Abstract
The purpose of the present study was to investigate basic methodological issues related to the usage of an examination stress protocol in studies of psychoneuroendocrinology. In the present study, 57 undergraduate students served as participants. All subjects provided salivary samples and completed psychological inventories during a low examination stress period and again during a high examination stress period. Salivary samples were analyzed for cortisol.
Three major findings were observed. First, the examination stress protocol proved to be an effective trigger of elevations in both psychological measures of stress and in cortisol levels. Second, sex differences were observed in cortisol levels, such that males showed an elevation in cortisol during the high examination stress session whereas females did not. Finally, no significant correlations were observed between elevations in psychological measures of stress and elevations in cortisol levels.
These findings suggest that the examination stress protocol used in the present study effectively elevated both psychological stress and cortisol levels. Furthermore, these findings suggest that there are biological differences in how males and females respond to stress. Finally, no evidence was found to suggest a relationship between psychological and hormonal levels of stress. Together, these findings suggest the need to better define and consider the implications of both the specific measures of stress being used and individual differences in the subject samples in psychoendocrine studies.
Introduction
Numerous studies have investigated the role of acute and chronic levels of psychological stress and stress hormone levels on several aspects of physiological and cognitive functioning (e.g. McEwen and Sapolsky Citation1995; Lupien and McEwen Citation1997). Specifically, with regard to acute elevations in the levels of the stress hormone cortisol, the vast majority of studies have used laboratory stressors (e.g. the trier social stress test—TSST, or hydrocortisone or dexamethasone challenge) in order to trigger transient elevations. However, it remains an open question as to whether increases in secretion of cortisol and other stress hormones in response to laboratory stressors strongly predict inncreases in the same stress hormones in response to naturalistic stressors (Cohen and Hammick Citation2003; Kamarck and Lovallo Citation2003). Therefore, the results of paradigms using laboratory stressors may misrepresent the extent to which naturally occurring stressors increase cortisol sercetion and in doing so may also misrepresent the extent to which the effects of naturalistic stressors are cortisol-dependent.
One common naturalistic stressor is examination stress (Stowell Citation2003). There is good evidence to suggest that the stress of examinations elicits elevated activity in the hypothalamic–pituitary–adrenal (HPA) axis and increased release of cortisol (Frankenhaeuser et al. Citation1978; Lovallo et al. Citation1986; Malarkey et al. Citation1995; Lacey et al. Citation2000; Lucini et al. Citation2002). However, there is some inconsistency in the literature, with some studies finding either no change in cortisol secretion or even decreased release of cortisol in the face of examinations (see Glaser et al. Citation1994; Vedhara et al. Citation2000). One explanation for the inconsistent findings is that the extent to which a stressor triggers an elevation in cortisol is dependent on a variety of factors, including novelty, uncertainty, negative emotions and ego-involvement (Mason Citation1968; Dickerson and Kemeny Citation2004 for a recent review). Another reason why cortisol reactivity may not be seen consistently across studies is that significant group and individual differences exist in reactivity (e.g. see Kemeny and Laudenslager Citation1999). For instance, numerous studies have suggested that the extent of a cortisol secretory response is dependent on the sex of the individual (Kudielka et al. Citation2000; Kajantie and Phillips Citation2006). Specifically, when sex differences are observed, males tend to show greater cortisol responses than do females.
However, the extent and direction of the sex differences in stress reactivity is also dependent both on the nature of the stressor itself and on the specific measure of stress reactivity that is used (Dickerson and Kemeny Citation2004; Kudielka and Kirschbaum Citation2005). With regard to the nature of the stressors, the vast majority of studies that investigated sex differences in cortisol responses have used laboratory stressors, such as the TSST (see Kudielka et al. Citation2000; Kajantie and Phillips Citation2006). While the TSST would appear to be an ecologically valid measure of stress due to its recreation of a realistic experience, because of its artificial nature, there is still the issue of it external validity. That is, it remains unclear to what extent results obtained from laboratory stressors generalize to stressful situations more commonly experienced. In contrast, the strength of measuring stress in the field, as is the case with examination stress protocols is its external validity. Consequently, one important issue to be resolved is whether sex-differences in cortisol reactivity occur in response to naturally occurring stressors. Indeed, very few studies have investigated sex differences in cortisol responses using an ecologically valid measure (see Kudielka et al. Citation2000; Kajantie and Phillips Citation2006). However, similar to studies using a laboratory stressor, studies using a examination stressor tend to find a greater cortisol (Frankenhaeuser et al. Citation1978; Ennis et al. Citation2001) or sympathetic-adrenal–medullary response (VanDoornen Citation1986) in males than do females.
With regard to the specific stress response measured, there is an abundance of evidence to suggest that males demonstrate greater cortisol reactivity in response to an acute laboratory stressor than do females (Stoney et al. Citation1988; Girdler et al. Citation1990; Spangler Citation1997; Kudielka et al. Citation2000). While similar findings have been reported regarding sex differences for adrenaline secretion, the effects are less consistent (see Kajantie and Phillips Citation2006). Moreover, when sex differences are found in psychological measures of stress (rather than hormonal measures), females tend to show greater reactions than do males (Mirowsky and Ross Citation1995; see Kudielka et al. Citation1998 for a review). Finally, the extent to which hormonal responses to stress are correlated to psychological responses to stress remains unclear (e.g. Peters et al. Citation2003; Schommer et al. Citation2003).
While the present study includes several experimental design characteristics consistent with earlier examination stress protocols, it also differs from the vast majority of these previous designs in three important ways. First, previous examination stress protocols tended to measure either psychological stress responses or stress hormone responses and make inferences about the non-measured response. In the present study, both hormonal and psychological measures of stress are assessed and the relationship between these stress measures is directly evaluated. Second, as described above, few examination stress studies have investigated sex differences in stress responses. In the present study, we investigate these differences. Finally, the vast majority of examination stress studies have used the same examination for each subject (Stowell Citation2003). However, this design is far less practical than would be an experimental design that simply required that all subjects be tested during a week of multiple examinations. The key here, then, is in simply establishing that such a manipulation is sufficient to obtain increases in stress-related psychological and cortisol responses.
The goal of the present study was to investigate individual differences in physiological and psychological stress in response to an examination stressor.
Methods
Subjects
Sixty seven 67 college students, ages 18–21 years (33 males and 34 females), served as participants. Exclusion criteria included: (i) smokers, (ii) left-handers, (iii) non-native English speakers, (iv) those with vision that was not corrected to normal, (v) antihistamine, glucocorticoid or asthma medication users, (vi) those with exposure to general anesthesia in the last year, (vii) those with a personal or first degree family diagnosis of a DSM-IV, Axis I disorder, and (viii) those with endocrine abnormalities. Originally, the intention was that all females would be tested during the midluteal stage of the menstrual cycle. However, it was not possible to test a sufficient number of females who were in the midluteal phase during a week of multiple examinations. Therefore, only information regarding oral contraceptive usage was collected and menstrual cycle stage was not recorded.
Design and procedures
All subjects participated in two behavioral sessions, one during a low examination stress period over the summer when students were not enrolled in classes and one during a high examination stress period when students were enrolled in classes. Therefore, low examination stress sessions occurred during a week when subjects had no examinations and no significant assignments due. High examination stress sessions occurred during a week when students had three or more examinations or significant assignments due.
The order of the low and high examination phases of the study were counterbalanced across subjects, such that Group A had their low examination session during the Summer of 2003 and their high examination session during Fall 2003 examinations. Group B had their high examination session during Spring of 2004 and their low examination session during Summer of 2004. Assignment into Group A and Group B occurred solely based on the timing of the prospective subject's response to the laboratory's recruitment requests.
After completing an online informed consent and exclusionary criteria survey, subjects who met the criteria were invited to attend recruitment meetings, one before the low examination session and the other before the high examination session. Subjects were asked in advance of their “high examination” meeting to bring documentation of their examination schedule for that academic term.
Behavioral testing sessions: Laboratory sampling. All subjects came into the laboratory individually, once during the low examination stress period and once during the high examination stress period. In order to avoid the sharp decline in cortisol level observed during the hours following morning awakening (Kirschbaum and Hellhammer Citation1994; Clow et al. Citation2004), all subjects were tested in the afternoon or early evening hours. A typical session started at either 3:30 pm or 5:30 pm. For any one individual, there was no more than a 1-h discrepancy between the commencement of the low stress and high stress testing sessions. The two sessions occurred approximately three months apart. Sessions were approximately 2 h in length.
At the beginning of the session, subjects completed a packet of inventories and provided one salivary sample. Subjects then participated in electrophysiological testing, which included a memory event-related potential (ERP) task, with a 30-min distracter period and a baseline EEG asymmetry task (Lewis et al. Citation2006). After electrophysiological testing, subjects provided a second salivary sample. The approximate time that elapsed between salivary samples was 1 h and 45 min. The study protocol was approved by the institutional review board of Pomona College.
Samples were immediately placed in a − 20 C freezer. After all samples had been collected, they were sent to Salimetrics, Inc in State College, PA to be analyzed via enzyme-linked immunosorbent assay (ELISA).
The laboratory sessions themselves were not intended to serve as stressors. Instead, the sessions were intended as a time to collect behavioral and ERP data, once when stress levels were low (i.e. during the “no-examination, low stress session”) and once when stress levels were high (i.e. during the “multiple-examination, high stress session”). Nevertheless, this assumption was also tested empirically below.
Inventories
The inventories included: (i) the Spielberger state–trait anxiety inventory (STAI) (both state and trait subscales), and (ii) the perceived stress inventory (PSS).
Spielberger state–trait anxiety inventory (STAI, Spielberger, Citation1983)
The state subscale
This 20-item subscale of the Spielberger STAI assesses anxiety at the time of testing. The subscale includes items such as, “I feel calm” and “I am presently worrying over possible misfortunes”. Subjects responded to each item using a 4-point scale in which 1 signifies “not at all” and 4 signifies “very much so” according to how they “feel right now, that is, at this moment”.
The trait subscale
This 20-item subscale of the Spielberger STAI assesses chronic anxiety. The subscale includes items such as, “I wish I could be as happy as others seem to be” and “I have disturbing thoughts”. Subjects responded to each item using a 4-point scale in which 1 signifies “almost never” and 4 signifies “almost always” according to how they “feel in general”.
Perceived stress scale (Cohen et al. Citation1983)
This 14-item inventory assesses the frequency of feelings of anxiety regarding certain potentially stressful events during the previous month. The 5-point scale includes items such as “In the last month, how often have you been angered because of things that happened that were outside of your control?” and “In the last month, how often have you felt difficulties were piling up so high that you could not overcome them?” Responses range from 0 indicating “never” to 4 indicating “very often.”
Statistical analysis
The hypotheses were tested through comparisons of means (t-tests), analyses of variance (ANOVAs) and Pearson product correlations (r) using SPSS for Macintosh, Version 11.03. Counterbalancing group (Group A, Group B) and sex served as grouping variables for most analyses. Dependent variables included demographic data (e.g. hours of sleep), cortisol concentrations and psychological measures of stress. For ANOVAs, both probability of significance and effect sizes were calculated for all effects.
Results
Demographics
Preliminary analyses were run on several demographic variables including: (i) body mass index (BMI), (ii) oral contraceptive usage, and (iii) hours of sleep during each of the two (low vs. high examination stress) sessions of the experiment. Standard deviations (SDs) are provided parenthetically following the means. First, average BMI was M = 22.6 (SD = 2.52), which is within the normal, healthy range. As expected, males showed significantly higher BMI scores (M = 23.3 (SD = 2.22)) than did females (M = 22.1 (SD = 2.78)) (t(62) = 2.11; p = 0.04). Second, 14 of the 35 women who participated in the study were on oral contraceptives. Their BMIs, hours of sleep and stress measures did not differ from those of the 21 women who were not on oral contraceptives. Third, subjects slept significantly more hours during the low examination phase (M = 7.5 h (SD = 0.75)) than during the high examination phase (M = 6.4 h (SD = 1.43)) of the experiment (t(61) = 6.42; p < 0.001). However, when sleep was entered as a covariate, it did not significantly alter any of the findings reported in this study.
Effect of examination period on stress measures
The purpose of the following set of ANOVAs was to investigate (i) whether examination stress-related elevations occurred in the one hormonal measure (cortisol) and in the three psychological stress measures (STAI-S, STAI-T and PSS), and (ii) whether sex differences were observed in these elevations. Because salivary cortisol samples were provided both at the beginning and the end of each session, we include separate cortisol analyses below, one with sample (beginning vs. end) as a within-subject variable and one where average cortisol concentration (the mean of the concentrations in the beginning and end of session samples) serves as the dependent variable.
Because order of session was counterbalanced across the participants such that Group A had the high examination stress session in the fall and Group B had the high examination stress session in the spring, all analyses were run with counterbalancing group as a between-subject factor to assure that order of phase/seasonality was not a confound in the present paradigm.
Inclusion of subjects. About 10 of the original 67 subjects were excluded because they had extreme values based on an outlier analysis of cortisol samples. More specifically, of the 10 subjects who were excluded, all had values at least 2.5 SDs above the mean. Eight of these subjects were male (leaving 25 males and 32 females in the final analysis). Five were from Group A and five were from Group B. Of the remaining subjects, one was excluded from the trait anxiety analysis because of incomplete data on that measure. In summary, 57 subjects were included in all but the trait anxiety analysis, which included 56 subjects ().
Examination-related changes in the stress measures
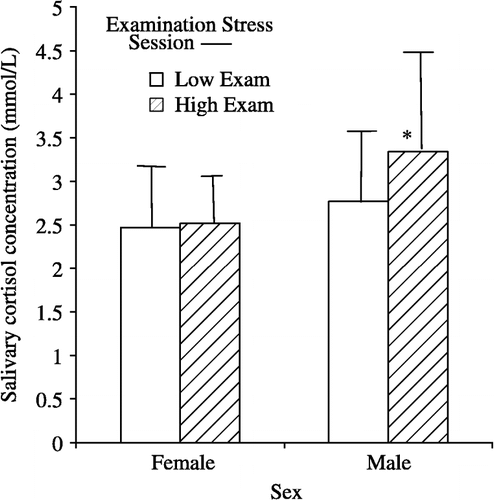
Cortisol. A 2 × 2 × 2 mixed ANOVA was performed with counterbalancing group (A,B) and sex (female, male) as between-subject factors and with Session (low examination period, high examination period) as the within-subject factor. Average cortisol concentration (that is, the average of the two samples that were taken at the beginning and end of each session) was the dependent variable. A main effect of sex (F(1,53) = 9.25; p = 0.004, η2 = 0.15) and a trend towards a main effect of session (F(1,53) = 3.55; p = 0.06, η2 = 0.06) were found, such that females had lower cortisol levels than did males and cortisol levels were higher during the high examination stress session than during the low examination stress session. Finally, a trend towards a two-way interaction was observed between sex and session (F(1,53) = 3.39; p = 0.07, η2 = 0.06) such that the increase in cortisol from low examination stress session to high examination stress session was significant for males (t(23) = 2.16; p = 0.04)) but not for females (t(32) = 0.22; p = ns)). No other significant main effects or interactions were observed. Importantly, there were no main effects or interactions involving counterbalancing group.
In order to test the assumption that the laboratory sessions themselves did not serve as stressors, a 2 × 2 × 2 × 2 mixed ANOVA was performed with counterbalancing group (A,B) and sex (female, male) as between-subject factors and with Session (low examination period, high examination period) and sample (beginning of session, end of session) as within-subject factors. Cortisol concentration was the dependent variable. Of greatest interest to the question of stressor, there was a significant main effect of sample (F(1,53) = 103.95; p = 0.000, η2 = 0.66), such that the cortisol sample taken at the end of the session was significantly lower (M = 2.08, SD 0.66 nmol/l) than was the cortisol sample taken at the beginning of the session (M = 3.31, SD 1.1 nmol/l). Furthermore, this effect did not significantly interact with session (F(1,53) = 1.99; p = ns, η2 = 0.04). Finally, there was a trend towards an interaction among sex, session and sample (F(1,53) = 3.09; p = 0.08, η2 = 0.06), such that the interaction between sex and session was significant for the first sample of the session (F(1,53) = 4.05; p = 0.05, η2 = 0.07), but not for the second sample of the session (F(1,53) = .44; p = ns, η2 = 0.01). As expected, the main effects and interactions observed in the last ANOVA were also replicated here. That is, there were main effects of sex and session, as well as an interaction between sex and session. Together, these findings argue against the role of the ERP and behavioral laboratory session as a stressor and against a significant role of the laboratory session in the interactions between sex and cortisol. Indeed, the findings suggest a significant decrease in salivary cortisol level across the two hours of the typical session, consistent with the unperturbed diurnal cycle.
In order to explore the possibility that use of the contraceptive pill might be a relevant factor in the sex difference, a 2 × 2 mixed ANOVA for females was performed with oral contraceptive usage (on OC, off OC) as the between-subject factor and session (low examination period, high examination period) as the within-subject factor. Average cortisol concentration was the dependent variable. No significant main effects or interactions were significant.
Psychological stress measures. 2 × 2 × 2 mixed ANOVAs were also performed for the three psychological stress measures with counterbalancing group (A,B) and sex (female, male) as between-subject factors and with session (low examination period, high examination period) as the within-subject factor. State anxiety and perceived stress showed significant main effects of session, with higher levels of stress reported during the high examination session than during the low examination session (for state anxiety (F(1,53) = 7.68; p = 0.008, η2 = 0.12), for PSS (F(1,53) = 13.34; p = 0.001, η2 = 0.20)). Further, a trend in the same direction was observed for trait anxiety (F(1,52) = 3.50; p = 0.07, η2 = 0.06). No significant main effects or interactions were observed with either counterbalancing group or sex (see ).
Table I. The influence of examination stress session (low vs. high) on the stress measures: For all subjects and for females and males separately.
Overall, both psychological and hormonal stress measures provided evidence for elevations with the examination stress protocol. However, in the case of cortisol, evidence was observed for sex differences, such that while males showed the predicted elevation in cortisol with examination stress, the females did not.
Reactivity across different stress measures
Correlations were used to establish the extent to which elevations related to examination stress based on one stress measure were predictive of elevations based on another and whether these relationships are different in the two sexes.
All subjects. Pearson product moment (r) correlations were computed between percentage change values for each of the three psychological stress measures (STAI-Spc, STAI-Tpc and PSSpc) and the cortisol measures across the two sessions. Here, we were most interested in the extent to which psychological and hormonal reactivity were related. No significant correlations were observed (r < 0.1), save those among different psychological stress measures.
Correlations by sex. Pearson product moment (r) correlations were computed separately for the two sexes between percentage change values for each of the three psychological stress measures (STAI-Spc, STAI-Tpc and PSSpc) and the cortisol measure across the two sessions. Again, we were most interested in the extent to which psychological and cortisol responses were related to one another. No significant correlations were observed (r < 0.12), save those among different psychological stress measures. These and no other significant correlations, occurred for both sexes.
In conjunction with the reported ANOVA findings, these results suggest that while the examination stress protocol triggered elevations in both psychological stress measures and cortisol levels, these elevations were unrelated to one another.
Discussion
An examination stress protocol was used in order to investigate individual and group differences in psychological and hormonal responses to a naturally occurring stressor. Three main findings were observed. First, the examination protocol was effective in raising levels of both psychological stress and cortisol. Second, while no sex differences were found in psychological stress elevations, increases in salivary cortisol concentrations were observed in males but not in females. Third, elevations in cortisol level were not predictive of elevations in psychological measures of stress for either sex.
Elevations in stress measures with examination period
As expected, an increased number of examinations and deadlines (from zero during the low examination session to three or more during the high examination session) were associated with increases in all measures of stress. This finding is consistent with the previous examination stress studies both in terms of psychological (e.g. Francis Citation1979; Wolf et al. Citation1995) and hormonal responses (e.g. Frankenhaeuser et al. Citation1978; Lovallo et al. Citation1986; Malarkey et al. Citation1995; Lacey et al. Citation2000; Lucini et al. Citation2002; but see Wolf et al. Citation1995) and suggests the efficacy of examination stress in triggering elevations in a variety of measures of stress. This efficacy is particularly important given the increased external validity of such protocols over either laboratory stressors or exogenous administrations (Stowell Citation2003).
Sex differences in elevations in stress measures with examination period
While less dramatic than in previous studies, the present finding of greater cortisol reactivity in males is also consistent with an abundance of other studies showing greater hormonal response to stress in males than in females both in laboratory stress studies and in naturalistic stressor studies (see Kudielka et al. Citation2000; Kajantie and Phillips Citation2006). With regard to a proposed mechanism, previous studies have suggested that sex hormone levels may be critical to the effect. More specifically, it has been argued that the presence of cortisol reactivity in females may be dependent on menstrual cycle stage and therefore on estrogen and progesterone levels (e.g. Kirschbaum et al. Citation1999). While menstrual cycle stage was not assessed in the present study, no effect was found for the use of oral contraceptives by females. To the extent that oral contraceptive usage affects sex hormone levels, these findings suggest that the sex differences observed in the present study are not simply sex hormone-dependent. The extent to which menstrual cycle stage plays a role in cortisol responses to naturalistic, rather than laboratory stressors remains unclear and in need of further investigation. Furthermore, there is some question regarding the extent to which the sex difference observed in the present study would generalize across other environmental stressors (see Lundberg Citation1996; Lundberg and Frankenhaeuser Citation1999). While compelling, these findings, in conjunction with the fact that no sex differences were found on psychological measures of stress, suggest that future studies need to use a broader array of physiological markers and stressors in order to capture multiple aspects of the stress response in the two sexes.
Examination-related responses in cortisol are not associated with examination-related responses in psychological stress measures
In the present study, there were no significant correlations between elevations in cortisol and elevations in psychological stress measures. Surprisingly, few studies have investigated the extent to which reactivity in one domain is predictive of reactivity in another. Of those studies that have, results have been inconsistent. While a few studies have found significant positive correlations between psychological and hormonal measures of stress (Kemeny and Laudenslager Citation1999), others have found no significant correlations between these measures (Schommer et al. Citation1999, Citation2003; deQuervain et al. Citation2000; Vedhara et al. Citation2002; Roy Citation2004), or even negative correlations between these measures (Roy Citation2004).
One explanation for this inconsistency and the general weakness of the relationship between these two categories of stress measures is the nature of the stressor itself (Dickerson and Kemeny Citation2004). For instance, some studies used a laboratory stressor to investigate this relationship and therefore (as described above) may not have provided accurate measures of either the psychological or the hormonal reaction one would see in response to an actual life stressor (Schommer et al. Citation1999; Vedhara et al. Citation2000). More specifically, Dickerson and Kemeny (Citation2004) argue that a situation may be psychologically stressful, but would not be expected to trigger a significant increase in cortisol unless that situation was also uncontrollable or included an ego evaluation.
The fact that elevations in psychological measures of stress appear to be independent of elevations in cortisol suggests that other hormonal or biochemical mechanisms are responsible for subserving the psychological stress effects. Indeed, there is an abundance of evidence to suggest that the relationship between psychological and hormonal levels of stress is related to the specific hormonal measure used (Peters et al. Citation2003; Schommer et al. Citation2003). One hormone that may show closer correlations with psychological measures of stress is adrenaline. While adrenaline was not measured in the current study, previous studies have repeatedly confirmed the relationship between psychological stress, stressor exposure and adrenaline level (Kamarck and Lovallo Citation2003). Indeed, Schommer et al. (Citation2003) have recently shown that while cortisol responses habituate to repeated psychosocial stressors, adrenaline responses do not (Gerra et al. Citation2000).
Another possibility is that “objective” measures of stress, as assessed by questionnaire, are not a good proxy for subjective stress levels. As an example, a study by Lupien and McEwen (Citation1998) revealed no association between cortisol levels and various standardized questionnaires on stress but revealed a significant association between cortisol levels and single-item, subjective feelings of stress. Consequently, some caution has to be applied when interpreting results of stress questionnaires in relation to cortisol levels in humans.
Finally, males may have less subjective awareness of their internal states than do females. Given this model, one might expect significant correlations between psychological and hormonal levels of stress in females but not males. Indeed, in the present study, we found significant correlations between psychological and cortisol responses in neither sex. This suggests that whatever the explanation for the lack of a correlation between the two different types of stress measures used here, the explanation does not appear to be sex-specific.
Conclusions
The findings of the present study argue for the high efficacy of examinations in triggering a stress response and also in detecting group and individual differences in this response. While examination stress is a commonly used experimental manipulation in psychoneuroimmunology studies of stress (Stowell Citation2003), it is rarely used in cognitive neuroscience studies of stress. The present study focuses on demonstrating the efficacy of the use of examinations to increase both psychological and hormonal levels of stress. The rationale for the use of examination stress is that it has greater external validity than do either of the more common laboratory manipulations used in cognitive neuroscience studies, including the TSST and exogenous administrations of HPA axis-stimulating drugs.
Furthermore, sex differences in the psychological and hormonal stress responses remain a compelling and worthy topic of further investigation. While sex differences have been well investigated in studies using laboratory stressors (Kudielka et al. Citation2000, Kudielka and Kirschbaum Citation2005; Kajantie and Phillips Citation2006), few studies have investigated these sex differences in responses to examination stress. Furthermore, the mechanisms responsible for such effects remain elusive. This absence is critical for a variety of reasons. For example, one current debate in the cognitive neuroscience literature relates to how sex differences affect the relationship between stress and memory (e.g. Wolf et al. Citation2001; Andreano and Cahill Citation2006). While these findings are compelling, we believe that the use of more naturalistic stressors is an important addition to the field. Indeed, inclusion of such studies may be key in delineating the mechanisms that lead to stress-related health issues in a variety of populations such as students, parents and workers.
Acknowledgements
This research was supported by a grant from the National Science Foundation (NSF-BCS-0224069) awarded to N. Weekes and R. Lewis. We acknowledge Salimetrics, LLC for performing the biological assays and thank Dennis Chang, Ambereen Kurwa, Erin Mohr, Rebecca Sheehan-Stross, Elizabeth Volkmann and Tracy Wang for their assistance in conducting this study.
References
- Adreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci 2006; 17: 466–470
- Cohen S, Hamrick N. Stable individual difference in physiological response to stressors: Implications for stress-elicited changes in immune related health. Brain Behav Immun 2003; 17: 407–414
- Cohen S, Kamarck T, Mermelstein RA. A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress 2004; 7: 29–37
- deQuervain DJ, Roozendahl B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long term declarative memory in humans. Nat Neurosci 2000; 3: 313–314
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull 2004; 136: 355–391
- Ennis M, Kelly KS, Lambert PL. Sex differences in cortisol excretion during anticipation of a psychological stressor: Possible support for the tend-and-befriend hypothesis. Stress Health 2001; 17: 253–261
- Francis KT. Psychologic correlates of serum indicators of stress in man: A longitudinal study. Psychosom Med 1979; 41: 617–628
- Frankenhaeuser M, von Wright M, Collins A, vonWright J, Sedvall, Swahn C. sex differences in psychoneuroendocrine reactions to examination stress. Psychosom Med 1978; 40: 334–343
- Gerra G, Zaimovic A, Mascetti GG, Gardini S, Zambelli U, Timpano M, Raggi MA, Brambilla F. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology 2000; 26: 91–107
- Girdler S, Hinderliter AL, Light KC. Gender differences in blood pressure control during a variety of behavioral stressors. Psychosom Med 1990; 52: 571–591
- Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein–Barr virus in response to examination stress. Psychoneuroendocrinology 1994; 19: 765–772
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006; 31: 151–178
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosom Med 2003; 65: 9–21
- Kemeny ME, Laundenslager ML. Beyond stress: The role of individual difference factors in psychoneuroimmunology. Brain Behav Immun 1999; 13: 73–75
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrinology research: Recent developments and applications. Psychoneuroendocrinology 1994; 19: 313–333
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamic–pituitary–adrenal axis. Psychosom Med 1999; 61: 154–162
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis response to stress: A review. Biol Psychol 2005; 69: 113–132
- Kudielka BM, Hellhammer J, Hellhammer DH, Wolf OT, Pirke KM, Varadi E, Pilz J, Kirschbaum C. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J Clin Endocrinol Metab 1998; 83: 1756–1761
- Kudielka BM, Hellhammer DH, Kirschbaum C. Sex differences in human stress response. Encyclopedia of stress, Academic Press, New York 2000; Vol. 3
- Lacey K, Zaharia MD, Griffiths J, Ravindran AV, Merali Z, Anisman H. A prospective study of neuroendocrine and immune alterations associated with the stress of an oral academic examination among graduate students. Psychoneuroendocrinology 2000; 25: 339–356
- Lewis RS, Weekes NY, Wang THY. 2006. The relationship among a naturalistic stressor, frontal asymmetry, stress, and health, Manuscript submitted for publication.
- Lovallo WR, Pincomb GA, Edwards GL, Brackett DJ, Wilson MF. Work pressure and the type A behavior pattern in exam stress in male medical students. Psychosom Med 1986; 48: 125–133
- Lucini D, Norbiato G, Clerici M, Pagani M. Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension 2002; 39: 184–188
- Lundberg U. The influence of paid and unpaid work on psychophysiological stress responses of men and women. J Occup Health Psychol 1996; 1: 117–130
- Lundberg U, Frankenhaeuser M. Stress and workload of men and women in high ranking positions. J Occup Health Psychol 1999; 4: 142–151
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognitive: Integration of animal and human model studies. Brain Res Rev 1997; 24: 1–27
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998; 1: 69–73
- Malarkey WB, Pearl DK, Demers LM, Kiecolt-Glaser JK, Glaser R. Influence of academic stress and season on 24-hour mean cortisol concentration of ACTH, cortisol, and β-endorphin. Psychoneuroendocrinology 1995; 20: 499–508
- Mason JW. A review of psychoendocrine research on the pituitary–adrenal cortical system. Psychosom Med 1968; 30: 576–607
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol 1995; 5: 205–216
- Mirowsky J, Ross C. Sex differences in distress: Real or artifact?. Am Sociol Rev 1995; 60: 449–468
- Peters ML, Godart GLR, Ballieux RE, Heijnen CJ. Moderation of physiological stress responses by personality traits and daily hassles: Less flexibility of immune system responses. Biol Psychol 2003; 65: 21–48
- Roy MP. Patterns of cortisol reactivity to laboratory stress. Hormones Behav 2004; 46: 618–627
- Schommer NC, Kudielka BM, Hellhammer DH, Kirschbaum C. No evidence for a close relationship between personality traits and circadian cortisol rhythm or a single cortisol stress response. Psychol Rep 1999; 84: 840–842
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the HPA-axis system and the sympathetic adrenal medullary system to repeated psychosocial stress. Psychosom Med 2003; 65: 450–460
- Spangler G. Psychological and physiological responses during an exam and their relation to personality characteristics. Psychoneuroendocrinology 1997; 22: 423–441
- Spielberger CD. State–trait anxiety inventory. Palo Alto, Mind Garden 1983
- Stoney CM, Davis MC, Matthews KA. Sex differences in physiological responses to stress and coronary heart disease: A causal link?. Psychophysiology 1988; 24: 127–131
- Stowell JR. Use and abuse of academic examination in stress research. Psychosom Med 2003; 65: 1055–1057
- VanDoornen LJP. Sex differences in physiological reactions to real life stress and their relationship to psychological variables. Psychophysiology 1986; 23: 657–662
- Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology 2000; 25: 535–549
- Wolf TM, Heller SS, Camp CJ. The process of coping with a gross anatomy examination during the first year of medical school. Br J Med Psychol 1995; 68: 85–87
- Wolf TM, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 2001; 26: 711–720