitrile containing 0.1% trifluoroacetate. Elution was monitored by absorbance at 220 nm. Isolation of collagen peptides was performed using a dual-piston pump preparative high-pressure liquid chromatography system (prep-HPLC, Gilson, Inc., Middleton, USA). The apparatus comprised Gilson 331/332 HPLC pumps, a GX-281 liquid handler, Prep ELSTM II detector, and a 155-UV-VIS detector. A Jupiter® 4 μm Proteo 90 Å LC column (250 × 212 mm) was applied for the major component isolation. The content of standard peptides, such as Gly-Pro, Pro-Hyp, and Gly-Pro-Hyp, was evaluated by LC-MS/MS (Thermo Fisher Scientific, Waltham, USA). Synthetic peptides (Gly-Pro, Pro-Hyp, and Gly-Pro-Hyp) were purchased from Anygen Co. Ltd. (Seoul, Korea).
Determination of matrix metalloproteinase-1 (MMP-1) production and type 1 procollagen synthesis
Human dermal fibroblast (HDF) cells were isolated from neonatal foreskins and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Paisley, UK) supplemented with 10% fetal bovine serum (Gibco-BRL), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco-BRL), in a 5% CO2-humidified incubator. The cells were exposed to ultraviolet B (UVB) irradiation (25 mJ/cm2), using a UVB cross-linker (6 × 8 W, 312 nm, Model CL-508M, Vilber Lourmat, Paris, France). After UVB irradiation, the cells (1 × 104 cells/well) were treated with CPNS (50 to 500 μg/mL), or Gly-Pro, Pro-Hyp or Gly-Pro-Hyp (50 and 100 μg/mL) for 24 h. In addition, HDF cells were treated with CPNS (50 to 500 μg/mL), or Gly-Pro, Pro-Hyp or Gly-Pro-Hyp (50 and 100 μg/mL) for 24 h. After incubation, the production of MMP-1 and type 1 procollagen was determined using commercial ELISA kits from Takara Biomedical (Shiga, Japan) and Amersham Life Science (Buckinghamshire, UK), respectively, according to the manufacturer’s instructions. CPNS-induced cytotoxicity was examined by the 3-(4,5-desethyithiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
In vivo absorption study
For the absorption of CPNS in the plasma, in vivo experiments were carried out according to a protocol described previously [Citation31]. Although previous reports demonstrated that purified collagen peptides with the concentration of 300 to 500 mg/kg/day were used for the absorption assay [Citation31,Citation32], a higher concentration of CPNS (1,000 mg/kg) was used for the absorption assay because CPNS was not further purified. Eight-week-old Sprague-Dawley rats were purchased from Samtaco Co. (Osan, Korea) and housed in a cage room maintained at a constant temperature of 23 ± 3°C, humidity of 55 ± 5%, and 12-h light-dark cycle. All rats were given access to food and water ad libitum. Each group of rats (n = 5) was orally administered with 1,000 mg/kg of CPNS suspended in saline. Blood was collected under anesthesia, through a small excision at the tip of the tail at 0, 10, 30, 60, 120, and 180 min, after CPNS administration. Blood samples were centrifuged to obtain plasma and then deproteinized using 5% (v/v) TCA, followed by centrifugation (13,500 rpm at 4°C for 5 min). Deproteinized plasma was analyzed for the concentrations of Gly-Pro, Pro-Hyp, and Gly-Pro-Hyp by using HPLC-MS/MS. These experiments were conducted according to the guidelines of the Committee on the Care and the Use of Laboratory Animals (17-R633) of Chemon, Inc. (Suwon, Korea).
HPLC-MS/MS (multiple reaction monitoring, MRM) analysis
Bioactive peptides in rat plasma were determined by LC-MS/MS, as described by Yamamoto et al. [Citation31] with a slight modification. Plasma samples (1 µL) were injected into a Shimadzu LC20AD, equipped with a Sciex API 4000 MS and a Luna HILIC column (150 × 3 mm, 3 µm) (Phenomenex, Torrance, CA, USA). The HPLC gradient elution was performed using a mixture of two different solvents: 10 mM ammonium formate in distilled water (solvent A) and 0.1% v/v formic acid (FA) in acetonitrile (solvent B). Peptides were eluted under the following condition of solvent A: 0.0–0.1 min, 10%; 0.1–1.5 min, 10–40%; 1.5–3.0 min, 40%; 3.0–3.1 min, 40–10%; 3.1–6.0 min, 10%, returning to the initial condition. The flow rate was 0.4 mL/min, and the column temperature was 40°C. The MS analytical conditions used were as follows: positive ion electrospray (ESI) mode at a capillary voltage of 5.5 kV, desolvation voltage of 10 V, curtain gas (nitrogen) at 12 l/h, 10 psi, and 600°C. The MRM acquisition mode was used, at a nitrogen gas pressure of 60 psi and collision voltage of 65 V. All data were acquired and processed using Analyst software (version 1.6.2; AB Sciex, Way-Carlsbad, USA).
Treatment with CPNS to UVB-irradiated mice
To investigate the protective effects of CPNS against UVB-induced photoaging in vivo, eight-week-old female hairless mice (CriOri:SKH1-Hrhr; Orient Bio, Inc., Seongnam, Korea) weighing 25–30 g were acclimatized for 1 week in the animal facility before the experiments and housed as described above. The animal experiments were approved by the Aestura Institutional Animal Care and Use Committee (IACUC16-047). Forty female hairless mice were randomly divided into four groups; normal group (control), UVB-irradiated group (UVB-control), UVB-irradiated and 300 mg/kg/day CPNS (300 CPNS), and UVB-irradiated and 500 mg/kg/day CPNS group (500 CPNS). The doses were determined on the basis of the results from previous studies [Citation33,Citation34]. Each group (n = 10) was housed in a cage, and all mice were given access to food and water ad libitum. Food consumption and body weight were recorded during the experimental periods. The mice of the UVB-irradiated group were exposed to a UVB lamp (Waldmann UV800, Villingen-Schwenningen, Germany) three times a week. The intensity of irradiation was gradually increased to afford the total dose of 126 minimal erythema dose (MED) over 12 weeks. The starting dose of UVB irradiation was 55 mJ/cm2 (equivalent to 1 MED) during the first week, and the dose was increased weekly by 1 MED until reaching 4 MED, which was maintained until 12 weeks. The mice were administered saline as a vehicle or CPNS via oral gavage for 12 weeks. After 12 weeks, mice were sacrificed under anesthesia by intraperitoneal injection of sodium pentobarbital (Entobar®, Hanlim Pharm Co., Yongin, Korea).
Evaluation of skin wrinkle formation
At the end of the experiment, skin replicas were made from the back skin of anesthetized hairless mice, using a Silflo impression material (Flexico Developments Ltd., Hertfordshire, UK). Photos were obtained with a CCD video camera, and a Visioline® VL 650 (Quantirides®; Courage & Khazaka, Cologne, Germany) device was used to assess the skin surface wrinkles.
Evaluation of TEWL and skin hydration
The TEWL, as a measure of epidermal skin barrier function, was evaluated by using a Delfin Vapometer® (Delfin Technologies Ltd., Kuopio, Finland), and stratum corneum (SC) hydration was determined by using a MoistureMeterSC® (Delfin Technologies Ltd., Kuopio, Finland). The measurements were conducted 48 h after the final irradiation, to allow recovery from the acute UV effect.
Histological analysis
At 48 h after the final irradiation, the mice were sacrificed, and biopsies were obtained from the central dorsal skin, perpendicular to the long axis of the trunk. The biopsies were fixed in 10% buffered formalin and prepared for optical microscopy. Hematoxylin and eosin staining was conducted for a routine examination of the tissue and to measure the epidermal and dermis thicknesses. Two contiguous histological sections, each photographed at the midpoint of the section, were quantitatively analyzed. The epidermal thickness per tissue section was determined from 10 representative sample measurements.
Statistical analysis
The results are presented as mean ± standard deviation. Statistical analysis was performed using SPSS 12.0. Data were analyzed by t-test and one-way ANOVA with Duncan’s multiple range tests, respectively, and P < 0.05 was accepted as statistically significant.
Results and discussion
Molecular weight and main peptide identification
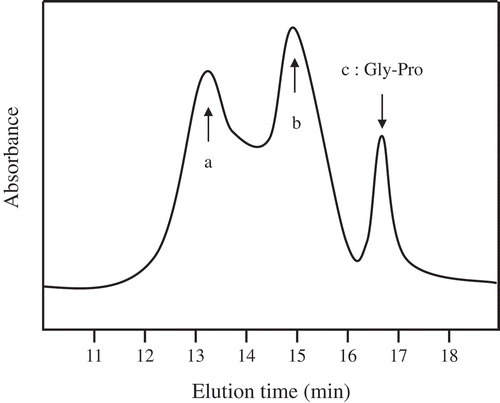
Gel permeation chromatography was utilized to examine the molecular size distribution of CPNS (). The CPNS is mainly constituted with dipeptides, a substantial quantity of longer oligopeptides, and a small fraction of amino acids. The average molecular weight of the CPNS was approximately 800 Da. The predominant collagen peptides of CPNS were isolated by using prep-HPLC. The low molecular weight peptide fractions below 500 Da were analyzed by LC-MS/MS to identify the specific peptide sequences. As a result, Gly-Pro dipeptide was revealed as a major constituent, whereas the other peptides were present in minor quantities. Prevalence of Gly-Pro in CPNS by LC-MS/MS analysis indicated approximately 4%. Moreover, other peptides, such as Gly-Gly, Pro-Ala, Pro-Ser, Phe-Hyp, Val-Pro, Gly-Pro-Pro, Gly-Pro-Arg, Gly-Pro-Gln, Gly-Pro-Ser, Gly-Pro-Gly-Pro, Ala-Phe-Val-Pro, Gly-Pro-Ser-Gly-Pro, was revealed as <0.3% in the low molecular weight peptides (data not shown). Enzymatically degraded collagen hydrolysates, including specific peptide profiles (Pro-Hyp and Gly-Pro-Hyp), have been identified as potential health food ingredients with several biological activities [Citation9]. Commercially marketed collagen hydrolysates, including Gly-Pro-Hyp, are usually manufactured by using bacterial collagenase-type proteases. Interestingly, we obtained low molecular collagen hydrolysates by using a cocktail of fungal peptidases to increase the quantity of bioactive peptides and improve the organoleptic quality of collagen hydrolysates. Unlike other developed collagen hydrolysates, the treatment with fungal proteinases increased the portion of low molecular weight collagen peptides containing bioactive peptides, and Gly-Pro dipeptide was revealed as a main peptide of CPNS.
Figure 1. Gel permeation chromatography profile of CPNS. CPNS prepared from fish scale was analyzed using an Ultra hydrogel column, and elution monitored by absorbance at 220 nm. Arrow indicates elution positions of the standard peptide. (a) peak of avg. MW 650 peptides, (b) peak of avg. MW 1,500 peptides, (c) peak of Gly-Pro dipeptide.
CPNS regulates the level of MMP-1 and type 1 procollagen in UVB-irradiated HDF cells
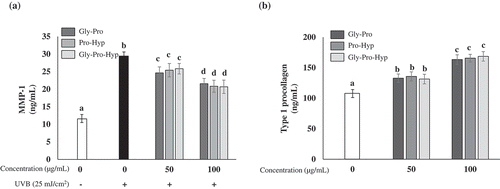
First, the effect of CPNS on the viability of HDF cells was detected by MTT assay. The viability of HDF cells was not significantly altered by CPNS at up to 500 μg/mL (). Since UV irradiation is responsible for the induction and activation of MMP-1 leading to the degradation of type 1 collagen in human skin [Citation3,Citation35], the influence of CPNS on the level of MMP-1 production in the culture supernatants from UVB-irradiated HDF cells was also determined. UVB irradiation significantly induced MMP-1 production, while CPNS significantly decreased the level of MMP-1 detected in a dose-dependent manner (). Furthermore, the 50 μg/mL CPNS treatment considerably increased the synthesis of type I procollagen in HDF cells and the increase was further augmented by CPNS at up to 500 μg/mL (). To examine whether bioactive peptides of CPNS regulate the levels of MMP-1 production and type 1 procollagen synthesis, HDF cells were treated with Gly-Pro, Pro-Hyp or Gly-Pro-Hyp (50 and 100 μg/mL) for 24 h. Gly-Pro, Pro-Hyp and Gly-Pro-Hyp significantly reduced MMP-1 production in UVB-irradiated HDF cells (). In contrast, they induced the production of type 1 procollagen in HDF cells at 50 and 100 μg/mL (). It is well known that Hyp-containing peptides, such as Pro-Hyp and Gly-Pro-Hyp, stimulate hyaluronic acid synthesis and cell proliferation in dermal fibroblasts [Citation21]. In contrast, the capacity of Gly-Pro to promote synthesis of MMP-1 and type 1 procollagen in dermal fibroblast cells has not been well elucidated. Our findings showed that the CPNS-derived peptide Gly-Pro plays an important role in the prevention of skin aging, similar to other bioactive peptides, such as those containing Hyp.
Figure 2. Effects of CPNS on the level of MMP-1 production and type 1 procollagen synthesis in UVB-irradiated HDF cells (25 mJ/cm2). (a) Effect of CPNS on HDF cell viability was determined by MTT assay (b) UVB-induced MMP-1 (ng/mL) concentrations, and (c) type 1 procollagen (ng/mL) concentrations were determined by ELISA. Symbols (-) and (+) designate the two control groups (unexposed and exposed to UVB, respectively).
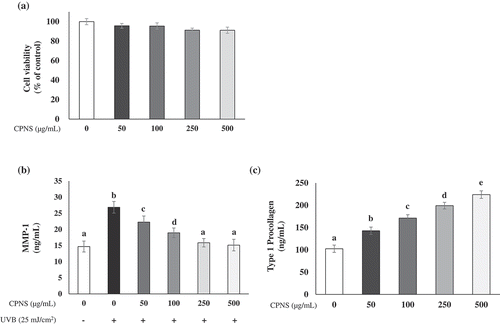
Figure 3. Effects of Gly-Pro, Pro-Hyp and Gly-Pro-Hyp on the level of MMP-1 production and type 1 procollagen synthesis in HDF cells. (a) UVB (25 mJ/cm2)-induced MMP-1 (ng/mL) concentrations, and (b) type 1 procollagen (ng/mL) concentrations were determined by ELISA. Symbols (-) and (+) designate the two control groups (unexposed and exposed to UVB, respectively).
Concentration of collagen peptides in the plasma after CPNS administration
Next, we performed LC-MS/MS to quantitate Gly-Pro, Pro-Hyp, and Gly-Pro-Hyp in the plasma of rats after oral administration of CPNS. The concentration of Gly-Pro in the plasma was rapidly increased within 30 min and gradually decreased thereafter (). Similar to Gly-Pro, Pro-Hyp dipeptide rapidly increased and reached maximum concentrations (Cmax) within 30 min. The Cmax of Pro-Hyp exceeded 16.3 ± 2.14 nmol/mL. In addition, the area under the curve (AUC) for Pro-Hyp (1,619.18 ± 271.62 min∙nmol/mL) was the highest compared to the other peptides, Gly-Pro and Gly-Pro-Hyp (240.28 ± 24.46 and 98.41 ± 13.58 minnmol/mL, respectively). The concentration then decreased gradually, reaching the lowest levels at 180 min after oral administration of CPNS. However, a small amount of Pro-Hyp remained at 180 min (). In contrast to Gly-Pro and Pro-Hyp, the concentration of Gly-Pro-Hyp did not significantly increase during treatment (up to 180 min) (). A previous report showed that intraperitoneal administration with collagen peptides containing Gly-Pro-Hyp and Gly-Pro-Ala resulted in the increased concentration of Gly-Pro-Hyp and Gly-Pro-Ala in the plasma of rats [Citation31]. However, unlike the previous study, Gly-Pro-Ala was not detected in CPNS in this study, presuming that only Gly-Pro-Hyp, but not Gly-Pro-Ala, was transiently observed in the plasma. Furthermore, these results suggest that Pro-Hyp was the most abundant peptide detected in the plasma after the oral administration of CNPS. Our study found that Gly-Pro was a major peptide (4%) in the CPNS while other peptides, such as Pro-Hyp and Gly-Pro-Hyp, were not detected. It has been previously verified that high concentrations of Pro-Hyp and Gly-Pro-Hyp are detected in the plasma when high quantities of Pro-Hyp and Gly-Pro-Hyp are administered [Citation32]. Thus, we hypothesized that ingestion of CPNS might result in a higher level of Gly-Pro in the plasma rather than Gly-Pro-Hyp or Pro-Hyp. However, the concentration of Gly-Pro was lower than that of Pro-Hyp in the plasma. Moreover, compared with other Gly-Pro-Hyp-containing collagen hydrolysates in the same absorption test, the Pro-Hyp of CPNS exhibited the highest plasma concentration (data not shown). Although Gly-Pro was revealed as a major peptide of CPNS, Pro-Hyp and Gly-Pro-Hyp were not detected in CPNS, after oral administration with CPNS, Pro-Hyp and Gly-Pro-Hyp together with Gly-Pro were presented in the plasma of rats. It is possibly explained that oligopeptides like X-Pro-Hyp-Y in CPNS are hydrolyzed by removing N-and/or C-terminal amino acid, and more stable structure of Pro-Hyp than other peptides is then consistently remained in the plasma [Citation26]. Furthermore, Pro-Hyp may be associated with the improvement of skin health. For instance, Pro-Hyp promoted the reproduction of skin by enhancing fibroblast proliferation and induced the production of hyaluronic acid in human skin fibroblasts [Citation21,Citation36], suggesting that Pro-Hyp plays a crucial role in maintaining skin health conditions. Therefore, although Gly-Pro was detected as a predominant peptide of CPNS, it is likely that CPNS was degraded by proteolytic enzymes in the gastrointestinal tract, leading to the high level of Pro-Hyp absorbed that may exert a beneficial effect on skin health.
Figure 4. Mean plasma concentration-time profiles of Gly-Pro (a), Pro-Hyp (b), and Gly-Pro-Hyp (c) after oral administration of CPNS to Sprague-Dawley rats. Each bar represents the mean ± standard deviation (n = 5).
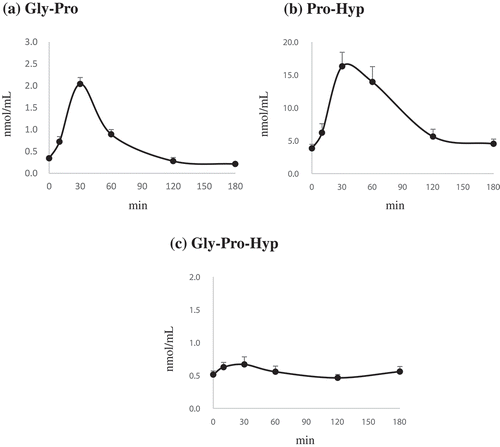
In the present study, the time to reach maximal concentration (Tmax) of Gly-Pro was rapid (30 min), although the plasma concentration of Gly-Pro was lower when compared with Pro-Hyp. According to Yamamoto et al. [Citation31], intraperitoneal administered Gly-Pro showed a significantly shorter Tmax than the other collagen-derived peptides, such as Gly-Pro-Hyp, Gly-Pro-Ala, Pro-Hyp, and Pro-Ala. This result suggests that the absorption of Gly-Pro into the bloodstream occurs rapidly and the dipeptide is then immediately transported to the organs because Gly-Pro possesses a much lower molecular weight than that of other collagen-derived peptides. Also, Gly-Pro increased the synthesis of type 1 procollagen and significantly attenuated MMP-1 production in HDF cells (). Gly-Pro might be produced by the carboxyl terminus degradation of Gly-Pro-containing oligopeptides (Gly-Pro-X). Moreover, it has been observed that Gly-Pro is not readily degraded to single amino acids by peptidases in the blood or intestine [Citation37]. These results indicate that like Pro-Hyp, Gly-Pro also plays important roles in improving skin health, as a dipeptide form. Accordingly, the high levels of both Pro-Hyp and Gly-Pro derived from CPNS in the plasma after oral administration could be beneficial to skin health maintenance.
Effect of oral administration of collagen peptide on UVB-induced photoaging
To further confirm whether bioactive peptides of CPNS, in particular, Pro-Hyp and Gly-Pro, exert a protective influence against UVB-induced photoaging in vivo, the dorsal skins of hairless mice were exposed to UVB and treated with low (300 mg/kg) and high (500 mg/kg) CPNS concentrations for 12 weeks. Throughout the experimental period, body weight and food intake were not significantly different among the groups (data not shown). Skin wrinkling is a typical characteristic of photoaging, resulting from chronic exposure to UVB irradiation [Citation38]. demonstrates that 12-week UVB irradiation, induced significant wrinkle formation on the dorsal skin of the UVB-control group, whereas, in comparison, wrinkle formation was attenuated in the UVB groups given 300 and 500 mg/kg of CPNS, respectively. Quantification of skin wrinkle severity was also investigated through the assessment of the total wrinkle area and number of wrinkles. After the 12-week administration, it was found that orally administered CPNS markedly inhibited UVB-induced wrinkle formation in a dose-dependent manner (). To investigate the skin barrier function, TEWL and skin hydration were evaluated. The oral administration with 300 mg/kg CPNS significantly reduced the UVB-induced TEWL (P < 0.05) by 20.3%. Although the oral administration with 500 mg/kg CPNS was likely to decrease the UVB-induced TEWL, no statistical significance was observed compared to UVB-control (). The oral administration of 500 mg/kg CPNS considerably restored the skin hydration by 52% (), suggesting that the oral administration with CPNS plays a protective role in UVB-induced skin damage.
Figure 5. Effect of CPNS supplementation on UVB-induced wrinkle formation. The dorsal skin surface of hairless mice was exposed to UVB three times a week for 12 weeks, and the mice were supplemented with saline or CPNS. At the end of the experiment, (a) skin replica images were obtained and (b) total wrinkle area and (c) number of wrinkles were analyzed by using a Visioline® VL 650 device.
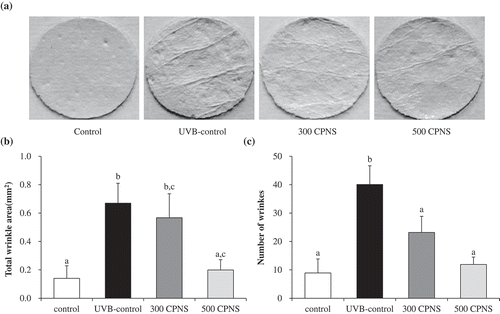
Figure 6. Effect of CPNS supplementation on UVB-induced skin barrier disturbance. The dorsal skin surface of hairless mice was exposed to UVB three times a week for 12 weeks, and the mice were supplemented with saline or CPNS. At the end of the experiment, (a) TEWL and (b) SC hydration were measured by using a Vapometer® and MoistureMeterSC®, respectively.
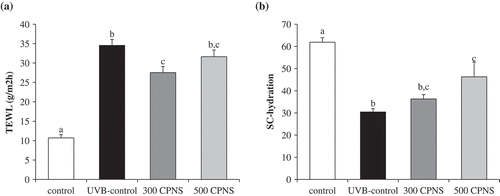
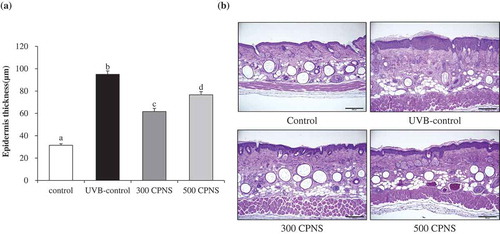
TEWL is the loss of water from the SC. While TEWL is typically used to assess the function of the SC barrier, the magnitude of the TEWL depends on the magnitude of the water gradient and permeability in the skin barrier membrane. In other words, the physiological conditions inside the skin and external environment determine the water gradient across the skin and permeability of the SC barrier [Citation39]. These dynamics of skin barrier function may explain the atypical results of the TEWL examination. Although oral administration of CPNS did not show a significant inhibitory effect on both TEWL and skin hydration in a dose-dependent manner, the results of the present study indicate that CPNS exhibited a preventive effect on UVB-induced skin barrier disturbance. Furthermore, distinctly evidenced that CPNS supplementation significantly attenuated UVB-induced epidermis thickness. Although a high epidermal thickness was observed in the UVB-control group, the oral administration of 300 and 500 mg/kg CPNS, respectively, significantly decreased the epidermal thickness, suggesting that CPNS administration was effective to reconstitute the epidermis. Our observation indicated that CPNS, which contained only 4% Gly-Pro, effectively acts on the improvement of skin health. Similar to our study, previous reports have shown that collagen peptides obtained from enzymatic degradation of collagens of sutchi catfish skin demonstrated anti-photoaging activities such as significant reduction of wrinkle formation and skin thickening. The collagen peptides contained 15% of bioactive tripeptides [Citation40]. Furthermore, it was also shown that commercially available collagen peptides improved skin hydration and elasticity in human clinical trial and the collagen peptides contained only 3% Gly-Pro-Hyp [Citation41]. Although CPNS contains only 4% Gly-Pro, it may be able to play a major role in the beneficial functions on the skin health. Although further studies are required to decipher the involvement of specific bioactive peptides in CPNS regarding their associations with improved skin health, it can be presumed that Gly-Pro, as a key bioactive peptide of CPNS, is associated with the enhancement of skin health.
Figure 7. Effect of CPNS supplementation on UVB-induced epidermis thickness. The dorsal skin surface of hairless mice was exposed to UVB three times a week for 12 weeks, and the mice were supplemented with saline or CPNS. At the end of the experiment, histological analysis was performed to evaluate (a) epidermis thickness. (b) Histological sections were stained with hematoxylin and eosin.
Conclusion
The present results demonstrate that CPNS is a beneficial supplement for the improvement in hydration, wrinkle inhibition, and roughness of skin. Moreover, Gly-Pro derived from CPNS may play a crucial role in the prevention of skin aging, together with Pro-Hyp and Gly-Pro-Hyp. It is the first time that a significant difference between commercially marketed collagen hydrolysates and a novel type of collagen hydrolysate with relatively higher concentrations of specific bioactive dipeptides, such as Gly-Pro, has been demonstrated in relation to the improvement of skin health. It can be supposed that CPNS, containing Gly-Pro, is a potential food supplement for the protection against skin aging.
Author contributions
H.-J.L., H.-L.J., D.-K.A., and H.-J.K. conceived, designed and performed the experiments. H.Y.J., D.B.S., J.-H.L., and J.K.C. performed the experiments. H.-J.L. and S.-S.K wrote the paper and discussed the results. All authors reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Imokawa G. Recent advances in characterizing biological mechanisms underlying UV-induced wrinkles: a pivotal role of fibrobrast-derived elastase. Arch Dermatol Res. 2008 Apr;300(Suppl 1):S7–20. PubMed PMID: 17968573.
- Boelsma E, Hendriks HF, Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr. 2001 May;73(5):853–864. PubMed PMID: 11333837.
- Brennan M, Bhatti H, Nerusu KC, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003 Jul;78(1):43–48. PubMed PMID: 12929747.
- Rosch R, Klinge U, Si Z, et al. A role for the collagen I/III and MMP-1/-13 genes in primary inguinal hernia? BMC Med Genet. 2002;3:2. PubMed PMID: 11872152; PubMed Central PMCID: PMCPMC65699
- Welgus HG, Jeffrey JJ, Eisen AZ. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. PubMed PMID: 6270089.
- Vaalamo M, Mattila L, Johansson N, et al. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997 Jul;109(1):96–101. PubMed PMID: 9204962.
- Matsuda N, Koyama Y, Hosaka Y, et al. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in the dermis. J Nutr Sci Vitaminol (Tokyo). 2006 Jun;52(3):211–215. PubMed PMID: 16967766.
- Deal CL, Moskowitz RW. Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate, and collagen hydrolysate. Rheum Dis Clin North Am. 1999 May;25(2):379–395. PubMed PMID: 10356424.
- Inoue N, Sugihara F, Wang X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J Sci Food Agric. 2016 Sep;96(12):4077–4081. PubMed PMID: 26840887.
- Iba Y, Yokoi K, Eitoku I, et al. Oral administration of collagen hydrolysates improves glucose tolerance in normal mice through GLP-1-dependent and GLP-1-independent mechanisms. J Med Food. 2016 Sep;19(9):836–843. PubMed PMID: 27540823.
- Zhang Z, Zhao M, Wang J, et al. Oral administration of skin gelatin isolated from Chum salmon (Oncorhynchus keta) enhances wound healing in diabetic rats. Mar Drugs. 2011;9(5):696–711. PubMed PMID: 21673883; PubMed Central PMCID: PMCPMC3111176.
- Zhang Z, Wang J, Ding Y, et al. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J Sci Food Agric. 2011 Sep;91(12):2173–2179. PubMed PMID: 21560132.
- Clark KL, Sebastianelli W, Flechsenhar KR, et al. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr Med Res Opin. 2008 May;24(5):1485–1496. PubMed PMID: 18416885.
- Gelse K, Poschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003 Nov 28;55(12):1531–1546. PubMed PMID: 14623400.
- Djagny VB, Wang Z, Xu S. Gelatin: a valuable protein for food and pharmaceutical industries: review. Crit Rev Food Sci Nutr. 2001 Sep;41(6):481–492. PubMed PMID: 11592686.
- Shrikant Sharma RS, Rana S. Bioactive peptides: a review. Int J Bioautomation. 2011 june 02;14(4):223–250.
- Ramshaw JA, Shah NK, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. PubMed PMID: 9724608 J Struct Biol. 1998;1221–2:86–91.
- Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A. 1978 Feb;75(2):871–875. PubMed PMID: 204938; PubMed Central PMCID: PMCPMC411359.
- Knight CG, Morton LF, Onley DJ, et al. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc Res. 1999 Feb;41(2):450–457. PubMed PMID: 10341844.
- Wisniewski K, Artemowicz B, Lutostanska A, et al. Central activity of peptide Gly-Pro-Hyp–the main component of collagen degradation products mixture. Acta Neurobiol Exp (Wars). 1994;54(1):33–38. PubMed PMID: 8023712.
- Ohara H, Ichikawa S, Matsumoto H, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol. 2010 Apr;37(4):330–338. PubMed PMID: 20507402.
- Nakatani S, Mano H, Sampei C, et al. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthritis Cartilage. 2009 Dec;17(12):1620–1627. PubMed PMID: 19615963.
- Iwai K, Hasegawa T, Taguchi Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005 Aug 10;53(16):6531–6536. PubMed PMID: 16076145.
- Ichikawa S, Morifuji M, Ohara H, et al. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int J Food Sci Nutr. 2010 Feb;61(1):52–60. PubMed PMID: 19961355.
- Shigemura Y, Akaba S, Kawashima E, et al. Identification of a novel food-derived collagen peptide, hydroxyprolyl-glycine, in human peripheral blood by pre-column derivatisation with phenyl isothiocyanate. Food Chem. 2011 Dec 1;129(3):1019–1024. PubMed PMID: 25212331.
- Yamamoto S, Deguchi K, Onuma M, et al. Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol Pharm Bull. 2016;39(3):428–434. PubMed PMID: 26934933.
- Backwell FR, Wilson D, Schweizer A. Evidence for a glycyl-proline transport system in ovine enterocyte brush-border membrane vesicles. Biochem Biophys Res Commun. 1995 Oct 13;215(2):561–565. PubMed PMID: 7487992.
- Ganapathy V, Mendicino JF, Leibach FH. Transport of glycyl-L-proline into intestinal and renal brush border vesicles from rabbit. J Biol Chem. 1981 Jan 10;256(1):118–124. PubMed PMID: 7451429.
- Ichimura T, Yamanaka A, Otsuka T, et al. Antihypertensive effect of enzymatic hydrolysate of collagen and Gly-Pro in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2009 Oct;73(10):2317–2319. PubMed PMID: 19809172.
- Asselin J, Knight CG, Farndale RW, et al. Monomeric (glycine-proline-hydroxyproline)10 repeat sequence is a partial agonist of the platelet collagen receptor glycoprotein VI. Biochem J. 1999 Apr 15;339(Pt 2):413–418. PubMed PMID: 10191274; PubMed Central PMCID: PMCPMC1220172.
- Yamamoto S, Hayasaka F, Deguchi K, et al. Absorption and plasma kinetics of collagen tripeptide after peroral or intraperitoneal administration in rats. Biosci Biotechnol Biochem. 2015;79(12):2026–2033. PubMed PMID: 26155906.
- Sontakke SB, Jung JH, Piao Z, et al. Orally available collagen tripeptide: enzymatic stability, intestinal permeability, and absorption of Gly-Pro-Hyp and Pro-Hyp. J Agric Food Chem. 2016 Sep 28;64(38):7127–7133. PubMed PMID: 27573716.
- Chen T, Hou H, Fan Y, et al. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J Photochem Photobiol B. 2016 Dec;165:34–41. PubMed PMID: 27768951.
- Tanaka M, Koyama Y, Nomura Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci Biotechnol Biochem. 2009 Apr 23;73(4):930–932. PubMed PMID: 19352014.
- Quan T, Qin Z, Xia W, et al. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009 Aug;14(1):20–24. PubMed PMID: 19675548; PubMed Central PMCID: PMCPMC2909639.
- Shigemura Y, Iwai K, Morimatsu F, et al. Effect of Prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J Agric Food Chem. 2009 Jan 28;57(2):444–449. PubMed PMID: 19128041.
- Ganapathy V, Leibach FH. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush-border membrane vesicles from the rabbit. Studies with L-carnosine and glycyl-L-proline. J Biol Chem. 1983 Dec 10;258(23):14189–14192. PubMed PMID: 6643475.
- Lee KO, Kim SN, Kim YC. Anti-wrinkle effects of water extracts of teas in hairless mouse. Toxicol Res. 2014 Dec;30(4):283–289. . PubMed PMID: 25584148; PubMed Central PMCID: PMCPMC4289929.
- Sparr E, Millecamps D, Isoir M, et al. Controlling the hydration of the skin though the application of occluding barrier creams. J R Soc Interface. 2013 Mar 6;10(80):20120788. PubMed PMID: 23269846; PubMed Central PMCID: PMCPMC3565729.
- Pyun HB, Kim M, Park J, et al. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev Nutr Food Sci. 2012 Dec;17(4):245–253. PubMed PMID: 24471092; PubMed Central PMCID: PMCPMC3866733.
- Choi SY, Ko EJ, Lee YH, et al. Effects of collagen tripeptide supplement on skin properties: a prospective, randomized, controlled study. J Cosmet Laser Ther. 2014 Jun;16(3):132–137. PubMed PMID: 24131075.

