ABSTRACT
The aim of present investigation was to elucidate the unrevealed beneficial role of diosgenin against an experimental model of TNBS (2,4,6-trinitrobenzenesufonic acid)-induced ulcerative colitis (UC). Colitis was induced in Sprague-Dawley rats by intrarectal administration of TNBS (in 50% ethanol). Then animals were treated with diosgenin (50, 100, and 200 mg/kg) for 14 days. Various biochemical, behavioral, molecular, and histological analysis was performed. Diosgenin significantly decreased (p < 0.05) TNBS-induced elevated colonic oxido-nitrosative damage, myeloperoxidase, hydroxyproline, mRNA expressions of proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IFN-γ) and inflammatory markers (iNOs and COX-2) induced by TNBS. Western blot analysis relevated that TNBS-induced up-regulated protein expressions of NF-κB, IκBα, Bax, and Caspase-1 were markedly decreased (p < 0.05) by diosgenin treatment. It also markedly ameliorated the histological insults induced in the colon by TNBS. In conclusion, diosgenin exerts its colon-protective efficacy probably through the inhibition of NF-κB/IkB-α and Bax/Caspase-1 signaling pathways to experimental TNBS-induced ulcerative colitis.
Abbreviations
ANOVA: Analysis of variance; 5-ASA: 5-aminosalicylic acid; Bax: Bcl-2-associated X protein; COX-2: Cyclooxygenase-2; DAI: Disease Activity Index; DMSO: Dimethyl sulfoxide; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; GSH: Glutathione; HP: Hydroxyproline; IAEC: International Animal Ethics Committee; IBD: Inflammatory Bowel Disease; IBS: Inflammatory Bowel Syndrome; IL’s: Interleukin’s; IFN-γ: Interferon-gamma; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha; iNOs: Inducible nitric oxide synthase; LTB4: Leukotriene B4; MDA: Malondialdehyde; MPO: Myeloperoxidase; NO: Nitric Oxide; NF-κB: Nuclear Factor-κB; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; TNBS: Trinitrobenzene Sulfonic Acid; TNF-α: Tumor necrosis factor-α
Graphical abstract
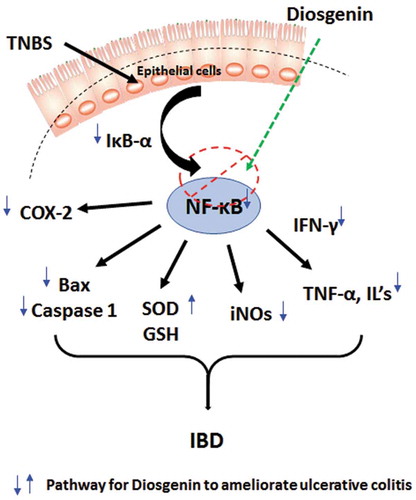
Diosgenin inhibited NF-κB/IkB-α pathway to ameliorate TNBS-induced ulcerative colitis
KEYWORDS:
Inflammatory bowel disease (IBD) is a chronic relapsing immune inflammatory disease of the gastrointestinal tract that exists in two major forms, including ulcerative colitis (UC) and Crohn’s disease (CD) [Citation1]. Amongst IBD, UC, which is an ulcerative condition mainly beset colon and rectum characterized by a disrupted epithelial barrier, the formation of mucous stool, rectal bleeding, and diarrhea [Citation2]. UC affects approximately 0.5–24.5 people per 100,000 people annually worldwide, imposes a significant burden of disease and thus affecting the quality of life [Citation2018]. Although the pathogenesis of UC has not been yet fully understood, however, alteration in immune response, modulation of interstitial microbial flora, genetic factors, and stimulus from external environment thought to play a vital role in the onset and progression of UC reactions [Citation4].
Numerous research studies have documented the important role of the immune-inflammatory system in the induction of UC via the release of an array of inflammatory mediators into the intestine [Citation4,Citation5]. Activation of PPARγ (peroxisome proliferator-activated receptor γ) and NF-κB (nuclear factor-κB) caused an influx of inflammatory mediators including the release of Th1 and Th17 cytokine, nitric oxide, and cyclooxygenase-2 (COX-2) [Citation6,Citation7]. Furthermore, stimulation of macrophages also produces an array of inflammatory cytokines such as Tumor necrosis factor-α (TNF-α) and Interleukins (IL’s such as IL-1β, IL-6) [Citation8,Citation9]. Studies suggested that elevated production of reactive oxygen species (ROS) leads to the generation of oxidative stress, which plays a significant role in the induction and maintenance of UC [Citation4]. It has been well documented that oxidative stress along with cytokines triggered the destruction of tight junctions of the intestinal barrier, which in turn increased its permeability caused tissue damage and thus resulted in the dysfunction of the gastrointestinal mucosal wall [Citation4,Citation5,Citation10].
Based on the understanding of this pathogenesis of UC, various therapeutic moieties have been originated for the management of UC. Many such anti-inflammatory agents, including corticosteroids and 5-aminosalicylates (5-ASA), have oriented toward inhibition of chronic intestinal inflammatory response [Citation11]. However, the clinical application of these treatment regimens remains limited due to the rapid increase of multidrug-resistant and recurrence of disease post these treatments [Citation11]. Additionally, an alternate choice of next-generation promising treatment, including anti-TNF-α monoclonal antibody and immunosuppressive agents, possess irreversible side effects, and its exorbitant costs restrict their long-term use. Thus, safe treatment for the management of IBD is still a major challenge, and there is an urge to identify potential molecules with effective, safer, and more reliable. Recently, the application of functional foods gained significant attention in the prevention and treatment of an array of diseases [Citation12–Citation15]. Therefore, the various animal model plays a vital role in identifying such moieties from functional food origin for the treatment of IBD [Citation16]. TNBS (2,4,6-trinitrobenzenesufonic acid)-induced colitis is one of such animal models for IBD, which is widely used, well standardized, and mimics most of the clinical characteristics of colitis including severe inflammation, ulceration and bloody stool [Citation17,Citation18].
Trigonella foenum-graecum L. is commonly known as fenugreek native to Asia, particularly in India, Sri Lanka, and China. Fenugreek seed has been widely used as a traditional condiment, and it’s one of the important functional food [Citation19]. In Ayurveda, fenugreek seed has been widely used for the treatment of an array of diseases, including diabetes, high cholesterol, wounds, inflammation, indigestion, baldness, and gastrointestinal ailments [Citation20]. Its wide range of pharmacological properties attributes to the presence of various chemical constituents in fenugreek seeds. The chemical analysis of fenugreek seed revealed the presence of flavonoids (quercetin, rutin, vitexin), alkaloids (trigonelline, betain), amino acids (isoleucine, 4-hydroxyisoleucine, L-tryptophan), steroidal sapinogens (yamogenin, diosgenin), fibers, lipids, mucilage, etc [Citation19]. Saponin contains furostanol glycosides as its major soluble fraction, which has two sugar chains, with one bonded at C3 and one attached through an ether linkage at C26 with a D-glucose. This fraction was shown to have various potential, including adaptogenic [Citation21] anabolic [Citation22], androgenic [Citation23] activity. Diosgenin ((3β,25 R)-spirost-5-en-3-ol), a steroidal sapogenin, has been shown to inhibit apoptosis in human colon and hepatic carcinoma in-vitro via modulation of caspase-3 activity [Citation24,Citation25]. It also significantly reduced allergen-induced intestinal inflammation via inhibition of COX-2 and IgE levels [Citation26]. A recent study also reported that diosgenin down-regulated TNF-α/NF-κB pathway to inhibit ischemic stroke-induced inflammatory diseases [Citation27]. Considering this diosgenin potential, the present investigation was designed to elucidate its unrevealed beneficial role against an experimental rat model of TNBS-induced ulcerative colitis.
Materials and method
Animals
Sprague-Dawley rats (adult male, 180–220 g) were procured from the Laboratory Animal Center of Nanchong Central Hospital. The housing conditions for rats throughout the experimental protocol were: temparature: 24 ± 1°C, relative humidity: 45–55%, dark/light cycle: 12:12 h, food: standard pellet chow, water: filtered (ad libitum). A time of 09:00 to 17:00 h were consider to carry out all the experiments protocol which was approved by the Institutional Animal Ethics Committee (IAEC, Second Clinical Medical College, Nanchong, Sichuan, China). A guidelines outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (http://www.nc3rs.org/ARRIVE) were followed to performed all the experiments.
Chemical and kits
Diosgenin and TNBS (Sigma Chemical Co., St Louis, MO, USA), Total RNA Extraction kit and One-step RT-PCR kit (MP Biomedicals India Private Limited, India), primary antibodies of Bax, Caspase-1, p-NF-κB, and p-IκBα (Abcam, Cambridge, MA, USA) were purchased from respective manufacturers.
Induction of colitis and drug treatment schedule
TNBS (100 mg/kg, in 50% ethanol) was used to induce colitis in overnight fasted rats according to method report elsewhere [Citation28] then, they were divided randomly into the various groups (n = 18) viz., Normal group (treated with 1% aqueous Dimethyl sulfoxide (DMSO)), TNBS control group (received DMSO), 5-ASA treated group (received 5-aminosalicylic acid (500 mg/kg, p.o.)), Diosgenin treated group (received diosgenin (50 or 100 or 200 mg/kg), Per se treated group (treated with diosgenin (200 mg/kg)). Rats were treated with either vehicle or diosgenin or 5-ASA for 14 days.
Macroscopic assessment
On day 15, the animals were sacrificed through cervical dislocation and colon was isolated for determination of colonic damage, ulcer area, and ulcer index according to previously reported methods [Citation29,Citation30]. A previously reported methods were used to evaluate the stool consistency, macroscopic score, and Disease Activity Index (DAI) [Citation29,Citation30].
Biochemical assays
The levels of myeloperoxidase (MPO), glutathione (GSH), superoxide dismutase (SOD), nitric oxide (NO content), lipid peroxidation (MDA content) and hydroxyproline (HP) were estimated in the colon tissue according to previously described methods [Citation29,Citation30].
Reverse transcriptase (RT)-PCR and Western blot assay
The messenger RNA (mRNA) expressions of iNOs (Inducible nitric oxide synthase), TNF-α, IL-1β, IL-6, IL-10, IFN-γ (Interferon-gamma), COX-2, LTB4 (leukotriene B4)and β-actin were analyzed in colon tissue using RT-PCR according to manufacturer’s instructions (MP Biomedicals India Private Limited, India). Whereas, protein expressions of Bax (Bcl-2-associated X protein) (E63, ab32503), Caspase-1 (EPR19672, ab238979), p-NF-κB (Phospho Nuclear Factor kappa B) (E379, ab207297) and p-IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha) (6A920, ab12134), and GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) (EPR16891, ab181602) were estimated in lung tissue according to method described elsewhere [Citation29,Citation30].
Histological analysis
Histopathological analysis of colon tissue was carried out using hematoxylin and eosin (H&E) stain and photographs were captured by a light compound microscope with a Zeiss intravital microscopy setup (Zeiss Axioscope A1, Carl Zeiss MicroImaging, Jena, Germany)as described previously [Citation31].
Statistical analysis
GraphPad Prism 5.0 software (GraphPad, San Diego, CA) was used to perform data analysis. Data are expressed as mean ± standard error mean (SEM) and analyzed by using One-Way ANOVA followed by Tukey’s multiple range post hoc analysis (for parametric tests) as well as Kruskal-Wallis test for post hoc analysis (non-parametric tests). A value of p < 0.05 was considered to be statistically significant.
Results
Disease activity, colonic damage, and ulceration
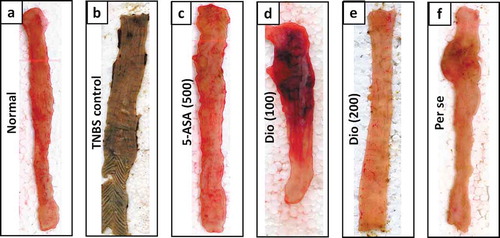
Disease activity was assessed by alteration in body weight, colon weight and colon weight to length ratio, ulcer area, and index as well as and stool consistency score, which was increased significantly (p < 0.05) in TNBS control rats as compared to normal rats. Findings from also depicted a significant elevation (p < 0.05) in the DAI and macroscopic score in TNBS control rats when compared with normal rats. However, these increased in body weight, colonic damage, ulceration, DAI, macroscopic and stool consistency score was markedly reduced (p < 0.05) in diosgenin (100 and 200 mg/kg) and 5-ASA treated rats in comparison with the TNBS control rats, however, these inhibitions were more significant (p < 0.05) in 5-ASA when compared with diosgenin treatment. There were no significant differences observed in these parameters among normal and per se treated group ( and ).
Table 1. Effect of diosgenin on TNBS induced alterations in body weight, colon weight, colon weight to length ratio, ulcer area, ulcer index, disease activity index, macroscopic score and stool consistency score in rats.
Colonic oxido-nitrosative damage
The levels of total protein, SOD, GSH, MDA, and NO were assessed in colon tissue to depict the oxido-nitrosative damage induced after intrarectal instillation of TNBS. Results suggest that the protein levels of GSH and SOD were markedly reduced (p < 0.05), whereas total protein, MDA, and NO levels were significantly elevated (p < 0.05) in TNBS control rats in contrast to the normal rats. Diosgenin (100 and 200 mg/kg) and 5-ASA treatment markedly inhibited (p < 0.05) these TNBS induced colonic oxido-nitrosative damage when compared with TNBS control rats. However, 5-ASA treatment, more notably, reduce (p < 0.05) elevated oxido-nitrosative damage in colon tissue as compared to diosgenin treatment. Furthermore, per se treatment did not make significant differences in colonic levels of total protein, SOD, GSH, MDA, and NO when compared with the normal rats. ()
Table 2. Effect of diosgenin on TNBS induced alterations in colonic SOD, GSH, MDA, NO, hydroxyproline, and MPO in rats.
Colonic MPO and hydroxyproline levels
The levels of colonic MPO and hydroxyproline was increased significantly (p < 0.05) in TNBS control rats in comparison with the normal rats. However, when compared with TNBS control rats, these elevated levels of MPO and hydroxyproline were markedly reduced (p < 0.05) by diosgenin (100 and 200 mg/kg) and 5-ASA treatment. However, TNBS induced an increase in colonic MPO, and hydroxyproline was more markedly reduce (p < 0.05) by 5-ASA treatment as compared to diosgenin treatment. Normal and per se treated group did not show any significant difference in colonic MPO and hydroxyproline levels ().
Cytokine levels
The colonic levels of proinflammatory cytokines, i.e., TNF-α, IL-1β, and IL-6 were markedly up-regulated (p < 0.05), whereas the level of the anti-inflammatory cytokine, i.e., IL-10 was markedly down-regulated (p < 0.05) in TNBS control rats contrast to the normal rats. The TNBS induced altered levels of proinflammatory and anti-inflammatory cytokines in the colon was significantly (p < 0.05) restored in diosgenin (100 and 200 mg/kg) and 5-ASA treatment in comparison with the TNBS control rats. However, 5-ASA treatment notably reduced (p < 0.05) up-regulated TNF-α, IL-1β and IL-6, and down-regulated IL-10 levels in the colon when compared with diosgenin treatment. There was no significant difference in the colonic TNF-α, IL-1β, IL-6, and IL-10 content between normal and per se treated group ().
Table 3. Effect of diosgenin on TNBS induced alterations in colonic TNF-α, IL-1β, IL-6, IL-10, IFN-γ, iNOs, COX-2 and LTB4 mRNA expressions in rats.
Inflammatory release
The inflammatory influx was evaluated by determining the mRNA expressions of iNOs, IFN-γ, COX-2, and LTB4 in colonic tissue and findings of the present study suggest that administration of TNBS caused significant elevation (p < 0.05) in these colonic inflammatory markers in TNBS control rats in contrast to the normal rats. However, the mRNA expressions of iNOs and COX-2 in the colon were markedly reduced (p < 0.05) by diosgenin (100 and 200 mg/kg) and 5-ASA as compared to TNBS control rats and diosgenin (200 mg/kg) was more significant in down-regulating iNOs and IFN-γ mRNA expressions in the colon when compared with 5-ASA. Treatment with diosgenin (50, 100, and 200 mg/kg) and 5-ASA did not make significant down-regulation in elevated colonic LTB4 levels when compared with the TNBS control counterpart. On the other hand, normal and per se treated groups did not show any significant differences among mRNA expressions of iNOs, IFN-γ, COX-2, and LTB4 in colonic tissue ().
Apoptosis and cell death
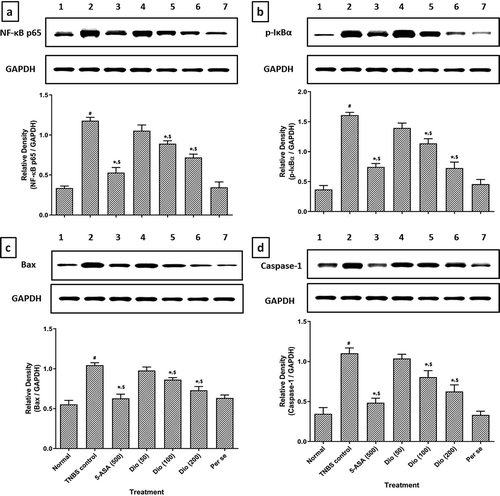
As shown in , findings suggested that colonic apoptosis (Bax and Caspase-1) and cell death (NF-κB and IκBα) was markedly upregulated (p < 0.05) in TNBS control rats after administration of TNBS as compared to normal rats. However, TNBS-induced elevated levels of Bax, Caspase-1, NF-κB and IκBα were markedly reduced (p < 0.05) by diosgenin (100 and 200 mg/kg) and 5-ASA when compared with TNBS control rats. The levels of this apoptotic protein, i.e., Bax, Caspase-1, NF-κB and IκBα in per se treated rats did not differ significantly when compared with normal rats.
Figure 2. Effect of diosgenin on TNBS induced alterations in colonic NF-κB (a), IκBα (b), Bax (c) and Caspase-1 (d) protein expression in rats. Data are expressed as mean ± S.E.M. (n = 6) and analyzed by one way ANOVA followed by Tukey’s multiple range test. *p < 0.05 as compared to TNBS control group, #p < 0.05 as compared to normal group and $p < 0.05 as compared to one another (diosgenin and 5-ASA). Representative protein expression of Normal (Lane 1), TNBS control (Lane 2), 5-ASA (500 mg/kg) (Lane 3), Diosgenin (50 mg/kg) (Lane 4), Diosgenin (100 mg/kg) (Lane 5), Diosgenin (200 mg/kg) (Lane 6) and Per Se (Lane 7) treated rats. TNBS: 2,4,6-Trinitrobenzenesulfonic acid; 5-ASA: 5-Aminosalicylic acid; Dio: Diosgenin; p-NF-κB: Phospho Nuclear Factor kappa B; p-IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha, Bax: Bcl-2-associated × protein and GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
Histopathology of colon
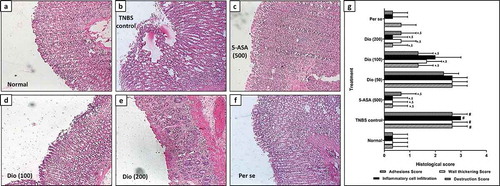
As depicted in ), the histological findings of colon tissue indicated that the architecture of colon tissue from normal rats is with mild edema, inflammatory infiltration, and necrosis. However, intrarectal administration of TNBS induced significant (p < 0.05) destruction of colon tissue reflected by the elevated influx of inflammatory cells, necrosis, and edema in TNBS control rats when compared with normal rats ()). These TNBS induced colonic damage was markedly attenuated by 5-ASA ()) and diosgenin (100 and 200 mg/kg) (,)) treatment denoted by significant decreased (p < 0.05) in edema, necrosis and inflammatory release in respective colon tissue. However, when compared with normal rats, colon tissue from per se treated rats did not show any significant alteration in edema, inflammatory infiltration, and necrosis (,g)).
Figure 3. Effect of diosgenin on TNBS induced alterations in colon histopathology. Photomicrograph of sections of colon tissue from Normal (a), TNBS control (b), 5-ASA (500 mg/kg) (c), Diosgenin (100 mg/kg) (d), Diosgenin (200 mg/kg) (e) and Per Se (f) treated rats stained with H&E stain. The quantitative representation of histological score (g). Data are expressed as mean ± S.E.M. (n = 3) and analyzed by one way ANOVA followed by the Kruskal-Wallis test was applied for post hoc analysis. *p < 0.05 as compared to TNBS control group, #p < 0.05 as compared to normal group and $p < 0.05 as compared to one another (diosgenin and 5-ASA). TNBS: 2,4,6-Trinitrobenzenesulfonic acid; 5-ASA: 5-Aminosalicylic acid; Dio: Diosgenin. Red arrow indicated inflammatory infiltration, and yellow arrow indicated necrosis. Images (×40 magnification) are typical and are representative of each study group.
Discussion
Ulcerative colitis (UC) is a chronic complicated immune disorder of the gastrointestinal system associated with inflammation and destruction of the intestinal mucosa, culminating in worsen the quality of life [Citation4]. Studies have suggested that inflammation and oxidative stress has great importance in the pathogenesis of colitis; thus, the investigation of the new therapeutic moiety is oriented toward amelioration of inflammatory influx and scavenging of elevated ROS [Citation1,Citation4,Citation5]. TNBS induces colitis via interaction with the colonic tissue proteins, which results in an inflammatory influx, which is similar to clinical colitis [Citation17,Citation18]. Thus, TNBS-induced colitis was selected as an experimental model of colitis in the present investigation to evaluate the potential of diosgenin. In the current investigation also, intrarectal administration TNBS results in cachexia, the release of inflammatory mediators, induction of oxidative stress, and extension of colonic damage with ulceration confirmed by histological analysis. However, administration of diosgenin significantly ameliorated TNBS-induced colitis via modulation of NF-κB/IkB-α pathway to inhibit elevated oxido-nitrosative stress, the release of proinflammatory cytokines (TNF-α, IL-1β, IL-6 and IFN-γ), inflammatory markers (iNOs, and COX-2) and apoptosis (Bax and Caspase-1) (Graphical abstract).
Numerous studies suggest that the administration of TNBS associated with various clinicopathological characteristics, including weight loss, altered stool consistency, and DAI [Citation17,Citation18,Citation32]. The covalent reaction between TNBS and tissue protein leads to activation of delayed-type hypersensitive response via stimulation of CD4 + T cell-mediated immune response [Citation33]. It has been well documented that a decrease in body weight reflects the immunocompromised disease state [Citation17,Citation18,Citation33], and in the present investigation also cachexia is a reflection of TNBS-induced immune-inflammatory response. Nevertheless, treatment with diosgenin significantly ameliorates TNBS-induced decreased body weight and elevated stool consistency with disease activity index, which may be by virtue of its anti-inflammatory potential.
Studies suggested that TNBS caused massive inflammatory influx and activation of innate immune cells, including monocytes, neutrophils, lymphocytes, and macrophages [Citation17,Citation18]. Further, neutrophilic infiltration into the colon leads to the production of various oxidant species, such as superoxide and hypochlorous acid, which caused tissue damage [Citation34,Citation35]. MPO is considered as an essential hallmark of neutrophil infiltration [Citation36,Citation37]. It is synthesized and transported intracellularly to the lysosomes. Activated neutrophils pass out of the circulation under stressful conditions, which then further enter into the inflamed mucosa and submucosa of the large intestine during acute inflammation. These consequences caused the overproduction of ROS that can contribute to intestinal injury [Citation38,Citation39]. In the current investigation, the administration of TNBS resulted in elevated levels of colonic MPO, which was significantly ameliorated by treatment with diosgenin. A recent study by Wang et al. (2018) also depicted the anti-inflammatory potential of diosgenin via inhibition of MPO levels [Citation40], and the result of the present study is in line with findings of the previous investigator [Citation40].
TNBS-induced ulcerative colitis exhibits multifactorial mechanisms, which include increased ROS levels, mitochondrial apoptosis, damage to DNA/RNA, lipid peroxidation, modulation of cellular energies, etc [Citation17,Citation28,Citation33]. Numerous literature has supported the direct link between oxidative stress and the pathogenesis of TNBS-induced colitis [Citation18,Citation33]. It has been documented that imbalance of pro-oxidant and antioxidant leads to the generation of free radicals and ROS, such as superoxide, peroxides, and hydroxyl radical [Citation41,Citation42]. The superoxide anion (O2−) is a principal free radical in tissues, and it’s converted to secondary oxidant H2O2 via SOD, which disrupted the integrity of the intestinal barrier leads to epithelial cells injury and inflammation [Citation43–Citation45]. GSH, a natural tripeptide, exhibits antioxidant activity, and its depletion reflected elevated oxidative stress [Citation46–Citation48]. Moreover, MDA is recognized as an end-product for polyunsaturated fatty acids oxidation, thus reflected as lipid peroxidation [Citation49,Citation50]. In the present study, the levels of three oxidative stress markers were altered significantly after the installation of TNBS, whereas treatment with diosgenin significantly inhibiting oxidative stress via attenuation of elevated MDA and decreased SOD and GSH levels.
Studies suggested that IL-6, which is a multifunctional cytokine, plays a central dogma role in the pathogenesis of IBD by inhibiting the function of regulatory T-cell and activation of T helper type 1 (Th1) cells [Citation18,Citation51]. Thus, patients with UC showed a significant correlation between serum levels of IL-6 and disease activity [Citation52]. Furthermore, activated Th1 cells caused secretion of IFN-γ that, in turn, increases mucosal permeability via modulating the structure of Tight Junction (TJ) protein [Citation18,Citation53]. Consistent with the previous researcher, the expression of NF-κB and IκBα was increased significantly in the colon tissue of TNBS control rats [Citation17,Citation54,Citation55]. However, the administration of diosgenin inhibited the phosphorylation and degradation of IκBα and thus suppressed the activation and nuclear translocation of NF‑κB which further attenuated downstream expressions of inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IFN-γ. The results of the present investigation are in line with the findings of the previous investigator, where diosgenin exerts its anti-inflammatory potential via down-regulation of proinflammatory cytokines and inhibition of NF-κB phosphorylation [Citation7,Citation40].
Literature reports that apoptosis occurred via either the intrinsic or extrinsic pathway, which is associated with mitochondria degradation or cell death [Citation56]. Bax, which is an apoptosis regulator protein responsible for alteration in mitochondrial cell membrane permeability that releases mitochondrial cytochrome C, which in turn activates caspases-1 and causes mitochondria cell apoptosis [Citation57–Citation59]. In the present study, it was found that TNBS administration associated with elevated expression of Bax and Caspases-1, which induces necrosis and apoptosis. This molecular alteration was further evident by histological findings of mucosal membrane from TNBS control rats, which showed prominent erosion and ulceration caused due to apoptosis. This histopathological aberration was significantly ameliorated by diosgenin treatment via inhibition of Bax and Caspases-1 induced apoptosis. Results of the present study are in line with the findings of Raju et al. (2004), where steroid saponin diosgenin from fenugreek modulate the expressions of apoptotic proteins to exerts its anticancer potential in HT-29 human colon cancer cell line in-vitro [Citation24].
Currently, 5-ASA has been generally considered as standard therapy for the management of UC [Citation11]. The researcher suggested the 5-ASA exerts its potential in colitis orchestrating multiple mechanisms, including inhibition of synthesis of leukotrienes and prostaglandins as well as the production of ROS and cytokines. However, this agent associated with potential side effects, including abdominal pain, loss of appetite, diarrhea, nephropathy, etc [Citation11,Citation60]. Nowadays, the number of IBD patients who preferred herbal medicine as an alternative treatment option has been increased significantly [Citation61]. Moreover, fenugreek has received much importance as a promising functional food and dietary supplement for the management of various clinical conditions [Citation62]. Additionally, a number of researchers have documented the potential of diosgenin, a steroid saponin from fenugreek against the treatment of colon cancer [Citation24,Citation63–Citation65]. Thus, further clinical research is needed on this natural compound diosgenin for possible consideration as a potential moiety in the management of IBD.
Conclusion
The present investigation demonstrates the potential of diosgenin against TNBS-induced colitis. Diosgenin exerts its colon-protective efficacy probably through inhibition of NF-κB/IkB-α and Bax/Caspase-1 signaling pathways to ameliorate production of oxido-nitrosative stress and proinflammatory cytokines (TNF-α, IL-1β, IL-6 and IFN-γ) and inflammatory markers (iNOs, and COX-2) during experimental colitis.
Author’s contribution
XT: Concepts, Design, Manuscript preparation, Manuscript editing, Manuscript review
GH: Experimental studies, Data acquisition, Manuscript editing, Manuscript review
TZ: Concepts, Design, Statistical analysis, Manuscript review
SL: Concepts, Manuscript preparation, Manuscript editing, Manuscript review
Disclosure statement
There is no conflict of interest between any of the authors.
Additional information
Funding
References
- Neurath MF. Cytokines in inflammatory bowel disease [Research Support, Non-U S Gov’t Review]. Nat Rev Immunol. 2014 May;14(5):329–342.
- Bernstein CN, Fried M, Krabshuis JH, et al. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010 Jan;16(1):112–124.
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018 Dec 23;390(10114):2769–2778.
- Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238.
- Lee SH, Kwon JE, Cho M-L. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018 Jan;16(1):26–42.
- Rosillo MA, Sanchez-Hidalgo M, Cardeno A, et al. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats Research Support, Non-U S Gov’t. Pharmacol Res. 2012 Sep;663:235–242.
- Shishodia S, Aggarwal BB. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, IκB kinase activation and NF-κB-regulated gene expression. Oncogene. 2006 Mar 9;25(10):1463–1473.
- Billiet T, Rutgeerts P, Ferrante M, et al. Targeting TNF-α for the treatment of inflammatory bowel disease Review. Expert Opin Biol Ther. 2014 Jan;141:75–101.
- Fukuda T, Majumder K, Zhang H, et al. Adenine inhibits TNF-α signaling in intestinal epithelial cells and reduces mucosal inflammation in a dextran sodium sulfate-induced colitis mouse model. J Agric Food Chem. 2016 Jun 1;64(21):4227–4234.
- Almeer RS, Mahmoud SM, Amin HK, et al. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem Toxicol. 2018 May;115:49–62.
- Lee HS, Park S-K, Park DI. Novel treatments for inflammatory bowel disease. Korean J Intern Med. 2018 Jan;33(1):20–27.
- Kandhare A, Phadke U, Mane A, et al. Add-on therapy of herbal formulation rich in standardized fenugreek seed extract in type 2 diabetes mellitus patients with insulin therapy: an efficacy and safety study Basic Research. Asian Pac J Trop Biomed. 2018 September 1;8(9):446.
- Kandhare AD, Rais N, Moulick ND, et al. Efficacy and safety of herbal formulation rich in standardized fenugreek seed extract as add-on supplementation in patients with type 2 diabetes mellitus on sulfonylurea therapy: A 12-week, randomized, double-blind, placebo-controlled, multi-center study. Pharmacogn Mag. 2018;14(57):393–402.
- Zhou Z, Kandhare AD, Kandhare AA, et al. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-β1/Smad3/AMPK and IκBα/NF-κB pathways. Excli J. 2019;18:723–745.
- Bodhankar S, Zhang L, Wu T, et al. Elucidation of the molecular mechanism of tempol in pentylenetetrazol-induced epilepsy in mice: role of gamma-aminobutyric acid, tumor necrosis factor-alpha, interleukin-1β and c-Fos Original Article. Pharmacogn Mag. 2018 December 1;14(59):520.
- Kandhare AD, Raygude KS, Ghosh P, et al. Patentability of animal models: india and the globe. Int J Pharm Biol Arc. 2011;2(4):1024–1032.
- Luo X, Yu Z, Deng C, et al. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci Rep. 2017 Nov 27;7(1):16374.
- Suzuki R, Katakura K, Fujiwara T, et al. Imiquimod-induced CCR9 ameliorates murine TNBS colitis. Fukushima J Med Sci. 2016 Dec 16;62(2):90–100.
- Kandhare AD, Thakurdesai PA, Wangikar P, et al. A systematic literature review of fenugreek seed toxicity by using ToxRTool: evidence from preclinical and clinical studies. Heliyon. 2019 Apr;5(4):e01536.
- Patil SP, Niphadkar PV, Bapat MM. Allergy to Fenugreek (Trigonella foenum graecum). Ann Allergy Asthma Immunol. 1997;78(3):297–300.
- Vasil’eva IS, Vaniushkin SA, Zinov’eva SV, et al. Photosynthetic pigments of tomato under conditions of biotic stress and effects of furostanol glycosides. Prikl Biokhim Mikrobiol. 2003 Nov-Dec;39(6):689–696.
- Dubinskaia VA, Strelkova LB, Vasil’eva IS, et al. Anabolic properties of furostanol glycosides from Dioscorea deltoidea wall. Biull Eksp Biol Med. 1998 Aug;126(8):178–181.
- Park S-W, Lee C-H, Shin D-H, et al. Effect of SA1, a herbal formulation, on sexual behavior and penile erection. Biol Pharm Bull. 2006 Jul;29(7):1383–1386.
- Raju J, Patlolla JM, Swamy MV, et al. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004 Aug;13(8):1392–1398.
- Li F, Fernandez PP, Rajendran P, et al. Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett. 2010 Jun 28;292(2):197–207.
- Huang C-H, Ku C-Y, Jan T-R. Diosgenin attenuates allergen-induced intestinal inflammation and IgE production in a murine model of food allergy. Planta Med. 2009 Oct;75(12):1300–1305.
- Zhu S, Tang S, Su F. Dioscin inhibits ischemic strokeinduced inflammation through inhibition of the TLR4/MyD88/NFkappaB signaling pathway in a rat model. Mol Med Rep. 2018 Jan;17(1):660–666.
- Li X, Yang X, Cai Y, et al. Proanthocyanidins from grape seeds modulate the NF-κB signal transduction pathways in rats with TNBS-induced ulcerative colitis Research Support, Non-U.S. Gov’t. Molecules. 2011 Aug 8;16(8):6721–6731.
- Kandhare AD, Ghosh P, Ghule AE, et al. Protective effect of Phyllanthus amarus by modulation of endogenous biomarkers and DNA damage in acetic acid induced ulcerative colitis: role of phyllanthin and hypophyllanthin. Apollo Medicine. 2013;10(1):87–97.
- Kumar VS, Rajmane AR, Adil M, et al. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28(2):132–145.
- Kandhare AD, Bodhankar SL, Mohan V, et al. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Biol Interact. 2015;237:151–165.
- Yang M, Lin H-B, Gong S, et al. Effect of astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis Research Support, Non-U S Gov’t . Cytokine. 2014 Dec;70(2):81–86.
- Antoniou E, Margonis GA, Angelou A, et al. The TNBS-induced colitis animal model: an overview. Ann Med Surg (Lond). 2016 Nov;11:9–15.
- Visnagri A, Kandhare AD, Kumar VS, et al. Elucidation of ameliorative effect of Co-enzyme Q10 in streptozotocin-induced diabetic neuropathic perturbation by modulation of electrophysiological, biochemical and behavioral markers. Biomed Aging Pathol. 2012;2(4):157–172.
- Yin H, Bodhankar S, Zhang G, et al. Ameliorative effect of morin, a plant flavonoid against Freund’s complete adjuvant-induced polyarthritis in rats Original Article. Pharmacogn Mag. 2019 January 1;15(60):43.
- Raygude KS, Kandhare AD, Ghosh P, et al. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012 Dec;20(6):331–341.
- Kandhare AD, Ghosh P, Ghule AE, et al. Elucidation of molecular mechanism involved in neuroprotective effect of coenzyme Q10 in alcohol-induced neuropathic pain. Fundam Clin Pharmacol. 2013 Dec;27(6):603–622.
- Kruidenier L, Kuiper I, Van Duijn W, et al. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease Research Support, Non-U.S. Gov’t . J Pathol. 2003 Sep;2011:17–27.
- Kruidenier L, Verspaget HW. Oxidative stress as a pathogenic factor in inflammatory bowel disease - radicals or ridiculous? Review. Aliment Pharmacol Ther. 2002;16(12):1997–2015.
- Wang H-W, Liu H-J, Cao H, et al. Diosgenin protects rats from myocardial inflammatory injury induced by ischemia-reperfusion. Med Sci Monit. 2018 Jan 12;24:246–253.
- Adil M, Kandhare AD, Dalvi G, et al. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren Fail. 2016 Jul;38(6):996–1006.
- Kandhare AD, Raygude KS, Ghosh P, et al. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac J Trop Biomed. 2012 May;2(5):337–344.
- Ketkar S, Rathore A, Kandhare A, et al. Alleviating exercise-induced muscular stress using neat and processed bee pollen: oxidative markers, mitochondrial enzymes, and myostatin expression in rats. Integr Med Res. 2015 Sep;4(3):147–160.
- Visnagri A, Kandhare AD, Chakravarty S, et al. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 2014 Jul;52(7):814–828.
- Patil MVK, Bhise S, Kandhare A. Pharmacological evaluation of ameliorative effect of aqueous extract of Cucumis sativus L. fruit formulation on wound healing in Wistar rats. Chronicles of Young Scientists. 2011;2(4):207–213.
- Patil MVK, Kandhare AD, Bhise SD. Anti-inflammatory effect of Daucus carota root on experimental colitis in rats. Int J Pharm Pharm Sci. 2012;4(1):337–343.
- Adil M, Kandhare AD, Ghosh P, et al. Ameliorative effect of naringin in acetaminophen-induced hepatic and renal toxicity in laboratory rats: role of FXR and KIM-1. Ren Fail. 2016 Jul;38(6):1007–1020.
- Ghule AE, Kandhare AD, Jadhav SS, et al. Omega-3-fatty acid adds to the protective effect of flax lignan concentrate in pressure overload-induced myocardial hypertrophy in rats via modulation of oxidative stress and apoptosis. Int Immunopharmacol. 2015 Sep;28(1):751–763.
- Goswami S, Kandhare A, Zanwar AA, et al. Oral L-glutamine administration attenuated cutaneous wound healing in Wistar rats. Int Wound J. 2016 Feb;13(1):116–124.
- Honmore V, Kandhare A, Zanwar AA, et al. Artemisia pallens alleviates acetaminophen induced toxicity via modulation of endogenous biomarkers. Pharm Biol. 2015 Apr;53(4):571–581.
- Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer Research Support, N I H, Extramural Research Support, Non-U S Gov’t. Cancer Cell. 2009 Feb 3;15(2):103–113.
- Umehara Y, Kudo M, Nakaoka R, et al. Serum proinflammatory cytokines and adhesion molecules in ulcerative colitis. Hepatogastroenterology. 2006 Nov-Dec;53(72):879–882.
- Amasheh M, Grotjohann I, Amasheh S, et al. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: A novel model for studying the pathomechanisms of inflammatory bowel disease cytokines Research Support, Non-U S Gov’t. Scand J Gastroenterol. 2009;44(10):1226–1235.
- Han YM, Koh J, Kim JW, et al. NF-kappa B activation correlates with disease phenotype in Crohn’s disease. PLoS One. 2017;12(7):e0182071.
- Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023.
- Ghasemzadeh MR, Amin B, Mehri S, et al. Effect of alcoholic extract of aerial parts of Rosmarinus officinalis L. on pain, inflammation and apoptosis induced by chronic constriction injury (CCI) model of neuropathic pain in rats. J Ethnopharmacol. 2016 Dec 24;194:117–130.
- Visnagri A, Kandhare AD, Bodhankar SL. Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren Fail. 2015 Apr;37(3):482–493.
- Adil M, Kandhare AD, Visnagri A, et al. Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3, TGF-β, and TNF-α. Ren Fail. 2015;37(8):1396–1407.
- Cui J, Wang G, Kandhare AD, et al. Neuroprotective effect of naringin, a flavone glycoside in quinolinic acid-induced neurotoxicity: possible role of PPAR-γ, Bax/Bcl-2, and caspase-3. Food Chem Toxicol. 2018 Nov;121:95–108.
- Guo G, Shi F, Zhu J, et al. Piperine, a functional food alkaloid, exhibits inhibitory potential against TNBS-induced colitis via the inhibition of IκB-α/NF-κB and induces tight junction protein (claudin-1, occludin, and ZO-1) signaling pathway in experimental mice. Hum Exp Toxicol. 2020 Apr;39(4):477–491.
- Fabian A, Rutka M, Ferenci T, et al. The use of complementary and alternative medicine is less frequent in patients with inflammatory bowel disease than in patients with other chronic gastrointestinal disorders. Gastroenterol Res Pract. 2018;2018:9137805.
- Wani SA, Kumar P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci. 2018 April 1;17(2):97–106.
- Dong M, Meng Z, Kuerban K, et al. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018 Oct 10;9(10):1039.
- Lepage C, Léger D, Bertrand J, et al. Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett. 2011;301(2):193–202.
- Malisetty VS, Patlolla JM, Raju J, et al. Chemoprevention of colon cancer by diosgenin, a steroidal saponin constituent of fenugreek. Cancer Res. 2005;65(9 Sup):580–581.