?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Phenolic compounds (PCs) have neuroprotective effects with potential to prevent or slower the progression of Parkinson’s disease (PD). However, whether the PCs neuroprotective effects can be observed under their dietary concentrations remains unclear. Therefore, we searched for the most cited articles in density on PCs and PD in the Web of Science Core Collection and All-Database (WoS-CC/AD) and selected the articles based on our eligibility criteria. From these 81 articles selected, we extracted information on experimental design, compounds tested, concentration and/or dose administered, route of administration, and main results obtained. We compared the concentrations of PCs evaluated in vitro with the concentrations bioavailable in the human bloodstream. Further, after extrapolation to humans, we compared the doses administered to animals in vivo with the daily consumed amounts of PCs. Concentrations evaluated in 21 in vitro laboratory studies were higher than those bioavailable in the bloodstream. In the case of in vivo laboratory studies, only one study administered doses of PCs in normal daily amount. The results of the comparisons demonstrate that the neuroprotective effects of the selected articles are mainly associated with concentrations, amounts and routes of administration that do not correspond to the consumption of phenolic compounds through the diet.
Introduction
Phenolic compounds (PCs) are secondary metabolites of plants responsible for protection against ultraviolet (UV) rays and pathogens (Cheynier et al. Citation2013). More than 10,000 PCs are known and can be classified as flavonoids, phenolic acids, lignans, and stilbenes (Tsimogiannis and Oreopoulou Citation2019). PCs can be found in foods and beverages of vegetable origin, such as fruits, wines, products derived from cacao, and olives (de Araújo et al. Citation2021).
The term bioavailability refers to the steps occurring after the consumption of these compounds, including their transport, biotransformation, and absorption, until they reach the target organ (Crozier, del Rio, and Clifford Citation2010; Stromsnes et al. Citation2021). Bioavailability is influenced by factors such as the chemical structure, food matrix, association with other foods, and microbiota composition (Duda-Chodak et al. Citation2015).
PCs have demonstrated neuroprotective effects in experimental models of Parkinson’s disease (PD), that have been associated to different mechanisms including antioxidant activity, regulation of mitochondrial dysfunction, anti-inflammatory activity, as well as inhibition and disaggregation of α-synuclein fibrils (Hussain et al. Citation2018; Reglodi et al. Citation2017; Magalingam, Radhakrishnan, and Haleagrahara Citation2015). However, the neuroprotective effects of PCs are related to the concentration and chemical structure of the evaluated compound, which is dependent on its bioavailability in the human body (Holst and Williamson Citation2008).
In a common oversight, research evaluating the bioactive potential of natural compounds is conducted using concentrations higher than those consumable through diet and those bioavailable after absorption (Bo’ et al. Citation2019; Stefania et al. Citation2021; Sang, Lambert, and Yang Citation2006; Mancuso, Siciliano, and Barone Citation2011) which leads to a false-positive results, emergence of false therapeutic candidates, or presumed activity against a wide panel of diseases (Ferreira, Martins, and Barros Citation2017).
The number of studies evaluating the neuroprotective potential of PCs against PD has increased after the demonstration of neuroprotective effects in vitro. However, whether PCs concentrations that demonstrate neuroprotection against PD can be achieved in humans through a natural diet, digestion, and absorption remains unclear.
To answer this question, we selected the most cited articles addressing the effects of PCs on PD from the Web of Science Core Collection (WoS-CC) and All-Database (WoS-AD) and identified articles that evaluated isolated compounds belonging to the main PCs classes (phenolic acids, flavonoids, stilbenes, and lignans) along with the experimental design used.
We also compared the concentrations of the compounds evaluated in vitro with the concentration normally found in human blood circulation, and in the case of in vivo experiments with rodents, after extrapolating the doses administered in animals to humans, we compared the concentrations of compounds belonging to the same subclass tested at the normal concentrations consumed.
Therefore, the main aim of this review is to evaluate whether the PCs concentrations presenting neuprotective effects in experimental models of PD can be achieved by consuming diets with foods rich in PCs.
Material and methods
Search strategy
The WoS-CC and WoS-AD was searched using the strategy described in .
Table 1. Search strategy.
Articles published until December 2022 were searched without restrictions on language, year of publication, or methodology. The articles were organized in descending order according to the number of citations.
Eligibility criteria
The selection of articles was independently performed by two respondents. Articles were selected that contained some terms of the search strategy in the title, abstract or keywords and citation density (number of citations/time of publication) equal to or higher than ten, and included articles that evaluated pure compounds or extracts rich in natural PCS with defined concentrations and presented only model of PD. Disagreements were resolved using the agreement method. Conference papers were excluded.
Data extraction
The article title, citation count, publication year, pure compound(s) (up to nine) or extract (E), study design, concentration with neuroprotective effect tested, pathway administration (in case of in vivo laboratory studies), and neuroprotective mechanisms were recorded from the selected articles. The study designs were grouped as follows: clinic study (prospective cohort), laboratory studies (in vitro and in vivo) and literature review, (Cochrane Collaboration Glossary, 2019).
Extrapolation of doses administered in animals to humans
The doses administered in animals were extrapolated to humans using the body surface area (BSA) normalization method (Reagan‐Shaw, Nihal, and Ahmad Citation2008) from equation 1:
[Eq. 1]
[Eq. 1]
The Km factor, that is, body weight (kg) divided by BSA (m2), was used to convert the mg/kg dose used in the study to a mg/m2 dose.
Obtaining data for average daily consumption and bioavailability of PCs
Data on daily intake and bioavailability of PC in human blood circulation were obtained from articles published up to December 2022 in the WoS-CC database. These were used as parameters for later comparison with the concentrations administered in the selected studies.
Comparison between concentrations
The concentrations of tested compounds were compared with those bioavailable in the human bloodstream and the doses administered to animals were compared with the quantities consumed through diet.
The concentrations of the compounds tested in studies with an in vitro laboratory experimental design (μM) and the concentration of the subclass bioavailable in the bloodstream (μM) were compared based on the ratio between the two concentrations.
The PCs doses utilized in vivo experimental models of PD, after extrapolation to humans, and the quantity of the compound subclass that was consumed daily (mg/kg) were also compared based on the ratio between the two concentrations.
Results
Using the search strategy described in , 2,594 articles were initially obtained. After classifying the citations in descending order, only 2,000 articles were evaluated by the eligibility criterion, since from the 2,000th article the number of citations is less than ten, so the density is also less than ten. From the 2,000 articles evaluated, 1,919 articles were excluded for not presenting citation density equal to or greater than ten and not exclusively addressing PD and CPs, resulting in 81 articles on PD and CPs with at least ten citations and citation density equal to or greater than ten. The articles selected were organized according to the experimental design in the following order: clinical study (prospective cohort), laboratory studies (in vivo), laboratory studies (in vitro), laboratory studies (in vivo and in vitro) and literature review ().
Table 2. Classification of articles selected from the experimental design.
The quantities consumed daily and bioavailable in human blood circulation for each subclass of PCs extracted from the Phenol Explorer and Integrity databases are shown in . Notably, the quantity consumed daily and their bioavailability are not available for some subclasses.
Table 3. Concentration consumed daily and max concentration of PCs found in plasma.
Phenolic acid
Benzoic acids
Three studies evaluated benzoic acids in in vitro experimental design. The compounds evaluated were protocatechuic acid (Zhang et al. Citation2015) and gallic acid (Ardah et al. Citation2014; Chandrasekhar et al. Citation2018) ().
Figure 1. Articles that evaluated benzoic acids in experimental models of PD in vitro 
 .
.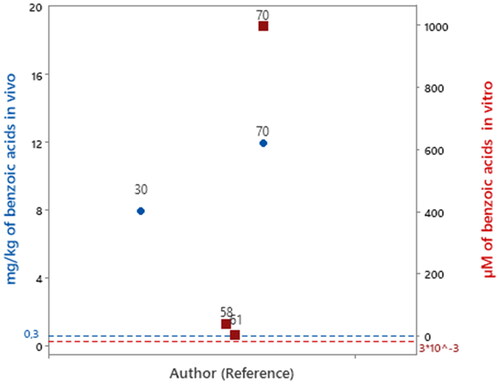
Protocatechuic acid was evaluated in concentration of 1000 μM (Zhang et al. Citation2015). Considering 0.003 μM as the concentration of benzoic acid subclass bioavailable in human blood circulation () that amount of protocatechuic acid evaluated is 33,300 times higher than the concentration of benzoic acid bioavailable in human blood. Likewise, gallic acid was evaluated at a concentration of 5 μM (Chandrasekhar et al. Citation2018) and 40 μM (Ardah et al. Citation2014) which is 1,666 times and 13,000 times higher than the reported bioavailable concentration of benzoic acids in human blood circulation, respectively.
Among the included studies, only two studies evaluated benzoic acids in vivo experimental design. The compounds evaluated were ellagic acid (Baluchnejadmojarad et al. Citation2017) and protocatechuic acid (Zhang et al. Citation2015). The dose administered to animals when extrapolated to humans was equal to 8 and 12 mg/kg/day, respectively, which was 26.6 and 40 times higher than the amount of benzoic acid subclass (0.3 mg/kg) consumed daily ().
Flavonoids
Flavones
In total, 13 articles were identified to evaluate flavones in an in vitro experimental design. The compounds evaluated included amentoflavone, apigenin, baicalein, chrysin, chrysoeriol, luteolin and vitexin ().
Figure 2. Articles that evaluated flavones in experimental models of PD in vitro 
 and intraperitoneal administration.
and intraperitoneal administration.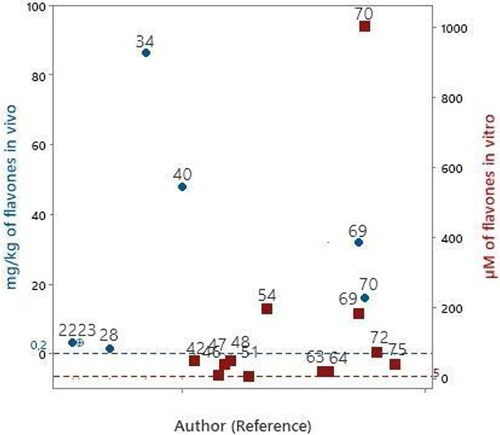
Baicalein was evaluated in six studies (Zhu et al. Citation2004; Caruana et al. Citation2011; Uversky et al. Citation2010; Zhang et al. Citation2012; Mu et al. Citation2009; Zhang et al. Citation2017). The lowest evaluated concentration of baicalein was 10 μM. Considering 5 μM as the concentration range of flavones bioavailable in human blood circulation (), the baicalein concentrations evaluated in these nine articles were 2–40 times above the concentration range bioavailable in human blood circulation.
Chrysin was evaluated in two studies (Mercer et al. Citation2005; Zhang et al. Citation2015). The lowest evaluated concentration of chrysin was 40 μM. Thus, the chrysin concentrations evaluated in these two articles were 8–200 times higher than the bioavailable range of flavones found in human blood circulation (5 μM).
Luteolin was evaluated in two studies (Wruck et al. Citation2007; Elmazoglu et al. Citation2020). The lowest evaluated concentration of luteolin was 5 μM, which can be found bioavailable in the human bloodstream. While the concentration tested in study 63 was 20 μM, which is 4 times higher than the flavone concentration that can be found bioavailable in the bloodstream (5 μM).
The compounds amentoflavone, chrysoeriol and vitexin were evaluated in each study. The concentrations evaluated were 75, 20 and 40 respectively, which are in the range of 4 - 15 times higher than the flavone concentration found bioavailable in human bloodstream.
Ten articles that evaluated flavones using an in vivo experimental laboratory design were identified. The evaluated compounds were amentoflavone, apigenin, baicalein, chrysin, luteolin and vitexin ().
Apigenin, and luteolin were evaluated by oral administration at a concentration of 3.2 mg/kg/day (Patil et al. Citation2014). Apigenin was also evaluated by intraperitoneal administration at a concentration of 3.2 mg/kg/day (Anusha, Sumathi, and Joseph Citation2017). Considering that the daily consumed quantity of flavones is 0.2 mg/kg (), the evaluated concentrations were 16 times higher than the daily amount consumed.
Baicalein was evaluated in four studies (Rui et al. Citation2020; Zhao et al. Citation2021; Mu et al. Citation2009; Zhang et al. Citation2017). The lowest evaluated concentration of the compound was 32 mg/kg/day, administered orally (Mu et al. Citation2009; Zhang et al. Citation2017). Thus, the evaluated concentration is 160 times higher than the daily quantity consumed (0.2 mg/kg/day). Baicalein was also orally administered to animals at a concentration of 48 and 86.4 mg/kg/day in two other studies (40andRui et al., 2020). which are in the range of 240–432 times higher than the daily quantity consumed (0.2 mg/kg/day).
Chrysin was evaluated in 2 articles (Goes et al. Citation2018; Zhang et al. Citation2015), and the lowest concentration of the compound, administered orally, was 1.6 mg/kg/day (Goes et al. Citation2018). Considering that the daily quantity of flavones consumed is 0.2 mg/kg (), the evaluated concentration was 8 times higher. Chrysin was also administered orally at 16 mg/kg/day (Zhang et al. Citation2015), which was 80 times higher than the daily quantity consumed.
The compounds amentoflavone and vitexin were administered in amounts 8 mg/kg/day orally in two studies (Cao et al. Citation2017; Hu, Li, and Wang Citation2018). The amount is 40 times higher than the daily consumed amount of flavone (0.2 mg/kg/day).
Isoflavones
Three studies evaluating isoflavones were identified. The compounds evaluated were puerarin, genistein, and calycosin ().
Figure 3. Articles that evaluated isoflavones in experimental models of PD in vitro 
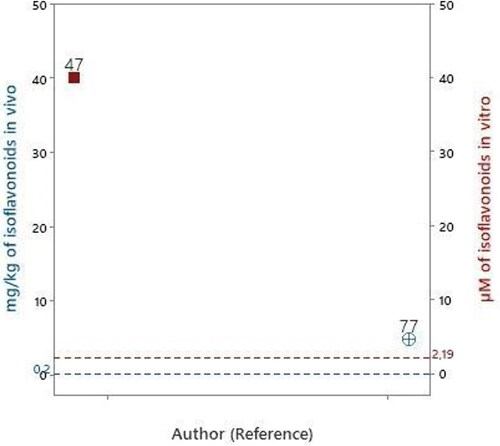
Puerarin and genistein compounds were evaluated at a concentration of 40 μM (Mercer et al. Citation2005). Compared with the concentration of isoflavone bioavailable in human blood circulation 2.19 μM, the evaluated concentration was 18 times higher.
Calycosin was evaluated in one study (Yang et al. Citation2019), but the concentration was not expressed and it was not possible to make comparisons with the concentration of isoflavone found bioavailable in human blood circulation.
Among the selected studied, only one study evaluated isoflavone in vivo (Yang et al. Citation2019). The compound evaluated, calycosin, was administered intraperitoneally at a concentration of 4.8 mg/kg/day. Compared with the daily quantity of isoflavonoids consumed (0.2 mg/kg, ), the evaluated concentration was 24 times higher ().
Flavanones
Among the articles included in this study, five evaluated flavanones using an in vitro experimental design ().
Figure 4. Articles that evaluated flavanones in experimental models of PD in vitro 
 .
.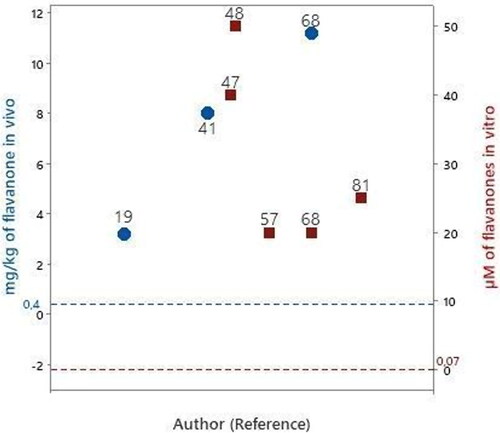
Naringenin was evaluated in three studies (Mercer et al. Citation2005; Lou et al. Citation2014; Kesh, Kannan, and Balakrishnan Citation2021). The lowest evaluated concentration of naringenin was 20 μM. Compared with the bioavailable concentration of flavanones in human blood circulation (0.07 μM), the evaluated concentrations of naringenin in these studies were 200 - 500 times higher.
Hesperidin was evaluated at 20 μM (Tamilselvam et al. Citation2013), which is 280 times higher than the bioavailable concentration of flavanones in human blood circulation and eriodictyol, was evaluated at 50 μM (Uversky et al. Citation2010), which is 700 times higher than the bioavailable concentration of flavone in human blood circulation.
Four articles evaluated flavanones in an in vivo experimental design using rats and zebrafish model. The evaluated compounds were naringenin and hesperidin ().
Naringenin was evaluated on zebrafish (Kesh, Kannan, and Balakrishnan Citation2021) and after oral administration on rats (Levites et al. Citation2001; Lou et al. Citation2014). The lowest evaluated concentration of naringenin was 3.2 mg/kg/day. The evaluated concentrations were in the range of 3.2 − 11.2 mg/kg/day. Compared with the daily quantity of flavonones consumed (0.4 mg/kg, ), the evaluated concentrations were 8 – 28 times higher ().
Hesperidin was evaluated in vivo using oral administration in an animal model at 8 mg/kg/day (Antunes et al. Citation2021), which was 20 times higher than the daily quantity of flavonones consumed ().
Flavonols
We found 8 articles that evaluated flavonols using an in vitro experimental design. The evaluated compounds were gossypetin, kaempferol, myricetin and quercetin ().
Figure 5. Articles that evaluated flavonols in experimental models of PD in vitro 
 and intraperitoneal administration.
and intraperitoneal administration.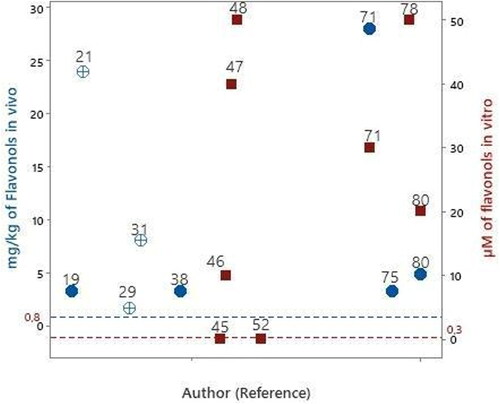
Quercetin was evaluated in five studies, and (Bureau, Longpré, and Martinoli Citation2008; Mercer et al. Citation2005; Bournival, Quessy, and Martinoli Citation2009; Ay et al. Citation2017; Wang et al. Citation2021) its lowest evaluated concentration was 0.1 μM (Bureau, Longpré, and Martinoli Citation2008; Bournival, Quessy, and Martinoli Citation2009). The concentration of flavonols that is bioavailable in human blood circulation is 0.3 μM. Thus, the quercetin concentrations evaluated in the two studies can be found bioavailable in human blood circulation. However, the concentrations of quercetin evaluated in other studies were 60 – 130 times higher than the bioavailable concentration in human blood circulation.
Myricetin was evaluated in one study at a concentration of 10 μM (Caruana et al. Citation2011) which is 30 times higher than the concentration of flavonol that can be found in human blood circulation. The compounds gossypetin and kaempferol were evaluated at a concentration of 50 μM (Uversky et al. Citation2010; Pan et al. Citation2020) which is greater than 100 times the concentration of flavonol found in blood.
Eight articles evaluated flavonols in an in vivo experimental design using rodents. The evaluated compounds were dihydromyricetin, fisetin, kaempferol and quercetin ().
Fisetin and quercetin were orally administered at concentrations of 3.2 mg/kg/day (Zbarsky et al. Citation2005; Kumar et al. Citation2020). Considering that the daily quantity of flavonols consumed is 0.8 mg/kg (), the evaluated concentrations were four times higher.
Quercetin was also evaluated in four other articles (Karuppagounder et al. Citation2013; El-Horany et al. Citation2016; Ay et al. Citation2017; Wang et al. Citation2021), by intraperitoneal administration at 8 - 24 mg/kg/day (Karuppagounder et al. Citation2013; El-Horany et al. Citation2016). Additionally, quercetin was administered orally at a concentration range of 4.8–28 mg/kg/day. Compared with the daily quantity of flavonols consumed (0.8 mg/kg, ), the evaluated concentrations were 6–35 times higher.
Dihydromyricetin and kaempferol were evaluated by intraperitoneal and orally administration at 1.6 and 3.2 mg/kg/day, respectively (Ren et al. Citation2016; Hu, Li, and Wang Citation2018). Compared with the daily quantity of flavonols consumed (0.8 mg/kg, ), the evaluated concentrations were in 2–4 times higher.
Flavanols
We identified 5 studies that evaluated flavanols. The compounds evaluated included catechin and epigallocatechin gallate ().
Figure 6. Articles that evaluated flavanols in experimental models of PD in vitro 
 .
.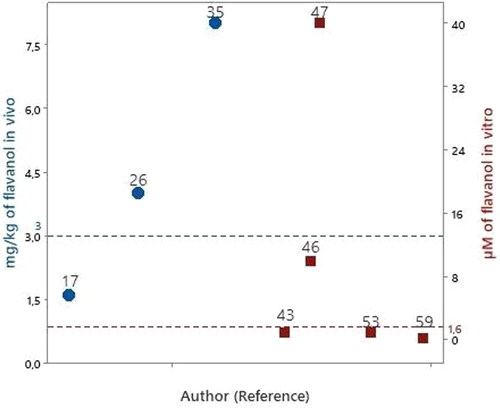
Catechin was evaluated in a study at a concentration of 40 µM (Mercer et al. Citation2005). Compared to the bioavailable concentration of flavanols in human blood circulation at 1.6 μM (), the concentrations evaluated in this study were 25 times higher.
Epigallocatechin gallate (EGCG) was evaluated in four studies (Levites, Youdim, et al. Citation2002; Caruana et al. Citation2011; Lorenzen et al. Citation2014; Xu et al. Citation2016). The lowest evaluated concentration of EGCG was 0.2 μM (Xu et al. Citation2016), and the concentrations evaluated in two studies (Levites, Youdim, et al. Citation2002; Lorenzen et al. Citation2014) and the concentrations evaluated in two studies (Levites, Youdim, et al. Citation2002; Lorenzen et al. Citation2014), can be found bioavailable in human blood circulation. The concentration evaluated in the other study was 6 times higher than the bioavailable flavanol concentration in human bloodstream.
Three studies evaluated EGCG in vivo using rats by oral administration (Levites et al. Citation2001; Xu et al. Citation2017; Zhou, Zhu, and Liang Citation2018), at 1.6 – 8 mg/kg/day (). Compared with the daily quantity of flavanols consumed, (3 mg/kg, ), the amounts evaluated in one article (Levites et al. Citation2001) were less than the amount of flavanols consumed daily, whereas the amount evaluated in other two (Xu et al. Citation2017; Zhou, Zhu, and Liang Citation2018) was 1–3 times higher than the daily consumed amount of flavanols.
Stilbenes
Seven studies evaluated stilbenes in vitro experimental design. The compounds evaluated were viniferin (Sergi et al. Citation2021), piceid (Bai et al. Citation2020) and resveratrol (Okawara et al. Citation2007; Bournival, Quessy, and Martinoli Citation2009; Zhang et al. Citation2010; Sergi et al. Citation2021; Zhang et al. Citation2018; Xia, Sui, and Zhang Citation2019). Viniferin was not evaluated in clear concentration in μM preventing comparisons between tested values and the amount consumed. On the other hand, piceid was evaluated at a concentration of 50 μM. Considering 0.02 μM as the concentration of stilbenes subclass bioavailable in human blood circulation (), that amount of piceid is 2,500 times higher than the reported bioavailable concentration of stilbenes in human blood circulation.
Resveratrol was extensively evaluated, in concentrations of 0.1 (Bournival, Quessy, and Martinoli Citation2009), 60 (Zhang et al. Citation2010), 100 (Okawara et al. Citation2007), 350 (Zhang et al. Citation2018) and 50,000 μM (Xia, Sui, and Zhang Citation2019). These concentrations of resveratrol evaluated are, respectively 5, 3,000, 5,000, 17,500 2,5.106 times higher than the concentration of stilbenes bioavailable in human blood.
Among the included studies, seven studies evaluated stilbenes in vivo experimental design. The compounds evaluated were piceid (Chen et al. Citation2015; Bai et al. Citation2020) and resveratrol (Khan et al. Citation2010; Abolaji et al. Citation2018; Abolaji et al. Citation2018; Zhang et al. Citation2018; Xia, Sui, and Zhang Citation2019; Bai et al. Citation2020). The piceid was evaluated in clear concentrations only in one study (Chen et al. Citation2015), with 22 mg/kg/day. Considering 0.04 mg/kg/day as the concentration of stilbenes subclass consumed in the human diet (), that amount of piceid is 550 times higher than the reported consumed amount of stilbenes consumed daily.
Resveratrol was extensively evaluated in five articles with dose administered to animals extrapolated to humans equal to 0.8, 1.6, 3.2, 9.6 and 22 mg/kg/day, respectively. These amounts are 20 - 550 times higher than the amount of stilbenes subclass consumed daily.
Tyrosols
Only one study evaluated a tyrosol in vitro experimental design. The compound evaluated was oleuropein (Palazzi et al. Citation2018) at a concentration of 700 μM. Considering 0.02 μM as the concentration of tyrosols subclass bioavailable in human blood circulation (), that amount tested of oleuropein is 35,000 times higher than the reported bioavailable concentration of tyrosols in human blood circulation.
Among the included studies, two studies evaluated tyrosols in vivo experimental design. The compounds evaluated were hydroxytyrosol and oleuropein (Brunetti et al. Citation2020; di Rosa et al. Citation2020). However, the amounts tested are not clearly described by the authors. In addition, the daily amounts consumed of tyrosols in the human diet are not found in the literature, preventing comparisons between tested values and the amount consumed.
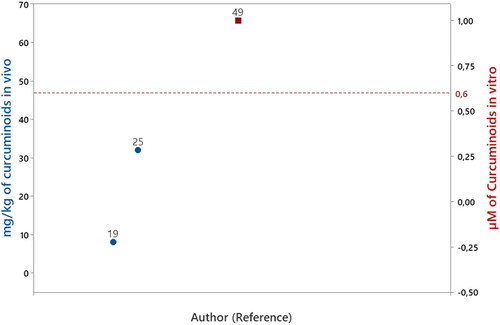
Curcuminoids
Only one study evaluated a curcuminoids in vitro experimental design. The compound evaluated was curcumin (Pandey et al. Citation2008) at a concentration of 1 μM. Considering 0.2 μM as the concentration of curcuminoid bioavailable in human blood circulation (), that amount tested of curcumin is 5 times higher than the reported bioavailable concentration of curcuminoids in human blood circulation.
Among the included studies, two studies evaluated curcuminoids in vivo experimental design. The compound evaluated was only curcumin (Zbarsky et al. Citation2005; Cui, Li, and Zhu Citation2016) at concentrations 8 and 32 mg/kg/day, respectively. On the other hand, the daily amounts consumed of curcuminoids in the human diet is not consolidated in the literature, preventing comparisons between tested amounts in the studies and the reference amount consumed ().
Discussion
Based on experimental designs, the 100 most-cited articles on PD and PCs included one prospective cohort study, 24 in vivo studies, 26 in vitro laboratory studies, 15 studies using both in vivo and in vitro assays and 15 literature reviews.
After extrapolation to human doses, only one group were found to be administered PCs at concentrations below the amounts daily consumed. Only five articles with in vitro assays were found to have evaluated PCs at concentrations below the bioavailable range in human blood circulation.
Therefore, approximately 96% of the studies with an experimental laboratory design evaluated PCs at higher concentrations than those normally found in the blood circulation and/or concentrations consumed in human diet.
In the 1970s and 1980s, David Sackett, David Eddy, and Archie Cochrane through evidence-based medicine (MBE), highlighted the need to strengthen the empirical practice of medicine and proposed the initial rules of evidence to guide clinical decisions (Djulbegovic and Guyatt Citation2017).
MBE suggests a pyramid of evidence focused on design and clinical studies to determine the reliability of results related to the effects of evaluated treatments. The evidence pyramid shows laboratory studies (in vitro and in vivo) and case series at the bottom, followed by case-control and cohort studies in the middle, followed by randomized controlled trials, systematic reviews, and meta-analyses at the top (Murad et al. Citation2016).
The neuroprotective potential of PCs in PD has been demonstrated in several studies; however, the scientific evidence remains limited because the results are derived from in vitro and in vivo experimental designs using PD models, which do not reproduce completely the complexity of the human disease.
In vitro experimental designs for PD are necessary to initially evaluate the off-target pharmacology, cytotoxicity, genetic toxicity, and neuroprotection of PCs, as well as for controlling the concentration and time of cellular exposure to neurotoxins. It is also possible to identify the role of pathological pathways in neurodegeneration (Dexter and Jenner Citation2013).
The selected articles that used an in vitro design demonstrated the neuroprotective effect of the tested PCs; however, these results should be considered with caution, because the in vitro models are not representative of the susceptibility of DN affected by PD in humans and because neuroprotection varies according to the bioavailability of each PCs (Ferreira, Martins, and Barros Citation2017; Dexter and Jenner Citation2013).
Upon oral consumption, PCs undergoes metabolic reactions and conjugations along the gastrointestinal tract and is then transported through the blood-brain barrier. Structural alterations caused by enzymes, which exist particularly at the blood-brain interfaces, where they influence the cerebral availability of toxic compounds, leading to the formation of metabolites, mainly glutathione and glucuronic acid derivatives, which localize in brain tissues nonspecifically at levels below of 1 nmol/g of tissue (Ferreira, Martins, and Barros Citation2017). Further, articles that used the in vitro experimental design tested pure compounds considering that they reached the DN without undergoing any structural changes.
Furthermore, the concentration of metabolites that crosses the blood-brain barrier and reaches the brain is higher than that of the parent compounds (Clancy Citation2002). In several cases, the concentrations of the tested pure compounds were 100 times higher than the bioavailable concentrations found in plasma.
After crossing the BBB, PCs can act to increase the expression of endogenous antioxidant enzymes, inhibit the expression of inflammatory cytokines, increase the activity of the mitochondrial complex and interact with the α-syn protein through covalent and non-covalent bonds, forming soluble oligomers that prevent aggregation and formation as described in .
The ideal experimental design for PD should include degeneration of dopaminergic and non-dopaminergic neurons, the central and peripheral nervous systems, and motor and non-motor symptoms; however, none of the currently available experimental models fulfill these characteristics.
The second most common experimental design among the articles involved in vivo animal models using rats, zebra-fish, and flies. In vivo experimental designs mimic the actual conditions of the organism, facilitating evaluation of the efficiency, efficacy, safety, and therapeutic dose of the phenolic matrix studied (Clancy Citation2002). Further, in vivo experimental models of PD contribute significantly to enhance the understanding of disease progression and identification of potential target therapeutics.
In vivo experimental PD models can be classified as either genetic or neurotoxic (Tieu Citation2011). Genetic models are created using known mechanisms causing the disease but without significant neurodegeneration and phenotypes. The limitations of these models can be complemented using neurotoxic models, which use different molecules to cause damage to the nigrostriatal pathway (Tieu Citation2011). Among the articles on WoS-CC and WoS-AD the neurotoxin models included 6-OHDA, MPTP, LSH and rotenone.
Another point to be reviewed in the experimental laboratory design in vivo is the route of PCs administration. The speed and amounts of compounds reaching the target organ (determinants of treatment effectiveness) depend on the route of administration. The routes of administration can be classified as enteral (oral, sublingual, rectal), parenteral (intravascular, intramuscular, subcutaneous, and inhalation), or topical (skin or mucus membranes). Each route has a specific purpose, advantage, and disadvantage (Mignani et al. Citation2013).
The oral route of administration has several advantages over other routes, including a low total dose, reduced gastrointestinal side effects, improved efficacy/safety ratio, and good patient acceptance and compliance; however, compounds administered via this route are not always fully absorbed (Mignani et al. Citation2013; Lambkin and Pinilla Citation2002).
Generally, absorption is affected by the structure of phenolic compounds. Before absorption, PCs with simpler structures are deglycosylated in the upper fraction of the gut, and those with more complex structures reach the colon, where they are transformed by the microbiota into low-molecular-weight metabolites for absorption (de Araújo et al. Citation2021).
Once absorbed, PCs undergo conjugation with enzymes in the liver. Hepatic metabolites (methylated, sulfated, or glucuronidated conjugates) can then be transported by the circulatory system to organs or be excreted, as these processes increase water solubility (Chen, Cao, and Xiao Citation2018).
Intraperitoneal administration is a type of parenteral administration, which results in rapid initial action on the target organ along with predictable and high bioavailability, suggesting that the administered amount of compounds is completely absorbed; however, this route presents risks of adverse effects owing to the rapid accumulation of high amounts of the administered compound and a risk of infection at the injection site (Chen, Cao, and Xiao Citation2018).
If the objective of studies is to evaluate the neuroprotective effects of PCs and their inclusion in the diet for the prevention of PD, the oral route of administration in in vivo experimental designs would be the most appropriate, as it would demonstrate neuroprotective effects based on the bioavailability of PCs from the diet.
In fact, the oral route was more used for the administration of PCs than other routes such as intraperitoneal (25, 7 studies, respectively). However, the results obtained in these studies should be considered with caution, as the extrapolation of the doses used in animals to humans revealed that the amount administered in 99% of the studies was higher than the daily amount consumed in CPs ingested through the diet. This suggests that the aim of the articles is focused on identifying therapies that could be administered orally as high-dose drugs.
Inappropriate translation of doses evaluated in animals to humans is one of the main causes of drug inefficacy in clinical studies. Extrapolation of the tested dose in animals to humans should use the most appropriate normalization based on body surface area (Reagan‐Shaw, Nihal, and Ahmad Citation2008).
The neuroprotective effects of PCs in humans from the diet depend on the amount ingested, which is estimated from the PCs concentration in food and their bioavailability PCs. The amount of PCs ingested PCS varies with individual food culture, sex, age, and other factors (Pinto and Santos Citation2017).
Consumption of PCs by the general population in Brazil is approximately 460.2 mg/day. This amount is lower than PCs consumed by the general population in the USA (900 mg/day), France (1,193.0 mg/day), and Japan (1,500 mg/day) (Bo’ et al. Citation2019). The doses evaluated in the experimental laboratory designs in vivo, when extrapolated to humans were 2 – 400 times higher than the daily amounts consumed.
Conclusion
The findings of the articles reviewed in this study indicate the neuroprotective potential of PCs on PD. The experimental designs of the studies evaluated in 80% are laboratory studies that suggest the neuroprotective effects of PCs not through the diet, but from the development of drugs with a high concentration of PCs for oral therapeutic use. However, it is necessary to validate the results obtained with the most promising PCs through highly controlled clinical studies, so that a diet enriched with these new products can be designed.
| Abbreviations | ||
| 5-HT | = | Serotonin |
| 6-OHDA | = | 6-Hydroxydopamine |
| α-syn | = | α-Synuclein |
| AChE | = | Acetylcholinesterase |
| Akb | = | Protein kinase B |
| ARE | = | Antioxidant response elements |
| Bax | = | BCL2 associated X |
| BBB | = | Blood-brain barrier |
| BCL2 | = | BCL2 apoptosis regulator |
| BDNF | = | Brain-derived neurotrophic factor |
| BTE | = | Black tea extract |
| CaMKII | = | Ca2+/calmodulin-dependent protein kinase II |
| CAT | = | Catalase |
| C-Jun | = | Protein encoded by the JUN gene |
| COMT | = | Catechol O-methyltransferase |
| Cyt c | = | Cytochrome c |
| DA | = | Dopamine |
| DARPP-32 | = | Dopamine- and cAMP-regulated phosphoprotein-32 |
| DN | = | Dopamine neurons |
| EGCG | = | Epigallocatechin gallate |
| ERK 1/2 | = | Extracellular signal-regulated kinases |
| GDNF | = | Glial cell-derived neurotrophic factor |
| GPx | = | Glutathione peroxidase |
| GSH | = | Glutathione |
| GSK-3β | = | Glycogen synthase kinase-3β |
| GTP | = | Green tea polyphenols |
| HMOX1 | = | Heme oxygenase-1 |
| HVA | = | Homovanillic acid |
| IFN-γ | = | Interferon-gamma |
| IL | = | Interleukin |
| iNOS | = | Nitric oxide synthase |
| I.P. | = | Intraperitonial |
| JNK 1/2 | = | c-Jun N-terminal kinase |
| Keap1 | = | Kelch-like ECH-associated protein 1 |
| LDH | = | Lactate dehydrogenase |
| MAO-Bis | = | monoamine oxidase type B inhibitors |
| MDA | = | Malondialdehyde |
| MMP | = | Mitochondrial membrane potential |
| MPP | = | 1-methyl-4-phenylpyridinium mRNA = Messenger ribonucleic acid |
| NDGA | = | Nordihydroguaiaretic acid |
| NF-ĸB | = | Nuclear factor-kappa B |
| NO | = | Nitric oxide |
| NQO1 | = | NAD(P)H:quinone oxidoreductase |
| Nrf-2 | = | Erythroid nuclear factor-2 related to factor 2 |
| O | = | orally |
| P38 | = | p38 mitogen-activated protein kinases |
| PCa | = | Carbonyl protein |
| PC | = | Phenolic compounds |
| PD | = | Parkinson’s disease |
| PIK-3 | = | Phosphoinositide 3-kinase |
| PKC | = | Protein kinase C |
| PLA2 | = | Phospholipase A2 |
| ROS | = | Reactive oxygen species |
| SMAC | = | Second mitochondria-derived activator of caspase |
| SOD | = | Superoxide dismutase |
| TBARS | = | Thiobarbituric acid reactive substances |
| TCM | = | Traditional Chinese Medicine |
| TH | = | Tyrosine hydroxylase |
| TH-ir | = | TH-immunoreactive |
| TNF-α | = | Tumor necrosis factor alpha |
| TRAP | = | Total reactive antioxidant potential |
| TS | = | Topic |
| VOSviewer | = | Visualization of Similarities Viewer |
| WoS-CC | = | Web of Science Core Collection |
Acknowledgements
José Messias Perdigão and Bruno José Brito Texeira and Vinicius Carvalho-da-Silva performed experiments, analyzed, interpreted the data and drafted the manuscript. Rui Daniel Prediger, Rafael Rodrigues Lima and Hervé Rogez formulated the study concept, designed the study, and made critical revisions of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Abolaji, A. O., A. O. Adedara, M. A. Adie, M. Vicente-Crespo, and E. O. Farombi. 2018. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced oxidative damage and behavioural deficits in drosophila melanogaster. Biochemical and Biophysical Research Communications 503 (2):1042–8. doi: 10.1016/j.bbrc.2018.06.114.
- Alemán-Jiménez, C., R. Domínguez-Perles, S. Medina, I. Prgomet, I. López-González, A. Simonelli-Muñoz, M. Campillo-Cano, D. Auñón, F. Ferreres, and Á. Gil-Izquierdo. 2021. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. European Journal of Nutrition 60 (2):905–15. doi: 10.1007/s00394-020-02295-0.
- Angelopoulou, E., E. S. Pyrgelis, and C. Piperi. 2020. Neuroprotective potential of chrysin in Parkinson’s disease: Molecular mechanisms and clinical implications. Neurochemistry International:(132)
- Antunes, M. S., F. V. L. Ladd, A. Ladd, A. L. Moreira, S. P. Boeira, and L. Cattelan Souza. 2021. Hesperidin protects against behavioral alterations and loss of dopaminergic neurons in 6-OHDA-lesioned mice: The role of mitochondrial dysfunction and apoptosis. Metabolic Brain Disease 36 (1):153–67. doi: 10.1007/s11011-020-00618-y.
- Anusha, C., T. Sumathi, and L. D. Joseph. 2017. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chemico-Biological Interactions 269:67–79. doi: 10.1016/j.cbi.2017.03.016.
- Aquilano, K., S. Baldelli, G. Rotilio, and M. R. Ciriolo. 2008. Role of nitric oxide synthases in Parkinson’s disease: A review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochemical Research 33 (12):2416–26. doi: 10.1007/s11064-008-9697-6.
- Ardah, M. T., K. E. Paleologou, G. Lv, S. B. Abul Khair, A. S. Kazim, S. T. Minhas, T. H. Al-Tel, A. A. Al-Hayani, M. E. Haque, D. Eliezer, et al. 2014. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Frontiers in Aging Neuroscience 6:197. doi: 10.3389/fnagi.2014.00197.
- Aryal, S., T. Skinner, B. Bridges, and J. T. Weber. 2020. The pathology of Parkinson’s disease and potential benefit of dietary polyphenols. Molecules 25:(19).
- Ay, M., J. Luo, M. Langley, H. Jin, V. Anantharam, A. Kanthasamy, and A. G. Kanthasamy. 2017. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and mitopark transgenic mouse models of Parkinson’s disease. Journal of Neurochemistry 141 (5):766–82. doi: 10.1111/jnc.14033.
- Bai, H., Y. Ding, X. Li, D. Kong, C. Xin, X. Yang, C. Zhang, Z. Rong, C. Yao, S. Lu, et al. 2020. Polydatin protects SH-SY5Y in models of Parkinson’s disease by promoting Atg5-mediated but parkin-independent autophagy. Neurochemistry International 134:104671. doi: 10.1016/j.neuint.2020.104671.
- Baluchnejadmojarad, T., N. Rabiee, S. Zabihnejad, and M. Roghani. 2017. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson’s disease: possible involvement of ERβ/Nrf2/HO-1 signaling. Brain Research 1662:23–30. doi: 10.1016/j.brainres.2017.02.021.
- Bo’, Bernardi, Marino, Porrini, Tucci, Guglielmetti, Cherubini, Carrieri, Kirkup, Kroon, et al. 2019. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 11 (6):1355. doi: 10.3390/nu11061355.
- Bournival, J., P. Quessy, and M. G. Martinoli. 2009. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cellular and Molecular Neurobiology 29 (8):1169–80. doi: 10.1007/s10571-009-9411-5.
- Brunetti, G., G. di Rosa, M. Scuto, M. Leri, M. Stefani, C. Schmitz-Linneweber, V. Calabrese, and N. Saul. 2020. Healthspan maintenance and prevention of Parkinson’s-like phenotypes with hydroxytyrosol and oleuropein aglycone in C. Elegans. International Journal of Molecular Science 21(7):1-23 doi: 10.3390/ijms21072588.
- Bureau, G., F. Longpré, and M. G. Martinoli. 2008. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. Journal of Neuroscience Research 86 (2):403–10. doi: 10.1002/jnr.21503.
- Cai, Z. Y., X. M. Li, J. P. Liang, L. P. Xiang, K. R. Wang, Y. L. Shi, R. Yang, M. Shi, J. H. Ye, J. L. Lu, et al. 2018. Bioavailability of tea catechins and its improvement. Molecules 23:(9).
- Calvo-Castro, L. A., C. Schiborr, F. David, H. Ehrt, J. Voggel, N. Sus, D. Behnam, A. Bosy-Westphal, and J. Frank. 2018. The oral bioavailability of trans-resveratrol from a grapevine-shoot extract in healthy humans is significantly increased by micellar solubilization. Molecular Nutrition & Food Research 62 (9):1701057. doi: 10.1002/mnfr.201701057.
- Cao, Q., L. Qin, F. Huang, X. Wang, L. Yang, H. Shi, H. Wu, B. Zhang, Z. Chen, and X. Wu. 2017. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicology and Applied Pharmacology 319:80–90. doi: 10.1016/j.taap.2017.01.019.
- Caruana, M., T. Högen, J. Levin, A. Hillmer, A. Giese, and N. Vassallo. 2011. Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Letters 585 (8):1113–20. doi: 10.1016/j.febslet.2011.03.046.
- Chandrasekhar, Y., G. Phani Kumar, E. M. Ramya, and K. R. Anilakumar. 2018. Gallic acid protects 6-OHDA induced neurotoxicity by attenuating oxidative stress in human dopaminergic cell line. Neurochemical Research 43 (6):1150–60. doi: 10.1007/s11064-018-2530-y.
- Chen, L., H. Cao, and J. Xiao. 2018. Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, recovery, and applications, 45–67. Austria: Elsevier. ISBN 9780128135723.
- Chen, Y., D. Q. Zhang, Z. Liao, B. Wang, S. Gong, C. Wang, M. Z. Zhang, G. H. Wang, H. Cai, F. F. Liao, et al. 2015. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Molecular Neurodegeneration 10:4. doi: 10.1186/1750-1326-10-4.
- Cheynier, V., G. Comte, K. M. Davies, V. Lattanzio, and S. Martens. 2013. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiology and Biochemistry: PPB 72:1–20. doi: 10.1016/j.plaphy.2013.05.009.
- Cirmi, S., A. Maugeri, G. E. Lombardo, C. Russo, L. Musumeci, S. Gangemi, G. Calapai, D. Barreca, and M. Navarra. 2021. A flavonoid-rich extract of mandarin juice counteracts 6-OHDA-induced oxidative stress in Sh-Sy5y cells and modulates Parkinson-related genes. Antioxidants 10:(4). doi: 10.3390/antiox10040539.
- Clancy, M. J. 2002. Overview of research designs. Emergency Medicine Journal 19 (6):546–9. doi: 10.1136/emj.19.6.546.
- Crozier, A., D. del Rio, and M. N. Clifford. 2010. Bioavailability of dietary flavonoids and phenolic compounds. Molecular Aspects of Medicine 31 (6):446–67. doi: 10.1016/j.mam.2010.09.007.
- Cui, Q., X. Li, and H. Zhu. 2016. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Molecular Medicine Reports 13 (2):1381–8. doi: 10.3892/mmr.2015.4657.
- Dabeek, W. M., and M. V. Marra. 2019. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 11:(10).
- de Araújo, F. F., D. de Paulo Farias, I. A. Neri-Numa, and G. M. Pastore. 2021. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chemistry 338:127535. doi: 10.1016/j.foodchem.2020.127535.
- DeRango-Adem, E. F., and J. Blay. 2021. Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Frontiers in Pharmacology: (12).
- Dexter, D. T., and P. Jenner. 2013. Parkinson Disease: From pathology to molecular disease mechanisms. Free radical Biology & Medicine 62:132–44.
- di Rosa, G., G. Brunetti, M. Scuto, A. T. Salinaro, E. J. Calabrese, R. Crea, C. Schmitz-Linneweber, V. Calabrese, and N. Saul. 2020. Healthspan enhancement by olive polyphenols in C. Elegans wild type and Parkinson’s models. International Journal of Molecualar Science 21:1–22. doi: 10.3390/ijms21113893.
- Djulbegovic, B., and G. H. Guyatt. 2017. Progress in evidence-based medicine: A quarter century on. The Lancet 390 (10092):415–23. doi: 10.1016/S0140-6736(16)31592-6.
- Duda-Chodak, A., T. Tarko, P. Satora, and P. Sroka. 2015. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. European Journal of Nutrition 54 (3):325–41. doi: 10.1007/s00394-015-0852-y.
- El-Horany, H. E., R. N. A. El-Latif, M. M. ElBatsh, and M. N. Emam. 2016. Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson’s disease: Modulating autophagy (quercetin on experimental Parkinson’s disease). Journal of Biochemical and Molecular Toxicology 30 (7):360–9. doi: 10.1002/jbt.21821.
- Elmazoglu, Z., A. S. Yar Saglam, C. Sonmez, and C. Karasu. 2020. Luteolin protects microglia against rotenone-induced toxicity in a hormetic manner through targeting oxidative stress response, genes associated with Parkinson’s disease and inflammatory pathways. Drug and Chemical Toxicology 43 (1):96–103. doi: 10.1080/01480545.2018.1504961.
- Ferreira, I., N. Martins, and L. Barros. 2017. Phenolic compounds and its bioavailability: In vitro bioactive compounds or health promoters? Advances in Food and Nutrition Research 82:1–44. doi: 10.1016/bs.afnr.2016.12.004.
- Gao, X., A. Cassidy, M. Schwarzschild, E. Rimm, S. A. Ascherio, and E. Ebr. 2012. Habitual intake of dietary flavonoids and risk of Parkinson Disease. Neurology 78 (15):1138–1145.
- Giuliano, C., S. Cerri, and F. Blandini. 2021. Potential therapeutic effects of polyphenols in Parkinson’s disease: In vivo and in vitro pre-clinical studies. Neural Regeneration Research 16 (2):234–41. doi: 10.4103/1673-5374.290879.
- Goes, A. T. R., C. R. Jesse, M. S. Antunes, F. v Lobo Ladd, A. A. B. Lobo Ladd, C. Luchese, N. Paroul, and S. P. Boeira. 2018. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: involvement of neuroinflammation and neurotrophins. Chemico-Biological Interactions 279:111–20. doi: 10.1016/j.cbi.2017.10.019.
- Han, X., S. Zhao, H. Song, T. Xu, Q. Fang, G. Hu, and L. Sun. 2021. Kaempferol alleviates LD-mitochondrial damage by promoting autophagy: Implications in Parkinson’s disease. Redox Biology 41:101911. doi: 10.1016/j.redox.2021.101911.
- Ho, L., D. Zhao, K. Ono, K. Ruan, I. Mogno, M. Tsuji, E. Carry, J. Brathwaite, S. Sims, T. Frolinger, et al. 2019. Heterogeneity in gut microbiota drive polyphenol metabolism that influences α-synuclein misfolding and toxicity. The Journal of Nutritional Biochemistry 64:170–81. doi: 10.1016/j.jnutbio.2018.10.019.
- Holst, B., and G. Williamson. 2008. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Current Opinion in Biotechnology 19 (2):73–82. doi: 10.1016/j.copbio.2008.03.003.
- Hu, M., F. Li, and W. Wang. 2018. Vitexin protects dopaminergic neurons in Mptp-induced Parkinson’s disease through Pi3k/Akt signaling pathway. Drug Design, Development and Therapy 12:565–73. doi: 10.2147/DDDT.S156920.
- Hussain, G., L. Zhang, A. Rasul, H. Anwar, M. U. Sohail, A. Razzaq, N. Aziz, A. Shabbir, M. Ali, and T. Sun. 2018. Role of plant-derived flavonoids and their mechanism in attenuation of Alzheimer’s and Parkinson’s diseases: An update of recent data. Molecules 23 (4):814. doi: 10.3390/molecules23040814.
- Jeong, S. H., J. H. Jang, H. Y. Cho, I. J. Oh, and Y. B. Lee. 2020. A sensitive UPLC–ESI–MS/MS method for the quantification of cinnamic acid in vivo and in vitro: Application to pharmacokinetic and protein binding study in human plasma. Journal of Pharmaceutical Investigation 50 (2):159–72. doi: 10.1007/s40005-019-00444-0.
- Joshi, R., Y. A. Kulkarni, and S. Wairkar. 2018. Pharmacokinetic, pharmacodynamic and formulations aspects of naringenin: An update. Life Sciences 215:43–56. doi: 10.1016/j.lfs.2018.10.066.
- Jude, S., A. Amalraj, A. B. Kunnumakkara, C. Divya, B. M. Löffler, and S. Gopi. 2018. Development of validated methods and quantification of curcuminoids and curcumin metabolites and their pharmacokinetic study of oral administration of complete natural turmeric formulation (CureitTM) in human plasma via UPLC/ESI-Q-TOF-MS spectrometry. Molecules 23(10). doi: 10.3390/molecules23102415.
- Jung, U. J., and S. R. Kim. 2018. Beneficial effects of flavonoids against Parkinson’s disease. Journal of Medicinal Food 21 (5):421–32. doi: 10.1089/jmf.2017.4078.
- Karuppagounder, S. S., S. K. Madathil, M. Pandey, R. Haobam, U. Rajamma, and K. P. Mohanakumar. 2013. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 236:136–48. doi: 10.1016/j.neuroscience.2013.01.032.
- Kesh, S., R. R. Kannan, and A. Balakrishnan. 2021. Naringenin alleviates 6-hydroxydopamine induced Parkinsonism in SHSY5Y cells and Zebrafish model. Comparative Biochemistry and Physiology. Toxicology & Pharmacology: CBP 239:108893. doi: 10.1016/j.cbpc.2020.108893.
- Khan, M. M., A. Ahmad, T. Ishrat, M. B. Khan, M. N. Hoda, G. Khuwaja, S. S. Raza, A. Khan, H. Javed, K. Vaibhav, et al. 2010. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Research 1328:139–51. doi: 10.1016/j.brainres.2010.02.031.
- Kim, S. J., H. Shin, S. M. Cheon, S. M. Ko, S. H. Ham, Y. D. Kwon, Y. B. Lee, and H. Y. Cho. 2017. A sensitive UHPLC–MS/MS method for the simultaneous quantification of three lignans in human plasma and its application to a pharmacokinetic study. Journal of Separation Science 40 (17):3430–9. doi: 10.1002/jssc.201700588.
- Kim, T. Y., E. Leem, J. M. Lee, and S. R. Kim. 2020. Control of reactive oxygen species for the prevention of Parkinson’s disease: The possible application of flavonoids. Antioxidants 9:1–28. doi: 10.3390/antiox9070583.
- Kumar, R., R. Kumar, N. Khurana, S. K. Singh, S. Khurana, S. Verma, N. Sharma, B. Kapoor, M. Vyas, R. Khursheed, et al. 2020. Enhanced oral bioavailability and neuroprotective effect of fisetin through its SNEDDS against rotenone-induced Parkinson’s disease rat model. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 144:111590. doi: 10.1016/j.fct.2020.111590.
- Kung, H. C., K. J. Lin, C. t Kung, and T. K. Lin. 2021. Oxidative stress, mitochondrial dysfunction, and neuroprotection of polyphenols with respect to resveratrol in Parkinson’s disease. Biomedicines 9(8).
- Lambkin, I., and C. Pinilla. 2002. Targeting approaches to oral drug delivery. Expert Opinion on Biological Therapy 2 (1):67–73. doi: 10.1517/14712598.2.1.67.
- Levites, Y., B. H. Youdim, G. Maor, and S. Mandel. 2002. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-KappaB (NF-B) activation and cell death by tea extracts in neuronal cultures. Biochemical Pharmacology 63 (1):21–29.
- Levites, Y., O. Weinreb, G. Maor, 2. Moussa, B. H. Youdim, and S. Mandel. 2001. Green tea polyphenol (±)-epigallocatechin-3-gallate prevents-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration. Journal of Neurochemistry 78 (5):1073–82. doi: 10.1046/j.1471-4159.2001.00490.x.
- Levites, Y., T. Amit, M. B. H. Youdim, and S. Mandel. 2002. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. The Journal of Biological Chemistry 277 (34):30574–80. doi: 10.1074/jbc.M202832200.
- Limboonreung, T., P. Tuchinda, and S. Chongthammakun. 2020. Chrysoeriol mediates mitochondrial protection via PI3K/Akt pathway in MPP + treated SH-SY5Y cells. Neuroscience Letters 714:134545. doi: 10.1016/j.neulet.2019.134545.
- Lorenzen, N., S. B. Nielsen, Y. Yoshimura, B. S. Vad, C. B. Andersen, C. Betzer, J. D. Kaspersen, G. Christiansen, J. S. Pedersen, P. H. Jensen, et al. 2014. How epigallocatechin gallate can inhibit α-synuclein oligomer toxicity in vitro. The Journal of Biological Chemistry 289 (31):21299–310. doi: 10.1074/jbc.M114.554667.
- Lou, H., X. Jing, X. Wei, H. Shi, D. Ren, and X. Zhang. 2014. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 79:380–8. doi: 10.1016/j.neuropharm.2013.11.026.
- Magalingam, K. B., A. K. Radhakrishnan, and N. Haleagrahara. 2015. Protective mechanisms of flavonoids in Parkinson’s disease. Oxidative medicine and Cellular Longevity 2015:314560. doi: 10.1155/2015/314560.
- Malar, D. S., M. I. Prasanth, J. M. Brimson, R. Sharika, B. S. Sivamaruthi, C. Chaiyasut, and T. Tencomnao. 2020. Neuroprotective properties of green tea (Camellia Sinensis) in Parkinson’s disease: A review. Molecules 25 (17):3926. doi: 10.3390/molecules25173926.
- Mancuso, C., R. Siciliano, and E. Barone. 2011. Curcumin and Alzheimer disease: This is not to be performed. Journal of Biological Chemistry:286(3). doi: 10.1074/jbc.L110.133520.
- Mercer, L. D., B. L. Kelly, M. K. Horne, and P. M. Beart. 2005. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: Investigations in primary rat mesencephalic cultures. Biochemical Pharmacology 69 (2):339–45. doi: 10.1016/j.bcp.2004.09.018.
- Mignani, S., S. el Kazzouli, M. Bousmina, and J. P. Majoral. 2013. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Advanced Drug Delivery Reviews 65 (10):1316–30. doi: 10.1016/j.addr.2013.01.001.
- Mohammad-Beigi, H., F. Aliakbari, C. Sahin, C. Lomax, A. Tawfike, N. P. Schafer, A. Amiri-Nowdijeh, H. Eskandari, I. M. Møller, M. Hosseini-Mazinani, et al. 2019. Oleuropein derivatives from olive fruit extracts reduce - synuclein fibrillation and oligomer toxicity. Journal of Biological Chemistry 294 (11):4215–32. doi: 10.1074/jbc.RA118.005723.
- Mu, X., G. He, Y. Cheng, X. Li, B. Xu, and G. Du. 2009. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental Parkinsonism in vivo and in vitro. Pharmacology, Biochemistry, and Behavior 92 (4):642–8. doi: 10.1016/j.pbb.2009.03.008.
- Murad, H., N. Asi, M. Alsawas, and F. Alahdab. 2016. New evidence pyramid. Evidence Based Medicine:(21). doi: 10.1136/ebmed.
- Mythri, R. B., and M. M. S. Bharath. 2012. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Current Pharmaceutical Design 18 (1):91–99.
- Okawara, M., H. Katsuki, E. Kurimoto, H. Shibata, T. Kume, and A. Akaike. 2007. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochemical Pharmacology 73 (4):550–60. doi: 10.1016/j.bcp.2006.11.003.
- Palazzi, L., E. Bruzzone, G. Bisello, M. Leri, M. Stefani, M. Bucciantini, and P. P. de Laureto. 2018. Oleuropein aglycone stabilizes the monomeric α-synuclein and favours the growth of non-toxic aggregates. Scientific Reports 8 (1):528–533. doi: 10.1038/s41598-018-26645-5.
- Pan, X., X. Liu, H. Zhao, B. Wu, and G. A. Liu. 2020. Anti-inflammatory and neuroprotective effect of kaempferol on rotenone-induced Parkinson’s disease model of rats and SH-S5Y5 cells by preventing loss of tyrosine hydroxylase. Journal of Functional Foods 74:104140. doi: 10.1016/j.jff.2020.104140.
- Pandey, N., J. Strider, W. C. Nolan, S. X. Yan, and J. E. Galvin. 2008. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathologica 115 (4):479–89. doi: 10.1007/s00401-007-0332-4.
- Patil, S. P., P. D. Jain, J. S. Sancheti, P. J. Ghumatkar, R. Tambe, and S. Sathaye. 2014. Neuroprotective and neurotrophic effects of apigenin and luteolin in MPTP induced Parkinsonism in mice. Neuropharmacology 86:192–202. doi: 10.1016/j.neuropharm.2014.07.012.
- Pinto, P., and C. N. Santos. 2017. Worldwide (poly)phenol intake: Assessment methods and identified gaps. European Journal of Nutrition 56 (4):1393–408. doi: 10.1007/s00394-016-1354-2.
- Reagan‐Shaw, S., M. Nihal, and N. Ahmad. 2008. Dose translation from animal to human studies revisited. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 22 (3):659–61. doi: 10.1096/fj.07-9574lsf.
- Reglodi, D., J. Renaud, A. Tamas, Y. Tizabi, S. B. Socías, E. Del-Bel, and R. Raisman-Vozari. 2017. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Progress in Neurobiology 155:120–48. doi: 10.1016/j.pneurobio.2015.10.004.
- Ren, Z. X., Y. F. Zhao, T. Cao, and X. C. Zhen. 2016. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacologica Sinica 37 (10):1315–24. doi: 10.1038/aps.2016.42.
- Rui, W., S. Li, H. Xiao, M. Xiao, and J. Shi. 2020. Baicalein attenuates neuroinflammation by inhibiting NLRP3/Caspase-1/GSDMD pathway in MPTP-induced mice model of Parkinson’s disease. The International Journal of Neuropsychopharmacology 23 (11):762–73. doi: 10.1093/ijnp/pyaa060.
- Sang, S., J. D. Lambert, and C. S. Yang. 2006. Bioavailability and stability issues in understanding the cancer preventive effects of tea polyphenols. Journal of the Science of Food and Agriculture 86 (14):2256–65. doi: 10.1002/jsfa.2660.
- Sergi, D., A. Gélinas, J. Beaulieu, J. Renaud, E. Tardif-Pellerin, J. Guillard, and M. G. Martinoli. 2021. Anti-apoptotic and anti-inflammatory role of trans ε-viniferin in a neuron–glia co-culture cellular model of Parkinson’s disease. Foods:10(3). doi: 10.3390/foods10030586.
- Singh, A., P. Tripathi, A. K. Yadawa, and S. Singh. 2020. Promising polyphenols in Parkinson’s disease therapeutics. Neurochemical Research 45 (8):1731–45. doi: 10.1007/s11064-020-03058-3.
- Singh, S., S. N. Sen Rai, H. Birla, W. Zahra, A. S. Rathore, and S. P. Singh. 2020. NF-ΚB-mediated neuroinflammation in Parkinson’s disease and potential therapeutic effect of polyphenols. Neurotoxicity Research 37 (3):491–507. doi: 10.1007/s12640-019-00147-2.
- Soyata, A., A. N. Hasanah, and T. Rusdiana. 2021. Isoflavones in soybean as a daily nutrient: The mechanisms of action and how they alter the pharmacokinetics of drugs. Turkish Journal of Pharmaceutical Sciences 18 (6):799–810. doi: 10.4274/tjps.galenos.2020.79106.
- Stefania, D. S., M. L. Clodoveo, M. Cariello, G. D’Amato, C. Franchini, M. F. Faienza, and F. Corbo. 2021. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Critical Reviews in Food Science and Nutrition 61 (11):1804–26. doi: 10.1080/10408398.2020.1765736.
- Strathearn, K. E., G. G. Yousef, M. H. Grace, S. L. Roy, M. A. Tambe, M. G. Ferruzzi, Q. L. Wu, J. E. Simon, M. A. Lila, and J. C. Rochet. 2014. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Research 1555:60–77. doi: 10.1016/j.brainres.2014.01.047.
- Stromsnes, K., R. Lagzdina, G. Olaso‐gonzalez, L. Gimeno‐mallench, and J. Gambini. 2021. Pharmacological properties of polyphenols: Bioavailability, mechanisms of action and biological effects in in vitro studies, animal models and humans. Biomedicines 9 (8):1074. doi: 10.3390/biomedicines9081074.
- Su, C. F., L. Jiang, X. W. Zhang, A. Iyaswamy, and M. Li. 2021. Resveratrol in rodent models of Parkinson’s disease: A systematic review of experimental studies. Frontiers in Pharmacology:12.
- Tamilselvam, K., N. Braidy, T. Manivasagam, M. M. Essa, N. R. Prasad, S. Karthikeyan, A. J. Thenmozhi, S. Selvaraju, and G. J. Guillemin. 2013. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease. Oxidative Medicine and Cellular Longevity 2013:1–11. doi: 10.1155/2013/102741.
- Tieu, K. 2011. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine 1:a009316. doi: 10.1101/cshperspect.a009316.
- Trapani, A., L. Guerra, F. Corbo, S. Castellani, E. Sanna, L. Capobianco, A. G. Monteduro, D. E. Manno, D. Mandracchia, S. di Gioia, et al. 2021. Cyto/biocompatibility of dopamine combined with the antioxidant grape seed-derived polyphenol compounds in solid lipid nanoparticles. Molecules 26 (4):916. doi: 10.3390/molecules26040916.
- Tsimogiannis, D., and V. Oreopoulou. 2019. Classification of phenolic compounds in plants. In Polyphenols in plants, 263–84. Massachusetts, EUA, Elsevier.
- Ullah, H., and H. Khan. 2018. Anti-Parkinson potential of silymarin: Mechanistic insight and therapeutic standing. Frontiers in Pharmacology 9: 1–10.
- Uversky, V. N., X. Meng, L. A. Munishkina, and A. L. Fink. 2010. Effects of various flavonoids on the -synuclein fibrillation process. Parkinson’s Disease 2010:650794. doi: 10.4061/2010/650794.
- Wang, W.-W., R. Han, H.-J. He, J. Li, S.-Y. Chen, Y. Gu, and C. Xie. 2021. Administration of quercetin improves mitochondria quality control and protects the neurons in 6-OHDA-lesioned Parkinson’s disease models. Aging 13 (8):11738–51. doi: 10.18632/aging.202868.
- Wang, X. S., Z. R. Zhang, M. M. Zhang, M. X. Sun, W. W. Wang, and C. L. Xie. 2017. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: A systematic experiment literatures review. BMC Complementary and Alternative Medicine 17 (1):1–10. doi: 10.1186/s12906-017-1922-x.
- Wruck, C. J., M. Claussen, G. Fuhrmann, L. Römer, A. Schulz, T. Pufe, V. Waetzig, M. Peipp, T. Herdegen, and M. E. Götz. 2007. Luteolin protects rat PC12 and C6 cells against MPP 1 induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. Journal of Neural Transmission 57–67.
- Wu, H., G. Oliveira, and M. A. Lila. 2022. Protein-binding approaches for improving bioaccessibility and bioavailability of anthocyanins. Comprehensive Reviews in Food Science and Food Safety (1):333–354. doi: 10.1111/1541-4337.13070
- Xia, D., R. Sui, and Z. Zhang. 2019. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/MiR-129/SNCA signaling pathway. Journal of Cellular Biochemistry 120 (4):4942–51. doi: 10.1002/jcb.27769.
- Xiong, S., W. Liu, Y. Zhou, Y. Mo, Y. Liu, X. Chen, H. Pan, D. Yuan, Q. Wang, and T. Chen. 2020. Enhancement of oral bioavailability and anti-parkinsonian efficacy of resveratrol through a nanocrystal formulation. Asian Journal of Pharmaceutical Sciences 15 (4):518–28. doi: 10.1016/j.ajps.2019.04.003.
- Xu, Q., M. Langley, A. G. Kanthasamy, and M. B. Reddy. 2017. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. The Journal of Nutrition 147 (10):1926–31. doi: 10.3945/jn.117.255034.
- Xu, Y., Y. Zhang, Z. Quan, W. Wong, J. Guo, R. Zhang, Q. Yang, R. Dai, P. L. McGeer, and H. Qing. 2016. Epigallocatechin Gallate (EGCG) inhibits alpha-synuclein aggregation: A potential agent for Parkinson’s disease. Neurochemical Research 41 (10):2788–96. doi: 10.1007/s11064-016-1995-9.
- Yang, J., M. Jia, X. Zhang, and P. Wang. 2019. Calycosin attenuates MPTP-induced Parkinson’s disease by suppressing the activation of TLR/NF-ΚB and MAPK pathways. Phytotherapy Research: PTR 33 (2):309–18. doi: 10.1002/ptr.6221.
- Zbarsky, V., K. P. Datla, S. Parkar, D. K. Rai, O. I. Aruoma, and D. T. Dexter. 2005. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radical Research 39 (10):1119–25. doi: 10.1080/10715760500233113.
- Zhang, F., J. S. Shi, H. Zhou, B. Wilson, J. S. Hong, and H. M. Gao. 2010. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Molecular Pharmacology 78 (3):466–77. doi: 10.1124/mol.110.064535.
- Zhang, L. F., X. L. Yu, M. Ji, S. Y. Liu, X. L. Wu, Y. J. Wang, and R. T. Liu. 2018. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food & Function 9 (12):6414–26. doi: 10.1039/C8FO00964C.
- Zhang, S., Z. Yu, J. Xia, X. Zhang, K. Liu, A. Sik, and M. Jin. 2020. Anti-Parkinson’s disease activity of phenolic acids from: Eucommia ulmoides oliver leaf extracts and their autophagy activation mechanism. Food & Function 11 (2):1425–40. doi: 10.1039/c9fo02288k.
- Zhang, X., L. Du, W. Zhang, Y. Yang, Q. Zhou, and G. Du. 2017. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Scientific Reports 7:1–14.doi: 10.1038/s41598-017-07442-y.
- Zhang, Z., G. Li, S. S. W. Szeto, C. M. Chong, Q. Quan, C. Huang, W. Cui, B. Guo, Y. Wang, Y. Han, et al. 2015. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radical Biology & Medicine 84:331–43. doi: 10.1016/j.freeradbiomed.2015.02.030.
- Zhang, Z., W. Cui, G. Li, S. Yuan, D. Xu, M. P. M. Hoi, Z. Lin, J. Dou, Y. Han, and S. M. Y. Lee. 2012. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. Journal of Agricultural and Food Chemistry 60 (33):8171–82. doi: 10.1021/jf301511m.
- Zhao, X., D. Kong, Q. Zhou, G. Wei, J. Song, Y. Liang, and G. Du. 2021. Baicalein alleviates depression-like behavior in rotenone- induced Parkinson’s disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 140:111556. doi: 10.1016/j.biopha.2021.111556.
- Zheng, J., H. Xiong, Q. Li, L. He, H. Weng, W. Ling, and D. Wang. 2019. Protocatechuic acid from chicory is bioavailable and undergoes partial glucuronidation and sulfation in healthy humans. Food Science & Nutrition 7 (9):3071–80. doi: 10.1002/fsn3.1168.
- Zhou, T., M. Zhu, and Z. Liang. 2018. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Molecular Medicine Reports 17 (4):4883–8. doi: 10.3892/mmr.2018.8470.
- Zhu, M., S. Rajamani, J. Kaylor, S. Han, F. Zhou, and A. L. Fink. 2004. The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. The Journal of Biological Chemistry 279 (26):26846–57. doi: 10.1074/jbc.M403129200.