ABSTRACT
Measures for protecting against PM2.5 (particulate matter with aerodynamic diameters ≤2.5 µm), which exacerbates respiratory diseases, have not been established. The present study investigated the effects of extracts of curry powder and its components on pro-inflammatory responses, extracellular and intracellular reactive oxygen species (ROS) production, induced by the PM2.5 component, DEP (diesel exhaust particles). Airway epithelial cells were exposed to DEP in the presence of curry powder, or its major and/or anti-inflammatory components, clove and turmeric. Curry powder, clove, and turmeric inhibited DEP-induced IL-6 release and extracellular ROS; in the absence of clove and turmeric, these effects of curry powder were mild but similar. Among the other curry spices, cinnamon decreased IL-6 and extracellular ROS, and coriander decreased IL-6 alone. This is the first report on the protective effects of extracts of curry powder and its components, against PM2.5-induced airway inflammation, which may be partly through inhibition of extracellular ROS.
GRAPHICAL ABSTRACT
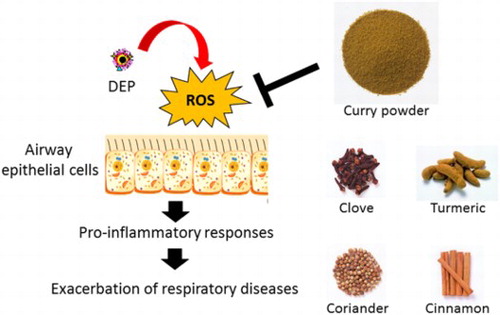
1. Introduction
Particulate matter with aerodynamic diameters ≤2.5 µm (PM2.5) is generated by anthropogenic activities, such as combustion of coal and other fossil fuels, and increases the health risk, especially in Asian countries (Cohen et al., Citation2017). Human exposure to PM2.5 occurs through inhalation, and its small size allows deep penetration into the respiratory tract. Some of the components of PM2.5 are known to contain hazardous substances, which can cause exacerbation of respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD) by disruption of the respiratory system (Schwarze et al., Citation2006).
The diesel exhaust particle (DEP), a representative component of PM2.5, is reported to affect respiratory inflammation. In previous in vivo studies on mice, DEP increased the number of inflammatory cells in bronchial alveolar lavage fluids (Ichinose, Furuyama, & Sagai, Citation1995), and enhanced the antigen-induced airway inflammation and local cytokine expression (Takano et al., Citation1997). In previous in vitro studies, DEP induced pro-inflammatory response via interleukin (IL)-6 release from human bronchial epithelial cells (Steerenberg et al., Citation1998), and increased oxidative stress in human airway epithelial cells, which can cause pro-inflammatory responses (Baulig et al., Citation2003; Li, Wang, Oberley, Sempf, & Nel, Citation2002). Therefore, DEP induces airway inflammation via cytokine release and reactive oxygen species (ROS) production from airway epithelial cells and can affect the respiratory diseases. It is hard to avoid invasion of PM2.5 into body due to the small size; however, measures against human health risks after inhalation of PM2.5 are effective. The effective measures are required to protect against cytokine release and ROS production.
“Curry” is a popular food in Asian countries and can be easily ingested on a daily basis. Epidemiological studies have shown that curry consumption improves cognitive performance of non-demented elderly Asians (Ng et al., Citation2006) and that consumption of a single serving of curry improves postprandial endothelial function in healthy male subjects (Nakayama et al., Citation2014). Efficacy of curry on respiratory system is known: it has been reported to improve pulmonary function in Asian elderly adults. Especially, forced expiratory volume in one second (FEV1) and FEV1/forced vital capacity (FVC) in smokers who consume curry were substantially higher than those in smokers who do not consume curry (Ng, Niti, Yap, & Tan, Citation2012). Therefore, curry powder possibly protects against respiratory degeneration after inhalation of PM2.5. Although the components of curry powder such as clove and turmeric are reported to have anti-inflammatory and anti-oxidative effects (Bachiega, de Sousa, Bastos, & Sforcin, Citation2012; Klawitter et al., Citation2012; Koo, Lee, Chung, Ko, & Kim, Citation2004; Yadav & Bhatnagar, Citation2007), there are few studies on the effects of curry powder itself.
In the present study, we investigated whether extracts of curry powder and its components protect against PM2.5-induced airway inflammation. We measured this based on IL-6 release from human airway epithelial cells, and extracellular and intracellular ROS production after exposure to DEP in the presence and absence of extracts of curry powder or the components.
2. Materials and methods
2.1. Cell culture
The BEAS-2B cell line, which is derived from human bronchial epithelial cells and is transformed by an adenovirus (12-SV40 hybrid virus), was purchased from European Collection of Cell Cultures (Salisbury, Wiltshire, UK). Airway epithelial cells were seeded in 96-well or 12-well collagen I coated plates and incubated for 72 h to reach semi-confluence in a serum free-medium, LHC-9 (Life technologies, Carlsbad, CA, USA), at 37°C in a humidified atmosphere containing 5% CO2.
2.2. Preparation of DEP and extracts of curry powder and its components
DEP was collected as described previously (Nakamura et al., Citation2012). Briefly, DEP was collected at the National Institute for Environmental Studies (Tsukuba, Ibaraki, Japan). An 8 L-diesel engine (J08C, Hino Motors, Tokyo) that was not fitted with after-treatment devices was powered under steady-state conditions (speed = 2000rpm; engine torque = 0 Nm; diesel fuel = JIS No. 2) for 5 h. Particles were electrostatically (−27 kVolts) collected at a distance of ≈10 m from the engine onto dichloromethane washed gold discs at a flow rate of 20 L/min using a SSPM-100 sampler (Shimadzu, Kyoto, Japan). The DEP suspension was sonicated with an ultrasonic disrupter (UD-201, Tomy Seiko, Tokyo, Japan).
Powdered curry and the all components (clove, turmeric, ginger, black pepper, chenpi, cumin, coriander, fenugreek, cardamom, nutmeg, fennel, and cinnamon) were provided by House Foods Corporation (Yotsukaido, Chiba, Japan). Ten gram each of powdered curry and its components were extracted overnight with 50 mL of acetone. Acetone soluble fractions were centrifuged at 1,670 ×g for 10 min. The supernatants were passed through filters (Advantec No. 5B, Toyo Roshi Kaisha, Ltd., Tokyo, Japan), and the resultant crude extracts were evaporated at 30°C and then dried under a gentle stream of nitrogen gas. Blank was also prepared in the same manner. The dried extracts were resuspended in ethanol to give a final concentration of 200 mg/mL and stored at −80°C until assay.
2.3. Experimental protocol
After airway epithelial cells were grown to semi-confluence in LHC-9, cells were exposed to DEP in the presence and absence of each extract for 6 or 24 h. Cytokine production, cell viability, extracellular and intracellular ROS were examined using enzyme-linked immunosorbent assay (ELISA), Water Soluble Tetrazolium Salts (WST-1) assay, and a CM-H2DCFDA fluorescent probe, respectively.
2.4. Cytokine in the culture supernatants
After exposure to DEP, in the presence and absence of each extract for 24 h, the medium was harvested and centrifuged at 400 g for 5 min to remove the floating cells. The final supernatants were stored at −80°C until analysis. The level of IL-6 (Thermo Scientific, Waltham, MA, USA) in the culture supernatants was measured by ELISA according to the manufacturer’s instructions. Absorbance was measured using iMarkMicroplate Absorbance Reader (Bio-Rad Laboratories) at a wavelength of 450 nm and a reference wavelength of 550 nm. The detection limit of IL-6 was <1.5 pg/mL.
2.5. Cell viability
After exposure to DEP, in the presence and absence of each extract for 24 h, cell viability was measured by WST-1 assay using Premix WST-1 Cell Proliferation Assay System (TaKaRa Bio Inc., Shiga, Japan). In brief, 7 μL of WST-1 reagent was added to each well of the 96-well plate (the volume of WST-1 reagent was 1/10 of original culture medium) and mixed well by gently rocking the plate. Then the airway epithelial cells were incubated at 37°C for 3 h; absorbance was measured using iMarkMicroplate Absorbance Reader (Bio-Rad Laboratories, Hercules, CA, USA) at a wavelength of 450 nm and a reference wavelength of 630 nm. Results were expressed as the percentage of viable cells, compared to untreated cells (vehicle).
2.6. ROS generation
We used a fluorescent probe, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA), to measure the production of intracellular and extracellular ROS. For measuring intracellular ROS, the cells were incubated with 5 μM of CM-H2DCFDA for 30 min after exposure to DEP, in the presence or absence of each extract for 3 h, which allowed the dye to enter the cells. The cells were then washed to remove the extracellular dye, and were exposed to DEP, in the presence or absence of each extract again. The fluorescence intensity during 0–3 h (excitation at 485 nm, emission at 530 nm) was measured.
For measuring extracellular ROS, 1 mM CM-H2DCFDA was hydrolyzed with 0.01 M NaOH at 37°C under protection from light for 30 min, to react with ROS under cell-free conditions. In the presence or absence of each extract, DEP was mixed with 5 μM of CM-H2DCFDA. The fluorescence intensity during 0–3 h (excitation at 485 nm, emission at 530 nm) was measured.
2.7. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) for each experimental group (n = 3−4). Differences among groups were analyzed using the Tukey multiple comparison test (Excel Statistics 2012, Social Survey Research Information, Tokyo). A p-value <0.05 was accepted as significant.
3. Results
3.1. Effects of curry powder, clove, and turmeric extracts on DEP-induced pro-inflammatory responses and cellular viability of human airway epithelial cells
To evaluate whether extract of curry powder has protective effect against DEP-induced pro-inflammatory responses in airway epithelial cells, we examined IL-6 release and cell viability after 24-h exposure.
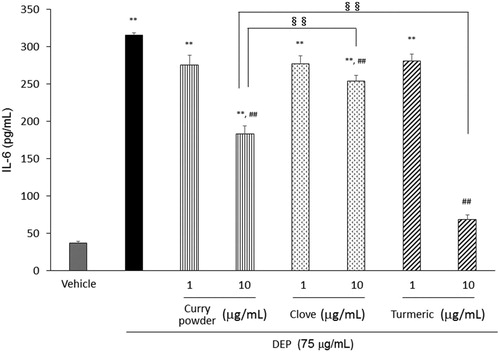
Exposure to DEP resulted in a significant increase in IL-6 release, compared to that in the vehicle. Curry powder, clove, and turmeric significantly attenuated the IL-6 release caused by DEP (). The level of efficiency was in order of turmeric>curry powder>clove.
Figure 1. Effects of curry powder, clove, and turmeric extracts on DEP-induced IL-6 release in human airway epithelial cells. Cells were exposed to the indicated concentrations of DEP and each extract for 24 h. IL-6 release was measured by ELISA. Data are represented as mean ± SE of three individual cultures. **p < 0.01, versus vehicle; ##p < 0.01, versus DEP; §§ p < 0.01, vs each other.
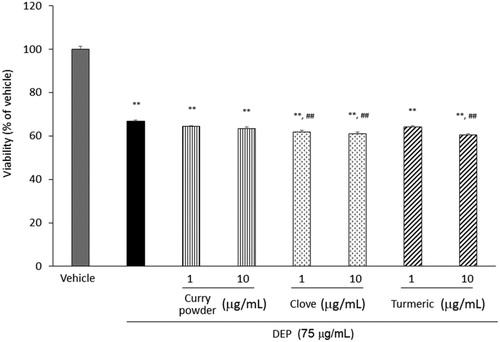
At a dose of 75 µg/mL, DEP significantly decreased the viability of airway epithelial cells. Exposure to DEP in the presence of curry powder, clove, and turmeric extracts showed similar viability, however, DEP in the presence of clove and turmeric extracts slightly decreased the cell viability compared to DEP alone ().
Figure 2. Effects of curry powder, clove, and turmeric extracts on DEP-induced viability of human airway epithelial cells. Cells were exposed to the indicated concentrations of DEP and each extract for 24 h. Viability was measured by WST-1 assay. Data are represented as mean ± SE of four individual cultures. **p < 0.01, versus vehicle; ##p < 0.01, versus DEP.
3.2. Effects of curry powder, clove, and turmeric extracts on DEP-induced ROS generation
3.2.1. Extracellular ROS
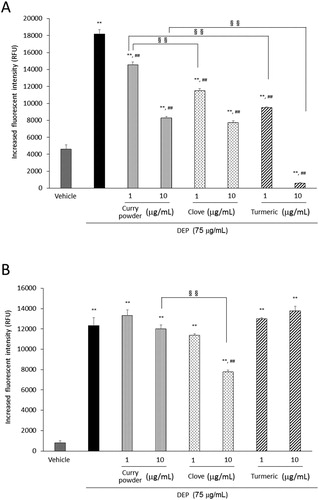
Exposure to DEP increased ROS production, compared to that of the vehicle. Curry powder, clove, and turmeric significantly decreased DEP-induced ROS ((A)). Turmeric showed the highest level of efficiency. At a dose of 10 µg/mL, curry powder and clove showed similar inhibitory responses.
Figure 3. Effects of curry powder, clove, and turmeric extracts on DEP-induced extracellular (A) and intracellular (B) ROS. The extracellular levels of ROS were measured after exposure to DEP and each extract reacted with CM-H2DCFDA fluorescent probe for 3 h. Cells were exposed to DEP and each extract, and intracellular levels of ROS after 6 h exposure were also measured. Data are represented as mean ± SE of four individual cultures. **p < 0.01, versus vehicle; ##p < 0.01, versus DEP; §§ p < 0.01, vs each other.
3.2.2. Intracellular ROS
We examined ROS generation after 6-h exposure to DEP; it increased ROS generation, compared to that of the vehicle. Only clove extract significantly attenuated the DEP-induced ROS ((B)).
3.3. Effects of curry powder extract on DEP-induced pro-inflammatory response and ROS generation in human airway epithelial cells, in the absence of clove and turmeric
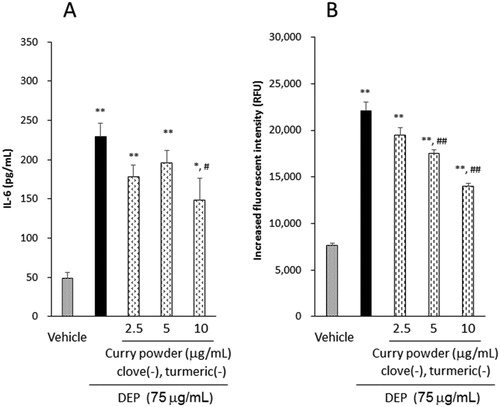
To identify the effects of spices, except clove and turmeric, we examined the effect of curry powder extract on DEP-induced IL-6 release and ROS generation, in absence of clove and turmeric. Curry powder, in the absence of clove and turmeric, mildly but similarly attenuated the IL-6 release and extracellular ROS generation induced by DEP ((A, B)) and we observed a decrease of approximately 0.9 times in IL-6 release and 0.7 times in ROS level, compared to curry powder in the presence of clove and turmeric.
Figure 4. Effects of curry powder extract on DEP-induced IL-6 release (A) and extracellular ROS (B) in the absence of clove and turmeric. Cells were exposed to the indicated concentrations of DEP and extract for 24 h. IL-6 release was measured by ELISA. The extracellular levels of ROS were measured after DEP and extract reacted with CM-H2DCFDA fluorescent probe for 3 h. Data are represented as mean ± SE of three or four individual cultures. **p < 0.01, *p < 0.05, versus vehicle; ##p < 0.01, #p < 0.05, versus DEP.
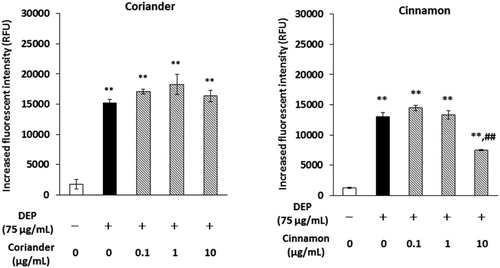
Curry powder includes a variety of spices such as ginger, black pepper, chenpi, cumin, coriander, fenugreek, cardamom, nutmeg, fennel, and cinnamon. We examined the effects of extracts of these spices on DEP-induced IL-6 releases and ROS generation. Among them, coriander and cinnamon significantly attenuated the DEP-induced IL-6 release (). Cinnamon decreased extracellular DEP-induced ROS generation, whereas coriander did not ().
Figure 5. Effects of various extracts of curry powder components on DEP-induced IL-6 release in human airway epithelial cells. Cells were exposed to the indicated concentrations of DEP and each extract for 24 h. IL-6 release was measured by ELISA. Data are represented as mean ± SE of three individual cultures. **p < 0.01, *p < 0.05, versus vehicle; ##p < 0.01, #p < 0.05, versus DEP.
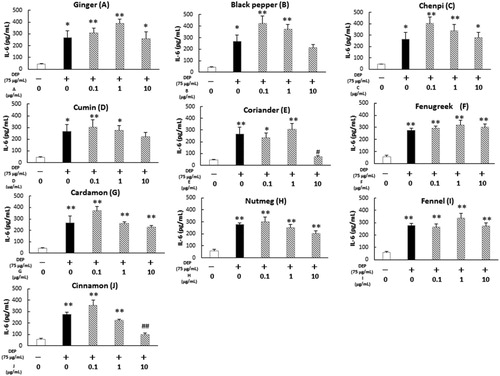
Figure 6. Effects of coriander or cinnamon extract on DEP-induced extracellular ROS. The extracellular level of ROS was measured after DEP and each extract reacted with CM-H2DCFDA fluorescent probe for 3 h. Data are represented as mean ± SE of four individual cultures. **p < 0.01, versus vehicle; ##p < 0.01, versus DEP.
4. Discussion
In the present study, we determined the effects of extracts of curry powder and its components on DEP-induced respiratory dysfunction. Clove and turmeric are known to have anti-inflammatory and anti-oxidative effects (Bachiega et al., Citation2012; Klawitter et al., Citation2012; Koo et al., Citation2004; Yadav & Bhatnagar, Citation2007), and they are ingredients of curry powder. Therefore, the effects of curry powder were compared to those of clove and turmeric. We demonstrated that DEP affected cell viability and elevated IL-6 release from airway epithelial cells and that curry powder, and clove and turmeric, attenuated DEP-induced pro-inflammatory responses and extracellular ROS production. Similarly, in the absence of clove and turmeric, curry powder attenuated DEP-induced pro-inflammatory responses and extracellular ROS. Cinnamon significantly attenuated both of them, whereas coriander decreased only pro-inflammatory responses.
For the first time, we found that curry powder extract can attenuate DEP-induced airway inflammation. Airway epithelial cells are the sources of pro-inflammatory cytokines such as IL-6, which is induced in response to environmental insults and plays an important role during the inflammation of the respiratory system by stimulating lymphocytes and up-regulating mucin secretion (Chen et al., Citation2003; Thacker, Citation2006). Various air pollutants including diesel exhaust particles, ambient PM2.5, extracted PM2.5, and Asian sand dust particles, stimulate IL-6 releases from airway epithelial cells (Honda et al., Citation2014, Citation2017; Onishi et al., Citation2018; Steerenberg et al., Citation1998). Previous studies have reported that food components such as curcumin from turmeric, rosmarinic acid from Lamiaceae herbs, proanthocyanidins from cacao, emodin from the Chinese herb, Radix et Rhizoma Rhei, decreased DEP-induced pro-inflammatory responses, such as IL-6, TNF-α, IL-1β, or ICAM-1 release (Nemmar, Al-Salam, Yuvaraju, Beegam, & Ali, Citation2015; Nemmar, Subramaniyan, & Ali, Citation2012; Sanbongi et al., Citation2003; Yasuda et al., Citation2008). Eugenol in clove has also been reported to inhibit DEP-induced pulmonary inflammation, which is characterized by higher polymorphonuclear cell infiltration and alveolar collapse (Zin et al., Citation2012). Although previous studies have focused on the pure chemicals present in foods, for the first time we identified the effect of curry powder as mixture of various food components against DEP, and found that the inhibition of IL-6 release does not come from cytotoxicity of spices because DEP in the presence of spices showed similar viability compared to DEP alone ().
As the mechanisms by which curry powder extract attenuated DEP-induced IL-6 releases are unknown, the present study suggested that curry powder can scavenge extracellular ROS. DEP has a carbon core to which organic chemical components, including polycyclic aromatic hydrocarbons (PAHs), and metals are adsorbed (Berube et al., Citation1999). These chemicals produce extra- and intracellular ROS. Baulig et al. (Citation2003) have reported that the antioxidants, mannitol, and N-acetylcysteine (NAC) reduce ROS production that is induced by organic extracts of DEP (OE-DEP) in human airway epithelial cells. Mannitol and NAC are known to be extracellular and intracellular antioxidants, respectively. For example, carbon black as the carbon core of DEP has produced ROS both in non-biological systems and in cell lines (Diabaté, Bergfeldt, Plaumann, Ubel, & Weiss, Citation2011). Quinone derivatives in DEP have been reported to be responsible for the production of free radicals under the cell-free conditions (Valavanidis, Fiotakis, Vlahogianni, Papadimitriou, & Pantikaki, Citation2006), and to be involved in indirect free radical production associated with the activity of enzymes like NADPH-cytochrome P-450 reductase in the cells (Kumagai et al., Citation1997). Benzo(a)pyrene, as a PAH, has induced oxidative stress through the aryl hydrocarbon receptor signaling pathway in human keratinocytes (Tsuji et al., Citation2011). Metals such as Pt and Cr have been shown to induce ROS in the airway epithelial cells (Schmid, Zimmermann, Krug, & Sures, Citation2007). Based on the present study, curry powder extract decreased the DEP-induced exogenous ROS. In brief, curry powder can be effective against the chemicals adsorbed to DEP, which have the ability to produce free radicals directly under cell-free conditions.
Curry powder is a mixture of cloves, turmeric, and other spices. Eugenol in clove and curcumin in turmeric are well known for their anti-inflammatory and anti-oxidative effects (Srinivasan, Citation2014). Furthermore, clove and/or turmeric extract has also decreased LPS- or IL-1β-induced IL-6 level (Bachiega et al., Citation2012; Klawitter et al., Citation2012) and oxidative stress (Koo et al., Citation2004; Yadav & Bhatnagar, Citation2007), which is in accordance with the present study. Interestingly, in the absence of clove and turmeric, curry powder extract also had inhibitory efficiency against DEP-induced pro-inflammatory response and extracellular ROS. There is a possibility that the other spices or the synergistic effect caused by various spices in curry powder extract contributed to the anti-inflammatory and anti-oxidative effects.
Because of examination of the effects of other spices, for the first time we found that cinnamon and/or coriander extracts significantly attenuated the DEP-induced IL-6 release. To our knowledge, no other reports show that the extracts of cinnamon and/or coriander decrease PM2.5-induced pro-inflammatory responses. Previous studies have indicated that cinnamon and/or coriander extracts attenuate LPS-induced pro-inflammatory responses (Chao et al., Citation2005; Wu et al., Citation2010) and that they protect against carbon tetrachloride- (Moselhy & Ali, Citation2009; Sreelatha, Padma, & Umadevi, Citation2009), bisphenol A- and octylphenol- (Morgan, El-Ballal, El-Bialy, & El-Borai, Citation2014), titanium dioxide nanoparticles- (Shakeel et al., Citation2018), thioacetamide- (Moustafa, Ali, Moselhey, Tousson, & El-Said, Citation2014), and lead-induced toxicity (Velaga, Yallapragada, Williams, Rajanna, & Bettaiya, Citation2014) via their anti-oxidative effect. Our findings proved that protection against DEP-induced toxicity can also be added to the list of efficacies of cinnamon and/or coriander. The present study showed that cinnamon extract decreases extracellular ROS production induced by DEP, in the same way as the curry powder extract did, whereas coriander did not. Therefore, coriander may decrease pro-inflammatory response via other mechanisms but not by targeting ROS.
Among the 12 spices (clove, turmeric, ginger, black pepper, chenpi, cumin, coriander, fenugreek, cardamom, nutmeg, fennel, and cinnamon), clove, turmeric, cinnamon, and coriander showed anti-inflammatory responses. The complex mixture of spices in curry powder may exert synergistic effects. For example, cinnamaldehyde from cinnamon trees with eugenol from clove has shown excellent antifungal properties (Yen & Chang, Citation2008). In addition, Resveratrol from grapes, which is known to have anti-inflammatory efficacy, and the mono-carbonyl analogs of curcumin (MCAs) hybrids have effectively inhibited the LPS-induced production of IL-6 and TNF-α in macrophages (Pan et al., Citation2017). Dimethoxycurcumin, a curcumin analogue, shows a more potent anticancer activity than curcumin via inhibition of cell proliferation and induction of apoptosis (Tamvakopoulos et al., Citation2007). A new chemical substance, as a complex, reacts with the various cell surfaces or intracellular molecules and then inhibits IL-6 release induced by DEP. Even if the components are ineffective by themselves, they may show anti-inflammatory effect as a combination.
In conclusion, curry powder extract inhibited the effects of DEP, which include inflammatory responses via IL-6 release from airway epithelial cells. Cinnamon and coriander with clove and turmeric or in synergy with various spices present in curry powder extract may be responsible for the anti-inflammatory effect of curry powder extract. These protective effects may be partly through inhibition of extracellular ROS production induced by DEP. These results show that curry can protect against PM2.5-induced exacerbation of respiratory diseases. Further investigation is necessary to elucidate its benefits.
Acknowledgments
We thank Kiyoe Itoi, Yufuko Kobayashi, Yusuke Takashima, Mari Tsuboi, and Megumi Nagao for their technical assistances.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bachiega, T. F., de Sousa, J. P., Bastos, J. K., & Sforcin, J. M. (2012). Clove and eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. Journal of Pharmacy and Pharmacology, 64(4), 610–616. doi: 10.1111/j.2042-7158.2011.01440.x
- Baulig, A., Garlatti, M., Bonvallot, V., Marchand, A., Barouki, R., Marano, F., & Baeza-Squiban, A. (2003). Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology, 285(3), L671–L679. doi: 10.1152/ajplung.00419.2002
- Berube, K. A., Jones, T. P., Williamson, B. J., Winters, C., Morgan, A. J., & Richards, R. J. (1999). Physicochemical characterisation of diesel exhaust particles: Factors for assessing biological activity. Atmospheric Environment, 33, 1599–1614. doi: 10.1016/S1352-2310(98)00384-7
- Chao, L. K., Hua, K. F., Hsu, H. Y., Cheng, S. S., Liu, J. Y., & Chang, S. T. (2005). Study on the antiinflammatory activity of essential oil from leaves of Cinnamomum osmophloeum. Journal of Agricultural and Food Chemistry, 53(18), 7274–7278. doi: 10.1021/jf051151u
- Chen, Y., Thai, P., Zhao, Y. H., Ho, Y. S., DeSouza, M. M., & Wu, R. (2003). Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. Journal of Biological Chemistry, 278, 17036–17043. doi: 10.1074/jbc.M210429200
- Cohen, A. J., Brauer, M., Burnett, R., Anderson, H. R., Frostad, J., Estep, K., … Forouzanfar, M. H. (2017). Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the global burden of diseases study 2015. Lancet, 389(10082), 1907–1918. doi: 10.1016/S0140-6736(17)30505-6
- Diabaté, S., Bergfeldt, B., Plaumann, D., Ubel, C., & Weiss, C. (2011). Anti-oxidative and inflammatory responses induced by fly ash particles and carbon black in lung epithelial cells. Analytical and Bioanalytical Chemistry, 401(10), 3197–3212. doi: 10.1007/s00216-011-5102-4
- Honda, A., Fukushima, W., Oishi, M., Tsuji, K., Sawahara, T., Hayashi, T., … Takano, H. (2017). Effects of components of PM2.5 collected in Japan on the respiratory and immune systems. International Journal of Toxicology, 36(2), 153–164. doi: 10.1177/1091581816682224
- Honda, A., Matsuda, Y., Murayama, R., Tsuji, K., Nishikawa, M., Koike, E., … Takano, H. (2014). Effects of Asian sand dust particles on the respiratory and immune system. Journal of Applied Toxicology, 34(3), 250–257. doi: 10.1002/jat.2871
- Ichinose, T., Furuyama, A., & Sagai, M. (1995). Biological effects of diesel exhaust particles (DEP). II. Acute toxicity of DEP introduced into lung by intratracheal instillation. Toxicology, 99(3), 153–167. doi: 10.1016/0300-483X(94)03013-R
- Klawitter, M., Quero, L., Klasen, J., Gloess, A. N., Klopprogge, B., Hausmann, O., … Wuertz, K. (2012). Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. Journal of Inflammation (London, England), 9(1), 29. doi: 10.1186/1476-9255-9-29
- Koo, B. S., Lee, W. C., Chung, K. H., Ko, J. H., & Kim, C. H. (2004). A water extract of Curcuma longa L. (Zingiberaceae) rescues PC12 cell death caused by pyrogallol or hypoxia/reoxygenation and attenuates hydrogen peroxide induced injury in PC12 cells. Life Sciences, 75(19), 2363–2375. doi: 10.1016/j.lfs.2004.07.003
- Kumagai, Y., Arimoto, T., Shinyashiki, M., Shimojo, N., Nakai, Y., Yoshikawa, T., & Sagai, M. (1997). Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPH-cytochrome P450 reductase and involvement of the bioactivation in the DNA damage. Free Radical Biology & Medicine, 22, 479–487. doi: 10.1016/S0891-5849(96)00341-3
- Li, N., Wang, M., Oberley, T. D., Sempf, J. M., & Nel, A. E. (2002). Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. The Journal of Immunology, 169(8), 4531–4541. doi: 10.4049/jimmunol.169.8.4531
- Morgan, A. M., El-Ballal, S. S., El-Bialy, B. E., & El-Borai, N. B. (2014). Studies on the potential protective effect of cinnamon against bisphenol A- and octylphenol-induced oxidative stress in male albino rats. Toxicology Reports, 1, 92–101. doi: 10.1016/j.toxrep.2014.04.003
- Moselhy, S. S., & Ali, H. K. (2009). Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biological Research, 42(1), 93–98. doi: 10.4067/S0716-97602009000100009
- Moustafa, A. H., Ali, E. M., Moselhey, S. S., Tousson, E., & El-Said, K. S. (2014). Effect of coriander on thioacetamide-induced hepatotoxicity in rats. Toxicology and Industrial Health, 30(7), 621–629. doi: 10.1177/0748233712462470
- Nakamura, R., Inoue, K., Fujitani, Y., Kiyono, M., Hirano, S., & Takano, H. (2012). Effects of nanoparticle-rich diesel exhaust particles on IL-17 production in vitro. Journal of Immunotoxicology, 9(1), 72–76. doi: 10.3109/1547691X.2011.629638
- Nakayama, H., Tsuge, N., Sawada, H., Masamura, N., Yamada, S., Satomi, S., & Higashi, Y. (2014). A single consumption of curry improved postprandial endothelial function in healthy male subjects: A randomized, controlled crossover trial. Nutrition Journal, 13, 67. doi: 10.1186/1475-2891-13-67
- Nemmar, A., Al-Salam, S., Yuvaraju, P., Beegam, S., & Ali, B. H. (2015). Emodin mitigates diesel exhaust particles-induced increase in airway resistance, inflammation and oxidative stress in mice. Respiratory Physiology & Neurobiology, 215, 51–57. doi: 10.1016/j.resp.2015.05.006
- Nemmar, A., Subramaniyan, D., & Ali, B. H. (2012). Protective effect of curcumin on pulmonary and cardiovascular effects induced by repeated exposure to diesel exhaust particles in mice. PLoS One, 7(6), e39554. doi: 10.1371/journal.pone.0039554
- Ng, T. P., Chiam, P. C., Lee, T., Chua, H. C., Lim, L., & Kua, E. H. (2006). Curry consumption and cognitive function in the elderly. American Journal of Epidemiology, 164(9), 898–906. doi: 10.1093/aje/kwj267
- Ng, T. P., Niti, M., Yap, K. B., & Tan, W. C. (2012). Curcumins-rich curry diet and pulmonary function in Asian older adults. PLoS One, 7(12), e51753. doi: 10.1371/journal.pone.0051753
- Onishi, T., Honda, A., Tanaka, M., Chowdhury, P. H., Okano, H., Okuda, T., … Takano, H. (2018). Ambient fine and coarse particles in Japan affect nasal and bronchial epithelial cells differently and elicit varying immune response. Environmental Pollution, 242(Pt B), 1693–1701. doi: 10.1016/j.envpol.2018.07.103
- Pan, J., Xu, T., Xu, F., Zhang, Y., Liu, Z., Chen, W., … Liang, G. (2017). Development of resveratrol-curcumin hybrids as potential therapeutic agents for inflammatory lung diseases. European Journal of Medicinal Chemistry, 125, 478–491. doi: 10.1016/j.ejmech.2016.09.033
- Sanbongi, C., Takano, H., Osakabe, N., Sasa, N., Natsume, M., Yanagisawa, R., … Yoshikawa, T. (2003). Rosmarinic acid inhibits lung injury induced by diesel exhaust particles. Free Radical Biology & Medicine, 34(8), 1060–1069. doi: 10.1016/S0891-5849(03)00040-6
- Schmid, M., Zimmermann, S., Krug, H. F., & Sures, B. (2007). Influence of platinum, palladium and rhodium as compared with cadmium, nickel and chromium on cell viability and oxidative stress in human bronchial epithelial cells. Environment International, 33(3), 385–390. doi: 10.1016/j.envint.2006.12.003
- Schwarze, P. E., Ovrevik, J., Låg, M., Refsnes, M., Nafstad, P., Hetland, R. B., & Dybing, E. (2006). Particulate matter properties and health effects: Consistency of epidemiological and toxicological studies. Human and Experimental Toxicology, 25, 559–579. doi: 10.1177/096032706072520
- Shakeel, M., Jabeen, F., Iqbal, R., Chaudhry, A. S., Zafar, S., Ali, M., … Asghar, M. S. (2018). Assessment of titanium dioxide nanoparticles (TiO2-NPs) induced hepatotoxicity and ameliorative effects of cinnamomum cassia in sprague-dawley rats. Biological Trace Element Research, 182(1), 57–69. doi: 10.1007/s12011-017-1074-3
- Sreelatha, S., Padma, P. R., & Umadevi, M. (2009). Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food and Chemical Toxicology, 47(4), 702–708. doi: 10.1016/j.fct.2008.12.022
- Srinivasan, K. (2014). Antioxidant potential of spices and their active constituents. Critical Reviews in Food Science and Nutrition, 54(3), 352–372. doi: 10.1080/10408398.2011.585525
- Steerenberg, P. A., Zonnenberg, J. A., Dormans, J. A., Joon, P. N., Wouters, I. M., van Bree, L., … Van Loveren, H. (1998). Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Experimental Lung Research, 24(1), 85–100. doi: 10.3109/01902149809046056
- Takano, H., Yoshikawa, T., Ichinose, T., Miyabara, Y., Imaoka, K., & Sagai, M. (1997). Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. American Journal of Respiratory and Critical Care Medicine, 156(1), 36–42. doi: 10.1164/ajrccm.156.1.9610054
- Tamvakopoulos, C., Dimas, K., Sofianos, Z. D., Hatziantoniou, S., Han, Z., Liu, Z. L., … Pantazis, P. (2007). Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clinical Cancer Research, 13(4), 1269–1277. doi: 10.1158/1078-0432.CCR-06-1839
- Thacker, E. L. (2006). Lung inflammatory responses. Veterinary Research, 37, 469–486. doi: 10.1051/vetres:2006011
- Tsuji, G., Takahara, M., Uchi, H., Takeuchi, S., Mitoma, C., Moroi, Y., & Furue, M. (2011). An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. Journal of Dermatological Science, 62(1), 42–49.
- Valavanidis, A., Fiotakis, K., Vlahogianni, T., Papadimitriou, V., & Pantikaki, V. (2006). Determination of selective quinones and quinoid radicals in airborne particulate matter and vehicular exhaust particles. Environmental Chemistry, 3, 118–123. doi: 10.1071/EN05089
- Velaga, M. K., Yallapragada, P. R., Williams, D., Rajanna, S., & Bettaiya, R. (2014). Hydroalcoholic seed extract of Coriandrum sativum (Coriander) alleviates lead-induced oxidative stress in different regions of rat brain. Biological Trace Element Research, 159(1-3), 351–363. doi: 10.1007/s12011-014-9989-4
- Wu, T. T., Tsai, C. W., Yao, H. T., Lii, C. K., Chen, H. W., Wu, Y. L., … Liu, K. L. (2010). Suppressive effects of extracts from the aerial part of Coriandrum sativum L. on LPS-induced inflammatory responses in murine RAW 264.7 macrophages. Journal of the Science of Food and Agriculture, 90(11), 1846–1854.
- Yadav, A. S., & Bhatnagar, D. (2007). Modulatory effect of spice extracts on iron-induced lipid peroxidation in rat liver. Biofactors, 29(2-3), 147–157. doi: 10.1002/biof.552029205
- Yasuda, A., Takano, H., Osakabe, N., Sanbongi, C., Fukuda, K., Natsume, M., … Yoshikawa, T. (2008). Cacao liquor proanthocyanidins inhibit lung injury induced by diesel exhaust particles. International Journal of Immunopathology and Pharmacology, 21(2), 279–288. doi: 10.1177/039463200802100204
- Yen, T. B., & Chang, S. T. (2008). Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresource Technology, 99(1), 232–236. doi: 10.1016/j.biortech.2006.11.022
- Zin, W. A., Silva, A. G., Magalhães, C. B., Carvalho, G. M., Riva, D. R., Lima, C. C., … Faffe, D. S. (2012). Eugenol attenuates pulmonary damage induced by diesel exhaust particles. Journal of Applied Physiology, 112, 911–917. doi: 10.1152/japplphysiol.00764.2011

