 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Subjective cognitive symptoms are common after mild traumatic brain injury (mTBI), and are associated with important outcome factors including return to work. This study examined self-reported cognitive symptoms in mTBI and trauma controls (TCs), and explored psychological distress and gender as predictors of these symptoms. Pre-morbidly healthy adults with mTBI (n = 68) and general trauma (n = 40) were prospectively recruited from inpatient hospital wards and assessed 6–10 weeks post-injury. Primary measures included self-reported cognitive symptoms, post-concussion symptoms, and psychological distress. Groups were matched on all background variables, including objective cognitive performance. Within this context, subjective cognitive symptoms were significantly elevated after mTBI relative to TCs (t = 3.396, p = .001). In contrast, there was no difference in post-concussion symptoms between groups (t = 1.275, p = .206). Psychological distress (β = .536, p < .001) and gender (β = .253, p = .012) predicted subjective cognitive symptoms in mTBI, with females and those with higher distress reporting greater symptoms. Unlike general post-concussion symptoms, subjective cognitive symptoms were elevated after mTBI relative to TCs, suggesting that mTBI-specific factors underly this elevation. Females and individuals with high psychological distress are important subgroups to consider for potential intervention following mTBI.
Mild traumatic brain injury (mTBI) is the most common type of traumatic brain injury, occurring at a rate of 100–300 hospital-evaluated cases per 100,000 worldwide, not accounting for those who do not present to hospital (Cassidy et al., Citation2004). Mild TBI is a major public health concern, because of its high incidence, and the failure of a subset of patients to recover successfully (McMahon et al., Citation2014).
Instances of mTBI often lead to a constellation of subjective cognitive, affective and/or somatic post-concussion symptoms (PCS) in the acute post-injury period (Levin & Diaz-Arrastia, Citation2015). While many individuals with mTBI make a full recovery in the initial weeks and months after injury, a subgroup of individuals continues to report symptoms in the chronic phase (>3 months) following injury (Cassidy et al., Citation2014; Machamer et al., Citation2022). The aetiology of late and persisting symptoms has been a point of debate in the literature (Silverberg & Iverson, Citation2011).
The cognitive symptom component of PCS, which comprise self-reported deficits in memory, attention, processing speed, and executive function, are very common after mTBI (Clarke et al., Citation2012). These subjective cognitive symptoms are associated with key outcome factors, including return to work and quality of life (Phillips et al., Citation2017; Theadom et al., Citation2017; Voormolen et al., Citation2019). These symptoms may additionally increase the likelihood of repeated health care presentations, contributing to the substantial economic burden of mTBI (Te Ao et al., Citation2014).
Despite the importance of subjective cognitive symptoms, these symptoms have received limited direct attention in mTBI research. Rather, most research focuses on post-concussion symptoms generically, with little emphasis on particular domains (Cassidy et al., Citation2014). Further, the limited research that has addressed subjective cognitive symptomatology as a distinct entity, has typically evaluated cognitive symptoms in terms of a small number of cognitive items that are typically incorporated in generic post-concussion measures (Ngwenya et al., Citation2018). As these measures only contain three to four cognition-related items, this approach limits the reliability and validity of inferences that can be made about cognitive symptoms specifically (Anderson, Citation2021). Only a couple of studies have evaluated these symptoms using more comprehensive measures comprised of multiple items for each cognitive construct commonly associated with subjective cognitive impairment after mTBI (i.e., processing speed, attention, memory and executive function; Anderson, Citation2021; Clarke et al., Citation2012).
In contrast to generic post-concussion symptoms, which can be reported at similar levels in mTBI and non-mTBI groups (Cassidy et al., Citation2014; Dean et al., Citation2012), preliminary findings suggest that subjective cognitive symptoms may be elevated in mTBI relative to controls (de Koning et al., Citation2016; Ponsford et al., Citation2011). A recent systematic review and meta-analysis examined this question and found that self-reported cognitive symptoms were elevated after mTBI relative to a combination of healthy and injured controls (Levy et al., Citation2022). Although this indicates that post-mTBI cognitive symptoms warrant attention, it is not yet clear whether cognitive symptoms are elevated in mTBI patients compared to trauma controls (TCs). Factors associated with general trauma, such as pain (Cnossen et al., Citation2018) and fatigue (Anderson & Jordan, Citation2021; Stulemeijer et al., Citation2007), can contribute to elevated self-reported symptoms. For this reason, there is a need for research that compares subjective cognitive symptoms between individuals with mTBI and TCs, who have equivalent levels of general trauma-related consequences, in order to control for these potential confounding factors.
Recent studies have also demonstrated that females (Levin et al., Citation2021) and those with increased psychological distress (Hromas et al., Citation2021; Stillman et al., Citation2019) report greater subjective cognitive symptoms after mTBI. However, to date no studies have employed a comprehensive measure of cognitive symptoms to determine whether these findings are specific to mTBI or common to all individuals who have suffered a traumatic injury. Indeed, findings to date suggest that gender and psychological distress may not be associated with self-reported cognitive symptoms in some control groups (Anderson, Citation2021; Levin et al., Citation2021). Consequently, the relative specificity of these relationships to mTBI is currently unknown, and our understanding of the mechanisms contributing to the relationships between psychological distress, gender and cognitive symptoms is therefore limited. This is particularly problematic because lack of mechanistic knowledge about the factors contributing to outcome after mTBI deleteriously impacts clinical intervention and management choices for individuals with mTBI.
The primary aims of this study were to comprehensively investigate self-reported cognitive symptoms in pre-morbidly healthy adults with mTBI and TCs. It was hypothesized that approximately 8 weeks after injury: (1) self-reported cognitive symptoms would be greater in the mTBI group than TCs; and (2) subjective cognitive symptoms would be significantly predicted by gender and psychological distress in the mTBI group. A secondary aim of this study was to evaluate whether different cognitive symptom measures have similar utility for evaluating these symptoms in mTBI.
Methods
Participants
Study participants were recruited from trauma wards at The Alfred hospital and Royal Melbourne Hospital between December 2016 and January 2020. Potential participants were allocated to either the mTBI or TC group based on the presence or absence of mTBI. The definition of mTBI was based on the World Health Organization (WHO) criteria, i.e., (1) presence of at least one of the following: confusion or disorientation, loss of consciousness ≤30 min, post-traumatic amnesia ≤24 h, and/or other transient neurological abnormalities; and (2) Glasgow Coma scale score of 13–15 after 30 min or more post-injury (Carroll et al., Citation2004). Individuals who had not sustained any impact or injury to the head were assigned to the TC group. Exclusion criteria for both groups included: age <18 or >60 years; current or recent mood/anxiety disorder; history of severe psychological condition; previous moderate-severe head injury; previous neurological history; current or prior intravenous drug use; current or prior heavy alcohol use; or lack of English proficiency. An additional exclusion criterion for the TC group was a history of previous head injury. Further detail on participant recruitment and eligibility has been published previously (Anderson & Cockle, Citation2021). Participants provided informed consent, and the study was approved by the Human Research Ethics Committees at The Alfred Hospital and Royal Melbourne Hospital.
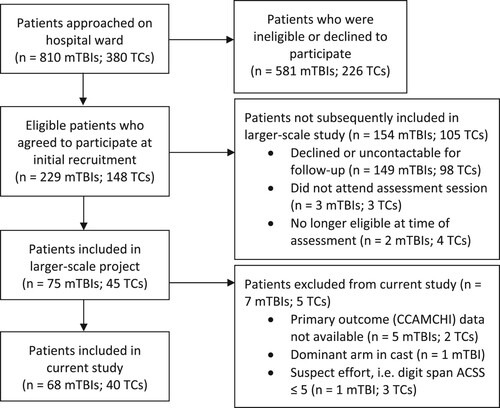
This study was part of a larger-scale project (Anderson, Citation2021; Anderson & Cockle, Citation2021). The current study excluded participants with primary outcome (i.e., CCAMCHI) data unavailable (n = 5 mTBIs, 2 TCs), those who displayed suboptimal effort (n = 1 mTBI, 3 TCs), and those with their dominant arm in a cast such that it was likely to affect motor performance on the SDMT (n = 1 mTBI; see ). A flow diagram of participant recruitment is provided in .
Figure 1. Flow chart of participant recruitment for mild traumatic brain injury (mTBI) and trauma control (TC) patients between December 2016 and January 2020.
Measures
Cognitive symptoms – primary measure
Subjective cognitive symptoms were assessed using the Cognitive Complaint After Mild Closed Head Injury (CCAMCHI) scale (Anderson, Citation2021; Clarke et al., Citation2012). The CCAMCHI is a 30-item questionnaire that assesses subjective beliefs about individuals’ cognitive abilities in the domains of attention, memory, processing speed, and executive function (Anderson, Citation2021). Participants with mTBI were asked to rate their current cognitive abilities compared to their abilities prior to their injury. Responses were made on a Likert scale from one (e.g., “much better”) to five (e.g., “much worse”). Possible scores ranged from 30 to 150. A score of 90 indicates no change in subjective cognitive abilities; a score above 90 indicates worse cognitive functioning relative to pre-injury; a score below 90 indicates better cognitive functioning relative to pre-injury. The control version of the questionnaire asked participants to rate their current cognitive abilities relative to their abilities 4 weeks ago. The CCAMCHI demonstrated excellent internal consistency (α = 0.90) in this sample, which is consistent with previous samples (Clarke et al., Citation2012).
The CCAMCHI was employed as the primary measure of subjective cognitive symptoms in this study, given that it is: (1) specifically developed for use in mTBI, and thus assesses the key cognitive domains impacted by mTBI (Anderson, Citation2021); and (2) is a comprehensive, 30-item measure, which is likely to provide higher reliability than cognitive subscales from postconcussion symptom measures, which typically consist of only three to four items (Anderson, Citation2021). The CCAMCHI is similar in length to other clinically used measures (Rush et al., Citation1996).
Cognitive symptoms – secondary measures
The A-B Neuropsychological Assessment Schedule (ABNAS; Aldenkamp et al., Citation1995; Brooks et al., Citation2001) was employed as a secondary measure of subjective cognitive symptoms in order to explore whether differing measures have similar utility for assessing these symptoms in mTBI. The ABNAS was originally developed to assess subjective cognitive side effects in individuals taking antiepileptic drugs (Brooks et al., Citation2001), and has since been employed in other clinical populations (Lamb et al., Citation2013). The 24-item measure assesses symptoms across the following domains: fatigue, cognitive slowing, memory, concentration, motor coordination, and language. The ABNAS has demonstrated good validity and excellent reliability in clinical and non-clinical samples (Brooks et al., Citation2001).
The cognitive subscale of the Rivermead Post-Concussion Symptoms Questionnaire (RPQ; King et al., Citation1995) was also employed as a secondary measure of subjective cognitive symptoms, in order to allow for comparison to previous studies. The cognitive subscale consists of the three cognition-related items from the RPQ: forgetfulness, poor concentration, and taking longer to think (Potter et al., Citation2006).
Post-concussion symptoms
The total score of the RPQ was employed as a measure of general post-concussion symptoms after injury (King et al., Citation1995). The 16-item scale assesses the presence and severity of various cognitive, affective, and somatic symptoms commonly experienced after mTBI. This tool is widely used in mTBI research and shows high reliability (King et al., Citation1995).
Psychological distress
Symptoms of depression, anxiety, and post-traumatic stress were assessed using the Inventory of Depressive Symptomatology (IDS; 30-item version; Rush et al., Citation1996), the Beck Anxiety Inventory (BAI; Beck et al., Citation1988), and the PTSD Checklist for DSM-5 (PCL-5; Weathers et al., Citation2013). Each of these measures have strong psychometric properties (Beck et al., Citation1988; Blevins et al., Citation2015; Rush et al., Citation1996) and have been used previously in mTBI populations (Levin et al., Citation2021; Stillman et al., Citation2019; Suhr & Gunstad, Citation2002). A psychological distress index was created by converting scores on the PCL-5 to a four-point scale to create equivalence with the IDS and BAI, and then summing scores on the three measures (Anderson & Jordan, Citation2021). Total possible scores ranged from zero (no symptoms experienced) to 207 (every symptom experienced to an extreme degree).
Pain and fatigue
Pain and fatigue were measured using the Short-Form McGill Pain Questionnaire (SF-MPQ-2; Dworkin et al., Citation2009) and the Multidimensional Fatigue Inventory (MFI; Smets et al., Citation1995), respectively. Both measures have been validated for use in clinical populations and have been used previously in TBI research (Anderson, Citation2020; Lovejoy et al., Citation2012; Manoli et al., Citation2020).
Objective cognitive performance
Premorbid intellectual functioning
The Weschler Test of Adult Reading (WTAR; Wechsler, Citation2001) was used to estimate premorbid intellectual abilities. This task requires individuals to read irregularly-spelled words aloud, with the total score being the number of words pronounced correctly. This task has been shown to provide stable estimates of premorbid abilities in controls and in individuals with mTBI (Steward et al., Citation2017). This is a more accurate method of estimating premorbid intellectual function compared to estimates based on demographic information (Bright & van der Linde, Citation2020).
Speed of information processing
Speed of information processing was assessed using the written version of the Symbol Digit Modalities Test (SDMT; Smith, Citation1982). This is a timed task that requires individuals to fill in numbers that correspond to specific symbols. This measure is sensitive to changes in processing speed after mTBI (Anderson & Cockle, Citation2021). The variable of interest on the task was the total number of items completed in 120 s.
Attention
The Digit Span subtest from the Weschler Adult Intelligence Scale, Fourth Edition (WAIS-IV) was employed as a measure of attention and working memory (Wechsler, Citation2008). On this task, individuals are required to repeat digit sets of increasing length in their original order of presentation, reverse order, and ascending order, respectively. Total raw score was used as the variable of interest, with age-scaled scores avoided in order to enable comparison with other cognitive tasks for which age-scaled scores were not available. This measure has good validity and reliability, and is commonly used in mTBI (Wechsler, Citation2008; Wilde et al., Citation2010).
Memory
Memory was assessed using the Rey Auditory Verbal Learning Test (RAVLT; Schmidt, Citation1996). On this task, individuals learn a 15-word list over repeated trials and then recall these words after a distraction and delay period. The variable of interest was the total number of items recalled after a 25-minute delay (Trial A7). The RAVLT is commonly used in mTBI research and has good psychometric properties (D’Souza et al., Citation2019; Wilde et al., Citation2010).
Executive functioning
The Trail Making Test-Part B (TMT-B) completion time (Reitan, Citation1992) and the Controlled Oral Word Association Test (COWAT) – letters F, A, and S (Benton et al., Citation1994) were used to assess executive functioning. The TMT-B incorporates various cognitive domains including mental flexibility and set shifting, and the COWAT is a measure of verbal fluency (Strauss et al., Citation2006). Both tasks are recommended for assessing outcome after TBI (Wilde et al., Citation2010).
Performance validity
The Digit Span subtest from the WAIS-IV was used as a measure of performance validity (Wechsler, Citation2008). Suboptimal effort was defined as an age-corrected standard score (ACSS) of five or below, a cutoff which maintains high specificity (≥90%) while maximizing sensitivity, including in TBI groups (Babikian & Boone, Citation2007).
Procedure
Group membership and eligibility were determined through patient interview and hospital records. Individuals who provided consent to be contacted for follow up were subsequently assessed at The Alfred Hospital 6–10 weeks after injury. Measures were completed in the following order: SDMT, WTAR, RAVLT, DS, TMT-B, COWAT-FAS, RPQ, ABNAS, CCAMCHI, MFI, IDS, BAI, PCL-5, and SF-MPQ-2.
Statistical analysis
Statistical assumptions were explored prior to all analyses. Groups were compared using Chi-square tests of independence and Welch two sample t-tests for categorial and continuous data, respectively (Field, Citation2012). The exception was the RPQ cognitive subscale, which is a continuous variable but was highly skewed and contained a large number of zero responses. For these reasons, this data was converted into a binary variable (0 = no symptom endorsement; 1 = any degree of symptom endorsement) and analyzed using odds ratios. To assess the relationships between the four symptom variables, Spearman’s rho correlations were employed. Multiple linear regression was used to examine predictors of cognitive and post-concussion symptoms. These analyses were conducted using the CCAMCHI, due to its large number of mTBI-specific items. Given the small number of females in each group, we additionally calculated bootstrapped confidence intervals for the regression models (Field, Citation2012). There was no more than one outlier (z score > 3.29; Tabachnick & Fidell, Citation2013), in each group for any variable. When outliers were present analyses were additionally run with Winsorized data; in all cases there were no changes to the results. Small, medium, and large effect sizes were defined as 0.2, 0.5, and 0.8 for Cohen’s d, and .01, .09, and .25 for r2, respectively (Ellis, Citation2010). Statistical analysis was conducted in R version 3.6.1 (R Core Team, Citation2019), using the following packages: tidyverse (Wickham et al., Citation2019), rstatix (Kassambara, Citation2021), Hmisc (Harrell, Citation2021), effect size (Ben-Shachar et al., Citation2020), lm.beta (Behrendt, Citation2014), confintr (Mayer, Citation2022), boot (Canty & Ripley, Citation2021; Davison & Hinkley, Citation1997), epiR (Stevenson et al., Citation2022), and DescTools (Signorell, Citation2023).
Results
A final sample of 68 mTBI participants (age M = 36.8, SD = 14.8; 22% female) and 40 TC participants (age M = 37.1, SD = 12.8; 18% female) was included in the current study. Demographic data and injury characteristics for included participants are presented in . The groups were well matched on all demographic and injury variables, with no significant between-group differences on any variable.
Table 1. Demographic information and injury characteristics.
Cognitive performance
Given that a key focus of this study was to compare subjective cognitive symptom reporting between mTBI and TC participants, groups were first compared on measures of objective cognitive performance. There was no difference in cognitive performance between groups on any cognitive measure ().
Table 2. Group comparisons on cognitive performance measures.
Cognitive and post-concussion symptoms
Summary statistics and between-group comparisons for each symptom measure are provided in . Subjective cognitive symptoms, as assessed by the CCAMCHI, were reported to a significantly greater degree in the mTBI group than the TC group (large effect). On this measure, the mTBI group tended towards reporting worse cognitive functioning relative to before injury (score >90), whereas the TC group reported approximately no change in cognitive functioning (score ∼90). There was also a significant difference between groups in cognitive symptom reporting on the RPQ. Due to the binary coding of this variable, this indicates that these symptoms were reported at a higher frequency in the mTBI group than in the TC group (small-medium effect). Of note, this was not a robust finding, given that the confidence interval for the odds ratio verged on including 1.0 (the value that indicates no differences between groups). In contrast, there was no difference between groups in the reporting of cognitive symptoms as assessed by the ABNAS, or in the reporting of general post-concussion symptoms on the RPQ.
Table 3. Group comparisons of cognitive and post-concussion symptom measures.
Correlations between subjective symptom variables in mTBI and TC groups are provided in . All four symptom variables were significantly correlated with each other in the mTBI group. In the TC group, only responses on the RPQ and the ABNAS were correlated with each other; responses on the CCAMCHI were not correlated with either the ABNAS, the RPQ total score, or the RPQ cognitive subscale.
Table 4. Correlations between symptom variables within each group.
Predictors of cognitive and post-concussion symptoms
provides the results of the regression analyses. Gender and psychological distress were each significant independent predictors of subjective cognitive symptoms in mTBI, with females and those with increased psychological distress reporting greater symptoms (small and medium-large effects, respectively). The overall model was significant, = .39, F(2, 64) = 21.89, p < .001. In the TC group, neither variable predicted cognitive symptoms, and the overall model was not significant,
= −.06, F(2, 35) = 0.02, p = .985.
Table 5. Predictors of cognitive and post-concussion symptoms within each group.
Psychological distress was also a significant predictor of general post-concussion symptoms in both the mTBI and TC groups, with medium-large effect sizes. Gender was a significant predictor of post-concussion symptom reporting in the mTBI group, with female gender associated with greater symptoms. Gender was not a significant predictor of cognitive symptoms in the TC group, although estimates of effect size were similar across both groups (small effects). The overall prediction model was significant in both the mTBI group, = .46, F(2, 64) = 28.92, p < .001, and the TC group,
= .36, F(2, 35) = 11.52, p < .001.
Discussion
Consistent with the hypotheses, this study demonstrated that in the context of equivalent levels of post-concussion symptom reporting and objective cognitive performance between groups, subjective cognitive symptoms were significantly elevated in mTBI participants relative to TCs. This difference in self-reported symptoms was clearly evident on a comprehensive, mTBI-based measure of cognitive symptoms (CCAMCHI). In contrast, findings were less robust when a three-item measure was employed (RPQ-cognitive subscale), and no discrepancy was present when a non-mTBI based measure was employed (ABNAS). This study also found that gender and psychological distress were significantly and independently predictive of self-reported cognitive symptoms in mTBI, with females and those with higher psychological distress reporting greater symptoms. In contrast, neither variable was predictive of cognitive symptom reporting in TCs.
The finding of elevated self-reported cognitive symptoms in mTBI relative to TCs indicates that these symptoms are not simply a result of general trauma. This provides evidence for the specific elevation of cognitive symptoms in mTBI, in contrast with post-concussion symptoms which have been shown to be non-specific for post-acute mTBI (Cassidy et al., Citation2014; Polinder et al., Citation2018). These findings expand on a recent meta-analysis showing that subjective cognitive symptoms are elevated in mTBI groups relative to combined healthy and injured controls (Levy et al., Citation2022). Findings are also consistent with a recent study that found elevated self-reported cognitive symptoms in mTBI patients relative to orthopedic TCs specifically (Levin et al., Citation2021).
The discrepancy in self-reported cognitive symptoms between mTBI and TC groups was found despite equivalent objective cognitive performance between groups, suggesting that the elevation in subjective symptoms in mTBI may not be driven by underlying cognitive deficits in this group. This finding may reflect poor sensitivity of traditional cognitive performance measures in individuals with mTBI (Bigler, Citation2013), which is an important topic for future research. Nevertheless, current findings align with existing research suggesting that subjective cognitive complaints often do not correspond with objective cognitive performance in mTBI (Anderson, Citation2021; Stulemeijer et al., Citation2007). Additionally, both groups in this study consisted of premorbidly healthy individuals, and groups were matched on various potentially confounding factors, including psychological distress, pain, fatigue, and litigation status. This suggests that none of these factors account for the differences in cognitive symptom reporting between groups.
This study also explored gender and psychological distress as predictors of subjective cognitive symptoms in mTBI and TC groups. Increased psychological distress was a significant predictor of greater cognitive symptoms in the mTBI group, but not in TCs. These findings extend those of a recent study which found that self-reported cognitive symptoms were associated with psychological distress in mTBI but not in healthy controls (Anderson, Citation2021). These findings suggest that it is something unique about the experience of an mTBI, over and above general trauma-related factors, that results in a linear association between psychological distress and subjective cognitive symptoms. One possible explanation for this finding is the hypothesis that symptoms after mTBI are initially attributable primarily to organic causes, and are later maintained by psychological factors such as catastrophizing or symptom preoccupation (Raymont & Fleminger, Citation2022). This concept has previously been applied to cognitive symptoms in mTBI, where a “subjective cognitive dysfunction loop” is triggered by early objective cognitive deficits, maintained by psychological factors (Kay et al., Citation1992). These concepts might also explain why there is no relationship between psychological distress and subjective cognitive symptoms in the TC group, as significant early cognitive deficits are not expected in this group.
Similarly, female gender was associated with increased subjective cognitive symptom reporting in the mTBI group, but not in the TC group. This finding suggests that females have a particular vulnerability to cognitive symptoms after mTBI, rather than after trauma more generally. Interestingly, this pattern was seen even when controlling for psychological distress, and therefore cannot be explained by greater psychological distress in this group (Altemus et al., Citation2014). Other factors that have been proposed to underlie gender differences in symptom reporting include biological sex differences in symptom perception, or social differences in attention to symptoms or in the willingness to report symptoms (Barsky et al., Citation2001). Within this framework, it may be possible that if there are limited internal/external cognitive symptom cues (e.g., after general trauma), a gender difference in symptom reporting may be less likely to be seen. That is, gender differences may not have been seen in the TC group because in this population acute cognitive changes do not occur with the same frequency and/or severity that occurs after mTBI (Landre et al., Citation2006). Further research is necessary to explore these possible explanations.
Consistent with previous research, this study found no difference in the reporting of general post-concussion symptoms after mTBI relative to general trauma (Cassidy et al., Citation2014; Dean et al., Citation2012). Additionally, this study found that both psychological distress and gender predicted post-concussion symptom reporting in mTBI participants, a finding that is also consistent with previous literature (Cnossen et al., Citation2018; Silverberg & Iverson, Citation2011).
The findings of the current study are clinically relevant given the importance of being able to identify individuals at risk of poor outcome following mTBI. Research has shown that some interventions can be effective at reducing symptoms following mTBI, but that these interventions should directly target those at risk of poor outcome (Ghaffar et al., Citation2006; Ponsford et al., Citation2002; Teo et al., Citation2020). Our findings implicate females and individuals with high psychological distress as subgroups of mTBI patients that particularly warrant clinical attention to determine whether intervention is required.
Our findings also highlight important differences in the relationships between psychological distress, gender, and subjective symptoms in mTBI patients versus TCs. While both psychological distress and gender predicted cognitive symptom reporting in the mTBI group, neither variable predicted self-reported cognitive symptoms in TCs. This suggests that the experience of mTBI may affect which factors underlie cognitive symptom reporting after injury, and may have implications for clinical intervention. Additionally, there was a relationship between subjective cognitive and post-concussion symptoms in mTBI participants but not in TC participants, suggesting that the experience of mTBI may impact the relationships between various symptom types. This raises the possibility that elevated cognitive symptoms in mTBI versus control groups may not necessarily be due to group differences in background variables (e.g., levels of psychological distress). Instead, these differences may be at least in part due to differences in how these factors contribute to cognitive symptom reporting within groups. Further research is needed to investigate these possibilities.
The primary cognitive symptom scale used in our study was a comprehensive 30-item scale specifically developed for use after mTBI (Anderson, Citation2021). When subjective cognitive symptoms were measured using just three cognitive items from a post-concussion symptom scale (RPQ), findings were less robust, potentially due to the limited reliability of using a small number of items to measure cognitive symptoms (Anderson, Citation2021). Further, when cognitive symptoms were measured using a scale not developed for mTBI (ABNAS), no elevations in cognitive symptoms were evident relative to TCs. These findings indicate that self-reported cognitive symptoms are not indiscriminately raised after mTBI, and instead cognitive symptom elevation occurs specifically in those domains that are objectively affected by mTBI. Thus, the use of a targeted, mTBI-based measure of cognitive symptoms is crucial when examining these symptoms. Additionally, comprehensive measures of cognitive symptoms may provide a more reliable method for detecting these symptoms after mTBI.
The primary limitation of this study is the modest sample size, particularly in the TC group. Limited sample sizes can result in true effects being missed (Field, Citation2012). To address this issue, measures of effect size were included for all primary analyses, as these measures are independent of sample size (Sullivan & Feinn, Citation2012). As recommended, effect size estimates were considered together with tests of statistical significance when interpreting the results of this study (Ellis, Citation2010). Discrepancies between statistical significance and effect size estimates were found in one instance, and a conservative inferential approach was taken in interpreting these findings. The statistical significance and effect size estimates were otherwise highly consistent, supporting the generalizability of these findings.
Conclusions
This study examined subjective cognitive symptoms in pre-morbidly healthy adults with mTBI and general trauma approximately 8 weeks after injury. Unlike general post-concussion symptom reporting, cognitive symptoms were found to be greater in individuals with mTBI than in TCs. This suggests that the experience of mTBI, rather than general trauma, is responsible for cognitive symptom elevation in the subacute period after mTBI. Further research is warranted to determine whether neurological and/or psychological factors underlie this mTBI-specific elevation. Nevertheless, these findings suggest mTBI-specific management and intervention may be warranted for individuals with elevated cognitive symptoms. This study also found that in the subacute period following mTBI, both psychological distress and gender were significant predictors of self-reported cognitive symptoms, with females and those with higher psychological distress reporting greater symptoms. In contrast, neither factor was associated with cognitive symptom reporting in TCs. These findings implicate females and individuals with high psychological distress as important subgroups to consider for potential intervention following mTBI. Further research is needed to understand the mTBI-specific mechanisms by which female gender and greater psychological distress lead to increased subjective cognitive symptoms in this group.
Acknowledgments
We would like to acknowledge the following individuals who assisted with data collection on this project: Lucy Oehr, Nicola Singleton, Courtney Lewis, Katie Priestley, Emily Cockle, Patrick Summerell, Lana Higson, and Emma Gust. We would also like to acknowledge Sue Finch (Melbourne Statistical Consulting Platform), who provided statistical consulting on this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Aldenkamp, A. P., Baker, G., Pieters, M. S. M., Schoemaker, H. C., Cohen, A. F., & Schwabe, S. (1995). The Neurotoxicity Scale: The validity of a patient-based scale, assessing neurotoxicity. Epilepsy Research, 20(3), 229–239. https://doi.org/10.1016/0920-1211(94)00082-8
- Altemus, M., Sarvaiya, N., & Epperson, C. N. (2014). Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology, 35(3), 320–330. https://doi.org/10.1016/j.yfrne.2014.05.004
- Anderson, J. F. I. (2020). The association between pain type, cognition and complaint after mild traumatic brain injury in prospectively studied premorbidly healthy adults admitted to hospital. Neuropsychology, 34(1), 53–62. https://doi.org/10.1037/neu0000585
- Anderson, J. F. I. (2021). Cognitive complaint and objective cognition during the post-acute period after mild traumatic brain injury in pre-morbidly healthy adults. Brain Injury, 1–11. https://doi.org/10.1080/02699052.2020.1859613
- Anderson, J. F. I., & Cockle, E. (2021). Investigating the effect of fatigue and psychological distress on information processing speed in the postacute period after mild traumatic brain injury in premorbidly healthy adults. Archives of Clinical Neuropsychology, 1–12. https://doi.org/10.1093/arclin/acaa123
- Anderson, J. F. I., & Jordan, A. S. (2021). Sex predicts post-concussion symptom reporting, independently of fatigue and subjective sleep disturbance, in premorbidly healthy adults after mild traumatic brain injury. Neuropsychological Rehabilitation, 1–16. https://doi.org/10.1080/09602011.2021.1993274
- Babikian, T., & Boone, K. B. (2007). Intelligence tests as measures of effort. In K. B. Boone (Ed.), Assessment of feigned cognitive impairment: A neuropsychological perspective (pp. 103–127). The Guilford Press.
- Barsky, A., Peekna, H., & Borus, J. (2001). Somatic symptom reporting in women and men. Journal of General Internal Medicine, 16(4), 266–275. https://doi.org/10.1046/j.1525-1497.2001.016004266.x
- Beck, A. T., Brown, G., Kiyosaki, R. T., & Lechter, S. L. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. https://doi.org/10.1037/0022-006X.56.6.893
- Behrendt, S. (2014). lm.beta: Add standardized regression coefficients to lm-objects (Version 1.5-1). R Package. https://cran.r-project.org/package=lm.beta
- Ben-Shachar, M., Lüdecke, D., & Makowski, D. (2020). Effectsize: Estimation of effect size indices and standardized parameters. Journal of Open Source Software, 5(56), 2815. https://doi.org/10.21105/joss.02815
- Benton, A. L., Hamsher, K. de S., & Sivan, A. B. (1994). Multilingual aphasia examination (3rd ed.). AJA Associates.
- Bigler, E. D. (2013). Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychology Review, 23(3), 169–209. https://doi.org/10.1007/s11065-013-9237-2
- Blevins, C. A., Weathers, F. W., Davis, M. T., Witte, T. K., & Domino, J. L. (2015). The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. https://doi.org/10.1002/jts.22059
- Bright, P., & van der Linde, I. (2020). Comparison of methods for estimating premorbid intelligence. Neuropsychological Rehabilitation, 30(1), 1–14. https://doi.org/10.1080/09602011.2018.1445650
- Brooks, J., Baker, G. A., & Aldenkamp, A. P. (2001). The A-B Neuropsychological Assessment Schedule (ABNAS): The further refinement of a patient-based scale of patient-perceived cognitive functioning. Epilepsy Research, 43(3), 227–237. https://doi.org/10.1016/S0920-1211(00)00198-4
- Canty, A., & Ripley, B. (2021). Boot: Bootstrap R (S-plus) functions (Version 1.3-28). R Package.
- Carroll, L. J., Cassidy, J. D., Holm, L., Kraus, J., & Coronado, V. G. (2004). Methodological issues and research recommendations for mild traumatic brain injury: The WHO collaborating centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine, 36(Suppl. 43), 113–125. https://doi.org/10.1080/16501960410023877
- Cassidy, J. D., Cancelliere, C., Carroll, L. J., Côté, P., Hincapié, C. A., Holm, L. W., Hartvigsen, J., Donovan, J., Nygren-De Boussard, C., Kristman, V. L., & Borg, J. (2014). Systematic review of self-reported prognosis in adults after mild traumatic brain injury: Results of the international collaboration on mild traumatic brain injury prognosis. Archives of Physical Medicine and Rehabilitation, 95(3, Suppl. 2), S132–S151. https://doi.org/10.1016/j.apmr.2013.08.299
- Cassidy, J. D., Carroll, L. J., Peloso, P. M., Borg, J., Von Holst, H., Holm, L., Kraus, J., & Coronado, V. G. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine, 36(Suppl. 43), 28–60. https://doi.org/10.1080/16501960410023732
- Clarke, L. A., Genat, R. C., & Anderson, J. (2012). Long-term cognitive complaint and post-concussive symptoms following mild traumatic brain injury: The role of cognitive and affective factors. Brain Injury, 26(3), 298–307. https://doi.org/10.3109/02699052.2012.654588
- Cnossen, M. C., van der Naalt, J., Spikman, J. M., Nieboer, D., Yue, J. K., Winkler, E. A., Manley, G., von Steinbuechel, N., Polinder, S., Steyerberg, E. W., & Lingsma, H. (2018). Prediction of persistent post-concussion symptoms following mild traumatic brain injury. Journal of Neurotrauma, 35(22), 2691–2698. https://doi.org/10.1089/neu.2017.5486
- Davison, A. C., & Hinkley, D. v. (1997). Bootstrap methods and their applications. Cambridge University Press.
- Dean, P. J. A., O’Neill, D., & Sterr, A. (2012). Post-concussion syndrome: Prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Injury, 26(1), 14–26. https://doi.org/10.3109/02699052.2011.635354
- de Koning, M. E., Gareb, B., El Moumni, M., Scheenen, M. E., van der Horn, H. J., Timmerman, M. E., Spikman, J. M., & van der Naalt, J. (2016). Subacute posttraumatic complaints and psychological distress in trauma patients with or without mild traumatic brain injury. Injury, 47(9), 2041–2047. https://doi.org/10.1016/j.injury.2016.04.036
- D’Souza, A., Mollayeva, S., Pacheco, N., Javed, F., Colantonio, A., & Mollayeva, T. (2019). Measuring change over time: A systematic review of evaluative measures of cognitive functioning in traumatic brain injury. Frontiers in Neurology, 10(May). https://doi.org/10.3389/fneur.2019.00353
- Dworkin, R. H., Turk, D. C., Revicki, D. A., Harding, G., Coyne, K. S., Peirce-Sandner, S., Bhagwat, D., Everton, D., Burke, L. B., Cowan, P., Farrar, J. T., Hertz, S., Max, M. B., Rappaport, B. A., & Melzack, R. (2009). Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain, 144(1–2), 35–42. https://doi.org/10.1016/j.pain.2009.02.007
- Ellis, P. D. (2010). The essential guide to effect sizes: Statistical power, meta-analysis, and the interpretation of research results. Cambridge University Press.
- Field, A. P. (2012). Discovering statistics using R. Sage.
- Ghaffar, O., McCullagh, S., Ouchterlony, D., & Feinstein, A. (2006). Randomized treatment trial in mild traumatic brain injury. Journal of Psychosomatic Research, 61(2), 153–160. https://doi.org/10.1016/j.jpsychores.2005.07.018
- Harrell, F. E. (2021). Hmisc: Harrell miscellaneous (Version 4.6-0). R Package.
- Hromas, G. A., Houck, Z. M., Asken, B. M., Svingos, A. M., Greif, S. M., Heaton, S. C., Jaffee, M. S., & Bauer, R. M. (2021). Making a difference: Affective distress explains discrepancy between objective and subjective cognitive functioning after mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 36(3), 186–195. https://doi.org/10.1097/HTR.0000000000000618
- Kassambara, A. (2021). rstatix: Pipe-friendly framework for basic statistical tests (Version 0.7.0). R Package. https://cran.r-project.org/package=rstatix
- Kay, T., Newman, B., Cavallo, M., Ezrachi, O., & Resnick, M. (1992). Toward a neuropsychological model of functional disability after mild traumatic brain injury. Neuropsychology, 6(4), 371–384. https://doi.org/10.1037/0894-4105.6.4.371
- King, N. S., Crawford, S., Wenden, F. J., Moss, N. E. G., & Wade, D. T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. Journal of Neurology, 242(9), 587–592. https://doi.org/10.1007/BF00868811
- Lamb, F., Anderson, J., Saling, M., & Dewey, H. (2013). Predictors of subjective cognitive complaint in postacute older adult stroke patients. Archives of Physical Medicine and Rehabilitation, 94(9), 1747–1752. https://doi.org/10.1016/j.apmr.2013.02.026
- Landre, N., Poppe, C. J., Davis, N., Schmaus, B., & Hobbs, S. E. (2006). Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Archives of Clinical Neuropsychology, 21(4), 255–273. https://doi.org/10.1016/j.acn.2005.12.007
- Levin, H. S., & Diaz-Arrastia, R. R. (2015). Diagnosis, prognosis, and clinical management of mild traumatic brain injury. The Lancet Neurology, 14(5), 506–517. https://doi.org/10.1016/S1474-4422(15)00002-2
- Levin, H. S., Temkin, N. R., Barber, J., Nelson, L. D., Robertson, C., Brennan, J., Stein, M. B., Yue, J. K., Giacino, J. T., McCrea, M. A., Diaz-Arrastia, R., Mukherjee, P., Okonkwo, D. O., Boase, K., Markowitz, A. J., Bodien, Y., Taylor, S., Vassar, M. J., Manley, G. T., … Zafonte, R. (2021). Association of sex and age with mild traumatic brain injury-related symptoms: A TRACK-TBI study. JAMA Network Open, 4(4), 1–17. https://doi.org/10.1001/jamanetworkopen.2021.3046
- Levy, A. M., Saling, M. M., & Anderson, J. F. I. (2022). Frequency and extent of cognitive complaint following adult civilian mild traumatic brain injury: A systematic review and meta-analysis. Brain Impairment, 1–24. https://doi.org/10.1017/BrImp.2022.19
- Lovejoy, T. I., Turk, D. C., & Morasco, B. J. (2012). Evaluation of the psychometric properties of the revised Short-Form McGill Pain Questionnaire (SF-MPQ-2). The Journal of Pain, 13(12), 1250–1257. https://doi.org/10.1016/j.jpain.2012.09.011
- Machamer, J., Temkin, N., Dikmen, S., Nelson, L. D., Barber, J., Hwang, P., Boase, K., Stein, M. B., Sun, X., Giacino, J., McCrea, M. A., Taylor, S. R., Jain, S., Manley, G., Badjatia, N., Bodien, Y., Diaz-Arrastia, R., Duhaime, A.-C., Feeser, V. R., … Zafonte, R. (2022). Symptom frequency and persistence in the first year after traumatic brain injury: A TRACK-TBI study. Journal of Neurotrauma, 39(5–6), 358–370. https://doi.org/10.1089/neu.2021.0348
- Manoli, R., Chartaux-Danjou, L., Delecroix, H., Daveluy, W., & Moroni, C. (2020). Is Multidimensional Fatigue Inventory (MFI-20) adequate to measure brain injury related fatigue? Disability and Health Journal, 13(3), 100913. https://doi.org/10.1016/j.dhjo.2020.100913
- Mayer, M. (2022). confintr: Confidence intervals (Version 0.1.2). R Package.
- McMahon, P. J., Hricik, A., Yue, J. K., Puccio, A. M., Inoue, T., Lingsma, H. F., Beers, S. R., Gordon, W. A., Valadka, A. B., Manley, G. T., Okonkwo, D. O., Casey, S. S., Cooper, S. R., Dams-O’Connor, K., Menon, D. K., Sorani, M. D., Yuh, E. L., Mukherjee, P., Schnyer, D. M., … Vassar, M. J. (2014). Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective TRACK-TBI study. Journal of Neurotrauma, 31(1), 26–33. https://doi.org/10.1089/neu.2013.2984
- Ngwenya, L. B., Gardner, R. C., Yue, J. K., Burke, J. F., Ferguson, A. R., Huang, M. C., Winkler, E. A., Pirracchio, R., Satris, G. G., Yuh, E. L., Mukherjee, P., Valadka, A. B., Okonkwo, D. O., & Manley, G. T. (2018). Concordance of common data elements for assessment of subjective cognitive complaints after mild-traumatic brain injury: A TRACK-TBI pilot study. Brain Injury, 32(9), 1071–1078. https://doi.org/10.1080/02699052.2018.1481527
- Phillips, D., Ngwenya, L., Huang, M., & Saeng Hong, O. (2017). Subjective cognitive complaints in mild traumatic brain injury and 6-month return to work prediction: A track-TBI study. Journal of Clinical and Translational Science, 1(Suppl. 1), 29. https://doi.org/10.1017/cts.2017.112 https://search-proquest-com.ezproxy.lib.rmit.edu.au/docview/1908297810?accountid=13552
- Polinder, S., Cnossen, M. C., Real, R. G. L., Covic, A., Gorbunova, A., Voormolen, D. C., Master, C. L., Haagsma, J. A., Diaz-Arrastia, R., & Von Steinbuechel, N. (2018). A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Frontiers in Neurology, 9(December), 1–14. https://doi.org/10.3389/fneur.2018.01113
- Ponsford, J., Cameron, P., Fitzgerald, M., Grant, M., & Mikocka-Walus, A. (2011). Long-term outcomes after uncomplicated mild traumatic brain injury: A comparison with trauma controls. Journal of Neurotrauma, 28(6), 937–946. https://doi.org/10.1089/neu.2010.1516
- Ponsford, J., Willmott, C., Rothwell, A., Cameron, P., Kelly, A. M., Nelms, R., & Curran, C. (2002). Impact of early intervention on outcome following mild head injury in adults. Journal of Neurology Neurosurgery and Psychiatry, 73(3), 330–332. https://doi.org/10.1136/jnnp.73.3.330
- Potter, S., Leigh, E., Wade, D., & Fleminger, S. (2006). The Rivermead Post Concussion Symptoms Questionnaire: A confirmatory factor analysis. Journal of Neurology, 253(12), 1603–1614. https://doi.org/10.1007/s00415-006-0275-z
- Raymont, V., & Fleminger, S. (2022). Alwyn Lishman’s contribution to the neuropsychiatry of head injury (traumatic brain injury); two key papers. Cognitive Neuropsychiatry, 27, 289–295. https://doi.org/10.1080/13546805.2022.2047631
- R Core Team. (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://doi.org/10.1007/978-3-540-74686-7; https://www.R-project.org/
- Reitan, R. M. (1992). Trail Making Test: Manual for administration and scoring. Reitan Neuropsychological Laboratory.
- Rush, A. J., Gullion, C. M., Basco, M. R., Jarrett, R. B., & Trivedi, M. H. (1996). The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine, 26(3), 477–486. https://doi.org/10.1017/S0033291700035558
- Schmidt, M. (1996). Rey Auditory-Verbal Learning Test. Western Psychological Services.
- Signorell, A. (2023). DescTools: Tools for descriptive statistics (Version 0.99.48). R Package. https://cran.r-project.org/package=DescTools
- Silverberg, N. D., & Iverson, G. L. (2011). Etiology of the post-concussion syndrome: Physiogenesis and psychogenesis revisited. NeuroRehabilitation, 29(4), 317–329. https://doi.org/10.3233/NRE-2011-0708
- Smets, E. M. A., Garssen, B., Bonke, B., & De Haes, J. C. J. M. (1995). The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research, 39(5), 315–325. https://doi.org/10.1016/0022-3999(94)00125-O
- Smith, A. (1982). Symbol Digit Modalities Test (SDMT) manual. Western Psychological Services.
- Stevenson, M., Sergeant, E., Nunes, T., Heuer, C., Marshall, J., Sanchez, J., Thornton, R., Reiczigel, J., Robison-Cox, J., Sebastiani, P., Solymos, P., Yoshida, K., Jones, G., Pirikahu, S., Firestone, S., Kyle, R., Popp, J., Jay, M., Reynard, C., … Rabiee, A. (2022). epiR: Tools for the analysis of epidemiological data (Version 2.0.54). R Package.
- Steward, K. A., Novack, T. A., Kennedy, R., Crowe, M., Marson, D. C., & Triebel, K. L. (2017). The Wechsler Test of Adult Reading as a measure of premorbid intelligence following traumatic brain injury. Archives of Clinical Neuropsychology, 32(1), 98–103. https://doi.org/10.1093/arclin/acw081
- Stillman, A. M., Madigan, N., Torres, K., Swan, N., & Alexander, M. P. (2019). Subjective cognitive complaints in concussion. Journal of Neurotrauma, 311, 305–311. https://doi.org/10.1089/neu.2018.5925
- Strauss, E., Sherman, E. M. S., & Spreen, O. (2006). A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed.). Oxford University Press.
- Stulemeijer, M., Vos, P. E., Bleijenberg, G., & van der Werf, S. P. (2007). Cognitive complaints after mild traumatic brain injury: Things are not always what they seem. Journal of Psychosomatic Research, 63(6), 637–645. https://doi.org/10.1016/j.jpsychores.2007.06.023
- Suhr, J. A., & Gunstad, J. (2002). “Diagnosis threat”: The effect of negative expectations on cognitive performance in head injury. Journal of Clinical and Experimental Neuropsychology, 24(4), 448–457. https://doi.org/10.1076/jcen.24.4.448.1039
- Sullivan, G. M., & Feinn, R. (2012). Using effect size—or why the P value is not enough. Journal of Graduate Medical Education, 4(3), 279–282. https://doi.org/10.4300/JGME-D-12-00156.1
- Tabachnick, B., & Fidell, L. (2013). Cleaning up your act: Screening data prior to analysis. In B. Tabachnick & L. Fidell (Eds.), Using multivariate statistics (6th ed., pp. 94–152). Pearson Education.
- Te Ao, B., Brown, P., Tobias, M., Ameratunga, S., Barker-Collo, S., Theadom, A., McPherson, K., Starkey, N., Dowell, A., Jones, K., & Feigin, V. L. (2014). Cost of traumatic brain injury in New Zealand: Evidence from a population-based study. Neurology, 83(18), 1645–1652. https://doi.org/10.1212/WNL.0000000000000933
- Teo, S. H., Fong, K. N. K., Chen, Z., & Chung, R. C. K. (2020). Cognitive and psychological interventions for the reduction of post-concussion symptoms in patients with mild traumatic brain injury: A systematic review. Brain Injury, 34(10), 1305–1321. https://doi.org/10.1080/02699052.2020.1802668
- Theadom, A., Barker-Collo, S., Jones, K., Kahan, M., Te Ao, B., McPherson, K., Starkey, N., & Feigin, V. (2017). Work limitations 4 years after mild traumatic brain injury: A cohort study. Archives of Physical Medicine and Rehabilitation, 98(8), 1560–1566. https://doi.org/10.1016/j.apmr.2017.01.010
- Voormolen, D. C., Polinder, S., von Steinbuechel, N., Vos, P. E., Cnossen, M. C., & Haagsma, J. A. (2019). The association between post-concussion symptoms and health-related quality of life in patients with mild traumatic brain injury. Injury, 50(5), 1068–1074. https://doi.org/10.1016/j.injury.2018.12.002
- Weathers, F. W., Litz, B. T., Keane, T. M., Palmieri, P. A., Marx, B. P., & Schnurr, P. P. (2013). The PTSD checklist for DSM-5 (PCL-5). www.ptsd.va.gov
- Wechsler, D. (2001). Wechsler Test of Adult Reading. The Psychological Corporation.
- Wechsler, D. (2008). Wechsler Adult Intelligence Scale (4th ed.). The Psychological Corporation.
- Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L., François, R., Grolemund, G., Hayes, A., Henry, L., Hester, J., Kuhn, M., Pedersen, T., Miller, E., Bache, S., Müller, K., Ooms, J., Robinson, D., Seidel, D., Spinu, V., … Yutani, H. (2019). Welcome to the tidyverse. Journal of Open Source Software, 4(43), https://doi.org/10.21105/joss.01686
- Wilde, E. A., Whiteneck, G. G., Bogner, J., Bushnik, T., Cifu, D. X., Dikmen, S., French, L., Giacino, J. T., Hart, T., Malec, J. F., Millis, S. R., Novack, T. A., Sherer, M., Tulsky, D. S., Vanderploeg, R. D., & Von Steinbuechel, N. (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Archives of Physical Medicine and Rehabilitation, 91(11), 1650–1660.e17. https://doi.org/10.1016/j.apmr.2010.06.033

