Abstract
Triclosan is an antimicrobial agent used in a range of consumer products, such as deodorants, oral care, clothing, and household items. As with many consumer products, triclosan can be rinsed down the drain and transported to wastewater treatment plants. While most is eliminated during activated sludge sewage treatment by biodegradation and adsorption, some triclosan enters the aquatic environment and may expose wildlife. Given the potential for exposure to both humans and wildlife, resolving whether triclosan is endocrine active is important due to growing concerns about potential adverse public health and environmental effects of endocrine-disrupting substances. A weight of evidence (WoE) analysis focusing on specific hypotheses related to interaction with estrogen, androgen, and thyroid hormone pathways, and steroidogenesis was applied to triclosan. This WoE procedure involved systematic consideration of each endpoint, focused on screening level studies in the US Endocrine Disruptor Screening Program, as well as those in levels 1 through 5 of the OECD Conceptual Framework. This was followed by a semiquantitative relevance weighting of each endpoint to a given hypothesis to reach scientifically justified conclusions. Use of all relevant and reliable information and consistent observations in multiple studies strengthen support for or against each mode of action hypothesis. Using data from multiple animal species and in vitro systems, this systematic and transparent WoE assessment indicated that triclosan is not acting as an agonist or antagonist within the estrogen, androgen, thyroid, or steroidogenic pathways and is not impacting endocrine pathways as a lead or primary mode of toxicity.
Introduction and background
Triclosan (CAS No. 3380–34-5) () is an antimicrobial agent used in a wide range of personal care and consumer products, such as deodorants, oral care, clothing, and household items. As with most personal care products, triclosan can be rinsed down the drain and transported to wastewater treatment plants. While most of the triclosan is eliminated during activated sludge sewage treatment plant processes by both biodegradation and sorption, some triclosan enters the aquatic environment and may expose ecological receptors (Federle et al. Citation2002). Given the wide range of public health and environmental concerns raised regarding endocrine disruptive chemicals and the potential exposure of humans and wildlife to triclosan, it is important to regulators, industry, and the public to resolve whether triclosan has the potential to exhibit endocrine activity under foreseeable conditions of exposure.
To efficiently address the large number of chemicals that might exhibit endocrine activity, the US Environmental Protection Agency has developed a two-tiered screening and testing approach. Under the Agency’s Endocrine Disruptor Screening Program (USEPA EDSP), Tier 1 screening level studies are used to identify those substances that have the potential to be endocrine active (USEPA Citation2011). These Tier 1 studies have been developed through a decade-long international collaboration, and correspond to levels 2 and 3 in the OECD Conceptual Framework (OECD Citation2012a). The USEPA has issued guidance to evaluate and integrate all relevant scientific data from these screening level studies using a weight-of-evidence (WoE) approach described as “…a collective evaluation of all pertinent information so that the full impact of biological plausibility and coherence is adequately considered” (USEPA Citation2011).
A WoE evaluation uses data from all relevant sources collectively to enable a conclusion that may not be evident based on an individual study or a few data points. Because such data are likely to vary widely in type and quality, a clearly documented, transparent, and systematic approach to their evaluation and integration is necessary to avoid weaknesses identified in much of the literature (Weed Citation2005). Elements critical in a WoE evaluation include (1) reliability of the information (e.g. quality of a study, validity of the method used, clarity, and transparency of the reporting of results); (2) relevance of the information (e.g. the extent the tests and data are appropriate for the question being asked); (3) adequacy (or usefulness) of the information for the intended purpose (e.g. regulatory decision-making); and (4) consistency of the information (the extent to which the collection of data supports a particular hypothesis). In the arena of regulatory toxicology, hypothesis-based WoE approaches have been developed that incorporate those critical elements (Boobis et al. Citation2008; Borgert et al. Citation2011; Rhomberg et al. Citation2013).
The term hypothesis-based WoE generally describes a process or method by which conclusions are reached by considering all scientific evidence relevant to the status of a hypothesis or set of alternative hypotheses. Here, the term “weight” implies that all data do not contribute equally to addressing a particular hypothesis. Thus, “weighting” involves a careful consideration of the specific hypothesis to be evaluated and how each particular measurement (data) informs that hypothesis. In order for hypothesis-based WoE methodologies to be robust, the hypotheses to be tested and the process used to weight the various types of data must be clearly articulated and the weightings must be derived a priori and applied consistently (McCarty et al. Citation2012).
Ideally, weight would be assigned quantitatively to each piece of data (Borgert et al. Citation2011) based on objective measurements of predictive power, false-positive and false-negative detection rates, and potency or strength of the response. This would avoid the biases inherent in methods based solely on professional judgments. In practice, however, quantitative rankings are seldom possible because the predictive capacity of most toxicological assays for potential adverse human health and ecological effects is associated with varying degrees of uncertainty, and this is particularly the case for endocrine-mediated toxicity. Therefore, qualitative rankings are necessary and appropriate, acknowledging that some reliance on professional judgment is unavoidable. Nonetheless, objectivity and transparency are the overriding goals. Borgert et al. (Citation2011, Citation2014) ranked the 56 endpoints evaluated in the 11 Tier 1 assays for relevance in testing eight hypotheses (agonist and antagonist) for the specific endocrine pathways under review (estrogen, androgen, thyroid, steroidogenesis).
WoE methods also require evaluating the reliability or soundness of the available data (Borgert et al. Citation2011 – supplemental material; McCarty et al. Citation2012; Lynch et al. Citation2016). To evaluate the data on triclosan, a transparent and consistent framework was applied (Klimisch et al. Citation1997; Schneider et al. Citation2009). Studies included in this WoE evaluation were those considered consistent with the Tier 1 studies from the USEPA EDSP, as well as those that had endpoints included in those screening studies but that were assessed using different, typically chronic, test guidelines (e.g. testes weight measurements in a rodent chronic study). Studies identified as having flawed study designs, confounded results when compared to controls, unknown relevance of reported results, not meeting the intent of the Tier 1 study requirements, or endpoints not considered relevant for the hypotheses under review were not included (e.g. those with Klimisch 3 or 4 ratings), whether they supported or refuted the hypothesis being tested. The reasons for the exclusions are documented in the following sections.
Methods
Literature search
The first step in conducting the WoE analysis for endocrine activity of triclosan was to gather all potentially relevant information. Google Scholar was searched for triclosan alerts using “triclosan” as the search term. Other searches used both SciFinder (ACS) and Science Direct (Elsevier) using triclosan#, chloro(3A)(dichlorophenoxyphenol# OR dichlorophenoxy(3A)phenol# OR (dichloro(3A)phenoxy)(3A)phenol#), and trichloro(3A)(hydroxyphenol# OR hydroxy(2A)phenyl#) OR irgasan# as the search terms. The collected literature was then filtered for studies on triclosan that followed methods outlined in EDSP Tier 1 guidelines or that were conceptually consistent. The literature search identified more than 35 peer-reviewed studies on triclosan that were potentially relevant for an assessment of endocrine activity. Applying the framework proposed by Borgert et al. (Citation2011, Citation2014), a WoE analysis was conducted, focusing on specific hypotheses related to agonist and antagonist interactions with estrogen, androgen, and thyroid hormone pathways, and steroidogenesis. This WoE procedure involved systematic consideration of each endpoint observed in one or more study designs, focused on the screening level studies in the USEPA EDSP, as well as those in levels 1 through 5 of the OECD Conceptual Framework (OECD Citation2012a), followed by a semiquantitative weighting of relevance of each endpoint to a given hypothesis to reach scientifically justified conclusions based on the currently available evidence.
Data quality assessment
The USEPA guidance for conducting a WoE evaluation for endocrine activity includes a provision on the “soundness” of the data for the intended purpose (USEPA Citation2011). In evaluating the “soundness” of the data, the USEPA provides the following considerations:
Adequacy of test methods to detect the effect of interest;
Conduct of studies according to the scientific method of hypothesis development and testing through observation, experimentation, and verification;
Ability to distinguish between a specific versus a nonspecific outcome according to the intended purpose of the study;
Interpretation of results and conclusions that are statistically significant, biologically plausible, and consistent with the data.
Following identification of potentially relevant literature for assessing endocrine activity, a data quality evaluation was performed on each study by applying methods described by Klimisch et al. (Citation1997) and using the ToxRTool created by the European Center for the Validation of Alternative Methods (Schneider et al. 2009). Studies were reviewed and assigned a Klimisch score, a numeric rating of 1 to 4, based on the adequacy and reliability of the study methods and reporting. Studies with a Klimisch 1 and 2, considered reliable without and with restrictions, respectively, form the basis of the weight-of-evidence analysis. Klimisch 3 and 4 studies, considered not reliable and not assignable, respectively, are reviewed for relevance and discussed, but not included in the WoE analysis.
Weight-of-evidence analysis methods
The hypothesis-driven WoE approach used to evaluate triclosan data is based on a transparent and systematic methodology described by Borgert et al. (Citation2011, Citation2014). This methodology has been cited in EPA and OECD guidance (USEPA Citation2011; OECD Citation2012a) and used elsewhere to assess the endocrine activity potential of other substances (de Peyster & Mihaich Citation2014). A total of eight hypotheses are tested in this WoE assessment, based on the Tier 1 assays in the USEPA EDSP and other scientifically relevant information. The Tier 1 screening battery was designed to determine if a substance has the potential to interact with the estrogen, androgen, or thyroid pathways or steroidogenesis. The WoE framework used in this assessment of triclosan takes the endpoints from the studies in Tier 1, and ranks them as to their relevance and specificity for each of the pathways and hypotheses. The more specific and less likely to be confounded by other toxicities, such as overt systemic toxicity, the more relevant the endpoint is to the hypothesis. The ranking criteria as set forth in Borgert et al. (Citation2014) are as follows:
Rank 1: Endpoints that are sensitive and specific for the hypothesis being evaluated. These endpoints are rarely confounded by nonspecific activity and can be interpreted without clarification from other endpoints. Currently, Rank 1 endpoints are in vivo measurements only as they incorporate metabolic processes.
Rank 2: These endpoints are also sensitive and specific for the hypothesis being evaluated but may be subject to alternate modes of action or other confounding influences and so are less informative. Both in vitro and in vivo data are included in Rank 2.
Rank 3: Endpoints that are relevant for the hypothesis being evaluated but only when they are corroborative of endpoints in Ranks 1 and 2. These are generally endpoints that respond to many modes of toxicity but could be informative given a particular pattern of response in the more specific endpoints.
The concepts set forth by Borgert et al. (Citation2011, Citation2014) reference standard assays defined by one or more regulatory guidelines. These guideline methods represent validated study designs demonstrated capable of detecting an effect on the relevant endpoints with accuracy. It is important to note that lack of adherence to a guideline method was not grounds for exclusion of a study from the WoE evaluation. To this point, a large number of studies used in this WoE evaluation were from the open, peer-reviewed literature and were not conducted according to a regulatory guideline or good laboratory practices (GLP). Both GLP-compliant and noncompliant investigational research from the peer-reviewed literature have a role in regulatory decision-making, and the relative strengths of each have been discussed (Borgert et al. Citation2016). Consideration was given to the design of each study and the presence of any confounding factors. This level of scrutiny was applied equally to all studies irrespective of whether they would support or refute a given hypothesis. Each study was summarized and the data included in the WoE analysis clearly identified. Where a study has been excluded from the WoE analysis, the rational for exclusion is discussed.
For each hypothesis, the results are reported in a tabular format (). Each endpoint is shaded to indicate the overall strength of the data. An un-shaded (white) cell indicates that the change of interest was not observed in this study or that data in support of that endpoint are reliable (from a well-designed study employing a validated method). A shaded cell indicates that data in support of a particular endpoint are from a study with design deficiencies or other confounding factors (e.g. uterus weight in an adult female rat without information on stage of estrous, or a non-dose-dependent response), although the study was deemed sufficiently reliable (Klimisch 1 or 2) and included. For ease of reading, the references for each endpoint appear in a column to the right in . All references used in the WoE evaluation for that endpoint are included. Those references noted as {bold} demonstrated a relevant effect for that endpoint, while others noted as [number] had no effect for that hypothesis and endpoint.
Table 1. Ranked endpoints for relevant triclosan data for the estrogen agonist hypothesis.
Table 2. Ranked endpoints for relevant triclosan data for the estrogen antagonist hypothesis.
Table 3. Ranked endpoints for relevant triclosan data for the androgen agonist hypothesis.
Table 4. Ranked endpoints for relevant triclosan data for the androgen antagonist hypothesis.
Table 5. Ranked endpoints for relevant triclosan data for the thyroid agonist hypothesis.
Table 6. Ranked endpoints for relevant triclosan data for the thyroid antagonist hypothesis.
Table 7. Ranked endpoints for relevant triclosan data for the steroidogenesis induction hypothesis.
Table 8. Ranked endpoints for relevant triclosan data for the steroidogenesis inhibition hypothesis.
Health effects studies of triclosan with endpoints relevant to endocrine activity
High-throughput assays
In vitro studies can provide useful information on potential mode of action for substances, which is why the USEPA included five in vitro assays in the EDSP (see next section for description). However, even these in vitro assays are time-consuming and require the use of animal tissues. Recently, high-throughput assays have been developed and optimized to provide information on bioactivity of a substance in both endocrine and alternative pathways that allow for thousands of substances to be screened for activity in a very short period of time. Triclosan is one of many substances that has been evaluated in the USEPA’s ToxCast™ program [http://www.epa.gov/chemical-research/toxicity-forecasting], a collection of high-throughput bioassays used to screen substances for different types of biological activity. A subset of these high-throughput screens has specific relevance for the estrogen, androgen, and thyroid pathways, although it is the totality of the responses that can provide clarity as to potentially the most relevant mechanisms of action. Triclosan was evaluated in a total of 1086 assays in the October 2015 release of ToxCast (Download date 10 January 2016). Triclosan was active in 241 assays (∼22%), including 2 of 18 estrogen receptor (ER) assays, 7 of 11 androgen receptor (AR) assays, and 1 of 5 thyroid receptor (ThR) assays. However, all 10 of these positive results had AC50 values (the half maximal activity response) above the lower cytotoxicity limit of 4.45 μM (1.29 mg/L) (). The cytotoxicity limit was identified using a collection of up to 35 assays in the ToxCast battery that are designed to detect cytoxicity or nonspecific cell loss that could result in false positive activity (Judson et al. Citation2015).
Figure 2. ToxCast concentration distribution for assays active for triclosan. The endocrine-specific receptor assays are estrogen (ER), androgen (AR), thyroid (ThR), and aromatase. The solid line represents the median cytotoxicity limit and the dashed line is the lower bound (5%) cytotoxicity limit.
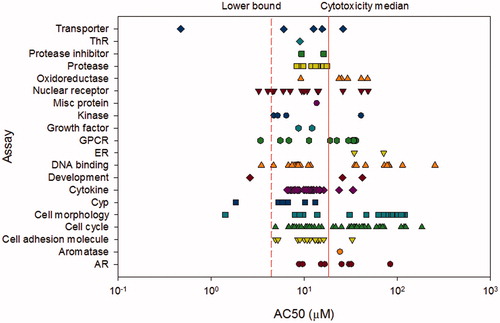
Biological activity above the cytotoxicity limit is not informative for characterizing a substance’s endocrine activity since general cytotoxic responses are expected to cascade to many biological targets (Judson et al. Citation2015). The majority of the biological activity identified in the ToxCast assays for triclosan was unrelated to these specific hormonal pathways. Below the lower cytotoxicity limit, pathways related to cell morphology, cytochrome P450 (Cyp) and cytokine activity, development, DNA binding, G-protein coupled receptors (GPCR), non-ER/AR/ThR nuclear receptors, and transporters were affected. While this biological activity information is not included in the WoE tables () it is essentially an initial screen that suggests that the effects noted in in vivo studies could be a result of some of these other biological activities occurring below the cytotoxicity limit. However, these high-throughput methodologies are limited in their metabolic capacity so must be considered only as part of the corroborative information in the WoE analysis.
In mammals, triclosan is primarily metabolized to sulfate and glucuronide conjugates and excreted primarily in urine for humans, hamsters, and rabbits, and via the fecal route in mice, rats, and dogs (Fang et al. Citation2010). Hydroxylation via cytochromes P450 has also been documented and can lead to 2,4-dichlorophenol and 4-chlorocatechol, which can be further metabolized. Although limited information is available for fish and other animals, in vitro data from catfish liver and intestine demonstrate that triclosan is rapidly glucuronidated by microsomal preparations and sulfonated by cytosolic preparations isolated from both tissues (James et al. Citation2012). Overall, the ratio between sulfonation and glucuronidation may vary between species and impact the primary route of excretion of triclosan. However, based on the information available, it is reasonable to assume that triclosan is likely to be readily biotransformed and eliminated in both mammals and fish.
In vitro studies
There are five in vitro assays in the Tier 1 battery of the EDSP. These in vitro assays would be considered in level 2 of the OECD Conceptual Framework (OECD Citation2012a). Two of the assays specifically address estrogen and androgen receptor binding (USEPA Citation2009b, Citation2009d). The estrogen receptor-binding assay (ERB) uses rat uterine cytosol to assess the ability of radiolabeled estradiol to interact with the estrogen receptor in the presence of a test substance. Similarly, the androgen receptor-binding assay (ARB) uses rat prostate cytosol to measure radioligand competitive binding in the presence of increasing concentrations of the test substance. Currently, neither of these assays distinguishes between agonist and antagonist responses as the antagonist portion has not been validated for the purposes of the EDSP.
Only one study was deemed sufficiently reliable to be considered in the WoE analysis. Gee et al. (Citation2008) describe an ERB-like assay that is not entirely consistent with all of the requirements of the ERB guideline as it uses MCF7 human breast cancer cell cytosol rather than rat uterine cytosol. Competitive binding was evaluated with 0.8 nM estradiol incubated with triclosan at concentrations ranging from 0.8 nM to 8 mM (a 1 to 10 million-fold molar excess of triclosan relative to estradiol). Estradiol binding was unchanged by an excess of triclosan in the range of 1- to 10,000-fold, although the authors report a decrease in estradiol binding of 25.2% to 72.2% between 100,000- and 10,000,000-fold excess. However, the highest concentration tested (8 mM) was above the recommended high concentration in the ERB guideline (1 mM). As reported by Laws et al. (Citation2006), high concentrations of chemicals in binding assays are likely to disrupt the biochemical stability of the test system, for example, by impacting receptor integrity, by forming aggregates, by precipitating out of solution, or by changing the pH. Laws et al. (Citation2006) also noted that ethanol used as a solvent can denature the receptor and reduce binding capacity of radiolabeled estradiol. According to the guideline, no more than 3% ethanol should be used in the test system. Gee et al. (Citation2008) used ethanol in the preparation of stock solutions, however, the concentration was not reported. The ER competitive binding curve in of Gee et al. (Citation2008) is nonsigmoidal. Nonsigmoidal binding curves should be considered with caution given the potential confounders listed above when testing excessively high concentrations (Laws et al. Citation2006). In addition, no weak reference estrogen (e.g. norethynodrel) was tested to determine relative binding affinity. Due to the lack of appropriate controls and the excessively high concentrations tested that may have impacted estradiol binding through nonspecific mechanisms, Gee et al. (Citation2008) is not included in the WoE analysis. However, given the concordance between ToxCast assays and the ERB (Browne et al. Citation2015), where it was reported that the accuracy of the ToxCast ER model predictions in comparison to ERB results is 86 to 93%, the lack of response in the estrogen pathway high-throughput assays () strongly suggests that triclosan does not bind to the estrogen receptor.
Gee et al. (Citation2008) also reported on an ARB assay, concluding that binding of radiolabeled testosterone was inhibited by an average of 49% to 77% by 1000- to 10,000-fold molar excess of triclosan, relative to testosterone. However, the study did not include evaluations of the relative affinity of the receptor preparation to the radiolabeled testosterone and did not include a reference androgen (e.g. methyltrienolone) to determine the relative binding affinity. Similar to the ERB by the same authors, this study is not included in the WoE analysis.
The estrogen receptor transactivation study (ERTA) measures the ability of a chemical to bind to the receptor and activate transcription (USEPA Citation2009e). Unlike the ERB assay, the transactivation screen can distinguish between agonist and antagonist activity. No studies were located that are consistent with all of the requirements set forth in the ERTA. However, two studies were identified that measured relevant endpoints. Ahn et al. (Citation2008) describe an ERTA assay in an ovarian cancer cell line with stable constructs for an estrogen responsive firefly luciferase plasmid. Cell lines were incubated with 0.1 μM, 1 μM, and 10 μM triclosan. Results were normalized to luciferase activity induced by 1 nM estradiol. Louis et al. (Citation2013) describe an ERTA assay in a breast cancer cell line (T47D-KBluc), also with stable constructs for an estrogen responsive firefly luciferase plasmid. Cell lines were incubated with triclosan at concentrations of 0.03 to 100 μM. In both studies, estrogen receptor transactivation indicative of estrogen agonism was unchanged with the triclosan-exposed cells. In order to assess antagonist activity, Ahn et al. (Citation2008) co-incubated triclosan and estradiol and the reduction in luciferase activity was compared to estradiol only induced activity. Unfortunately, known antagonists or negative compounds were not tested concurrently and there was no apparent assessment of cytotoxicity, making it difficult to interpret the reported results. Thus, this portion of the study was not included in the WoE analysis.
Androgren receptor-dependent signaling in a human ductal breast epithelial tumor cell line with an androgen receptor responsive element (a stably integrated firefly luciferase reporter gene plasmid) was also reported in the study by Ahn et al. (Citation2008). Cells were incubated with ethanol at 1% as the carrier solvent and 10 μM testosterone or triclosan was dissolved in DMSO at 1 or 10 μM. Luciferase activity was reported relative to percent induction by 10 μM testosterone, but the study did not include positive and negative controls or apparent cytotoxicity measurements. In addition, other than noting antiandrogenic activity for triclosan in this test system in a table in the paper, there was no data presented to evaluate the response. Given that the androgen receptor transactivation assay is not included in the USEPA EDSP, because it has only recently been validated (OECD Citation2016), along with the deficiencies noted in the Ahn et al. study concerning the responsiveness of the test system and results for triclosan, this study is not included in the WoE analysis for the androgen pathway.
Another of the in vitro assays is the steroidogenesis assay, an in vitro screening assay in a human adrenocortical carcinoma cell line (H295R) intended to identify substances that effect the steroidogenic pathway from gonadotropin receptor binding to steroid synthesis (USEPA Citation2009j; OECD Citation2011). Specifically, changes in estradiol and testosterone secretion over a range of noncytotoxic cell concentrations are evaluated and a statistically greater fold-induction with estradiol or testosterone than that observed for the solvent control is considered a positive effect. In a study described by Forgacs et al. (Citation2012), BLT-1 cells (a cell line derived from mouse Leydig cell tumor) were exposed to triclosan at concentrations of 0.1–30 μM. While estradiol was not assessed in this assay, triclosan exposure did not alter basal testosterone production. In order to simulate a luteinizing hormone stimulation of steroidogenesis, recombinant chorionic gonadotropin (rhCG) applied to the cultures at 3 ng/ml increased the production of testosterone approximately fourfold from basal levels. While triclosan did not alter basal testosterone levels, rhCG-induced testosterone biosynthesis was reduced by about 25% after a 4 h incubation period in cultures treated with 30 μM triclosan. In this steroidogenesis assay, cytotoxicity was assessed in a concentration range from 1 to 600 μM triclosan. Cell viability was ≥90% from 1 to 30 μM triclosan, but cytotoxicity was noted at concentration greater than 30 μM triclosan. Information that is included in the WoE analysis is that incubation of the mouse Leydig cell (BLT-1) cultures with 30 μM triclosan did not alter testosterone production over a 4-h period.
One study in human choriocarcinoma placental (JEG-3) cells employed methods generally comparable to the guideline and reported relevant endpoints (Honkisz et al. Citation2012), although the results were not included in the WoE analysis. The three highest concentrations of 10, 50, and 100 μM triclosan were cytotoxic as indicated by significant increases in lactate dehydrogenase release at 24 h incubation time, confounding the interpretation of the data. The statistically significant increase in estradiol at 1 μM at 24 h was not reported in terms of a fold-increase relative to the solvent control making it unclear as to the interpretation of the data in the context of the guideline. In a study described by Kumar et al. (Citation2008), primary cultures of adult rat Leydig cells were exposed to triclosan at concentrations of 0.001–10 μM. Triclosan exposure at 1 μM (only concentration tested) did not alter basal testosterone production, although triclosan did decrease luteinizing hormone-induced testosterone production in a dose-dependent manner from 0.01 to 10 μM triclosan. The positive control (forskolin; concentration not reported) did not yield a significant increase in testosterone production suggesting that there may be issues with the responsiveness of the test system. The study does not indicate that triclosan alters steroid biosynthesis, however, because of a lack of response in the positive control, this study was excluded from the WoE evaluation.
Stoker et al. (Citation2014) describe an in vitro study to examine the effect of triclosan on testosterone production using the H295R cell line. Forskolin and prochloraz were used as positive controls as specified in the USEPA OPPTS 890.1550 guideline (USEPA Citation2009j). The information available is currently limited to a poster presentation at a scientific meeting which does not provide sufficient information to evaluate the study (Klimisch 4). However, forskolin and prochloraz are reported to have produced the expected increase and decrease in testosterone production, respectively. Triclosan from 0.01 to 3.0 μM did not alter testosterone concentration so it was without effect in the study. The authors reported that 10 μM triclosan induced 20% cytotoxicity in this system. As this is an unpublished study and limited to a poster presentation the data has not been included in the WoE analysis. However, the reported lack of effect on testosterone concentrations are in line with the results of Forgacs et al. (Citation2012). No other steroidogenesis-like studies with triclosan were identified in the literature search.
The last in vitro assay included in the USEPA EDSP Tier 1 battery is the aromatase inhibition assay (USEPA Citation2009c), it is an in vitro screening assay in microsomes containing recombinant human aromatase enzyme, intended to identify substances that may affect the endocrine system by inhibiting catalytic activity of aromatase. No studies were located that assessed the aromatase inhibiting potential of triclosan. There was one aromatase assay in the ToxCast battery resulting in activity greater than the lower cytotoxicity limit ().
Endocrine-focused studies in female and male rodent models
The US EPA EDSP Tier 1 assays include four rodent assays in the battery of assays to identify potential interactions with the endocrine system. The uterotrophic study (OECD Citation2007; USEPA Citation2009k) is an in vivo screening assay in immature females (after weaning or prior to puberty) or in young adult females after ovariectomy. A minimum of two treatment groups receive the test substance daily by oral gavage or subcutaneous injection for a period of three consecutive days. Twenty-four hours after the last dose, the animals are necropsied and mean uterine weight determined. There were two studies identified that were consistent with the requirements set forth in the uterotrophic study guideline. Stoker et al. (Citation2010) describe a study of uterine effects of triclosan in immature rats conducted per requirements set forth in the OECD 440 guideline (OECD Citation2007). Immature female Wistar rats were administered triclosan in corn oil by oral gavage on day 19–21 post-natal age at one of nine doses between 1–300 mg/kg and terminated 6 h following the final dose and uterine weights were determined. Similarly, Louis et al. (Citation2013) describe a study of uterine effects of triclosan in immature female Wistar rats administered triclosan in corn oil by oral gavage on day 19–21 post-natal age at a single dose of 37.5 mg/kg. Twenty-four hours following the last dose, the animals were euthanized and the uterine weights were recorded. Uterine weight was unchanged compared to controls in both studies with triclosan exposure alone. However, triclosan, appears to increase the uterotrophic response of ethinyl estradiol alone when coadministered with the synthetic estrogen, but only at doses of triclosan that are significantly higher than potential human exposure (Stoker et al. Citation2010; Louis et al. Citation2013).
Two other uterotrophic-like studies were identified but not included in the WoE analysis. In the study by Rodriguez and Sanchez (Citation2010), only a single animal per dose group was assessed for uterine weight. In addition, the dosing of the animals is in question. The reported water solubility of triclosan at 20 °C is 12 mg/L (per OECD 105 (water solubility) study) (ECHA Citation2015). Assuming triclosan was dissolved in water at the maximum achievable concentration, the high dose of 50 mg/kg/day would correspond to a consumption rate of 220 ml water per day; which is approximately four times the body weight of the weanling rats used in this study. Jung et al. (Citation2012) reported their results as uterine weight/body weight ratio with no reporting of individual uterine weights or body weight, providing insufficient information with which to evaluate the results.
The Hershberger Bioassay (OPPTS 890.1400, OECD 441) (OECD Citation2009b; USEPA Citation2009g) is “a mechanistic in vivo screening assay for androgen agonists, androgen antagonists, and 5α-reductase inhibitors” evaluating the accessory tissues of the reproductive tract of castrated peripubertal male rats. These five androgen-dependent tissues (glans penis, ventral prostate, seminal vesicle, levator ani-bulbocavernosus (LABC), and Cowper’s gland) exhibit characteristic increases and decreases in absolute weight in response to androgens and antiandrogens. A recent conference abstract by Stoker et al. (Citation2014) describes a Hershberger study run in accordance with OPPTS 890.1400. While it is summarized here, it is not included in the results tables as the study is only available in an abstract and poster presentation at this point and thus received a Klimisch rating of 4 (nonassignable). In the study, castrated peripubertal male Wistar rats were administered triclosan in corn oil by oral gavage on day 52 to 62 post-natal age at 50 mg/kg or 200 mg/kg and terminated on day 62. Trunk blood, liver, thyroid, and the five accessory tissues of the reproductive tract were collected at necropsy. As reported in the poster, the weights of the five accessory sex tissues were unchanged by triclosan.
The male and female rat pubertal development and thyroid assays (OPPTS 890.1450, OPPTS 890.1500) (USEPA Citation2009h, Citation2009i) are two EDSP Tier 1 assays with both mechanistic and apical endpoints. Exposure to the test substance in rats occurs prior to the onset of puberty. Age and weight at preputial separation is an androgen-dependent biomarker of puberty onset in the male rat while vaginal opening is an estrogen-dependent biomarker in females. Starting on post-natal day 30 in males and 22 in females, rats are examined daily for preputial separation or vaginal opening. For males at necropsy, general growth, blood chemistry including serum testosterone, thyroxine (T4) and thyroid-stimulating hormone (TSH), as well as organ weights including testes, seminal vesicle and coagulating gland, ventral and dorsolateral prostate, LABC muscle complex, epididymis, thyroid, liver, kidney, pituitary, and adrenal weights are recorded and samples retained for histopathology. For females, the endpoints are similar but include the assessment of uterus and ovaries in place of the male specific organs previously listed.
Zorrilla et al. (Citation2009) describe a study of pubertal effects of triclosan in immature male rats that is generally consistent but not fully compliant with requirements set forth in OPPTS 890.1500. Groups of 8 to 10 immature male Wistar rats were administered triclosan in corn oil by oral gavage on days 23 to 53 post-natal age at 0, 3, 30, 100, 200, or 300 mg/kg. Age and body weight at preputial separation were unchanged in triclosan-exposed animals. There was a non-dose-dependent decrease in serum testosterone at 200 mg/kg, but not at 3, 30, 100, or 300 mg/kg. Thyroid weight was not reported but there was a statistically significant decrease in serum T4 at all but the lowest dose, with no difference compared to controls in serum TSH and no changes in follicular epithelial height in the thyroid gland. Colloid depletion in the thyroid was only noted at the highest dose. Liver weight was increased at the three highest doses while other organ weights, except the pituitary, remained unchanged. The non-dose-related increase noted in pituitary weight at the lowest (3 mg/kg) and highest (300 mg/kg) dose but not at 30, 100, or 200 mg/kg has not been included in the WoE analysis results. Notably, the USEPA Integrated Summary Report for the Tier 1 validation of OPPTS 890.1500 (USEPA Citation2007) cautions on the covariance of the pituitary with body weight and recommends that any analysis of organ weight is normalized to body weight. In the absence of detailed body weight data, the relevance of the reported observations is unclear.
Triclosan has also been evaluated in a female pubertal study reported by Stoker et al. (Citation2010). Immature female Wistar rats were administered triclosan in corn oil by gavage on days 21–42 post-natal age at 0, 9.4, 37.5, 75 or 150 mg/kg. Animals were observed for vaginal patency and weighed daily. Age at onset of vaginal opening was reduced at the highest dose (150 mg/kg) compared to controls, although body weight at vaginal opening was not recorded. Thyroid weight and histopathology were not reported but, similar to the Zorrilla et al. (Citation2009) study, there was a statistically significant decrease in serum T4 at all but the lowest dose, with no difference compared to controls in serum TSH. Uterine weight was increased at the highest dose (150 mg/kg) with no changes in body weight relative to controls noted. The relevance of this result is unclear as the estrous stage at necropsy was not reported and the authors note that females were terminated at different stages of the estrous cycle. Stump et al. (Citation2014) noted that uterine weights in the female pubertal study can vary by threefold depending on the stage of estrous. However, since the weight increase occurred at the highest dose, the result is included in the WoE analysis.
Rodriguez and Sanchez (Citation2010) describe a study of pubertal effects of triclosan in immature offspring of Wistar rat dams exposed to triclosan throughout mating and gestation. Triclosan was delivered to dams and offspring in drinking water at concentrations implied equivalent to doses of 1, 10, and 50 mg/kg/day. At weaning, four females from each litter were randomly assigned to control and treated groups. Each group, containing nine female rats, was exposed to vehicle or triclosan in drinking water from post-natal day 22 to 50 and terminated on day 50. In contrast to the reduced age at vaginal opening reported by Stoker et al. (Citation2010), a delay in vaginal opening was noted by Rodriguez and Sanchez (Citation2010). Due to the pre- and post-natal exposure design of the study, the ability to randomize across litters was limited. In addition, although significant changes in body weights at vaginal opening are reported in the study, baseline body weights were not reported, confounding the ability to fully evaluate the relevance of the time to vaginal opening. The impact of body weight and litter effects on vaginal opening is well documented in the literature (Marty et al. Citation1999). As previously noted, the apparent drinking water concentration requiring the animals to consume four times their body weight is unusual. Interestingly, the cited method for preparation of stock triclosan solutions (Greenman et al. Citation1997) was a microbiology study in which triclosan was dissolved in a serum protein rich culture media. In the absence of analytical confirmation of dosing solution concentrations, it is not clear what exposures were achieved. The reported changes for age and body weight at onset of vaginal opening are of unknown relevance in light of methodological limitations. This study was not included in the WoE analysis.
Mammalian general toxicology, reproduction, and developmental studies
While Tier 1 of the US EPA EDSP is limited to specific shorter-term mammalian screening studies to identify the potential of a substance to interact with the endocrine system, studies fitting levels 3, 4, and 5 of the OECD conceptual framework (OECD Citation2012a) can provide other scientifically relevant information in the WoE assessment.
Kumar et al. (Citation2009) reported on a 60-day oral gavage study in male Wistar rats approximately 10 weeks of age at the start of the study, with reported body weight of 165–169 g. Doses of 5, 10, and 20 mg/kg/day were delivered in a volume of 200 μL. The authors report significant reductions in testes weight in rats treated with 10 and 20 mg/kg/day. The authors also report “histological malformations” at the 20 mg/kg/day dose, but these observations were not described further. While reduced sperm density in the lumina of the epididymal tubule from rats treated with 20 mg/kg/day was also noted, the basis for this conclusion is not clear from the data provided. In addition, although the study reports a significant change in testes weight relative to the concurrent control, the body weight gain across all of the treatment groups was consistent but unusually small. Specifically, the controls demonstrated only a 19 g increase over a 60 day period. The lack of detail in the histological reporting and minor body weight gain across dose groups, including the solvent control, brings questions to the interpretation of any findings. In addition, in a report from the Scientific Committee on Consumer Safety (SCCS Citation2011) uncertainty with regards to the results of this study was described due to the question of impurities in the test compound (purity approximately 98%). This study was excluded from the WoE analysis.
Pubertal female Long-Evans rats (27–29 days of age) were exposed to triclosan via oral gavage (in corn oil) at doses of 0, 10, 30, 100, 300, 1000 mg/kg/day for four consecutive days (Crofton et al. Citation2007). Rats were terminated 24 h after the last dose and trunk blood was collected for serum thyroxine (T4) analysis. Serum T4 concentrations were significantly decreased in a dose-related manner at doses of 100, 300, and 1000 mg/kg; 1000 mg/kg was also associated with significant increases in liver weight and liver to body weight ratio. The authors indicate that this effect on thyroid homeostasis is related to the increase in clearance mechanisms, including the increases in sulfonation and glucuronidation activity reported by others and the known upregulation of cytochrome P450 2B isozymes. Induction of cytochrome P450 activity was noted below the cytotoxicity limit in the relevant ToxCast assays (). Although there was not a significant change in body weight gain, there was a significant difference in liver weight at 1000 mg/kg and the published maximum tolerated dose for rats is less than 300 mg/kg (Rodricks et al. Citation2010) suggesting that the two highest doses may be in excess of the maximum tolerated dose. However, the study did not include functional measures of thyroid integrity (e.g. histology, thyroid weight). The decrease in T4 observed at doses up to the maximum tolerated dose is consistent with the observations of Stoker et al. (Citation2010).
As a follow on to Crofton et al. (Citation2007), Paul et al. (Citation2010) report a study in which pubertal female Longs-Evans rats (23–27 days of age) were exposed to triclosan via gavage (in corn oil) at doses of 0, 10, 30, 100, 300, and 1000 mg/kg for four consecutive days. Rats were terminated 24 h after the final treatment and trunk blood and liver were collected. Four daily oral doses of triclosan in pubertal female rats were associated with a significant reduction in serum T4 at doses of 100 mg/kg and higher, a significant reduction in serum T3 at doses of 300 mg/kg and higher, with no change in TSH. Significant increases in liver weight and liver to body weight ratio were noted at 1000 mg/kg. These results are consistent with those reported by Stoker et al. (Citation2010). Hepatic enzyme activity (CYP 2B) was significantly increased at dose of 300 mg/kg and 1000 mg/kg while there was no change in CYP 1A1 activity. mRNA was increased for CYP 2B2 at 300 mg/kg and for CYP 3A1 at 100 and 300 mg/kg. Activity but not mRNA was increased for uridine 5′-diphospho-glucuronosyltransferase (UGT) at 1000 mg/kg. This study supports the hypothesis that reduction in T4 may be partially due to increased catabolism in the liver.
Extending the previous study, Paul et al. (Citation2012) administered triclosan to time-pregnant Long-Evans rats via gavage at 0, 10, 20, 100, and 300 mg/kg daily from gestation day 6 through postnatal day 21. Blood and liver were collected from different groups of dams at gestation day 20 and post-natal day 22 and from fetuses at gestation day 20 and post-natal day 4, 14, and 21. Serum T4, T3, TSH, and liver enzyme activities were quantified. There were no effects of triclosan exposure on reproductive parameters including gestation length, litter size, viability index, or sex ratio. There were no fetal abnormalities or changes in viability of the offspring and the day of eye opening was not changed. Serum T4 was significantly decreased in dams and fetuses on gestation day 20 at 300 mg/kg, in pups at post-natal day 4 at 300 mg/kg and in dams at post-natal day 22 at 100 and 300 mg/kg. There were no effects on T3 or TSH in dams or offspring at any dose tested. Enzyme activity in dams and pups indicated that upregulated hepatic catabolism may contribute to hypothyroxemia during development in triclosan-exposed animals.
Rodriguez and Sanchez (Citation2010) measured T3 and T4 in tail vein samples collected through gestation and lactation in a study with female Wistar rats exposed to triclosan in drinking water at doses of 0, 1, 10, and 50 mg/kg for 8 days prior to mating to lactation day 21. Serum T4 levels were significantly reduced at all timepoints in dams exposed to 10 or 50 mg/kg triclosan. Serum T3 levels were decreased at gestation day 10, 15, and 20 and lactation day 5 and 10; decreases were significant at all doses of triclosan except at gestation day 10 where decreases were significant at 10 and 50 mg/kg doses only. Although this study does not include analytical verification of concentrations and no dosimetry information is provided, these results are consistent with the observations of Stoker et al. (Citation2010).
In a similar study in dams and their offspring, Axelstad et al. (Citation2013) exposed time-mated Wistar rats by gavage to 0, 75, 150, or 300 mg/kg/day triclosan starting at gestation day 3 through post-natal day 16. On the day after delivery, the pups were evaluated, including measures of anogenital distance. All animals were terminated at postnatal day 16. Among other endpoints assessed, post mortem evaluations included weight and histopathology of prostate and thyroid in male rats, as well as measures of nipple retention. Retention of thoracic nipples in male pups is potentially indicative of an antiandrogenic effect (USEPA Citation1998). Triclosan exposure at 300 mg/kg was associated with a statistically significant decrease in maternal body weight gain. However, there were no effects on litter size or viability, or on anogenital distance or nipple retention. Pre- and post-natal maternal exposure to triclosan up to doses causing moderate maternal toxicity did not impact sex ratio, anogenital distance, nipple retention or thyroid or prostate histopathology in male Wistar rat pups. Plasma T4 levels were significantly decreased in dams exposed to triclosan with no change in thyroid weight (histopathology not performed). Therefore, even at doses that are described by the authors as causing a moderate degree of maternal toxicity, there were no indications of antiandrogenic effects.
To evaluate whether direct oral exposure to young pups would result in changes to thyroid hormones that did not occur through lactation, Axelstad et al. (Citation2013) exposed pups from post-natal day 3 to 16 to 0, 50, or 150 mg/kg. The triclosan solutions were allowed to drip into the pups’ mouths. At post-natal day 16, the animals were terminated and trunk blood was collected for total T4 analysis. A dose-dependent reduction in serum T4 was observed in the study. The authors caution that the interpretation of the results (plasma T4 from trunk blood collected at post-natal day 16) was confounded by litter effect (all controls were from the same litter) and/or unusually high control T4 values. For the above reasons, the direct post-natal exposure data were not incorporated into the WoE evaluation.
Fish and other nonmammalian studies
The USEPA EDSP has two Tier 1 screening studies in nonmammalian wildlife. The Fish Short-Term Reproduction Assay (FSTRA) (OECD Citation2012b; USEPA Citation2009f) is an in vivo screening assay of sexually mature male and spawning female fish. Mating pairs are exposed for 21 days at which point vitellogenin protein is measured in the liver or plasma. Vitellogenin is a protein normally found in ovarian follicles of female fish whose production in the liver is prompted by estrogen stimulus. Vitellogenin can be detectable at low levels in young and adult male fish if they are exposed to natural estrogens or estrogen-like compounds (Tyler et al. Citation1999). Secondary sexual characteristics are also evaluated. This assay also includes histology of gonads and quantitative measures of the apical endpoints of fecundity, fertility, and gonadosomatic index. No studies were located that were consistent with all of the requirements set forth in the FSTRA guidelines. However, one study was run in accordance with a similar guidance (BASF Citation2012) and was included in the WoE analysis. Two additional studies were identified and included in the WoE analysis as they measured some of the relevant endpoints (Ishibashi et al. Citation2004; Schultz et al. Citation2012).
BASF (Citation2012) reported on a study in fathead minnow (Pimephales promelas) conducted in accordance with EPA/600/R-01/067 (USEPA Citation2002) that is substantively similar to the FSTRA guidelines. The concentration range tested was based on results from an early life-stage fish study in rainbow trout, and taking into consideration concentration-setting guidance from the OECD 229 guideline (OECD Citation2012b). Four replicates of four fish (three females, one male) were exposed to measured concentrations of 3.7, 6.8, or 13.5 μg/L triclosan (nominal concentrations 6.3, 12.5, or 25 μg/L) for 21 days. No changes compared to controls in plasma vitellogenin, secondary sexual characteristics (tubercle score), fecundity, fertility, gonadosomatic index, and plasma estradiol and testosterone concentrations were reported in the study.
A second study in fathead minnow was reported by Schultz et al. (Citation2012) with measured concentrations of 0.17 and 0.45 μg/L. The study exposed 12 males and 10 females separately per replicate with two replicates per sex per treatment, rather than the required four males and two females in four replicates in the FSTRA guideline. Additionally, only two, not the recommended three, test concentrations were tested. The male low dose (0.17 μg/L) triclosan-treated fish exhibited a 20% mortality which may confound the results as toxicity would not be expected at such low test concentrations (BASF Citation2012). No changes compared to controls in plasma vitellogenin, secondary sexual characteristics (tubercle score), gonadal histopathology, and male behavior were reported in the study.
Japanese medaka was the test species in a study reported by Ishibashi et al. (Citation2004), with measured triclosan concentrations of 12.8, 60.8, and 136.9 μg/L in the 21-day exposure. Endpoints assessed included vitellogenin, fecundity, fertility, and gonadosomatic index. Hepatic vitellogenin was increased in male fish exposed to the lowest concentrations (12.8 and 60.8 μg/L) but not the highest concentration (136.9 μg/L) of triclosan. This non-dose-related change has been included in the WoE analysis since the observation was present in more than one concentration. Both male and female gonadosomatic index were increased compared to controls in the study. However, no supportive changes in fecundity or fertility compared to controls were noted at any concentration tested.
Given the mechanistic utility of an assessment of vitellogenin, the literature was reviewed for studies reporting this endpoint that were not consistent with the FSTRA guideline but might provide other scientifically relevant information. Vitellogenin levels following triclosan exposure have been monitored in male South African clawed frogs (Xenopus laevis) (Matsumura et al. Citation2005) and western mosquitofish (Gambusia affinis) (Raut & Angus Citation2010).
Matsumura et al. (Citation2005) report no changes in vitellogenin expression in male South African clawed frogs (Xenopus laevis) following a 14-day exposure to triclosan in water at 20, 100, and 200 μg/L. However, of note is the plasma vitellogenin level of 0.3 μg/mL in solvent (DMSO) control animals, which was clearly above the limit of detection (1 ng/ml); normally vitellogenin is nondetectable in the plasma of male frogs (Mitsui et al. Citation2003). The plasma vitellogenin concentration in the DMSO control was also higher than that in the 17β-estradiol positive control (approximately 0.2 μg/mL). Due to possible confounding factors, including the lack of a valid positive control response, these data are excluded from the WoE analysis.
Raut and Angus (Citation2010) measured vitellogenin gene expression in male western mosquitofish (Gambusia affinis) following a 35-day exposure to triclosan at concentrations of 29–101 μg/L. The authors report a significant increase in vitellogenin hepatic mRNA at the highest concentration, but the 20% control mortality in this study confounds interpretation of the results. Additionally, this study was deemed not reliable due to the following limitations: (1) The paper does not appear to report the number of animals per group; and (2) The specificity of the mRNA primer is suspect and no information is given as to what steps were taken to validate the primer other than the inclusion of an estrogen positive control. This is important since the vitellogenin gene has not been sequenced in this species and as such the primer was designed using the published sequence for rainbow trout (Oncorhynchus mykiss). For the above reasons, this study was not incorporated into the WoE evaluation.
Tubercle score in male fathead minnow is a required endpoint in the FSTRA guideline. Other than the studies by BASF (Citation2012) and Schultz et al. (Citation2012) no additional studies were located that report tubercle score in male fish. However, a study in Japanese medaka (Oryzias latipes) by Foran et al. (Citation2000) reported dorsal and anal fin lengths, which are indicators of secondary sexual characteristics. This study found no significant changes following exposure of fry to triclosan at 1, 10 and 100 μg/L for 14 days. This study has been excluded from the WoE analysis because the positive control (estradiol) was not significantly different from the solvent (ethanol) control for this endpoint. In addition, there was no replication or analytical confirmation of concentration. Given the lack of a valid positive control response, the relevance of these observations is unknown.
The other Tier 1 wildlife screen is the Amphibian Metamorphosis Assay (AMA) (EPA OPPTS 890.1100; OECD 231) (OECD Citation2009a; USEPA Citation2009a), an in vivo screening assay to identify substances which may interfere with the normal function of the hypothalamic-pituitary-thyroid (HPT) axis. Amphibian metamorphosis is a well-studied, thyroid-dependent process and the AMA is the only guideline assay that detects thyroid activity in an animal undergoing morphological development. In the assay, African clawed frog (Xenopus laevis) tadpoles at developmental stage 51 NF (Nieuwkoop & Faber Citation1994) are exposed to the test article or vehicle control for 21 days with measured endpoints of hind limb length, snout to vent length, developmental stage, wet weight, thyroid histopathology, and daily observations of mortality. The assay is not gender specific, therefore, the endpoints specified in and indicate that changes in either males or females are relevant findings.
In a study by Fort et al. (Citation2010), performed according to the OECD 231 guideline, NF developmental stage 51 X. laevis larvae were exposed to triclosan in water at 0.6, 1.5, 7.2, or 32.3 μg/L for 21 days. Primary endpoints were survival, hind limb length, body length (whole; snout to vent), development stage, wet whole body weight, and thyroid histology. Thyroxine (T4) levels and thyroid receptor-beta expression were also assessed. Developmental stage, synchronicity of development within the organism, and thyroid histopathology were unchanged between triclosan exposed and control frogs. Whole body length and weight, hind limb length, and snout-vent length in frogs exposed to 1.5 μg/L triclosan were all reduced compared to controls. This non-concentration-related change was not considered treatment related. In addition, no changes were observed in T4 or thyroid receptor-beta expression at any concentration compared to controls.
A second study by Fort et al. (Citation2011) exposed premetamorphic (NF developmental stage 47 larvae) to water or triclosan at 0.3, 1.3, 5.9, or 29.6 μg/L (measured concentrations) for 32 days (approximately NF developmental stage 59–60) in order to help distinguish between effects on metamorphosis from the effects on growth. Triclosan exposure was not associated with differences in survival, developmental stage, hind limb length, T4 expression in thyroid tissue, T3 or T4 concentrations in plasma, and expression of thyroid hormone receptor β and type II and type III deiodinase in larvae relative to controls. Body length and weight in organisms exposed to triclosan at 0.3, 5.9, and 29.6 μg/L were significantly greater than in controls. Thyroid histopathology in triclosan-exposed animals revealed minimal thyroid hypertrophy with no overall change in thyroid structure (e.g. follicle count, follicular area, colloid content/tadpole, colloid content/follicle); the thyroid glands were generally larger than in control specimens, consistent with the larger body size of the triclosan-treated larvae. This study is supportive of the conclusions set forth by Fort et al. (Citation2010) and suggests that triclosan does not alter metamorphosis. The increase in growth, with no impact on developmental stage, thyroid hormone concentrations, or hind limb length suggests that the effect on these growth parameters is not related to an endocrine mechanism. Although the study started with pre-metamorphic larvae, which is not in accordance with the AMA guideline, it was considered a Klimisch 1 study and included in the WoE analysis.
Three additional nonguideline studies for evaluating amphibian metamorphosis are also summarized and the reasons for exclusion enumerated. Marlatt et al. (Citation2013) reported on a study in the Pacific tree frog (Pseudacris regilla) in which premetamorphic (Gosner stage 26–28; approximately equal to NF stage 48–50) tadpoles were exposed to triclosan in water for 21 days at nominal concentrations of 0.3, 3.0, and 30 μg/L or a water control. The authors indicated that the methods employed were mainly consistent with OECD 231, although they acknowledged that they did not include thyroid histopathology or analytical confirmation of test concentrations, and the egg masses were collected from the environment and thus not taken from a standardized breeding culture. The study included a positive (T4) control that resulted in forelimb emergence in 50% of treated tadpoles by day 21. The study also included coexposures of triclosan and thyroxine (T4). In the coexposure experiments, mortality in the T4 control, as well as in the low- and mid-concentration triclosan treatments, was >10% by day 17. According to the guideline, mortality >10% in the control does not meet the specified performance criteria, and could mean the test system is compromised, so the relevance of the results of the coexposure experiments cannot be determined. While the Gosner staging scheme (Gosner Citation1960) is a well-established method, it is defined by a single external measure of development for the hind limb, thus precluding evaluation of asynchronous development, a rank 1 endpoint (Borgert et al. Citation2014). By comparison, the equivalent NF stages are characterized by no fewer than two external criteria involving both hind limb and forelimb, as well as length and multiple internal criteria. Although triclosan did not have an effect on developmental stage compared to control in this 21-d study, and had no consistent or concentration-related changes in body length, snout to vent length, or wet weight, the study was not included in the WoE analysis. While there was no mortality in the two highest triclosan exposure concentrations, there was >10% mortality in the controls by day 21. The effect on survival, along with the lack of analytical confirmation of concentration, and the developmental staging method employed in this nonvalidated model, that does not appear to define developmental milestones for tissues other than hind limb for the phases of development evaluated in the guideline, precludes its use in the WoE analysis.
Veldhoen et al. (Citation2006) describe a study in the North American bullfrog (Rana catesbeiana) in which premetamorphic (Gosner stage 31–33; approximately equal to NF stage 52) tadpoles were exposed to triclosan in water for four days at nominal concentrations of 0.3, 3.0, and 30 μg/L (measured concentrations at 48 h of 0.16, 0.89, and 11.2 μg/L) or a water control. On day 4, tadpoles then received either vehicle (NaOH) or T3 (3,3′-triiodo-L-thyronine) by injection into the peritoneal cavity at a volume of 1 μg/g body weight for a dose of 0.01 nM T3 through day 18. On day 6, only one replicate of animals was euthanized and the developmental stage determined and measured (snout/vent and tail length). On day 18, an additional single replicate of animals was euthanized and subject to the same measurements as on day 6. A significant decrease in body weight in the high dose triclosan exposure group relative to control at day 4 (prior to administration of T3) was observed, although this reduction in body weight was transient and not observed at later time points. Premetamorphic animals exposed to triclosan alone did not exhibit a change in development over the 18-day treatment period when compared to the control tadpoles. Premetamorphic animals treated with T3 alone did not appreciably advance in developmental stage over the 18 day period. However, animals exposed to a mixture of triclosan and T3 through day 18 exhibited statistically significant increases in developmental stage relative to T3 controls alone. The OECD detailed review paper on the amphibian metamorphosis assay (OECD Citation2004) discourages the use of R. catesbeiana as a test species due to the long length of development, although this alone does not preclude the use of these results in the WoE analysis. The lack of a positive control as well as the low number of replicates used complicates interpretation of the relevance of the findings, particularly with respect to co-exposure with T3, in this nonstandard model. This is compounded by the differences between the developmental staging technique by Gosner (Gosner Citation1960) and the Xenopus-specific technique described by Nieuwkoop and Faber (Nieuwkoop & Faber Citation1994) previously noted, as well as the fact that tadpoles, with and without T3, did not progress through metamorphosis during the study. Although there were no effects with triclosan exposure alone, except for a transient decrease in body weight at day 4, the lack of metamorphic progression as expected with the established guideline procedures preclude the use of this information in the WoE analysis.
The endpoints reported by Hinther et al. (Citation2011) are not comparable to in vitro and ex vivo assays under consideration by OECD for the identification of modulators of thyroid hormone signaling (OECD Citation2014). Hinther et al. (Citation2011) employed the cultured premetamorphic American bullfrog tadpole tail fin biopsy assay to assess the effects of triclosan on thyroid hormone signaling after a 48-h exposure. Biomarkers of cellular stress (heat shock protein 30 and catalase) were also evaluated in the 48-h exposure. Triclosan did not affect thyroid hormone responsive transcripts at concentrations from 290 ng/L to 290 μg/L (1–1000 nM). Triclosan exposure caused increases in both cellular stress biomarkers, but these responses did not follow a clear dose-response relationship (e.g. heat shock protein 30 increased at 0.3 μg/L and 2.9 μg/L triclosan but was not significantly different from the control at 29 μg/L). The relevance of these findings is unknown. This study is not included in the WoE evaluation.
Weight of evidence results
summarize the data used in the WoE analysis for each of the eight hypotheses.
Estrogen agonist hypothesis
Seven studies were found to report endpoints relevant to test the hypothesis that triclosan exhibits the potential to interact as an agonist with components of estrogen pathways.
An increase in uterine weight in an uterotrophic study and an induction of vitellogenin in male fish in a FSTRA are the two rank one endpoints for the estrogen agonist hypothesis (). There are three studies that essentially followed the FSTRA guidelines (OECD Citation2012b; USEPA Citation2009f), assessing the potential for triclosan to interact with the endocrine system of Japanese medaka and fathead minnow (Ishibashi et al. Citation2004; BASF Citation2012; Schultz et al. Citation2012). There was no statistically significant difference compared to controls in vitellogenin in the two studies with fathead minnow (BASF Citation2012; Schultz et al. Citation2012). However, there was a non-dose-dependent increase in vitellogenin in male medaka compared to controls in the study by Ishibashi et al. (Citation2004). The increase in vitellogenin was in the two lower concentrations (12.8 and 60.8 μg/L) but not in the highest concentration (136.9 μg/L). As it was not dose-responsive, this endpoint was considered of equivocal reliability. Comparing the concentrations across the studies, the Ishibashi et al. study was performed with a higher top concentration than the other two studies, however, the lowest concentration where vitellogenin was increased (12.8 μg/L) overlapped with the top concentration (13.5 μg/L) in the BASF (Citation2012) study. Secondary sexual characteristics as measured by nuptial tubercle number, a rank 2 endpoint, were only assessed in the two studies with fathead minnow since medaka do not visibly display this endpoint. Anal fin papillary processes are the secondary sex characteristic identified in the OECD 230 guideline for medaka, but they were not assessed in the Ishibashi et al. (Citation2004) study. No decrease in nuptial tubercles compared to controls was observed in either study. In addition, there was no change in gonadal histopathology or changes in male behavior noted (Schultz et al. Citation2012), both rank 2 endpoints. The only rank 3 FSTRA endpoint that was changed was female gonadosomatic index in one of two studies. However, in the study by Ishibashi et al. (Citation2004), the increase was not dose-responsive and a similar increase was noted in the male fish which is not what would be expected of an estrogen agonist. Reductions in gonadosomatic index in treated male and female fish, compared to controls, were noted in fathead minnows exposed for three weeks to the synthetic estrogen, 17α-ethinylestradiol (Pawlowski et al. Citation2004). No impacts on the other rank 3 endpoints of fecundity, fertility, or histopathology were noted in any of the studies where these endpoints were measured.
While uterine weight is a common endpoint in chronic rodent studies, the assessment of uterine weight in a uterotrophic study (OECD Citation2007; USEPA Citation2009k) is considered the rank 1 endpoint for a determination of the potential for estrogenic agonist activity. Studies by Louis et al. (Citation2013) and Stoker et al. (Citation2010) were identified that report on an evaluation of uterine weight following procedures similar to those described in the uterotrophic guidelines. In both studies, there was no statistically significant increase in uterine weight compared to the control group. Also reported in Stoker et al. (Citation2010), there was an increase in uterine weight in a female pubertal rat study. Uterine weight in cycling adult female rodents, as would be the case in a pubertal study, is highly variable (Stoker & Zorrilla Citation2010) so it is only considered a rank 3 corroborative endpoint.
Of the nine endpoints that are considered to be sensitive and specific enough to rate a rank 2 designation, a decreased age at vaginal opening in female rats was the only statistically significant response relevant for the estrogen agonist hypothesis (). Vaginal opening is an estrogen-dependent process occurring at the onset of puberty. This endpoint is sensitive to body weight at weaning (Goldman et al. Citation2000); hence the emphasis in OPPTS 890.1450 to ensure that all groups have similar mean body weight and variances and that littermates are not placed in the same group where possible. Stoker et al. (Citation2010) describe a decreased age at vaginal opening in female Wistar rats at 150 mg/kg (but not at 9.4, 37.5, or 75 mg/kg) triclosan. Body weight was not measured at vaginal opening but there were no changes in body weight at post-natal day 30. This endpoint was designated of equivocal reliability due to the lack of body weight data at the time of vaginal opening and because the difference in time to vaginal opening was only two days between control and treated animals (O’Connor et al. Citation2002). Vaginal opening will vary depending on organ/body weights so it has been recommended that only changes greater than two days be considered compound related (Marty et al. Citation1999).
As shown in , the estrogen agonist hypothesis includes 27 endpoints and data for 23 of them were identified from studies with triclosan. Only two endpoints of equivocal reliability support the estrogen agonist hypothesis: an increase in VTG in one of three fish studies from rank 1 and a decrease in age of vaginal opening in a female pubertal study from rank 2 (). Two endpoints in rank 3 could be corroborative, but they were also of equivocal reliability. These responses were not supported by more mechanistic endpoints of ER transcriptional activity or an increase in uterine weight, both hallmarks of estrogen agonism, or the results from the ToxCast screening of triclosan (). Thus, the WoE analysis evaluating endpoints relevant for estrogen pathway interaction does not support the hypothesis that triclosan exhibits the potential to interact as an agonist with components of the estrogen pathway.
Estrogen antagonist hypothesis
As described in Borgert et al. (Citation2014), none of the endpoints in the current USEPA EDSP Tier 1 assays are considered sufficiently sensitive and specific to assign them rank 1 status for testing the estrogen antagonist hypothesis. However, it was noted that the antagonist mode of the uterotrophic study could be sufficiently specific and interpretable to be considered a rank 1 endpoint once validated. Both available uterotrophic studies did include a coadministration with ethinyl estradiol to assess antagonistic properties (Stoker et al. Citation2010; Louis et al. Citation2013). While triclosan alone did not impact uterine weight, triclosan in combination with ethinyl estradiol increased uterine weight above that observed with ethinyl estradiol alone. An estrogen antagonist would be expected to reduce the response of the uterus observed after exposure to a known estrogenic compound (Ashby et al. Citation2002). While there are a number of mechanisms, both endocrine and nonendocrine that could result in the observed response, the relative increase in uterine weight observed after co-administration of ethinyl estradiol and triclosan, rather than the expected decrease, demonstrates that triclosan is not acting as an estrogen antagonist in this test system.
In the FSTRA studies, female vitellogenin was not decreased in any of the three studies, gonad histopathology was unchanged, fecundity and fertilization success were not impacted and steroid hormone measurements were comparable to controls, as mentioned previously (). Given that the findings in the study by Ishibashi et al. (Citation2004) is a rank 3 corroborative endpoint and no changes in other fish endpoints were noted, the observed change in both male and female gonadosomatic index compared to the controls is not supportive of the antagonist hypothesis.
While uterine weights were not included in the Borgert et al. (Citation2014) framework, uterine weights, along with ovary weights as noted in , would be expected to decrease with exposure to estrogen antagonists (Ashby et al. Citation2002). However, both uterine and ovarian weights can be variable in cycling rodents (Stoker & Zorrilla Citation2010) making these rank 3 corroborative endpoints. Ovary weights were unchanged, but uterine weights were increased in the study by Stoker et al. (Citation2010) (). The increase in uterine weight, as opposed to a decrease in weight, does not agree with the expected directionality of the response for an estrogen antagonist so is not supportive of the hypothesis.
Thus, with respect to the estrogen antagonist hypothesis and the expected directionality of the responses for these endpoints with an estrogen antagonist, the WoE analysis does not support the potential for triclosan to interact as an antagonist with the estrogen pathway.
Androgen agonist hypothesis
For the androgen agonist hypothesis, accessory sex organ weights in a Hershberger study and secondary sex characteristics in fish are the rank 1 endpoints. No studies were identified that met the requirements of a Hershberger study; however, some of the same accessory sex organ weights were assessed in a male pubertal rodent study (Zorrilla et al. Citation2009). No changes in any endpoints relevant for the androgen agonist hypothesis in the pubertal male rat were observed ().
Female fish do not normally express male specific tubercles, a secondary sex characteristic common in male cyprinid fish such as fathead minnow. In the presence of androgens or androgen-like compounds, female fathead minnow can develop such tubercles making this a sensitive and specific endpoint for androgen exposure (Ankley et al. Citation2001). One of the three fish studies evaluated tubercles and found no change compared to controls in the female fish (BASF Citation2012) (). There was also no change in female vitellogenin or gonad histopathology, both rank 2 endpoints but with similar sensitivity to androgens (Pawlowski et al. Citation2004).
Since there were no ranks 1 or 2 endpoints that were impacted, the rank 3 responses are not considered informative (Borgert et al. Citation2014). However, there were three rank 3 endpoints that resulted in a statistically different result from controls () and they are discussed here for the sake of transparency. The first was a decreased age at vaginal opening in a female rat pubertal study (Stoker et al. Citation2010). The decrease in age was only 2 days different from the controls and within the normal age for the species. There was also an increase in uterine weight in the same study, although the stage of estrous at necropsy was not noted and uterine weights in cycling female rodents are extremely variable. The third was a nondose responsive increase in gonadosomatic index in one of two studies in both male and female fish (Ishibashi et al. Citation2004).
The androgen agonist hypothesis includes 32 endpoints and there was data available with triclosan for 22 endpoints. Notably, of the 15 ranks 1 and 2 endpoints, data specific to triclosan were located for 11, none of which support the androgen agonist hypothesis. In addition, while 7 of the 11 AR assays in ToxCast were positive, the AC50s were all above the lower bound cytotoxicity limit where general cytotoxic responses could occur (). Thus, the weight of the evidence does not support the hypothesis that triclosan exhibits the potential to interact as an agonist with components of androgen pathways.
Androgen antagonist hypothesis
Ranks 1 and 2 endpoints for the mammalian in vivo studies in the androgen antagonist hypothesis are the same for the androgen agonist hypothesis (). There was no Hershberger study available with triclosan so the rank 1 endpoint is not fulfilled. Zorrilla et al. (Citation2009) did measure a number of the androgen-dependent organ weights in a pubertal study with triclosan. Data were available for 8 of the 9 rank 2 pubertal endpoints and there were no changes compared to controls in any of them (). There were also no effects on female vitellogenin, secondary sex characteristics or gonad histopathology, all rank 2 endpoints in fish.
Responses in rank 3 corroborative endpoints are not relevant when there are no endpoint responses in ranks 1 or 2, thus the observed change in both male and female gonadosomatic index compared to the controls in the study by Ishibashi et al. (Citation2004) () is not supportive of the antagonist hypothesis.
As shown in , the androgen antagonist hypothesis includes 23 endpoints; data specific to triclosan were available for 16 endpoints. No ranks 1 or 2 endpoints support the androgen antagonist hypothesis. Thus, the weight of the evidence does not support the hypothesis that triclosan exhibits the potential to interact as an antagonist with components of the androgen pathway.
Thyroid agonist hypothesis
Rank 1 endpoints for the thyroid agonist hypothesis are asynchronous development and thyroid histopathology evaluated in the amphibian metamorphosis assay. Similarly, the rank 2 amphibian endpoints are an increase in development stage and hindlimb length. All of the ranks 1 and 2 endpoints for amphibians were unchanged compared to controls in the study by Fort et al. (Citation2010) and Fort et al. (Citation2011) ().
The results summarized in identify the rank 2 endpoint of changes in thyroid weight in male rats, and serum T4 and TSH in male and female rats in the pubertal assay. Zorrilla et al. (Citation2009) and Stoker et al. (Citation2010) both report a statistically significant dose-related decrease in serum T4 in rats in studies similar to the pubertal rat guideline. However, there was no change in thyroid weight or TSH noted in the studies.
As previously noted, rank 3 endpoints are only corroborative of ranks 1 and 2 endpoints. This is because rank 3 endpoints are easily confounded by general toxicity and are not necessarily specific for a particular mode of action. There was one rank 3 endpoint that resulted in a statistically different result from controls. Stoker et al. (Citation2010) reported a decreased age at vaginal opening in a female rat pubertal study. The decrease in age was only 2 days different from the controls and within the normal range for the species. O’Connor et al. (Citation2002) suggest that only alternations of greater than 2 days be considered to potentially be compound related. While snout-vent length and wet weight were increased in the study by Fort et al. (Citation2011), a thyroid agonist would be expected to decrease these parameters if it was impacting metamorphosis (Borgert et al. Citation2014), so they are not included as positive endpoint responses in .
Considering the WoE framework, the lack of any effect on rank 1 endpoints and the change in T4 with no accompanying change in TSH or thyroid weight suggests that the effect is not likely caused by a thyroid agonist interaction.
Thyroid antagonist hypothesis
Thyroid weight and histopathology in male and female rodents and asynchronous development in metamorphosis and thyroid histopathology in amphibians are rank 1 endpoints for the thyroid antagonist hypothesis. In the studies by Fort et al. (Citation2010, Citation2011) synchronicity of development and thyroid histopathology in X. laevis were unchanged compared to controls (). In male rodents, thyroid histopathology in the study by Zorrilla et al. (Citation2009) revealed no significant change in follicular epithelial cell height in any triclosan-exposed group, but a significant decrease in colloid area in the thyroid gland at 300 mg/kg only was noted.
A number of parameters in the male and female pubertal rat studies are included in rank 2 for this hypothesis. In male rodents, liver weight, serum T4, and TSH are identified as rank 2 endpoints, while in females age and weight at vaginal opening, ovary weight, as well as serum T4 and TSH are informative for the hypothesis. Serum T4 was decreased in both male and female rodents while serum TSH was not changed compared to controls (). There was also no change compared to controls in ovary weight in females. Liver weight in male rodents was increased in triclosan-exposed animals in a dose-related manner. The results in identify equivocal data for the endpoint of changes in age and weight at vaginal opening in the study by Stoker et al. (Citation2010). In the Stoker et al. study, age at vaginal opening was reduced compared to controls, although body weight at that time point was not reported. As was discussed previously, the age at vaginal opening observed in this study is within the normal historical control range for this species and there was only a 2-day difference in age which has been suggested is likely not compound related (O'Connor et al. Citation2002). Data were available for 8 of the 9 rank 3 endpoints and none were identified as supporting the thyroid antagonist hypothesis.
As shown in , the thyroid antagonist hypothesis includes 22 endpoints and data specific for triclosan were identified for 18 of them. Amphibian development and thyroid histopathology were not changed by exposure to triclosan. The rank 1 change in thyroid histopathology in the male rodent study was considered of uncertain relevance, as only colloid area was reduced and thyroid weights were not assessed. Colloid depletion absent follicular hypertrophy and hyperplasia should be evaluated cautiously as tissue processing and staining must be carefully controlled to prevent errors in interpretation (O’Connor et al. Citation1999). The increase in liver weight with the decrease in serum T4 and no change in TSH suggests that alternative mechanisms of action, including an increase in metabolic clearance of T4 could be occurring (Borgert et al. Citation2014). No rank 3 corroborative endpoints were different from controls. In summary, the WoE analysis does not support the thyroid antagonist hypothesis.
Steroidogenesis induction hypothesis
The steroidogenesis hypothesis targets the synthesis of steroids in the estrogen and androgen pathways. To address the possibility that triclosan has the potential to induce steroidogenesis, two studies were found that report endpoints relevant to this hypothesis. No endpoints were considered sufficiently sensitive, specific, and interpretable to be considered as rank 1 for this hypothesis (Borgert et al. Citation2014) (). The two steroid hormone endpoints, testosterone and estradiol, measured in the in vitro steroidogenesis study (USEPA Citation2009j) are considered rank 2. Forgacs et al. (Citation2012) reported that triclosan exposure from 0.1 to 30 μM did not alter basal testosterone production. No studies that measured estradiol concentrations were identified. Serum testosterone levels in the male pubertal rat study are considered a rank 3 endpoint. Zorrilla et al. (Citation2009) reported that serum testosterone was unchanged in triclosan-exposed pubertal rats compared to controls. Recently, the OECD guideline 456 steroidogenesis study (OECD Citation2011) was modified for use as a high-throughput assay to evaluate the effects of chemicals on the steroidogenic pathway by measuring a suite of 13 hormones (Karmaus et al. Citation2016). Triclosan did not impact any of the hormones in this high-throughput assay. While the steroidogenesis induction hypothesis does not have as many endpoints as some of the other hypotheses discussed, the lack of response after exposure to triclosan in the two relevant studies, along with the lack of response in the high-throughput assay, does suggests that triclosan is not interacting as an agonist of the steroidogenic pathway.
Steroidogenesis inhibition hypothesis
For the steroidogenesis inhibition hypothesis, a reduction in uterine weight in the rat pubertal female study was considered a rank 1 endpoint. Uterine weights would be expected to be reduced by a potent aromatase inhibitor, although the endpoint’s sensitivity to a weak inhibitor is likely not as diagnostic (Marty et al. Citation1999). There was one study with triclosan that evaluated uterine weight in a pubertal female assay (Stoker et al. Citation2010). In this study, uterine weights were increased in the highest dose tested, relative to the controls, which is inconsistent with the expected directionality of the response for an inhibitor of steroidogenesis, so it is listed as no effect in . Of the five endpoints identified as rank 2, there were four studies that tested relevant endpoints. There were no responses in the rank 2 endpoints that supported a steroidogenesis antagonist hypothesis (). Rank 3 endpoints are only corroborative of ranks 1 and 2 endpoints (Borgert et al. Citation2014) since they are easily confounded by general toxicity and are not necessarily specific for a particular mode of action. While there were no ranks 1 or 2 endpoints that supported the hypothesis, for completeness the one rank 3 endpoint that resulted in a statistically different result from controls is reported. Gonadosomatic index was increased in one of two studies in both male and female fish (Ishibashi et al. Citation2004; BASF Citation2012). However, in the study by Ishibashi et al. (Citation2004) the increase was not dose-responsive and the noted increase occurred in both males and females. While steroidogenesis inhibitors have been shown to increase the gonadosomatic index in male fish, in female fish there is either no response or a decrease in gonad size relative to the body weight (Ankley et al. Citation2007), contrary to what was observed in the Ishibashi study. In line with the majority of the responses for this hypothesis, the aromatase activity noted in the ToxCast evaluation occurs well above the cytotoxicity limit suggesting that the response is nonspecific ().
As shown in , the steroidogenesis antagonist hypothesis includes 15 endpoints and data specific to triclosan were located for 13 of those endpoints. Using the available data, no ranks 1 or 2 endpoints support the steroidogenesis antagonist hypothesis, thus triclosan does not exhibit the potential to interact as an antagonist of the steroidogenic pathway.
Discussion
Criteria for identifying and potentially categorizing endocrine disruptors are the topic of much discussion internationally, although there is general agreement that the evaluation of endocrine disruption should be based on the World Health Organization/International Programme on Chemical Safety definition (WHO/IPCS Citation2002). This definition states: “An endocrine disruptor is an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse effects in an intact organism, or its progeny, or (sub)populations.” This definition embodies the key elements of adversity, endocrine mode of action, and a causal link between endocrine activity and adverse effect. Employing a WoE process in the evaluation of available data supports the development of lines of evidence that can lead to an understanding of the linkages between adverse effect and endocrine mode of action. When there are no strong connections in the lines of evidence or there is a lack of consistency across endpoints it is important to consider the data underpinning the assessment to determine if the findings of adversity or the strength of association with an endocrine mode of action are in question.
For example, there are three Tier 1 type fish studies with triclosan. Two of the three had no change in vitellogenin (BASF Citation2012; Schultz et al. Citation2012), which is a sensitive and specific response, particularly in male fish to an estrogen (Ankley et al. Citation2001). However, in the third study there was an increase in vitellogenin in males (Ishibashi et al. Citation2004), but only in the two lower concentrations (12.8 and 60.8 μg/L). Comparing the concentrations across the studies, Ishibashi et al. (Citation2004) was performed with a higher top concentration (136.9 μg/L) than the other two studies (0.45 μg/L and 13.5 μg/L), however, the concentrations where vitellogenin was increased were comparable to the highest concentration in the BASF (Citation2012) study (13.5 μg/L), suggesting a lack of consistency across studies. While the species were different in two of the three studies, there is no data to suggest that one species is more sensitive than the other with respect to estrogenic responses. Finally, in a WoE framework, supporting evidence both within and across studies should be evaluated to determine if there are other suggestions of estrogenic activity. While there was a nondose responsive increase in female gonadosomatic index, a rank 3 endpoint, there was a similar increase in the male fish which would not be the expected response in a male fish to an estrogen agonist, suggesting that the response of this endpoint is of equivocal reliability for this hypothesis. As a decrease in gonadosomatic index in both male and female fish was observed in a study with 17α-ethinylestradiol (Pawlowski et al. Citation2004), it is clear that a robust WoE evaluation is needed to assign a specific mode of action to observed responses. For example, fertility and fecundity, as well as secondary sexual characteristics, were also evaluated in these studies. None of these other endpoints were impacted in any of the three studies, again suggesting that the empirical evidence is very weak in support of the hypothesis.
One endpoint in support of a number of the hypotheses, but most importantly the estrogen agonist hypothesis discussed above, is the decrease in age at onset of vaginal opening in female rats at the highest dose in a study generally consistent with guideline methods (Stoker et al. Citation2010). However, this endpoint was considered to be of equivocal reliability because, although the authors detected a statistically significant difference between control and triclosan-treated female rats, the age at vaginal opening observed in this study is within the normal historical control range for this species and there is only a 2-day difference in age. O’Connor et al. (Citation2002) suggest that only alternations of greater than 2 days be considered to potentially be compound related because of the variability inherent in the endpoint.
The statistically significant dose-related decrease in serum T4 in male rats in a pubertal study by Zorrilla et al. (Citation2009) and in female rats in a pubertal study reported by Stoker et al. (Citation2010) is further supported by the findings of Crofton et al. (Citation2007) in a short-term (4-day) exposure of pubertal female rats. Serum T4 was also reduced in the study by Paul et al. (Citation2012), although, as in other studies the hypothyroxemia noted in triclosan-exposed animals was not associated with adverse growth or reproductive outcomes. Crofton et al. (Citation2007) assert that the observed effect on thyroid homeostasis is related to the increase in clearance mechanisms, including the increases in sulfonation and glucuronidation activity reported by others and the known upregulation of cytochrome P450 2B isozymes.
With the lack of changes in thyroid weights or histopathology, caution must be employed when interpreting the changes in thyroid hormone levels as they are single point measurements and could be influenced by stress at necropsy, stage of estrous, decreased body weight or decreased body weight gain, or compromised nutritional status (Stump et al. Citation2014). The USEPA has indicated that the biological/toxicological significance of these changes will be interpreted using a WoE approach that includes the thyroid hormone and histopathology data from the amphibian metamorphosis assay (USEPA Citation2011). To that end, it is relevant to note that this perturbation of thyroid hormone clearance observed in rats is not observed in amphibians as reported in the study by Fort et al. (Citation2010). No significant differences compared to control in T4 expression in thyroid tissue or plasma or thyroid histopathology were observed in X. laevis tadpoles exposed to triclosan for 21 days. An identical result was reported by Fort et al. (Citation2011) upon exposure of premetamorphic (NF developmental stage 47) X. laevis tadpoles to triclosan for 32 days. In that study, there were no significant differences in T4 expression in thyroid tissue or in plasma T3 or T4 concentrations in triclosan-exposed tadpoles relative to control. Thyroid histopathology indicated increased mean thyroid area corresponding with increased overall tadpole growth, but did not indicate any changes of thyroid function or the onset of metamorphosis.
In humans, no changes in thyroid hormone levels were seen at doses of triclosan that are in the relevant range encountered from the use of consumer products. A study by Cullinan et al. (Citation2012) reported that the use of a triclosan-containing toothpaste by volunteers for 5 years did not affect thyroid function. A study by Allmyr et al. (Citation2009) complements the Cullinan study in that they found that an estimated exposure to 0.01 mg/kg/day of triclosan for 2 weeks in human volunteers (five men and seven women) resulted in no changes in thyroid function. Therefore, both short-term and long-term use of triclosan has been shown to not disrupt thyroid function in humans.
A cross-sectional study using data obtained from the 2007–2008 National Health and Nutrition Examination Survey (NHANES) by Koeppe et al. (Citation2013) found no correlation between urinary triclosan and serum endpoints of the thyroid system including free and total T3 and T4, thyroglobulin, and TSH in adults. The authors did report a modest positive association between urinary TCS levels and T3 in adolescents without changes in other thyroid parameters. However, the positive association was small and was acknowledged by the authors that it could have been due to residual confounding or chance. In addition, an elevation of T3 is not consistent with the animal data and does not have a plausible physiological explanation. Moreover, no other association between urinary triclosan and thyroid function endpoints was observed for adolescents or adults such as changes in TSH and T4 levels.
As indicated in the recently finalized guidance from the US Food and Drug Administration (USFDA Citation2015), changes in histopathology can be indicative of potential endocrine-related effects. In addition, changes in thyroid, detected histologically, were reported to appear to be less sensitive to confounders and may provide a better assessment of thyroid function than serum hormone concentrations (DeVito et al. Citation1999). Furthermore, other international guidelines for repeat-dose testing for chemicals with potential endocrine activity support that definitive identification of thyroid-active chemicals is considered more reliable by histopathological analysis rather than hormone levels (OECD Citation2008). In the study by Zorrilla et al. (Citation2009), there was no significant change in follicular epithelial height in any triclosan-exposed group, although there was a decrease in colloid area in the thyroid gland at the highest dose tested (300 mg/kg). A case study prepared by USEPA and presented to the Scientific Advisory Panel, July 30–August 1 2013, described a decrease in colloid area for compound J, a coded substance described as a triazole fungicide, as an effect “considered to be due to normal homeostatic response of the thyroid hormone system” (USEPA Citation2013). Numerous subchronic and chronic studies in various mammalian species (mice, rats, dogs, hamsters, and baboons) with histopathological evaluation of thyroid showed no evidence of any pathological lesion at various doses of triclosan ().
Table 9. Histopathological examination of thyroid in repeat dose studies with various species, duration, and route of exposure to triclosan.
In contrast to the responses observed in the rodent pubertal studies with triclosan (Zorrilla et al. Citation2009; Stoker et al. Citation2010), the expected response of a known thyroid antagonist, propylthiouracil, presents a consistent pattern of responses for comparison and context. In the male pubertal assay, propylthiouracil yielded a statistically significant decrease in body weight gain, testes, prostate, and seminal vesicle weights and serum T4 with significant increases in serum TSH, liver, thyroid, and epididymis weight, and age at preputial separation (Marty et al. Citation2001). In comparison, no changes in body weight, testes, prostate, seminal vesicle and epididymis weights, serum TSH, or age at preputial separation were observed with exposure to triclosan in the male pubertal study (Zorrilla et al. Citation2009). In the female pubertal assay, propylthiouracil was associated with a decrease in body weight gain, body weight at time of vaginal opening (with no change in age at vaginal opening) and increased ovarian and thyroid weights (Marty et al. Citation1999). Another study describes a delay in onset of vaginal opening on propylthiouracil-treated rats (O'Connor et al. Citation1999). Triclosan exposure resulted in no changes in body weight or ovarian weight. While body weight at the time of vaginal opening was not assessed, the change in age at vaginal opening was a decrease (but only by 2 days), not an increase, as was noted in the study by O'Connor et al. (Citation1999).
In summary, using the hypothesis-testing framework developed by Borgert et al. (Citation2014), endpoint responses from studies equivalent to Tier 1 of the USEPA EDSP or similar were ranked according to their diagnostic utility and specificity for indicating potential endocrine activity. While there are a few endpoints that show effects across the hypotheses tested (), using this data from multiple animal species and in vitro systems, this systematic and transparent WoE assessment indicated that triclosan is not acting as an agonist or antagonist within the estrogen, androgen, thyroid, or steroidogenic pathways.
Declaration of interest
The employment affiliation of the authors is as shown on the cover page. Dr Ellen Mihaich is the owner of Environmental and Regulatory Resources, a private consulting firm providing services to the private and public sectors. Her work on this paper was funded by the Colgate-Palmolive Company. Colgate-Palmolive formulates consumer products with triclosan. This review paper was prepared during the normal course of employment of the authors. The authors have sole responsibility for the writing and content of this paper.
Acknowledgements
The authors gratefully acknowledge the comments provided by six reviewers selected by the Editor and anonymous to the authors. The comments were helpful to the authors in revising the paper.
References
- Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DP, et al. 2008. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect. 116:1203–1210.
- Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. 2009. Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Basic Clin Pharmacol Toxicol. 105:339–344.
- Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. 2001. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environ Toxicol Chem. 20:1276–1290.
- Ankley GT, Jensen KM, Kahl MD, Makynen EA, Blake LS, Greene KJ, Johnson RD, Villeneuve DL. 2007. Ketoconazole in the fathead minnow (Pimephales promelas): reproductive toxicity and biological compensation. Env Toxicol Chem. 26:1214–1223.
- Ashby J, Owens W, Deghenghi R, Odum J. 2002. Concept evaluation: an assay for receptor-mediated and biochemical antiestrogens using pubertal rats. Reg Toxicol Pharmacol. 35:393–397.
- Axelstad M, Boberg J, Vinggaard AM, Christiansen S, Hass U. 2013. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem Toxicol. 59: 534–540.
- BASF. 2012. Robust study summary: evaluation of reproductive toxicity to fathead minnow (Pimephales promelas) using 21-d fish reproduction screen (EPA/600/R-01/067) [Internet]. [cited 2015 Dec 18]. In: ECHA 2015, Triclosan REACH dossier. Available from: http://echa.europa.eu/information-on-chemicals/registered-substances.
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Muss S, Ruchirawat M, Schlatter J, Seed J, Vickers C. 2008. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38:87–96.
- Borgert CJ, Mihaich EM, Ortego LS, Bentley KS, Holmes CM, Levine SL, Becker RA. 2011. Hypothesis-driven weight of evidence framework for evaluating data within the US EPA's Endocrine Disruptor Screening Program. Reg Toxicol Pharmacol. 61:185–191.
- Borgert CJ, Stuchal LD, Mihaich EM, Becker RA, Bentley KS, Brausch JM, Coady K, Geter DR, Gordon E, Guiney PD, et al. 2014. Relevance weighting of tier 1 endocrine screening endpoints by rank order. Birth Defects Res B Dev Reprod Toxicol. 101:90–113.
- Borgert CJ, Becker RA, Carlton BD, Hanson M, Kwiatkowski PL, Marty MS, McCarty LS, Quill TF, Solomon K, Van Der Kraak G, et al. 2016. Does GLP enhance the quality of toxicological evidence for regulatory decisions?. Toxicol Sci. 151:206–213.
- Browne P, Judson RS, Casey W, Kleinstreuer N, Thomas R. 2015. Screening chemicals for estrogen receptor bioactivity using a computational model. Environ Sci Technol. 49:8804–8814.
- Crofton KM, Paul KB, Devito MJ, Hedge JM. 2007. Short-term in vivo exposure to the water contaminant triclosan: evidence for disruption of thyroxine. Environ Toxicol Pharmacol. 24:194–197.
- Cullinan MP, Palmer JE, Carle AD, West MJ, Seymour GJ. 2012. Long term use of triclosan toothpaste and thyroid function. Sci Total Environ. 416:75–79.
- de Peyster A, Mihaich E. 2014. Hypothesis-driven weight of evidence analysis to determine potential endocrine activity of MTBE. Regul Toxicol Pharmacol. 69:348–370.
- DeVito M, Biegel L, Brouwer A, Brown S, Brucker-Davis F, Cheek AO, Christensen R, Colborn T, Cooke P, Crissman J, et al. 1999. Screening methods for thyroid hormone disruptors. Environ Health Perspect. 107:407–415.
- ECHA [Internet]. 2015. Triclosan REACH dossier. [cited 2016 Feb 15]. Available from: http://echa.europa.eu/information-on-chemicals/registered-substances.
- Fang JL, Stingley RL, Beland FA, Harrouk W, Lumpkins DL, Howard P. 2010. Occurrence, efficacy, metabolism, and toxicity of triclosan. J Environ Sci Health, Part C: Environ Carcin Ecotoxicol Rev. 28:147–171.
- Federle TW, Kaiser SK, Nuck BA. 2002. Fate and effects of triclosan in activated sludge. Environ Toxicol Chem. 21:1330–1337.
- Foran CM, Bennett ER, Benson WH. 2000. Developmental evaluation of a potential non- steroidal estrogen: triclosan. Mar Environ Res. 50:153–156.
- Forgacs AL, Ding Q, Jaremba RG, Huhtaniemi IT, Rahman NA, Zacharewski TR. 2012. BLTK1 murine Leydig cells: a novel steroidogenic model for evaluating the effects of reproductive and developmental toxicants. Toxicol Sci. 127:391–402.
- Fort DJ, Rogers RL, Gorsuch JW, Navarro LT, Peter R, Plautz JR. 2010. Triclosan and anuran metamorphosis: no effect on thyroid-mediated metamorphosis in Xenopus laevis. Toxicol Sci. 113:392–400.
- Fort DJ, Mathis MB, Hanson W, Fort CE, Navarro LT, Peter R, Buche C, Unger S, Pawlowski S, Plautz JR. 2011. Triclosan and thyroid-mediated metamorphosis in anurans: differentiating growth effects from thyroid-driven metamorphosis in Xenopus laevis . Toxicol Sci. 121:292–302.
- Gee RH, Charles A, Taylor N, Darbre PD. 2008. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol. 28:78–91.
- Goldman JM, Laws SC, Balchak SK, Cooper RL, Kavlock RJ. 2000. Endocrine-disrupting chemicals: prepubertal exposures and effects on sexual maturation and thyroid activity in the female rat. A focus on the EDSTAC recommendations. Crit Rev Toxicol. 30:135–196.
- Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 16:183–190.
- Greenman J, McKenzie C, Nelson DG. 1997. Effects of triclosan and triclosan monophosphate on maximum specific growth rates, biomass and hydrolytic enzyme production of Streptococcus sanguis and Capnocytophaga gingivalis in continuous culture. J Antimicrob Chemoth. 40:659–666.
- Hinther A, Bromba CM, Wulff JE, Helbing CC. 2011. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol. 45:5395–5402.
- Honkisz E, Zieba-Przybylska D, Wojtowicz AK. 2012. The effect of triclosan on hormone secretion and viability of human choriocarcinoma JEG-3 cells. Reprod Toxicol. 34:385–392.
- Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K. 2004. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol. 67:167–179.
- James MO, Marth CJ, Rowland-Faux L. 2012. Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquat Toxicol. 124–125:72–82.
- Judson RS, Magpantay FM, Chickarmane V, Haskell C, Tania N, Taylor J, Xia M, Huang R, Rotroff DM, Filer DL, et al. 2015. Integrated model of chemical perturbations of a biological pathway using 18 in vitro high-throughput screening assays for the estrogen receptor. Toxicol Sci. 148:137–154.
- Jung EM, An BS, Choi KC, Jeung EB. 2012. Potential estrogenic activity of triclosan in the uterus of immature rats and rat pituitary GH3 cells. Toxicol Lett. 208:142–148.
- Karmaus AL, Toole CM, Filer DL, Lewis KC, Martin MT. 2016. High-throughput screening of chemical effects on steroidogenesis using H295R human adrenocortical carcinoma cells. Toxicol Sci. 150: 323–332.
- Klimisch HJ, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol. 25:1–5.
- Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. 2013. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007-2008. Sci Total Environ. 445–446:299–305.
- Kumar V, Balomajumder C, Roy P. 2008. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: probable mechanism of action. Toxicology. 250:124–131.
- Kumar V, Chakraborty A, Kural MR, Roy P. 2009. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod Toxicol. 27:177–185.
- Laws SC, Yavanhxay S, Cooper RL, Eldridge JC. 2006. Nature of the binding interaction for 50 structurally diverse chemicals with rat estrogen receptors. Toxicol Sci. 94:46–56.
- Louis GW, Hallinger DR, Stoker TE. 2013. The effect of triclosan on the uterotrophic response to extended doses of ethinyl estradiol in the weanling rat. Reprod Toxicol. 36:71–77.
- Lynch HN, Goodman JE, Tabony JA, Rhomberg LR. 2016. Systematic comparison of study quality criteria. Regul Toxicol Pharmacol. 76:187–198.
- Marlatt VL, Veldhoen N, Lo BP, Bakker D, Rehaume V, Vallee K, Haberl M, Shang D, van Aggelen GC, Skirrow RC, et al. 2013. Triclosan exposure alters postembryonic development in a Pacific tree frog (Pseudacris regilla) Amphibian Metamorphosis Assay (TREEMA). Aquat Toxicol. 126:85–94.
- Marty MS, Crissman JW, Carney EW. 1999. Evaluation of the EDSTAC female pubertal assay in CD rats using 17beta-estradiol, steroid biosynthesis inhibitors, and a thyroid inhibitor. Toxicol Sci. 52:269–277.
- Marty MS, Crissman JW, Carney EW. 2001. Evaluation of the male pubertal assay's ability to detect thyroid inhibitors and dopaminergic agents. Toxicol Sci. 60:63–76.
- Matsumura N, Ishibashi H, Hirano M, Nagao Y, Watanabe N, Shiratsuchi H, Kai T, Nishimura T, Kashiwagi A, Arizono K. 2005. Effects of nonylphenol and triclosan on production of plasma vitellogenin and testosterone in male South African clawed frogs (Xenopus laevis). Biol Pharm Bull. 28:1748–1751.
- McCarty LS, Borgert CJ, Mihaich EM. 2012. Information quality in regulatory decision making: peer review versus good laboratory practice. Environ Health Perspect. 120:927–934.
- Mitsui N, Tooi O, Kawahara A. 2003. Sandwich ELISAs for quantification of Xenopus laevis vitellogenin and albumin and their application to measurement of estradiol-17 beta effects on whole animals and primary-cultured hepatocytes. Comp Biochem Physiol, Toxicol Pharmacol. 135c:305–313.
- Nieuwkoop PD, Faber J. 1994. Normal table of Xenopus laevis (daudin). New York (NY): Garland Publishing Inc.
- O'Connor JC, Frame SR, Davis LG, Cook JC. 1999. Detection of thyroid toxicants in a tier I screening battery and alterations in thyroid endpoints over 28 days of exposure. Toxicol Sci. 51:54–70.
- O’Connor JC, Cook JC, Marty MS, Davis LG, Kaplan AM, Carney EW. 2002. Evaluation of Tier 1 screening approaches for detecting endocrine-active compounds (EACs). Crit Rev Toxicol. 32:521–549.
- OECD. 2004. Detailed review paper on amphibian metamorphosis assay for the detection of thyroid active substances (No. 46). ENV/JM/MONO(2004)17. Paris, France: OECD.
- OECD [Internet]. 2007. OECD 440: Uterotrophic bioassay in rodents: a short-term screening test for oestrogenic properties. [cited 2016 Jan 30]. Available from: http://www.oecd-ilibrary.org/environment/test-no-440-uterotrophic-bioassay-in-rodents_9789264067417-en.
- OECD [Internet]. 2008. OECD 407: Repeated dose 28-day oral toxicity study in rodents. [cited 2016 Feb 4]. Available from: http://www.oecd-ilibrary.org/docserver/download/9740701e.pdf?expires=1466203290&id=id&accname=guest&checksum=09DB9333B32C06647F3E126C9B5A1E93.
- OECD [Internet]. 2009a. OECD 231: The Amphibian Metamorphosis Assay. [cited 2016 Jan 30]. Available from: http://www.oecd-ilibrary.org/environment/test-no-231-amphibian-metamorphosis-assay_9789264076242-en.
- OECD [Internet]. 2009b. OECD 441: Hershberger Bioassay in Rats: A Short-term Screening Assay for Anti(Androgenic) Properties. [cited 2016 Feb 2]. Available from: http://www.oecd-ilibrary.org/environment/test-no-441-hershberger-bioassay-in-rats_9789264076334-en.
- OECD [Internet]. 2011. OECD 456: H295R Steroidogenesis Assay. [cited 2016 Feb 3]. Available at http://www.oecd-ilibrary.org/docserver/download/9745601e.pdf?expires=1477918612&id=id&accname=guest&checksum=9B7463B356CBFE6D9D348CDACCEDF2BE.
- OECD. 2012a. Guidance document on standardised test guidelines for evaluating chemicals for endocrine disruption, Series on Testing and Assessment No. 150. ENV/JM/MONO(2012)22. Paris, France: OECD.
- OECD [Internet]. 2012b. OECD 229: Fish short term reproduction assay. [cited 2016 Jan 20]. Available from: http://www.oecd-ilibrary.org/environment/test-no-229-fish-short-term-reproduction-assay_9789264076211-en.
- OECD. 2014. New scoping document on in vitro and ex vivo assays for the identification of modulators of thyroid hormone signalling, No. 207. ENV/JM/MONO(2014)23. Paris, France: OECD.
- OECD [Internet]. 2016. OECD 458: Stably transfected human androgen receptor transcriptional activation assay for detection of androgenic agonist and antagonist activity of chemicals. [cited 2016 Nov 10]. Available from: http://www.oecd-ilibrary.org/docserver/download/9716131e.pdf?expires=1477907747&id=id&accname=guest&checksum=F96B8209F22062030CF634201CBD65C4
- Paul KB, Hedge JM, DeVito MJ, Crofton KM. 2010. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in Young Long-Evans rats. Toxicol Sci. 113:367–379.
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, Crofton KM. 2012. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 9300:31–45.
- Pawlowski S, Sauer A, Shears JA, Tyler CR, Braunbeck T. 2004. Androgenic and estrogenic effects of the synthetic androgen 17α-methyltestosterone on sexual development and reproductive performance in the fathead minnow (Pimephales promelas) determined using the gonadal recrudescence assay. Aquat Toxicol. 68:277–291.
- Raut SA, Angus RA. 2010. Triclosan has endocrine-disrupting effects in male western mosquitofish, Gambusia affinis. Environ Toxicol Chem. 29:1287–1291.
- Rhomberg LR, Goodman JE, Bailey LA, Prueitt RL, Beck NB, Bevan C, Honeycutt M, Kaminski NE, Paoli G, Pottenger LH, et al. 2013. A survey of frameworks for best practices in weight-of-evidence analyses. Crit Rev Toxicol. 43:753–784.
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. 2010. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 40:422–484.
- Rodriguez PE, Sanchez MS. 2010. Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in Wistar rat offspring. J Toxicol Environ Health A. 73:1678–1688.
- SCCP [Internet]. 2009. Scientific Committee on Consumer Products: Opinion on Triclosan. [cited 2016 Mar 15]. Available from: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_166.pdf, European Commission.
- SCCS [Internet]. 2011. Scientific Committee on Consumer Safety Opinion on Triclosan: Addendum to the SCCP Opinion on Triclosan (SCCP/1192/08) from January 2009, 22 March 2011. [cited 2016 Mar 15]. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_054.pdf
- Schneider K, Schwarz M, Burkholder I, Kopp-Schneider A, Edler L, Kinsner-Ovaskainen A, Hartung T, Hoffmann S. 2009. “ToxRTool”, a new tool to assess the reliability of toxicological. Toxicol Lett. 189:138–144.
- Schultz MM, Bartell SE, Schoenfuss HL. 2012. Effects of triclosan and triclocarban, two ubiquitous environmental contaminants, on anatomy, physiology, and behavior of the fathead minnow (Pimephales promelas). Arch Environ Contam Toxicol. 63:114–124.
- Stoker TE, Zorrilla L. 2010. The effects of endocrine disrupting chemicals on pubertal development in the rat. In: Eldridge JC, Stevens JT, editors. Endocrine toxicology. 3rd ed. New York (NY): CRC Press. p. 27–81.
- Stoker TE, Gibson EK, Zorrilla LM. 2010. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol Sci. 117:45–53.
- Stoker TE, Hallinger DR, Louis G. 2014. The effects of triclosan on the male reproductive system of the rat. Poster 1713d Presented at the Society of Toxicology Annual Meeting. Phoenix, AZ.
- Stump DG, O'Connor JC, Lewis JM, Marty MS. 2014. Key lessons from performance of the U.S. EPA Endocrine Disruptor Screening Program (EDSP) Tier 1 male and female pubertal assays. Birth Defects Res, Part B, Develop Reprod Toxicol. 101:43–62.
- Tyler CR, van Aerle R, Hutchinson TH, Maddix S, Trip H. 1999. An in vivo testing system for endocrine disruptors in fish early life stages using induction of vitellogenin. Environ Toxicol Chem. 18:337–347.
- USEPA [Internet]. 1998. OPPTS 870.3800: reproduction and fertility effects. [cited 2016 Feb 2]. Available from: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/epa/epa_870_3800.pdf
- USEPA [Internet]. 2002. A short-term test method for assessing the reproductive toxicity of endocrine-disrupting chemicals using the fathead minnow (Pimephales promelas). EPA/600/R-01/067. [cited 2016 Nov 10]. Available from: https://nepis.epa.gov/Exe/tiff2png.cgi/30002HBA.PNG?-r+75+-g+7+D%3A%5CZYFILES%5CINDEX%20DATA%5C00THRU05%5CTIFF%5C00000374%5C30002HBA.TIF.
- USEPA [Internet]. 2007. Integrated summary report for validation of a test method for assessment of pubertal development and thyroid function in juvenile male rats as a potential screen in the endocrine disruptor screening program tier-1 battery. [cited 2016 Feb 20]. Available from: https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0012-0017.
- USEPA [Internet]. 2009a. OPPTS 890.1100: Amphibian Metamorphosis (Frog). [cited 2016 Jan 30]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009b. OPPTS 890.1150: Androgen Receptor Binding (Rat Prostate Cytosol). [cited 2016 Jan 3]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009c. OPPTS 890.1200: Aromatase (human recombinant) assay. [cited 2016 Jan 3]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009d. OPPTS 890.1250: Estrogen receptor binding assay using rat uterine cytosol (ER-RUC). [cited 2016 Jan 5]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009e. OPPTS 890.1300: Estrogen receptor transcriptional activation (Human Cell Line (HeLa-9903). [cited 2016 Jan 2]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009f. OPPTS 890.1350: Fish short-term reproduction assay. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009g. OPPTS 890.1400: Hershberger bioassay. [cited 2016 Feb 2]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009h. OPPTS 890.1450: Pubertal development and thyroid function in intact juvenile/peripubertal female rats. [cited 2016 Feb 5]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009i. OPPTS 890.1500: Pubertal development and thyroid function in intact juvenile/peripubertal male rats. [cited 2016 Feb 5]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009j. OPPTS 890.1550: Steroidogenesis (Human Cell Line –H295R). [cited 2016 Feb 3]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2009k. OPPTS 890.1600: Uterotrophic assay. EPA 740-C-09-0010. [cited 2016 Jan 30]. Available from: http://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program.
- USEPA [Internet]. 2011. Weight-of-evidence: evaluating results of EDSP tier 1 screening to identify the need for tier 2 testing. [cited 2016 Mar 1]. Available from: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2010-0877-0021.
- USEPA [Internet]. 2013. SAP Review of weight-of-evidence: evaluating results of EDSP Tier 1 Screening. [cited 2016 Nov 10]. Available from: http://www.regulations.gov/#!docketDetail;D=EPA-HQ-OPP-2013-0230.
- USFDA [Internet]. 2015. Guidance for industry. Nonclinical evaluation of endocrine-related drug toxicity. [cited 2016 Mar 10]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm369043.pdf.
- Veldhoen N, Skirrow RC, Ji L, Domanski D, Bonfield ER, Bailey CM, Helbing CC. 2006. Use of heterologous cDNA arrays and organ culture in the detection of thyroid hormone- dependent responses in a sentinel frog, Rana catesbeiana. Comp Biochem Physiol Part D: Genom Proteom. 1:187–199.
- Weed DL. 2005. Weight of evidence: a review of concept and methods. Risk Anal. 25:1545–1557.
- WHO/IPSC [Internet]. 2002. Global assessment of the state-of-the-science of endocrine disruptors. World Health Organization publication. WHO/PCS/EDC 02.2. [cited 2016 Jan 3]. Available from: http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/.
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE. 2009. The effects of triclosan on puberty and thyroid hormones in male Wistar rats. Toxicol Sci. 107:56–64.