ABSTRACT
Background: EarlyNutrition (www.project-earlynutrition.eu) is an international research consortium investigating the effects of early nutrition on metabolic programming. Objective: To summarize current evidence and standards, recommendations, guidelines, and regulations on nutrition or supplements in lactating women with emphasis placed on long-term health effects in offspring, including cardiovascular disease, hypertension, overweight/obesity, metabolic syndrome, diabetes, or glucose intolerance. Methods: Medline, Embase, selected databases and websites were searched for documents published between 2010 and 2015. Results: Thirteen documents met the inclusion criteria. Effects of maternal long-chain polyunsaturated fatty acid (LC-PUFA) supplementation on overweight/obesity or hypertension in offspring were assessed in 10 studies. One study described the effect of maternal vitamin D supplementation on overweight/obesity, and the remaining 2 studies assessed the effects of maternal probiotic/synbiotic supplementation during lactation on overweight/obesity or metabolic syndrome in their infants. Forty-one documents contained dietary recommendations on various macro- and micronutrients for lactating women, but without consideration of our long-term health outcomes in infants. Conclusion: Literature on nutrition of lactating women and its effect on their infants' later health with respect to metabolic programming outcomes appeared to be scarce, and focused mostly on supplementation of LC-PUFA's. No recent guidelines or recommendations were available, highlighting the significant research gaps regarding this topic.
Introduction
Both fetal and early postnatal life are periods of rapid growth and development during which imbalanced nutrition might result in metabolic or body composition alterations (Gluckman and Hanson, Citation2008). Emerging evidence specifically suggests that increased risk of overweight or obesity later in life is programmed by nutrition during early life (Barker, Citation2004; McMillen and Robinson, Citation2005; Gluckman and Hanson, Citation2008). This relation is thought to be multifactorial and is likely to be U-shaped, with increased risks of adverse health outcomes both for early-life undernutrition as well as overnutrition (Gluckman and Hanson, Citation2008). Obesity in children is associated with adolescent and adult onset of noncommunicable diseases, such as type 2 diabetes mellitus, cardiovascular disease and hypertension (Barouki et al., Citation2012; Agostoni et al., Citation2013).
Given the well-known short- and long-term advantages of breastfeeding for infant and maternal health (vanRossum et al., Citation2005; Agostoni et al., Citation2009), leading health authorities such as the World Health Organization (WHO), the American
Academy of Pediatrics (AAP) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) highly recommend breastfeeding as the preferred source of postnatal nutrition. In the context of the fetal-infant programming hypothesis, maternal diet during lactation is thought to contribute to desirable long-term health advantages in children (Rush, Citation2001; Luoto et al., Citation2010) including the quality of growth in later life.
EarlyNutrition (www.project-earlynutrition.eu) is an international research project, sponsored by the European Union 7th Framework Programme (Koletzko et al., Citation2014b). The project's objectives include providing evidence of the effects of early nutrition on metabolic programming and their consequent health impacts. This project brings together an international consortium of experts in various research fields. The aim is to form a multilateral partnership for the enhancement of knowledge on early nutrition and metabolic programming and its impact on obesity and the risk of related disorders in adulthood.
The primary objective of the current systematic review, prepared as part of the EarlyNutrition project, was to summarize current evidence from systematic reviews and randomized controlled trials (RCTs) and standards, recommendations, guidelines, and regulations on nutrition in lactating women with emphasis on EarlyNutrition project outcomes. These include the effects on later health of the offspring with respect to adiposity overweight or obesity, cardiovascular disease, hypertension, metabolic syndrome, diabetes, or glucose intolerance. The secondary objective was to identify potential research gaps, as this will enable to develop research agendas.
Methods
Search strategy
Bibliographic databases, i.e. Ovid MEDLINE, EMBASE (both http://ovid.com), Cochrane Central Register of Controlled Trials (CENTRAL), and several selected guideline databases or websites of relevant professional organizations that may have produced guidelines were searched in May 2015 (). In addition, references were obtained by consultation of experts in the field (partners of the EarlyNutrition project). Searches in EMBASE and MEDLINE combined groups of key-words related to our target population, different nutrition components, and the type of preferred documents or study design. The detailed search strategy is provided in . All other databases, if possible, were searched with a combination of the phrases (infant* or child*) AND (lactat* OR breastfe* OR “breast milk”) AND (nutrition OR diet). Alternatively, they were simply hand searched.
Table 1. Sources searched.
Table 2. Medline and Embase search strategy.
Searches were limited to human studies published in the last five years (2010 and onwards), and restricted to English-language publications.
Selection of documents
Studies and documents were eligible for inclusion if they were relating to diet and nutrition for lactating women. In addition the documents had to consider the effect of this dietary exposure/intervention on EarlyNutrition health outcomes in the offspring, i.e. adiposity, overweight or obesity, cardiovascular disease, hypertension, metabolic syndrome, diabetes, or glucose intolerance.
We excluded documents focusing exclusively on prevention or treatment of a particular disease, such as allergic diseases or iron deficiency anemia that were not related to the outcomes mentioned above, or that focused on the period of pregnancy only. In addition, scientific documents other than randomized controlled trials, systematic reviews and meta-analysis, and documents dedicated to a local community, without national or international outreach were excluded.
Two authors (MDW and either SK, PCE or JCL) searched the provided sources and screened titles, abstracts and then full-text reports for inclusion independently. The total list of titles was additionally screened by BB. Any discrepancies were resolved through discussion or by consultation with an expert in the field.
Data extraction process
For each eligible study MDW, SK, PCE, and JCL independently extracted the following data: general information (title, author, year of publication, type of document: scientific trial or guideline, recommendation or consensus statement), document characteristics (scientific trial: design, participants characteristics, intervention and control regimens), the impact on defined health outcomes, and authors' suggestions for further research. Other documents: report type, target population, brief recommendation, the impact on defined health outcomes, the level of evidence (if stated by the authors), and authors' suggestions for further research. Inconsistencies were checked and resolved through discussion.
We did not perform any formal methodological assessment of the included documents' quality, and we did not attempt to evaluate the level of evidence of each recommendation if not done so by the authors.
Determining research gaps
Research gaps, associated with EarlyNutrition outcomes, were extracted from the identified documents if recognized by the authors of the documents.
Results
Selection of documents
shows a detailed description of the study selection process. The search strategy yielded 4218 documents, 3902 of which were excluded based on title or abstract. Full text evaluation of the remaining 316 records identified 13 documents that met our selection criteria. None of these were guidelines or clinical protocols.
Figure 1. Flowchart of study selection process. 1No access to this database outside Great-Britain. 2Duplicates within one database or at one website were identified and excluded from the number of screened records, duplicates between databases or websites were not.
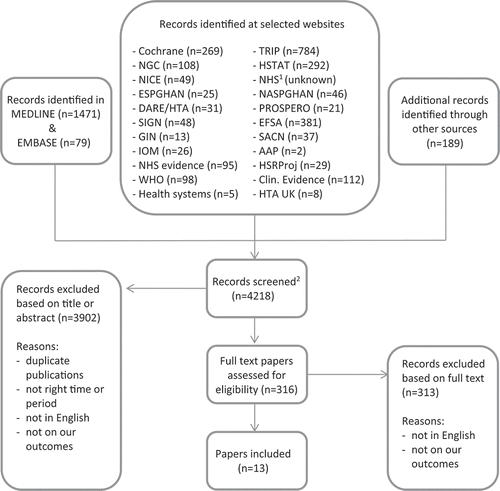
The effect of maternal fatty acid supplementation was assessed in 10 of the included studies, 1 study described the effect of Vitamin D supplementation and the remaining 2 assessed the effects of maternal probiotics/synbiotic supplementation during lactation. An outline of all included studies is provided in .
Table 3. Summary description of documents included.
Forty-one of the excluded documents did contain dietary recommendations on various macro- and micronutrients for lactating women (), but without consideration of our EarlyNutrition outcomes.
Table 4. Dietary recommendations in excluded documents.
Long-chain polyunsaturated fatty acid supplementation during lactation
Seven of the included publications on maternal long-chain polyunsaturated fatty acid (LC-PUFA) supplementation were systematic reviews (Muhlhausler et al., Citation2010; Campoy et al., Citation2012; Rodriguez et al., Citation2012; Martinez-Victoria and Yago, Citation2012; Koletzko et al., Citation2014b; Stratakis et al., Citation2014; Delgado-Noguera et al., Citation2015), one of which a Cochrane review (Delgado-Noguera et al., Citation2015). The remaining 3 documents were original reports of randomized controlled trials (RCTs) (Jensen et al., Citation2010; Bergmann et al., Citation2012; Hauner et al., Citation2012) ().
The seven systematic reviews enclosed a total of 10 publications (describing outcomes of five randomized trials), including the three RCTs we identified. The remaining seven publications were published before the year 2010, and were therefore not individually identified by our search (Helland et al., Citation2001; Ulbak et al., Citation2004; Jensen et al., Citation2005; Lauritzen et al., Citation2005; Lucia Bergmann et al., Citation2007; Helland et al., Citation2008; Asserhoj et al., Citation2009). Their results were however included in the current review, since the previous reviews were based upon them ().
The (updated) Cochrane review was the most recently published document and included all 10 publications. The remaining systematic reviews included part, but not all, of the publications ().
The effects of LC-PUFA supplementation exclusively during lactation was described in 1 systematic review, whereas two others additionally included supplementation during both pregnancy and lactation. The remaining four reviews also included supplementation only during pregnancy. Since this subject did not fall within the scope of the current review, we did not recite results exclusively on this topic.
Doses of n-3 LCPUFA in the described intervention groups ranged from 200 mg docosahexaenoic acid (DHA) to 1183 mg DHA plus 803 mg eicosapentaenoic acid (EPA) (with a total of 2494 mg n-3 LC-PUFA), and were derived from fish oil or algal oils.
Intervention periods extended from 15, 18, or 21 weeks of pregnancy to 3 or 4 months after delivery or exclusively during the first 4 months postpartum.
The EarlyNutrition outcome “overweight/obesity” was assessed in nine publications, at various time-points, and with various parameters (i.e. weight, body mass index, and fat mass and fat distribution) (Helland et al., Citation2001; Jensen et al., Citation2005; Lauritzen et al., Citation2005; Lucia Bergmann et al., Citation2007; Helland et al., Citation2008; Asserhoj et al., Citation2009; Jensen et al., Citation2010; Bergmann et al., Citation2012; Hauner et al., Citation2012).
summarizes the results, which were categorized into short-term (up to 12 months), medium-term (up to 24 months), and long-term (beyond 24 months) outcomes according to Delgado et al. (Delgado-Noguera et al., Citation2015).
Table 5. Documents on LC-PUFA supplementation during lactation—summary of study results.
All included reviews came to the same conclusion that evidence on the potential relationship between maternal n-3 LC-PUFA intake and infant growth or later body composition was inconclusive. Supplementation with LC-PUFA did not seem to exert a clear and consistent short- or long-term benefit in offspring, and any transient early differences disappeared in subsequent assessments.
The EarlyNutrition outcome “hypertension” was assessed in two of the publications based on the same RCT (Ulbak et al., Citation2004; Asserhoj et al., Citation2009). At 2.5 years of age no difference in either systolic or diastolic blood pressure (SBP and DBP) between groups was observed. At seven years of age, children in the LC-PUFA supplemented group had a higher unadjusted mean SBP and mean arterial pressure (MAP). After adjustment for covariates SBP did not differ between the randomized groups anymore. Due to an interaction between intervention and sex (p = 0.027 for DBP and p = 0.026 for MAP), adjusted ANOVA was performed separately for the two sexes. Among boys, both DBP and MAP differed between the randomized groups, but blood pressure of girls did not.
Vitamin D supplementation during lactation
The one publication on vitamin D intake during lactation referred to the EarlyNutrition outcome overweight/obesity, and was a prospective, double blinded, randomized controlled trial of vitamin D supplementation during lactation in both mother and infant (Czech-Kowalska et al., Citation2014).
Examined outcomes in the infants were weight, length, head circumference, and body composition (fat mass) by dual-energy X-ray absorptiometry (DXA) measurements at three time points: three weeks after delivery (baseline visit), and three and six months after delivery. The maternal intervention group received 1200 IU/d of cholecalciferol, the control group 400 IU/d. All breastfed infants received 400 IU/d. No significant differences in body composition and bone mass as examined by DXA were found between the intervention groups at any time point. However, serum hydroxy-vitamin D status (25(OH)D) was significantly higher in the 1200 IU/d group although the recommended level (>20 ng/mL by the Institute of Medicine (IOM) or >30 ng/mL (Holick et al., Citation2011; Pludowski et al., Citation2013b; Pludowski et al., Citation2013a) was still not reached in many women. This might have been due to the low baseline levels at study entry (65% of postpartum vitamin D deficiency) which highly depends on the maternal diet before birth and to sun exposure.
Probiotic and synbiotic supplementation during lactation
Two publications of RCTs referring to probiotic or synbiotic (both pre-and probiotic) supplementation in lactating mothers, assessing the EarlyNutrition outcomes overweight/obesity and metabolic syndrome were identified. (Aaltonen et al., Citation2011; Ostadrahimi et al., Citation2013). In the first RCT, by Aaltonen et al. 256 pregnant women in Finland were randomized to receive dietary counseling + a probiotic supplement, dietary counseling + placebo, or no counseling + placebo, starting in the first trimester until six months after delivery. Anthropometrics of the offspring were measured at three study visits (first trimester, third trimester, and six months postdelivery). In addition, infants' breastfeeding status was recorded and their metabolic status was evaluated, by serum 32–33 split proinsulin. This is a novel marker of adverse metabolic status in infancy, and has previously been shown to be a well-characterized predictor for insulin resistance in adults and older children (Mykkanen et al., Citation1997). The authors found that the infants' risk of high 32–33 split proinsulin concentration was significantly lower in the dietary counseling + probiotics and dietary counseling + placebo groups, compared to the control group. In a multivariate analysis, the independent effect of dietary counseling on the infants' 32–33 split proinsulin was still statistically significant (with breastfeeding and mother's glucose level at 6 months, and weight gain during pregnancy). However, there was no effect of the probiotic supplementation in this population.
The second trial, by Ostradahimi et al. in 2013, was conducted in lactating mothers and their exclusively breastfed infants in Iran. The authors examined the effect of a synbiotic supplement on infant weight and growth (synbiotic: n = 40, placebo, n = 40). They found that the administration of synbiotics may prevent weight loss in undernourished lactating mothers, enhance breastfeeding duration and infant weight gain.
Research gaps
Research gaps as identified and described by the authors of the 13 included studies were summarized in . All included systematic reviews on maternal LC-PUFA supplementation pointed out that there was a wide variation in interventions, outcome parameters and follow-up periods between trials and that most trials were prone to methodological limitations. In addition, identified research gaps comprised a lack of control for nutritional status and intakes at baseline, as well as for genetic variation. The latter was based on the identification of polymorphisms in the fatty acid desaturase (FADS) gene cluster (Glaser et al., Citation2011; Lattka et al., Citation2011). This gene plays a role in the conversion of n-3 LC-PUFA's from their precursors, and it was demonstrated that pregnant and lactating women with the less common genotypes of the FADS gene cluster had very low conversion rates (Koletzko et al., Citation2011; Lattka et al., Citation2011; Steer et al., Citation2013).
Table 6. Research gaps identified by the authors of documents included.
For vitamin D supplementation, the authors stated that taking into account previous studies, vitamin D supplementation seems to be a more effective source for supplementation than additional input from diet and endogenous skin synthesis in many contemporary industrialized populations with changing lifestyles (sun exposure, obesity, unhealthy diet). Yet, they feel that more studies are needed in various settings, specifically addressing infants' long-term outcomes.
The identified research gap for studies on pro- and synbiotic supplementation was the need for targeted studies to evaluate the independent effect of synbiotic supplementation in lactating mothers on infants' health outcomes.
Discussion
The EarlyNutrition project was designed to answer clinically important questions and to help in building foundations of future evidence-based recommendations. To our knowledge, this is the first systematic review assessing the effect of nutrition of lactating women on their infants' later health with respect to adiposity, overweight or obesity, cardiovascular disease, hypertension, metabolic syndrome, diabetes, or glucose intolerance. Literature on this topic appeared to be scarce, and focused mostly on the supplementation of LC-PUFA's. In the past five years, no guidelines or recommendations on nutrition in breastfeeding women relating to our defined effects on their offsprings' health were published.
The lack of literature and guidelines was unsuspected, since the programming hypothesis of early-life nutrition now is a pertinent topic. The hypothesis proposes that overweight or obesity, impaired glucose metabolism, and heightened blood pressure are modulated by environmental cues that originate in developmental plasticity, including nutritional aspects (Barker, Citation2004).
In both developed and less developed countries, excess body weight is now a major health problem; more than 20% of children and adolescents in Europe and more than 30% in the United States suffer from overweight or obesity (www.IASO.org). Becoming obese earlier in life clearly amplifies certain health risks such as type 2 diabetes. Based on the fetal-infant programming hypothesis it has been suggested that attempts to prevent obesity should start during early life. Alteration of early exposure to certain nutrients via maternal diet during lactation is such an attempt. However, our current review showed that (human) evidence for the effectiveness of this is so far scarce and limited to interventions with LC-PUFAs, vitamin D and probiotics/synbiotics.
Acquisition of fat cells early in life appears to be an irreversible process, and early exposure to n-3 LC-PUFA's is suggested to have the potential to limit adipose tissue deposition, mainly by limiting the production of prostacyclin which has been shown to enhance adipogenesis. A deficiency in vitamin D during breastfeeding has been shown to be associated with a range of negative health outcomes in mother and child, such as cardiovascular disease, hypertension and diabetes (Pludowski et al., Citation2013a). Finally, research in animals and humans has shown that probiotics have an effect on the host energy metabolism by altering the gut microbiota. The combination of probiotics and prebiotics, referred to as synbiotics, may affect the host by improving the survival and the implementation of live bacterial strains in the gastrointestinal tract, thus exerting potentiated positive health effects. Our results showed that there is little evidence to support a favorable programming effect by maternal n-3 LC-PUFA or vitamin D supplementation during lactation. One small trial suggested that there might be a possible positive effect of maternal probiotic/synbiotic supplementation on infants' weight gain and body composition. Yet, much more evidence is needed to support these findings and to formulate evidence-based recommendations.
A major part of the documents excluded from this review focused on nutrition in lactating women, and some of them did include recommendations (). However, most of these recommended intakes were extrapolated from known losses of nutrients in milk, sometimes with adjustment for bioavailability, and not on possible health effects for the infants. Studies analyzing those losses also emerged from our literature search. Although these studies did not fall within the scope of our review, they can be considered crucial early stages for the documents that we were searching for, since effects of nutrients on health of offspring can only be expected if certain levels in breastmilk will be reached. Research on the extent to which the nutrient composition of human milk can be affected by maternal status and intake is on the other hand scarce (Allen, Citation2012). In the past, nutrients have been categorized into 2 groups during lactation. Poor maternal status of group I nutrients (thiamin, riboflavin, vitamin B-6, vitamin B-12, choline, retinol, vitamin A, vitamin D, selenium and Iodine) results in low concentrations of these nutrients in breast milk whereby the infant becomes depleted, whereas group II nutrients (folate, calcium, Iron, copper, and zinc) in breast milk are relatively unaffected by maternal intake or status; the mother gradually becomes more depleted when intake is less than the amount secreted in milk, but milk concentrations are maintained (Allen, Citation2012). Future research focusing on supplementation of group I nutrients in lactating women is therefore to be expected to show the clearest results on infants' health.
The major strength of the current review is the extensive search strategy. Limitations were the restriction to English-language publications leading to the possibility that eligible documents in other languages may have been omitted, and the restriction on year of publication. To ensure that we included the latest and most up-to-date documents we only considered documents published from 2010 onwards, in order to be consistent with other systematic reviews performed as part of the EarlyNutrition project. It is therefore possible that we have not included some potentially relevant documents. On the other hand, as systematic reviews were included, with studies originating more than 5 years ago, we are quite confident that the important studies are included. Another limitation is that we did not set out to critically appraise the documents that we identified, in terms of their quality, nor did we directly assess any of the information that the documents and conclusions were based upon.
Conclusions
This systematic review of current literature and recommendations on nutrition of lactating women and its effects on later health outcomes in their offspring showed that current evidence is scarce and many aspects of nutrition need further elucidation. This review can form a foundation for further research based on gaps provided by the authors of included documents and identified during the process of this review.
Funding
The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007–2013), project EarlyNutrition under grant agreement n°[289346].
References
- Aaltonen, J., Ojala, T., Laitinen, K., Poussa, T., Ozanne, S. and Isolauri, E. (2011). Impact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: A prospective randomized controlled study. Eur. J. Clin. Nutr. 65(1):10–19.
- Agostoni, C., Baselli, L. and Mazzoni, M. B. (2013). Early nutrition patterns and diseases of adulthood: A plausible link? Eur. J. Intern. Med. 24(1):5–10.
- Agostoni, C., Braegger, C., Decsi, T., Kolacek, S., Koletzko, B., Michaelsen, K. F., Mihatsch, W., Moreno, L. A., Puntis, J., Shamir, R., Szajewska, H., Turck, D. and van Goudoever, J. (2009). Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 49(1):112–125.
- Allen, L. H. (2012). B vitamins in breast milk: Relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 3(3):362–369.
- Asserhoj, M., Nehammer, S., Matthiessen, J., Michaelsen, K. F. and Lauritzen, L. (2009). Maternal fish oil supplementation during lactation may adversely affect long-term blood pressure, energy intake, and physical activity of 7-year-old boys. J. Nutr. 139(2):298–304.
- Barker, D. J. (2004). The developmental origins of chronic adult disease. Acta. Paediatr. Suppl. 93(446):26–33.
- Barouki, R., Gluckman, P. D., Grandjean, P., Hanson, M. and Heindel, J. J. (2012). Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 11:42.
- Bergmann, R. L., Bergmann, K. E., Richter, R., Haschke-Becher, E., Henrich, W. and Dudenhausen, J. W. (2012). Does docosahexaenoic acid (DHA) status in pregnancy have any impact on postnatal growth? Six-year follow-up of a prospective randomized double-blind monocenter study on low-dose DHA supplements. J. Perinat. Med. 40(6):677–684.
- Campoy, C., Escolano-Margarit, M. V., Anjos, T., Szajewska, H. and Uauy, R. (2012). Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br. J. Nutr. 107(Suppl 2):S85–S106.
- Czech-Kowalska, J., Latka-Grot, J., Bulsiewicz, D., Jaworski, M., Pludowski, P., Wygledowska, G., Chazan, B., Pawlus, B., Zochowska, A., Borszewska-Kornacka, M. K., Karczmarewicz, E., Czekuc-Kryskiewicz, E. and Dobrzanska, A. (2014). Impact of vitamin D supplementation during lactation on vitamin D status and body composition of mother-infant pairs: A MAVID randomized controlled trial. PLoS One 9(9):e107708.
- Delgado-Noguera, M. F., Calvache, J. A., Bonfill Cosp, X., Kotanidou, E. P. and Galli-Tsinopoulou, A. (2015). Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 7:CD007901.
- Glaser, C., Lattka, E., Rzehak, P., Steer, C. and Koletzko, B. (2011). Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern. Child Nutr. 7(Suppl 2):27–40.
- Gluckman, P. D. and Hanson, M. A. (2008). Developmental and epigenetic pathways to obesity: An evolutionary-developmental perspective. Int. J. Obes. (Lond.) 32(Suppl 7):S62–S71.
- Hauner, H., Much, D., Vollhardt, C., Brunner, S., Schmid, D., Sedlmeier, E. M., Heimberg, E., Schuster, T., Zimmermann, A., Schneider, K. T., Bader, B. L. and Amann-Gassner, U. (2012). Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: An open-label randomized controlled trial. Am. J. Clin. Nutr. 95(2):383–394.
- Helland, I. B., Saugstad, O. D., Smith, L., Saarem, K., Solvoll, K., Ganes, T. and Drevon, C. A. (2001). Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 108(5):E82.
- Helland, I. B., Smith, L., Blomen, B., Saarem, K., Saugstad, O. D. and Drevon, C. A. (2008). Effect of supplementing pregnant and lactating mothers with n-3 very-long-chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 122(2):e472–e479.
- Holick, M. F., Binkley, N. C., Bischoff-Ferrari, H. A., Gordon, C. M., Hanley, D. A., Heaney, R. P., Murad, M. H., Weaver, C. M. and Endocrine, S. (2011). Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(7):1911–1930.
- Jensen, C. L., Voigt, R. G., Llorente, A. M., Peters, S. U., Prager, T. C., Zou, Y. L., Rozelle, J. C., Turcich, M. R., Fraley, J. K., Anderson, R. E. and Heird, W. C. (2010). Effects of early maternal docosahexaenoic acid intake on neuropsychological status and visual acuity at five years of age of breast-fed term infants. J. Pediatr. 157(6):900–905.
- Jensen, C. L., Voigt, R. G., Prager, T. C., Zou, Y. L., Fraley, J. K., Rozelle, J. C., Turcich, M. R., Llorente, A. M., Anderson, R. E. and Heird, W. C. (2005). Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am. J. Clin. Nutr. 82(1):125–132.
- Koletzko, B., Boey, C. C., Campoy, C., Carlson, S. E., Chang, N., Guillermo-Tuazon, M. A., Joshi, S., Prell, C., Quak, S. H., Sjarif, D. R., Su, Y., Supapannachart, S., Yamashiro, Y. and Osendarp, S. J. (2014a). Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 65(1):49–80.
- Koletzko, B., Brands, B., Chourdakis, M., Cramer, S., Grote, V., Hellmuth, C., Kirchberg, F., Prell, C., Rzehak, P., Uhl, O. and Weber, M. (2014b). The Power of Programming and the EarlyNutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Ann. Nutr. Metab. 64(3–4):187–96.
- Koletzko, B., Lattka, E., Zeilinger, S., Illig, T. and Steer, C. (2011). Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: Findings from the Avon Longitudinal Study of Parents and Children. Am. J. Clin. Nutr. 93(1):211–219.
- Lattka, E., Rzehak, P., Szabo, E., Jakobik, V., Weck, M., Weyermann, M., Grallert, H., Rothenbacher, D., Heinrich, J., Brenner, H., Decsi, T., Illig, T. and Koletzko, B. (2011). Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am. J. Clin. Nutr. 93(2):382–391.
- Lauritzen, L., Hoppe, C., Straarup, E. M. and Michaelsen, K. F. (2005). Maternal fish oil supplementation in lactation and growth during the first 2.5 years of life. Pediatr. Res. 58(2):235–242.
- Lucia Bergmann, R., Bergmann, K. E., Haschke-Becher, E., Richter, R., Dudenhausen, J. W., Barclay, D. and Haschke, F. (2007). Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy?. J. Perinat. Med. 35(4):295–300.
- Luoto, R., Kalliomaki, M., Laitinen, K. and Isolauri, E. (2010). The impact of perinatal probiotic intervention on the development of overweight and obesity: Follow-up study from birth to 10 years. Int. J. Obes. (Lond.) 34(10):1531–1537.
- Martinez-Victoria, E. and Yago, M. D. (2012). Omega 3 polyunsaturated fatty acids and body weight. Br. J. Nutr. 107(Suppl 2):S107–S116.
- McMillen, I. C. and Robinson, J. S. (2005). Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 85(2):571–633.
- Muhlhausler, B. S., Gibson, R. A. and Makrides, M. (2010). Effect of long-chain polyunsaturated fatty acid supplementation during pregnancy or lactation on infant and child body composition: A systematic review. Am. J. Clin. Nutr. 92(4):857–863.
- Mykkanen, L., Haffner, S. M., Hales, C. N., Ronnemaa, T. and Laakso, M. (1997). The relation of proinsulin, insulin, and proinsulin-to-insulin ratio to insulin sensitivity and acute insulin response in normoglycemic subjects. Diabetes 46(12):1990–1995.
- Ostadrahimi, A., Nikniaz, L., Mahdavi, R., Hejazi, M. A. and Nikniaz, Z. (2013). Effects of synbiotic supplementation on lactating mothers' energy intake and BMI, and infants' growth. Int. J. Food Sci. Nutr. 64(6):711–714.
- Pludowski, P., Holick, M. F., Pilz, S., Wagner, C. L., Hollis, B. W., Grant, W. B., Shoenfeld, Y., Lerchbaum, E., Llewellyn, D. J., Kienreich, K. and Soni, M. (2013a). Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 12(10):976–989.
- Pludowski, P., Karczmarewicz, E., Bayer, M., Carter, G., Chlebna-Sokol, D., Czech-Kowalska, J., Debski, R., Decsi, T., Dobrzanska, A., Franek, E., Gluszko, P., Grant, W. B., Holick, M. F., Yankovskaya, L., Konstantynowicz, J., Ksiazyk, J. B., Ksiezopolska-Orlowska, K., Lewinski, A., Litwin, M., Lohner, S., Lorenc, R. S., Lukaszkiewicz, J., Marcinowska-Suchowierska, E., Milewicz, A., Misiorowski, W., Nowicki, M., Povoroznyuk, V., Rozentryt, P., Rudenka, E., Shoenfeld, Y., Socha, P., Solnica, B., Szalecki, M., Talalaj, M., Varbiro, S. and Zmijewski, M. A. (2013b). Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 64(4):319–327.
- \Rodriguez, G., Iglesia, I., Bel-Serrat, S. and Moreno, L. A. (2012). Effect of n-3 long chain polyunsaturated fatty acids during the perinatal period on later body composition. Br. J. Nutr. 107(Suppl 2):S117–S128.
- Rush, D. (2001). Maternal nutrition and perinatal survival. J. Health Popul. Nutr. 19(3):S217–S264.
- Steer, C. D., Lattka, E., Koletzko, B., Golding, J. and Hibbeln, J. R. (2013). Maternal fatty acids in pregnancy, FADS polymorphisms, and child intelligence quotient at 8 y of age. Am. J. Clin. Nutr. 98(6):1575–1582.
- Stratakis, N., Gielen, M., Chatzi, L. and Zeegers, M. P. (2014). Effect of maternal n-3 long-chain polyunsaturated fatty acid supplementation during pregnancy and/or lactation on adiposity in childhood: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 68(12):1277–1287.
- Ulbak, J., Lauritzen, L., Hansen, H. S. and Michaelsen, K. F. (2004). Diet and blood pressure in 2.5-y-old Danish children. Am. J. Clin. Nutr. 79(6):1095–1102.
- van Rossum, C. T. M., Büchner, F. L. and Hoekstra, J. A. (2005). Quantification of Health Effects of Breastfeeding: Review of the Literature and Model Simulation. pgs. 15–24. RIVM Available at http://www.rivm.nl/dsresource?objectid=7ccfaa02-db10-42c0-aefa-c3d797cf6108&type=org&disposition=inline.

