Abstract
Polarized epithelial cells of multicellular organisms confront the environment with a highly specialized apical cell membrane that differs in composition and function from that facing the internal milieu. In the case of absorptive cells, such as the small intestinal enterocyte and the kidney proximal tubule cell, the apical cell membrane is formed as a brush border, composed of regular, dense arrays of microvilli. Hydrolytic ectoenzymes make up the bulk of the microvillar membrane proteins, endowing the brush border with a huge digestive capacity. Several of the major enzymes are localized in lipid rafts, which, for the enterocyte in particular, are organized in a unique fashion. Glycolipids, rather than cholesterol, together with the divalent lectin galectin-4, define these rafts, which are stable and probably quite large. The architecture of these rafts supports a digestive/absorptive strategy for nutrient assimilation, but also serves as a portal for a large number of pathogens. Caveolae are well-known vehicles for internalization of lipid rafts, but in the enterocyte brush border, binding of cholera toxin is followed by uptake via a clathrin-dependent mechanism. Recently, ‘anti-glycosyl’ antibodies were shown to be deposited in the enterocyte brush border. When the antibodies were removed from the membrane, other carbohydrate-binding proteins, including cholera toxin, increased their binding to the brush border. Thus, anti-glycosyl antibodies may serve as guardians of glycolipid-based rafts, protecting them from lumenal pathogens and in this way be part of an ongoing ‘cross-talk’ between indigenous bacteria and the host.
Introduction
A hallmark of epithelial cells in multicellular organisms is their simultaneous and direct contact with two very different types of environments; on the one side they face the interior of the organism and on the other, they are exposed to the external milieu or the lumen of an internal organ. Because of this duality epithelial cells are architecturally and functionally polarized. The inward-facing (basolateral) surface needs to be equipped with a full complement of receptors enabling the epithelial cell to decode and execute all instructions emanating from the control centers of the organism regarding growth, differentiation, migration, and apoptosis. Consequently, the main priority for the basolateral surface is to be dynamic and adaptable to rapid changes related to the generation/termination of signaling cascades and membrane remodeling. In contrast, the main functional priority for the apical cell surface facing the exterior is to act as a selective filter: Firstly, it must constitute a permeability barrier protecting the organism from hazardous agents, including pathogens, and secondly, it must be capable of extracting nutrients from the environment. Both these demands call for a cell surface that is stable rather than dynamic and in addition is sufficiently robust to withstand external challenges of various kinds, such as an acid pH, degradative enzymes (proteases, lipases, glycosidases), detergents (bile acids), and invading pathogens. Work over the last few years has revealed a unique lipid raft organization related to the function of the apical membrane in epithelial cells and this new insight will be the subject of the present review.
The apical membrane architecture: A microvillar brush border
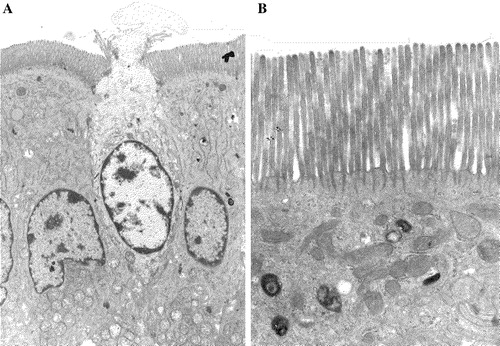
Although microvillar protrusions can be found on many cell types they are particularly plentiful at the apical surface of epithelial cells. Architecturally, they are defined by a longitudinal actin-based cytoskeleton in the core of the microvillus together with short, actin-binding cross filaments connected transversely with the cytoplasmic leaflet of the microvillar membrane and the core actin filaments (Louvard et al. [Citation1992], Mooseker et al. [Citation1983]). These radiate vertically as microvillar rootlets into the so-called terminal web region, a myosin-rich filamentous structure that may extend up to 1 µm into the cell. A high density of microvilli (up to 3000/cell) constitutes a brush border, which is typically seen at the apical surface of epithelial cells specifically designed for high-throughput absorptive functions, such as the kidney proximal tubule cell, the placental syncytiotrophoblast and the small intestinal enterocyte (). Another distinct anatomical feature of brush borders is deep apical tubules, situated between adjacent microvilli. These structures, which can be visualized by electron dense membrane impermeable markers, such as Ruthenium Red, are often seen in close contact with the microvillar actin rootlets, have a diameter of 50-100 nm and extend up to 1 µm into the cytoplasm (Hansen et al. [Citation2003], Maunsbach, [Citation1973], Maunsbach [Citation1976]). Functionally, the deep apical tubules are likely areas of the apical cell surface specialized in endo/exocytotic membrane trafficking, because they bridge the terminal web area which otherwise sterically excludes larger membraneous structures such as mitochondria, lysosomes and endosomes ().
Figure 1. Epithelial cells with an apical brush border. (A) Tall, columnar small intestinal enterocytes with a brush border facing the lumen of the gut. The apoptotoic cell in the middle is in the process of being extruded from the epithelium. (B) A closer view of the apical region of an enterocyte showing a dense array of microvilli with rootlets of actin filaments extending into the underlying cytoplasm. Notice that organelles such as mitochondria, lysosomes and endosomes are excluded from this uppermost terminal web region of the cytoplasm.
Although still controversial (see below), lipid rafts are now generally believed to exist in most if not all membranes of eukaryotic cells, and the raft- or ‘membrane cluster’ hypothesis originally emerged from studies on the asymmetric transport and distribution of membrane lipids in MDCK cells (Simons & van Meer [Citation1988], van Meer et al. [Citation1987]). The liquid-ordered (lo phase) state characteristic of raft microdomains is generally believed to be caused by the clustering of the raft-forming membrane lipids cholesterol and sphingolipids (Brown & London [Citation1998], Simons & Ikonen [Citation1997]), but it is worth noting that the relative amounts of these constituents may vary considerably, particularly in apical membranes of epithelial cells. Thus, a typical plasma membrane of a mammalian cell contains about 20% cholesterol, 15–20% sphingomyelin and about 5% glycolipids, and in line with this composition, cholesterol and sphingomyelin together comprise about 65% of the total lipid of detergent resistant membranes from kidney proximal tubule cells (Parkin et al. [Citation2001]). By comparison, glycolipids are by far the predominant raft-promoting lipids of the small intestinal brush border, making up > 30% of the total lipid, whereas cholesterol and sphingomyelin are only present in modest amounts (about 10- and 5%, respectively) (Christiansen & Carlsen [Citation1981], Hauser et al. [Citation1980], Kawai et al. [Citation1974]). In view of this unusual lipid composition, it is not surprising that lipid rafts from small intestinal brush borders have proven to be largely resistant to cholesterol depletion (Hansen et al. [Citation2001]), and that caveolin-1, a cholesterol-binding marker for the caveolar-type of rafts (Okamoto et al. [Citation1998], Schlegel et al. [Citation2000]), is largely detergent-soluble in the small intestinal brush border (Hansen et al. [Citation2001]). This significant difference in lipid microdomain environment may also help explain why a number of transmembrane peptidases (aminopeptidases N- and A and dipeptidyl peptidase IV) which are predominantly detergent-soluble in the kidney (Hooper & Bashir [Citation1991], Hooper & Turner [Citation1988]) are largely resistant to detergent in the intestine (Alfalah et al. [Citation1999], Alfalah et al. [Citation2002], Danielsen [Citation1995], Mirre et al. [Citation1996]). Nevertheless, one should be careful not to view the small intestinal brush border as simply one gigantic raft: some of the major digestive enzymes, such as lactase and maltase-glucoamylase, are prominent examples of proteins not associated with lipid rafts.
Detergent resistant membranes (DRM's) and lipid rafts: A true picture?
Despite the surge in raft papers of recent years and the inclusion of the raft concept in all major textbooks of cell biology, most seasoned membrane biologists are well aware of the pitfalls within the field of raftology in which the most serious is the shortage of evidence for the bona fide existence of lipid rafts in the membranes of living cells (Munro [Citation2003]). So far, most of the experimental evidence concerning lipid rafts is indirect. Thus, the most widely used assay for raft existence is based on the observation that when cell membranes are extracted with the nonionic detergent Triton X-100 at 4°C, only a subset of the components is solubilized. When the membrane extract is subsequently layered at the bottom of a density gradient and subjected to centrifugation, detergent-resistant raft membranes (DRMs) will float and thus separate from the ‘non-raft’ components (Brown & Rose [Citation1992]). The major concern with this type of experiment is the possibility of nonphysiological rearrangements arising from the temperature-dependence of lipid phase behaviour. In other words, lipid rafts might simply be low-temperature artifacts. Another concern is the compositional asymmetry of the inner and outer leaflets, which through detergent-induced formation of holes in the membrane might become mixed during the extraction procedure. Finally, differential sensitivity of the two membrane bilayers to Triton X-100 might result in transient unstable structures such as monolayers (Munro, [Citation2003]).
Relevant as they are, at least some of these caveats have been addressed. Thus, the near monopoly status of Triton X-100 as detergent for raft analysis has been broken by a number of studies using a variety of nonionic detergents. One detergent in particular, Brij 98, has attracted interest by its ability to isolate rafts from a number of different cell types at physiological temperature (Braccia et al. [Citation2003], Danielsen & Hansen [Citation2003], Drevot et al. [Citation2002], Holm et al. [Citation2003], Munoz et al. [Citation2003], Schuck et al. [Citation2003]). From these works, it is fair to conclude that lipid rafts per se are not artifacts created in vitro simply by temperature-induced phase transition of the membrane lipids. Of equal importance, it can also be concluded that although the lipid-lipid and lipid-protein interactions defining raft microdomains are generally weak, they are sufficiently strong to form at the surface of living cells at 37°C.
As shown in , a close examination by electron microscopy of the ultrastructure of lipid rafts from small intestinal brush borders does not lend support to the notion that components from the two bilayers mix during detergent extraction. Thus, immunogold labeling for the ectoenzyme aminopeptidase N is generally observed only along one of the two membrane leaflets. In addition, high magnification images of rafts reveal an intact bilayer structure.
Figure 2. Bilayer membrane structure of lipid rafts. Lipid rafts prepared from intestinal microvillar membranes, using Brij 98 (A, B) or Triton X-100 (C) (Braccia et al. [Citation2003]). (A) Immunogold labeling for aminopeptidase N. Note that the labeling is confined mainly to one side of the membrane. (B, C) High magnification electron micrographs of raft membranes. The two leaflets of the bilayer are visible regardless of choice of detergent. Bars: 200 nm.
![Figure 2. Bilayer membrane structure of lipid rafts. Lipid rafts prepared from intestinal microvillar membranes, using Brij 98 (A, B) or Triton X-100 (C) (Braccia et al. [Citation2003]). (A) Immunogold labeling for aminopeptidase N. Note that the labeling is confined mainly to one side of the membrane. (B, C) High magnification electron micrographs of raft membranes. The two leaflets of the bilayer are visible regardless of choice of detergent. Bars: 200 nm.](/cms/asset/cf10f7c5-d9e3-4f03-9fd5-4ac89bb21a24/imbc_a_144543_f0002_b.jpg)
No doubt questions concerning lipid raft size, stability and functional relevance will continue to be debated. However, few membrane biologists today will question that functional protein-protein interactions occurring at the surface of living cells can be influenced by the lipid microdomain environment in which they take place.
Galectin-4: An organizer and stabilizer of glycolipid-based lipid rafts in the brush border
Galectin-4 belongs to the galectin family of β-galactoside-binding proteins and members of the family have been found in a variety of tissues and cell types and been implicated in a host of diverse physiological functions (Barondes et al. [Citation1994], Drickamer & Taylor [Citation1993], Huflejt & Leffler [Citation2004]). Galectin-4, originally discovered in rat intestinal extracts (Leffler et al. [Citation1989]), is a 36-kDa protein and comprises two carbohydrate recognition domains that both bind lactose with a similar affinity but have differential affinities for other saccharides. This divalency makes galectin-4 a natural cross-linker, but in a modified sense because the two carbohydrate recognition domains have separate subset of ligands. Its expression is confined to the entire length of the gastrointestinal tract both during development and in normal adult tissue, but in addition galectin-4 expression is induced in cancers from other tissues (Huflejt & Leffler [Citation2004]). Like other members of the family, galectin-4 is synthesized without a signal for membrane translocation, but by a poorly understood process of ‘nonclassical’ secretion (Nickel [Citation2003], Nickel [Citation2005]), it is targeted to the extracellular side of the small intestinal brush border where it is firmly associated with lipid rafts and binds to other proteins, including the major brush border enzymes aminopeptidase N and sucrase-isomaltase (Danielsen & van Deurs [Citation1997]). In a recent study, galectin-4 was shown to associate with a wide range of sulfated glycolipids and the carcinoembryonic antigen in patches on the surface of human colonadenocarcinoma cells (Ideo et al. [Citation2005]). It thus seems fair to conclude that galectin-4 indeed has the ability to cross-link a broad repertoire of glycoconjugates. Furthermore, that galectin-4 is not just a passive raft component but probably serves a major role as a raft organizer/stabilizer (Braccia et al. [Citation2003]) is indicated by the following observations: (i) Release of galectin-4 from the membrane by lactose also releases other raft-associated proteins, such as aminopeptidase N and alkaline phosphatase; (ii) By sequential extraction with Triton X-100 at increasing temperature (0, 20,- and 37°C), a fraction of ‘superrafts’ (membranes resisting extraction with Triton X-100 at physiological temperature) could be obtained in which galectin-4 is greatly enriched relative to other raft components. Together, these properties indicate that galectin-4 is a core constituent of glycolipid-based rafts.
The general question concerning size and stability of lipid rafts in situ has been a highly disputed topic among ‘raftologists’ for a long time (Anderson & Jacobson [Citation2002], Edidin [Citation2001], Hooper [Citation1998]), but a consensus seems to be emerging that rafts are typically rather small (maybe as small as a single protein molecule surrounded by a cluster of raft lipid molecules) and transient. This concept of very small and dynamic rafts or ‘shells’ (Anderson & Jacobson [Citation2002]) that may be triggered to assemble into larger functional microdomains fits well with the formation of signal transduction complexes (signalosomes) following activation of cell surface receptors (Harder [Citation2004], He et al. [Citation2005], Pike [Citation2003], Simons & Toomre [Citation2000], Werlen & Palmer [Citation2002]). However, this trendy concept does not agree well with the properties described above concerning the glycolipid-based rafts containing galectin-4 as a cross-linker of glycoconjugated membrane lipids and proteins. That lipid rafts in the small intestinal brush border are stable, rather than transient, and also of a substantial size has also been indicated by the possibility to separate microvillar membrane vesicles (which are about 100 nm in diameter) into raft-rich and raft-poor types without the use of detergents (Hansen et al. [Citation2001]). With regard to size they may therefore be examples of naturally occurring microdomains that can only be mimicked experimentally with other types of cell membranes when raft proteins are forced to coalesce by antibody-induced ‘copatching’ (Harder et al. [Citation1998]).
Functional roles of glycolipid-based rafts
The small intestinal brush border is designed to function as a digestive surface that is maximally prepared at all times for processing dietary nutrients into small, non-hazardous molecules that can be safely absorbed by membrane transporters (Trier [Citation1968]). This digestive/absorptive strategy implies that apical fluid-phase uptake of nutrients by endocytosis in the intestine must be kept at a minimal rate. In contrast, the kidney proximal tubule cell, which morphologically resembles the small intestinal enterocyte, mainly relies on endocytosis followed by digestion in the lysosomes (Maunsbach [Citation1976]). Therefore, it may well be that the organization into glycolipid-based microdomains stably cross-linked by galectin-4 and possibly by other members of the galectin family in the small intestine serves as a mechanism to limit endocytosis. In support of this notion, it has previously been shown that another type of large lipid raft domains, caveolae, represent highly stable plasma membrane compartments not involved in constitutive endocytosis (Hommelgaard et al. [Citation2005], Thomsen et al. [Citation2002]). Furthermore, Myo1a, the brush border myosin that links the membrane to the microvillar actin cytoskeleton, is associated with lipid rafts and has been proposed to be required for the retention of the raft protein sucrase-isomaltase in the brush border (Tyska & Mooseker [Citation2004]), and in Myo1a knockout mice, the brush border localization of galectin-4 and other raft-associated proteins was affected (Tyska et al. [Citation2005]).
In a recent paper, galectin-4 was shown to play a functional role in apical trafficking in enterocyte-like cells (Delacour et al. [Citation2005]). Thus, the lectin was detected on post-Golgi carrier vesicles of HT-29 5M12 cells, and a knockdown of galectin-4 expression by 80% using RNA interference (RNAi) caused apical membrane markers to accumulate intracellularly while the localization of a basolateral marker was unaffected. The same group had previously shown that a glycosylation inhibitor, 1-benzyl-2-acetamido-2-deoxy-α-D-galactopyranoside, likewise perturbs apical trafficking in this cell type (Delacour et al. [Citation2003]). Sulfatides with long chain-hydroxylated fatty acids were prominent constituents of DRM's isolated from these cells, and as also reported by another group (Ideo et al. [Citation2005]), this particular class of glycolipids were identified as high-affinity ligands for galectin-4, forming ‘superraft’ complexes resisting solubilization with Triton X-100 at 37°C (Delacour et al. [Citation2005]).
Annexin A2 (annexin II) is another cytosolic, lipid raft-associated protein (Harder & Gerke [Citation1994]) that like galectin-4 is translocated to the lumenal side of the small intestinal brush border membrane by nonclassical secretion (Danielsen et al. [Citation2003]). Like galectin-4, it was recently shown to be present on apically destined, exocytic lipid raft vesicles, and a knockdown of annexin A2 expression by RNAi caused these vesicles to accumulate in the submembraneous periphery of MDCK cells (Jacob et al. [Citation2004]).
Taken together, these findings highlight the importance of stable, glycolipid-based raft microdomains in an intracellular de novo assembly and apical targeting of preapical domains, but the exact stage where galectin-4 as well as annexin A2 enter the exocytic biosynthetic pathway and translocate across the membrane bilayer remains to be defined.
Lipid rafts as portals for pathogen invasion
However important lipid rafts are as an organizing principle for the cell membrane, it has become increasingly clear over the past years that they may also serve a less beneficial function, namely as target sites for pathogens during adhesion to/invasion of target cells. Thus, an impressive number of pathogens, including bacteria, viruses, fungi, parasites and toxins specifically recognize raft components when making their initial contact with the target cell (reviewed in (Duncan et al. [Citation2002], Manes et al. [Citation2003], Rosenberger et al. [Citation2000], Shin & Abraham [Citation2001]). This apparent preference for rafts most likely reflects their essential property which is to cluster a specific subset of membrane components, in this case pathogen receptors, within a confined area of the cell surface. In addition, the lipid rafts may harbour, or be able to recruit, the signaling capacity needed to provide an entry into the cell (Duncan et al. [Citation2002]). With regard to mechanisms of entry, lipid rafts are often thought to promote internalization by a clathrin-independent mechanism, as exemplified by uptake via caveolae (Duncan et al. [Citation2002], Nichols [Citation2003], Sharma et al. [Citation2004]).
In comparison with other types of cell membranes the glycolipid-based raft organization of the small intestinal brush border described above should be expected to be particularly vulnerable for exploitation by pathogens (Taieb et al. [Citation2004]). Cholera toxin (CT) of Vibrio cholera recognizes the ganglioside GM1, a widespread raft glycolipid, and probably the one most frequently used by many investigators (Sandvig & van Deurs [Citation2002]). In the small intestinal epithelial cell line Caco-2, CT was internalized by a cholesterol-dependent mechanism involving caveolae-like domains (Orlandi & Fishman [Citation1998]). However, studies of CT uptake in Caco-2 cells by another group indicated that the toxin may enter the cell both by clathrin-dependent- and independent mechanisms (Torgersen et al. [Citation2001]). Since the small intestinal epithelium is the prime natural target for CT, we recently investigated how CT crosses the enterocyte brush border (Hansen et al. [Citation2005a]). As observed with other cell types, CT associated tightly with microvillar DRMs, but surprisingly the toxin rapidly induced the formation of numerous apical clathrin-coated pits- and vesicles, indicating a ‘classical’ clathrin-dependent mechanism of endocytosis in native small intestinal epithelial cells. Furthermore, cholesterol depletion with methyl-β-cyclodextrin had no measurable effect on CT binding and uptake. This observation underscores the point that endocytosis may well be both lipid raft- and clathrin-dependent, as well as cholesterol-independent. This notion is somewhat at odds with current beliefs on endocytosis via lipid rafts (Nichols & Lippincott-Schwartz [Citation2001]), but it makes sense for a cell membrane like the small intestinal brush border that contains only low amounts of caveolin (Badizadegan et al. [Citation2000]), and relies on glycolipids rather than cholesterol for raft integrity and stability (Danielsen & Hansen [Citation2003]).
Anti-glycosyl antibodies: Guardians of glycolipid-based rafts
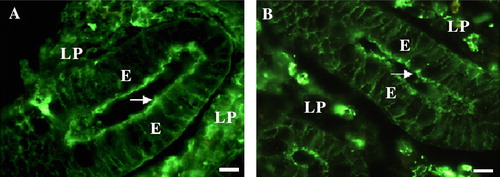
Anti-glycosyl antibodies, which comprise about 1% of the total amount of circulating antibodies in humans (Galili et al. [Citation1984]), are defined as antibodies induced in the host by a glycosyl antigen and which combine with a specific carbohydrate moiety of that antigen (Pazur et al. [Citation1978]). They were originally isolated by affinity chromatography on ‘lactoseagarose’ from antisera of rabbits immunized with nonviable cells of Streptococcues faecalis, which contain an antigenic diheteroglycan of glucose and galactose in its cell wall. Two sets of anti-glycosyl antibodies were characterized, one combining with the terminal galactose residues (anti-galactose antibodies) and another set combining with terminal lactose residues (anti-lactose antibodies) of the same antigen (Pazur et al. [Citation1978]). The anti-glycosyl antibodies include both antibody classes IgG and IgM with the former showing the highest affinity and being of the anti-lactose type whilst IgM are of the anti-galactose type (Mandal et al. [Citation1984]). By ‘lactoseagarose’ chromatography, anti-glycosyl antibodies were recently shown to be the major soluble lectin-like proteins in the small intestine of the pig with affinity towards lactose (Hansen et al. [Citation2005b]). Surprisingly for this organ, they included substantial amounts of IgM and IgG in addition to IgA, otherwise considered the principal class of antibodies produced by the gut (Mostov [Citation1994], Neutra et al. [Citation2001], Rojas & Apodaca [Citation2002]). Depositions of IgM, IgG, and IgA at the small intestinal brush border were mainly localized in microvillar DRM's and could be released by a brief wash with lactose, implying that a fraction of the anti-glycosyl antibodies are targeted to lipid raft microdomains at the apical surface of epithelial cells (). Interestingly, a lactose wash releasing the anti-glycosyl antibodies simultaneously increased the binding to the brush border of lectin PNA, a galactosyl-binding plant lectin (Lotan et al. [Citation1975]), as well as cholera toxin B which binds to ganglioside GM1, a glycolipid containing a terminal galactose residue (Cuatrecasas [Citation1973], Holmgren et al. [Citation1973]). Taken together, these observations led to the idea that the anti-glycosyl antibodies, by competing with pathogenic molecules for the galactosyl/lactosyl binding sites at the brush border, serve as guardians of apical lipid rafts (Hansen et al. [Citation2005b]).
Figure 3. IgG and IgA in the enterocyte brush border. Cryosections of the crypt region of small intestinal mucosa labeled for IgG (A) or IgA (B). Both immunoglobulin classes are deposited in the brush border of enterocytes (arrows). Labeling is also seen along the basolateral surface of the enterocytes (E), as well as in plasma cells of the lamina propria (LP). Bars: 10 µm. This figure is reproduced in colour in Molecular Membrane Biology online.
Host-bacterial ‘crosstalk’
The intestine is host to an immense number of commensal microrganisms that live in harmony with their host organ. In contrast, it takes only 10–100 single organisms of a pathogen such as Shigella to destroy this peaceful coexistence and cause disease (Kohler et al. [Citation2003]). Maintaining health, as well as causing disease, depends upon interactive processes taking place between the intestinal epithelium and the bacteria and are commonly referred to as ‘crosstalk’.
One interesting aspect of the host-bacterial crosstalk is the ability of microorganisms to modulate the glycosylation pattern of the gut epithelium. Early studies comparing germ-free and conventional mice showed that the indigeneous Bacteroides thetaiotaomicron induces epithelial surface fucosylation, supposedly in order to match the host carbohydrate structures as substrates for the bacterial glycosidases (Hooper et al. [Citation2002]). In addition, a soluble factor from B. thetaiotaomicron was shown to specifically increase the surface galactosylation of the intestinal cell line HT29-MTX (Freitas et al. [Citation2001]). Subsequently, the same group has made the observation that other indigeneous bacteria, Lactobacillus casei and Lactobacillus acidophilus in turn generate different patterns of host surface glycosylation and proposed that this crosstalk should be seen as a potential strategy to reduce receptor recognition by pathogens (Freitas et al. [Citation2003]). In this context the ‘coating’ of the stable lipid rafts of the brush border by anti-glycosyl antibodies mentioned above can be viewed as yet another manifestation of the ongoing host-bacterial crosstalk.
Conclusion and future perspectives
The aim of this review has been to describe the special type of lipid raft organization in epithelial brush borders that is rather different from our general concept of these membrane microdomains. Thus, lectins, examplified by galectin-4, seem to play a key role in conferring stability to these mainly glycolipid-based rafts. In the near future, it would be interesting to define more clearly how galectins and similar lectin-like proteins may play a role also in the intracellular assembly and apical targeting of preapical domains. To tackle this problem, we will need to learn more about how and where soluble galectins manage to translocate the cell membrane and gain access to their ligands. Much the same holds for annexin A2, which, as described above, is another interesting candidate protein for playing a pivotal role in the apical targeting. Like galectin-4, annexin A2 is a soluble protein with divalent, raft-binding properties that exits the cytoplasm by a nonclassical mechanism. Unfortunately, nonclassical secretion of proteins remains an ill-defined concept despite the fact that the phenomenon has been known for many years (Nickel [Citation2003], Nickel [Citation2005]). Galectins and annexins might hold the clue to solve this mystery concept.
This paper was first published online on prEview on 24 January 2006.
Gillian Danielsen is thanked for a critical discussion of the manuscript. The authors wish to thank The Danish Medical Research Council, The Novo-Nordic Foundation, The Beckett Foundation and the Augustinus Foundation for financial support.
References
- Alfalah M, Jacob R, Naim HY. Intestinal dipeptidyl peptidase IV is efficiently sorted to the apical membrane through the concerted action of N- and O-glycans as well as association with lipid microdomains. J Biol Chem 2002; 277: 10683–10690
- Alfalah M, Jacob R, Preuss U, Zimmer KP, Naim H, Naim HY. O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr Biol 1999; 9: 593–596
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 2002; 296: 1821–1825
- Badizadegan K, Dickinson BL, Wheeler HE, Blumberg RS, Holmes RK, Lencer WI. Heterogeneity of detergent-insoluble membranes from human intestine containing caveolin-1 and ganglioside G(M1). Am J Physiol Gastrointest Liver Physiol 2000; 278: G895–G904
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem 1994; 269: 20807–20810
- Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrom BT, Hansen GH, Danielsen EM. Microvillar membrane Microdomains exist at physiological temperature – Role of galectin-4 as lipid raft stabilizer revealed by ‘superrafts’. J Biol Chem 2003; 278: 15679–15684
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 1998; 14: 111–136
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992; 68: 533–544
- Christiansen K, Carlsen J. Microvillus membrane vesicles from pig small intestine. Purity and lipid composition. Biochim Biophys Acta 1981; 647: 188–195
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry 1973; 12: 3547–3558
- Danielsen EM. Involvement of detergent-insoluble complexes in the intracellular transport of intestinal brush border enzymes. Biochemistry 1995; 34: 1596–1605
- Danielsen EM, Hansen GH. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim Biophys Acta 2003; 1617: 1–9
- Danielsen EM, van Deurs B. Galectin-4 and small intestinal brush border enzymes form clusters. Mol Biol Cell 1997; 8: 2241–2251
- Danielsen EM, van Deurs B, Hansen GH. “Nonclassical” secretion of annexin A2 to the lumenal side of the enterocyte brush border membrane. Biochemistry 2003; 42: 14670–14676
- Delacour D, Gouyer V, Leteurtre E, Ait-Slimane T, Drobecq H, Lenoir C, Moreau-Hannedouche O, Trugnan G, Huet G. 1-benzyl-2-acetamido-2-deoxy-alpha-D-galactopyranoside blocks the apical biosynthetic pathway in polarized HT-29 cells. J Biol Chem 2003; 278: 37799–37809
- Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, Andre S, Le Bivic A, Gabius HJ, Manninen A, Simons K, Huet G. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol 2005; 169: 491–501
- Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP, Lasserre R, He HT. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J 2002; 21: 1899–1908
- Drickamer K, Taylor ME. Biology of animal lectins. Annu Rev Cell Biol 1993; 9: 237–264
- Duncan MJ, Shin JS, Abraham SN. Microbial entry through caveolae: variations on a theme. Cell Microbiol 2002; 4: 783–791
- Edidin M. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol 2001; 11: 492–496
- Freitas M, Cayuela C, Antoine JM, Piller F, Sapin C, Trugnan G. A heat labile soluble factor from Bacteroides thetaiotaomicron VPI-5482 specifically increases the galactosylation pattern of HT29-MTX cells. Cell Microbiol 2001; 3: 289–300
- Freitas M, Tavan E, Cayuela C, Diop L, Sapin C, Trugnan G. Host-pathogens cross-talk. Indigenous bacteria and probiotics also play the game. Biol Cell 2003; 95: 503–506
- Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med 1984; 160: 1519–1531
- Hansen GH, Dalskov SM, Rasmussen CR, Immerdal L, Niels-Christiansen LL, Danielsen EM. Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry 2005a; 44: 873–882
- Hansen GH, Immerdal L, Thorsen E, Niels-Christiansen LL, Nystrom BT, Demant EJ, Danielsen EM. Lipid rafts exist as stable cholesterol-independent microdomains in the brush border membrane of enterocytes. J Biol Chem 2001; 276: 32338–32344
- Hansen, GH, Pedersen, ED, Immerdal, L, Niels-Christiansen, LL, Danielsen, EM. 2005b. Anti-glycosyl antibodies in lipid rafts of the enterocyte brush border: A possible host defense against pathogens. Am J Physiol Gastrointest Liver Physiol, 289:G1100–G1107.
- Hansen GH, Pedersen J, Niels-Christiansen LL, Immerdal L, Danielsen EM. Deep-apical tubules: dynamic lipid-raft microdomains in the brush-border region of enterocytes. Biochem J 2003; 373: 125–132
- Harder T. Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr Opin Immunol 2004; 16: 353–359
- Harder T, Gerke V. The annexin II2p11(2) complex is the major protein component of the triton X-100-insoluble low-density fraction prepared from MDCK cells in the presence of Ca2+. Biochim Biophys Acta 1994; 1223: 375–382
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 1998; 141: 929–942
- Hauser H, Howell K, Dawson RM, Bowyer DE. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim Biophys Acta 1980; 602: 567–577
- He HT, Lellouch A, Marguet D. Lipid rafts and the initiation of T cell receptor signaling. Semin Immunol 2005; 17: 23–33
- Holm K, Weclewicz K, Hewson R, Suomalainen M. Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55(gag) associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100. J Virol 2003; 77: 4805–4817
- Holmgren J, Lonnroth I, Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun 1973; 8: 208–214
- Hommelgaard AM, Roepstorff K, Vilhardt F, Torgersen ML, Sandvig K, van Deurs B. Caveolae: stable membrane domains with a potential for internalization. Traffic 2005; 6: 720–724
- Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 2002; 22: 283–307
- Hooper NM. Membrane biology: do glycolipid microdomains really exist?. Curr Biol 1998; 8: R114–R116
- Hooper NM, Bashir A. Glycosyl-phosphatidylinositol-anchored membrane proteins can be distinguished from transmembrane polypeptide-anchored proteins by differential solubilization and temperature-induced phase separation in Triton X-114. Biochem J 1991; 280: 745–751
- Hooper NM, Turner AJ. Ectoenzymes of the kidney microvillar membrane. Differential solubilization by detergents can predict a glycosyl- phosphatidylinositol membrane anchor. Biochem J 1988; 250: 865–869
- Huflejt ME, Leffler H. Galectin-4 in normal tissues and cancer. Glycoconj J 2004; 20: 247–255
- Ideo H, Seko A, Yamashita K. Galectin-4 binds to sulfated glycosphingolipids and carcinoembryonic antigen in patches on the cell surface of human colon adenocarcinoma cells. J Biol Chem 2005; 280: 4730–4737
- Jacob R, Heine M, Eikemeyer J, Frerker N, Zimmer KP, Rescher U, Gerke V, Naim HY. Annexin II is required for apical transport in polarized epithelial cells. J Biol Chem 2004; 279: 3680–3684
- Kawai K, Fujita M, Nakao M. Lipid components of two different regions of an intestinal epithelial cell membrane of mouse. Biochim Biophys Acta 1974; 369: 222–233
- Kohler H, McCormick BA, Walker WA. Bacterial-enterocyte crosstalk: cellular mechanisms in health and disease. J Pediatr Gastroenterol Nutr 2003; 36: 175–185
- Leffler H, Masiarz FR, Barondes SH. Soluble lactose-binding vertebrate lectins: a growing family. Biochemistry 1989; 28: 9222–9229
- Lotan R, Skutelsky E, Danon D, Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem 1975; 250: 8518–8523
- Louvard D, Kedinger M, Hauri HP. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol 1992; 8: 157–195
- Mandal C, Mandal C, Karush F. Restriction in IgM expression–V. Fine structure analysis in the anti-lactose system. Mol Immunol 1984; 21: 895–900
- Manes S, del Real G, Martinez A. Pathogens: raft hijackers. Nat Rev Immunol 2003; 3: 557–568
- Maunsbach AB. Ultrastructure of the proximal tubule. Handbook of physiology, SR Geiger. American Physiological Society, Washington, DC 1973; 31–79
- Maunsbach AB. Cellular mechanisms of tubular protein transport. Int Rev Physiol 1976; 11: 145–167
- Mirre C, Monlauzeur L, Garcia M, Delgrossi MH, Le Bivic A. Detergent-resistant membrane microdomains from Caco-2 cells do not contain caveolin. Am J Physiol 1996; 271: C887–C894
- Mooseker MS, Keller TC III, Hirokawa N. Regulation of cytoskeletal structure and contractility in the brush border. Ciba Found Symp 1983; 95: 195–215
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol 1994; 12: 63–84
- Munoz P, Navarro MD, Pavon E, Salmeron J, Malavasi F, Sancho J, Zubiaur M. CD38 signaling in T cells is initiated within a subset of membrane rafts containing Lck and CD3-zeta subunit of the T cell antigen receptor. J Biol Chem 2003; 278: 50791–50802
- Munro S. Lipid rafts. Elusive or illusive?. Cell 2003; 115: 377–388
- Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol 2001; 2: 1004–1009
- Nichols BJ. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr Biol 2003; 13: 686–690
- Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol 2001; 11: 406–412
- Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem 2003; 270: 2109–2119
- Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic 2005; 6: 607–614
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes’ at the plasma membrane. J Biol Chem 1998; 273: 5419–5422
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol 1998; 141: 905–915
- Parkin ET, Turner AJ, Hooper NM. Differential effects of glycosphingolipids on the detergent- insolubility of the glycosylphosphatidylinositol-anchored membrane dipeptidase. Biochem J 2001; 358: 209–216
- Pazur JH, Dresher KL, Forsberg LS. Anti-glycosyl antibodies. Two sets of isoantibodies with specificity for different carbohydrate moieties of the same glycosyl antigen. J Biol Chem 1978; 253: 1832–1837
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 2003; 44: 655–667
- Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol 2002; 3: 944–955
- Rosenberger CM, Brumell JH, Finlay BB. Microbial pathogenesis: lipid rafts as pathogen portals. Curr Biol 2000; 10: R823–R825
- Sandvig K, van Deurs B. Membrane traffic exploited by protein toxins. Annu Rev Cell Dev Biol 2002; 18: 1–24
- Schlegel A, Pestell RG, Lisanti MP. Caveolins in cholesterol trafficking and signal transduction: implications for human disease. Front Biosci 2000; 5: D929–D937
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 2003; 100: 5795–5800
- Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell 2004; 15: 3114–3122
- Shin JS, Abraham SN. Caveolae as portals of entry for microbes. Microbes Infect 2001; 3: 755–761
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1: 31–39
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry 1988; 27: 6197–6202
- Taieb N, Yahi N, Fantini J. Rafts and related glycosphingolipid-enriched microdomains in the intestinal epithelium: bacterial targets linked to nutrient absorption. Adv Drug Deliv Rev 2004; 56: 779–794
- Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell 2002; 13: 238–250
- Torgersen ML, Skretting G, van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci 2001; 114: 3737–3747
- Trier, JJ. 1968. Morphology of the epithelium of the small intestine. In:. Handbook of physiology – alimentary canal. Washington, DC, American Physiological Society. pp, 1125–1176.
- Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 2005; 16: 2443–2457
- Tyska MJ, Mooseker MS. A role for myosin-1A in the localization of a brush border disaccharidase. J Cell Biol 2004; 165: 395–405
- van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol 1987; 105: 1623–1635
- Werlen G, Palmer E. The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr Opin Immunol 2002; 14: 299–305
