ABSTRACT
This review aimed to gather information about the biological effects of bovine colostrum (BC) supplementation for improving the respiratory health in humans and the potential role of bioactive molecules from BC as adjunctive therapy for SARS-CoV-2 infection (Coronavirus Disease 2019 – COVID-19). Several studies have shown that BC supplementation is effective against infections and respiratory allergies, as well as in attenuating immunosuppression caused by intense exercise in high-performance athletes. The major immune system modulation proteins present in BC are immunoglobulins, lactoferrin and transforming growth factor-β (TGF-β). Studies have revealed that lactoferrin is effective in combating SARS-CoV-2. Hyperimmune colostrum may constitute an alternative way to produce specific antibodies against COVID-19. Based on the immune system boosting ability as well as anti-inflammatory, antioxidant and antiviral/antibacterial activities, we suggest that well designed, randomized, placebo-controlled, multicenter clinical trials should be done to verify the safety and effectiveness of BC supplements against SARS-CoV-2 infection.
1. Introduction
Bovine colostrum (BC) is the first secretion of the mammary glands during the first 2–4 days postpartum (Godhia & Patel, Citation2013). It is rich in many bioactive nutrients and immunological substances, which are required to nourish the newborn and help in the infant’s growth and development (Marnila & Korhonen, Citation2011). With the exception of lactose amount, colostrum has higher levels of nutrients than the mature milk (Abd El -Fattah et al., Citation2012). The immunoglobulins present in colostrum lay the foundation of life-long immunity in the newborn. Colostrum’s bioactive ingredients help the maturation of the gastrointestinal tract, prevent infections, promote differentiation of bone marrow stem cells, increase lean muscle mass and decrease body fat in the newborn (Bagwe et al., Citation2015).
Due to the high levels of immunomodulatory nutrients present in BC (Buttar et al., Citation2017), several investigators have demonstrated beneficial effects of BC consumption in improving the respiratory health of humans (). The results found so far have been promising in controlling allergies and fighting respiratory infections in adults and children (Ramesh Menon et al., Citation2010; Ulfman et al., Citation2018). BC compounds seem to benefit athletes, who are more susceptible to upper respiratory tract infections after intense physical training and competitions, especially in endurance exercises. Although the mechanisms for this condition are not yet fully understood, supplementation with colostrum has shown positive effects in treating these infections (Glówka et al., Citation2020; Jones et al., Citation2016).
Figure 1. Bovine Colostrum (BC) composition includes bioactive molecules such as lactoferrin, immunoglobulins and transforming growth factor-β (TGF-β), which have been related to protective effects against respiratory diseases. Those health benefits might be extended over SARS-CoV-2 infection and Covid-19 clinical presentation. Created with Biorender.com.
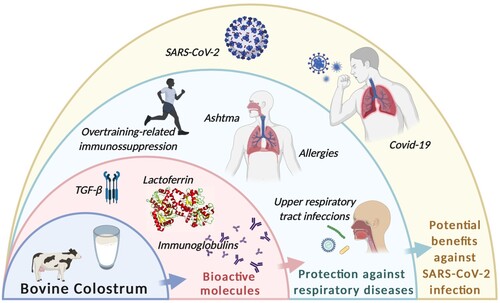
The immunomodulatory function of BC seems to be associated with its chemical composition which is rich in proteins and biologically active peptides such as immunoglobulins, lactoferrin and transforming growth factor-β (TGF-β) (Godhia & Patel, Citation2013; Korhonen, Citation2013; Perdijk et al., Citation2018; Ulfman et al., Citation2018). Given this bioactive profile, it is possible that colostrum may also assist in treating patients affected by the severe acute respiratory syndrome caused by the coronavirus (SARS-CoV-2), which is causing the current Coronavirus Disease 2019 pandemic (COVID-19). Infection with this virus is related to a great heterogeneity of clinical manifestations ranging from asymptomatic, mild cases (fever, cough, dyspnea, fatigue, myalgia and headache, lymphopenia) to Severe Acute Respiratory Syndrome (SARS) (pneumonia, difficulty breathing, respiratory distress, cardiac, neurological and renal damage), and may culminate in multiple organ failure. Studies show that the main cause of worsening cases is Cytokine Release Syndrome, which consists of a large amount of pro-inflammatory cytokines and chemokines released into the circulation in the acute phase, causing systemic effects due to over activation of the immune system (Conti et al. (Citation2020), which can be fatal (Tay et al. (Citation2020). Due to its proven immunomodulatory effects, BC presents itself as a possible strategy to be studied and then inserted in the clinical management of COVID-19 as an economically viable natural therapeutic adjuvant with (possibly) fewer side effects than other immunomodulators.
Therefore, this critical review will address studies which have investigated, in the last 20 years, the implications of BC supplementation on the health of the human respiratory system and related diseases. Furthermore, the main components of colostrum involved in modulating the immune system and which may be mediators of potential therapeutic effects in respiratory diseases will be discussed. The possible contributions of BC to clinical management of COVID-19 will also be addressed herein based on the current clinical findings on the subject.
2. The main immunomodulating components of milk and bovine colostrum
According to van Neerven et al. (Citation2012) and Sozańska (Citation2019) the consumption of bovine milk in childhood may be one of the factors responsible for the lower incidence of allergies, upper tract infections and asthma in children. The authors report this effect appears to be even greater in children born on farms, who consume raw milk, leading them to speculate that this protective effect may be related to milk components that are sensitive to heat, as in the case of proteins.
Although not yet fully elucidated, studies have shown that there is a relationship between the immune responses triggered in the intestine and the respiratory system immunity (Maijó et al., Citation2012a; Maijó et al., Citation2012b; Perdijk et al., Citation2018). Transforming growth factor-β (TGF-β), lactoferrin and immunoglobulins are components in cow milk which are related to several immune protection reactions, as well as to maintaining the functioning of the epithelial barrier, consisting of one of the main innate immunity mechanisms (Perdijk et al., Citation2018; van Neerven, Citation2014).
It is noteworthy that the concentration of these nutrients in colostrum is much higher than in bovine milk, making colostrum an excellent food source for immunomodulatory substances (McGrath et al., Citation2016). Considering that colostrum has a low coagulation temperature and cannot be heated to temperatures above 65 °C, the thermal treatment to be applied in colostrum must be “low temperature, long time pasteurization” (vat pasteurization). In this context, less losses of bioactive substances such as immunoglobulin G (IgG) and TGF-β occur in this temperature range (Das & Seth, Citation2017). Thus, heat-treated BC would be more nutritious and safer than raw bovine milk. However, more studies need to be carried out to verify the impact and efficiency of heat treatment on BC quality parameters and bioactive composition.
2.1. Transforming growth factor-β (TGF-β)
The transforming growth factor beta (TGF-β) is a cytokine produced by different types of cells regulating various cellular functions such as suppression of immune response, cell proliferation and oncogenesis (Ihara et al., Citation2017). It has an immunomodulatory and anti-inflammatory function, also acting on the intestinal epithelial barrier (Kelly et al., Citation2017; van Neerven, Citation2014). In addition to the epithelium, TGF-β can modulate the immune cells and the luminal microbiota functions in the intestine, thus contributing to maintaining intestinal homeostasis (Ihara et al., Citation2017).
TGF-β participates in regulating innate immunity and can induce or suppress adaptive immune responses (Kelly et al., Citation2017; Travis & Sheppard, Citation2014). This protein can control or inhibit airway inflammation and the hyper-reactivity caused by effector T helper 2 cells, which play an important regulatory role in asthma development (Hansen et al., Citation2000). Evidence was gathered that this protein acts in developing and maintaining the immune response in children, protecting them from allergies and inflammation (Oddy & Mcmahon, Citation2011; Oddy & Rosales, Citation2010).
2.2. Lactoferrin
Lactoferrin is a glycoprotein quite abundant in milk, and its levels are even higher in colostrum. This protein has the ability to bind to iron and interact with pathogens, being the first line of defense in the body’s mucous membranes. Among other functions, it has antibacterial, antiviral, antifungal, antiparasitic and anti-inflammatory capacities and it is involved in modulating innate and adaptive immunity (Korhonen, Citation2013; Siqueiros-Cendón et al., Citation2014). Thus, the scientific community is increasingly interested in the benefits of its use as dietary supplement due to its multifunctionality (Dzik et al., Citation2018).
The antiviral activity of BC consists mainly on inhibiting the viruses from binding to the host cells, inhibiting viral replication and improving immune functions, while the antibacterial activity is associated with deprivation of iron, which is essential for bacterial growth (Serrano et al., Citation2020; Wakabayashi et al., Citation2014). Furthermore, animal studies have shown protection and maintenance of intestinal integrity during endotoxemia. Lactoferrin can also mediate tissue pathophysiology, attenuating the pathological damage in elicited airway inflammation due to allergens and pulmonary damage during tuberculosis. According to Kruzel et al., by limiting the pathology and tissue damage postinfection, lactoferrin could be considered in therapeutic protocols aiming to reduce community transmission (Kruzel et al., Citation2017).
2.3. Immunoglobulins
Immunoglobulins in bovine milk also play an active role in modulating the human immune system, especially IgG. They remain intact and functionally active when passing through the gastrointestinal tract, binding directly to viruses, pathogenic bacteria or food and respiratory allergens in the human body. Thus, IgG influences the function of the epithelial barrier and increase elimination of pathogens (Ulfman et al., Citation2018; van Neerven, Citation2014).
There are differences between the immunoglobulin levels in human and bovine colostrum. Imunoglobulin A (IgA) is predominant in human colostrum, while it is less predominant in BC. IgG represents about 75% of all immunoglobulins in the latter, as shown in . However, it is speculated that bovine immunoglobulins, mainly IgG, could be used to overcome the deficiency of IgA in children fed with milk formulas (lacking immunoglobulins) due to their immunomodulatory effect. Studies are needed to evaluate this hypothesis (Ulfman et al., Citation2018).
Table 1. Comparison of immunoglobulins’ levels in human and bovine colostrum and milk.
3. Bovine colostrum application in asthma and respiratory allergies
Studies have shown that breastfeeding can be a protective factor against developing respiratory diseases in childhood, such as asthma (Mosconi et al., Citation2010; Ulfman et al., Citation2018). Bovine milk consumption has also been associated with reduced allergies in children, especially when consumed raw. Sozańska speculates that intact milk proteins play important role in allergies’ protection, since heating denatures them (Sozańska, Citation2019). Considering that BC has higher amounts of protein when compared to milk (van Neerven, Citation2014), this feature could also make BC efficient in protecting against allergies if submitted to proper processing.
The protective capacity of BC against allergies may also be related to its composition being rich in proline-rich polypeptide (PRP). PRP regulates the thymus and inhibits overproduction of lymphocytes and T cells, mitigating the symptoms of allergies and autoimmune diseases (Bagwe et al., Citation2015). However, few studies have investigated the effectiveness of colostrum intake in symptoms moderation in respiratory allergies in humans, especially in asthma and allergic rhinitis.
A randomized, double-blind study with 38 children, aged 7–18 years, who had bronchial asthma and allergic rhinitis, showed that BC supplementation for three months was significantly effective in reducing nasal symptoms and improving lung function. In this clinical trial there was no difference regarding asthma control and severity (Wong, Citation2016). However, another study showed that BC supplementation significantly improved nasal symptoms and lung function in monosensitized children, while asthma control and severity scores were significantly better in polysensitized children (Oloroso-Chavez et al., Citation2017).
4. Bovine colostrum protects upper respiratory tract against infections
Upper respiratory tract infections (URTIs) are infections which affect the mouth, nose, throat, larynx and trachea, provoking nasorafingitis (common cold), sinusitis, pharyngitis, laryngitis and laryngotracheitis (Grief, Citation2013). The positive effects of oral BC supplementation against URTIs are evident for both children and adults. Studies investigating this association show that BC was able to reduce the incidence of URTI and decrease the duration of self-reported symptoms, as shown in . However, the mechanisms for such effects are not fully elucidated (Brinkworth & Buckley, Citation2003; Jones et al., Citation2013; Patel & Rana, Citation2006; Saad et al., Citation2016).
Table 2. Effects of bovine colostrum oral supplementation on upper respiratory tract infections in humans.
The effects of colostrum in the diet have been tested in laboratory animals against Human Syncytial Virus (HRSV), which is one of the most common causes of respiratory infection in children (Xu et al., Citation2015). Previous studies show that immune system cells, called CD8 T, are inhibited in HRSV infections, which hinders the fight against infection (Rossey et al., Citation2014). In a study with mice, Xu et al. (Citation2015) demonstrated that colostrum was effective in inhibiting HRSV, in improving the infection symptoms caused by the virus and in increasing the response of CD8 T cells. In view of these results, studies are needed to investigate the effects of BC in humans affected by HRSV (Xu et al., Citation2015).
In humans, a cohort study showed that BC supplementation was effective in significantly reducing URTI and diarrhea episodes, as well as the number of hospitalizations due to URTI and diarrhea in children aged 1–6 years. It is worth mentioning that this study consisted of 160 children with a recurrent history of URTI and diarrhea, who received from 3.0 g (up to 2 years old) to 6.0 g (over 2 years old) of supplement for 4 weeks (Saad et al., Citation2016).
A commercial supplement containing BC (SinergaTM) was evaluated in another cohort study (Nigro et al., Citation2014). The supplement proved to be efficient in reducing the number of respiratory infections episodes that required intervention with antibiotics in children. Nonetheless, as SinergaTM contains other substances in its composition, the beneficial effects observed cannot be directly attributed to colostrum. These results, however, suggest that BC regular consumption could improve the respiratory health in children, weakening the severity of airway infections.
In a non-comparative study, other BC-based supplement (Pedimune®) was shown to be effective in reducing the number of upper respiratory tract infection, diarrhea and hospitalization episodes for URTI and diarrhea in children aged 1–8 years. The study included 605 children who received 3.0 g of the supplement for 12 weeks. In addition to improving general well-being, 91.19% and 86.60% of the patients had a reduction in URTI and diarrhea episodes at the end of the therapy, respectively (Patel & Rana, Citation2006).
Considering that recurrent respiratory tract infections may be linked to immunoglobulin A (IgA) deficiency, Patiroǧlu and Kondolot investigated whether BC supplementation would increase salivary IgA in patients with serum IgA deficiency (Patiroǧlu & Kondolot, Citation2013). In this clinical trial, sixteen children received 42 mg of Igazym® per day, a commercial supplement of BC and egg white (as source of the antimicrobial enzyme lysozyme). Although the severity score of the URTI viral infection was significantly lower in the test group, there was no correlation with salivary IgA levels, showing that the immunomodulatory function of colostrum in the respiratory system may be driven by another mechanism (Patiroǧlu & Kondolot, Citation2013). Studies with a larger sample size need to be carried out to confirm these findings.
A clinical trial with healthy adult men showed that 60 g/day of concentrated BC protein powder supplementation compared to 60 g/day of whey protein powder significantly decreased the incidence of URTIs, although there was no statistical difference in the duration of self-reported symptoms between groups (Brinkworth & Buckley, Citation2003). A similar result was described by Jones et al. (Citation2013), in which they observed that daily supplementation with 20 g of BC for 12 weeks reduced the number of URTI episodes, as well as the duration of symptoms. The fact that the study by Jones et al. (Citation2013) observed a reduction in the duration of symptoms in contrast to the study by Brinkworth and Buckley (Citation2003) may be due to the type of supplement used in the placebo group, which in the latter was a bovine milk derivative, while in the former study it was an isoenergetic/isomacronutrient. Whey protein is known to have several bioactive peptides, which could be responsible to positive effects on the parameters studied.
Systematic reviews and meta-analyzes concluded that supplementation with bovine colostrum can reduce the incidence rate and the days of upper respiratory symptoms in athletes and physically active people. However, they emphasize that many articles are biased and there is a gap in scientific knowledge about what mechanisms are involved in this improvement. Therefore, they recommend that new randomized control clinical trials should be carried out to determine the mechanisms of action and a consensus on the best supplementation strategy (Glówka et al., Citation2020; Jones et al., Citation2016).
Altogether, the evidence summarized herein points to the adjuvant potential of BC in mitigating lower respiratory tract infections in healthy children and adult men, as well as in rats infected with HRSV. Studies investigating the effects of colostrum on respiratory tract infections, especially in the older adult population and in adult women are needed in order to evaluate the therapeutic potential of BC supplementation in individuals of both genders and of all ages.
5. Bovine colostrum ameliorates symptoms and prevents respiratory infections caused by influenza viruses
Influenza is an acute viral infectious respiratory disease. The genera of influenza viruses which affect humans are type A and B, but type C and D viruses are also known. Their symptoms can range from a mild infection in the upper respiratory tract, fever, sore throat, runny nose, coughing, headache, muscle pain and fatigue, to a scenario of severe pneumonia which can be aggravated by a secondary bacterial infection. Transmission occurs from person to person, mainly through droplets and aerosols of the respiratory secretions of infected individuals (Krammer et al., Citation2018).
Studies highlight the intake of BC as highly effective in preventing infection by the influenza virus. Cesarone et al. (Citation2007) compared the effect of the vaccine in preventing influenza infection with the daily intake of 400 mg of lyophilized defatted BC. To do so, 144 healthy adults aged 30–80 were divided into four groups (no prophylaxis; vaccine and colostrum; only colostrum; only vaccine against the influenza virus). The groups of participants who received either colostrum alone or colostrum plus vaccine had significantly less flu episodes and fewer days with the disease symptoms than groups with no colostrum supplementation. Moreover, in the same study, the authors observed that end-stage coronary patients, patients with pulmonary hypertension and patients with severe cardiovascular problems registered fewer flu episodes and fewer hospital admissions when supplemented with colostrum or colostrum and vaccine compared to the group which only received vaccine. These results suggest that the use of colostrum can be combined with the annual influenza vaccine in specific patient groups (Cesarone et al., Citation2007).
Some studies have investigated the mechanisms by which colostrum works to fight infections by the influenza virus. Uchida et al. (Citation2012) showed that supplementation with 2000µg/g of skim BC by body weight in mice was effective in increasing the activity of Natural Killer (NK) cells in Peyer’s plaque cells, splenocytes and lung cells, indicating that administration oral BC activates systemic and local immunity and protects mice against infection by the influenza virus.
Similar outcomes were reported by Wong et al. (Citation2014), when mice infected with the influenza A (H1N1) virus and supplemented with 1 g/kg of BC by body weight developed a milder infectious condition, with less reduction in body weight and increased NK cell activity. It is worthy to mention that there was an increase in IgA in the small intestine and lung of mice supplemented with colostrum in this study (Wong et al., Citation2014).
Studies suggest that the bioactive function of colostrum is due to its protein composition. Xu et al. (Citation2013) investigated whether supplementation with the isolated acidic protein fraction of BC is effective in combating infection by the Influenza A virus (H1N1) in mice. The result was that 100% of the animals supplemented with colostrum protein survived, whereas survival in the group supplemented with phosphate buffered saline (PBS) was only 33% after infection with H1N1. The group treated with BC also had a lower reduction in body weight when compared to the control, and these results were similar to those achieved by animals treated with the Oseltamivir antiviral drug (Xu et al., Citation2013).
Colostrum from cows immunized with virus or bacteria strains and which contains antibodies specific to these pathogens in its composition is called “hyperimmune colostrum”. Its use has been studied as a more effective therapeutic possibility against influenza than common colostrum due to the passive transfer of specific antibodies. In fact, a study with mice showed that colostrum from cows vaccinated with the influenza A virus proved to be highly effective in preventing and fighting viral infection (Ng et al., Citation2010). Human studies investigating the effects of hyperimmune bovine colostrum are needed to elucidate if the findings with animal models are translated to humans regarding the impact of hyperimmune BC on influenza virus infection prevention. Considering that regular bovine colostrum (non-hyperimmune) already yielded important results against influenza viral infections in humans, it is likely that hyperimmune BC may also be useful in this type of health condition.
Therefore, according to the findings summarized herein, orally administered bovine colostrum may be effective in preventing and treating patients affected by influenza and, in some cases, showing to be equally successful in comparison to standard-of-care interventions.
6. Bovine colostrum and its benefits to athletes’ respiratory system
Athletes submitted to long duration exercises regularly, which require a lot of effort, such as cyclists, runners, swimmers and triathletes, have an increased risk of respiratory tract infections. This is due to the fact that strenuous and prolonged exercises, mainly associated with overtraining (insufficient body recovery), can cause mild immunosuppression, resulting in an increased risk of infections (Davison, Citation2013; Williams et al., Citation2018).
Respiratory infection can compromise physical performance which can be a serious problem, especially for elite and professional athletes. These athletes inevitably need to undergo regular training in order to achieve good performance in competitions, which makes necessary the adoption of strategies to attenuate the side-effects of excess of exercise on immunity (Davison, Citation2013; Williams et al., Citation2018).
BC has been studied as a therapeutic option to increase athletes’ immunity (Gleeson & Pyne, Citation2016). Several studies have investigated the effects of BC intake on the respiratory system and immune markers in different types of sports, as shown in . In fact, oral BC intake appears to improve innate immunity in athletes. Davison and Diment (Citation2010) observed that 20 g of BC supplementation for 4 weeks in active men promoted faster recovery of the innate immune system after prolonged exercise by accelerating the recovery of neutrophilic function and attenuating decreased salivary lysozyme. In another study, colostrum supplementation increased the sensitivity of the immune response to a new antigen in the post-exercise period, although it did not identify effects on the total or differential leukocyte count (Jones et al., Citation2019). It was also seen that there was less salivary bacterial load in active adult men supplemented with colostrum and fewer episodes of upper respiratory tract infection (Jones et al., Citation2014). These results corroborate the hypothesis that colostrum can attenuate the immunosuppression caused by prolonged exercise.
Table 3. Effects of bovine colostrum oral supplementation on athletic performance and immune system.
Crooks et al. observed that supplementation with a drink containing BC for 12 weeks significantly increased salivary IgA levels in long-distance runners, making this a possible mechanism for improving the immune system (Crooks et al., Citation2006). However, similar studies have found no effects of BC supplementation on salivary IgA levels (Davison & Diment, Citation2010; Jones et al., Citation2014).
Supplementation with 10 g of colostrum increased performance and reduced exercise fatigue in highly trained cyclists, in addition to avoiding a reduction in the ventilatory threshold of athletes (Shing et al., Citation2006). It also reduced the incidence of upper tract infections, but there was no difference in the duration of symptoms (Shing et al., Citation2007). However, the findings were limited regarding immunological markers. No changes were found in the leukocyte and salivary IgA levels (Jones et al., Citation2015; Shing et al., Citation2007), although colostrum supplementation prevented the decrease in the IgG2 concentration in the post-exercise period (Shing et al., Citation2007).
There were also no effects of colostrum supplementation on plasma and salivary levels of albumin and IgG, IgA and IgM immunoglobulins in swimmers. However, a reduction in the incidence of upper respiratory tract infection symptoms and a decrease in the number of days with symptoms was observed in the group that received colostrum supplementation (Crooks et al., Citation2010).
Despite the limited number of studies that evaluated the effects of BC in athletes and the low number of individuals recruited in these studies, the positive effects of BC intake on the immune system of athletes are promising, especially when it comes to reducing upper respiratory tract infections and strengthening immunological health. However, the mechanisms for such effects remain controversial and require further investigation, ideally with larger samples.
7. Can bovine colostrum assist in therapy against covid-19?
The pandemic of Coronavirus Disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), emerged in late 2019 and has since plagued the world, causing thousands of deaths in several countries (Ge et al., Citation2020). With this event, a massive race began worldwide in the search for effective treatments and vaccines to prevent infection by the newly known virus.
An adequate immune system response is able to suppress the infection in some cases. However, a dysfunctional immune system response in several cases leads to an exaggerated release of pro-inflammatory cytokines, causing severe acute respiratory syndrome (Tay et al., Citation2020).
Thus, considering the literature data gathered and discussed, it is suggested that the strong antiviral activity of BC provided by its bioactive components, especially lactoferrin, could help to slow the disease progression. The potential application of BC on clinical management of COVID-19 is not only based on the anti-inflammatory, antibacterial and antiviral functions (Carvalho et al., Citation2017; Yadav et al., Citation2016), but also on its capacity in strengthening the human innate and adaptive immune system.
Yadav et al. (Citation2016) have reported that BC possesses strong antimicrobial activity against both Gram negative e Gram positive strains. The minimal inhibitory concentration (MIC) of colostrum was found to be 100 µg/ml against E. coli, S. aureus, P. vulgaris, E. aerogenes, and S. typhi. It is possible that BC might have viricidal effects against COVID-19 virus (Yadav et al., Citation2016). This hypothetical idea may be worth pursuing!
Currently, the antiviral effects of several components of BC are being explored against SARS-CoV-2. We will, briefly, highlight the use of lactoferrin and hyperimmune colostrum in the context of SARS-CoV-2 infection.
7.1. Lactoferrin
Lactoferrin may be a potential therapeutic agent against COVID-19 due to its strong antiviral (Chang et al., Citation2020; Kell et al., Citation2020; Wakabayashi et al., Citation2014) and anti-inflammatory activity (Serrano et al., Citation2020; Siqueiros-Cendón et al., Citation2014). Preliminary results from an in vitro study reported that lactoferrin was effective against the SARS-CoV-2 virus (Carvalho et al., Citation2020, preprint), as well as being effective against the Zika and Chikungunya viruses (Carvalho et al., Citation2017).
The anti-inflammatory activity of lactoferrin can be potentially useful in modulating the cytokine storm activation characteristic of severe COVID-19 which is responsible for the acute respiratory distress syndrome that leads to pulmonary edema, pulmonary failure and hepatic, cardiac and renal impairment (Kell et al., Citation2020). In addition, lactoferrin can also act to prevent infection by inhibiting the SARS-CoV-2 virus from binding to host cells (Kell et al., Citation2020).
Serrano et al. (Citation2020) investigated the effects of oral and topical lactoferrin supplementation (Lactyferrin ForteTM) in 75 positive COVID-19 patients for 10 days. There was a reduction in the incidence of dry cough, headache and diarrhea, and an improvement in the shortness of breath, muscle pain, tiredness, anosmia and ageusia. The daily lactoferrin dose varied from 256 to 384 mg/day for those treated. A total of 256 people who had contact with the infected patients were also treated with a lower dose of the supplement (128–192 mg/day), which demonstrated a preventive role against infection by the virus. These results suggest that lactoferrin seems to be effective in preventing and treating people affected by COVID-19 (Serrano et al., Citation2020).
It is interesting to note that the lactoferrin concentration in BC is 1.5–5.0 mg/mL, which is much higher than the concentration of 0.02–0.75 mg/mL in milk (McGrath et al., Citation2016), thus making it an important dietary source of lactoferrin for the human diet.
7.2. Hyperimmune colostrum
Another treatment for COVID-19 under investigation is the administration of hyperimmune immunoglobulins (specific antibodies) from patients previously infected with the virus as a way to fight the SARS-CoV-2 infection (De Alwis et al., Citation2020; Valk et al., Citation2020). A recent study found that the breast milk of women previously infected with COVID-19 showed IgA reactivity against SARS-CoV-2, showing that milk has specific immune activity against COVID-19, thus constituting a factor which could help in preventing and treating infection by this virus (Fox et al., Citation2020, preprint).
Nevertheless, the use of bovine hyperimmune colostrum has shown positive results against viruses and bacteria (). Its production consists of vaccinating cows against virus or bacteria strains and subsequently collecting colostrum rich in specific immunoglobulins against those pathogens (Steele et al., Citation2013). The results have been promising against the Influenza A virus and the bacteria Escherichia coli, Clostridium difficile and Helicobacter pylori ().
Table 4. Hyperimmune bovine colostrum in the prevention and treatment of infectious diseases.
Civra et al. (Citation2019) showed that the conventional bovine rotavirus vaccine was able to promote production of colostral antibodies against human rotavirus, possibly due to epitope sharing between the two pathogens. This result makes this procedure a possible technique for preventing and treating rotavirus in humans, which is a major cause of severe diarrhea in children. Similar findings showed that vaccination of cows with HIV-1 (Human immunodeficiency virus 1) gp140 resulted in a hyperimmune colostrum rich in anti-gp140 IgG with a neutralizing, fast and potent activity against HIV-1 infection (Kramski et al., Citation2012a; Kramski et al., Citation2012b). In addition to the antiviral properties, hyperimmune colostrum also displayed anti-inflammatory activity in mice affected with rheumatoid arthritis (Hung et al., Citation2018).
Farm animals are being studied to produce hyperimmune milk and serum (Jawhara, Citation2020; Zylberman et al., Citation2020). However, so far, no scientific evidence was gathered to confirm whether cows immunized with the inactivated virus or with viral particles of the SARS-CoV-2 could provide hyperimmune colostrum with high levels of specific IgG to combat COVID-19. If confirmed, this could be a cheaper and faster alternative to obtaining antibodies.
8. Conclusions and future perspectives
Data summarized herein demonstrated that ingesting bovine colostrum is beneficial for the immunity of the human respiratory system. However, the mechanisms for its effects are still unknown and further studies are needed.
Current evidence indicates that colostrum and its components may contribute as a non-pharmacological alternative for the clinical management of COVID-19. As BC was able to prevent virus infections and reduce the symptoms severity, perhaps BC supplementation could also have some positive impact as a prophylactic measure against SARS-CoV-2 infection. This will only be accessed through reliable and carefully designed investigations, preferably randomized, double-blinded, placebo-controlled clinical trials and longitudinal studies. That way the effects of BC supplementation in healthy individuals and in patients affected by COVID-19 could be better examined and elucidated. Further studies on the effects of colostrum consumption on the health of the lower respiratory tract and asthma are also needed.
Apart from BC benefits related to COVID-19, which is still under scientific scrutiny, there is a solid indication of BC supplementation as an important source of bioactive molecules able to provide health improvements. Therefore, the incorporation of BC in human eating habits is desirable. Dairy derivatives with BC as an ingredient could be a natural form of consumption, considering that the origin of colostrum is the same as that of animal milk on the farm. Under proper professional supervision, foods or supplements containing BC could be proposed as adjuvants in therapy for patients with infections and respiratory allergies, as well as those who are immunosuppressed.
Hence, the development of BC products or its use as food ingredient should be better studied due to its potential application in the healthcare context, especially in a public health emergency, when serious efforts are necessary to provide and/or improve the population’s health. This also represents an underexplored commercial opportunity for the food industry worldwide.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abd El -Fattah, A. M., Abd Rabo, F. H. R., EL-Dieb, S. M., & Elkashef, H. A. S. (2012). Changes in composition of colostrum of Egyptian buffaloes and Holstein cows. BMC Veterinary Research, 8, 19. https://doi.org/10.1186/1746-6148-8-19
- Bagwe, S., Tharappel, L. J. P., Kaur, G., & Buttar, H. S. (2015). Bovine colostrum: An emerging nutraceutical. Journal of Complementary and Integrative Medicine, 12(3), 1–11. https://doi.org/10.1515/jcim-2014-0039
- Brinkworth, G. D., & Buckley, J. D. (2003). Concentrated bovine colostrum protein supplementation reduces the incidence of self-reported symptoms of upper respiratory tract infection in adult males. European Journal of Nutrition, 42(4), 228–232. https://doi.org/10.1007/s00394-003-0410-x
- Buckley, J. D., Abbott, M. J., Brinkworth, G. D., & Whyte, P. B. D. (2002). Bovine colostrum supplementation during endurance running training improves recovery, but not performance. Journal of Science and Medicine in Sport, 5(2), 65–79. https://doi.org/10.1016/S1440-2440(02)80028-7
- Buttar, H. S., Bagwe, S. M., Bhullar, S. K., & Kaur, G. (2017). Health benefits of bovine colostrum in children and adults. In R. R. Watson, R. J. Collier, & V. R. Preedy (Eds.), Dairy in human health and disease across the lifespan (pp. 3–20). Academic Press. https://doi.org/10.1016/B978-0-12-809868-4.00001-7.
- Carvalho, C. A. M., Casseb, S. M. M., Gonçalves, R. B., Silva, E. V. P., Gomes, A. M. O., & Vasconcelos, P. F. C. (2017). Bovine lactoferrin activity against chikungunya and zika viruses. Journal of General Virology, 98(7), 1749–1754. https://doi.org/10.1099/jgv.0.000849
- Carvalho, C. A. M., Matos, A. R., Caetano, B. C., Sousa Junior, I. P., Campos, S. P. C., & Geraldino, B. R. (2020, preprint). In vitro inhibition of SARS-CoV-2 infection by bovine lactoferrin. bioRxiv preprint. Available from: https://www.biorxiv.org/content/10.1101/2020.05.13.093781v1
- Casswall, T. H., Nilsson, H. O., Björck, L., Sjöstedt, S., Xu, L., Nord, C. E., Borén, T., Wadstrom, T., & Hammarström, L. (2002). Bovine anti-helicobacter pylori antibodies for oral immunotherapy. Scandinavian Journal of Gastroenterology, 37(12), 1380–1385. https://doi.org/10.1080/003655202762671242
- Cesarone, M. R., Belcaro, G., Di Renzo, A., Dugall, M., Cacchio, M., Ruffini, I., Pellegrini, L., Boccio, G. D., Fano, F., Ledda, A., Bottari, A., Ricci, A., Stuard, S., & Vinciguerra, G. (2007). Prevention of influenza episodes with colostrum compared with vaccination in healthy and high-risk cardiovascular subjects: The epidemiologic study in San Valentino. Clinical and Applied Thrombosis/Hemostasis, 13(2), 130–136. https://doi.org/10.1177/1076029606295957
- Chang, R., Ng, T. B., & Sun, W.-Z. (2020). Lactoferrin as potential preventative and adjunct treatment for COVID-19. International Journal of Antimicrobial Agents, 56(3), 1–7. https://doi.org/10.1016/j.ijantimicag.2020.106118
- Civra, A., Altomare, A., Francese, R., Donalisio, M., Aldini, G., & Lembo, D. (2019). Colostrum from cows immunized with a veterinary vaccine against bovine rotavirus displays enhanced in vitro anti-human rotavirus activity. Journal of Dairy Science, 102(6), 4857–4869. https://doi.org/10.3168/jds.2018-16016
- Conti, P., Ronconi, G., Caraffa, A., Gallenga, C. E., Ross, R., Frydas, I., & Kritas, S. K. (2020). Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: Anti-inflammatory strategies., 34, 11–15. https://doi.org/10.23812/conti-e
- Crooks, C., Cross, M. L., Wall, C., & Ali, A. (2010). Effect of bovine colostrum supplementation on respiratory tract mucosal defenses in swimmers. International Journal of Sport Nutrition and Exercise Metabolism, 20(3), 224–235. https://doi.org/10.1123/ijsnem.20.3.224
- Crooks, C. V., Wall, C. R., Cross, M. L., & Rutherfurd-Markwick, K. J. (2006). The effect of bovine colostrum supplementation on salivary IgA in distance runners. International Journal of Sport Nutrition and Exercise Metabolism, 16(1), 47–64. https://doi.org/10.1123/ijsnem.16.1.47
- Das, A., & Seth, R. (2017). Studies on quality attributes of skimmed colostrum powder. International Journal of Chemical Studies, 5(3), 17–20.
- Davison, G. (2013). Bovine colostrum and immune function after exercise. Medicine Sport Science, 59, 62–69. https://doi.org/10.1159/000341966
- Davison, G., & Diment, B. C. (2010). Bovine colostrum supplementation attenuates the decrease of salivary lysozyme and enhances the recovery of neutrophil function after prolonged exercise. British Journal of Nutrition, 103(10), 1425–1432. https://doi.org/10.1017/S0007114509993503
- De Alwis, R., Chen, S., Gan, E. S., & Ooi, E. E. (2020). Impact of immune enhancement on COVID-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine, 55, 1–7. https://doi.org/10.1016/j.ebiom.2020.102768
- Dzik, S., Miciński, B., Miciński, J., Mituniewicz, T., & Kowalski, P. M. (2018). Therapeutic properties of lactoferrin. Polish Annals of Medicine, 25, 158–161. https://doi.org/10.29089/2017.17.00020
- Fox, A., Marino, J., Amanat, F., Krammer, F., Hahn-Holbrook, J., Zolla-Pazner, S., & Powell, R. L. (2020, preprint). Evidence of a significant secretory-IgA-dominant SARS-CoV-2 immune response in human milk following recovery from COVID-19. medRxiv preprint doi: https://doi.org/10.1101/2020.05.04.20089995. Available from: https://www.medrxiv.org/content/10 .1101/2020.05.04.20089995v1
- Ge, H., Wang, X., Yuan, X., Xiao, G., Wang, C., Deng, T., Yuan, Q., & Xiao, X. (2020). The epidemiology and clinical information about COVID-19. European Journal of Clinical Microbiology & Infectious Diseases, 39(6), 1011–1019. https://doi.org/10.1007/s10096-020-03874-z
- Gleeson, M., & Pyne, D. B. (2016). Respiratory inflammation and infections in high-performance athletes. Immunology & Cell Biology, 94(2), 124–131. https://doi.org/10.1038/icb.2015.100
- Glówka, N., Durkalec-Michalski, K., & Woźniewicz, M. (2020). Immunological outcomes of bovine colostrum supplementation in trained and physically active people: A systematic review and meta-analysis. Nutrients, 12(4), 1–24. https://doi.org/10.3390/nu12041023
- Godhia, M. L., & Patel, N. (2013). Colostrum - Its composition, benefits as a nutraceutical: A review. Current Research in Nutrition and Food Science Journal, 1(1), 37–47. https://doi.org/10.12944/CRNFSJ.1.1.04
- Grief, S. N. (2013). Upper respiratory infections. Primary Care: Clinics in Office Practice, 40(3), 757–770. https://doi.org/10.1016/j.pop.2013.06.004
- Hansen, G., McIntire, J. J., Yeung, V. P., Berry, G., Thorbecke, G. J., Chen, L., Deekruyff, R. H., & Umetsu, D. T. (2000). CD4+ t helper cells engineered to produce latent TGF-β1 reverse allergen-induced airway hyperreactivity and inflammation. Journal of Clinical Investigation, 105(1), 61–70. https://doi.org/10.1172/JCI7589
- Hung, L. H., Wu, C. H., Lin, B. F., & Hwang, L. S. (2018). Hyperimmune colostrum alleviates rheumatoid arthritis in a collagen-induced arthritis murine model. Journal of Dairy Science, 101(5), 3778–3787. https://doi.org/10.3168/jds.2017-13572
- Hutton, M. L., Cunningham, B. A., Mackin, K. E., Lyon, S. A., James, M. L., Rood, J. I., & Lyras, D. (2017). Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative. Scientific Reports, 7(1), 1–13. https://doi.org/10.1038/s41598-017-03982-5
- Ihara, S., Hirata, Y., & Koike, K. (2017). TGF-β in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. Journal of Gastroenterology, 52(7), 777–787. https://doi.org/10.1007/s00535-017-1350-1
- Jawhara, S. (2020). Can drinking microfiltered raw immune milk from cows immunized against SARS-CoV-2 provide short-term protection against COVID-19? Frontiers in Immunology, 11, 1–5. https://doi.org/10.3389/fimmu.2020.01888
- Jones, A. W., Cameron, S. J. S., Thatcher, R., Beecroft, M. S., Mur, L. A. J., & Davison, G. (2014). Effects of bovine colostrum supplementation on upper respiratory illness in active males. Brain, Behavior, and Immunity, 39, 194–203. https://doi.org/10.1016/j.bbi.2013.10.032
- Jones, A. W., March, D. S., Curtis, F., & Bridle, C. (2016). Bovine colostrum supplementation and upper respiratory symptoms during exercise training: A systematic review and meta-analysis of randomised controlled trials. BMC Sports Science, Medicine and Rehabilitation, 8(1), 1–10. https://doi.org/10.1186/s13102-016-0047-8
- Jones, A. W., March, D. S., Thatcher, R., Diment, B., Walsh, N. P., & Davison, G. (2019). The effects of bovine colostrum supplementation on in vivo immunity following prolonged exercise: A randomised controlled trial. European Journal of Nutrition, 58(1), 335–344. https://doi.org/10.1007/s00394-017-1597-6
- Jones, A. W., Thatcher, R., March, D. S., & Davison, G. (2015). Influence of 4 weeks of bovine colostrum supplementation on neutrophil and mucosal immune responses to prolonged cycling. Scandinavian Journal of Medicine & Science in Sports, 25(6), 788–796. https://doi.org/10.1111/sms.12433
- Jones, A. W., Thatcher, R., Mur, L. A. J., Cameron, S. J. S., Beecroft, M., & Davison, G. (2013). Exploring the mechanisms behind the effects of chronic bovine colostrum supplementation on risk of upper respiratory tract infection. International Journal of Exercise Science, 10(1), ISSN 1939-795X. Available from: https://digitalcommons.wku.edu/ijesab/vol10/iss1/11.
- Kell, D. B., Heyden, E. L., & Pretorius, E. (2020). The biology of lactoferrin, an iron-binding protein that Can help defend against viruses and bacteria. Frontiers in Immunology, 11, 1–15. https://doi.org/10.3389/fimmu.2020.01221
- Kelly, A., Houston, S. A., Sherwood, E., Casulli, J., & Travis, M. A. (2017). Regulation of innate and adaptive immunity by TGFβ. Advances in Immunology, 134, 137–233. https://doi.org/10.1016/bs.ai.2017.01.001
- Korhonen, H. J. (2013). Production and properties of health-promoting proteins and peptides from bovine colostrum and milk. Cell Mol Biol (Noisy-le-grand), 58(1), 26–38. https://doi.org/10.1170/T915
- Krammer, F., Smith, G. J. D., Fouchier, R. A. M., Peiris, M., Kedzierska, K., Doherty, P. C., Palese, P., Shaw, M. L., Treanor, J., Webster, R. G., & García-Sastre, A. (2018). Influenza. Nature Reviews Disease Primers, 4(1), 1–2. https://doi.org/10.1038/s41572-018-0002-y
- Kramski, M., Center, R. J., Wheatley, A. K., Jacobson, J. C., Alexander, M. R., Rawlin, G., & Purcell, D. F. J. (2012a). Hyperimmune bovine colostrum as a low-cost, large-scale source of antibodies with broad neutralizing activity for HIV-1 envelope with potential use in microbicides. Antimicrobial Agents and Chemotherapy, 56(8), 4310–4319. https://doi.org/10.1128/AAC.00453-12
- Kramski, M., Lichtfuss, G. F., Navis, M., Isitman, G., Wren, L., Rawlin, G., Center, R. J., Jaworowski, A., Kent, S. J., & Purcell, D. F. J. (2012b). Anti-HIV-1 antibody-dependent cellular cytotoxicity mediated by hyperimmune bovine colostrum IgG. European Journal of Immunology, 42(10), 2771–2781. https://doi.org/10.1002/eji.201242469
- Kruzel, M. L., Zimecki, M., & Actor, J. K. (2017). Lactoferrin in a context of inflammation-induced pathology. Frontiers in Immunology, 8, 1–15. https://doi.org/10.3389/fimmu.2017.01438
- Larcombe, S., Hutton, M. L., & Lyras, D. (2019). Hyperimmune bovine colostrum reduces gastrointestinal carriage of uropathogenic Escherichia coli. Human Vaccines & Immunotherapeutics, 15(2), 508–513. https://doi.org/10.1080/21645515.2018.1528836
- Maijó, M., Miró, L., Polo, J., Campbell, J., Russell, L., Crenshaw, J., Weaver, E., Moretó, M., & Pérez-Bosque, A. (2012a). Dietary plasma proteins attenuate the innate immunity response in a mouse model of acute lung injury. British Journal of Nutrition, 107(6), 867–875. https://doi.org/10.1017/S0007114511003655
- Maijó, M., Miró, L., Polo, J., Campbell, J., Russell, L., Crenshaw, J., Weaver, E., Moretó, M., & Pérez-Bosque, A. (2012b). Dietary plasma proteins modulate the adaptive immune response in mice with acute lung inflammation. The Journal of Nutrition, 142(2), 264–270. https://doi.org/10.3945/jn.111.149070
- Marnila, P., & Korhonen, H. (2011). Milk: Colostrum. Encyclopedia of Dairy Sciences, 2, 591–597. https://doi.org/10.1016/B978-0-12-374407-4.00322-8
- McGrath, B. A., Fox, P. F., McSweeney, P. L. H., & Kelly, A. L. (2016). Composition and properties of bovine colostrum: A review. Dairy Science & Technology, 96(2), 133–158. https://doi.org/10.1007/s13594-015-0258-x
- Mosconi, E., Rekima, A., Seitz-Polski, B., Kanda, A., Fleury, S., Tissandie, E., Monteiro, R., Dombrowicz, D. D., Julia, V., Glaichenhaus, N., & Verhasselt, V. (2010). Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunology, 3(5), 461–474. https://doi.org/10.1038/mi.2010.23
- Ng, W. C., Wong, V., Muller, B., Rawlin, G., & Brown, L. E. (2010). Prevention and treatment of influenza with hyperimmune bovine colostrum antibody. PLoS One, 5, 1–10. https://doi.org/10.1371/journal.pone.0013622
- Nigro, A., Nicastro, A., & Trodella, R. (2014). Retrospective observational study to investigate sinerga, a multifactorial nutritional product, and bacterial extracts in the prevention of recurrent respiratory infections in children. International Journal of Immunopathology and Pharmacology, 27(3), 455–460. https://doi.org/10.1177/039463201402700318
- Oddy, W. H., & Mcmahon, R. J. (2011). Milk-derived or recombinant transforming growth factor-beta has effects on immunological outcomes: A review of evidence from animal experimental studies. Clinical & Experimental Allergy, 41(6), 783–793. https://doi.org/10.1111/j.1365-2222.2011.03762.x
- Oddy, W. H., & Rosales, F. (2010). A systematic review of the importance of milk TGF-β on immunological outcomes in the infant and young child. Pediatric Allergy and Immunology, 21(1-Part-I), 47–59. https://doi.org/10.1111/j.1399-3038.2009.00913.x
- Oloroso-Chavez, K., Andaya, P., & Wong, C. (2017). OR082 bovine colostrum supplementation in respiratory allergies according to sensitization: Subgroup analysis of randomized controlled trial. Annals of Allergy, Asthma & Immunology, 119(5), S11–S12. https://doi.org/10.1016/j.anai.2017.08.062
- Otto, W., Najnigier, B., Stelmasiak, T., & Robins-Browne, R. M. (2011). Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scandinavian Journal of Gastroenterology, 46(7-8), 862–868. https://doi.org/10.3109/00365521.2011.574726
- Patel, K., & Rana, R. (2006). Pedimune in recurrent respiratory infection and diarrhoea- The Indian experience-the pride study. The Indian Journal of Pediatrics, 73(7), 585–591. https://doi.org/10.1007/BF02759923
- Patiroǧlu, T., & Kondolot, M. (2013). The effect of bovine colostrum on viral upper respiratory tract infections in children with immunoglobulin A deficiency. The Clinical Respiratory Journal, 7(1), 21–26. https://doi.org/10.1111/j.1752-699X.2011.00268.x
- Perdijk, O., van Splunter, M., Savelkoul, H. F. J., Brugman, S., & van Neerven, R. J. J. (2018). Cow’s milk and immune function in the respiratory tract: Potential mechanisms. Frontiers in Immunology, 9, 1–14. https://doi.org/10.3389/fimmu.2018.00143
- Ramesh Menon, P., Lodha, R., & Kabra, S. K. (2010). Bovine colostrum in pediatric respiratory diseases: A systematic review. The Indian Journal of Pediatrics, 77(1), 108–109. https://doi.org/10.1007/s12098-009-0257-0
- Rossey, I., Sedeyn, K., De Baets, S., Schepens, B., & Saelens, X. (2014). CD8+ t cell immunity against human respiratory syncytial virus. Vaccine, 32(46), 6130–6137. https://doi.org/10.1016/j.vaccine.2014.08.063
- Saad, K., Abo-Elela, M. G. M., El-Baseer, K. A. A., Ahmed, A. E., Ahmad, F. A., Tawfeek, M. S. K., El-Houfey, A., About_Khair, M. D., Abdel-Salam, A. M., Abo-elgheit, A., Qubaisy H., Ali A. M., & Abdel-Mawgoud E. (2016). Effects of bovine colostrum on recurrent respiratory tract infections and diarrhea in children. Medicine, 95, 1–5. https://doi.org/10.1097/MD.0000000000004560
- Savarino, S. J., McKenzie, R., Tribble, D. R., Porter, C. K., O’Dowd, A., Sincock, S. A., Poole, S. T., DeNearing, B., Woods, C. M., Kim, H., Grahek S. L., Brinkley C., Crabb J. H., & Bourgeois A. L.. (2019). Hyperimmune bovine colostral anti-CS17 antibodies protect against enterotoxigenic Escherichia coli diarrhea in a randomized, doubled-blind, placebo-controlled human infection model. The Journal of Infectious Diseases, 220(3), 505–513. https://doi.org/10.1093/infdis/jiz135
- Serrano, G., Kochergina, I., Albors, A., Diaz, E., Oroval, M., Hueso, G., & Serrano, J. M. (2020). Liposomal lactoferrin as potential preventative and cure for COVID-19. International Journal of Research in Health Sciences, 8(1), 8–15. https://doi.org/10.5530/ijrhs.8.1.3
- Shing, C. M., Jenkins, D. G., Stevenson, L., & Coombes, J. S. (2006). The influence of bovine colostrum supplementation on exercise performance in highly trained cyclists. British Journal of Sports Medicine, 40(9), 797–801. https://doi.org/10.1136/bjsm.2006.027946
- Shing, C. M., Peake, J., Suzuki, K., Okutsu, M., Pereira, R., Stevenson, L., Jenkins, D. G., & Coombes, J. S. (2007). Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. Journal of Applied Physiology, 102(3), 1113–1122. https://doi.org/10.1152/japplphysiol.00553.2006
- Siqueiros-Cendón, T., Arévalo-Gallegos, S., Iglesias-Figueroa, B. F., García-Montoya, I. A., Salazar-Martínez, J., & Rascón-Cruz, Q. (2014). Immunomodulatory effects of lactoferrin. Acta Pharmacologica Sinica, 35(5), 557–566. https://doi.org/10.1038/aps.2013.200
- Sozańska, B. (2019). Raw Cow’s milk and its protective effect on allergies and asthma. Nutrients, 11(2), 1–11. https://doi.org/10.3390/nu11020469
- Sponseller, J. K., Steele, J. A., Schmidt, D. J., Kim, H. B., Beamer, G., Sun, X., & Tzipori, S. (2015). Hyperimmune bovine colostrum as a novel therapy to combat clostridium difficile infection. Journal of Infectious Diseases, 211, 1334–1341. https://doi.org/10.1093/infdis/jiu605
- Steele, J., Sponseller, J., Schmidt, D., Cohen, O., & Tzipori, S. (2013). Hyperimmune bovine colostrum for treatment of GI infections: A review and update on clostridium difficile. Human Vaccines & Immunotherapeutics, 9(7), 1565–1568. https://doi.org/10.4161/hv.24078
- Tawfeek, H. I., Najim, N. H., & Al-Mashikhi, S. (2003). Efficacy of an infant formula containing anti-Escherichia coli colostral antibodies from hyperimmunized cows in preventing diarrhea in infants and children: A field trial. International Journal of Infectious Diseases, 7(2), 120–128. https://doi.org/10.1016/S1201-9712(03)90007-5
- Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A., & Ng, L. F. P. (2020). The trinity of COVID-19: Immunity, inflammation and intervention. Nature Reviews Immunology, 20(6), 363–374. https://doi.org/10.1038/s41577-020-0311-8
- Tran, C. D., Kritas, S., Campbell, M. A. F., Huynh, H. Q., Lee, S. S., & Butler, R. N. (2010). Novel combination therapy for the eradication of Helicobacter pylori infection in a mouse model. Scandinavian Journal of Gastroenterology, 45(12), 1424–1430. https://doi.org/10.3109/00365521.2010.506245
- Travis, M. A., & Sheppard, D. (2014). TGF-β activation and function in immunity. Annual Review of Immunology, 32(1), 51–82. https://doi.org/10.1146/annurev-immunol-032713-120257
- Uchida, K., Hiruta, N., Yamaguchi, H., Yamashita, K., Fujimura, K., & Yasui, H. (2012). Augmentation of cellular immunity and protection against influenza virus infection by bovine late colostrum in mice. Nutrition, 28, 442–446. https://doi.org/10.1016/j.nut.2011.07.021
- Ulfman, L. H., Leusen, J. H. W., Savelkoul, H. F. J., Warner, J. O., & van Neerven, R. J. J. (2018). Effects of bovine immunoglobulins on immune function, allergy, and infection. Frontiers in Nutrition, 5, 1–20. https://doi.org/10.3389/fnut.2018.00052
- Valk, S. J., Piechotta, V., Chai, K. L., Doree, C., Monsef, I., Wood, E. M., Lamikanra, A., Kimber, C., McQuilten, Z., So-Osman, C., Estcourt, L. J., & Skoetz, N. (2020). Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A rapid review. Cochrane Database of Systematic Reviews, 5, 1–134. https://doi.org/10.1002/14651858.CD013600
- van Neerven, R. J. J. (2014). The effects of milk and colostrum on allergy and infection: Mechanisms and implications. Animal Frontiers, 4(2), 16–22. https://doi.org/10.2527/af.2014-0010
- van Neerven, R. J. J., Knol, E. F., Heck, J. M. L., & Savelkoul, H. F. J. (2012). Which factors in raw cow’s milk contribute to protection against allergies? Journal of Allergy and Clinical Immunology, 130(4), 853–858. https://doi.org/10.1016/j.jaci.2012.06.050
- Wakabayashi, H., Oda, H., Yamauchi, K., & Abe, F. (2014). Lactoferrin for prevention of common viral infections. Journal of Infection and Chemotherapy, 20(11), 666–671. https://doi.org/10.1016/j.jiac.2014.08.003
- Wheeler, T. T., Hodgkinson, A. J., Prosser, C. G., & Davis, S. R. (2007). Immune components of colostrum and milk - A historical perspective. Journal of Mammary Gland Biology and Neoplasia, 12(4), 237–247. https://doi.org/10.1007/s10911-007-9051-7
- Williams, N. C., Killer, S. C., Svendsen, I. S., & Jones, A. W. (2018). Immune nutrition and exercise: Narrative review and practical recommendations. European Journal of Sport Science, 19(1), 49–61. https://doi.org/10.1080/17461391.2018.1490458
- Wong, C. (2016). O033 bovine colostrum as an adjunct therapy in the control of allergic respiratory disease in children. Annals of Allergy, Asthma & Immunology, 117(5), S12. https://doi.org/10.1016/j.anai.2016.09.393
- Wong, E. B., Mallet, J. F., Duarte, J., Matar, C., & Ritz, B. W. (2014). Bovine colostrum enhances natural killer cell activity and immune response in a mouse model of influenza infection and mediates intestinal immunity through toll-like receptors 2 and 4. Nutrition Research, 34(4), 318–325. https://doi.org/10.1016/j.nutres.2014.02.007
- Xu, M. L., Kim, H. J., Chang, D. Y., & Kim, H. J. (2013). The effect of dietary intake of the acidic protein fraction of bovine colostrum on influenza A (H1N1) virus infection. Journal of Microbiology, 51(3), 389–393. https://doi.org/10.1007/s12275-013-2683-y
- Xu, Mei Ling, Kim, Hyoung Jin, Wi, Ga Ram, & Kim, Hong-Jin. (2015). The effect of dietary bovine colostrum on respiratory syncytial virus infection and immune responses following the infection in the mouse. Journal of Microbiology, 53(9), 661–666. https://doi.org/10.1007/s12275-015-5353-4
- Xu, M. L., Kim, H. J., Wi, G. R., & Kim, H. J. (2015). The effect of dietary bovine colostrum on respiratory syncytial virus infection and immune responses following the infection in the mouse. Journal of Microbiology, 53(9), 661–666. https://doi.org/10.1007/s12275-015-5353-4
- Yadav, R., Angolkar, T., Kaur, G., & Buttar, H. S. (2016). Antibacterial and antiinflammatory properties of bovine colostrum. Recent Patents on Inflammation & Allergy Drug Discovery, 10(1), 49–53. https://doi.org/10.2174/1872214810666160219163118
- Zylberman, V., Sanguineti, S., Pontoriero, A. V., Higa, S. V., Cerutti, M. L., Seijo, S. M. M., Pardo, R., Muñoz, L., Intrieri, M. E. A., Alzogaray, V. A., & Avaro, M. M. (2020). Development of a hyperimmune equine serum therapy for covid-19 in Argentina. Medicina (B.Aires), 80(Suppl. 3), 1–6.


