Abstract
Lipid rafts are specialized plasma membrane microdomains in which glycosphingolipids and cholesterol are major structural components. Their relative insolubility to nonionic detergents is the most widely used method to purify these structures. Several signalling proteins are associated with these microdomains in T lymphocytes, including receptors for growth factors and cytokines. ProTα is a highly conserved and widely distributed protein whose physiological functions remain elusive. In previous works we identified, by means of affinity cross-linking, affinity chromatography and fluorescence microscopy, a set of binding proteins for ProTα in human lymphoblasts. Now, this work goes deeply in that ProTα receptor description revealing, by different experimental approaches, its presence in lipid rafts. Moreover, our results fit a model in which a tyrosine phosphorylation signalling cascade confined to rafts is initiated upon ProTα receptor recognition, which represents an important and promising finding in the research for elucidating the molecular mechanisms underlying the immunomodulatory functions of ProTα.
| Abbreviations | ||
| MβCD | = | methyl beta cyclodextrin |
| ProTα | = | Prothymosin α |
| GPI | = | glycosylphosphatidylinositol |
| TX-100 | = | Triton X-100 |
| CTB | = | cholerae toxin B subunit |
| HRP | = | horseradish peroxidase |
| PE | = | phycoerythrin |
| FITC | = | fluorescein isothiocyanate |
| DAB | = | 3,3′-diaminobenzidine |
| GM1 | = | ganglioside M1 |
| mAb | = | monoclonal antibody |
| PHA | = | phytohemagglutinin |
| AP | = | alkaline phosphatase |
Introduction
The current view of the three-dimensional plasma membrane organization includes specific interactions between membrane lipids and proteins, producing a physical and functional compartmentation in dynamic microdomains also called rafts Citation[1,2]. Rafts are enriched in extracellular GPI-anchored as well as cytoplasmic acylated proteins. However, transmembrane receptors or cytoskeletal proteins have been also detected by different means Citation[3]. These microdomains deeply affect membrane functionality since the clustering of all these proteins (and associated molecules) in a very small area renders supramolecular complexes with new functions related with vesicular trafficking, signal transduction, viral gemation and cell motility Citation[4–6]. However, despite the major advances made, the presence of rafts in vivo, their dynamics and stability as well as their actual dimensions are still under discussion Citation[7–10].
The raft model has received much attention after the discovery of their role in the functionality of the lymphocyte, the central cell of the Immune System. This is because a crucial event in its biology, the signalling through the TCR/BCR receptors, is closely related with these membrane structures. According to a recent model both TCR and BCR are weakly associated with or even excluded from rafts during the resting state, and only upon triggering of these receptors (for example, after antibody-crosslinking) they move inside these microdomains Citation[11–15], where Src kinases accumulate. The number of signalling processes related with proliferation and differentiation where it was found a link to rafts is increasing daily. Many cytokine and growth factor receptors are located in this region, including IL-2α Citation[16], EGFR Citation[17], PDGF Citation[18], FGF Citation[19] and insulin Citation[20]. The current goal is not only to find out the signalling mechanisms triggered by these structures, but also the functional consequences that a membrane compartmentation could have on these transduction pathways.
Prothymosin α (ProTα) is a highly acidic and small protein of only 109 amino acids with an unusual primary structure. Based on its wide distribution or high conservation degree amongst mammals one would expect ProTα to play an essential role in the organism Citation[21]. However, despite the number of effects described for this protein (e.g., intracellular modulator of nuclear processes Citation[22], inhibitor of apoptosome formation Citation[23] or extracellular functions; reviewed in Piñeiro et al. Citation[21]) none of them have been really accepted as its actual physiological role.
The immunoregulatory properties of ProTα have been described both in vivo and in vitro. Perhaps, the most outstanding in vivo assays are the ones showing an anticancer activity for ProTα in an experimental tumour model, prolonging the survival of DBA/2 mice inoculated intraperitoneally with syngenic L1210 leukemic cells Citation[24,25]. In vitro, ProTα has been shown to increase allo- and auto-mixed lymphocyte responses in multiple sclerosis and systemic lupus erythematosus patients Citation[26,27]. Likewise, ProTα regulates IL-2 and PGE2 secretion in bulk mononuclear cell-mediated lympholytic activities in cancer patients Citation[28]. These effects are also observed in normal donors, where there is an enhancement of the PHA-induced proliferation of PBMC and an increased NK activity Citation[29–33]. Finally, Eckert et al. opened the way for clinical applications of ProTα in melanoma and colorectal tumour patients Citation[34,35].
Taking into account all the above mentioned biological responses to ProTα and after detecting a number of binding sites on the plasma membrane of lymphoid cells Citation[36–38] our group started a study to broach the ProTα receptor characterization. As a result, affinity crosslinking and affinity chromatography experiments uncovered the existence of three binding proteins for ProTα of 31, 29 and 19 kDa on the cell surface of human lymphoblasts Citation[39].
On the other hand, a basic criterion that a putative receptor for a specific ligand should met is that it must be present not only in all tissues and cell types showing pharmacological activity, but also located in a membrane region where adequately carry out its activity. In this report we demonstrate that different ProTα binding proteins (ProTα receptor) are detected on rafts from human lymphocytes and also that, upon ProTα receptor recognition by its ligand, there is a change in the tyrosine phosphorylation pattern of proteins exclusively located in rafts. Thus, our data point toward a ProTα receptor being responsible for the activities described for its ligand on the Immune System and reinforce the information gathered during the last years suggesting that the main part of the signalling processes related with cell proliferation and differentiation arise in these lipid rafts microdomains.
Material and methods
Cell isolation and culture
Buffy coats were kindly provided by the Centro de Transfusiones de Galicia, Santiago de Compostela, Spain. Human PBMCs (Peripheral Blood Mononuclear Cells) were isolated by Ficoll Paque PLUS (Amersham Biosciences) density gradient centrifugation as described Citation[39]. Cells were cultured at 1×106 PBMCs/ml in RPMI 1640 (Sigma, Spain) supplemented with 10% inactivated FBS (Invitrogen, Spain), 100 µg/ml streptomycin and 100 IU/ml penicillin (Sigma), in a humidified atmosphere with 5% CO2 at 37°C and activated with 2.5 µg/ml PHA-P (Sigma) Citation[39]. After three days of culture, lymphoblasts (more than 90% CD3+ T cells) were collected. Cell viability, assessed by trypan blue exclusion and flow cytometry analysis, was always higher than 90%.
Biotinylation
ProTα isolated from calf thymus and tested for purity by amino acid analysis and high voltage electrophoresis was kindly provided by Thymoorgan GmbH (Vieneenburg, Germany). ProTα (1 mg) was dissolved in 1 ml 0.1 M bicarbonate buffer pH 8.3 and subsequently conjugated with biotin-succinimidyl ester as previously described by Piñeiro et al. Citation[39].
Isolation of TX-100 resistant membranes/rafts by equilibrium density gradient centrifugation
All the following steps were carried out at 4°C unless indicated and basically as described in Ilangumaran et al. Citation[40–42]. PHA-lymphoblasts (50×106) were washed twice in PBS pH 7.4 and once in TKM buffer (50 mM Tris-HCl, pH 7.5, 25 mM KCl, 5 mM MgCl2, and 1 mM EDTA). Detergent lysates were prepared in TKM containing 0.5% Triton X-100 (TX-100) and the protease inhibitors Pefabloc SC (Roche Diagnotics, Barcelona; 2 mM), leupeptin (Sigma; 10 µg/ml) and aprotinin (Sigma; 5 µg/ml) for 20 min on ice. For equilibrium gradient centrifugation, cell extracts were adjusted to 40% sucrose and loaded into SW55Ti (Beckman L8-M) tubes. Next, 2.7 ml of 36% sucrose and finally 1.575 ml of 5% sucrose, both solutions prepared in TKM buffer, were successively added. After centrifugation at 200,000×g for 18 h, 450 µl fractions were collected from top to bottom, numbered (1–11) and stored at −20°C. Proteins of discontinuous gradient density fractions were evaluated by dot-blot immunoassay.
Affinity chromatography
The affinity matrix was prepared by coupling ProTα to a 1 ml NHS-activated HiTrap column (Amersham-Biosciences Europe GmbH, Barcelona, Spain). To this end, 1 ml of 0.2 M NaHCO3, 0.5M NaCl pH 8.3 (coupling buffer) containing 6 mg of ProTα was injected into the column and the incubation was carried out for 4 h at 4°C. According to the manufacturer's instructions after the incubation the amount of protein bound to the column was determined by a BCA test. Several wash steps with buffers A (0.5 M ethanolamine, 0.5 M NaCl pH 8.3) and B (0.1 M acetate, 0.5 M NaCl pH 4) were performed in order to block any remaining active groups and remove the ligand excess. Column was equilibrated with TNE buffer (20 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA) and coupled to an ÄKTA Purifier 10 (Amersham-Biosciences Europe GmbH, Barcelona, Spain) under the control of UNICORN 3.00 software. TX-100 resistant membranes (rafts) from PHA-activated lymphocytes were purified by equilibrium density gradient ultracentrifugation as described before, pooled and dialysed against TNE buffer. Samples (10–12 ml) was injected stepwise (2 ml each) into the column with a 0.5 ml/min flux. Non specific proteins were washed away with 30 ml of TNE buffer and elution was carried out with 0.1 M glycine pH 2.5 (0.5 ml/min flux). 1 ml fractions were collected, neutralised with 1 M Tris pH 9, concentrated (centricon-10, Millipore Corp., Bedford, MA, USA) and analysed by SDS-PAGE and silver staining.
Affinity cross-linking
Aliquots of 2×106 lymphoblasts were incubated with 40 µg biotin-ProTα at room temperature for 30 min. Chemical cross-linking was carried out in a 20 µl volume containing 1 mM BS3 (Pierce Biotechnology, Inc, Rockford, USA) for 30 min at 4°C. Reaction was stopped by the addition of 1 ml TES buffer (10 mM Tris, pH 7.4, 1 mM EDTA, 250 mM sucrose) and cells extensively washed in ice-cold PBS pH 7.4.
Immunostaining and immunofluorescence
Cells were cross-linked with biotin-ProTα and stained with streptavidin-PE and/or anti CD59-FITC (clon p282; BD-Biosciences, Madrid) and anti CD71-FITC (clon M-A712; BD-Biosciences). The percentage of cells positive for the Ag was evaluated by setting proper negative controls. For studies of detergent resistant proteins associated with rafts we have adapted the work of Janes et al. Citation[43] to flow cytometry. Briefly, cells were treated with 1% TX-100 for 5 min on ice, 10 mM methyl-β-cyclodextrin (MβCD) (Sigma) for 15 min at 37°C, or MβCD followed by TX-100 extraction, before fixation pH (3% paraformaldehyde-60 mM sucrose in PBS Ph 7.4 for 30 min at RT). Subsequently, cells were washed with PBS-20 mM glycine, blocked with PBS 5% BSA and stained as described above. Samples were processed on a Becton Dickinson FACScalibur flow cytometer and WinMDI software (a kind gift of J. Trotter, Scripps Institute, La Jolla, CA) was used to analyse the data.
Protein-depletion from rafts with CTB-HRP/DAB/H2O2
Depletion of microdomain-linked proteins was carried out, with modifications, according to Cheng et al. Citation[44,45]. After a 3-day culture period with 2.5 µg/ml PHA human lymphoblasts were washed twice in ice-cold modified HBSS+ (13 mM CaCl2, 50 mM KCl, 5 mM MgCl2, 4 mM MgSO4, 1.38 M NaCl, 56 mM glucose and 200 mM Hepes, pH 7.4). Aliquots of 2×106 cells were incubated with cholera toxin B subunit (CTB; 0.01, 0.05, 0.1 or 0.1 µg/ml; Sigma) for 30 min at 37°C and washed with ice-cold HBSS+. Samples were then resuspended at 4°C in 1 ml HBSS+ containing diaminobencidine (DAB, Sigma; 0.5 mg/ml or 0.1 mg/ml), in the absence or presence of H2O2 (0.01, 0.05, or 0.1%), for 45 min. After two washes with cold HBSS+, cell lysis was performed for 30 min on ice with TKM/0.5%TX-100/protease inhibitors and nuclei, debris and DBA cross-linked proteins eliminated after centrifugation at 13,000 rpm for 15 min (4°C). Once all the conditions for this protocol were set up aliquots of 2×106 lymphoblasts, with or without biotin-ProTα (15 µM) bound to the cell surface receptors with BS3, were treated with CTB (0.05 µg/ml) and incubated in the absence or presence of DAB (0.5 mg/ml) and H2O2 (0.01%) as indicated.
Dot blot analysis
For these experiments two kinds of serially diluted samples were handled: (a) postnuclear lysates, normalized for total protein or for an even number of cells, from biotin-ProTα (15 µM) labelled lymphoblasts, where rafts microdomains were depleted with the CTB-HRP based method described above; (b) equal volumes of samples from discontinuous density gradient fractions. Proteins were applied to the wells of a dot-blot apparatus (Bio-Rad Laboratories, Inc, CA, USA) to be transferred to nitrocellulose filters (HybondECL, Amersham) for analysis with appropriate antibodies: anti CD59 (clon p282; BD-Biosciences), anti CD71 (clon M-A712; BD-Biosciences) or anti biotin-HRP (Sigma). Goat anti mouse (GAM)-HRP (Sigma) was used as a secondary Ab to reveal anti CD59 or anti CD71 binding to the membranes. Detection was carried out by using a nonisotopic chemiluminescent system (ECL+, Amersham Biosciences) in all cases. When measuring alkaline phosphatase (AP) levels bromochloroindolyl phosphate/nitro blue tetrazolium was used as a substrate (BCIP/NBT; BioRad Laboratories). Spots were quantified by scanning the filters and densitometry (ImageMaster ID, Amersham Biosciences Europe GmbH).
Confocal microscopy
For confocal microscopy, aliquots of 2×106 PHA activated lymphoblasts were cross-linked with 1 mM BS3 in the presence or absence of 15 µM biotin-ProTα. Cells were incubated with streptavidin-PE to detect ProTα receptor expression and stained with anti CD59 or anti CD71 mAbs at 4°C. In order to induce either rafts (with anti-CD59) or non-raft (with anti-CD71) protein clustering and detect both CD59 and CD71 antigens, a further incubation step at RT (i.e., allowing free lateral movements) with a FITC-labelled anti-IgG Ab was performed. After washing with PBS pH 7.4 and SlowFade equilibrium buffer, cells were finally resuspended in a antifading Slowfade solution (Molecular Probes, Inc., OR, USA). Observations were made with a Leica TCS 4D confocal scanning laser microscope adapted to an inverted Leitz DMIRBE microscope (Leica Lasertechnik GmbH, Heidelberg, Germany). Colocalization analysis was performed by means of the MultiColour software (version 2.0; Leica Lasertechnik GmbH).
Western blotting
To detect tyrosine phosphorylation of proteins in total cell lysates, human lymphoblasts (5×106) were placed in 100 µl of RPMI-1640 and incubated at 37°C for 10 min. Then, two 5 µl aliquots (controls) were collected and the rest was divided in two identical (2×106 cells) samples that were treated with 45 µl of RPMI-1640 containing or not ProTα (5 µg/ml final concentration). 10 µl samples were removed at different times and cellular lysis was carried out immediately by the addition of an equal volume of 2× SDS-PAGE Laemmli sample buffer. In another set of experiments, lymphoblasts (50×106) obtained as above and treated or not for 1 min at 37°C with 5 µg/ml of ProTα were subjected to equilibrium density gradient centrifugation as previously indicated. Insoluble (3–6) and soluble (10–11) fractions were pooled, protein concentration of every sample determined and either the same volume or the same amount of protein loaded in a 7.5% SDS-PAGE. After transferring to PVDF (HybondP) or nitrocellulose (HybondECL) membranes (Amersham Biosciences Europe GmbH) blots were blocked with 1% BSA in TBS-T (20 mM Tris-HCl pH 7.6, 137 mM NaCl, 0.1% Tween-20) 1 h at RT, hybridized with anti phosphotyrosine (clon PY20, BD-Biosciences) in blocking buffer 30 min at RT, washed extensively with TBS-T and incubated with GAM-HRP under the same conditions. Bands were detected using ECL+ and revealed with X-OMAT XAR 5 (Eastman-Kodak, Sigma).
Results
ProTα receptor isolated from detergent-resistant membrane fractions
We recently demonstrated by fluorescence microscopy and biotin-ProTα cross-linking to human lymphoblasts that there is a set of ProTα binding proteins heterogeneously distributed on the cell surface to form a cap at one of the poles Citation[39]. Taking into account that the same capping phenomenon has been described for many important receptors we came to the conclusion that a study on the membrane distribution of this receptor would be interesting. Therefore, PHA-activated lymphocytes were cultured and lipid rafts isolated based on their nonionic detergent insolubility at low temperature and their density when submitted to a discontinuous sucrose gradient ultracentrifugation step. Eleven fractions, collected and numbered from the top to the bottom of the tube, were analysed for protein concentration and AP activity () in order to be confident about the conditions used in our raft purification protocol Citation[46,47].
Figure 1. ProTα receptor is located in rafts microdomains. After BS3-mediated crosslinking of biotin-ProTα to surface receptors on human lymphoblasts (50×106) cell lysis was carried out in TKM buffer containing 0.5% TX-100. The cell lysate was then adjusted to 40% sucrose and subjected to equilibrium density gradient centrifugation in a SW55Ti rotor. After a overnight centrifugation at 200,000×g, 11 fractions (0.45 ml/each) were collected from top to bottom. Serial dilutions of the fractions were dotted on and analysed for total protein (BCA assay; a), alkaline phosphatase activity (BCIP/NBT-based assay; a and b), and the presence of ProTα receptor (b), CD59 (c) and CD71 (c). Films were scanned, subjected to densitometry, and data shown as raw arbitrary units (b) or expressed as a percentage of the total amount for the respective antigen (a, c). (d) Analysis by SDS-PAGE of pooled fractions obtained after running a raft membrane extract from human lymphoblasts through a ProTα affinity column.
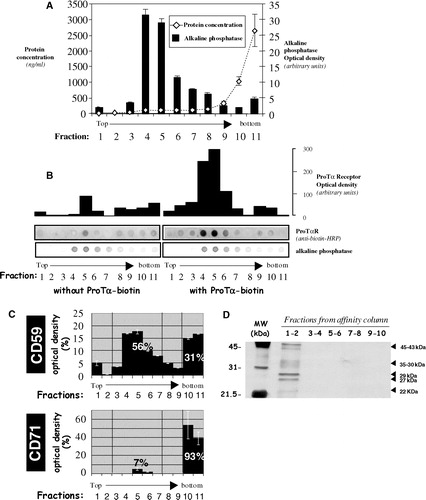
Next, we carried out the same kind of experiments but using human lymphoblasts cross-linked with biotin-ProTα accordingly with the protocol we had previously described Citation[39]. Analysis of those cells by flow cytometry showed activated lymphoblasts exhibiting the correct cell morphology and being, in a vast majority (>90%), positive for the ProTα receptor (data not shown). To study the presence of the receptor in membrane fractions isolated by discontinuous sucrose density gradient ultracentrifugation, dot-blot analyses were performed. We used in this set of experiments peroxidase-conjugated monoclonal antibody against biotin. As observed in , most of the labelling from anti biotin-antibody was detectable on fractions 4–6, the ones with the highest levels of AP activity. We also analysed () the presence of both CD59 (as raft marker) and CD71 (a protein normally excluded from rafts) in order to attain the highest stringent conditions when purifying the different membrane fractions. These controls also pointed out to the 4–6 fractions as the ones containing exclusively raft resident proteins. On the other hand, experiments designed to characterize and purify the Prothymosin α receptor (manuscript in preparation) allowed us to detect in rafts, by affinity chromatography, several protein species of about 35–30, 29–27 and 22–19 kDa (), providing strong evidences about a ProTα interaction with proteins located within lipid rafts.
ProTα receptor is present on lipid rafts from living cells
It has been described that the method based on the insolubility in nonionic detergents for the analysis of raft domains suffers from some technical limitations Citation[48]. Taking that into account, we employed other techniques that could reflect, in a more reliable way, the actual situation of the ProTα receptor in living cells.
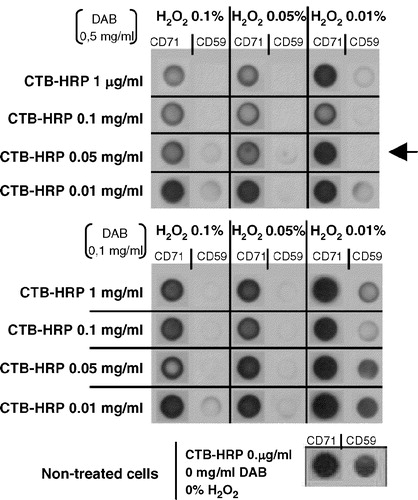
Cholera toxin B subunit (CTB) exhibits a great affinity for ganglioside GM1, a glycosphyngolipid present almost exclusively in raft regions Citation[10]. Cell surface labelling with CTB linked to peroxidase (CTB-HRP) allows a specific cross-linking of surrounding proteins in the presence of hydrogen peroxide (H2O2) and 3,3′-diaminobenzidine (DAB). Any protein at close proximity to CTB-HRP will be polymerized into insoluble aggregates when DAB and H2O2 are both present, but not in the absence of H2O2. Hence, only proteins displayed within the area where GM1 is present in vivo will precipitate after cell lysis and centrifugation. This technique has been designed to work with closed cellular microenvironments, such as organelles or vesicles Citation[49]. The adaptation to our experimental system was carried out using two well-established raft- and soluble- markers (CD59 and CD71, respectively) and different concentrations of CTB-HRP, DAB and H2O2 to induce chemical cross-linking. As observed in , the different concentrations of reagents generate a differential drag of the components analysed, being some conditions so aggressive that eliminate even the transferrin receptor (CD71). On the contrary, other conditions were observed to be totally ineffective in eliminating CD59. Hence, the amounts of reagents considered to be optimal were 0.05 µg/ml CTB-HRP, 0.01% H2O2 and 0.5 mg/ml DAB (, arrow).
Figure 2. Improvement of a CTB-HRP-based method for the specific depletion of raft proteins. Lymphoblasts were separated in different samples, incubated with various amounts of cholera toxin (CTB; 0.01, 0.05, 0.1 or 1 µg/ml) for 30 min at 37°C and then washed several times with HBSS+. Next, cells were placed in 1 ml HBSS+ and the 3,3′-diaminobenzidine (DAB) crosslinker, either at 0.5 or 0.1 mg/ml, added in the presence of different concentrations of H2O2 (0.01, 0.05 or 0.1%) for 45 min at 4°C. After this incubation step, lymphoblasts were washed twice with cold HBSS+ and cell lysis performed with TKM/0.5% TX-100 for 30 min on ice. A postnuclear supernatant was obtained after centrifugation at 13,000 rpm for 15 min (4°C) to eliminate, amongst others, nuclei and rafts proteins. Soluble proteins (non raft proteins) were dotted on a nitrocellulose membrane (HybondECL, Amersham-Biosciences Europe, GmbH) and the presence of CD59 (raft marker) and CD71 (non raft marker) analysed as in by Immunoblotting combined with ECLPlus (Amersham-Biosciences Europe, GmbH). Initial expression of both CD59 and CD71 was evaluated in non-treated cells.
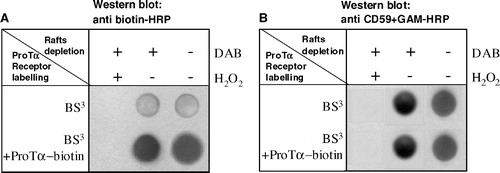
The next step was to evaluate the capacity of the chemical cross-linking of proteins residing at the same microenvironment as GM1 to eliminate the ProTα receptor. clearly shows how after the chemical cross-linking and elimination of raft proteins induced by CTB-HRP, H2O2 and DAB both ProTα receptor () and CD59 () were no longer detectable in the sample. Besides, it is also observed how the signal of the samples treated with DAB in the absence of H2O2 is the same as the one of cells incubated exclusively with CTB-HRP. Taking into account all these results we can state that the ProTα receptor is associated to raft microdomains in vivo and not as a consequence of experimental manipulations or artefacts.
Figure 3. Raft protein crosslinking and depletion from lymphoblasts leads also to a ProTα receptor removal. Aliquots of 2×106 PHA-activated cells were cross-linked with 20 µl mM 1 BS3, in the absence or presence of 15 µM biotin-ProTα. To eliminate raft proteins all samples were treated with CTB-HRP (0.05 µg/ml) and incubated (+) or not (−) with DAB (0.5 µg/ml) in the absence (−) or presence (+) of H2O2 (0.01%) as indicated. After lysis and centrifugation, samples were dotted on a nitrocellulose membrane and the presence of ProTα receptor (a) and CD59 (b) detected with anti biotin-HRP and anti CD59 + GAM-HRP, respectively.
ProTα receptor location corresponds to membrane areas highly resistant to MβCD and TX-100
The effect of cholesterol removal on rafts integrity was assessed by treating biotin-ProTα cross-linked lymphoblasts with either TX-100, a non ionic detergent which eliminates non-raft proteins, or MβCD (methyl β-cyclodextrin), which efficiently extracted cholesterol from cells. shows the results from a typical experiment obtained after biotin-ProTα cross-linking, detergent treatment and immunofluorescent detection with streptavidin-PE. It can be observed that none of the conditions eliminate the ProTα receptor labelling. To test the efficiency of the treatment a well-known TX-100 sensitive and MβCD-resistent cell surface marker, CD71, was also used (), which allowed us to be sure about the experimental conditions. Once again we can conclude that our results are consistent with an in vivo ProTα receptor location within rafts.
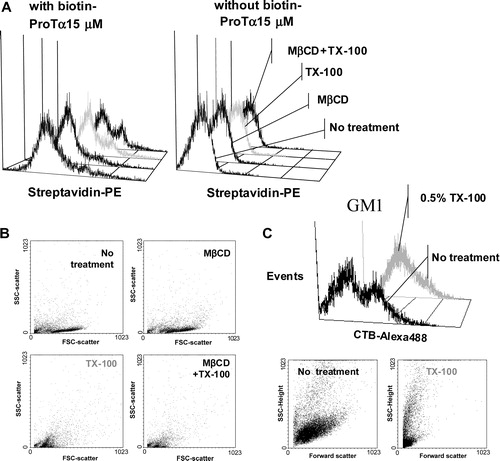
Figure 4. Flow cytometry analysis of the ProTα receptor association with raft regions on human lymphoblasts. Cells (2×106) were cross-linked with BS3 in the absence (to check for background fluorescence; see also , right) or the presence of 15 µM of biotin-ProTα, and then treated with 1% TX-100, 10 mM MβCD or both as indicated in Material and Methods. After paraformaldehyde fixation, ProTα receptor and CD71 expression was revealed by staining with streptavidin-PE and anti CD71-FITC, respectively. In order to know the percentage of CD71+ cells and to place the histogram marker as shown, a FITC-labelled IgG2aκ isotype antibody was used as a negative control. Data acquisition was done on a Becton Dickinson FACScalibur flow cytometer, while WinMDI software was used to analyse the data. This experiment is representative of several with similar results.
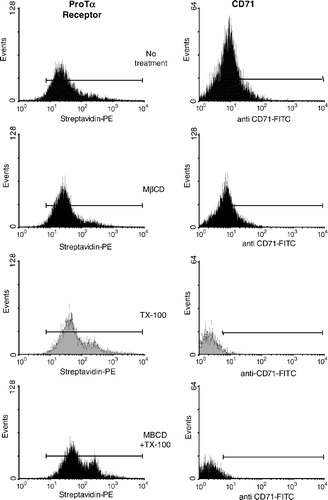
If we observe the histogram profiles () where TX-100 has been used to extract part of the lipid fraction of the membrane it easily follows that ProTα receptor signal is not reduced but increased. The effect of these treatments on cell size and microstructure of the plasma membrane was also analysed. Thus, cells treated with TX-100 showed a decrease in size due to the loss of a great part of their lipid content in plasma membrane (). But, at the same time, its structure likely presents a relaxation that could allow somehow the entrance of streptavidin-PE inside cells. Further controls, subjected to the same cross-linking treatment but in the absence of biotin-ProTα, allowed us to rule out a non-specific binding of streptavidin-PE to intracellular components as responsible for that enhanced receptor fluorescence (). Therefore, the most plausible explanation was that streptavidin-PE is detecting an internalized ProTα-receptor complex. In fact, the same kind of observation can be made with the GM1 ganglioside, a raft marker with important intracellular stores, detected by CTB-Alexa 488 immunofluorescent staining, and whose expression is enhanced upon TX-100 treatment (). Thus, these results gave more support to our hypothesis of internalization for the ProTα receptor Citation[36–38], a phenomenon normally associated with signalling through growth factors receptors Citation[50].
Figure 5. High resistance to non ionic detergent extraction is consistent with an in vivo location of ProTα receptor within rafts. Cells from samples described in were analysed by flow cytometry for streptavidin-PE (ProTa receptor) immunofluorescence (a) and for forward and right-angle light scattering (b). In (a), negative control cells (right) were not incubated with biotin-ProTα in order to show the non specific binding of streptavidin-PE. Dot plot (forward versus right-angle scattering) and histograms (CTB-Alexa 488 versus events) shown in figure (c) represent human PHA-lymphoblasts from a different donor treated with or without 1% TX-100 and stained with CTB-Alexa 488 to reveal GM1 ganglioside expression.
ProTα receptor is colocalized with aggregated rafts microdomains generated by surface cross-linking of CD59
To obtain confocal images showing the above mentioned ProTα receptor inclusion in raft regions from biotin-ProTα cross-linked lymphoblasts, we compared the ProTα receptor surface location with CD59 or CD71 distribution. In order to carry out these experiments either CD59 or CD71 antigens from biotin-ProTα cross-linked lymphoblasts were labelled with anti CD59-FITC or anti CD71-FITC and patching induced with a FITC-labelled anti IgG. To allow free lateral movements the last incubation was performed at room temperature.
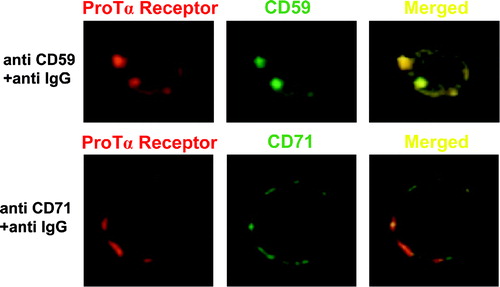
As observed in , both CD71 and CD59 markers present a distribution with a dotted appearance, which indicates that the antibody cross-linking was effective in generating aggregates. To determine the correspondence between the physical locations of these molecules on biotin-ProTα cross-linked lymphoblasts, double-immunofluorescence analyses were carried out with either anti CD59-FITC/Streptavidin-PE or anti CD71/Streptavidin-PE combinations. In the double labelled anti CD71-FITC/Streptavidin-PE samples the signal detected by confocal microscopy corresponded with separate areas of the membrane (). However, anti CD59-FITC/Streptavidin-PE labelled PHA-stimulated lymphocytes showed that both CD59 and ProTα receptor molecules were colocalized in rafts (). Furthermore, since these images have been taken using a 63×objective, with a resolution limit for the z-axis of 0.23 µm, it is likely that both proteins, ProTα receptor and CD59, are confined in an area with a diameter ≤230 nm.
Figure 6. ProTα receptor and CD59 show a similar distribution pattern on plasma membrane from human lymphoblasts. Aliquots of 2×106 cells were cross-linked with 1 mM BS3 in the presence (figure) or absence (not shown) of 15 µM biotin-ProTα. Lymphoblasts were incubated with streptavidin-PE to reveal ProTα receptor expression (red) and also, in order to induce either raft or non-raft proteins clustering, stained with anti CD59 or anti CD71 mAbs respectively at 4°C and incubated at RT (to allow free lateral movements of proteins) with a FITC-labelled anti-IgG Ab to cross-link any antibody on the cell surface. Both CD59 and CD71 expression are shown in green, while colocalization level (merged) of any of these markers with ProTα receptor is presented in yellow.
Increased tyrosine phosphorylation in response to ProTα takes place in raft microdomains from T lymphoblasts
Next we investigated the possible transduction mechanisms promoted by the interaction between ProTα and its receptor on PHA-lymphoblasts. As a first approach, whole lysates from cells treated with 5 µg/ml ProTα for various times were analysed, showing that ProTα strongly induced a transitory tyrosine phosphorylation of multiple proteins after 30 s incubation, with a maximum at 15 min ().
Figure 7. ProTα induces a tyrosine phosphorylation event confined to rafts in lymphoblasts. (a) Cells were incubated or not with 5 µg/ml of ProTα for different times and their lysates run on a 7.5% SDS-PAGE electrophoresis gel, transferred to a PVDF membrane and the presence of phosphotyrosines revealed with an anti-phosphotyrosine mAb (clone PY20, BD-Biosciences) in combination with GAM-HRP and ECLPlus (Amersham Biosciences Europe, GmbH). (b) Proteins from cells incubated or not with 5 µg/ml of ProTα for 1 min at 37°C were extracted as indicated in . Raft fractions (3–6) and soluble fractions (10–11) were pooled and protein concentration determined (BCA, Pierce Biotechnology, Inc). Identical amounts of protein (2 µg; c) and volume (10 µl; d) from both pooled insoluble and soluble fractions were run on a 7.5% SDS-PAGE gel and detection of phosphotyrosines performed as before. In a and c protein loading controls were established after membrane stripping and incubation with an anti β-actin mAb.
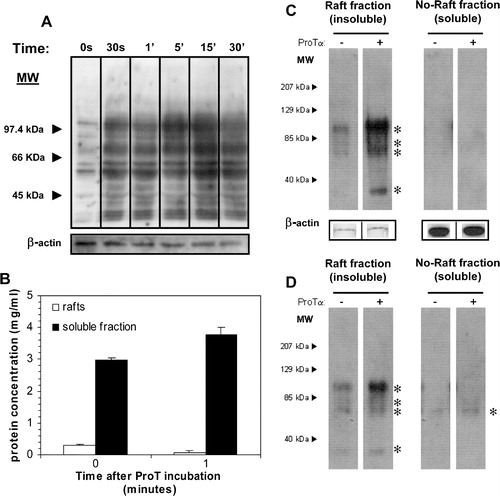
An additional support for the ProTα receptor presence in rafts microdomains was obtained when we evaluated the distribution of these tyrosine phosphorylated proteins among different membrane fractions. Cells were incubated or not for 1 min with 5 µg/ml ProTα and fractions from discontinuous sucrose density gradients, corresponding to either TX-100 insoluble (fractions 3–6; lipid rafts) or soluble membranes (fractions 10–11), pooled. Protein quantification of all the samples was carried out because of the substantial differences between raft and non-raft fractions and the very fast protein distribution we have observed upon one minute incubation with ProTα (), which could alter our perception about the magnitude of the tyrosine phosphorylation taking place. Based on that, we decided to carry out several experiments loading SDS-PAGE gels with either the same amount of membrane protein () or identical fractions volumes (). In both cases, it was apparent an induced tyrosine phosphorylation in several rafts resident proteins from ProTα-treated versus untreated cells, being even more evident when equal amounts of proteins were resolved by SDS-PAGE (). On the contrary, tyrosine phosphorylated species were hardly detected in TX-100-soluble membrane fractions, and only when the same sample volume was loaded (). We should mention that all these phosphorylated proteins (asterisks in Western blot of ) were consistently present in raft fractions from ProTα-treated cells and were similar in size to some of the phosphorylated proteins detected in the whole cell lysates (). Therefore, our results definitively involve a tyrosine phosphorylation cascade taking place in a raft microdomain environment as a response to the interaction of ProTα with its receptor, which agree with our previous results showing a proliferative effect of ProTα Citation[29].
Discussion
Due to all the in vitro and in vivo immunoregulatory properties previously shown by ProTα is of fundamental importance to elucidate the molecular mechanisms underlying these functions and, therefore, to understand the nature of the ProTα interaction with the cell surface. Recently, we demonstrated by affinity cross-linking and affinity chromatography the existence of binding sites for ProTα in human lymphoblasts, detecting also by fluorescence microscopy a heterogeneous distribution of this ProTα receptor in a cap-like structure at one of the cell poles Citation[39]. To test whether this particular receptor polarization had also some type of relationship with a specific location in differentiated plasma membrane compartments we started purifying, by discontinuous sucrose density gradients, both raft (TX-100 insoluble) and non-raft (TX-100 soluble) membrane fractions. In agreement with other authors Citation[47] rafts proteins (7%) were detected in detergent insoluble fractions (3–6), while most of the protein (85%) appeared in the soluble area (10–11). ProTα receptor was found in strong association with raft microdomains, as well as several well-known raft proteins (alkaline phosphatase, CD59). Thus, both ProTα receptor and alkaline phosphatase (AP) showed a normal distribution between fractions 3–6 Citation[46], while CD59, a GPI-anchored protein whose presence in rafts is prominent, revealed also a percentage of CD59 (31%) still present in non-raft fractions. This last finding could result from its weak association with these microdomains, the extraction procedure or the maturity degree of the protein (presence or not of the GPI-anchorage) Citation[40]. On the contrary CD71, a transmembrane protein accessible for nonionic detergents, proved to be a good marker for non-raft membrane areas and showed a different membrane distribution when compared with ProTα receptor.
However, in spite of nonionic detergents insolubility and discontinuous density gradient ultracentrifugation have been shown to be a good starting point to detect raft proteins and several studies prove its validity Citation[51–53], it has been also published that this technique presents some limitations Citation[48]. Thus, it is currently under debate if data obtained with this purification method really reflects the actual in vivo situation or it is merely an artefact. As well, there are some reasonable doubts whether this technique is adequate for detecting raft proteins whose association to rafts is weak Citation[40,43]. To sort all these problems out, we decided to use a different experimental approach. Cholera toxin B subunit (CTB) colocalization is equivalent to the association of the protein under study with rafts in intact cells Citation[43]. Therefore, we wondered if, under very controlled conditions, the capacity of the peroxidase (HRP) linked to CTB to catalyze a massive protein cross-linking in the presence of DAB and H2O2 would delete ProTα receptor from cells. Once again, our results probed, in agreement with our data obtained from sucrose gradients, that both CD59 and ProTα receptor were located at the same raft microenvironment.
Cyclodextrins are non invasive tools of extracting cholesterol in cell membranes. Because cholesterol is essential for raft integrity Citation[52] many proteins associated with these microdomains are extracted with MβCD or acquire sensitivity to solubilization with nonionic detergents after treatment with MβCD Citation[40]. Thus, by using nonionic detergents and cyclodextrins prior to flow cytometry analysis, we wanted to test the exact position of ProTα receptor within the plasma membrane. The ProTα receptor labelling persistence upon TX-100 and/or MβCD treatment was consistent with its presence in rafts microdomains of human lymphoblasts.
Studies carried out by chemical cross-linking of markers Citation[54] and FRET Citation[55,56] have demonstrated that raft domains are dynamic structures whose mean diameter is around 70 nm in living cells. For such a reason they are outside the limits of resolution of conventional optical microscopy techniques. However, lipids and proteins exposed extracellularly can be laterally cross-linked by means of antibodies or multivalent bacterial toxins (e.g., CTB), which causes its aggregation on the cell surface Citation[57]. Therefore, cross-linking of known raft components has allowed us to sort out the above mentioned problem and determine the suspected association of some proteins with rafts Citation[43,58]. By using this approach ProTα receptor was also found to be close to CD59 in cholesterol/esfingolipids enriched membrane microdomains. However, it has been also described that many receptors migrate to a raft region only after binding to its specific ligand, and vice versa. Due to the fact that in our experiments the detection of the receptor by means of either confocal microscopy or flow cytometry can only be achieved after binding ProTα, its location should correspond to rafts when the receptor has been engaged. Therefore, purification and identification of all the acceptor molecules for ProTα with different techniques and strategies will allow us to clarify their location before or after the binding of a specific ligand. In this sense, affinity chromatography experiments carried out to characterize and purify the ProTα receptor led to the detection in rafts preparations of several protein species of about 35–30, 29–27 and 22–19 kDa (the present data and manuscript in preparation). However, since it is known that not all the rafts proteins come from the lymphocyte plasma membrane [48 and our data] and ProTα seems to have both intracellular and extracellular functions Citation[21], it is likely that not all these binding proteins are linked to the actual surface receptor. Studies are currently being carried out to gain insights on this regard.
As mentioned above, confocal microscopy provided us with data supporting the fact that both ProTα receptor and CD59 were close to each other when the former is aggregated by its ligand and probably located in an area (clustered rafts) with a diameter no bigger than 230 nm (the maximum distance to see colocalization between two proteins). Therefore, we have a receptor not only situated in cells which the immunoregulatory properties of ProTα have been described for, but also a receptor placed in a plasma membrane area (rafts) where many receptors known to have similar biological activities reside and where the signalling processes for differentiation and proliferation seem to be more active. In agreement with that we provided experimental evidence supporting the fact that a phosphotyrosine pathway is initiated upon ProTα receptor recognition and that such a signalling cascade is confined to rafts. It has been previously reported by other authors as well as ourselves that ProTα may act as a co-stimulator in the proliferation process Citation[28–30]. Our data are relevant because they show a ProTα receptor and a ligand induced tyrosine phosphorylation located in rafts from human lymphoblasts. This allows us to feel more confident about the idea that this receptor possesses the necessary requirements to form part of the mechanism of action of ProTα as a biological response modifier in the Immune System. Therefore, an aspect that should be studied, as soon as the sequence become available, is the possible secondary modification responsible for its association to raft regions. The search for that modification on the receptor and the identification of all the participants in the signalling cascade triggered by ProTα will lead us to a better understanding at a molecular level of this peculiar protein.
This paper was first published online on prEview on 21 April 2005.
We are grateful to Thymoorgan Gmbh Pharmazie and Co KG (Vienenburg, Germany) for kindly supply us with ProTα. We also wish to thank Dr Begoña Bujía and Dr Pilar Arias for their invaluable help, the Centro de Transfusiones de Galicia for the buffy coats provided and Dr. J. Trotter (Scripps Institute, La Jolla, CA) for the WindMDI software. This work was supported by grant PGDIT99BIO201 (Xunta de Galicia). Alicia Piñeiro, Juan Lojo, and Ana Canda-Sánchez are all recipients of a Xunta de Galicia predoctoral fellowship.
References
- Brown DA, London E. Structure of detergent-resistant membrane microdomains: does phase separation occur in biological membranes?. Biochem Biophys Res Commun 1997; 240: 1–7
- Anderson RG. The caveolae membrane system. Annu Rev Biochem 1998; 67: 199–225
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572
- Eramo A, Sargiacomo M, Ricci-Vitiani L, Todaro M, Stassi G, Messina CGM, Parolini I, Loti F, Sette G, Peschle C, De Maria R. CD95 death-inducing signaling complex formation and internalization occur in lipid rafts of type I and type II cells. Eur J Immunol 2004; 34: 1930–1940
- Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: Secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity 2004; 21: 461–465
- Panchal RG, Ruthel G, Kenny TA, Kallstrom GH, Lane D, Li L, Bavari S, Aman MJ. In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc Natl Acad Sci USA 2003; 100: 15936–15941
- Harder T, Simmons K. Caveolae, DIGs and the dinamic of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol 1997; 9: 534–542
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 2000; 275: 17221–17224
- Pralle A, Keller P, Florin EL, Simmons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol 2000; 148: 997–1008
- Laude AJ, Prior IA. Plasma membrane microdomains: Organization, function and trafficking. Mol Memb Biol 2004; 21: 193–205
- Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol 2000; 12: 23–24
- Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP, Lasserre R, He HT. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J 2002; 15: 1899–1908
- Viola A. The amplification of TCR signaling by dynamic membrane microdomains. Trends Immunol 2001; 22: 322–327
- Gupta N, DeFranco AL. Visualizing lipid rafts dynamics and early signalling events during antigen receptor-mediated B-lymphocyte activation. Mol Biol Cell 2003; 14: 432444
- Cheng PC, Brown BK, Song W, Pierce SK. Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling. J Immunol 2001; 166: 3693–3701
- Marmor MD, Julius M. Role for lipid rafts in regulating IL-2R signalling. Blood 2001; 98: 1489–497
- Roepstorff K, Thomsem P, Sandvig K, van Deurs B. Secuestration of EGF receptors in non-caveolar lipid rafts inhibits ligand binding. J Biol Chem 2000; 277: 18954–18960
- Liu P, Ying Y, Anderson RGW. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA 1997; 94: 13666–13670
- Davy A, Feuerstein C, Robbins SM. Signalling within a caveolae-like microdomain in human neuroblastoma cells in response to fibroblast growth factor. J Neurochem 2000; 74: 676–683
- Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Holmgren PK, Magnusson KE, Stralfors P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J 1999; 13: 1961–1971
- Piñeiro A, Cordero OJ, Nogueira M. Fifteen years of prothymosin alpha: contradictory past and new horizons. Peptides 2000; 9: 1433–1446
- Trumbore MW, Berger SL. Prothymosin a is a nonspecific facilitator of nuclear processes: Studies of Run-on transcription. Protein Expr Purif 2000; 20: 414–420
- Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science 2003; 299: 223–226
- Papanastasiou M, Baxevanis CN, Papamichail M. Promotion of murine antitumor activity by prothymosin alpha treatment: I Introduction of tumoricidal peritoneal cells producing high levels of tumour necrosis factor. Cancer Immunol Immunother 1992; 35: 145–150
- Baxevanis CN, Gritzapis AD, Spanakos G, Tsitsilonis OE, Papamichail M. Induction of tumor-specific T lymphocyte responses in vivo by prothymosin alpha. Cancer Immunol Immunother 1995; 40: 410–418
- Baxevanis CN, Frilingos S, Reclos GJ, Arsenis P, Katsiyiannis A, Anastasopoulos E, Seferiadis K, Tsolas D, Papamichail M. Enhancement of human T lymphocyte function by prothymosin alpha: increased production of interleukin-2 and expression of interleukin-2 receptors on normal human peripheral blood T lymphocytes. Immunopharmacol Immunotoxicol 1990; 12: 595–617
- Baxevanis CN, Reclos GJ, Papamichail M, Tsokos GC. Prothymosin alpha restores the depressed autologous and allogeneic mixed lymphocyte responses in patients with systemic lupus erythematosus. Immunopharmacol Immunotoxicol 1987; 9: 429–440
- Baxevanis CN, Spanakos G, Voutsas IF, Gritzapis AD, Tsitsilonis OE, Mamalaki A, Papamichail M. Increased generation of autologous tumor-reactive lymphocytes by anti-CD3 monoclonal antibody and prothymosin alpha. Cancer Immunol Immunother 1999; 48: 71–84
- Cordero OJ, Sarandeses CS, Lopez JL, Cancio E, Regueiro BJ, Nogueira M. Prothymosin alpha enhances interleukin-2 receptor expression in normal human T-lymphocytes. Int J Immunopharmacol 1991; 13: 1059–1065
- Cordero OJ, Sarandeses CS, Lopez JL, Nogueira M. Prothymosin alpha enhances human natural killer cell cytotoxicity: Role in mediating signals for NK activity. Lymphokine Cytokine Res 1992; 11: 277–285
- Cordero OJ, Sarandeses CS, Lopez-Rodriguez JL, Nogueira M. The presence and cytotoxicity of CD16+ CD2- subset from PBL and NK cells in long-term IL-2 cultures enhanced by Prothymosin alpha. Immunopharmacology 1995; 29: 215–223
- Lopez-Rodriguez JL, Cordero OJ, Sarandeses CS, Viñuela J, Nogueira M. Interleukin-2 killer cells: In vitro evaluation of combination with prothymosin alpha. Lymphokine Cytokine Res 1994; 13: 175–182
- Lopez JL, Czarnecki J, Cordero OJ, Nogueira M. Enhancement of cytoskeletal polarisation of NK cells upon conjugation with target cells by prothymosin alpha. Int J Thymol 1995; 3: 296–303
- Eckert K, Grunberg E, Garbin F, Maurer HR. Preclinical studies with prothymosin alpha 1 on mononuclear cells from tumour patients. Int J Immunopharmacol 1997; 19: 493–500
- Eckert K, Grunberg E, Immenschuh P, Garbin F, Kreuser ED, Maurer HR. Interleukin-2 activated killer cell activity in colorectal tumor patients: Evaluation of in vitro effects by prothymosin alpha 1. J Cancer Res Clin Oncol 1997; 123: 420–428
- Cordero OJ, Sarandeses CS, Nogueira M. Prothymosin alpha receptors on peripheral blood mononuclear cells. FEBS Letters 1994; 341: 23–27
- Cordero OJ, Sarandeses CS, Nogueira M. Prothymosin a receptors on lymphocytes. J Interferon Cytokine Res 1995; 15: 731–737
- Cordero OJ, Sarandeses CS, Nogueira M. Binding of 125I-prothymosin alpha to lymphoblasts through the non-thymosin alpha-1 sequence. Life Science 1996; 58: 1757–1770
- Piñeiro A, Bugía B, Arias MP, Cordero OJ, Nogueira M. Identification of receptors for Prothymosin a on human lymphocytes. Biol Chem 2001; 382: 1473–1482
- Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by ciclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J 1998; 335: 433–440
- Ilangumaran S, Briol A, Hoessli DC. Distinct interactions among GPI-anchored, transmembrane and membrane associated intracellular proteins, and sphingolipids in lymphocyte and endothelial cell plasma membranes. Biochim Biophys Acta 1997; 1328: 27–36
- Ilangumaran S, Arni S, van Echten-Deckert G, Borish B, Hoessli DC. Microdomain-dependent regulation of lck and Fyn protein-tyrosine kinases in T-lymphocyte plasma membranes. Mol Biol Cell 1999; 10: 891–905
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signalling via the T cell antigen receptor. J Cell Biol 1999; 147: 447–461
- Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Medicine 1999; 190: 1549–1560
- Salgado FJ, Lojo J, Alonso-Lebrero JL, Lluis C, Franco R, Cordero OJ, Nogueira M. A role for interleukin-12 in the regulation of T cell plasma membrane compartmentation. J Biol Chem 2003; 278: 24849–24857
- Hanada KM, Nishijima Y, Akamasatu Y, Pagano RE. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatilinositol-anchored protein, in mammalian membranes. J Biol Chem 1995; 270: 6254–6260
- Ostermeyer AG, Beckrich BT, Ivarson KA, Grove KE, Brown DA. Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells MβCD does not affect cell surface transport of a GPI-anchored protein. J Biol Chem 1999; 274: 34459–34466
- Magee AI, Parmryd I. Detergent-resistant membranes and the protein composition of lipid rafts. Genome Biol 2003; 4: 234–237
- Cuortoy PJ, Quintart J, Baudhuin P. Shift of equilibrium density induced by 3,3′-diaminobenzidine cytochemistry: a new procedure for the analysis and purification of peroxidase-containing organelles. J Cell Biol 1984; 98: 870–878
- Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: A current overview. Cell 1993; 73: 5–8
- Cerneus DP, Ueffing E, Posthuma G, Strous GJ, van der Ende A. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis Role of cholesterol. J Biol Chem 1993; 268: 3150–3155
- Schroeder R, London E, Brown D. Interactions between satured acyl chains confer detergent resistance to lipids and glycophosphatidylinositol-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behaviour. Proc Natl Acad Sci USA 1994; 91: 12310–12334
- Ahmed SN, Brown DA, London E. On the origin of sphingolipid cholesterol-rich detergent-insoluble cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent insoluble, liquid ordered phase in model membranes. Biochemistry 1997; 36: 10944–10953
- Friedrichson T, Kurzchalia T. Microdomains of GPI-anchored proteins in living cells revealed by cross-linking. Nature 1998; 394: 802–805
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 1998; 394: 798–801
- Hooper NM. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol Memb Biol 1999; 16: 145–156
- Spiegel S, Kassis M, Wilchek M, Fishman PH. Direct visualization of redistribution and capping of fluorescent gangliosides on lymphocytes. J Cell Biol 1984; 99: 1575–1581
- Harder T, Scheifelle P, Verkade P, Simmons K. Lipid domain structure revealed by patching of membrane components. J Cell Biol 1998; 141: 929–942
