Abstract
Endocannabinoids are involved in depressive and anxious symptoms and might play a role in stress-associated psychiatric disorders. While alterations in the endogenous cannabinoid system have been repeatedly found in patients with posttraumatic stress disorder (PTSD), this system has been mostly neglected in borderline personality disorder (BPD). However, there is first evidence for elevated serum levels of the endocannabinoids arachidonylethanolamide (AEA) and 2-arachidonyl-sn-glycerol (2-AG) in BPD patients compared to healthy controls and PTSD patients. In this study, hair endocannabinoids were analyzed, reflecting long-term endocannabinoid concentrations. We assessed AEA concentrations as well as 2-AG and the 2-AG main isomer 1-AG (1-AG/2-AG) in hair in women with BPD (n = 15) and age- and education-matched healthy women (n = 16). We found significantly reduced log AEA in BPD patients compared to healthy women (p = .03) but no differences in log 1-AG/2-AG concentrations. In addition, there was no association between 1-AG/2-AG and hair cortisol, but we found a non-significant correlation between hair concentrations of AEA and cortisol (p = .06). Our data indicate altered long-term release of endogenous cannabinoids in women with BPD depending on type of endocannabinoid. AEA has been suggested to modulate the basal activity of the endocannabinoid system and seems to attenuate depressive and anxious symptoms. Thus, chronically reduced AEA might contribute to psychiatric symptoms in BPD.
Introduction
Borderline personalitity disorder (BPD) is a severe psychiatric disorder characterized by intense and rapidly changing mood states as well as chronic feelings of emptiness, impulsivity, fear of abandonment, unstable relationships, and unstable self-image. Furthermore, non-suicidal self-injurious behavior is a core feature of the disorder. Patients with BPD often suffer from comorbid psychiatric disorders, predominantly major depressive disorder (MDD), anxiety disorders, and posttraumatic stress disorder (PTSD).
Endocannabinoids are involved in affect, pain, and stress regulation (Kirtley, O’Carroll, & O’Connor, Citation2015). Two important and frequently investigated endocannabinoids are arachidonylethanolamide (AEA) and 2-arachidonyl-sn-glycerol (2-AG) which might contribute to BPD psychopathology (Schaefer et al., Citation2014). So far only one study examined basal endocannabinoids in BPD patients (Schaefer et al., Citation2014). The authors found elevated serum concentrations of AEA and 2-AG in the BPD group compared to healthy controls and PTSD patients. However, circulating endocannabinoids are dynamic (Hillard, Citation2018) and only one single fasting blood sample was drawn in this study.
Compared to BPD, more studies on endocannabinoids have been conducted in people with PTSD (Hill, Campolongo, Yehuda, & Patel, Citation2017). One study analyzed endocannabinoids in hair, reflecting long-term endocannabinoid concentrations. The authors found reduced concentrations in PTSD patients (Wilker et al., Citation2016). Further studies also suggest a deficient endocannabinoid activation, i.e. reduced levels of AEA and 2-AG in plasma and elevated brain CB1 receptor availability in PTSD (Hill et al., Citation2013; Neumeister et al., Citation2013). The CB1 receptor is widely expressed throughout the brain and has been associated with the regulation of the stress response, anxiety and memory function (Katona & Freund, Citation2012). AEA and 2-AG bind to CB1 receptors. While AEA modulates the basal tonic activation of the endocannabinoid system, 2-AG is involved in mediating synaptic plasticity and, thus, represents the more phasic and reactive signal (Hill et al., Citation2018; Katona & Freund, Citation2012).
In the current study, we investigated whether long-term endocannabinoid concentrations are altered in BPD. To do so, we measured AEA as well as 2-AG and the 2-AG main isomer 1-AG (1-AG/2-AG) concentrations in hair, representing a cumulative measure over several months (depending on the length of the hair segment). Based on findings in PTSD patients, we expected to find reduced long-term concentrations of endocannabinoids in BPD as well. Given the close relationship between the endocannabinoid system and the hypothalamic pituitary adrenal axis (Riebe & Wotjak, Citation2011), we additionally analyzed – based on results one of our former studies (Dettenborn et al., Citation2016) – whether hair endocannabinoids and hair cortisol are correlated.
Methods
Subjects
Women with BPD (n = 15) and age and education-matched healthy women (HC) (n = 16) were recruited from the Department of Psychiatry and Psychotherapy, Charité Campus Benjamin Franklin, Berlin, Germany and Department of Psychosomatic Medicine and Psychotherapy, Schön Klinik Hamburg Eilbek, or via local advertisement (posters at the University Hospital and via the Department home page) and received financial remuneration. All subjects provided written informed consent. Physical health status was assessed by medical history and careful clinical examination by a staff physician.
Exclusion criteria were a history of severe somatic diseases (e.g. neurological diseases), metabolic diseases (e.g. diabetes), endocrine disorders (e.g. Cushing’s syndrome), autoimmune diseases, or current infections. Furthermore, pregnancy, current anorexia, current or lifetime schizophrenia, current alcohol or drug dependence, bipolar disorder, schizoaffective disorder, major depression with psychotic symptoms, attention deficit hyperactivity disorder, and cognitive impairment resulted in exclusion. In the control group, Beck Depression Inventory-I (BDI-I) scores over 10 also lead to exclusion. The study was approved by the Ethics committee of the Chamber of Physicians, Hamburg, Germany.
Clinical assessments
Patients and controls underwent structured clinical interviews for DSM-IV BPD, MDD, and PTSD administered by trained psychologists (Structured Clinical Interview for DSM-IV Disorders; SCID). Furthermore, all participants completed the BDI-I and the childhood trauma questionnaire (CTQ). In addition, the patients also completed the posttraumatic stress diagnostic scale (PDS).
Hair analysis
Hair strands were cut with scissors as close as possible to the scalp at a posterior vertex position. Endocannabinoids concentrations were determined from the proximal 3 cm hair segment. Based on an average hair growth rate of 1 cm/month, the 3 cm segment used for analysis represents hair grown over the three months period prior to hair sampling. Hair strands were washed twice by shaking in 2.5 mL isopropanol for 3 min at room temperature. Then they were allowed to dry under a fume hood for at least 12 h. 7.5 mg of whole hair was carefully weighed out and sliced into small pieces. After this, 20 μL internal standard and 1.8 mL methanol were added and the hair was incubated for 18 h at room temperature for endocannabinoids extraction. 1.6 mL of the clear supernatant was transferred into a new tube, and was evaporated at 50 °C under a constant stream of nitrogen until the samples were completely dried (duration: approximately 20 min). The dry residue was resuspended using 120 µL methanol/water (v/v = 50/50), 100 µL of which were used for LC–MS/MS analysis following a recently published analytical protocol (Krumbholz, Anielski, Reisch, Schelling, & Thieme, Citation2013), which was established and validated for our laboratory. We measured AEA as well as 1-AG and 2-AG levels. Due to much higher levels of 1-AG detected in hair compared with 2-AG, we used 1-AG/2-AG as the name of summation. Detection limit for AEA and 1-AG/2-AG was 0.016 pg/mg.
Statistics
Statistical analyses were performed using SPSS Version 22.0. Demographic data were analyzed using Pearson’s Chi2-test for categorical data and Student’s t-test for continuous data. AEA and 1-AG/2-AG concentrations were not normally distributed (Shapiro–Wilk-Test p < .001). Thus, log transformed data were used for statistical analysis (t-test). We calculated Cohen's d for AEA and 1-AG/2-AG to estimate the effect sizes. Pearson's correlations were performed between AEA and 1-AG/2-AG (log value) and CTQ and BDI, respectively, as well as AEA, 1-AG/2-AG, and cortisol (log value).
Results
Patients with BPD and HC did not differ significantly with respect to age, body mass index, and smoking (see ). BPD patients had higher depression and childhood trauma scores. All patients fulfilled the DSM-IV criteria for BPD. In addition, four were diagnosed with PTSD and seven BPD patients met criteria for current MDD. Two BPD patients had both comorbidities. HC were free of psychiatric disorders according to DSM-IV SCID I and II.
Table 1. Sample characteristics and hair endocannabinoid and cortisol concentrations (mean/SD).
Four of the BPD patients were taking psychotropic medication (two had more than one drug). These included atypical antipsychotics (quetiapine n = 1; olanzapine n = 1, and promethazine n = 1) as well as antidepressive treatment (selective serotonin reuptake inhibitors: citalopram n = 1, fluoxetine n = 1; tricyclic antidepressants: trimipramine n = 1; serotonin and norepinephrine reuptake inhibitors, venlafaxine n = 1).
BPD patients had significantly lower hair AEA levels compared to controls (Cohen's d = 0.66; see also ). Mean values and statistics are presented in . There were no differences between BPD patients and healthy control women in hair 1-AG/2-AG levels (Cohen's d = 0.34). When taking hair weight into account (ANCOVA with hair weight as covariate) the results did not change (log AEA p = .02, AEA raw values p = .03; no significant effects concerning 1-AG/2-AG).
Figure 1. (a) Hair AEA values (pg/mg) in healthy females and BPD patients (data are presented as mean and standard deviations); (b) correlation (r = −0.36, p = .059) between log AEA and cortisol: white circles = controls, black circles = BPD patients.
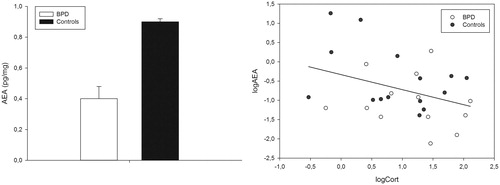
As reported previously, there were no group differences in hair cortisol in this sample (Dettenborn, et al., Citation2016). Hair 1-AG/2-AG levels did not correlate with CTQ nor BDI scores (both p > 0.72). Correlation coefficients between AEA and CTQ (r = −0.23, p = .25) as well as AEA and BDI (r = −0.31, p = .10) also did not reach significance.
Furthermore, there was neither a significant correlation between 1-AG/2-AG and cortisol (r = 0.26, p = .17), nor hair concentrations of AEA and cortisol (r = −0.36, p = .059, see also ).
Discussion
Our preliminary data indicate altered long-term secretion of endocannabinoids in female patients with BPD. In line with our hypothesis, we found decreased hair AEA concentrations in BPD. In contrast, 1-AG/2-AG levels were not significantly different between BPD patients and HCs, although they were numerically lower in BPD patients. Interestingly, AEA has been reported to attenuate depressive and anxious symptoms (Katona & Freund, Citation2012; Mechoulam & Parker, Citation2013). Thus, chronically reduced AEA might contribute to psychopathology in BPD such as depressive symptoms or chronic feelings of emptiness.
Our data contradict one earlier study that found high AEA and 2-AG levels in BPD patients compared to PTSD patients and HCs in a single assessment in serum (Schaefer, et al., Citation2014). However, serum endocannabinoid levels are known to fluctuate significantly depending on acute stress exposure, time of day, exercise, inflammation, or tissue injury (Hillard, Citation2018). Carefully controlling for such factors in an animal study with pharmacological manipulation of endocannabinoid levels, Bedse et al. showed a good agreement between serum and brain levels of endocannabinoids (Bedse et al., Citation2017). However, since there are no published data comparing brain, systemic, and hair levels of endocannabinoids, a systematic investigation is warranted.
Compared to BPD more studies on the role of endocannabinoids have been conducted in PTSD, suggesting decreased endocannabinoid concentrations in these patients (Hill et al., Citation2018). However, most studies had a cross-sectional design and, therefore, do not allow to draw conclusion on causality. Interestingly, animal data propose that deficits in endocannabinoid signaling represent a vulnerability marker to develop psychopathology after trauma (Bluett et al., Citation2017; Bosch-Bouju, Larrieu, Linders, Manzoni, & Laye, Citation2016). In line, many BPD patients report traumatic stress (Cattane, Rossi, Lanfredi, & Cattaneo, Citation2017). Neuroimaging data are in line with our results. Reduced endocannabinoid concentrations have been linked to hyperactivity of the amygdala, hypoactivity of the medial prefrontal cortex, and increased functional coupling of both regions (Hill et al., Citation2018). All of these findings have been repeatedly described in PTSD and BPD patients (Bandelow et al., Citation2017; Krause-Utz, Winter, Niedtfeld, & Schmahl, Citation2014).
An interesting by-product of our study is the (non-significant) negative association between hair AEA and cortisol, which fits nicely to the literature (Riebe & Wotjak, Citation2011). Of note, many psychiatric disorders including BPD and MDD are characterized by alterations of the HPA axis, suggesting hyperactivity of the system, albeit the literature is not consistent (Wingenfeld & Wolf, Citation2015). In the presented sample, there were no differences between patients and controls concerning hair cortisol. However, reduced endocannabinoid concentrations might contribute to an overactive HPA axis activity. This hypothesis has to be investigated in larger, clinical well-characterized samples.
There are some limitations of the study. Most importantly, the sample was small and there were some missing data. Thus, our study was underpowered to find small effects. Furthermore, many patients exhibited comorbid psychiatric disorders such as MDD and many patients received psychotropic medication. Unfortunately, the sample was too small to conduct subgroup analyses. We included only women in this study and, thus, no conclusions can be drawn with regard to men. Finally, further studies may include additional measures of psychopathology, self-injurious behavior and pain sensitivity.
In sum, our results provide first evidence for altered long-term incorporation of endocannabinoids in hair in women with BPD. Reduced endocannabinoid concentrations have been reported not only for PTSD (Hill et al., Citation2018) but also for MDD (Hillard & Liu, Citation2014), and these deficits might contribute to BPD symptomatology. Furthermore, endocannabinoids are involved in self-injurious behavior (Kirtley et al., Citation2015), which is a prominent feature in BPD. In sum, there are many reasons to further study this important multi-functional system in patients with BPD. Future studies should analyse subgroups of BPD patients with different comorbid psychiatric disorders, such as PTSD and MDD.
Disclosure statement
There was no conflict of interest.
References
- Bandelow, B., Baldwin, D., Abelli, M., Bolea-Alamanac, B., Bourin, M., Chamberlain, S.R., &Cinosi, E. (2017). Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World Journal of Biological Psychiatry, 18, 162–214. doi:10.1080/15622975.2016.1190867.
- Bedse, G., Hartley, N.D., Neale, E., Gaulden, A.D., Patrick, T.A., Kingsley, P.J., et al. (2017). Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biological Psychiatry, 82, 488–499. doi:10.1016/j.biopsych.2017.03.002.
- Bluett, R.J., Baldi, R., Haymer, A., Gaulden, A.D., Hartley, N.D., Parrish, W.P., et al. (2017). Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nature Communications, 8, 14782. doi:10.1038/ncomms14782.
- Bosch-Bouju, C., Larrieu, T., Linders, L., Manzoni, O.J., & Laye, S. (2016). Endocannabinoid-mediated plasticity in nucleus accumbens controls vulnerability to anxiety after social defeat stress. Cell Reports, 16, 1237–1242. doi:10.1016/j.celrep.2016.06.082.
- Cattane, N., Rossi, R., Lanfredi, M., & Cattaneo, A. (2017). Borderline personality disorder and childhood trauma: exploring the affected biological systems and mechanisms. BMC Psychiatry, 17, 221. doi:10.1186/s12888-017-1383-2.
- Dettenborn, L., Kirschbaum, C., Gao, W., Spitzer, C., Roepke, S., Otte, C., et al. (2016). Increased hair testosterone but unaltered hair cortisol in female patients with borderline personality disorder. Psychoneuroendocrinology, 71, 176–179. doi:10.1016/j.psyneuen.2016.05.026.
- Hill, M.N., Bierer, L.M., Makotkine, I., Golier, J.A., Galea, S., McEwen, B.S., et al. (2013). Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology, 38, 2952–2961. doi:10.1016/j.psyneuen.2013.08.004.
- Hill, M.N., Campolongo, P., Yehuda, R., & Patel, S. (2018). Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology, 43, 80–107. doi:10.1038/npp.2017.162.
- Hillard, C.J. (2018). Circulating endocannabinoids: From whence do they come and where are they going? Neuropsychopharmacology, 43, 155–177. doi:10.1038/npp.2017.130.
- Hillard, C.J., & Liu, Q.S. (2014). Endocannabinoid signaling in the etiology and treatment of major depressive illness. Current Pharmaceutical Design, 20, 3795–3811.
- Katona, I., & Freund, T.F. (2012). Multiple functions of endocannabinoid signaling in the brain. Annual Review of Neuroscience, 35, 529–558. doi:10.1146/annurev-neuro-062111-150420.
- Kirtley, O.J., O'Carroll, R.E., & O'Connor, R.C. (2015). The role of endogenous opioids in non-suicidal self-injurious behavior: methodological challenges. Neuroscience Biobehavioral Reviews, 48, 186–189. doi:10.1016/j.neubiorev.2014.11.007.
- Krause-Utz, A., Winter, D., Niedtfeld, I., & Schmahl, C. (2014). The latest neuroimaging findings in borderline personality disorder. Current Psychiatry Reports, 16, 438. doi:10.1007/s11920-014-0438-z.
- Krumbholz, A., Anielski, P., Reisch, N., Schelling, G., & Thieme, D. (2013). Diagnostic value of concentration profiles of glucocorticosteroids and endocannabinoids in hair. Therapeutic Drug Monitoring, 35, 600–607. doi:10.1097/FTD.0b013e3182953e43.
- Mechoulam, R., & Parker, L.A. (2013). The endocannabinoid system and the brain. Annual Review of Psychology, 64, 21–47. doi:10.1146/annurev-psych-113011-143739.
- Neumeister, A., Normandin, M.D., Pietrzak, R.H., Piomelli, D., Zheng, M.Q., Gujarro-Anton, A., et al. (2013). Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Molecular Psychiatry, 18, 1034–1040. doi:10.1038/mp.2013.61.
- Riebe, C.J., & Wotjak, C.T. (2011). Endocannabinoids and stress. Stress (Amsterdam, Netherlands), 14, 384–397. doi:10.3109/10253890.2011.586753.
- Schaefer, C., Enning, F., Mueller, J.K., Bumb, J.M., Rohleder, C., Odorfer, T.M., et al. (2014). Fatty acid ethanolamide levels are altered in borderline personality and complex posttraumatic stress disorders. European Archives of Psychiatry and Clinical Neuroscience, 264, 459–463. doi:10.1007/s00406-013-0470-8.
- Wilker, S., Pfeiffer, A., Elbert, T., Ovuga, E., Karabatsiakis, A., Krumbholz, A., et al. (2016). Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology, 67, 198–206. doi:10.1016/j.psyneuen.2016.02.010.
- Wingenfeld, K., & Wolf, O.T. (2015). Effects of cortisol on cognition in major depressive disorder, posttraumatic stress disorder and borderline personality disorder – 2014 Curt Richter Award Winner. Psychoneuroendocrinology, 51, 282–295. doi:10.1016/j.psyneuen.2014.10.009.

