?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Magnesium (Mg), green tea and rhodiola extracts have, in isolation, been shown to possess stress and anxiety relieving effects. Green tea and rhodiola have been shown to modulate EEG oscillatory brain activity associated with relaxation and stress perception. The combined capacity of these ingredients to confer protective effects under conditions of acute stress has yet to be examined. We tested the hypothesis that a combination of Mg (with B vitamins) + green tea + rhodiola would acutely moderate the effects of stress exposure.
Methods
A double blind, randomised, placebo controlled, parallel group design was employed (Clinicaltrials.gov:NCT03262376; 25/0817). One hundred moderately stressed adults received oral supplementation of either (i) Mg + B vitamins + green tea + rhodiola; (ii) Mg + B vitamins + rhodiola; (iii) Mg + B vitamins + green tea; or (iv) placebo. After supplementation participants were exposed to the Trier Social Stress Test. The effects of the study treatments on electroencephalogram (EEG) resting state alpha and theta, subjective state/mood, blood pressure, heart rate variability and salivary cortisol responses after acute stress exposure were assessed.
Results
The combined treatment significantly increased EEG resting state theta (p < .02) – considered indicative of a relaxed, alert state, attenuated subjective stress, anxiety and mood disturbance, and heightened subjective and autonomic arousal (p < .05).
Conclusions
Mg, B vitamins, rhodiola and green tea extracts are a promising combination of ingredients that may enhance coping capacity and offer protection from the negative effects of stress exposure.
Trial registration: ClinicalTrials.gov identifier: NCT03262376.
Introduction
Stress and anxiety are distinct, yet highly comorbid, affective states with the capacity to potentiate each other’s negative effects. Both have profound consequences for the quality of life of individuals and incur substantial social and economic costs for society. Stress, anxiety or depression accounted for 44% of all UK cases of work-related ill health and 54% of working days lost to illness in 2018/19 [Citation1] and 74% of UK respondents have felt ‘overwhelmed or unable to cope’ at some point in the past year [Citation2]. A global poll of 140 countries found over a third had experienced stress (35%) or worry (39%) ‘a lot of the day yesterday’ [Citation3].
Magnesium (Mg) has long been proposed to offer therapeutic action with evidence of mood stabilising effects (e.g. depression [Citation4], mania [Citation5]). Mg is a particularly relevant nutrient in the treatment of stress and anxiety since Mg status is closely aligned with stress levels: exposure to psychosocial stress increases Mg excretion, resulting in Mg deficiency [Citation6] which increases endocrine stress reactivity [Citation7], further depleting Mg levels. Dietary levels of Mg intake are also modestly inversely associated with subjective anxiety [Citation8]. Further, Mg supplementation can reduce anxiety-related symptomology in anxiety vulnerable populations (e.g. premenstrual syndrome) [Citation9]; particularly when administered with additional ingredients such a B vitamins which are also depleted by stress and associated with stress alleviation [Citation10,Citation11].
Rhodiola has been consumed for centuries to alleviate stress, anxiety and fatigue. Animal models demonstrate a significant stress-reducing capacity of rhodiola after acute and chronic stress [Citation12]. The efficacy of rhodiola in the reduction of stress and anxiety in humans has predominantly been demonstrated after chronic intake (e.g. 14 days [Citation13]). However, subjective stress alleviation is evident after 3 days intake [Citation14]. Further, acute rhodiola intake is proposed to increase stress coping capacity. For example, acute intake improves endurance and coping capacity under condition of physical stress [Citation15], and increases subjective vigour when faced with a stressor [Citation16]. Acute rhodiola intake has also been shown to attenuate cardiovascular responses (pulse pressure [Citation17]) to stress.
Epidemiological studies show an association between green tea consumption and reduced psychological distress [Citation18]. Examination of the constituent nutrients of green tea – specifically flavonoids (e.g. epigallocatechin gallate [EGCG]) and L-theanine – have demonstrated a range of functional benefits of acute consumption on subjective, physiological and cognitive states under conditions of acute stress provocation. These include increased subjective relaxation and calmness [Citation19], improved subjective mood [Citation20], and attenuated subjective stress [Citation19,Citation21,Citation22], anxiety [Citation22], cortisol [Citation21] and heart-rate [Citation22] responses.
Oscillatory brain activity measured by electroencephalogram (EEG) is an emerging marker for assessing the functional benefits of nutrient intake. Both green tea constituents and rhodiola have been demonstrated to acutely modulate oscillatory brain activity. Acute intake of L-theanine increases resting alpha oscillatory power – considered to be an index of an increased relaxed state without drowsiness [Citation23,Citation24]. Similarly, the green tea constituent EGCG increases alpha and theta oscillatory activity during a rested state, predominantly in the midline frontal and central brain regions [Citation19]; regions associated with a meditative, relaxed state. Acute rhodiola intake has been shown to increase theta band oscillatory brain activity during the viewing of emotionally stressful stimuli [Citation25] suggesting that rhodiola also has the capacity moderate neural responses in a stress context.
Existing evidence of the capacity of Mg, B vitamins, green tea and rhodiola to moderate the cascade of physiological and psychological responses activated under condition of stress creates a strong case to examine the efficacy of these ingredients administered in combination to offer synergistic functional benefits above those shown in isolation. The capacity of rhodiola and green tea to acutely affect stress responses suggests the synergistic contribution of these ingredients are most worthy of exploration. Further, given emerging evidence of the capacity of rhodiola and green tea to acutely modulate neurophysiological processes, the effects of the combined treatment on oscillatory EEG activity associated with a relaxed state is of primary interest. This randomised, placebo controlled trial assessed the functional efficacy of Mg (combined B vitamins), green tea and rhodiola administered in combination to acutely moderate the effects of laboratory stress exposure on EEG oscillatory brain activity during the rested state. The capacity of the combined treatment to attenuate the effects of stress exposure on subjective, autonomic and endocrine responses was also assessed. The fully combined treatment was compared to placebo, and Mg (+B vitamins) combined with either rhodiola or green tea in isolation. The combined treatment was expected to offer greater functional benefit above and beyond the comparison treatments under acute stress provocation.
Methods
Study design
The study conformed to a double blind, randomised, placebo controlled, parallel group design comprising 4 treatment arms: (i) COMBINED: Mg + vitamins B6, B9, B12 + green tea + rhodiola extract; (ii) GREEN TEA: Mg + vitamins B6, B9, B12 + green tea; (iii) RHODIOLA: Mg + vitamins B6, B9, B12 + rhodiola extract; (iv) PLACEBO. The study design and primary and secondary outcomes were pre-registered at Clinicaltrials.gov (NCT03262376; August 25th 2017).
Primary outcomes
The effect of treatment on EEG alpha and theta oscillatory brain activity during the rested state and during completion of attentional tasks under conditions of stress exposure. The EEG outcomes associated with cognitive task performance are reported separately elsewhere (manuscript in preparation).
Secondary outcomes
The effect of treatment on subjective stress, mood, anxiety, salivary cortisol, cardiovascular parameters (BP, HRV), cognitive performance and attentional event related potentials (ERPs). Cognitive performance and ERP’s results are reported elsewhere in the cognitive task related EEG paper (manuscript in preparation).
Participants
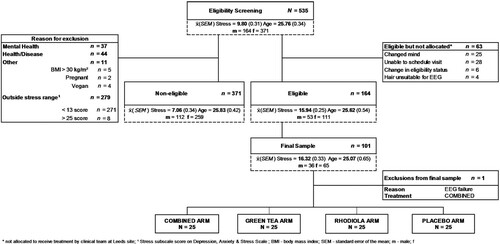
One hundred healthy adults (men = 36; women = 64) were recruited from the University of Leeds campus and local community. The final sample was selected from respondents completing an initial online self-report screening questionnaire (N = 535) to confirm the trial eligibility criteria (CONSORT diagram shown in ). The study inclusion and exclusion criteria are shown below:
Inclusion criteria
Reporting moderate subjective stress levels (score of ≥13 to ≤25 on the stress subscale of the Depression Anxiety and Stress Scale [DASS] [Citation26])
≥18 to ≤50 years of age (premenopausal if female indicated by self-reported lack of menopause symptoms or associated absence of period for 12 months+)
BMI ≥ 18 and ≤30 kg/m2
Healthy (free from significant physical or psychiatric disorders [self-report])
Exclusion criteria
Intake of prescribed medication except contraceptives
Hypertension (self-report or resting blood pressure >160/95 mmHg)
Intake of regular nutritional supplementation
Smoking > 5/day
Pregnant or lactating
Previous exposure to a laboratory stress protocol
Night-working/shift work
Recreational drug use
Previous brain surgery or severe brain injury requiring hospital treatment
Any form of neurological condition (e.g. Epilepsy)
Respondents fulfilling the eligibility criteria were invited to a face to face screening to confirm eligibility. Written informed consent was attained from all included participants. The study was approved by The University of Leeds School of Psychology Research Ethics Committee (17-0235; granted October 30th 2017) and conducted according to the principles of Good Clinical Practice and The Declaration of Helsinki. An honorarium of £60 was paid for completion of the study.
Treatments
Treatments were administered with water in a single oral dose comprising one tablet and 2 capsules. Mg (150 mg elemental) + vitamins B6 (0.7 mg), B9 (0.1 mg), B12 (0.00125 mg) were administered in a single pressed tablet form. The B vitamin combination was selected based on the association of these forms with the maintenance of brain function, and impairment in stress and mood related biochemistry (e.g. homocysteine balance, serotonin and catecholamine production) in cases of subclinical deficiency [Citation28]. A combined green tea/rhodiola extract formulation (Teadiola®; Sanofi-Aventis Groupe) was employed. For the purposes of blinding, the manufacturer was requested to split the formulation and the green tea (125 mg containing 40% L-theanine [50 mg]) and rhodiola (222 mg) treatments were administered in separate opaque capsules. Identical placebo equivalent forms (cellulose crystalline) were produced for all treatments. Consumption was witnessed and checked by the experimenter. All treatments were manufactured by an affiliate of Sanofi-Aventis Groupe.
Randomisation
The experimenter created 150 unique randomly generated 6 digit/string ID codes (www.random.org). Participants were sequentially allocated an ID code by the experimenter at the face to face screening and subsequently randomly allocated to treatment by an independent statistician. To ensure a balanced representation of age and gender across treatment arms, eligible participants were divided into six strata (male or female and (i) 18–30, (ii) 31–40, or (iii) 41–50 years old). An adaptive randomisation scheme was employed that used the existing balance of the covariates gender and age group across treatment arms to balance the randomisation of subsequent participants to treatment [Citation29].
Blinding
Four treatment codes were generated by an independent technician at Sanofi-Aventis. The independent statistician employed the blinded treatment codes to allocate participants to treatment arms. To ensure the experimenters were blind to the treatment codes, one member of the Leeds research team, not involved in experimental testing, liaised with the statistician to prepare the treatments for administration at the Leeds site. These treatments were identifiable to the experimenter only by the packing number. Treatments were administered in identical capsule forms to ensure participants were also blind to treatment.
The Leeds research team and statistician remained blinded until the data were locked and primary statistical analysis completed. The treatments codes were broken to complete the a priori treatment comparisons as per the fixed hierarchical statistical analysis approach adopted (described in the statistical analysis section below).
Measures
EEG methodology
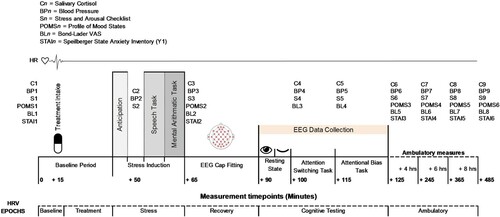
EEG was recorded continuously with a BioSemi ActiveTwo EEG system (BioSemi©, Amsterdam) from 64 electrodes at a 1024 Hz sampling rate. Four electrooculograms (EOG) electrodes were used to record eye movements and were placed lateral and dorsal to each eye. Two EOGs were placed on the mastoids. EEG recordings comprised two separate blocks: resting state eyes open (290s), resting state eyes closed (290s). Tasks were completed in sequential order in one session.
Prior to analysis, EEG data were pre-processed in Matlab using both EEGLAB [Citation30] and Fieldtrip [Citation31]. Data were initially down sampled to 512 Hz. All participants’ data were checked by eye and exceptionally bad channels removed. Continuous data was then re-referenced to the average of all 64 channels (with exception of removed bad channels) and data destined for spectral analysis were high-pass finite impulse response (FIR) filtered using 1 Hz cut-off. Independent component analysis (ICA) [Citation32] was used to identify eye-movement components in the data which were subsequently removed by hand. Resting state data were segmented into epochs between −0.1 and 1.8 s (no baseline period).
After defining epochs a Matlab implementation of the Fully Automated Statistical Thresholding for EEG Artefact Rejection (FASTER [Citation33]) was used to remove artefacts in the data. This included (1) thresholded interpolation of bad channels via identification of global and local artefacts in channel data and (2) removal of bad trials on the basis of remaining eye and muscle movement noise and voltage spikes. Spectral power was estimated across each epoch in the range of 2–40 Hz in 0.1 Hz steps using a Hann taper with a variable time window (four cycles at each frequency). Frequencies of specific interest – alpha (8–12 Hz) and theta (4–7 Hz) – were extracted for statistical analysis. Resting State (eyes open and closed) Alpha and theta values were extracted from 0.6 to 1.2 s within each epoch and averaged to give a mean resting state value.
Subjective measures
Stress and arousal checklist (SACL) [Citation34] is a 30-item adjective checklist measuring self-reported stress (e.g. ‘apprehensive’; possible range 18–72) and arousal (e.g. ‘stimulated’; 12–48). Responses were made on a four-point Likert scale. The long scoring method was employed [Citation34] and alternative versions administered at each time point.
The profile of mood states – short form (POMS-SF) [Citation35] is a 37-item adjective checklist measuring six affective mood states: Tension-anxiety (possible range 0–24), Depression-dejection (0–32), Anger-hostility (0–28), Vigour-activity (0–24), Fatigue-inertia (0–20), Confusion-bewilderment (0–20); and Total Mood Disturbance Score (TMD score [0–124] which is the sum of all subscales minus Vigour-activity).
Bond-lader mood scale [Citation36] comprises 16 100 mm visual analogue scales (VAS) of three mood scores: alertness, contentedness, and calmness. The VAS are anchored by the antonyms of specific subjective states (e.g. drowsy/alert).
Spielberger state-trait anxiety inventory (STAI) [Citation21]. The STAI trait subscale STAIY2 was completed at screening to control for dispositional anxiety by including this as a covariate in statistical analyses. The state subscale (STAI-Y1) was administered to assess transitory subjective anxiety levels during the study protocol.
Cortisol
Cortisol was determined from saliva collected using a Salivette® device (Sarstedt, Nümbrecht, Germany). Saliva was extracted from the Salivette by centrifugation (2500 rpm, 5 min) and frozen at −20°C until assay. Salivary cortisol levels were assayed by an independent laboratory (Daacro’s Saliva Lab Trier, Germany) using a high sensitivity salivary cortisol competitive immunoassay kit (Salimetrics, LLC, U.S.A.). After assay completion, optical density was read on a BioTeK ELx808 microplate reader at 450 nm (with correction at 490 nm). The intra- and inter-assay CVs were 3.54% and 4.39% respectively.
Blood pressure (BP)
Systolic and diastolic BP was measured using a Spacelabs 90207 (Spacelabs Medical Inc., U.S.A.) automated oscillometric upper arm ambulatory BP monitor (ABPm). The monitor was worn throughout the test protocol.
Heart rate variability (HRV)
RR interval data were recorded using a V800 Polar heart rate monitor (HRM) with a Polar H10 chest strap (Polar®, Stockholm, Sweden) at a sampling frequency of 1000 Hz. HRV parameters were determined using the Kubios HRV Premium software (Ver. 3.1.0; Kubios Oy, Kuopio, Finland). Artefacts were detected by the Kubios automatic correction algorithm [Citation37] and detrended using the smoothness priors method ([Citation38]; λ = 500). Baseline HRV data were recorded whilst seated. HRV data were epoched and averaged into 6 time periods (shown in relation to procedural timeline in ): Time and frequency domain outcomes were produced for each epoch. Low (0.04–0.15 Hz), and high (HF, 0.15–0.4 Hz) frequency bands were calculated using Fourier transformation.
Stress protocol
Acute stress was experimentally induced by The Trier Social Stress Test (TSST). The TSST is an extensively validated and standardised paradigm [Citation39]. Briefly, participants gave an extemporaneous 5-minute speech presenting themselves as a job candidate to an unresponsive, social-evaluative panel of two female confederates. Upon completion of the speech, participants completed a 5-minute mathematics subtraction task in front of the panel.
Procedure
The study was undertaken at the Human Appetite Research Unit (HARU), University of Leeds, UK. Testing was restricted to between 1100 and 1500 h to avoid the cortisol awakening response and ensure comparable diurnal cortisol levels. Female participants were tested during the luteal phase to account for variation in cortisol responsivity during the menstrual cycle. Participants fasted one hour and avoided alcohol and vigorous exercise for 24 h prior to study commencement. The procedural timeline is shown in . Upon arrival participants were fitted with the chest strap and HRV monitor and ABPm and seated in a testing cubicle to complete baseline measures (see ). After 15 min the allocated treatment was administered. Participants relaxed in the cubicle for 30 min before being taken to a separate room and introduced to the TSST. Participants returned to the testing cubicle for a 5 min anticipation period. After TSST completion participants were taken to the EEG laboratory for fitting of the BioSemi ActiveTwo system. Once a clear signal was established, participants completed two resting EEG recordings, one eyes open and one eyes closed lasting five minutes each. Participants were instructed to sit as still as possible and limit eye blinks and facial movements. An attention switching task and attentional bias task were then undertaken in sequential order. Upon completion of tasks the EEG cap was removed and participants were free to leave the laboratory. Instructions for collecting ambulatory measures at 3 intervals of 2 h from the time of leaving were provided. Participants were instructed to avoid exercise and alcohol during the collection period. All measures were returned the following day.
Statistical analyses
The primary outcomes were alpha and theta oscillatory brain activities during the rested stated. The sample size was justified upon significant modification of EEG alpha activity following intake of a constituent of green tea. L-theanine has been demonstrated to induce significant changes in EEG resting state and attentional performance related alpha power [Citation23,Citation40–42]. The effect sizes of L-theanine on EEG alpha activity in these studies ranged from 0.803 to 1.141 (Cohen’s d). Assuming an effect size of 0.9, α = 0.05, and a power of 80%, it was estimated that a sample of 25 participants per arm (total sample size = 100) was required to detect an effect of this magnitude. The primary analyses were performed in the randomised population, defined as all randomised subjects who received at least one dose of the treatment and for whom a reliable EEG signal could be obtained.
All statistical analyses were performed using SAS (Statistical Analysis System, Version 9.2; SAS Institute, Inc., Cary, NC). Residual plots were inspected for deviations from normality. Cortisol data were skewed and normalised using logarithmic transformations. One participant was removed and replaced due to a fault in the EEG system. Eight participants’ HRV data were removed from the ambulatory epoch due to recorded data being less than the 6 hrs required (exclusions: COMBINED: n = 2; RHODIOLA: n = 1; GREEN TEA: n = 1; PLACEBO: n = 4). LSMeans and SEM are presented for primary and secondary outcomes.
A mixed-effects ANCOVA was employed with treatment and gender entered as fixed model effects and participant ID entered as a random effect. For repeated measures outcomes, values at time 1 (HRV: Baseline epoch) were entered as a covariate in analyses to control for baseline effects. Age, gender, BMI, trait anxiety (STAIY2), and DASS stress score were entered as covariates in all models and removed or retained based on the Akaike Information Criterion (AIC). To control the family-wise type 1 error rate, a fixed sequence testing procedure (six-steps) was used to assess the effect of each treatment arm within each primary outcome. The six-step sequence adhered to the following order: (1) COMBINED vs. PLACEBO; (2) COMBINED vs. RHODIOLA; (3) GREEN TEA vs. PLACEBO; (4) RHODIOLA vs. PLACEBO; (5) COMBINED vs. GREEN TEA; (6) RHODIOLA vs. GREEN TEA. The significance at a level of 0.05 was tested only if the results of the previous step reached significance. Secondary endpoints related to the secondary objectives were also tested using the same fixed sequence procedure. For repeated measures secondary outcomes, significant treatment x time interactions necessitated the analysis of outcomes by time point. If a comparison in the six-sequence procedure reached significance at a particular time point on an outcome, the corresponding level of comparisons is reported for all time points on this outcome. Exploratory (post hoc) analyses of the comparisons not reached in the fixed sequence due to non-significance of previous steps are supplied as supplementary materials and briefly summarised in the discussion for exploratory purposes only and to aid future investigation of the ingredients.
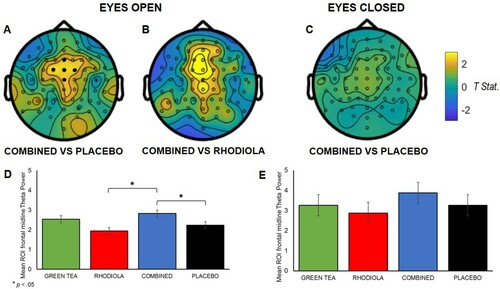
For analyses of primary EEG outcomes during the rested state two approaches were adopted. The effect of L-theanine on resting Alpha shows diffuse activation across multiple neural regions [Citation23]. Therefore a whole brain approach was employed to capture this activity. An independent sample t-test (Monte Carlo approach 500 random permutations for all electrodes) was employed in Fieldtrip [Citation31] to compare oscillatory Alpha power magnitude (µV) outcome differences between the treatments following the six-step fixed sequence. An a priori ROI approach was employed to examine resting state theta; specifically, theta activity in the frontal midline region (comprising channels: ‘Cz’, ‘Fz’, ‘FCz’, ‘FC1’, ‘FC2’ indicated in (A)) which is associated with a focussed, relaxed state and anxiolytic action [Citation43]. Power values (µV) were extracted from these electrodes, averaged, and analysed by mixed effect ANCOVA as per the procedure outlined above.
Results
A descriptive summary of the final sample is shown in . One way ANOVAs revealed no significant differences between the study treatments across the participant characteristics of Age (F(3,96) = 2.318, p = .08), BMI (F = <1), STAI-Y2 ([trait anxiety]; F(3,96) = 2.185, p = .10), and DASS stress score (F = <1).
Table 1. Summary characteristics of the sample receiving each treatment.
Primary outcomes
Resting state EEG
Resting alpha
No significant whole brain comparison differences were observed in Alpha band activity between COMBINED and PLACEBO treatments during the eyes open and eyes closed resting state (p >.05; spectral maps are shown in supplementary materials). Accordingly, no further comparisons are reported.
Resting theta
Frontal midline theta (Fmθ) ROI analysis revealed intake of the COMBINED treatment significantly increased theta power vs. PLACEBO, t(96) = 2.3; p = .02; (A), and vs. RHODIOLA, t(96) = 3.44; p = .001, in the eyes open rested state ((B)). No significant difference in theta power was revealed between GREEN TEA and PLACEBO treatments in the eyes open rested state and between the COMBINED and PLACEBO treatments in the eyes closed condition ((C)). Accordingly, no further comparisons are reported.
Figure 3. Maps depicting comparison of resting state theta power by treatment (maps A, B, and C; positive values indicate COMBINED > effect in comparison). ROI midline frontal electrodes shown by • in A. (A): t-Statistic for comparison of theta band activity for eyes open COMBINED vs. PLACEBO; (B): and eyes open COMBINED vs. RHODIOLA; (C): t-Statistic for comparison of theta band activity for eyes closed COMBINED vs. PLACEBO. (D): Mean averaged ROI (SEM) midline frontal theta power eyes open; (E) and closed by treatment.
Secondary outcomes
Stress and arousal checklist (SACL)
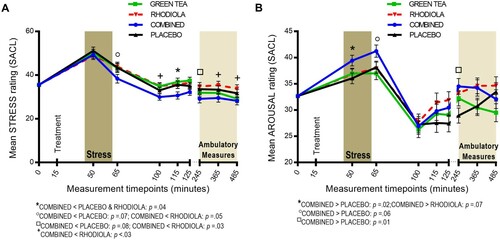
Stress: The COMBINED treatment significantly attenuated subjective stress vs. PLACEBO during the recovery period (+115 min; t(90)= −2.11; p = .04; (A)) and by trend after stressor cessation (+65 min; t(90)= −1.83; p = .07) and during the ambulatory period (+245 min: t(89)= −1.74; p = .08). The COMBINED treatment also significantly attenuated stress vs. RHODIOLA across the response profile: +65 min: t(90)= −2.01; p = .05; +100 min: t(90)= −2.47; p = .02; +115 min: t(90)= −2.12; p = .04; +245 min: t(89)= −2.23; p = .03; +365 min: t(88)= −2.25; p = .03; and +485 min: t(87)= −2.23; p = .03. GREEN TEA did not significantly differ from PLACEBO at these time points. Accordingly, no further comparisons are reported.
Arousal: The COMBINED treatment significantly increased subjective arousal in anticipation of stress vs. PLACEBO (+50 min, t(91) = 2.38; p = .02; (B)). Heightened arousal in the COMBINED treatment was maintained after stressor cessation vs. PLACEBO by trend (+65 min, t(91) = 1.87; p = .06) and significantly reactivated during the ambulatory period (+245 min, t(90) = 2.7; p = .01). COMBINED did not significantly differ from RHODIOLA at these time points. Accordingly, no further comparisons are reported.
Profile of mood states (POMS)
POMS subscale figures are shown in .
Figure 5. POMS subscale response profiles: (A) Tension-Anxiety; (B) Depression-Dejection; (C) Anger-Hostility; (D) Vigour-Activity; (E) Fatigue-Inertia; (F) Total Mood Disturbance score.

Tension-Anxiety: The COMBINED treatment significantly attenuated tension-anxiety during the ambulatory period vs. PLACEBO at +245 min (t(88)= −2.32; p = .02) and by trend at +485 min (t(87)= −1.89; p = .06). The COMBINED treatment also significantly attenuated tension-anxiety vs. RHODIOLA across the response profile: +125 min: t(89)= −2.02; p = .05; +245 min: t(88)= −3.23; p = .002; +365 min: t(88)= −2.1; p = .04; +485 min: t(87)= −2.16; p = .03. GREEN TEA did not significantly differ from PLACEBO at these time points. Accordingly, no further comparisons are reported.
Depression-Dejection: The COMBINED treatment significantly reduced depression-dejection vs. PLACEBO at +245 min (t(89)= −2.86; p = .01), and vs. RHODIOLA at +125 min (t(90)= −2.61; p = .01), and +245 min (t(89)= −2.23; p = .03). GREEN TEA did not significantly differ from PLACEBO at these time points. Accordingly, no further comparisons are reported.
Anger-Hostility: The COMBINED treatment significantly attenuated anger-hostility vs. PLACEBO (t(90)= −3.05; p = .003) and RHODIOLA (t(90)= −2.7; p = .03) at +245 min. GREEN TEA initially significantly increased anger-hostility at +65 min (t(91) = 2.41; p = .02), then attenuated ratings at +245 min (t(90)= −2.44; p = .02) vs. PLACEBO. RHODIOLA did not significantly differ from PLACEBO at this time point. Accordingly, no further comparisons are reported.
Vigour-Activity: The COMBINED treatment significantly increased vigour-activity vs. PLACEBO (t(91) = 2.47; p = .02) and RHODIOLA (t(91) = 2.89; p = .005) after stressor cessation (+65 min). GREEN TEA did not significantly differ from PLACEBO at this time point. Accordingly, no further comparisons are reported.
Fatigue-Inertia: The COMBINED treatment significantly attenuated fatigue-inertia vs. PLACEBO during the ambulatory period at +245 min (t(90)= −2.33; p = .02) and +365 min (t(90)= −1.99; p = .05). COMBINED did not significantly differ from RHODIOLA at these time points. Accordingly, no further comparisons are reported.
Confusion-Bewilderment: No significant differences between COMBINED vs. PLACEBO. Accordingly, no further comparisons are reported.
Total Mood Disturbance score: The COMBINED treatment significantly attenuated TMD score during the ambulatory period at +245 min vs. PLACEBO (t(89)= −3.21; p = .002) and RHODIOLA (t(89)= −2.3; p = .02). GREEN TEA did not significantly differ from PLACEBO at this time point. Accordingly, no further comparisons are reported.
Bond-lader mood scale
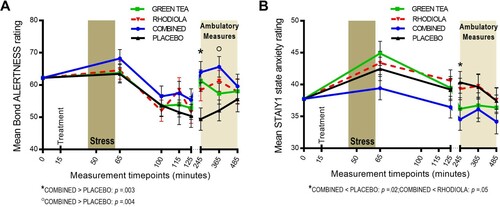
The COMBINED treatment significantly increased subjective alertness vs. PLACEBO during the ambulatory period at +245 min (t(89) = 3.05; p = .003) and +365 min (t(90) = 2.99; p = .004; (A)). COMBINED did not significantly differ from RHODIOLA at these time points. No significant differences between COMBINED and PLACEBO were revealed for contentedness or calmness VAS scales. Accordingly, no further comparisons are reported.
Spielberger state-trait anxiety inventory (STAI – Y1)
The COMBINED treatment significantly attenuated subjective anxiety vs. PLACEBO (t(88) = −2.43; p = .02) and RHODIOLA (t(88)= −1.99; p = .05) during the ambulatory period at +245 min ((B)). GREEN TEA did not significantly differ from PLACEBO at this time point. Accordingly, no further comparisons are reported.
Salivary cortisol
No significant differences between COMBINED vs. PLACEBO. Accordingly, no further comparisons are reported. The salivary cortisol response profile is shown in supplementary materials.
Blood pressure
The COMBINED treatment significantly raised diastolic BP during the ambulatory period at +485 min ( = 75.03, SEM = 1.55 mmHg) vs. PLACEBO (
= 69.33, SEM = 1.66 mmHg, t(86) = 2.52; p = .01). The COMBINED treatment did not significantly differ from RHODIOLA at this time point. No significant differences between COMBINED and PLACEBO were revealed for systolic BP response. Accordingly, no further comparisons are reported. BP response profiles are shown in supplementary materials.
Heart rate variability
The COMBINED treatment ( = 2.81, SEM = 0.22) significantly raised the Low Frequency:High Frequency ratio (LF:HF) during the recovery HRV epoch vs. PLACEBO (
= 2.09, SEM = 0.22, t(91) = 2.37; p = .02). LF:HF was also significantly raised in COMBINED vs. RHODIOLA during the stress (
= 3.80, SEM = 0.22 vs.
= 3.00, SEM = 0.22, t(91) = 2.56; p = .01) and recovery (
= 2.81, SEM = 0.22 vs.
= 1.85, SEM = 0.22, t(91) = 3.14; p = .002) HRV epochs. GREEN TEA did not significantly differ from PLACEBO at these time points. No significant differences were revealed between COMBINED and PLACEBO treatments for Mean RR, SDNN, Mean Heart Rate (HR), Mean SDHR or Low and High Frequency Power (ms2). Accordingly, no further comparisons are reported.
Discussion
To determine the capacity of bioactive ingredients to confer stress and anxiety relieving effects the effects of these ingredients need to be examined under the conditions of stress. Whilst the COMBINED treatment did not significantly moderate the salivary cortisol response or attenuate cardiovascular response to stress, despite existing evidence of the capacity of these ingredients to do so (e.g. [Citation21,Citation27]), elevations in both salivary cortisol and BP across all treatments confirmed that the TSST successfully established a stress context. Under these conditions of acute stress the COMBINED treatment demonstrated significantly superior functional capacity across a number of primary and secondary outcomes.
The COMBINED treatment significantly increased Fmθ during the eyes open resting state compared to both PLACEBO and RHODIOLA treatments. Increased theta activity in the frontal midline region is associated with relaxation and modulation of anxious states. Increases in Fmθ are shown during sustained internalised attention or meditation [Citation44], controlled breathing [Citation45] and biofeedback [Citation46] relaxation. Further, Fmθ activation is positively associated with level of meditation expertise [Citation44]. Fmθ activation is also an established neural effect of anxiolytic pharmaceuticals – GABA-A and 5-HT1A receptor specific anxiolytics increase Fmθ [Citation43] – and is associated with reduced symptomology in clinical samples [Citation43]. These data suggest the COMBINED treatment elicited a focussed and relaxed state under conditions of stress. This effect may be reflective of an anxiety reducing capacity of the COMBINED treatment. Alternatively, this may also indicate a protective capacity against the impairing effect of stress since stress is known to decrease frontal theta activity [Citation47]. Post hoc analyses revealed GREEN TEA significantly increased eyes open Fmθ power vs. RHODIOLA. Further, the COMBINED treatment was not significantly superior to GREEN TEA. This suggests a greater contribution of green tea over rhodiola to the heightened Fmθ observed in the COMBINED treatment. However, GREEN TEA was not superior to PLACEBO so the combination of the ingredients appears crucial.
Heightened resting state Fmθ in the COMBINED treatment did not retain significance in the eyes closed condition. Eyes closed and open resting state measures are commonly used interchangeably or combined as a unified measure. However, increasing evidence suggests the two states should be considered in isolation [Citation48]. The exteroceptive and interoceptive mental state hypothesis [Citation49] considers the eyes open state reflective of attentional and visual motor activity and the eyes closed state of imagination and multisensory activity [Citation48]. Therefore, the capacity of COMBINED treatment to influence neural state may be specific to attentional ‘online’ activity and reflective of a relaxed, yet attentionally focussed, state.
The COMBINED treatment did not moderate resting state alpha activity despite containing equivalent doses of green tea constituents previously shown increase alpha activation [Citation23,Citation40]. Post hoc analyses revealed no significant effects on resting state Alpha across any treatment comparisons. The reason for the failure to replicate these findings is not clear. One possibility is the stress context in which the treatments were examined; this is the only study to examine the rested state after stress induction. Further, green tea was not given in isolation therefore the interaction of the ingredients may also have negated the effects previously reported.
The COMBINED treatment was efficacious in the alleviation of subjective states associated with stress perception (SACL-stress, POMS-tension-anxiety) compared to PLACEBO and RHODIOLA treatments. The COMBINED treatment also significantly reduced subjective anxiety compared to PLACEBO and RHODIOLA during the ambulatory period, with lower ratings across the response profile reaching significance at +245 min. The capacity of the COMBINED treatment to attenuate stress perception and alleviate anxiety indicates a potential to enhance coping when faced with acute stress. Further, post hoc analyses show the superiority of the COMBINED treatment vs. GREEN TEA for stress and anxiety alleviation. This suggests green tea and rhodiola have capacity to affect subjective state when taken together rather than in isolation.
The COMBINED treatment increased indices of arousal consistently across a number of measures both acutely and temporally removed from stress induction. SACL arousal and POMS vigour rating in anticipation of, and immediately post-stressor, were significantly heightened in the COMBINED treatment compared to PLACEBO. Both arousal related subscales are primarily measures of energetic arousal exemplified by the adjectives: ‘activated’, ‘vigorous’, ‘energetic’, and therefore can be considered evidence of an invigorating or energising effect of treatment in the face of a stressful challenge. Further, the COMBINED treatment restored arousal levels during the ambulatory period (SACL arousal; +245 min) compared to PLACEBO. This is supported further by significantly reduced POMS fatigue ratings and increased attentional alertness (Bond-Lader VAS) compared to PLACEBO across the ambulatory period. This demonstrates the capacity of the COMBINED treatment to maintain subjective levels of arousal and alertness up to 5 hrs after stress cessation following a demanding testing session.
Post hoc analyses also indicated significantly heightened arousal in the COMBINED compared to the GREEN TEA treatment. Further, RHODIOLA significantly increased arousal towards the end of the response profiles vs. PLACEBO and GREEN TEA which indicates a potential greater contribution of rhodiola to the arousing effects of the COMBINED treatment. However, RHODIOLA and GREEN TEA were both superior to PLACEBO on the Bond-Lader Alertness subscale during the ambulatory period suggesting a contribution of both ingredients to the superiority of the COMBINED treatment.
Further evidence of the capacity of the COMBINED treatment to impact arousal was shown by a significantly increased HRV LF:HF ratio compared to PLACEBO and RHODIOLA treatments during stress period. The LF:HF ratio is considered to reflect sympathovagal balance, with an increased LF:HF ratio indicative of increased autonomic response. The LF:HF ratio increase is suggestive of a heightened sympathovagal arousal state and temporally corresponds with heightened subjective arousal outcomes during stress exposure. The isolated heightened diastolic BP reading in COMBINED vs. PLACEBO treatment 7hrs after stress cessation was not corroborated by any alteration in the more reactive systolic BP. This isolated finding is suggestive of a measurement artefact resultant from participants self-collecting data at this time point. Furthermore, the level of diastolic BP reached in the COMBINED condition kept within the normal, healthy diastolic BP range.
The COMBINED treatment demonstrated significant protective effects on mood under stress conditions. POMS depressive mood and anger ratings were both significantly reduced in the COMBINED treatment compared to PLACEBO and RHODIOLA towards the end of the laboratory visit and into the ambulatory period. This capacity to offer protection from the effects of stress on mood in the hours after stress exposure is further supported by significantly attenuated overall mood disturbance (POMS TMD) during the ambulatory period. Many of the enhanced subjective mood effects in the COMBINED treatment reached significance during the later stages of the response profiles. This suggests a temporal effect with the greatest functional mood benefits of the COMBINED treatment emerging up to 1 hr after stress and effects evident up to 5 and 7 hrs after stress exposure. Post hoc analyses showed the superiority of the COMBINED treatment over PLACEBO and RHODIOLA in the attenuation of POMS negative mood states was also shown vs. GREEN TEA; particularly in the early stress recovery period when GREEN TEA appeared to increase POMS negative mood states. This was also evident for subjective contentedness (Bond-Lader VAS) which was significantly heightened by the COMBINED treatment vs. GREEN TEA immediately after stress cessation (+65 min).
A number of potential mechanistic pathways have been described which may account for the relationship between the examined ingredients and observed effects. For example, animal models show that Mg reduces neuronal hyperexcitability by inhibiting NMDA receptor activity and plays a pivotal role in the function of the GABA(γ-aminobutyric acid)ergic system [Citation50,Citation51]; GABA is the main inhibitory neurotransmitter in the mammalian brain. Constituents of green tea and rhodiola also influence the production and activity of neural GABA, and additionally, serotonin, and dopamine [Citation12,Citation52]. The effects of the COMBINED treatment on subjective measures were elicited in the absence of any consistent activation of physiological markers of stress (e.g. cortisol, BP). This is not surprising since psychophysiological correspondence between acute stress responses is often weak [Citation53]. Further, evidence of potential brain neurochemistry mechanistic pathways suggest stress-reducing effect can occur via direct routes of action rather than being the indirect product of attenuated physiological responses.
Limitations and future directions
The establishment of baseline and post-treatment Mg, B vitamins, and relevant green tea and rhodiola constituent levels would have provided further insight into the observed effects. The collection of relevant biochemical nutritional markers to assess baseline levels of supplemented nutritional ingredients would have been the gold standard approach to establish this accurately. However, the number of assays that would be required was prohibitive in an RCT of this size and would require venepuncture; which itself can act as a stressor. Future studies of the observed effects should consider recording daily food intake prior to participation to assess any impact of basal dietary status.
The exploratory nature of the study, and existing evidence of the diverse effects of the ingredients on stress responses, informed the choice to examine a full spectrum of stress response outcomes assessed via several measures. Interest in the potential contribution of specific individual ingredients, often neglected in the literature, also informed the administration of multiple treatments to gain greater insight into the potential of the combined ingredients to confer functional benefit. However, these approaches increased the complexity of the findings reported. Significant findings arising from this study should be used in future examination of these ingredients to inform more focussed hypothesis generation and in the prioritising and planning of comparisons to reduce the number of outcomes and simplify the results reported.
Conclusions
This double-blind, placebo-controlled trial examined the capacity for a combination of ingredients shown to have stress and anxiety reducing potential in isolation to confer synergistic benefits when combined. In a moderately stressed population, the combining of Mg and B6, B9, B12 vitamins with selected rhodiola and green tea extracts conferred significant functional benefits when faced with an acute stressor compared to a placebo and the non-fully combined treatment. The combined treatment promoted a relaxed, focussed state – as indexed by EEG –, reduced stress perception, and increased energetic arousal in anticipation and in the immediate recovery from stress exposure. The combined treatment was also superior in the attenuation of stress perception across the later recovery profile and up to 7 h post stress, and offered protection from negative mood disturbance, reduced subjective anxiety, and heightened subjective energetic arousal and alertness between 2 and 7 h after stress exposure. Mg, B vitamins, rhodiola and green tea extracts therefore represents a promising combination of ingredients that may enhance coping capacity and offer protection from the negative effects of stress exposure that is worthy of further investigation. If confirmed, there is a significant practical benefit of a non-pharmaceutical method of reducing the negative impact of stress considering the associated profound detriment to the quality of life of individuals and substantial social and economic societal costs.
Supplemental Material
Download MS Word (565.3 KB)Acknowledgements
The authors express their gratitude to Lionel Noah and Etienne Pouteau (Sanofi) for assistance in the preparation of this manuscript. The statistical advice of Frits Quadt and Beatrice Bois-de-Fer (Sanofi) is also gratefully appreciated. The authors acknowledge the contribution of Fiona Croden (HARU lab manager) and stress panel confederates to conducting the trial and all the participants that generously gave their time.
Disclosure statement
No potential conflict of interest was reported by the author(s). Louise Dye has received consultancy and honoraria for work in the area of stress from Sanofi.
Data availability statement
Due to its proprietary nature the study data cannot be made openly available.
Additional information
Funding
Notes on contributors
Neil Bernard Boyle
Dr Neil Boyle is a Research Fellow in the School of Psychology, University of Leeds. Dr Boyle's research interests include the relationship between diet, stress and health.
Jac Billington
Dr Jac Billington is an Associate Professor in the School of Psychology, University of Leeds. Dr Billington's areas of interest include neuroscience; perception; action; motor control; DCD; vision; driving; looming; collision; pain; and multisensory.
Clare Lawton
Dr Clare Lawton is an Associate Professor in biopsychology in the School of Psychology, University of Leeds. Dr Lawton's research interests include appetite; satiety; body weight; wellbeing; cognition; diet; obesity; overweight; glucoregulation; stress; breakfast; food choice; eating behaviour; cognition; memory; attention; mood; and functional foods.
Frits Quadt
Dr Frits Quadt is an independent statistician consultant with 30 years experience of the use of advanced statistical methodologies in food industry related research.
Louise Dye
Dr Louise Dye is the N8 Chair and Professor of Nutrition and Behaviour in the University of Leeds Human Appetite Research Unit, based in the School of Psychology, University of Leeds. She is also an academic lead for the N8 Agrifood Programme at the University of Leeds. Professor Dye's research interests include nutrition and cognitive function across the lifespan; systematic reviews of food and food components on cognition; stress; breakfast & cognition/academic outcomes; digestive function; wellbeing.
References
- Health & Safety Executive. Work-related stress, anxiety or depression statistics in Great Britain, 2019. UK GOV: London; 2019; https://www.hse.gov.uk/statistics/causdis/stress.pdf
- Mental Health Foundation. Stress: are we coping? London: Mental Health Foundation; 2018; https://www.mentalhealth.org.uk/publications/stress-are-we-coping
- Gallup. Global emotions report. Washington, DC; 2019; https://www.gallup.com/analytics/248909/gallup-2019-global-emotions-report-pdf.aspx
- Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypoth. 2006;67:362–370. doi:10.1016/j.mehy.2006.01.047.
- Pavlinac D, Langer R, Lenhard L, Deftos L. Magnesium in affective-disorders. Biol Psychiatry. 1979;14:657–661.
- Grases G. ,Pérez-Castelló A, Sanchis P, Casero A, Perelló J, Isern B, Rigo E, Grases F. Anxiety and stress among science students. study of calcium and magnesium alterations. Magnes Res. 2006;19:102–106.
- Durlach J, Bac P, Bara M, Guiet-Bara A. Physiopathology of symptomatic and latent forms of central nervous hyperexcitability due to magnesium deficiency: a current general scheme. Magnes Res 2000;13:293–302.
- Jacka FN. Overland S, Stewart R, Tell GS, Bjelland I, Mykletun A. Association between magnesium intake and depression and anxiety in community-dwelling adults: the Hordaland Health Study. Aust N Z J Psychiatry. 2009;43:45–52. doi:10.1080/00048670802534408.
- Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress – a systematic review. Nutrients. 2017;9; doi:10.3390/nu9050429.
- Stough C. Scholey A, Lloyd J, Spong J, Myers S, Downey LA. The effect of 90 day administration of a high dose vitamin B-complex on work stress. Hum Psychopharmacol. 2011;26:470–476. doi: 10.1002/hup.1229
- Pouteau E., Kabir-Ahmadi M., Noah L., Mazur A., Dye L., Hellhammer J., Pickering G., Dubray C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: a randomized, single-blind clinical trial. PloS one. 2018;13:e0208454. doi:10.1371/journal.pone.0208454.
- Mattioli L, Funari C, Perfumi M. Effects of Rhodiola rosea L. extract on behavioural and physiological alterations induced by chronic mild stress in female rats. J Psychopharmacol. 2008;23:130–142. doi:10.1177/0269881108089872.
- Cropley M, Banks AP, Boyle J. The effects of Rhodiola rosea L. extract on anxiety, stress, cognition and other mood symptoms. Phytother Res. 2015;29:1934–1939. doi:10.1002/ptr.5486.
- Edwards D, Heufelder A, Zimmermann A. Therapeutic effects and safety of Rhodiola rosea extract WS® 1375 in subjects with life-stress symptoms – results of an open-label study. Phytother Res 2012;26:1220–1225. doi:10.1002/ptr.3712.
- De Bock K, Eijnde BO, Ramaekers M, Hespel P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab. 2004;14:298–307. doi:10.1123/ijsnem.14.3.298.
- Duncan MJ, Clarke ND. The effect of acute Rhodiola rosea ingestion on exercise heart rate, substrate utilisation, mood state, and perceptions of exertion, arousal, and pleasure/displeasure in active men. J Sports Med. 2014;8. doi:10.1155/2014/563043.
- Shevtsov VA. Zholus BI, Shervarly VI, Vol'skij VB, Korovin YP, Khristich MP, Roslyakova NA, Wikman G. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine. 2003;10:95–105. doi:10.1078/094471103321659780.
- Hozawa A. Kuriyama S, Nakaya N, Ohmori-Matsuda K, Kakizaki M, et al. Green tea consumption is associated with lower psychological distress in a general population: the Ohsaki Cohort 2006 Study. Am J Clin Nutr. 2009;90:1390–1396. doi:10.3945/ajcn.2009.28214.
- Scholey A. Downey LA, Ciorciari J, Pipingas A, Nolidin K, Finn M, Wines M, Catchlove S, Terrens A, Barlow E, Gordon L, Stough C. Acute neurocognitive effects of epigallocatechin gallate (EGCG). Appetite. 2012;58:767–770. doi:https://doi.org/10.1016/j.appet.2011.11.016.
- Yoto A, Motoki M, Murao S, Yokogoshi H. Effects of L-theanine or caffeine intake on changes in blood pressure under physical and psychological stresses. J Physiol Anthropol. 2012;31:28–28. doi:10.1186/1880-6805-31-28.
- White DJ. De Klerk S, Woods W, Gondalia S, Noonan C, Scholey AB. Anti-stress, behavioural and magnetoencephalography effects of an l-theanine-based nutrient drink: a randomised, double-blind, placebo-controlled, crossover trial. Nutrients. 2016;8:53. doi:10.3390/nu8010053.
- Kimura K, Ozeki M, Juneja LR, Ohira H. L-Theanine reduces psychological and physiological stress responses. Biol Psychol. 2007;74:39–45. doi:10.1016/j.biopsycho.2006.06.006.
- Juneja LR, Chu DC, Okubo T, Nagato Y, Yokogoshi H. L-theanine – a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci Technol. 1999;10:199–204. doi:10.1016/s0924-2244(99)00044-8.
- Nobre AC, Rao A, Owen GN. L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac J Clin Nutr 2008;17(Suppl. 1):167–168.
- Dimpfel W. Neurophysiological effects of Rhodiola rosea extract containing capsules (a double-blind, randomised, placebo-controlled study). Int J Nutr Food Sci. 2014;3:157–165.
- Lovibond SH, Lovibond PF. Manual for the depression and anxiety stress scales. Psychology Foundation; 1995.
- World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi:10.1001/jama.2013.281053.
- Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem. 2013;24:725–743. doi:10.1016/j.jnutbio.2013.01.002.
- Colavincenzo J. SAS® Global Forum SF: Doctoring Your Clinical Trial with Adaptive Randomization: SAS® Macros to Perform Adaptive Randomization. Pharmaceutical SAS User Group (PharmaSUG).
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi:10.1016/j.jneumeth.2003.10.009.
- Oostenveld R, Fries P, Maris E, Schoffelen JM. Fieldtrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;156869; doi:10.1155/2011/156869.
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34:1443–1449. doi:10.1016/j.neuroimage.2006.11.004.
- Nolan H, Whelan R, Reilly RB. FASTER: fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods. 2010;192:152–162. doi:10.1016/j.jneumeth.2010.07.015.
- Mackay C, Cox T, Burrows G, Lazzerini T. Inventory for measurement of self-reported stress and arousal. Br J Soc Clin Psychol. 1978;17:283–284. doi: 10.1111/j.2044-8260.1978.tb00280.x
- Shacham S. A shortened version of the profile of mood states. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14.
- Bond A, Lader M. Use of analog scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. https://doi.org/10.1111/j.2044-8341.1974.tb02285.x
- Lipponen JA, Tarvainen MP. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J Med Eng Technol. 2019;43:173–181. doi:10.1080/03091902.2019.1640306.
- Tarvainen MP, Ranta-Aho PO, Karjalainen PA. An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng. 2002;49:172–175. doi:10.1109/10.979357.
- Kudielka, B. M., Hellhammer, D. H., Kirschbaum, C. Ten years of research with the Trier Social Stress Test (TSST)–—revisited. In Harmon-Jones E, Winkielman P, editors. Social neuroscience: integrating biological and psychological explanations of social behavior. The Guilford Press: New York; 2007. p. 56-83.
- Nobre AC, Rao A, Owen GN. L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac J Clin Nutr. 2008;17:167–168.
- Gomez-Ramirez M. Higgins BA, Rycroft JA, Owen GN, Mahoney J, Shpaner M, Foxe JJ. The deployment of intersensory selective attention: a high-density electrical mapping study of the effects of theanine. Clin Neuropharmacol 2007;30:25–38. doi:10.1097/01.wnf.0000240940.13876.17.
- Gomez-Ramirez M, Kelly SP, Montesi JL, Foxe JJ. The effects of L-theanine on alpha-band oscillatory brain activity during a visuo-spatial attention task. Brain Topogr. 2009;22:44–51. doi:10.1007/s10548-008-0068-z.
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86:156–185. doi:https://doi.org/10.1016/j.pneurobio.2008.09.005.
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Lett. 2001;310:57–60. doi:10.1016/s0304-3940(01)02094-8.
- Kubota Y. Sato W, Toichi M, Murai T, Okada T, Hayashi A, Sengoku A.Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Cogn Brain Res. 2001;11:281–287. doi:10.1016/s0926-6410(00)00086-0.
- Prinsloo GE, Rauch HG, Karpul D, Derman WE. The effect of a single session of short duration heart rate variability biofeedback on EEG: a pilot study. Appl Psychophysiol Biofeedback. 2013;38:45–56. doi:10.1007/s10484-012-9207-0.
- Gärtner M, Rohde-Liebenau L, Grimm S, Bajbouj M. Working memory-related frontal theta activity is decreased under acute stress. Psychoneuroendocrinology. 2014;43; doi:10.1016/j.psyneuen.2014.02.009.
- Wei J. Chen T, Li C, Liu G, Qiu J, Wei D. Eyes-open and eyes-closed resting states with opposite brain activity in sensorimotor and occipital regions: multidimensional evidences from machine learning perspective. Front Hum Neurosci. 2018;12; doi:10.3389/fnhum.2018.00422.
- Marx E. Stephan A, Nolte A, Deutschländer A, Seelos KC, Dieterich M, Brandt T. Eye closure in darkness animates sensory systems. NeuroImage. 2003;19:924–934. doi:10.1016/S1053-8119(03)00150-2.
- Coan EJ, Collingridge GL. Magnesium ions block an N-methyl-D-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci Lett. 1985;53:21–26. doi:10.1016/0304-3940(85)90091-6.
- Papadopol, V., Nechifor, M. Magnesium in Neuroses and Neuroticism. In Vink R, Nechifor M, editors. Magnesium and the central nervous system. University of Adelaide Press: Adelaide; 2011.
- Nathan PJ, Lu K, Gray M, Oliver C. The neuropharmacology of L-theanine(N-ethyl-L-glutamine): a possible neuroprotective and cognitive enhancing agent. J Herb Pharmacother. 2006;6:21–30.
- Campbell J, Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010