ABSTRACT
Helicobacter pylori (H. Pylori) is a potential pathogen found in approximately 50% of the human population, although many infected by it remain asymptomatic. The current treatment regime of it has become less effective because of antibiotic resistance. Accordingly, an alternative treatment with reasonable cost is essential. Urease is an important enzyme that enables the acclimation to harsh acidic conditions in the stomach. IgY implemented therapy is an attractive approach to preventing infection of H. pylori. The present study evaluated the anti-urease IgY effect on the prevention of H. pylori infection. To generate IgY, a 100 ng/ml mixture of purified urease A and B used to immunize Hyline brown hens. Anti-urease IgY was purified from egg yolks and orally administered to SPF C57BL/6 female mice that had been infected with H. pylori. The results demonstrated that anti-urease IgY has greater potential to prevent infection in mice, suggesting a rational therapeutic approach to H. pylori.
1. Introduction
H. pylori, which is closely associated with chronic active gastritis and gastric malignancies, is a key factor of recurrence and aggravation of ulcers as well as gastric malignancies (Backert, Neddermann, Maubach, & Naumann, Citation2016). H. pylori aggravates ulcers by continuously stimulating gastric secretion (Czinn & Blanchard, Citation2011; Kabir, Citation2007; Sutton & Lee, Citation2011). Thus, elimination of H. pylori is a key point for ulcer treatment in adults exhibiting a high incidence of H. pylori infection (Backert, Tegtmeyer, & Selbach, Citation2010; Tatematsu, Tsukamoto, & Mizoshita, Citation2005; Yong et al., Citation2015). Various therapies composed of proton-pump inhibitors and antibiotics have been recommended for the treatment of H. pylori-mediated gastritis and ulcers (Plummer, Franceschi, Vignat, Forman, & de Martel, Citation2015; Svennerholm & Lundgren, Citation2007; Zajac, Schubert, Dyek, & Oelkrung, Citation2017). However, the antibiotics used for the treatment of H. pylori are becoming ineffective because of rapidly-increasing resistance to antibiotics (Aebischer, Schmitt, Walduck, & Meyer, Citation2005; Ayala, Escobedo-Hinojosa, de la Cruz-Herrera, & Romero, Citation2014; Milani, Sharifi, Rahmati, Somi, & Akbarzadeh, Citation2015; Shin et al., Citation2002; Svennerholm & Lundgren, Citation2007). Worldwide abuse of antibiotics is also leading to the failure of the standard triple therapy against H. pylori by resistant strains. Therefore, there is a great demand for alternative treatments for H. pylori infections.
New treatments using natural resources such as probiotics and natural plant extracts have been investigated (Hong et al., Citation2018). Among these, anti-H. pylori immunoglobulin from chicken egg yolk (IgY) has been recognized as an inexpensive source of antibodies with potential therapeutic effects. The idea of oral administration of immunoglobulins specific to host pathogens is not new, but the therapeutic usefulness of IgY for passive immunization through oral ingestion of IgY enriched food products is a novel alternative approach to prevention and treatment of intestinal infections caused by entero-toxigenic pathogens (Plummer et al., Citation2015; Suzuki et al., Citation2004). Eggs are an everyday food product with a low risk of toxic side effects. Additionally, there is no concern of allergic reactions to eggs as the final IgYs products do not contain allergenic albumin, and IgY itself does not activate the mammalian complement system or interact with rheumatoid factors, proteins A and G, or mammalian Fc receptors (Plummer et al., Citation2015). Accordingly, the use of IgYs specifically generated against H. pylori would be beneficial for the prevention and treatment of such infections. Previous studies have shown that IgY against H. pylori whole-cell lysates could be used to inhibit the growth of H. pylori and reduce gastric inflammatory cell accumulation in H. pylori-infected Mongolian gerbils, suggesting a novel treatment mortality against H. pylori-associated gastric mucosal diseases (Ki et al., Citation2014; Rahman, Van, Icatlo Jr, Umeda, & Kodama, Citation2013; Shin et al., Citation2002; Suzuki et al., Citation2004; Wang, Yang, Cao, Wang, & Pan, Citation2014). Because of the risk of cross-reactivity with other bacteria and normal human intestinal flora, IgY produced by whole-cell lysates are questionable because of the possibility of decreasing efficiency and specificity (Plummer et al., Citation2015; Shin et al., Citation2003; Suzuki et al., Citation2004). Accordingly, immunization using a selective antigen is required (Foegeding, Caston, McClain, Ohi, & Cover, Citation2016; Michetti et al., Citation1994; Palframan, Kwok, & Gabriel, Citation2012; Rahman et al., Citation2013; Suzuki et al., Citation2004; Wang et al., Citation2014; Zajac et al., Citation2017). Among the immunodominant proteins recognized by IgY against H. pylori, whole-cell lysate urease and HSP60 have been studied as vaccine antigens against H. pylori infection (Suzuki et al., Citation2004).
Urease activity is considered an important activity for evaluation of the virulence of H. pylori pathogens. The urease activity and flagella-mediated motility first facilitate its survival and movement to lower mucus, then several adhesins, binding proteins and outer membrane proteins facilitate the antigen receptors on the host cells (Berger et al., Citation2016; Clyne, Dolan, & Reeves, Citation2007; Israel & Peek, Citation2001; Kao, Sheu, & Wu, Citation2016; Kwak, Choi, Cho, Lee, & Kim, Citation2014; Michetti et al., Citation1994; Shiota, Suzuki, & Yamaoka, Citation2013). Following successful colonization, various toxins, cytotoxin-associated gene A and vacuolating cytotoxin A are involved in damage to the host tissue and intracellular replication. Urease regulates H. pylori macrophage interactions and is essential for H. pylori survival in macrophages; thus, the inhibition of urease activity would compromise its ability to colonize the stomach and therefore provide a target for the prevention or eradication of H. pylori infection (Kao et al., Citation2016). There are two subunits of urease enzyme, UreA and B, which both contain the active site for enzyme activity. In an animal model, neutralization of urease activity after its interaction with administered antibodies reduced the H. pylori population and it was not able to survive in the acidic pH of the gastric lumen and mucosa. The anti-urease IgY might decrease the activity of urease from H. pylori. Therefore, in this study, we investigated the potential effects of anti-Urease IgY produced from immunized egg yolks with recombinant protein of UreA and UreB to prevent H. pylori infection in a mouse model. The effects of anti-urease IgY were assessed through serological and histopathological changes in gastric tissues.
2. Material and methods
2.1. Culture of H. pylori strain
Helicobacter pylori (Strain 43504) were obtained from ATCC. The bacteria were scraped onto blood agar using a sterile cotton swab, then placed in a pouch containing a gas pack. After incubation for 3 days at 37°C in a CO2 incubator at 37°C, samples were inoculated in Brucella broth (Difco Laboratories, USA) with 5% horse serum in a shaking incubator at 37°C under 10% CO2, then inactivated by formalin 0.1% treatment. The cells were subsequently inactivated for 2 days at room temperature. After centrifugation at 6000 rpm for 20 min, the pellet was suspended in PBS (pH 7.2), treated again with formalin 0.1%, stored in the refrigerator and used as an antigen.
2.2. Production of recombinant proteins
2.2.1. Genomic DNA extraction, gene amplification and expression
DNA was extracted using a DNeasy Blood and tissue kit (Qiagen, Venlo (Pays Bas), Germany). Primers were then synthesized to amplify the UreA and UreB genes using genomic DNA as a template (data not shown), after which PCR was conducted using the extracted DNA as a template with a BioFACT Lamp Taq DNA polymerase kit (BioFACT, Daejun, Korea). PCR products were cloned into pGEM-T easy vector (Promega, USA), after which each gene was identified by sequencing.
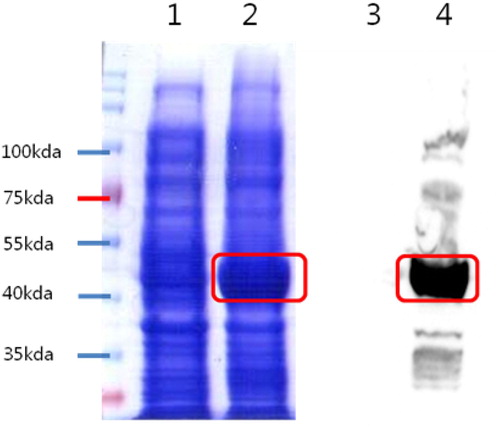
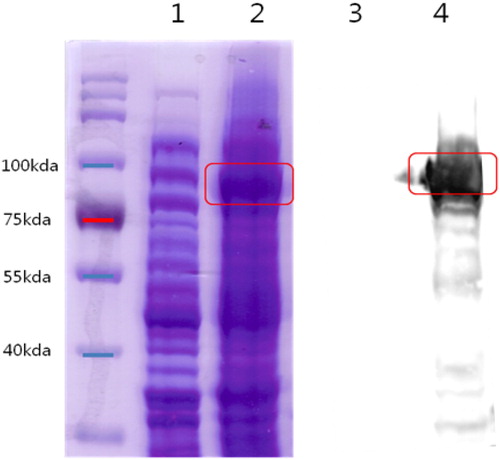
The pET32a (+) vector contains the thioredoxin protein and six histidines at the N-terminus and has the T7 promoter for expression of each gene in pET32a (+) vector (Novagen). The UreA and UreB proteins were fused to these proteins to produce recombinant proteins. E. coli strain BL21 (DE3) transformed with the recombinant protein gene was obtained and inoculated in 5 ml of LB liquid medium (100 μg/ml ampicillin) and cultured at 37°C for 16 h. Next, each transformant strain was inoculated into 50 ml of LB liquid medium (100 μg/ml ampicillin) at a ratio of 1/100 and incubated at 37°C, after which the absorbance was measured using a spectrophotometer. Liquid culture was conducted until the absorbance (A600) reached 0.4–0.6. (0, 0.25, 0.5, 0.75, 1 mM), and then was added to the T7 promoter of the expression system and 1 ml of the culture was added at 1 h intervals, The isopropyl-β-D-thio-galactopyranoside for induction of recombinant protein (IPTG; Sigma, USA). After centrifugation, conditions for inducing protein expression were examined. After 4 h of incubation, the cells were centrifuged at 10,000 rpm and 4°C. The resulting 50 ml total cell pellet was then resuspended in 5 ml of lysis buffer (50 mM Tris-HCl, 0.5 M NaCl, pH 8.0), after which it was treated with 1% Triton X-100 and 40 μg/ml phenylmethylsulfonyl fluoride (PMSF) and allowed to react at room temperature for 20 min. Next, 100 μg/ml lysozyme was added and mixed well. After 1 h of reaction on ice, E. coli was lysed by freezing and thawing three times using liquid nitrogen. Next, centrifugation was conducted at 10,000 rpm and 4°C for 15 min to separate the soluble portion and the insoluble portion, after which the protein expression was confirmed by SDS-PAGE.
2.2.2. Production and identification of recombinant proteins
Luria–Bertani (LB) broth was prepared and used for antigen-specific recombinant proteins, and ampicillin was added at a concentration of 50 µg/ml. Cell colonies were inoculated on LB agar plates on the previous day. After that, E coli inoculated in LB and incubated at 37°C in a shaking incubator at 200 rpm for 4 to 5 h. At 3 h after the inoculation, the absorbance was measured at an OD of 600 nm, and when OD reached at 0.6, 500 µM of IPTG was added to overexpress the protein. After the addition of IPTG, cells were cultured for an additional 6 h, then centrifuged at 6000 rpm for 30 min. Cells overexpressed by IPTG were subsequently resuspended in cell lysis buffer (50 mM Tris-HCl (pH 8.0), 1 mM EDTA, 100 mM NaCl) and 1 mg/ml DNAse for 1 h.
Next, cells were disrupted using a sonicator and centrifuged at 6000 rpm for 30 min, after which the pellet was collected. The above procedure was then repeated with lysozyme. The pellet obtained after lysozyme treatment was sonicated using urea buffer (100 mM Tris-HCl (pH 8.0), 5 mM EDTA, 2 M Urea, 2% Triton X-100) for 30 min, then crushed, after which it was dialyzed with PBS to remove urea and the collected protein was confirmed by SDS-PAGE.
2.2.3. Measurement of recombinant protein activity
To confirm the enzymatic activity of urease A and B, their activity was measured using an activity kit (BioVision, USA) according to the manufacturer’s instructions. The results were expressed as the urease activity (mU/mg protein) divided by the protein concentration of the sample multiplied by the reaction time.
2.3. Immunization
Hyline brown hens (n = 12) were immunized intramuscularly with 100 µg/ml mixtures of purified urease A and B () emulsified with an equal volume of Freund’s incomplete adjuvant. Three booster injections were administered at 2 week intervals following the first injection. Two months after immunization, eggs were collected, first weekly for 1 month and then daily. The egg-yolk was stored after separation from the egg white, then washed with distilled water, pooled, and freeze-dried.
Table 1. Urease activity after anti-urease IgY treatment.
2.4. Purification of recombinant proteins for ELISA antibody titres
To confirm the activity of the antibody by ELISA, each recombinant protein was separated and purified as follows using a Ni-NTA column (QIAGEN, USA). To separate and purify the protein, the recombinant protein antigen production method was performed by dissolving the protein in the inclusion body collected with 8M Urea buffer through the IPTG overexpression process and the lysozyme lysis process. SDS-PAGE of each protein collected by elution buffer was conducted to confirm the separation purity. Protein quantification was performed according to the test method of the BCA protein quantification kit and the amount of the protein was confirmed.
2.5. Isolation of anti-urease antibodies from yolk
Eggs were collected and the egg whites and yolks were removed using a yolk separator. Next, egg yolk diluted with distilled water at 4× the yolk volume (w/v) was stirred for 1 h and then adjusted to pH 5 using 1 M HCl. This egg yolk solution was stored at −70°C for one day, after which it was allowed to increase to room temperature. The supernatant was then obtained by centrifugation at 9000 rpm and 4°C for 10 min, after which it was filtered through Whatman No. 1 filter paper using a vacuum pump. Next, ammonium sulfate was added to the supernatant at 25% (v/w) slowly under ice conditions. The ammonium sulfate-treated solution was then held overnight at 4°C, after which the pellet was collected by centrifugation at 9000 rpm and 4°C for 30 min and resuspended in 1× PBS.
2.6. ELISA test method for measuring the activity of anti-urease IgY antibody
The specificity of IgY was measured by the indirect ELISA method. First, samples were diluted in carbonate coating buffer at the concentration set for each antigen, after which 100 μl aliquots were added to wells in a 96-well polystyrene plate and held overnight at 4°C. After washing with PBS-T (phosphate buffer saline, 0.05% Tween 20, pH 7.4), cells were blocked with PBS buffer containing 3% BSA for 1 h at 37°C and washed as above. Negative controls, positive controls and samples were then diluted 2× each, after which 100 µl aliquots were added to individual wells and allowed to stand at 37°C for 1 h. Next, samples were washed three times with PBS, after which 100 μl aliquots of secondary antibody (anti-chicken: Sigma, USA) diluted in PBS were added to each well and incubated at 37°C for 1 h. After washing three times, 100 μl of TMB solution was added to each well and incubated at room temperature for about 10 min. The reaction was then stopped by adding 100 μl of 1.6 N H2SO4 to each well, at which time the absorbance was measured and expressed as an ELISA value.
2.7. Identification of anti-urease IgY antibody
The separated egg yolk antibodies were identified by Western-blot. SDS-PAGE was performed by 12% SDS-PAGE. Blotting membrane was incubated with anti-Chicken-IgY conjugate HRP in PBS-T containing 3% skim milk, ECL analysis was performed to detect anti-urease IgY antibodies.
2.8. In vitro potency assay of anti- H. pylori IgY
2.8.1. Agglutination test for H. pylori
To evaluate the effects of inhibiting the growth of H. pylori by IgY anti-urea, H. pylori was collected, diluted in PBS buffer and analyzed to determine the OD at 405 nm. Next, 100 μl aliquots of prepared antibiotics were placed in a 96-well plate and incubated at room temperature for 2 h. Next, 100 μl aliquots of IgY anti-ureas diluted 2–22 times in PBS were added to the wells and incubated for 2 h. After 2 h, the degree of aggregation was observed with an optical microscope at 200×.
2.8.2. Effects of anti-urease IgY activity on inhibition of urease production by H. pylori in a cell model
The specificity of IgY was measured by indirect ELISA method. First, purified IgY antibodies were coated with carbonate coating buffer on a 96-well polystyrene plate overnight at 4°C. After washing with PBS-T (phosphate buffer saline, 0.05% Tween 20, pH 7.4), purified recombinant Ure A or B proteins in PBS containing 3% BSA were incubated for 1 h at 37°C and washed as above. Next, anti-Ure A or B antibodies incubated for 1 h at 37°C and then washed 3 times with PBS-T. Anti-rabbit alkaline phosphatase secondary antibody was incubated at 37° C for 1 h. After washing three times, 100 μl of TMB solution was added to each well and incubated at room temperature for 10 min. For stop reaction, 100 μl of 1.6 N H2SO 4 was added to each well. Absorbance was measured for ELISA value.
The effects of H. pylori infection on the development of the human gastric cancer (AGS) cell line were confirmed. AGS cell lines were cultured in RPMI1640 medium supplemented with 10% FBS (Fetal Bovine Serum), after which the cells were detached using 0.25% trypsin-EDTA for adhesion. The AGS cell line was divided into 100 μl aliquots at a concentration of 1 × 105/ml and pre-cultured for 24 h to form a single layer of AGS. Next, H. pylori was resuspended in PBS at a concentration of 1 × 108 CFU/ml and then added to the monolayer of pre-incubated AGS. The anti-urease IgY was subsequently diluted in RPMI1640 medium to a and added to cell culture, incubated at 37°C and 10% CO2 for a minute. Upon completion of the reaction, the culture medium was collected and added to 96 wells of urea detection medium (2% urea, 0.03% phenol red) at 1: 1 and incubated at 37°C for 30 min. Next, the absorbance at 550 mM was measured to determine the amount of H. pylori-secreted urease.
2.9. Animal model confirmation of H. pylori infection
2.9.1. Animal experimental design
Eight-week-old specific pathogen-free male C57BL/6 wild-type mice were purchased from Orient Bio (Seongnam, Korea) and maintained under a 12-h light–dark schedule while being fed standard food pellets and water ad libitum for one week before experimental use.
2.9.2. H. pylori infection and treatment with IgY
After 24-hours of fasting, the mice (n = 10/group) were inoculated with H. pylori (1 × 108 CFU/200 µl/mouse) using oral gavage three times with a 2-day interval. Mice were orally administered IgY at 50, 100, 200 or 500 mg/kg twice a day for 18 days. One day after the final administration, mice were sacrificed and their gastric mucosa was collected for the detection of H. pylori using CLO kits according to the manufacturer’s instructions. All animal experiments and euthanasia procedures were conducted according to the Animal Care and Experiment Rules of the Chuncheon Bio-Industry Foundation (CBF IACUC No. 2017-010).
2.10. Statistics
The results were expressed as the means ± standard deviation (SD) for data in each indicated treated group. Differences among groups were analyzed by analysis of variance with post-hoc comparison of the means. Student’s t-tests were used to identify statistically significant differences between two groups. Differences were considered significant at p < 0.05.
3. Results
3.1. Confirmation of recombinant protein expression in E. coli
The proteins yielded from the lysate of the E. coli cells were confirmed by SDS-PAGE followed by western blot analysis. Overexpression of UreA and UreB was found upon SDS-PAGE and western blot analysis ( and ).
3.2. Anti-urease activity of IgY
Because we produced anti-urease IgY, we evaluated the IgY capacity to neutralize urease. The results showed that urease A and B were 7.54 and 6.46 mU/mg protein, respectively ().
3.3. Purity of anti-urease IgY antibody
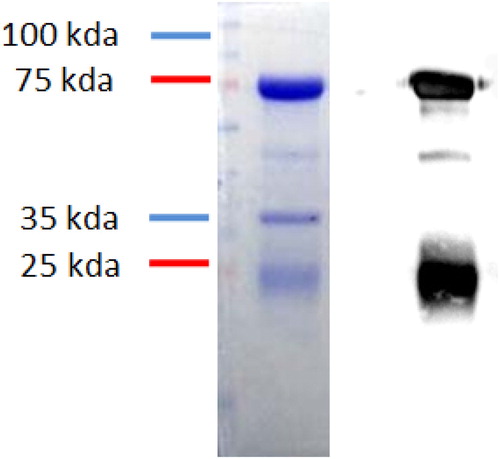
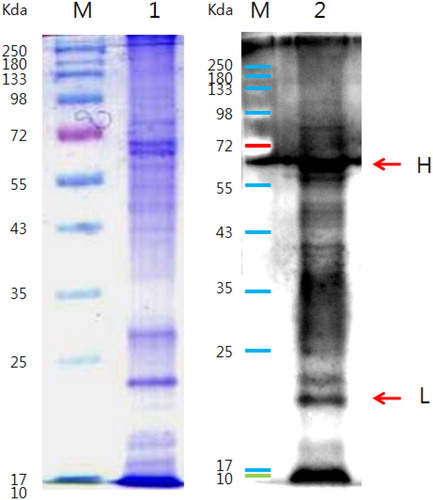
The anti-urease IgY was purified and it was confirmed by SDS-PAGE and western blot. The results revealed that the protein in 75 kda (heavy chain) and 25 kda (light chain) clearly shown in the SDG-PAGE The IgY properly bonded anti-chicken-IgY-HRP secondary antibody in western blot (). This result indicated the IgY properly purified and had functionally active.
3.4. Purity and binding affinity of anti-urease IgY antibody
The IgY antibody function is dependent on its purification, and the anti-urease IgY antibody has two chains, a heavy chain (75 kda) and a light chain (21 kda). The purity of the antibody was confirmed by SDS-page and western blot (). The results also indicated that antibody properly bound with the H. pylori by both chains of the antibody. Taken together, these findings confirmed that the anti-urease IgY has the potential to bind to H. pylori ().
3.5. In vitro potency of anti-urease IgY assay
3.5.1. Coagulation reaction confirmation test
Analysis of the purified anti-urease IgY at 40 mg/ml diluted from 2 to 22 times showed that the aggregation reaction occurred up to 0.155 mg/ml (; ). This aggregation reaction was the result of binding of the antibody to H. pylori, which inhibits the growth of H. pylori.
Figure 5. Coagulation reaction confirmation test result. Notes: Cohesion test results. IgY sample 40 mg/ml starting, 2-fold dilution, optical microscopy at 200× magnification.
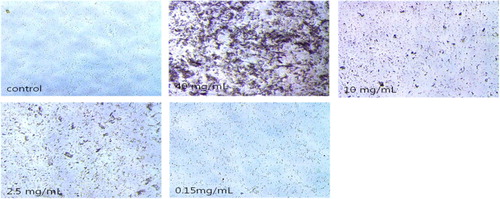
Table 2. Test results for coagulation by IgY concentration.
3.5.2. Urease inhibition
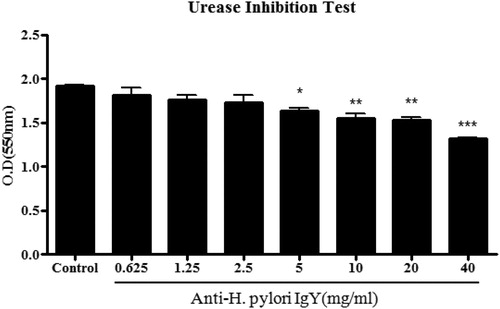
To investigate the effects of urease inhibition activity in vitro, anti-urease A-IgY and anti-urease B-IgY, H. pylori were infected into AGS cells, after which the urease inhibition activity was checked.
When H. pylori and anti-urease IgY were simultaneously treated with AGS cells and as observed with a microscope after 96 h of incubation for 2 h, the concentration of egg yolk antibody was found to have increased. These results showed that the OD of the group treated with the anti-urease IgY simultaneously decreased compared to the group treated with H. pylori alone, and a significant decrease was observed from 5 mg/ml (). These results suggest that H. pylori binds directly to anti-urease IgY, which inhibits its growth and infection of stomach cells. Anti-urease-IgY seems to be inhibited urease activity first and then inhibited the growth of H. pylori.
3.6. Evaluation of in vivo efficacy of anti-urease IgY
3.6.1. Weight change in H. pylori infected animal model
The body weight of each mouse was checked to see if there was any effect of H. pylori infection and treatment period (). No differences were observed among groups throughout the experimental period.
Table 3. Test animal weight change results.
3.6.2. Inhibitory effect of anti-urease IgY on H. pylori infection
At the end of the study, the gastric tissues were removed from the mouse and H. pylori infection was confirmed using a CLO kit. The results showed that H. pylori infection was prevented in a dose-dependent manner (). In addition, H. pylori also decreased in a dose-dependent manner when isolated from the infected mouse after anti-urease IgY treatment (). These results indicate that anti-urease IgY has the potential to prevent H. pylori infection in the humanized mouse model.
Table 4. Reactivity in CLO Kit of H pylori infected mice gastric mucosa after treatment with anti-urease IgY.
Table 5. Identification of H pylori through culture of gastric mucosa from infected mice after treatment with anti-urease IgY.
4. Discussion
IgY is a good alternative for analysis of mammalian immunoglobulins for various immunological diagnostic purposes as well as in functional foods for prophylactic and therapeutic purposes (De Meulenaer & Huyghebaert, Citation2001; Diraviyam et al., Citation2014). IgY has low lipid so that it influences on the immune modulation in the murine model (Nelson, Katayama, Mine, & Duarte, Citation2007). IgY is also more stable than mammalian immunoglobulins at different pH, temperature and proteolytic enzyme (Jaradat & Marquardt, Citation2010; Punyokun, Hongprayoon, Srisapoome, & Sirinarumitr, Citation2013). Taken together, these findings indicate IgY would be suitable for use as a functional food.
H. pylori has remarkable acid-resistance in the gastric environment via its production of urease (Huang, Sweeney, Guillemin, & Amieva, Citation2017). Thus, antibodies against urease in H. pylori disrupt H. pylori activity and infection. Most studies have investigated anti-IgY against whole H. pylori (Shin et al., Citation2002). The results of such studies have shown that IgY have a compromised potential to decrease H. pylori activity because of a lack of specificity and cross-reaction with intestinal normal flora (Malekshahi, Gargari, Rasooli, & Ebrahimizadeh, Citation2011). Normally, urease enzyme help to growth the H.pylori in the gastric mucosa. The present study was conducted to develop a method for production of egg yolk anti-urease IgY against H. pylori for the prevention and treatment of H. pylori infection in humans. Currently available treatment regimes have been found to be ineffective against H. pylori, especially antibiotics; therefore, it is necessary to develop alternative methods for the control and prevention of H. pylori infection. The results of the present study indicate that IgY is a good alternative to currently available methods as a passive immunization and therapeutic for H. pylori. IgY was neutralized the heat labile toxin of E coli (Akita, Li-Chan, & Nakai, Citation1998).
The results of the present study demonstrated the effects of anti-urease IgY prepared from egg yolks by immunized hens with recombinant H. pylori-UreA and UreB antigen. Moreover, the results revealed a potential advantage of using IgY with anti-H. pylori activity to control H. pylori associated gastric diseases. Most studies conducted to date have focused on the production of IgY against H. pylori whole cells and H. pylori lysates. In this study, our purified recombinant enzyme proteins (UreA and UreB) were used to immunize hens, after which anti-H. pylori isolated from the egg yolks of the immunized hens was purified to increase the antibody activity toward specific virulent factors of this pathogen. As a result, the IgY can be used to inhibit H. pylori infection in humans (Ky & Ahn, Citation2007; Shin et al., Citation2002). Both in vitro and in vivo results revealed that the investigated recombinant enzyme proteins (UreA and UreB) have the potential for use as antigens in vaccines against H. pylori (Malekshahi et al., Citation2011). Moreover, the anti-H. pylori urease IgY could be used for therapeutic intervention of H. pylori associated gastric diseases (Dubois et al., Citation1998; Koesling, Lucas, Develioglou, Aebischer, & Meyer, Citation2001; Michetti et al., Citation1994; Suzuki et al., Citation2004).
To evaluate the effects of aggregation reaction to inhibit the growth of H. pylori, the effects of anti-urease IgY antibody at concentrations of 40–0.155 mg/ml were investigated. The results showed that anti-urease IgY appears to be inhibited by preventing propagation of H. pylori activity first, followed by inhibition of its growth. The results also showed that the concentration of OD in the group treated with the anti-urease IgY antibody simultaneously decreased compared to the group treated with H. pylori alone, with a significant decrease observed from 5 mg/ml. In a previous study conducted by Phadis et al., the extracellular urease of H. pylori gradually increased with time under bacterial culture conditions (Phadnis et al., Citation1996).
Following administration of anti-urease IgY antibody, the gastric tissues of mice were removed and H. pylori infection was confirmed using a CLO kit. The results revealed concentration-dependent effects in the treated group. Additionally, the results of the CLO kit were partially positive, and a positive result was found in the group not infected with H. pylori. Furthermore, extracellular urease detected from H. pylori appeared to be very strong and the efficacy of the test was believed to be high as well; however, some partial positive specimens were found indicating cross reactivity with other urease producing pathogens in the gastric mucosa. To overcome this, other suitable markers should be included in future studies.
The main limitation of the study is that no marker enzymes other than urease (e.g. oxidase, catalase, nitrate reduction or H2S production) were observed in H. pylori infected stomach tissues. Additionally, even though we also checked the histopathological findings and the microscopic observations showed that H. pylori infected samples showed some infiltration of immune cells into the gastric mucosa, no significant differences from normal tissues were observed. In addition, there were no significant differences between the group administered anti H. pylori egg yolk IgY antibody and the group administered H. pylori alone, except at the highest concentration.
5. Conclusion
Taken together, the in vitro and in vivo results of this study indicated that anti-urease IgY antibody would be a reasonable alternative approach for passive immunization using hens immunized with recombinant H. pylori urease. This indicates that further research investigating recombinant protein enzyme H. pylori IgY production for management and treatment of H. pylori infection is warranted. In addition, IgY has the potential for use as an alternative antibiotic and functional food or health food for the prevention and treatment of H. pylori infection in humans. However, numerous problems must be resolved prior to clinical use.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aebischer, T., Schmitt, A., Walduck, A. K., & Meyer, T. F. (2005). Helicobacter pylori vaccine development: Facing the challenge. International Journal of Medical Microbiology, 295, 343–353. doi: 10.1016/j.ijmm.2005.06.005
- Akita, E. M., Li-Chan, E. C. Y., & Nakai, S. (1998). Neutralization of enterotoxigenic Escherichia coli heat-labile toxin by chicken egg yolk immunoglobulin Y and its antigen-binding fragments. Food and Agricultural Immunology, 10(2), 161–172. doi: 10.1080/09540109809354979
- Ayala, G., Escobedo-Hinojosa, W. I., de la Cruz-Herrera, C. F., & Romero, I. (2014). Exploring alternative treatments for Helicobacter pylori infection. World Journal of Gastroenterology, 20, 1450–1469. doi: 10.3748/wjg.v20.i6.1450
- Backert, S., Neddermann, M., Maubach, G., & Naumann, M. (2016). Pathogenesis of Helicobacter pylori infection. Helicobacter, 21, 19–25. doi: 10.1111/hel.12335
- Backert, S., Tegtmeyer, N., & Selbach, M. (2010). The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter, 15, 163–176. doi: 10.1111/j.1523-5378.2010.00759.x
- Berger, H., Marques, M. S., Zietlow, R., Meyer, T. F., Machado, J. C., & Figueiredo, C. (2016). Gastric cancer pathogenesis. Helicobacter, 21, 34–38. doi: 10.1111/hel.12338
- Clyne, M., Dolan, B., & Reeves, E. P. (2007). Bacterial factors that mediate colonization of the stomach and virulence of Helicobacter pylori. FEMS Microbiology Letters, 268, 135–143. doi: 10.1111/j.1574-6968.2007.00648.x
- Czinn, S. J., & Blanchard, T. (2011). Vaccinating against Helicobacter pylori infection. Nature Reviews Gastroenterology & Hepatology, 8, 133–140. doi: 10.1038/nrgastro.2011.1
- De Meulenaer, B., & Huyghebaert, A. (2001). Isolation and purification of chicken egg yolk immunoglobulins: A review. Food and Agricultural Immunology, 13(4), 275–288. doi: 10.1080/09540100120094537
- Diraviyam, T., Zhao, B., Wang, Y., Schade, R., Michael, A., & Zhang, X. (2014). Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: A systematic review and meta-analysis. PLoS One, 9((5)), e97716. doi: 10.1371/journal.pone.0097716
- Dubois, A., Lee, C. K., Fiala, N., Kleanthous, H., Mehlman, P. T., & Monath, T. (1998). Immunization against natural Helicobacter pylori infection in nonhuman primates. Infection and Immunity, 66, 4340.
- Foegeding, N., Caston, R., McClain, M., Ohi, M., & Cover, T. (2016). An overview of Helicobacter pylori VacA toxin biology. Toxins, 8, 173. doi: 10.3390/toxins8060173
- Hong, K. S., Ki, M. R., Ullah, H. M. A., Lee, E. J., Kim, Y. D., Chung, M. J., … Jeong, K. S. (2018). Preventive effect of anti-VacA egg yolk immunoglobulin (IgY) on Helicobacter pylori-infected mice. Vac, 36(3), 371–380.
- Huang, J. Y., Sweeney, E. G., Guillemin, K., & Amieva, M. R. (2017). Multiple acid Sensors control Helicobacter pylori colonization of the stomach. PLOS Pathogens, 13(1), e1006118. doi: 10.1371/journal.ppat.1006118
- Israel, D. A., & Peek, R. M. (2001). Review article: Pathogenesis of Helicobacter pylori-induced gastric inflammation. Alimentary Pharmacology and Therapeutics, 15, 1271–1290. doi: 10.1046/j.1365-2036.2001.01052.x
- Jaradat, Z. W., & Marquardt, R. R. (2010). Studies on the Stability of chicken IgY in different sugars, complex carbohydrates and food materials. Food and Agricultural Immunology, 12(4), 263–272. doi: 10.1080/09540100020008137
- Kabir, S. (2007). The current status of Helicobacter pylori vaccines: A review. Helicobacter, 12, 89–102. doi: 10.1111/j.1523-5378.2007.00478.x
- Kao, C. Y., Sheu, B. S., & Wu, J. J. (2016). Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomedical Journal, 39(1), 14–23. doi: 10.1016/j.bj.2015.06.002
- Ki, M. R., Hwang, M., Kim, A. Y., Lee, E. M., Lee, E. J., & Lee, M. M. (2014). Role of vacuolating cytotoxin VacA and cytotoxin-associated antigen CagA of Helicobacter pylori in the progression of gastric cancer. Molecular and Cellular Biochemistry, 396, 23–32. doi: 10.1007/s11010-014-2138-8
- Koesling, J., Lucas, B., Develioglou, L., Aebischer, T., & Meyer, T. F. (2001). Vaccination of mice with live recombinant Salmonella ryphimurium aro A against H. Pylori: Parameters associated with prophylactic and therapeutic vaccine efficacy. Vac, 12(413), 420.
- Kwak, H. W., Choi, I. J., Cho, S. J., Lee, J. Y., & Kim, C. G. (2014). Characteristics of gastric cancer according to Helicobacter pylori infection status. Journal of Gastroenterology and Hepatology, 29, 1671–1677. doi: 10.1111/jgh.12605
- Ky, K., & Ahn, D. U. (2007). Preparation of immunoglobulin Y from egg yolk using ammonium sulfate precipitation and ion exchange chromatography. Poultry Science, 86(2), 400–407. doi: 10.1093/ps/86.2.400
- Malekshahi, Z. V., Gargari, S. L., Rasooli, I., & Ebrahimizadeh, W. (2011). Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Microbial Pathogenesis, 51(5), 366–372. doi: 10.1016/j.micpath.2011.06.002
- Michetti, P., Corthésy-Theulaz, I., Davin, C., Haas, R., Vaney, A.-C., Heitz, M., … Blum, A. (1994). Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology, 107, 1002–1011. doi: 10.1016/0016-5085(94)90224-0
- Milani, M., Sharifi, Y., Rahmati, Y. M., Somi, M. H., & Akbarzadeh, A. (2015). Immunology and vaccines and nano vaccines for Helicobacter pylori infection. Expert Review of Vaccines, 14, 833–840. doi: 10.1586/14760584.2015.1008460
- Nelson, R., Katayama, S., Mine, Y., & Duarte, J. (2007). Immunomodulating effects of egg yolk low lipid peptic digests in a murine model. Food and Agricultural Immunology, 18(1), 1–15. doi: 10.1080/09540100601178623
- Palframan, S. L., Kwok, T., & Gabriel, K. (2012). Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Frontiers in Cellular and Infection Microbiology, 2, 92. doi: 10.3389/fcimb.2012.00092
- Phadnis, S. H., Parlow, M. H., Levy, M., Ilver, D., Caulkins, C. M., Connors, J. B., & Dunn, B. E. (1996). Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infection and Immunity, 64, 905–912.
- Plummer, M., Franceschi, S., Vignat, J., Forman, D., & de Martel, C. (2015). Global burden of gastric cancer attributable to Helicobacter pylori. International Journal of Cancer, 136, 487–490. doi: 10.1002/ijc.28999
- Punyokun, K., Hongprayoon, R., Srisapoome, P., & Sirinarumitr, T. (2013). The production of anti-vibrio harveyi egg yolk immunoglobulin and evaluation of its stability and neutralisation efficacy. Food and Agricultural Immunology, 24(3), 279–294. doi: 10.1080/09540105.2012.684203
- Rahman, S., Van, N. S., Icatlo Jr, F. C., Umeda, K., & Kodama, Y. (2013). Oral passive IgY-based immune therapeutics. A novel solution for prevention and treatment of alimentary tract diseases. Human Vaccines & Immunotherapeutics, 9, 1039–1048. doi: 10.4161/hv.23383
- Shin, J. H., Nan, S. W., Kim, J. T., Yoon, J. B., Bang, W. G., & Roe, I. H. (2003). Identification of immunodominant Helicobacter pylori proteins with reactivity to H. Pylori-specific egg-yolk immunoglobulin. Journal of Medical Microbiology, 52, 217–222. doi: 10.1099/jmm.0.04978-0
- Shin, J. H., Yang, M., Nam, S. W., Kim, J. T., Myung, N. H., Bang, W. G., & Roe, I. H. (2002). Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of H. Pylori infection. Clinical and Diagnostic Laboratory Immunology, 9, 1061–1066.
- Shiota, S., Suzuki, R., & Yamaoka, Y. (2013). The significance of virulence factors in Helicobacter pylori. Journal of Digestive Diseases, 14, 341–349. doi: 10.1111/1751-2980.12054
- Sutton, P., & Lee, A. (2011). Helicobacter pylori vaccines-the current status. Alimentary Pharmacology and Therapeutics, 14, 1107–1118. doi: 10.1046/j.1365-2036.2000.00825.x
- Suzuki, H., Nomura, S., Masaoka, T., Goshima, H., Kamata, N., Kodama, Y., … Hibi, T. (2004). Effect of dietary anti-Helicobacter pylori-urease immunoglobulin Y on Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics, 20, 185–192. doi: 10.1111/j.1365-2036.2004.02027.x
- Svennerholm, A. M., & Lundgren, A. (2007). Progress in vaccine development against Helicobacter pylori. FEMS Immunology & Medical Microbiology, 50, 146–156. doi: 10.1111/j.1574-695X.2007.00237.x
- Tatematsu, M., Tsukamoto, T., & Mizoshita, T. (2005). Role of Helicobacter pylori in gastric carcinogenesis: The origin of gastric cancers and heterotopic proliferative glands in Mongolian gerbils. Helicobacter, 10, 97–106. doi: 10.1111/j.1523-5378.2005.00305.x
- Wang, B., Yang, J., Cao, S., Wang, H., & Pan, X. (2014). Preparation of specific anti-Helicobacter pylori yolk antibodies and their antibacterial effects. International Journal of Clinical and Experimental Pathology, 7, 6430–6637.
- Yong, X., Tang, B., Li, B. S., Xie, R., Hu, C. J., Luo, G., … Yang, S. M. (2015). Helocobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Comm Sig, 30, 13.
- Zajac, J., Schubert, A., Dyek, T., & Oelkrung, C. (2017). Igy antibodies for the prevention and treatment of Helicobacter pylori infections. Journal of Medical Microbiology & Diagnosis, 6, 2–5. doi: 10.4172/2161-0703.1000254